Abstract
We herein present a case study of a patient with heart failure with a preserved ejection fraction and severe symptoms who underwent interatrial shunt device implantation and follow-up at a tertiary care heart failure clinic. The interatrial shunt device implantation was successful. No adverse events occurred, and the device prevented hospitalization for heart failure during long-term follow-up.
Keywords: Heart failure with preserved ejection fraction, interatrial shunt device, long-term follow-up, hospitalization for heart failure, adverse outcomes, case report
Introduction
We herein present a case study of a patient with heart failure with a preserved ejection fraction (HFpEF) and severe symptoms who underwent implantation of an interatrial shunt device (IASD; Corvia Medical, Inc., Tewksbury, MA, USA) in 2015 as a participant in the Reduce Elevated Left Atrial Pressure in Patients with Heart Failure (REDUCE LAP-HF) study. 1
Case Report
This case involved a 75-year-old woman with a history of cardiovascular and non-cardiac diseases. In 2013, she had been diagnosed with paroxysmal atrial fibrillation and non-ST segment elevation myocardial infarction. Coronary angiography showed three-vessel disease with critical stenosis of the left circumflex artery requiring percutaneous coronary angioplasty with a drug-eluting stent and subsequent triple coronary artery bypass.
The patient’s other medical history was significant for arterial hypertension, type 2 diabetes mellitus, and chronic obstructive pulmonary disease. Other significant comorbidities included obesity (body mass index of 36 kg/m2), chronic kidney disease with an estimated glomerular filtration rate of 69 mL/minute/1.73 m2, and chronic anemia with a hemoglobin level of 11.5 g/dL. The patient was an ex-smoker. Her heart failure history started after the occurrence of myocardial infarction with moderate symptoms (New York Heart Association (NYHA) functional class III) despite medical therapy including bisoprolol at 5 mg once a day, perindopril at 4 mg once a day, furosemide at 60 mg once a day, spironolactone at 25 mg once a day, and hydrochlorothiazide at 12.5 mg once a day. Other medical therapy included rosuvastatin at 10 mg once a day, apixaban at 5 mg twice a day, ciclesonide inhaler at 160 mcg/dose once a day, tiotropium bromide monohydrate at 2.5 mcg once a day, and oral potassium supplementation at 1 g once a day.
The patient was referred to a heart failure clinic for further evaluation and possible treatment. Transthoracic echocardiography revealed a normal left ventricular (LV) ejection fraction (60%) with normal LV kinetics, elevated LV filling pressure, normal right ventricular dimensions and function, left atrial enlargement, trivial mitral and tricuspid regurgitation, and mild pulmonary hypertension. Repeated coronary angiography showed patent coronary grafts. Cardiac magnetic resonance imaging revealed normal ejection function of the left and right ventricles, and late gadolinium enhancement showed subendocardial scarring of the lateral segments affecting at least 50% of the myocardial wall but with preserved wall motion.
The patient was asked to participate in the REDUCE LAP-HF trial. After providing written informed consent, she underwent screening procedures including a hemodynamic study at rest and during stress in accordance with the study protocol. After assessment of her resting hemodynamics, the patient exercised by supine cycle ergometry at a cycling cadence of 60 rotations per minute starting at a 20-W workload and increasing by 10-W or 20-W increments in 3-minute stages to the maximum tolerated level.
The patient met the inclusion criteria for the study. The inclusion and exclusion criteria for the REDUCE LAP-HF trial have been previously reported. 1 Briefly, the main inclusion criteria for the study were a history of chronic heart failure with an LV ejection fraction of ≥40%, NYHA class of ≥II, and pulmonary capillary wedge pressure (PCWP) of ≥15 mmHg at rest or ≥25 mmHg during a stress hemodynamic study. The main exclusion criteria were right atrial pressure of >14 mmHg at rest or >20 mmHg during stress and severe pulmonary hypertension.
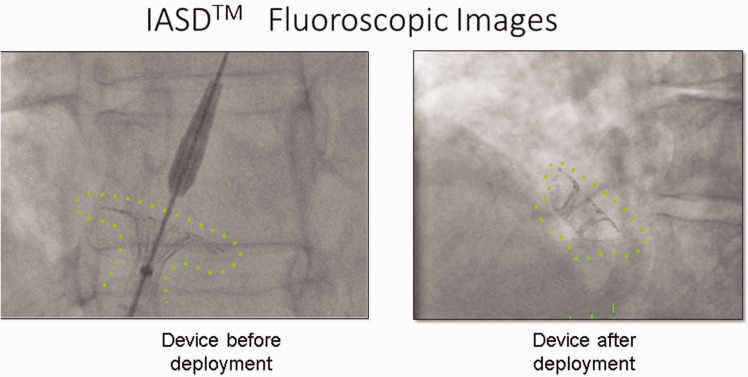
The IASD is a nitinol implant with a 19-mm diameter, and it is inserted percutaneously to create a permanent 8-mm atrial septal shunt. In the present case, the procedure was performed under sedation and the access site was the right femoral vein. The atrial septum was punctured using a standard technique and sheath. The fluoroscopic view of the IASD is shown in Figure 1.
Figure 1.
Fluoroscopic images of interatrial shunt device. Left: device before deployment, Right: device after deployment.
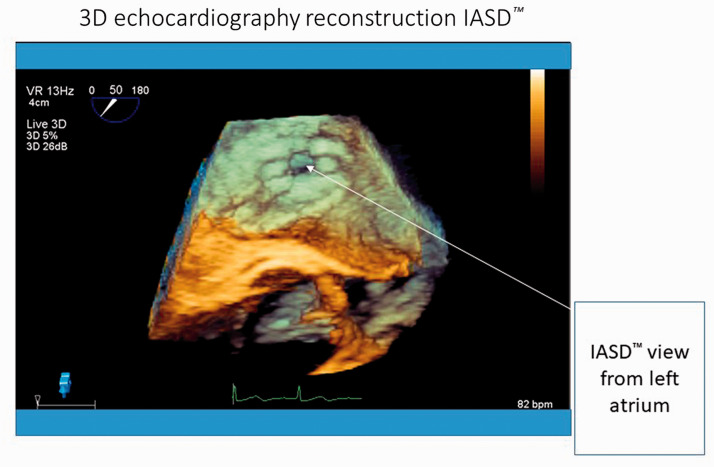
The IASD was successfully implanted. In accordance with the study protocol, the patient was followed up for 3 years and then on a routine basis at the tertiary heart failure clinic. The patient’s symptoms and stress tolerance improved after the procedure. Her NYHA functional class decreased from III to II, and her 6-minute walk test distance improved from 198 m before the procedure to 210 m after 1 month and to 252 m after 6 months of follow-up. Compared with the baseline hemodynamic study, implantation of the IASD was associated with a smaller increase in the PCWP during stress at the 6-month follow-up. The N-terminal pro-brain natriuretic peptide concentration decreased from 1050 pg/mL at baseline to 625 pg/mL at the 1-month follow-up and remained stable at 6 months. After shunt implantation, the patient required no hospitalization for worsening heart failure. She had one episode of recurrent atrial fibrillation with successful electrical cardioversion and underwent successful atrial fibrillation ablation (elective hospital stay). Follow-up examinations revealed a patent interatrial shunt with left-to-right flow and no adverse findings. Three-dimensional transesophageal echocardiography reconstruction of the IASD is shown in Figure 2.
Figure 2.
Three-dimensional echocardiography reconstruction of interatrial shunt device.
The patient’s symptoms remained stable for 4 years (NYHA class II) with slight worsening and the need to gradually increase the diuretic dose to reach euvolemic status. The daily furosemide dose was increased from 60 to 250 mg, and the daily spironolactone dose was increased from 25 to 100 mg. The daily dose of hydrochlorothiazide remained unchanged. The patient’s symptoms were influenced partly by her chronic comorbidities: chronic obstructive pulmonary disease, chronic kidney disease, and anemia.
Because of worsening symptoms (return to NYHA class III), new cardiac catheterization including coronary angiography and right heart catheterization were required. Coronary angiography showed patent grafts without progressive coronary atherosclerosis compared with the previous study.
The results of the baseline (before shunt implantation) and 6-year follow-up hemodynamic studies together with the results of the baseline and follow-up echocardiographic studies and cardiac magnetic resonance study (baseline and 6 months after shunt implantation) are shown in Table 1. The table shows cardiac output measured by the thermodilution technique and the ratio of pulmonary blood flow to systemic blood flow calculated by the Fick formula immediately after IASD implantation and at the follow-up visits.
Table 1.
Baseline and follow-up parameters after IASD implantation.
| Parameter | Baseline (March 2015) | Follow-up (April 2021) |
|---|---|---|
|
Hemodynamic study |
20 March 2015 |
7 April 2021 |
| Mean RA, mmHg | 10 | 7 |
| Mean PAP, mmHg | 28 | 27 |
| Mean PCWP, mmHg | 18 | 17 |
| Systolic Ao, mmHg | 148 | 137 |
| Diastolic Ao, mmHg | 58 | 47 |
| Mean Ao, mmHg | 81 | 80 |
| PAR, dyn | 118.4 | 76 |
| PAR, Wood units | 1.5 | 1.0 |
| CO, thermodilution, L/minute | 6.75 | 10.52 |
| CI, L/minute/m2 | 3.02 | 4.93 |
| Qp/Qs | 1.18 after implantation of IASD | |
| Qp/Qs | 1.22 (at 6 months) | |
| Qp/Qs | 1.36 (at 6 years) | |
|
CMR |
9 March 2015 |
2 September 2015 |
| LVEF | 61% | 71% |
| LVEDV, mL | 142 | 139 |
| LVESV, mL | 56 | 59 |
| LVEDVi, mL/m2 | 64 | 63 |
| LVESVi, mL/m2 | 56 | 59 |
| RVEF | 65% | 68% |
| RVEDV, mL | 137 | 139 |
| RVESV, mL | 49 | 59 |
| RVEDVi, mL/m2 | 61 | 63 |
| RVESVi, mL/m2 | 22 | 32 |
| LA, mL | 81 | 79 |
| RA, mL | 65 | 72 |
| LAi, mL/m2 | 36 | 35 |
| RAi, mL/m2 | 22 | 32 |
|
TTE |
11 March 2015 |
6 April 2021 |
| LVEF | 60% | 60% |
| LVEDD, mm | 51 | 54 |
| LVESD, mm | 36 | 39 |
| RVEDD, mm | 27 | 30 |
| TAPSE, mm | 17 | 20 |
| PW Doppler E wave, cm/s | 80 | 90 |
| PW Doppler A wave, cm/s | 50 | 60 |
| TDI É sept, cm/s | 5 | 5 |
| TDI É lat, cm/s | 7 | 10 |
| E/A | 1.6 | 1.5 |
| E/É | 13.3 | 12 |
| MR grade | 1+ | 1+ |
| TR grade | 1+ | 1+ |
| TRI gradient | 32 | 37 |
| IVC – expiration, mm | 20 | 21 |
|
Oral diuretic dose, mg/day |
Baseline |
Follow-up |
| Furosemide | 60 | 250 |
| Spironolactone | 25 | 100 |
| Hydrochlorothiazide | 12.5 | 12.5 |
IASD, interatrial shunt device; RA, right atrial pressure; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; Ao, aortic pressure; PAR, pulmonary arterial resistance; CO, cardiac output; CI, cardiac index; Qp/Qs, ratio of pulmonary blood flow to systemic blood flow; CMR, cardiac magnetic resonance imaging; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEDVi, left ventricular end-diastolic volume index; LVESVi, left ventricular end-systolic volume index; RVEF, right ventricular ejection fraction; RVEDV, right ventricular end-diastolic volume; RVESV, right ventricular end-systolic volume; RVEDVi, right ventricular end-diastolic volume index; RVESVi, right ventricular end-systolic volume index; LA, left atrial volume; LAi, left atrial volume index; RA, right atrial volume; RAi, right atrial volume index; TTE, transthoracic echocardiography; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; RVEDD, right ventricular end-diastolic diameter; TAPSE, tricuspid annulus posterior systolic excursion; PW, pulsed wave; E wave, velocity of early filling at diastole in left ventricle; A wave, velocity of left atrial contraction; TDI, tissue Doppler imaging; É sept, velocity of septal mitral annulus assessed by TDI; É lat, velocity of lateral mitral annulus assessed by TDI; E/A, ratio of E and A waves; E/É, ratio of E wave and mean of É sept and É lat; MR, mitral regurgitation; 1+, trivial; TR, tricuspid regurgitation; TRI gradient, systolic gradient of tricuspid regurgitation; IVC, inferior vena cava.
We predicted the patient’s outcome using Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) calculator at baseline and at follow-up (Table 2). 2 As shown in Table 2, the risk of death at baseline was >12% in 1 year and >29% in 3 years.
Table 2.
Predicting survival in heart failure – MAGGIC calculator.
| Parameter | Baseline | Six-year follow-up |
|---|---|---|
| Age, years | 70 | 75 |
| Height, cm | 169 | 169 |
| Weight, kg | 104 | 104 |
| BMI, kg/m2 | 36.46 | 36.46 |
| LVEF | 60% | 60% |
| sBP, mmHg | 148 | 137 |
| Creatinine, µmol/L | 81 | 108 |
| eGFR (CKD-EPI equation), mL/minute/1.73 m2 | 69 | 42 |
| NYHA functional class | III | III |
| Smoker | No | No |
| Diabetes mellitus | Yes | Yes |
| COPD on therapy | Yes | Yes |
| HF history ≥18 months ago | Yes | Yes |
| BB use | Yes | Yes |
| ACEI use | Yes | Yes |
| Total score, points | 22 | 26 |
| Risk of 1-year mortality | 12.2% | 17.5% |
| Risk of 3-year mortality | 29.2% | 39.7% |
BMI, body mass index; LVEF, left ventricular ejection fraction; sBP, systolic blood pressure (invasive); eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; HF, heart failure; BB, beta-blocker; ACEI, angiotensin-converting enzyme inhibitor.
The reporting of this study conforms to the CARE consensus-based clinical case reporting guidelines. 3
Discussion
HFpEF is common, and the treatment options are limited. Heart failure is a progressive clinical syndrome, and because of its natural history, it is associated with the risk of hospitalization for heart failure, the development of comorbidities, and premature death.
In the present case, the use of an experimental interatrial shunt in a patient with HFpEF and advanced symptoms was associated with a delay of adverse outcomes and a reduced risk of hospitalization for heart failure. Unfortunately, we had to gradually increase the patient’s diuretic dose to maintain her euvolemic status. The main effect of the IASD is a reduction of the left atrial pressure during stress, and the creation of a small left-to-right shunt may cause some degree of volume overload.
As shown in Table 1, the 6-year follow-up after IASD implantation revealed no adverse effects of the shunt on right heart function, pulmonary artery pressures, or pulmonary vascular resistance.
The efficacy and safety of IASD implantation have been investigated in several studies. The pilot study showed that IASD implantation was safe and had a beneficial effect on left atrial pressure reduction together with improvement of symptoms and exercise tolerance. The resting PCWP decreased significantly, and the right atrial pressure and systolic pulmonary artery pressure were unchanged. The patient’s symptoms improved and her functional NYHA class decreased. 4
The proof-of-concept REDUCE LAP-HF study revealed a beneficial effect of IASD implantation on PCWP during stress. 1 The beneficial hemodynamic effect of IASD implantation was confirmed in a randomized sham-controlled study. 5 The effects of IASD implantation on mortality, morbidity, and major adverse cardiovascular and renal events are being tested in a large multicenter randomized sham-controlled study. 6
Our calculation at the 6-year follow-up revealed a dramatic increase in the 3-year risk of death to >40%. This increased risk was caused by advanced age and worsening kidney function.
We conclude that IASD implantation may have beneficial effects on symptoms and prevention of hospitalization for heart failure during short-term follow-up. Long-term effects may be influenced by volume overload caused by left-to-right shunting with the need for increased diuretic doses. Heart failure events including hospital admissions, unscheduled outpatient visits for intravenous diuretic therapy, or intensification of oral diuretic therapy are the components of the primary composite endpoint of the ongoing randomized controlled trial.
Declaration of conflicting interest: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Libor Dujka received institutional research grants RVO (NNH, 00023884), 170501. Filip Málek received institutional research grants RVO (NHH, 00023884), IG160502. Vivek Reddy owns stock in Corvia Medical, Inc. Jan Komtebedde is an employee of Corvia Medical, Inc. Petr Neužil, Martin Mates, Tomáš Mráz, and Jiří Weichet declare that they have no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by institutional research grant RVO (NNH, 00023884), 170501, and 160502.
Ethics and consent
Approval for this study was granted by the Local Board Ethics Committee of Na Homolce Hospital (24.4.2014/4). The patient provided written informed consent for treatment and publication of this case.
ORCID iD
Filip Málek https://orcid.org/0000-0002-8176-2191
References
- 1.Hasenfuß G, Hayward C, Burkhoff D, et al. REDUCE LAP-HF study investigators. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet 2016; 387: 1298–1304. [DOI] [PubMed] [Google Scholar]
- 2.Pocock SJ, Ariti CA, McMurray JJV, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013; 34: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 3.Gagnier JJ, Kienle G, Altman DG, et al. ; CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 4.Søndergaard L, Reddy V, Kaye D, et al. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail 2014; 16: 796–801. [DOI] [PubMed] [Google Scholar]
- 5.Shah SJ, Feldman T, Ricciardi TM, et al. One-year safety and clinical outcomes of a transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction in the Reduce Elevated Left Atrial Pressure in Patients With Heart Failure (REDUCE LAP-HF I) trial: a randomized clinical trial. JAMA Cardiol 2018; 3: 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.REDUCE LAP-HF TRIAL II. https://clinicaltrials.gov/ct2/show/NCT03088033