SUMMARY
We estimated the true incidence of campylobacteriosis and salmonellosis in the European Union (EU) in 2009. The estimate was based on disease risks of returning Swedish travellers, averaged over the years 2005–2009, and anchored to a Dutch population-based study on incidence and aetiology of gastroenteritis. For the 27 EU member states the incidence of campylobacteriosis was about 9·2 (95% CI 2·8–23) million cases, while the incidence of salmonellosis was 6·2 (95% CI 1·0–19) million cases. Only 1/47 (95% CI 14–117) cases of campylobacteriosis and one 1/58 (95% CI 9–172) cases of salmonellosis were reported in the EU. The incidence rate of campylobacteriosis in EU member states varied between 30 and 13 500/100 000 population and was significantly correlated with the prevalence of Campylobacter spp. in broiler chickens. The incidence rate of salmonellosis in EU member states varied between 16 and 11 800/100 000 population and was significantly correlated with the prevalence of Salmonella Enteritidis in laying hens.
Key words: Campylobacter, gastroenteritis, infectious disease epidemiology, Salmonella, travellers' infection
INTRODUCTION
In 2009 there were 198 252 notified cases of campylobacteriosis and 108 614 notified cases of salmonellosis in the European Union (EU) [1]. The EU average incidence rate/100 000 population was 45·6 reported cases for campylobacteriosis and 23·7 reported cases for salmonellosis. It is widely accepted that case reporting is subject to considerable under-ascertainment and underreporting. This is because many cases with milder symptoms do not consult a physician and/or because the specific aetiology is not diagnosed [2]. There is little information on specific underreporting factors in different European countries. In order to support risk assessments of controlling zoonotic bacteria in the food chain, country-specific estimates of their true incidence are required.
Ekdahl & Andersson [3] and De Jong & Ekdahl [4] have published estimates on differential underreporting of campylobacteriosis and salmonellosis, based on the risk of illness in returning Swedish travellers for the period 1997–2003. Subsequently, EU countries have made considerable investments in Salmonella control programmes in different species of food animals (in particular poultry and pigs) and the reported incidence of salmonellosis has decreased significantly [1]. In contrast, efforts to control campylobacteriosis have been limited to some Nordic countries, and only addressed the broiler meat chain. While the reported incidence of salmonellosis has decreased in the EU, the reported incidence of campylobacteriosis is relatively stable [1]. Therefore, there is a need to update previous estimates of the incidence of both campylobacteriosis and salmonellosis in the EU. We present updated information on the risk for Swedish travellers in the EU, based on Swedish surveillance data from 2005 to 2009 and provide new incidence estimates for all EU member states (EU-27), by anchoring to a Dutch population-based study.
METHODS
Modelling approach
The true incidence (i.e. the number of new cases in a specified country in 2009) was estimated by combining several data sources, subject to a set of simplifying assumptions (see Discussion for more details). The risks in Swedish travellers were estimated by combining two databases, both containing data at the country level: reported cases of salmonellosis and campylobacteriosis in travellers returning from these countries and the number of journeys undertaken to these same countries, respectively. The true disease incidence rate (i.e. the number of incident cases in 2009 divided by the population at risk in each country) was then estimated by expressing all risks to Swedish travellers relative to the risk of travelling to The Netherlands, and multiplying this relative risk with the incidence rate from a population-based study from The Netherlands. The disease incidence was estimated by multiplying the incidence rate with the population in each country. Finally the underreporting factor per country was calculated as the ratio between the estimated true incidence and the reported incidence.
Disease risk in Swedish travellers
Diseases notifiable according to the Swedish Communicable Disease Act are reported to SmiNet, a computerized surveillance system used at the Swedish Institute for Communicable Disease Control since 1997. Information about a case, including diagnosis, age, sex and the probable country of infection is notified in the surveillance system by the treating physician and the causal pathogen is reported by the laboratory. The laboratory notification is automatically merged with the clinical notification in SmiNet with the help of a unique personal number given to every Swedish citizen at birth. Cases of campylobacteriosis and salmonellosis in Swedish travellers were enumerated in SmiNet and covered the years 2005–2009. Information on whether a case was travel-related or not, and the country of travel, was available for 91–97% of all reported cases with campylobacteriosis and 98–99% of all reported cases with salmonellosis. Records where the reported country of infection was either Sweden or unknown were excluded.
Travel data were obtained from the commercial Swedish Travel and Tourist Data Base (TDB, Resurs AB, Stockholm, Sweden); where 2000 randomly selected individuals are interviewed every month regarding their travel habits. The respondent information is weighted based on demographic information (age, sex, size of household and place of residence) and extrapolated to estimate the total number of journeys in the Swedish population by country. For more information on the survey methods and data processing see Ivarsson et al. [5]. Only journeys with at least one overnight stay in the country of destination were included in the model.
Disease incidence in The Netherlands
Estimates of the true incidence were anchored to population-based estimates from The Netherlands. These estimates were based on raw data from a Dutch population-based study [6], which estimated the incidence of gastroenteritis and an embedded case-control study to identify aetiological agents. The age-specific incidence rates of campylobacteriosis and salmonellosis from these studies were applied to the average population of 2005–2009 and scaled to the observed average of laboratory-confirmed cases [7] for these years in comparison to the year 1999 when the case-control study was performed. A full description of the simulation method is given in [8].
Disease incidence in the EU
For each EU member state, the risk of campylobacteriosis and salmonellosis for Swedish travellers to country C (STC) was calculated by dividing the number of reported cases among travellers to country C by the number of journeys to country C. Subsequently, all risks were expressed relative to the risk of travelling to The Netherlands, calculated as STC/STNL.
The estimated true incidence rate in all EU countries was then calculated as:
where IRC is the incidence rate/100 000 population of campylobacteriosis or salmonellosis in country C (The Netherlands for C = NL).
The incidence rates in Sweden cannot be calculated from this dataset, and were assumed to be the same as in Finland. The true incidence of campylobacteriosis or salmonellosis in a country was estimated by multiplying the incidence rate with the population size in 2009, as reported by Eurostat (http://epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS-QA-09-031/EN/KS-QA-09-031-EN.PDF). An underreporting factor (URF) was then calculated as the estimated true incidence (the product of the population size in a country and the incidence rate, IRC) divided by the reported incidence taken from [1]. To present the incidence rate on a map of the EU, shading density was based on the log scale of incidence rate values. Log values were classified in five equal interval classes (1–2, 2–3, 3–4, 4–5) and displayed using a colour ramp going from light (low values) to dark shades (high values). Labels showing the original values were used to indicate incidence rates for each country on the map.
Uncertainty analysis
Uncertainty in the disease risk in Swedish travellers was simulated by bootstrapping, assuming a Poisson process. Uncertainty in the Dutch age-specific incidence rate of gastroenteritis was simulated by gamma distributions, assuming a Poisson process. Uncertainty in the age-specific fraction of gastroenteritis cases attributed in The Netherlands to Campylobacter spp. or Salmonella spp. was simulated by beta distributions, assuming a binomial process [9]. Monte Carlo simulations were performed using @RISK 5.0 (Palisade Corporation, USA), an add-in to Microsoft Excel®. It should be noted that this approach ignores the uncertainty in the number of journeys. In the study conducted by Ekdahl & Andersson [3] uncertainty is estimated by a lognormal approximation, also taking sampling effects in the travel data into account. Such information was not currently available. Bootstrapping of data as reported previously [3] showed only slightly lower uncertainty margins for the bootstrap approach, therefore uncertainty in STC is most probably dominated by sampling effects in the case numbers (data not shown).
Evaluation
The risks to Swedish travellers in the period 2005–2009 were compared with those reported previously for the period 1997–2003 [3, 4] by linear regression analysis (intercept forced through the origin). Estimated true incidence rates of salmonellosis per country based on the 2005–2009 data were compared by regression to the incidence rates estimated by De Jong & Ekdahl [4] for the period 1997–2003. The campylobacteriosis incidence rate for each EU country was also compared by regression to the prevalence of Campylobacter on broiler carcasses using data from the EU-wide baseline survey conducted in 2008 [10, 11]. Bulgaria and Romania were deleted from the analysis because of high leverage. Salmonellosis incidence rates were also compared by regression to data on the Salmonella prevalence in laying hens in 2008 [12]. All regression analyses were performed in R 2.12.2 (R Foundation for Statistical Computing, Austria). Spatial clustering of dependent and independent variables, as well as in regression residuals was analysed by calculating Moran's I in ArcGis 9.2 (ESRI, USA).
RESULTS
Disease risk in Swedish travellers
A total of 7260 cases of campylobacteriosis and 3854 cases of salmonellosis with a history of foreign travel to any of the EU-27 countries (Sweden excluded) before disease onset were registered between 2005 and 2009; about 46 million journeys to the same countries were undertaken by Swedes (Table 1). The average number of journeys per year was 9·2 million, higher than reported for 1997–2003 (7·5 million). Nevertheless, the number of reported imported cases of campylobacteriosis decreased from 1786 to 1452 per year and from 1983 to 771 per year for salmonellosis. The average risk (per 100 000 journeys) of campylobacteriosis in Swedish travellers returning from the EU in 2005–2009 was 15·9 (95% CI 15·5–16·3), ranging from 0·40/100 000 for travellers returning from Finland to 182 for travellers returning from Bulgaria. For salmonellosis, the risk was 8·44 (95% CI 8·18–8·71), ranging from 0·13/100 000 for travellers returning from Finland to 94·3 for travellers returning from Bulgaria (Table 1).
Table 1.
Risks of campylobacteriosis and salmonellosis in returning Swedish travellers from EU-27, Norway and Switzerland, 2005–2009
| Country | Journeys (×100 000) | Campylobacteriosis | Salmonellosis | ||||
|---|---|---|---|---|---|---|---|
| Cases | Risk* | RR† | Cases | Risk | RR | ||
| Austria | 9·24 | 66 | 7·14 | 1·07 | 27 | 2·92 | 1·90 |
| Belgium | 6·20 | 47 | 7·58 | 1·14 | 5 | 0·81 | 0·52 |
| Bulgaria | 5·11 | 931 | 182 | 27·3 | 482 | 94·3 | 61·3 |
| Cyprus | 5·92 | 115 | 19·4 | 2·91 | 138 | 23·3 | 15·2 |
| Czech Republic | 6·52 | 193 | 29·6 | 4·44 | 151 | 23·1 | 15·1 |
| Denmark | 68·8 | 233 | 3·38 | 0·51 | 93 | 1·35 | 0·88 |
| Estonia | 11·3 | 25 | 2·20 | 0·33 | 30 | 2·64 | 1·72 |
| Finland | 76·7 | 31 | 0·40 | 0·06 | 10 | 0·13 | 0·08 |
| France | 26·4 | 604 | 22·9 | 3·44 | 63 | 2·39 | 1·55 |
| Germany | 51·8 | 236 | 4·56 | 0·68 | 155 | 2·99 | 1·95 |
| Greece | 23·4 | 601 | 25·7 | 3·86 | 823 | 35·2 | 22·9 |
| Hungary | 5·01 | 231 | 46·1 | 6·92 | 157 | 31·3 | 20·4 |
| Ireland | 3·08 | 50 | 16·2 | 2·43 | 1‡ | 0·32 | 0·21 |
| Italy | 26·7 | 211 | 7·91 | 1·19 | 106 | 3·97 | 2·58 |
| Latvia | 5·78 | 29 | 5·02 | 0·75 | 72 | 12·5 | 8·11 |
| Lithuania | 1·13 | 15 | 13·2 | 1·99 | 33 | 29·1 | 18·9 |
| Luxembourg | 0·84 | 5 | 5·92 | 0·89 | 1‡ | 1·18 | 0·77 |
| Malta | 1·57 | 61 | 38·8 | 5·82 | 84 | 53·4 | 34·7 |
| Poland | 9·07 | 470 | 51·8 | 7·78 | 185 | 20·4 | 13·3 |
| Portugal | 5·85 | 265 | 45·3 | 6·79 | 202 | 34·5 | 22·4 |
| Romania | 0·91 | 99 | 109 | 16·5 | 13 | 14·4 | 9·36 |
| Slovakia | 0·52 | 17 | 32·9 | 4·94 | 17 | 32·9 | 21·4 |
| Slovenia | 0·92 | 8 | 8·69 | 1·30 | 9 | 9·78 | 6·4 |
| Spain | 58·9 | 2430 | 41·3 | 6·19 | 948 | 16·1 | 10·5 |
| Sweden | n.a. | n.a. | n.a. | 0·06 | n.a. | n.a. | 0·08 |
| The Netherlands § | 7·80 | 52 | 6·66 | 1·00 | 12 | 1·54 | 1·00 |
| United Kingdom | 37·0 | 235 | 6·35 | 0·95 | 37 | 1·00 | 0·65 |
| EU-27 | 456·5 | 7260 | 159 | 2·39 | 3854 | 8·44 | 5·49 |
| Norway | 49·2 | 93 | 1·89 | 0·28 | 12 | 0·24 | 0·16 |
| Switzerland | 5·08 | 24 | 4·72 | 0·71 | 5 | 0·98 | 0·64 |
RR, Relative risk; n.a., not applicable.
Risk of campylobacteriosis and salmonellosis for Swedish travellers to country C (STC) per 100 000 journeys.
Risk relative to travelling to the Netherlands (STC/STNL).
No reported cases in Ireland and Luxembourg. For the model, one case was inserted.
Reference country.
Disease incidence in The Netherlands
In total, 81 300 (95% CI 24 600–202 000) cases of campylobacteriosis and 31 700 (95% CI 4990–95 200) cases of salmonellosis per annum were estimated to occur in The Netherlands in 2009, an incidence rate of 493 and 192/100 000 population, respectively.
Disease incidence in the EU
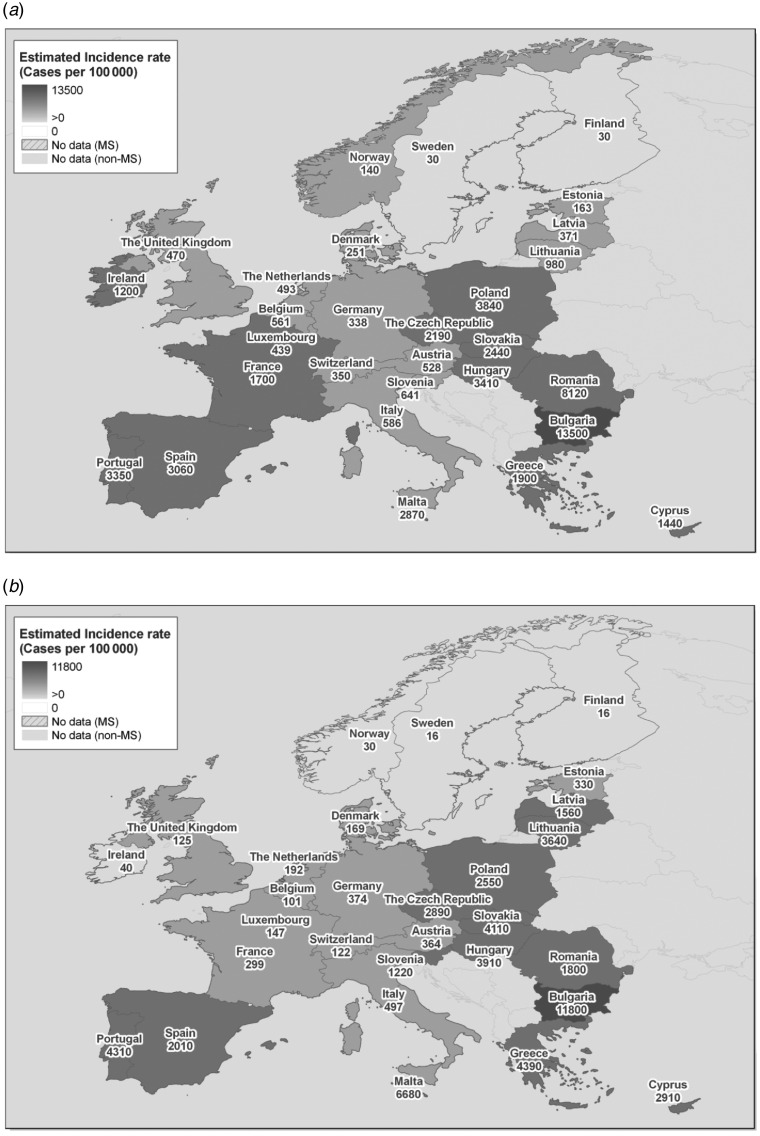
The incidence of campylobacteriosis in EU-27 in 2009 was about 9·25 (95% CI 2·79–23·1) million cases, and the incidence of salmonellosis in the same year was 6·25 (95% CI 0·98–18·7) million cases (Table 2). It should be noted the underreporting factor for campylobacteriosis in the EU as a whole was estimated at 46·7 (95% CI 14·1–117), but ranged from 0·4 for Finland and Sweden to almost 40 000 for Bulgaria. For salmonellosis, the underreporting factor was estimated at 57·5 (95% CI 9·0–172), but ranged from 0·4 for Finland to more than 2000 for Portugal. The incidence rates are visualized in Figure 1.
Table 2.
Estimated true incidence of human campylobacteriosis and salmonellosis in EU-27 (2005–2009), Norway and Switzerland and underreporting factors for 2009
| Country | Population 2009 (million) | Campylobacteriosis | Salmonellosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IRC* | True cases (×1000) | Reported cases | URF | IRC | True cases (×1000) | Reported cases | URF | ||
| Austria | 8·355 | 528 | 44·1 | 1516 | 29·1 | 364 | 30·5 | 2775 | 11·0 |
| Belgium | 10·667 | 561 | 59·8 | 5697 | 10·5 | 101 | 10·8 | 3113 | 3·5 |
| Bulgaria | 7·607 | 13 500 | 1030 | 26 | 39 400 | 11 800 | 896 | 1247 | 719 |
| Cyprus | 0·797 | 1440 | 11·5 | 37 | 310 | 2910 | 23·2 | 134 | 173 |
| Czech Republic | 10·468 | 2190 | 229 | 20 259 | 11·3 | 2890 | 303 | 10 480 | 28·9 |
| Denmark | 5·511 | 251 | 13·8 | 3353 | 4·1 | 169 | 9·3 | 2130 | 4·4 |
| Estonia | 1·340 | 163 | 2·2 | 170 | 12·8 | 330 | 4·4 | 261 | 17·0 |
| Finland | 5·326 | 29·9 | 1·6 | 4050 | 0·4 | 16·3 | 0·9 | 2329 | 0·4 |
| France | 64·351 | 1700 | 1090 | 3956 | 276 | 299 | 192 | 7153 | 26·9 |
| Germany | 82·002 | 338 | 277 | 62 331 | 4·4 | 374 | 307 | 31 395 | 9·8 |
| Greece | 11·260 | 1900 | 214 | No data | — | 4390 | 496 | 403 | 1230 |
| Hungary | 10·031 | 3410 | 342 | 6579 | 52·0 | 3910 | 392 | 5873 | 66·7 |
| Ireland | 4·450 | 1200 | 53·4 | 1810 | 29·5 | 40·4 | 1·8 | 335 | 5·4 |
| Italy | 60·045 | 586 | 352 | 531 | 662 | 497 | 298 | 4156 | 71·8 |
| Latvia | 2·261 | 371 | 8·4 | 0 | — | 1560 | 35·2 | 795 | 44·3 |
| Lithuania | 3·350 | 980 | 32·8 | 812 | 40·4 | 3640 | 122 | 2063 | 59·1 |
| Luxembourg | 0·494 | 439 | 2·2 | 551 | 3·9 | 147 | 0·7 | 162 | 4·5 |
| Malta | 0·414 | 2870 | 11·9 | 132 | 90·0 | 6680 | 27·6 | 124 | 223 |
| Poland | 38·136 | 3840 | 1460 | 357 | 4100 | 2550 | 973 | 8521 | 114 |
| Portugal | 10·627 | 3350 | 356 | No data | — | 4310 | 458 | 220 | 2080 |
| Romania | 21·499 | 8120 | 1740 | 254 | 6870 | 1800 | 386 | 1105 | 350 |
| Slovakia | 5·412 | 2440 | 132 | 3813 | 34·7 | 4110 | 222 | 4182 | 53·2 |
| Slovenia | 2·032 | 641 | 13·1 | 952 | 13·8 | 1220 | 24·8 | 616 | 40·3 |
| Spain | 45·828 | 3060 | 1400 | 5106 | 274 | 2010 | 922 | 4304† | 214 |
| Sweden | 9·256 | 29·9 | 2·8 | 7178 | 0·4 | 16·3 | 1·5 | 3054 | 0·5 |
| The Netherlands ¶ | 16·486 | 493 | 81·3 | 3739‡ | 21·7 | 192 | 31·6 | 1205§ | 26·3 |
| United Kingdom | 61·179 | 470 | 288 | 65 043 | 4·4 | 125 | 76·5 | 10 479 | 7·3 |
| EU-27 | 499·185 | 1860 | 9250 | 198 252 | 46·7 | 1260 | 6250 | 108 614 | 57·5 |
| Norway | 4·799 | 140 | 6·7 | 2848 | 2·4 | 30·5 | 1·5 | 1235 | 1·2 |
| Switzerland | 7·702 | 350 | 26·9 | 8154 | 3·3 | 122 | 9·4 | 1325 | 7·1 |
URF, Underreporting factor.
IRC = estimated true incidence rate per 100 000 population.
Surveillance system with coverage of: † 25%, ‡ 52%, § 64%.
Reference country.
Fig. 1.
[colour online]. Estimated true incidence rate of (a) human campylobacteriosis and (b) salmonellosis in EU-27, 2005–2009. Map shading is based on equal intervals on a log scale of incidence rate values.
Sensitivity analysis (Spearman's rank correlation) indicted that the uncertainty in the estimated disease incidence was most influenced by uncertainty in the Dutch case numbers. The uncertainty in the number of cases in returning Swedish travellers was important for countries with low case numbers.
Evaluation
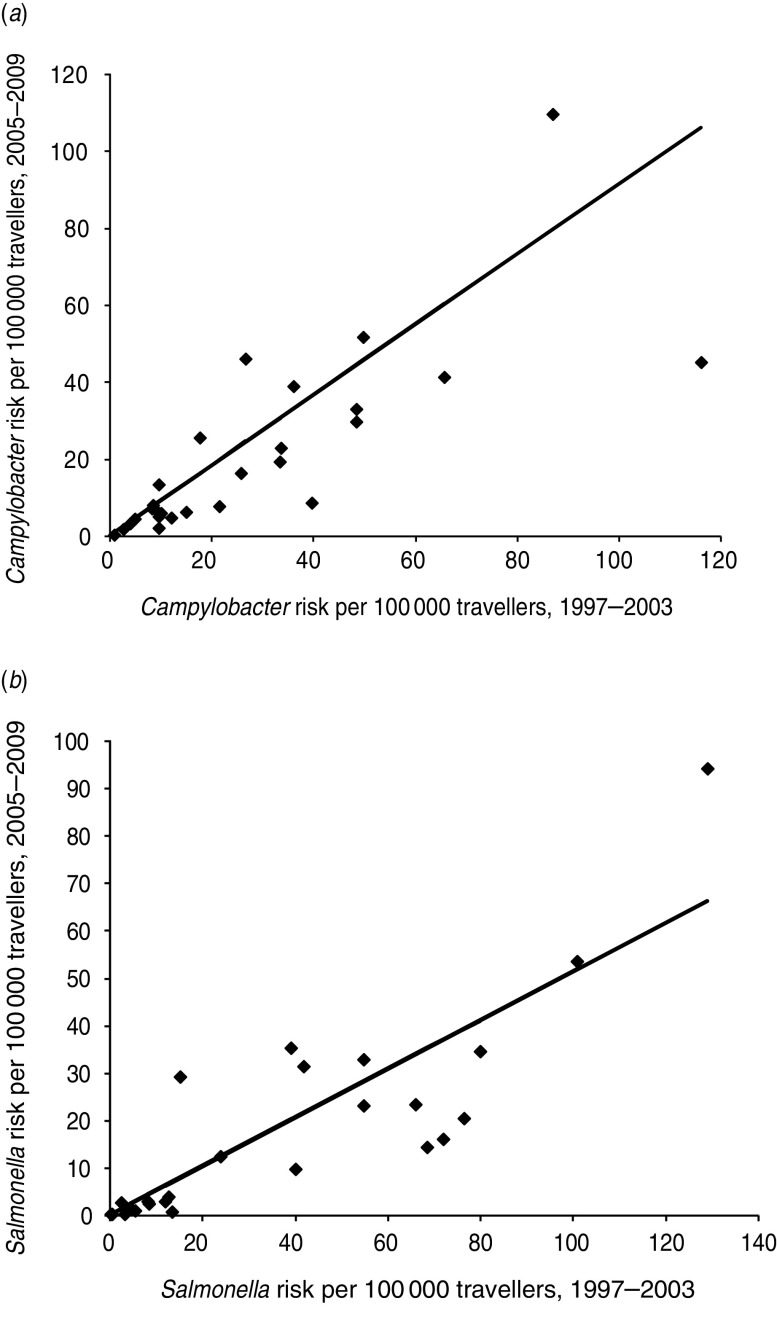
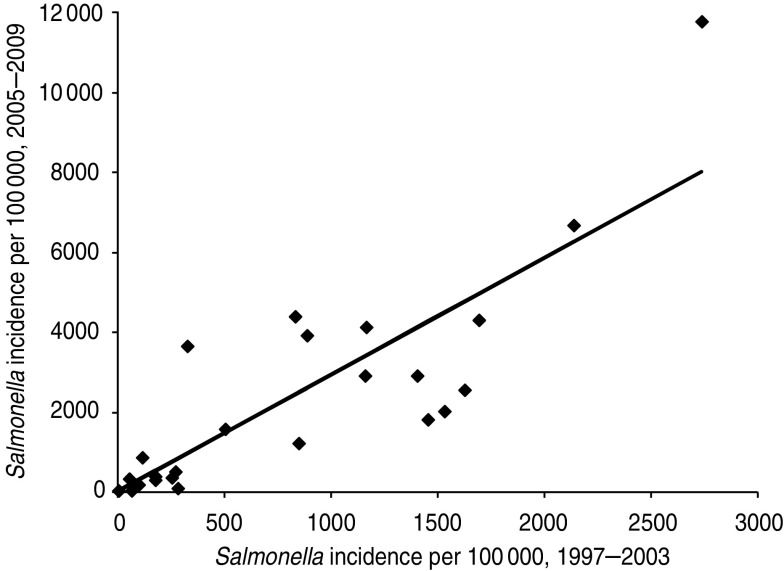
The risks to Swedish travellers in the period 2005–2009 were highly correlated (P≪0·001) with those reported previously for the period 1997–2003 for both Campylobacter spp. and Salmonella spp. (see Fig. 2). Estimates for campylobacteriosis in the 2005–2009 period were similar to those reported previously (regression coefficient 0·91), but for Salmonella spp., the risks in the 2005–2009 period were approximately half of those reported for 1997–2003 (regression coefficient 0·51). There was a significant correlation (r = 0·85, P≪0·001) between the salmonellosis incidence rate per country based on the 2005–2009 data and the estimates by De Jong & Ekdahl [4] for 1997–2003 (see Fig. 3). The average incidence rate for 2005–2009 was 2·3 times higher than for 1997–2003.
Fig. 2.
Comparison of (a) campylobacteriosis and (b) salmonellosis risks in Swedish travellers in two time periods. Each symbol (◆) represents one member state.
Fig. 3.
Comparison of estimated true incidence rate of human salmonellosis per 100 000 population in two time periods, according to two estimation methods. Each symbol (◆) represents one member state.
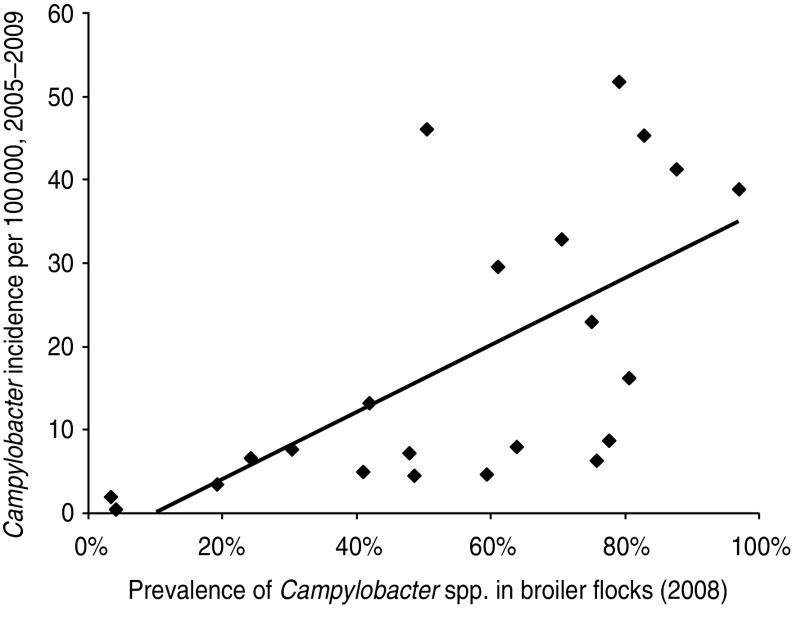
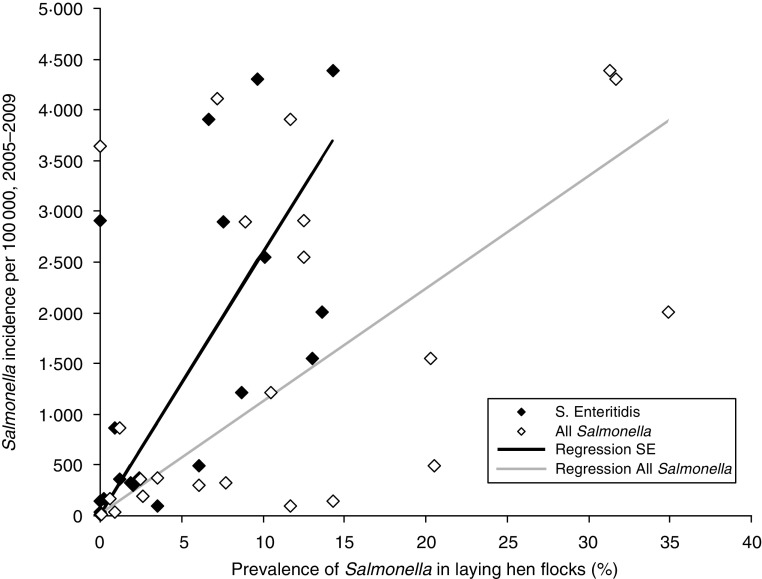
Although there was considerable scatter in the data, a significant correlation was found between the campylobacteriosis incidence rate per EU country and the prevalence of Campylobacter spp. on broiler chicken carcasses (r = 0·64, P = 0·001; Fig. 4). The data show that in countries with high prevalence of Campylobacter spp. on broiler carcasses, there is also a high risk of human campylobacteriosis, confirming that broiler meat is an important risk factor for campylobacteriosis. De Jong & Ekdahl demonstrated a strong correlation between their estimates of incidence rates of salmonellosis in EU member states and the prevalence of Salmonella spp. in laying hens as found in the EU-wide baseline survey [4]. Since the baseline study, EU-wide control programmes have been implemented, and have led to considerable decreases in the prevalence of Salmonella spp. in laying-hen flocks, even when the reduced sensitivity of the mandatory monitoring compared to the baseline study protocol is taken into account. The new disease incidence rates in humans were significantly correlated with Salmonella Enteritidis prevalence in laying hens in 2008 (P = 0·02) but not with the prevalence of all Salmonella serovars (P = 0·08) (see Fig. 5).
Fig. 4.
Comparison of estimated true incidence rate of human campylobacteriosis in the European Union member states with the prevalence of Campylobacter spp. on broiler chicken carcasses. Each symbol (◆) represents one member state.
Fig. 5.
Comparison of estimated true incidence rate of human salmonellosis per 100 000 population and prevalence of Salmonella spp. in flocks of laying hens in the European Union. Each symbol (◆) represents one member state.
There was significant spatial clustering (P = 0·02–0·001) in the risks of illness to Swedish travellers for both Campylobacter spp. and Salmonella spp. in both time periods and, as a consequence, in the estimated illness incidence rates in EU member states. Spatial clustering was not detected in any of the risk factors (P = 0·08–0·49) nor in any of the residuals of the regression analysis (P = 0·08–0·50). Further spatial analysis was therefore not undertaken.
DISCUSSION
The data presented above suggest a very high incidence of human campylobacteriosis and salmonellosis in the EU (each year, about one out of every 50 inhabitants will be affected by Campylobacter spp. and one out of every 80 by Salmonella spp.). The risk of salmonellosis to Swedish travellers in the EU has decreased by about 50% on average in the last decade, and is consistent with decreases in reported human salmonellosis and intensive control efforts in the food chain. Nevertheless, laying hens infected with S. Enteritidis continue to drive the epidemic of salmonellosis, particularly in high-incidence countries. No trend in risks of campylobacteriosis to Swedish travellers was observed and the importance of contaminated broiler meat as a risk factor was confirmed. Considerable public health benefits are expected by further controls of these zoonotic pathogens in the food chain.
The limitations and assumptions in the models need to be taken into consideration when interpreting these findings. The case data are extracted from the Swedish infectious disease surveillance system and rely on laboratories and physicians reporting diagnosed cases to the Swedish Institute for Communicable Disease Control. Clearly, only a fraction of all cases of illness will be reported. For this model, mainly the potential of differential reporting per country should be considered. Both cases and journeys are counted as such, without consideration of the duration of the stay abroad, the purpose of the visit (business or leisure). Day travels are excluded from the data. There are, for example, a very high number of journeys to Denmark and Finland, which may be mainly for business purposes and of short duration [13]. Hence, the duration of exposure may be shorter but on the other hand travellers who fall ill will most likely return to their home country and their illness is more likely to be reported in the Swedish public health system when seeking healthcare. On the other hand, trips to the Mediterranean area may be mainly for leisure purposes and last one or more weeks. Travellers may be exposed for longer time periods, but when ill may have recovered before returning home. It is difficult therefore to predict in which direction biases may occur. The estimated underreporting factor for Finland is <1, implying that there are fewer cases than actually reported, which is highly unlikely. This might indicate that for presumably short-term visits, the risks to travellers may be underestimated. Further biases may be introduced by seasonal travel patterns. It is likely that most journeys to the Mediterranean take place in summer, when the prevalence of Campylobacter in animals and food is highest. Health-seeking behaviour of travellers or medical decisions about stool cultures may be affected by the country of destination.
A second important assumption is that relative risks to Swedish travellers are predictive of risks for the local population. This assumption ignores any potential effects, e.g. of acquired immunity, and differences in eating habits between visitors and local residents, as well as differences between strains circulating in different parts of Europe. It is currently not possible to estimate the magnitude or even the direction of these biases.
We found that the average incidence rate of salmonellosis in 2005–2009 was 2·3 times higher than a previous estimate for 1997–2003. This does not imply that the incidence of salmonellosis has increased in recent years. The earlier estimates were arrived at by anchoring to the data reported by Norway, where no underreporting was assumed to occur. In contrast, the new estimates were anchored to a Dutch population-based survey, so the same average level of underreporting was not expected. Interestingly, the underreporting factor in Norway of 2·4 in Table 2 is very close to the previous estimate of 2·3.
The estimates for individual countries were found to correlate well with major risk factors in the food chain. Furthermore, for the UK, the underreporting factor for campylobacteriosis is estimated to be 4·4 (95% CI 1·3–11·0), and 7·3 (95% CI 1·1–22·4) for salmonellosis in this study. Independent estimates, based on the Infectious Intestinal Disease study carried out from 2008–2009 [14] were 9·3 (95% CI 6·0–14·4) and 4·7 (95% CI 1·2–18·2), respectively. The wide confidence bounds of both estimates overlap to a large degree. Even so, it may seem unexpected that the underreporting factor for salmonellosis is higher than for campylobacteriosis. In the UK, there has been an increase in the number of reported cases of campylobacteriosis, while the number of reported cases of salmonellosis has decreased [1].
ACKNOWLEDGEMENTS
This work was carried out during the preparation of two Opinions of the Panel on Biological Hazards of the European Food Safety Authority (EFSA, Parma, Italy), on ‘Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain’ (http://www.efsa.europa.eu/en/efsajournal/pub/2105.htm) and ‘Scientific Opinion on a quantitative estimation of the public health impact of setting a new target for the reduction of Salmonella in broilers’ (http://www.efsa.europa.eu/en/efsajournal/pub/2106.htm), respectively. The authors are grateful to the members of the ad-hoc working groups preparing these documents for discussions and feedback. EFSA provided financial support for obtaining travel data from Resurs AB, Stockholm, Sweden. The assistance of Francesca Riolo (Unit on Biological Monitoring, EFSA, Parma, Italy) in the preparation of Figure 1 is gratefully acknowledged. The authors thank Jeroen van Leuken (National Institute for Public Health and the Environment, Bilthoven, The Netherlands) for performing the spatial regression analyses and John Threlfall (Health Protection Agency, London, UK) for correction of English usage. They are grateful to the editor and three anonymous referees for constructive comments.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.European Food Safety Authority; European Centre for Disease Prevention and Control.Trends and sources of zoonoses and zoonotic agents in the European Union in 2009. EFSA Journal 2011; 9: 2090. [Google Scholar]
- 2.Borgdorff MW, Motarjemi Y.Surveillance of foodborne diseases: what are the options? Geneva: World Health Organization, 1997. Report No.: WHO/FSF/FOS/97.3. [PubMed] [Google Scholar]
- 3.Ekdahl K, Andersson Y. Regional risks and seasonality in travel-associated campylobacteriosis. BMC Infectious Diseases 2004; 4: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Jong B, Ekdahl K. The comparative burden of salmonellosis in the European Union member states, associated and candidate countries. BMC Public Health 2006; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivarsson S, Stålsmeden H, Löfdahl M.Unique Swedish travel data compared with surveillance data identifies trends in contracting food- and water-borne diseases abroad. Stockholm, Sweden: European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE), 6–8 November 2011.
- 6.De Wit MA, et al. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. American Journal of Epidemiology 2001; 154: 666–674. [DOI] [PubMed] [Google Scholar]
- 7.Van Pelt W, et al. Laboratory surveillance of bacterial gastroenteric pathogens in The Netherlands, 1991–2001. Epidemiology and Infection 2003; 130: 431–441. [PMC free article] [PubMed] [Google Scholar]
- 8.Havelaar AH, et al. Disease burden in the Netherlands due to infections with Shiga-toxin producing Escherichia coli O157. Epidemiology and Infection 2004; 132: 467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vose D. Quantitative risk analysis. In: A Guide to Monte Carlo Simulation Modelling. Chichester: John Wiley & Sons Ltd, 1996, pp. 171–173. [Google Scholar]
- 10.European Food Safety Authority. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of campylobacter and Salmonella on broiler carcasses in the EU, 2008 – Part A: Campylobacter and Salmonella prevalence estimates. EFSA Journal 2010; 8: 1503. [Google Scholar]
- 11.European Food Safety Authority. Analysis of the baseline survey on the prevalence of campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses, in the EU, 2008 – Part B: Analysis of factors associated with campylobacter colonisation of broiler batches and with campylobacter contamination of broiler carcasses; and investigation of the culture method diagnostic characteristics used to analyse broiler carcass samples. EFSA Journal 2010; 8: 1522. [Google Scholar]
- 12.European Food Safety Authority; European Centre for Disease Prevention and Control. The community summary report on trends and sources of zoonoses, zoonotic agents, antimicrobial resistance and foodborne outbreaks in the European Union in 2008. EFSA Journal 2010; 8: 1496. [Google Scholar]
- 13.Ekdahl K, Giesecke J. Travellers returning to Sweden as sentinels for comparative disease incidence in other European countries, Campylobacter and Giardia infection as examples. Eurosurveillance 2004; 9: 6–9. [PubMed] [Google Scholar]
- 14.Tam CC, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 2012; 61: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]