SUMMARY
Shigella flexneri 4a caused sustained outbreaks in a large long-stay psychiatric centre, Taiwan, 2001–2006. Trimethoprim-sulphamethoxazole (SXT) prophylaxis was administered in 2004. We recovered 108 S. flexneri 4a isolates from 83 symptomatic (including one caregiver) and 12 asymptomatic subjects (11 contacts, one caregiver). The isolates were classified into eight antibiogram types and 15 genotypes (six clusters) by using antimicrobial susceptibility testing and pulsed-field gel electrophoresis of NotI-digested DNA, respectively. These characteristics altered significantly after SXT prophylaxis (P < 0·05), with concomitant emergence of SXT-resistant isolates in two antibiogram types. P01 (n = 71), the predominant epidemic genotype, caused infection in two caregivers and five patients under their care; two P01 isolates were recovered from the same patient 6 months apart. These results indicate the importance of sustained person-to-person transmission of S. flexneri 4a by long-term convalescent, asymptomatic or caregiver carriers, and support the emergence of SXT-resistant strains following selective pressure by SXT prophylaxis.
Key words: Antibiotic resistance, infectious disease epidemiology, pulsed-field gel electrophoresis (PFGE), Shigella
INTRODUCTION
Shigellosis is the major cause of diarrhoea-related morbidity and mortality in developing countries. Worldwide, Shigella spp. cause 164·7 million illness episodes and 1·1 million deaths annually [1]. However, several recent studies have suggested that these figures are greatly underestimated [2, 3]. Shigella spp. are highly contagious due to their low infectious dose [4] and readily cause outbreaks in psychiatric institutions [5, 6]. In low- and medium-resource countries, S. flexneri has the highest isolation rate (60–68%), followed by S. sonnei (15–22%) [1, 7]. Of the 15 serotypes of S. flexneri, 2a is the most commonly detected serotype in Asia [1, 7]. In developed countries, most Shigella infections are caused by S. sonnei (71·7–77%), followed by S. flexneri (16–18·4%) [1, 8].
Effective antibiotic therapy reduces the duration of illness by reducing the excretion of the bacterium in the faeces, thus preventing further transmission and potentially lethal complications [9, 10]. Antimicrobial resistance in Shigella spp. limits the control of shigellosis [10–12]. Mobile genetic elements, including plasmids, transposons, and gene cassettes in integrons are important in the dissemination of multidrug resistance [13–16]. These reports [13–16] have highlighted one or more specific mechanisms, but probably do not represent the full complement of mechanisms that exist in nature.
In Taiwan, shigellosis is a notifiable disease, with an annual incidence of 0·6–5·8 cases/100 000 persons (1997–2006). The majority of Shigella isolates were of S. sonnei and S. flexneri [17]. S. dysenteriae and S. boydii were rare and isolated only from imported cases [18]. S. sonnei is responsible for most of the large shigellosis outbreaks in the developed community [19]. In contrast, S. flexneri circulates mainly among aboriginal tribes in the mountainous areas [20, 21], and was found in one instance in a psychiatric facility [6].
The Veteran Nursing Centre (VNC) is a large psychiatric institution in Eastern Taiwan. During 2001–2003, there were four outbreaks, involving 27 patients, and 11 sporadic cases with S. flexneri 1a or 4a [6]; 95% of the S. flexneri isolates were susceptible to trimethoprim-sulphamethoxazole (SXT) [6]. Thus, SXT was used internally for mass prophylaxis. To control nosocomial infections, the VNC also implemented several other control measures, including environmental surveillance, monitoring of water residual chlorine, investigation of contacts, stool culture screening of new incoming residents, and good hand hygiene practices. Despite all these measures, outbreaks occurred again in 2005 and 2006; these outbreaks were caused by both SXT-resistant as well as SXT-susceptible isolates. To investigate variations of Shigella strains isolated before and after SXT prophylaxis and their route of transmission, we characterized S. flexneri 4a isolates obtained from the VNC between 2001 and 2006 by using antimicrobial susceptibility testing (AST) and pulsed-field gel electrophoresis (PFGE).
METHODS
Setting
The VNC, accommodating more than 1600 institutionalized mentally disabled residents, is a semi-closed psychiatric institution located in Eastern Taiwan. Most of the long-term institutionalized residents were physically capable adults (age range 20–86 years) who showed inadequate or poor personal hygiene. The average duration of institutionalization is about 10 years. The VNC has two divisions (A and B). Division A comprises six chronic wards (A1, A2, A3, A4, A6, A16) and five acute wards (a1–a5). Division B provides occupational therapy and rehabilitation training for stable psychiatric residents. Wards for male and female residents are distinct, and person-to-person contact between residents from different wards is limited. For psychiatric treatment, residents might be transferred to different divisions and wards. A central kitchen provides meals for divisions A and B.
Epidemiological investigation and prophylaxis intervention
Investigation of contacts and source of infection was conducted by the staff of the Sixth Branch of Taiwan Centres for Disease Control (Taiwan CDC) and the Hualien County Health Bureau by using reporting forms of the infectious disease and reports of the internal epidemiological investigation, respectively. Stool samples of patients with shigellosis and of contacts or caregivers when epidemiologically indicated in the VNC were forwarded regularly to Taiwan CDC. In the prolonged outbreak of 2006, 147 new incoming residents and 72 food handlers were monitored by stool culture. Inanimate surfaces of suspected environmental specimens – bed armrests, toilet handles, water taps, cushions of fabric stools, hands of food handlers, and table surfaces – in nine infected wards were also sampled using swabs. The swabs were incubated in Gram-negative (GN) enrichment broth (Difco, USA) overnight at 37 °C and inoculated on Hektoen enteric (HE) agar (Difco, USA).
At the VNC, shigellosis patients were treated mainly with ampicillin (AMP) in designated wards and returned to their original wards after two consecutive negative stool cultures (collected at least 24 h apart and at least 48 h after antimicrobial therapy had stopped) were obtained. In December 2003, in order to control the sustained outbreak of S. flexneri 1a in 20 patients of ward A4 during 2001–2003 [6], a mass prophylaxis with SXT was administered to all 309 residents of ward A4. In October 2004, after the occurrence of S. flexneri 4a cases in ward A16, mass prophylaxis was administered to all 311 residents and 31 staff members of ward A16. Subjects were administered 160 mg trimethoprim and 800 mg sulphamethoxazole twice a day for 5 days.
Bacterial strains and serological identification
The 108 S. flexneri 4a isolates used in this study were recovered from stool samples of patients, contacts, or caregivers. All the S. flexneri isolates were confirmed by Gram staining, biochemical tests (API 20E test kit; bioMérieux, France), and slide agglutination tests with a commercially available polyclonal antiserum (Denka Seiken, Japan), according to the manufacturer's instructions.
Antimicrobial susceptibility testing
The AST of the isolates was performed using disc diffusion method with the following antimicrobials: amikacin (AMK, 30 μg), AMP (10 μg), cefazolin (CFZ, 30 μg), cefotaxime (CTX, 30 μg), cephalothin (CEF, 30 μg), chloramphenicol (CHL, 30 μg), gentamicin (GEN, 10 μg), nalidixic acid (NAL, 30 μg), ofloxacin (OFX, 5 μg), streptomycin (STR, 10 μg), SXT (25 μg), and tetracycline (TET, 30 μg) (Oxoid, UK). The testing was performed as recommended by the Clinical and Laboratory Standards Institute [formerly National Committee for Clinical Laboratory Standards (NCCLS), 2000]. The interpretive criteria for antimicrobial susceptibility were provided by the manufacturer. Escherichia coli ATCC 25922 was used as the reference strain.
PFGE and banding pattern analyses
The genomic DNAs from all S. flexneri isolates were digested with the NotI restriction enzyme and analysed by PFGE with a CHEF Mapper apparatus (Bio-Rad Laboratories, USA) at 6 V/cm and an angle of 120° with pulses of 2·16–54·17 s for 19 h. Estimation of genetic similarity was calculated as described previously [6]. Briefly, the PFGE banding patterns were compared for similarity using Bionumerics software (version 3.0; Applied Math, USA). On the basis of the Dice similarity coefficient, a dendrogram was constructed by the unweighted pair group method with arithmetic average clustering. Isolates that differed by one or more bands were assigned to different genotypes. Isolates that showed genetic similarities ⩾90% were categorized into the same cluster [6]. Salmonella enterica serovar Braenderup H9812 was used as the reference strain for PFGE analysis. PFGE patterns of Shigella isolates from the VNC were compared with those in the Taiwan Shigella Fingerprint Database (TSFD), constructed by Taiwan CDC and shared with other members of PulseNet International [22], for assessment of their genetic relatedness. The database contained PFGE patterns of 1071 S. flexneri isolates of various serotypes collected since 1994.
Statistical analysis
The χ2 test was used to compare the distributions of S. flexneri PFGE genotypes and antibiogram types in isolates obtained before and after the administration of SXT prophylaxis. All statistical analyses were performed using SAS 9.1 (SAS Institute, USA). A probability of <0·05 was considered statistically significant.
Results
Shigellosis occurrence and epidemiological surveillance in the VNC
S. flexneri 1a alone was responsible for the sustained outbreaks in ward A4 [6]. However, this strain was no longer isolated after SXT prophylaxis in the VNC in December 2003. SXT prophylaxis in ward A16 was implemented after an S. flexneri 4a outbreak occurred in October 2004; however, S. flexneri 4a infection persisted in 2005 and 2006. From 2001 to 2006, S. flexneri 4a was isolated from 83 symptomatic patients (including one caregiver) and 12 asymptomatic subjects (including 11 contacts and one caregiver). We recovered 108 isolates from the 95 subjects, including two isolates each from nine subjects and three isolates each from two subjects. S. flexneri 4a had spread more widely: from three wards in 2001 to nine wards in 2006 (Table 1). To implement further epidemiological investigation, we examined stool specimens from 72 food handlers and 147 new incoming residents as well as 275 environmental swab samples collected during the 2006 prolonged outbreak, and found that Shigella spp. was not detectable. Residue testing performed weekly indicated that the tap water contained 0·5–0·8 ppm of chlorine.
Table 1.
PFGE genotypes and antibiogram types of S. flexneri 4a isolates in the VNC during 2001–2006a
| Division A | Division B | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chronic ward | Acute ward | ||||||||||||
| A1 (M) | A2 (F) | A3 (M) | A4 (F) | A6 (M) | A16 (M) | a1 (M) | a2 (F) | a3 (F) | B1 (M) | B2 (M) | B3 (M) | ||
| 2001 | P10/E (1)b | P06/D (1) P08/D (4) |
P01/I (1) | 7 | |||||||||
| 2002 | P08/D (2) | P04/E (1) P05/E (1) |
P01/D (3) P09/D (1) |
P01/D (1) | 9 | ||||||||
| 2003 | P01/D (1) P03/H (1) |
2 | |||||||||||
| 2004c | P01/D (2) P07/D (1) P13/D (1) |
4 | |||||||||||
| 2005d | P01/D (8)e P01/L (1) | P11/J(2)f | P01/D (2) | 13 | |||||||||
| 2006d | P01/D (14)g | P01/D (2) | P15/D (1) | P01/D (18)h | P01/D (6) | P01/D (5)i | P11/J (3)f | P01/D (2) | P01/D (3) | 73 | |||
| P01/K (1) | P11/J(4)f, i | P02/F (1)f | P02/F (1)f | P11/D (3) | P12/J (1)f | P01/E (1) | |||||||
| P03/H (1) | P11/J (3)f | P11/J (2)f, i | |||||||||||
| P14/D (1) | |||||||||||||
| Total | 17 | 13 | 3 | 22 | 16 | 22 | 2 | 4 | 3 | 1 | 2 | 3 | 108 |
PFGE, Pulsed-field gel electrophoresis; VNC, Veteran Nursing Centre; M, male; F, female.
VNC is a large long-term psychiatric institution in Eastern Taiwan with two divisions (A and B). Division A has six chronic wards (A1, A2, A3, A4, A6, A16) and five acute wards (a1–a5). There were no shigellosis cases in wards a4 and a5 of division A.
Numerator/denominator indicates PFGE genotype/antibiogram type. Numbers in parentheses indicate the number of S. flexneri 4a isolates.
In October 2004, after the occurrence of S. flexneri 4a patients in ward A16, SXT prophylaxis was performed in all 311 residents and 31 staff members of ward A16.
There were 12 asymptomatic contacts epidemiologically indicated in 2005 and 2006.
Two individuals were female caregivers; one of the caregivers was asymptomatic.
There were 17 SXT-resistant isolates.
Five individuals were asymptomatic contacts.
Three individuals were asymptomatic contacts.
One of the individuals was an asymptomatic contact.
Susceptibility pattern analysis
On the basis of AST results, we categorized the 108 S. flexneri 4a isolates into the following eight antibiogram types: antibiogram type D (resistant to STR, n = 82), E (resistant to AMP, STR, n = 4), F (resistant to AMP, STR, SXT, n = 2), H (resistant to AMP, CEF, CTX, CFZ, STR, n = 2), I (resistant to AMP, CEF, CHL, GEN, CFZ, NAL, STR, SXT, TET, n = 1), J (resistant to STR, SXT, n = 15), K (resistant to STR, TET, n = 1), and L (resistant to CEF, STR, n = 1). The antibiogram type D, which contained 76% (82/108) of all tested isolates and appeared for 6 consecutive years, was sensitive to all antibiotics except STR (Fig. 1).
Fig. 1.
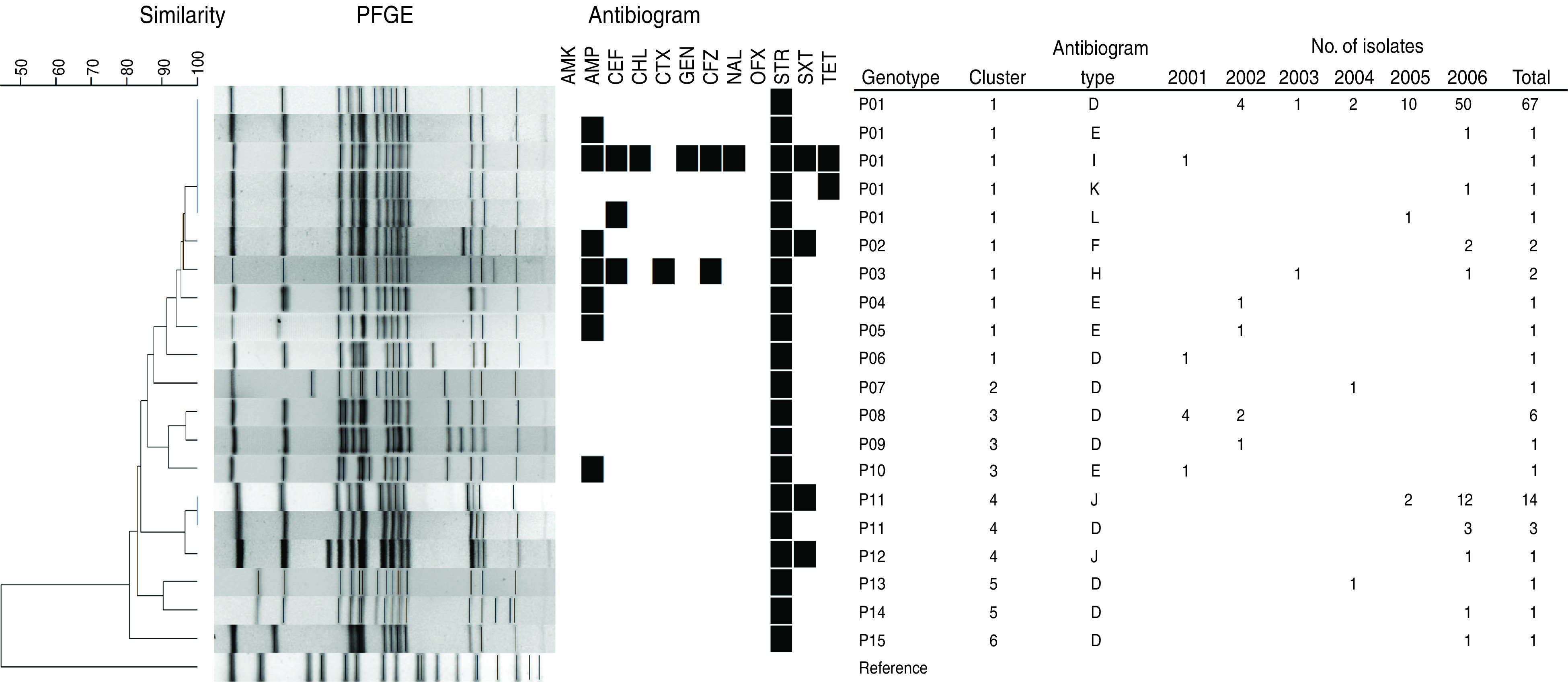
Dendrogram of S. flexneri 4a isolates determined on the basis of NotI-based PFGE patterns, the corresponding antibiogram type, and the number of isolates in the VNC during 2001–2006. The results for susceptibility (white) and resistance (black) are presented for the antimicrobials in the following sequence: amikacin (AMK), ampicillin (AMP), cephalothin (CEF), chloramphenicol (CHL), cefotaxime (CTX), gentamicin (GEN), cefazolin (CFZ), nalidixic acid (NAL), ofloxacin (OFX), streptomycin (STR), trimethoprim-sulphamethoxazole (SXT), and tetracycline (TET). Escherichia coli ATCC 25922 and Salmonella enterica serovar Braenderup H9812 were used as the reference strains for AST and PFGE analyses, respectively.
The χ2 test showed that the antibiogram types were significantly different before and after SXT prophylaxis (P = 0·011) (Table 2). The major difference was the emergence of the antibiogram types F and J, both of which exhibited resistance to SXT after SXT prophylaxis. Seventeen isolates of antibiogram types F and J were detected, and these were obtained from 15 different infected subjects. Of these 15 subjects, five were administered SXT prophylaxis in 2004. However, before SXT prophylaxis, only one isolate that emerged in 2001 was SXT resistant (antibiogram type I) (Fig. 1).
Table 2.
Antibiogram type, PFGE genotype, and cluster analysis of S. flexneri 4a strains isolated before and after SXT prophylaxisa
| Total (n = 108) | Before (n = 22) | After (n = 86) | χ2 test | P value | |
|---|---|---|---|---|---|
| Antibiogram type | 11·22 | 0·011 | |||
| D | 82 | 17 | 65 | ||
| F | 2 | 0 | 2 | ||
| J | 15 | 0 | 15 | ||
| Othersb | 9 | 5 | 4 | ||
| PFGE genotype | 42·70 | <0·0001 | |||
| P01 | 71 | 8 | 63 | ||
| P08 | 6 | 6 | 0 | ||
| P11 | 17 | 0 | 17 | ||
| Othersc | 14 | 8 | 6 | ||
| Cluster | 38·87 | <0·0001 | |||
| 1 | 78 | 12 | 66 | ||
| 3 | 8 | 8 | 0 | ||
| 4 | 18 | 0 | 18 | ||
| Othersd | 4 | 2 | 2 |
PFGE, Pulsed-field gel electrophoresis.
In October 2004, after the occurrence of S. flexneri 4a patients in ward A16, SXT prophylaxis was performed in all 311 residents and 31 staff members of ward A16.
Including antibiogram types E (n = 4), H (n = 2), and I, K, L (n = one each).
Including P02–P03 (n = 2), P04–07, P09–10, P12–15 (n = one in each group).
Including cluster 2 (n = 1), cluster 5 (n = 2), and cluster 6 (n = 1).
PFGE typing
The 108 S. flexneri 4a isolates were classified into 15 genotypes: P01 (including antibiogram types: D, n = 67; E, I, K, L, n = 1 for each), P02 (type F, n = 2), P03 (type H, n = 2), P04 (type E, n = 1), P05 (type E, n = 1), P06 (type D, n = 1), P07 (type D, n = 1), P08 (type D, n = 6), P09 (type D, n = 1), P10 (type E, n = 1), P11 (type D, n = 3; type J, n = 14), P12 (type J, n = 1), P13 (type D, n = 1), P14 (type D, n = 1), and P15 (type D, n = 1) (Fig. 1). The similarity among all genotypes was >80%. The relatedness analysis divided these genotypes into six clusters. Cluster 1 included genotypes P01–P06; clusters 2 and 6 included genotypes P07 and P15, respectively; cluster 3 included genotypes P08–P10; cluster 4 included genotypes P11 and P12; and cluster 5 included genotypes P13 and P14. Notably, SXT-resistant and SXT-susceptible isolates coexisted in clusters 1 and 4 (Fig. 1).
Statistical analysis revealed that the PFGE type and cluster distributions of S. flexneri 4a isolates changed significantly after SXT prophylaxis (both P < 0·0001) (Table 2). Isolates belonging to cluster 3 (n = 8, all sensitive to SXT) appeared only before SXT prophylaxis, while those belonging to cluster 4 (n = 18, 15 resistant to SXT) occurred only after SXT prophylaxis (Fig. 1, Table 2).
During 2001–2004, eight P01 genotype (antibiogram type D, n = 7; type I, n = 1) isolates were all obtained from men, and seven of these isolates were obtained from patients in ward A16. During 2005–2006, 63 P01 (type D, n = 60; E, K, L, n = 1 for each) isolates were identified in residents of eight different wards (five male and three female wards) and in two female caregivers (Table 1, Fig. 1). All 12 asymptomatic contacts epidemiologically identified, with the exception of one caregiver, were psychiatric residents from four different wards (two male and two female wards). These cases were infected with the P01 (type D, n = 10) and the P11 (type J, n = 2) genotypes (Table 1).
Patients from whom more than one isolate was recovered
Two or three isolates were recovered at varying intervals from 11 patients. Two isolates were recovered from patients 1 and 2 at intervals of 31 months and 27 months, respectively (Table 3). The isolates recovered from patient 1 shared only 86·2% PFGE pattern similarity but were of the same antibiogram type. The isolates recovered from patient 2 also shared relatively low pattern similarity (82·9%); however, the antibiogram type of these isolates changed from type D (resistance to STR) to type J (resistance to STR and SXT). Three S. flexneri 4a isolates were obtained from patient 3 at intervals of 6 months and 7 days: the first and the second isolates belonged to the same pulsotype and were of the same antibiogram type, but the third isolate showed additional resistance to AMP and SXT (type F). In patients 4–11, the isolates were recovered at intervals ranging between 1 and 27 days: no shift in the cluster was observed for these isolates. The isolates recovered from patients 4, 5, and 10 exhibited different resistance patterns. The second isolate from patients 4 and 5 acquired resistance to additional antibiotics; however, the second isolate from patient 10 lost resistance to two of three antibiotics, converting from type F (resistance to AMP, STR, SXT) to type D (resistance to STR).
Table 3.
Characteristics of 11 shigellosis patients who provided two or three S. flexneri 4a isolates
| Patient no. | Gender | Age (yr) | Ward | Date of the first isolate | Date of the second isolate | Date of the third isolate | Time interval | PFGE cluster/genotype/antibiogram type | Similarity (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 72 | A16 | 18 Mar. 2002 | 4 Oct. 2004 | 31 months | 1/P01/D, 2/P07/D | 86·2 | |
| 2 | M | 76 | A18 | 7 Nov. 2003 | 25 Feb. 2006a | 27 months | 1/P01/D, 4/P11/J | 82·9 | |
| 3 | M | 79 | A6 | 22 Jan. 2006 | 28 June 2006 | 5 July 2006 | 6 months, 7 days | 1/P01/D, 1/P01/D, 1/P02/F | 100·0, 96·6 |
| 4 | M | 76 | A1 | 13 Mar. 2006 | 21 Mar. 2006 | 8 days | 1/P01/D, 1/P03/H | 95·0 | |
| 5 | M | 48 | A1 | 30 Mar. 2006a | 10 Apr. 2006a | 11 days | 1/P01/D, 1/P01/K | 100·0 | |
| 6 | M | 26 | A16 | 4 Apr. 2006 | 11 Apr. 2006 | 7 days | 1/P01/D | 100·0 | |
| 7 | F | 30 | a2 | 19 June 2006 | 27 June 2006 | 28 June 2006 | 8 days, 1 day | 4/P11/J, 4/P12/J, 4/P11/J | 96·6 |
| 8 | F | 59 | A4 | 26 June 2006 | 3 July 2006 | 7 days | 1/P01/D | 100·0 | |
| 9 | M | 83 | A6 | 28 June 2006 | 4 July 2006 | 6 days | 1/P01/D | 100·0 | |
| 10 | F | 60 | A4 | 23 July 2006 | 26 July 2006 | 3 days | 1/P02/F, 1/P01/D | 96·6 | |
| 11 | F | 45 | A4 | 4 Aug. 2006 | 31 Aug. 2006 | 27 days | 1/P01/D | 100·0 |
Asymptomatic contact.
DISCUSSION
Shigellosis outbreaks occur mostly in facilities with crowded conditions and where personal hygiene is poor, such as mental institutions, schools, and daycare centres [5, 6, 11, 23]. The administrators of the VNC considered that the personal hygiene of these psychiatric patients was indeed poor, and that the wards were crowded. In order to control the sustained outbreaks, SXT prophylaxis was executed internally. This study paid particular attention to the consequences of such prophylactic intervention.
In this study, we examined the variations of S. flexneri 4a isolates in a large long-stay psychiatric institution during sustained outbreaks during 2001–2006. The data indicate that antibiogram types, PFGE patterns, and clustering significantly changed after SXT prophylaxis (Table 2). Variation in antimicrobial resistance profiles after SXT prophylaxis was also observed between the isolates recovered from patient 2, who was one of the patients administered SXT prophylaxis (Table 3). To our knowledge, this is the first study providing empirical evidence of mass prophylaxis-induced Shigella drug resistance.
Our results suggest that the emergence of SXT-resistant types F and J may have been elicited by such SXT prophylaxis. Several lines of evidence support this hypothesis. First, the relatively segregated environment of the VNC does not favour introduction of new resistant strains. The results of clustering analysis of PFGE patterns of the S. flexneri 4a strains recovered from the VNC and those from the TSFD indicate that the VNC strains were genetically distant from other S. flexneri 4a strains emerging outside the VNC. Thus, the SXT-resistant strains that emerged after SXT prophylaxis may have evolved from strains circulating in the VNC, most likely owing to selective pressure from the SXT prophylaxis. In fact, a similar pulsotype before and after prophylaxis along with the emergence of a newly acquired SXT-resistant phenotype seem to support this hypothesis. Second, stool culture monitoring of new incoming residents did not yield positive results; thus, silent transmission from new incoming residents is an unlikely cause of the emergence of resistance to SXT. Third, before the emergence of SXT resistance, shigellosis in the VNC was treated mainly with AMP; therefore, SXT could not have selected resistance before the prophylaxis.
The following points are of interest with regard to the sources of shigellosis. First, all environmental and tap-water samples and stool cultures of food handlers, sampled during the 2006 outbreak, were negative for Shigella spp. Therefore, contaminated water and food are unlikely vehicles of the outbreak. Second, two caregivers (one of whom was asymptomatic) and five patients under their care were infected with the same genotype, with very close dates of onset. Furthermore, most psychiatric patients had poor hygienic habits and required caregiver assistance for daily activities. It is possible that transmission within and across wards may have occurred through the VNC's caregivers. Third, 12 asymptomatic contacts, including one caregiver, were epidemiologically indicated. Such asymptomatic cases could have accounted for transmission in the VNC. Interestingly, two other cases of long-term asymptomatic carriers of shigellosis have been previously reported [24, 25]. Last, the interval between isolation of S. flexneri isolates of the same genotype from patient 3 during the widespread outbreak in 2006 was 6 months, suggesting the possibility of a long-term Shigella carrier state in VNC residents. This finding is similar to those of a previous study about two Shigella carriers with prolonged shedding, whose stools were culture positive for 16 or 17 months after clinical treatment [24].
In patients 1 and 2, the isolates recovered before and after the prophylaxis belonged to different clusters as determined by PFGE. The first and second isolates recovered from patient 1 belonged to clusters 1 and 2, respectively; those recovered from patient 2, to clusters 1 and 4, respectively. These findings indicate new infection with different strains of circulating S. flexneri 4a. The finding that multiple isolates from each of patients 3, 4, 7, and 10 showed slightly different PFGE patterns but still belonged to the same cluster (Table 3) suggests that genetic divergence of circulating S. flexneri 4a isolates occurred over time in the VNC. This finding is similar to a previous observation that some S. sonnei strains, a close relative of S. flexneri, derived from the primary epidemic strain emerged and then persisted for only a short period of time [18, 26], suggesting continuous genetic variation.
In summary, this study provided information on the pattern of sustained person-to-person transmission of a predominant epidemic strain, S. flexneri 4a, in a long-stay psychiatric centre by long-term convalescent carriers and/or asymptomatic or caregiver carriers, indicating that foodborne, waterborne, and fomite transmission may be less important. Notably, mass prophylaxis may select for a resistant clone or sub-population of the organism.
ACKNOWLEDGEMENTS
This study was supported by research grant DOH95-DC-2034 from the Taiwan Centres for Disease Control (Taiwan CDC). The authors thank Dr Wen-Chien Liu, Zhuo-An Liu, and the staff of the Hualien County Health Bureau for their assistance in the epidemiological investigation. The authors also thank Dr Yi-Hsiung Tseng and Dr Hin-Chung Wong for helpful discussions, and Ming-Ching Liu for technical assistance.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Kotloff KL, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bulletin of the World Health Organization 1999; 77: 651–666. [PMC free article] [PubMed] [Google Scholar]
- 2.Chompook P, et al. Estimating the burden of shigellosis in Thailand: 36-month population-based surveillance study. Bulletin of the World Health Organization 2005; 83: 739–746. [PMC free article] [PubMed] [Google Scholar]
- 3.Ram PK, et al. Part II. Analysis of data gaps pertaining to Shigella infections in low and medium human development index countries, 1984–2005. Epidemiology and Infection 2008; 136: 577–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DuPont HL, et al. Inoculum size in shigellosis and implications for expected mode of transmission. The Journal of Infectious Diseases 1989; 159: 1126–1128. [DOI] [PubMed] [Google Scholar]
- 5.Pillay DG, et al. Nosocomial transmission of Shigella dysenteriae type 1. Journal of Hospital Infection 1997; 37: 199–205. [DOI] [PubMed] [Google Scholar]
- 6.Lee YS, et al. Molecular epidemiology of Shigella flexneri in a long-stay psychiatric nursing center during 2001 to 2003. Journal of Clinical Microbiology 2005; 43: 1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seidlein LV, et al. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Medicine 2006; 3: 1556–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, et al. Laboratory-confirmed shigellosis in the United States, 1989–2002: epidemiologic trends and patterns. Clinical Infectious Diseases 2004; 38: 1372–1377. [DOI] [PubMed] [Google Scholar]
- 9.Salam MA, Bennish ML. Antimicrobial therapy for shigellosis. Reviews of Infectious Diseases 1991; 13 (Suppl. 4): S332–S341. [DOI] [PubMed] [Google Scholar]
- 10.Niyogi SK. Increasing antimicrobial resistance–an emerging problem in the treatment of shigellosis. Clinical Microbiology and Infection 2007; 13: 1141–1143. [DOI] [PubMed] [Google Scholar]
- 11.Chuang YY, Huang YC, Lin SY. Outbreak of Shigella sonnei gastroenteritis in northeastern Taiwan. Pediatric Infectious Disease Journal 2006; 25: 92–94. [DOI] [PubMed] [Google Scholar]
- 12.Haukka K, Siitonen A. Emerging resistance to newer antimicrobial agents among Shigella isolated from Finnish foreign travellers. Epidemiology and Infection 2008; 136: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe-Magnus DA, Guerout AM, Mazel D. Bacterial resistance evolution by recruitment of super-integron gene cassettes. Molecular Microbiology 2002; 43: 1657–1669. [DOI] [PubMed] [Google Scholar]
- 14.Stokes HW, Hall RM. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Molecular Microbiology 1989; 3: 1669–1683. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed AM, et al. Genetic characterization of multidrug resistance in Shigella spp. From Japan. Journal of Medical Microbiology 2006; 55: 1685–1691. [DOI] [PubMed] [Google Scholar]
- 16.Peirano G, et al. Occurrence of integrons and resistance genes among sulphonamide-resistant Shigella spp. from Brazil. Journal of Antimicrobial Chemotherapy 2005; 55: 301–305. [DOI] [PubMed] [Google Scholar]
- 17.Taiwan Centres for Disease Control. Statistics of communicable diseases and surveillance report in Taiwan area, 2007, pp. 117–119.
- 18.Wei HL, et al. Epidemiology and evolution of genotype and antimicrobial resistance of an imported Shigella sonnei clone circulating in central Taiwan. Diagnostic Microbiology and Infectious Disease 2007; 58: 469–475. [DOI] [PubMed] [Google Scholar]
- 19.Lin CS, et al. Analysis of relationships between several Shigella sonnei outbreaks in the Taoyuan area of Taiwan. Taiwan Epidemiology Bulletin 2001; 17: 83–97. [Google Scholar]
- 20.Chen JH, et al. Molecular epidemiology of Shigella in a Taiwan township during 1996 to 2000. Journal of Clinical Microbiology 2003; 41: 3078–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiou CS, et al. Molecular epidemiology of a Shigella flexneri outbreak in a mountainous township in Taiwan, Republic of China. Journal of Clinical Microbiology 2001; 39: 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swaminathan B, et al. Building PulseNet International: an interconnected system of laboratory networks to facilitate timely public health recognition and response to foodborne disease outbreaks and emerging foodborne diseases. Foodborne Pathogens and Disease 2006; 3: 36–50. [DOI] [PubMed] [Google Scholar]
- 23.Heymann DL. Control of Communicable Diseases Manual, 18th edn. Washington, DC: APHA, 2004, pp.700. [Google Scholar]
- 24.Levine MM, et al. Long-term Shigella-carrier state. New England Journal of Medicine 1973; 288: 1169–1171. [DOI] [PubMed] [Google Scholar]
- 25.Marcus U, et al. Shigellosis – a re-emerging sexually transmitted infection: outbreak in men having sex with men in Berlin. International Journal of STD & AIDS 2004; 15: 533–537. [DOI] [PubMed] [Google Scholar]
- 26.Garrett V, et al. A recurring outbreak of Shigella sonnei among traditionally observant Jewish children in New York City: the risks of daycare and household transmission. Epidemiology and Infection 2006; 134: 1231–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]


