Graphical abstract
Outcomes of cardiac surgery with left atrial appendage occlusion versus no occlusion, direct oral anticoagulants, and vitamin K antagonists
Keywords: Left atrial appendage occlusion, Direct oral anticoagulants, Vitamin K antagonists, Atrial fibrillation, Stroke, All-cause mortality
Abbreviations: AF, atrial fibrillation; CI, confidence interval; DOACs, direct oral anticoagulants; LA, left atrium; LAA, left atrial appendage; LAAO, left atrial appendage occlusion; MACE, major adverse cardiac events; NOACs, novel oral anticoagulants; OR, odds ratio; VKAs, vitamin K antagonists
Abstract
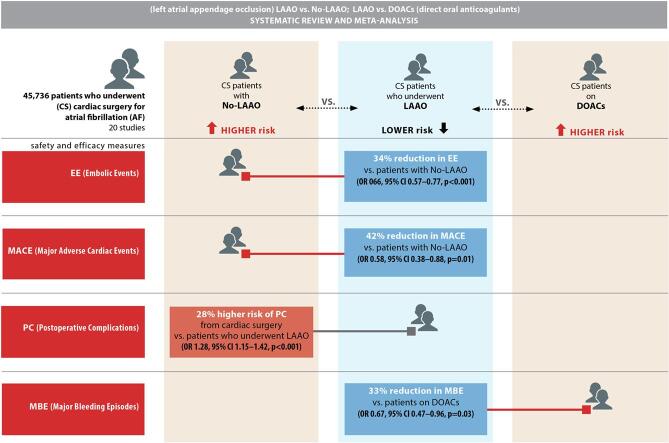
Surgical left atrial appendage occlusion (LAAO) is being used increasingly in the setting of atrial fibrillation but has been associated with procedural complications. This systematic review and meta-analysis compared the outcomes of surgical LAAO with those of no LAAO and the use of direct oral anticoagulants (DOACs) and vitamin K antagonists (VKAs) using the PRISMA guidelines. A literature search was undertaken for relevant studies published between January 1, 2003, and August 15, 2021. Primary clinical outcomes were all-cause mortality, embolic events, and stroke. Secondary clinical outcomes included major adverse cardiac events (MACE), postoperative atrial fibrillation, postoperative complications, reoperation for bleeding, and major bleeding. There was a statistically significant 34% reduction in incidence of embolic events (odds ratio [OR] 0.66, 95% confidence interval [CI] 0.57–0.77, p < 0.001) and a significant 42% reduction in risk of MACE (OR 0.58, 95% CI 0.38–0.88, p = 0.01) in patients who underwent LAAO.Surgical LAAO has the potential to reduce embolic events and MACE in patients undergoing cardiac surgery for atrial fibrillation. However, complete replacement of DOACs and warfarin therapy with surgical LAAO is unlikely despite its non-inferiority in terms of minimizing all-cause mortality, embolic events, MACE, major bleeding, and stroke in patients on oral anticoagulation therapies.
1. Introduction
Left atrial appendage occlusion (LAAO) is the treatment of choice for patients with atrial fibrillation (AF) undergoing cardiac surgery and in those on direct oral anticoagulants (DOACs) or vitamin K antagonists (VKAs) [1]. LAAO is also recommended for patients with AF and known contraindications to DOACs. LAAO begins with a fluoroscopically guided atrial transseptal puncture and seals the left atrial appendage (LAA) using well-positioned equipment [2]. LAAO is recommended in scenarios where the risk of procedural complications outweighs the possible incidence of ischemic/hemorrhagic stroke and transient ischemic attack [1]. The contemporary evidence suggests that LAAO is considered to be a safe and effective procedure for patients with non-valvular AF [3]. However, the reported LAAO complications including device-related embolism and pericardial effusion increase the risk of cardiovascular mortality [4]. the current evidence in the literature does not substantiate a net clinical benefit of surgical LAAO over pharmacotherapies and routine cardiac surgery [5]. Moreover, it is unclear in the medical literature, whether or not antithrombotic pharmacotherapy is necessary after LAAO [6]. Potential indications for surgical LAAO include hospitalization, thromboembolism, transient ischemic attack, ischemic stroke, and bleeding [7].
Recent evidence suggests that LAAO is an effective treatment for resistant LAA thrombus in patients with AF [8]. The American Heart Association (AHA) and American College of Cardiology (ACC) guidelines include a class IIB recommendation for use of percutaneous LAAO in patients in whom prolonged DOAC/VKA therapy is contraindicated and in those who are at increased risk of stroke [9]. The recently conducted LAAOS III trial advocates the potential of surgical LAAO in minimizing systemic embolism or systemic stroke events in AF patients who continue with antithrombotic therapies after cardiac surgery [10]. This benefit, however, is not confirmed in patients with AF who do not undergo cardiac surgeries. In addition, the PROTECT-AF trial demonstrates the non-inferiority of LAAO over oral anticoagulants [11]. A multicenter observational registry by Marroquin et al. indicates the lack of periprocedural embolic complications and a high procedural success rate in patients with LAA thrombus who received percutaneous LAA closure [12].
A recent meta-analysis reported the incidence of device-related thrombus that triggered ischemic events in 3.8% of patients who received LAAO [13]. In addition, the PROTECT AF trial reported a 0.3% incidence of device-related thrombotic stroke in LAA scenarios [14]. However, the current literature advocates WATCHMAN device-directed LAAO in patients requiring ablation of AF [15]. Incorrect positioning of the LAAO implant results in a patent LAA but does not significantly increase the risk of all-cause mortality or cardiovascular death [16]. The increasing global burden of AF and its association with stroke (4%), stroke-related death (8%), and all-cause mortality (30%) are further reasons for recommending LAAO in cardiac patients [17]. The benefit of LAAO in stroke prevention is attributed to its ability to exclude thrombogenic LAA tissue [18]. However, appropriate training is necessary to avoid the mechanical complications associated with LAAO that cause trauma to the LAA/left atrium (LA) and pericardial effusion in 89% of patients in whom perioperative/procedural mishaps are encountered [19]. Accordingly, recent studies advocate transesophageal echocardiography-guided LAAO to minimize the risk of procedural complications and their cardiovascular consequences [20].
A recent meta-analysis by Ibrahim et al. suggested a reduced incidence of ischemic stroke and all-cause mortality in patients with surgical LAAO compared to no LAAO [21]. The meta-analysis by Atti et al. further confirmed the potential of LAAO to minimize the risk of stroke and thromboembolic episodes in patients with AF and cardiac surgeries [22]. The network meta-analysis by Hanif et al. also emphasized the benefits of oral anticoagulation therapy in patients with AF who undergo LAAO [23]. The meta-analysis by Li et al. similarly underscored the benefits of LAAO in comparison with novel oral anticoagulant (NOAC) therapy in minimizing the incidence of hemorrhagic and thromboembolic events [24]. However, the current evidence is not sufficient to substantiate the complete replacement of DOACs and VKAs with LAAO and its integration with cardiac surgery in patients with and without AF. To the best of our knowledge, this is the first systematic review and meta-analysis to compare the safety and efficacy outcomes in patients who undergo LAAO during cardiac surgery with those in patients who do not receive LAAO or receive DOAC/NOAC/VKA therapies.
2. Methods
2.1. Search strategy
This systematic review and meta-analysis were performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines (Supplementary Fig. 1). On August 19, 2021, two authors searched the PubMed/Medline, Cochrane Central Register of Controlled Trials, and JSTOR databases for relevant studies published between January 1, 2003 and August 15, 2021. The following search terms were used: “LAAO AND no-LAAO; LAAO AND cardiac surgeries”; “LAA occlusion AND DOACs”; “LAAO AND VKAs”; “LAAO AND warfarin”; “LAAO AND NOACs”; and “LAAO OR no-LAAO OR DOACs or NOACs or VKAs AND cardiac surgeries”.
2.2. Study selection and data collection
Opinion papers, review articles, meta-analyses, systematic reviews, and scientific correspondence articles were excluded. Two investigators working independently analyzed the abstracts and titles of full-text studies to identify potentially eligible retrospective cohort analyses, randomized controlled trials, open-label randomized trials, randomized non-inferiority trials, and retrospective, case-control, cross-sectional, observational, prospective, and propensity score matching studies. In the event of disagreement regarding data extraction, consensus was reached by discussion.
2.3. Outcome variables
The meta-analysis was stratified as follows: LAAO vs no LAAO (14 studies); LAAO vs DOACs/NOACs (four studies); and LAAO vs VKA/warfarin therapy (two studies). The primary clinical outcomes were all-cause mortality, embolic events, and stroke. Secondary clinical outcomes were as follows: major adverse cardiac events (MACE; composite of death, cardiac perforation, myocardial infarction, air embolism, systemic embolism, stroke, transient ischemic attack, major bleeding, septicemia, pacemaker implantation, severe pericardial effusion, device dislodgment, device embolization, and cardiac tamponade); postoperative AF; postoperative complications; reoperation for bleeding; and major bleeding.
2.4. Statistical analysis
Two authors independently performed the meta-analysis using Cochrane Review Manager (version 5.4) [25]. The random-effects approach was used to calculate odds ratios (ORs) with 95% confidence intervals (CIs) [26]. A multivariate meta-regression was additionally performed to explore the potential confounding factors for each comparison. The assessment of the risk differences (RDs) between outcomes of interest, in addition to their odds ratios (ORs), strengthened statistical comparisons between LAAO vs. no LAAO/DOACs/VKAs. The meta-regression with RD calculation through random effects model delineated the observed risk of events between the study groups. Study heterogeneity was assessed by tau-squared, chi-squared, and I-squared tests. The I-squared values determined low (0–25%), moderate (26–50%), and high (51–100%) heterogeneity of findings. A two-tailed p-value of < 0.05 was considered statistically significant for heterogeneity and outcome variables.
2.5. Risk of bias
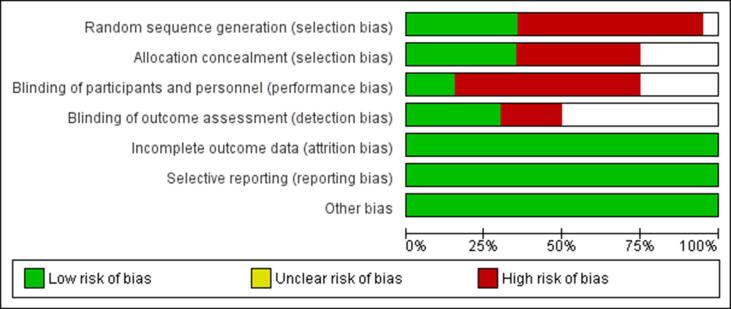
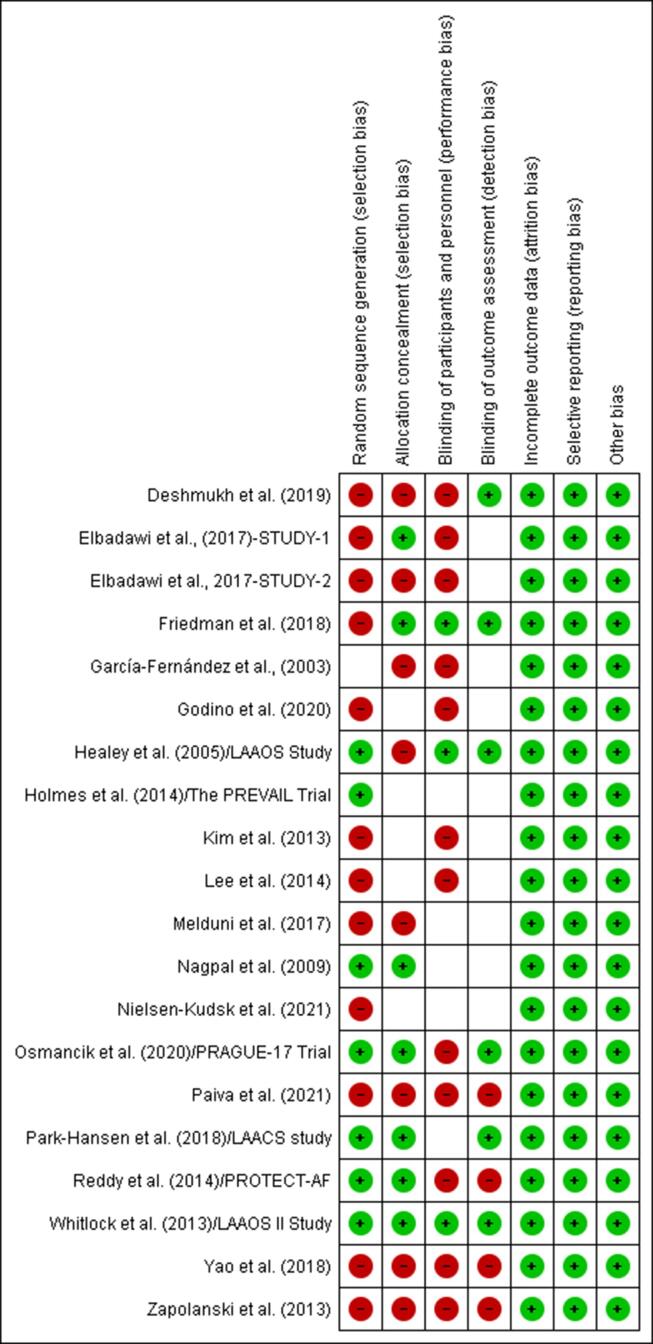
The risks of selection, performance, detection, attrition, and reporting biases in the systematic review and meta-analysis outcomes were determined using the Cochrane risk of bias graph and summary [27] (Fig. 1, Fig. 2a).
Fig. 1.
Risk of bias graph.
Fig. 2a.
Risk of bias summary.
3. Results
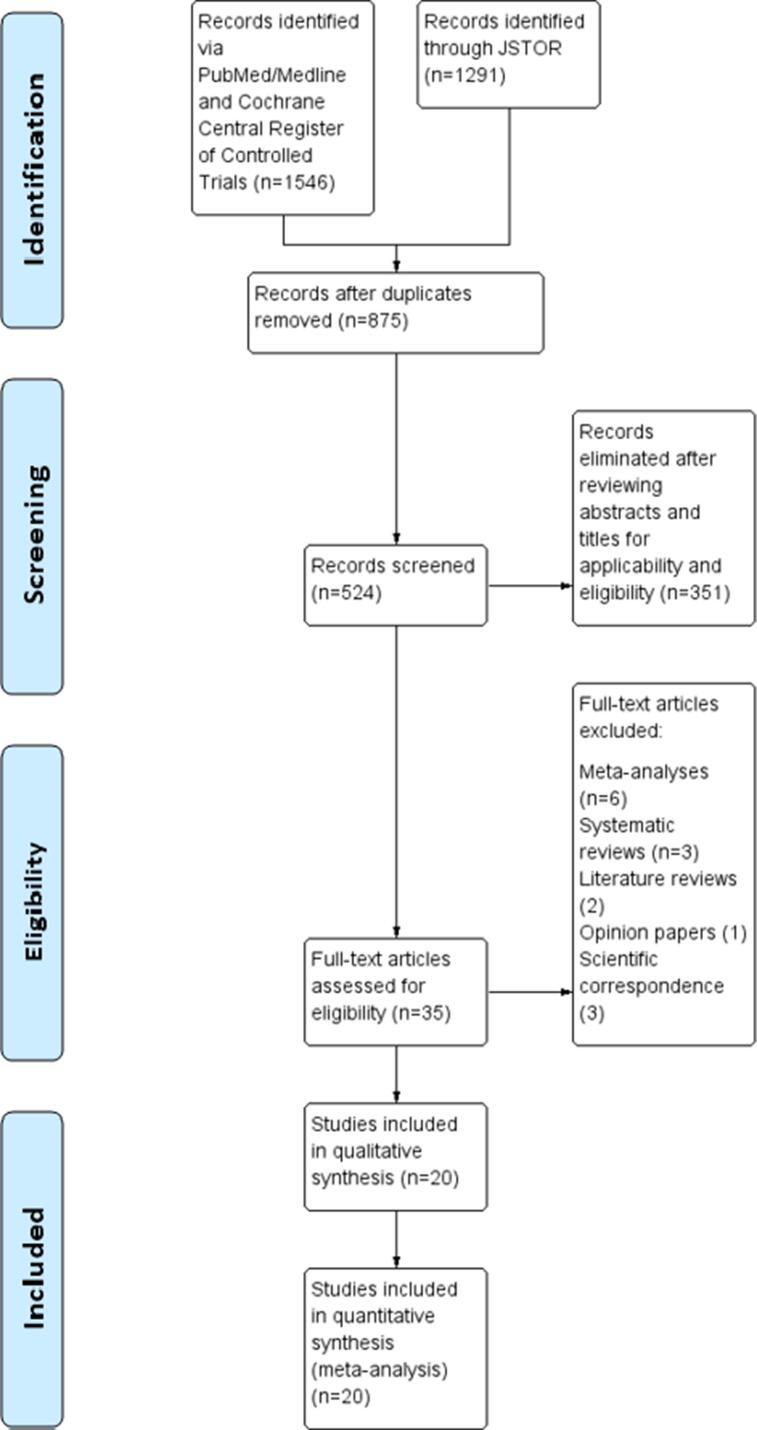
The search of the PubMed/Medline and Cochrane Central Register of Controlled Trials databases yielded 1546 articles of interest (Fig. 2b). A further 1291 articles of interest were retrieved from the JSTOR database. A total of 875 articles remained after removal of duplicates, 524 of which were potentially relevant studies. After elimination of 351 articles that were published only in abstract form and studies that were not comparable, 35 full-text articles were assessed for eligibility. Subsequent elimination of six meta-analyses, three systematic reviews, two literature reviews, one opinion paper, and three scientific correspondence articles, left 20 studies for systematic review and meta-analysis. The search results were finalized after 100% agreement was reached between the investigators.
Fig. 2b.
PRISMA Flow Diagram.
3.1. Study characteristics
Supplementary Table 1 summarizes the background characteristics of patients in the included studies. A total of 45,736 patients were included and comprised 26,878 who underwent LAAO, 1723 who received DOACs/NOACs, 382 who were treated with warfarin, and 16,753 who underwent cardiac surgery without LAAO. The mean patient age in the studies ranged from 50 years to 82 years. Supplementary Table 2 summarizes the sample size, publication year, design, and outcomes of the included studies [11], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]. Supplementary Table 3 summarizes the procedure-related complications, while Supplementary Table 4 elaborates on antithrombotic therapies between the study groups.
3.2. Clinical outcomes
3.2.1. LAAO vs no LAAO
Eight studies demonstrated a reduced risk of all-cause mortality in patients who underwent LAAO [28], [33], [34], [35], [36], [37], [39], [40]. One study indicated a higher incidence of all-cause mortality in patients who underwent cardiac surgery without LAAO [38]. There was a 58% reduction in all-cause mortality in patients who underwent LAAO (OR 0.75, 95% CI 0.47 – 1.18, p = 0.21; Fig. 2c). However, this finding was not statistically significant. The multivariate meta-regression finding (RD: −0.02 [95% CI −0.05, 0.01, p = 0.15]) confirmed the statistically insignificant RD for all-cause mortality between LAAO and non-LAAO groups (Supplementary Fig. 2).
Fig. 2c.
All-cause mortality (Forest plot).
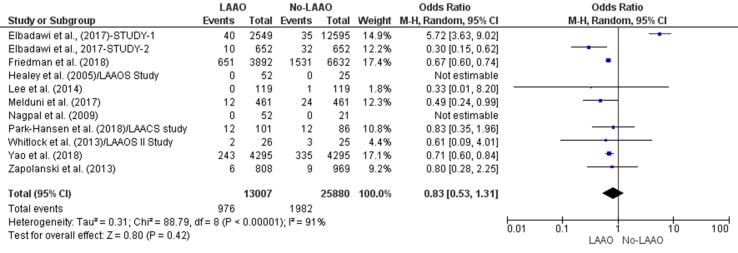
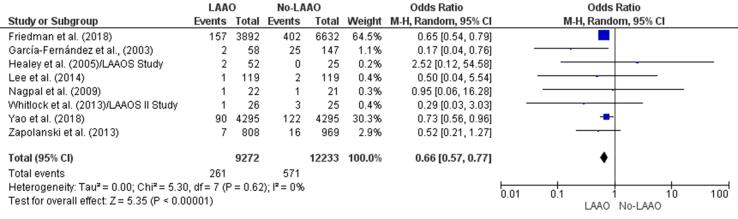
Seven studies [28], [30], [32], [33], [34], [35], [40] found a lower incidence of embolic events in patients who received LAAO and one [29] reported a higher risk of embolic episodes in patients who did not undergo LAAO. There was a statistically significant reduction (34%) in the incidence of embolic events in the LAAO group (OR 066, 95% CI 0.57–0.77, p < 0.001; Fig. 2d). The multivariate meta-regression findings confirmed the initial outcome with a statistically significant RD (-0.01, 95% CI −0.03, −0.00, p = 0.02) for embolic events between patients who underwent LAAO versus those who did not receive LAAO (Supplementary Fig. 3).
Fig. 2d.
Embolic events (Forest plot).
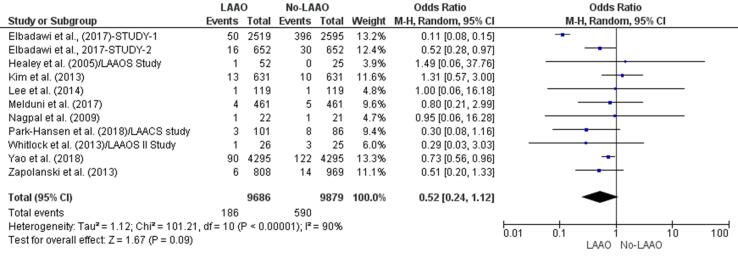
Seven studies [28], [33], [35], [36], [37], [38], [39] found that patients who underwent LAAO were less prone to stroke events and two [29], [31] reported a higher incidence of stroke in patients who did not receive LAAO [34]. The incidence of stroke events was 48% lower in the LAAO group (OR 0.52, 95% CI 0.24–1.12, p = 0.09; Fig. 2e). This finding was not statistically significant. In addition, the meta-regression further confirmed this outcome by revealing a statistically insignificant RD (-0.02, 95% CI −0.06, 0.01, p = 0.14) for stroke between LAAO and no-LAAO patients (Supplementary Fig. 4).
Fig. 2e.
Stroke (Forest plot).
Three studies [33], [37], [40] found a statistically significant reduction (42%) in risk of MACE in patients who underwent LAAO during cardiac surgery in comparison with those who did not (OR 0.58, 95% CI 0.38–0.88, p = 0.01; Fig. 2f). The meta-regression finding further revealed a statistically significant RD (-0.08, 95% CI −0.10, −0.06, p < 0.001) for MACE between patients who received LAAO versus those who were devoid of LAAO (Supplementary Fig. 5).
Fig. 2f.
MACE (Forest plot).
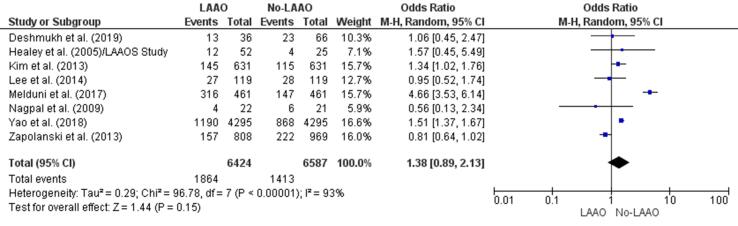
The incidence of postoperative AF was lower in patients who underwent LAAO in two studies [32], [35] and higher in patients who did not undergo LAAO in four studies [28], [29], [31], [36]. Overall, there was a 38% increase in postoperative AF events in patients who underwent cardiac surgery without LAAO (OR 1.38, 95% CI 0.89–2.13, p = 0.15; Fig. 3). This increase was not statistically significant. In addition, the finding from meta-regression confirmed a statistically insignificant RD (0.07, 95% CI −0.02, 0.16, p = 0.13) for postoperative AF among LAAO versus no-LAAO groups (Supplementary Fig. 6).
Fig. 3.
Postoperative atrial fibrillation (Forest plot).
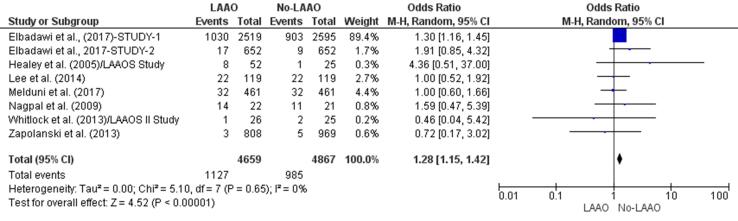
Three studies reported a higher incidence of postoperative complications in patients who underwent cardiac surgery that included LAAO [29], [32], [39] whereas two [33], [35] found that these patients were less prone to complications. There was a significant higher risk (28%) of postoperative complications in patients who did not undergo LAAO (OR 1.28, 95% CI 1.15–1.42, p < 0.001; Fig. 4a). The meta-regression finding; however, negated this result since the RD (0.02, 95% CI −0.03, 0.07, p = 0.39) for postoperative complications between LAAO and non-LAAO groups was found to be insignificant (Supplementary Fig. 7).
Fig. 4a.
Postoperative complications (Forest plot).
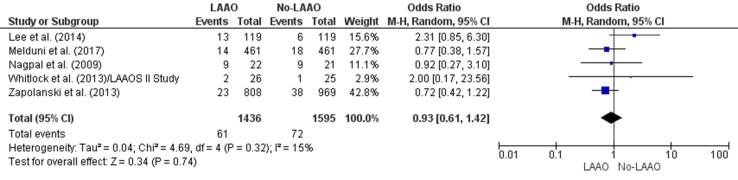
Three studies [32], [35], [36] found a lower incidence of reoperation for bleeding in patients who underwent LAAO during cardiac surgery and two studies [33], [34] did not. There was a 7% reduction in the risk of reoperation for bleeding in the LAAO group (OR 0.93, 95% CI 0.61–1.42, p = 0.74; Fig. 4b), which was not statistically significant. The meta-regression finding also did not produce statistically significant RD (-0.01, 95% CI 0.02, 0.01, p = 0.47) concerning reoperation for bleeding between patients with and without LAAO (Supplementary Fig. 8).
Fig. 4b.
Reoperation for bleeding (Forest plot).
3.2.2. LAAO vs NOACs/DOACs
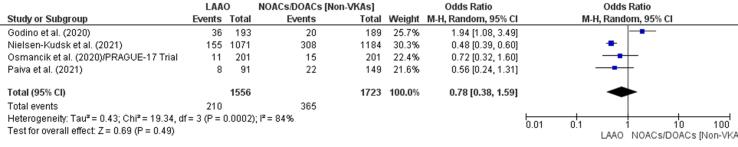
Three studies [43], [44], [45] reported a lower incidence of all-cause mortality in patients who underwent LAAO and one found a higher incidence in those who did not receive a NOAC or DOAC [42]. The incidence of all-cause mortality events was 31% lower in patients who underwent cardiac surgery with LAAO (OR 0.78, 95% CI 0.38–1.59, p = 0.49; Fig. 4c). The reduction was not statistically significant. The meta-regression supported the initial finding by confirming a non-significant RD (-0.03, 95% CI −0.12, 0.05, p = 0.48) for all-cause mortality between patients who received LAAO and those with NOACs/DOACs (Supplementary Fig. 9).
Fig. 4c.
All-cause mortality (Forest plot).
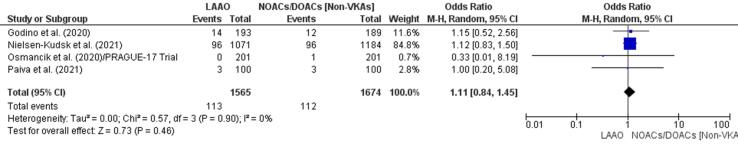
One study [43] found a lower incidence of embolic events in an LAAO group and another [42] found a higher incidence in a DOAC/NOAC group. Overall, there was a 54% increase in embolic episodes in patients who received DOAC therapy (OR 1.11, 95% CI 0.84–1.45, p = 0.46; Fig. 4d); this was not statistically significant. The meta-regression finding further ascertained a non-significant RD (-0.00, 95% CI −0.01, 0.01, p = 0.88) for embolic events between patients with LAA and NOACs/DOACs (Supplementary Fig. 10).
Fig. 4d.
Embolic events (Forest plot).
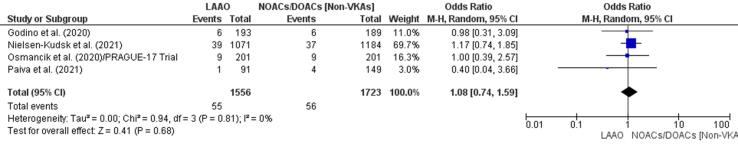
Three studies [42], [43], [44] found that neither LAAO nor DOAC therapy had an impact on stroke events. One study confirmed a lower incidence of stroke events in a LAAO group [45]. Overall, there was a 32% increase in stroke events in patients treated with a DOAC/NOAC (OR 1.08, 95% CI 0.74–1.59, p = 0.68; Fig. 4e). This finding was not statistically significant. The finding from meta-regression confirmed the initial result with a statistically insignificant RD (0.00, 95% CI −0.01, 0.01, p = 0.84) for stroke between the study groups (Supplementary Fig. 11).
Fig. 4e.
Stroke (Forest plot).
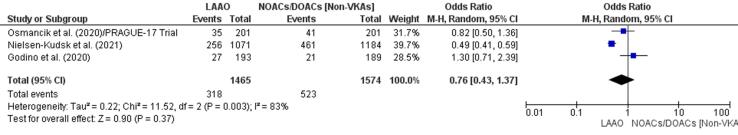
There was a lower incidence of MACE in the LAAO group in two studies [43], [44] and a higher incidence in the DOAC/NOAC group in another study [42]. Patients who underwent LAA showed a 24% reduction in MACE (OR 0.76, 95% CI 0.43–1.37, p = 0.37; Fig. 4f), which was statistically insignificant. The finding from meta-regression further negated RD (-0.05, 95% CI −0.18, 0.07, p = 0.39) for MACE between LAAO and NOAC/DOAC groups (Supplementary Fig. 12).
Fig. 4f.
MACE (Forest plot).
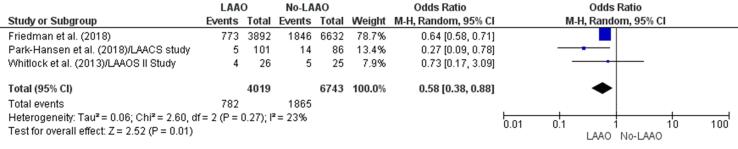
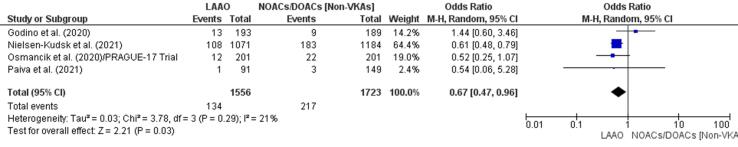
Major bleeding episodes were less common in the LAAO group in three studies [43], [44], [45] and more common in the DOAC/NOAC group in another study [42]. The finding of a 33% reduction in major bleeding episodes in patients who underwent LAAO (OR 0.67, 95% CI 0.47–0.96, p = 0.03; Fig. 5) was statistically significant. The meta-regression analysis; however, negated this outcome based on a statistically insignificant RD (-0.02, 95% CI −0.06, 0.01, p = 0.21) for major bleeding between the study groups (Supplementary Fig. 13).
Fig. 5.
Major bleeding (Forest plot).
3.2.3. LAAO vs VKAs
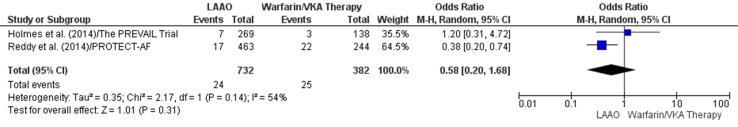
One study indicated no impact of LAAO or VKA therapy on all-cause mortality [46] and another found a lower incidence of all-cause mortality in the LAAO group [11]. Although there was a 42% decline in all-cause mortality in patients who underwent LAAO (OR: 0.58, 95% CI 0.20, 1.68, p = 0.31) (Fig. 6a); the decrease was not significant. This result was reconfirmed by a statistically insignificant RD (-0.02, 95% CI −0.09, 0.04, p = 0.47) through meta-regression (Supplementary Fig. 14).
Fig. 6a.
All-cause mortality (Forest plot).
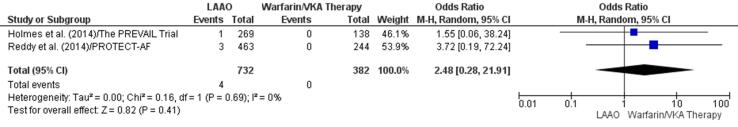
Two studies indicated a higher incidence of embolic events in patients who received a VKA [11], [46]. There was a 148% increase in embolic episodes in the VKA group (OR 2.48, 95% CI 0.28–21.91, p = 0.41; Fig. 6b); the increase was not significant. In addition, the RD (0.01, 95% −0.00, 0.01) for embolic events between the study groups was also found to be insignificant (p = 0.17) (Supplementary Fig. 15).
Fig. 6b.
Embolic events (Forest plot).
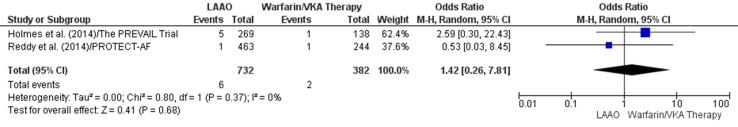
One study [46] found a higher incidence of stroke events in patients who received a VKA and another study [11] reported a lower incidence of these events in patients who underwent LAAO. There was a 42% increase in stroke episodes in patients receiving VKA therapy (OR 1.42, 95% CI 0.26–7.81, p = 0.68; Fig. 6c). However, this finding lacked statistical significance. The statistically insignificant RD (0.00, 95% CI −0.01, 0.02, p = 0.78) for stroke between LAA and VKA therapy groups reconfirmed the initial result (Supplementary Fig. 16).
Fig. 6c.
Stroke (Forest plot).
3.3. Assessment of heterogeneity
3.3.1. LAAO vs no LAAO
The findings for all-cause mortality, postoperative AF, and stroke were highly heterogenous (I-squared value, 51–100%) whereas those for embolic events, MACE, postoperative complications, and reoperation for bleeding showed low heterogeneity (I-squared value, 0–25%).
3.3.2. LAAO vs NOACs/DOACs
The findings for all-cause mortality and MACE showed high heterogeneity (I-squared value, 51–100%) whereas those for embolic events, major bleeding, and stroke showed low heterogeneity (I-squared value, 0–25%).
3.3.3. LAAO vs VKAs
Heterogeneity was high for all-cause mortality (I-squared value, 51–100%) and low for embolic events and stroke (I-squared value, 0–25%).
3.4. Risk of bias
The risk of bias was 65% for random sequence generation, 45% for allocation concealment, 55% for blinding of participants, and 30% for blinding of outcome assessment (Fig. 2a). However, the risk of bias assessment revealed a low risk of attrition and reporting bias in the outcomes data.
4. Discussion
This meta-analysis found a 34% reduction in embolic events [25], [27], [29], [30], [31], [32], [37] and a 42% decrease in MACE [30], [34], [37] in patients who underwent cardiac surgery and received LAAO compared with their counterparts who did not. Our findings negated the RD for all-cause mortality, postoperative AF, postoperative complications, reoperation for bleeding, and stroke in patients who underwent LAAO group than in those who did not [26], [29], [36]. They also ruled out RD for all-cause mortality, embolic events, MACE, major bleeding, and stroke in patients who underwent LAAO group compared with those who received DOAC/NOAC therapy [40], [41], [42]. No statistically significant difference in all-cause mortality, embolic events, or stroke was found between patients who underwent LAAO and those who received VKA/warfarin therapy, which confirms the non-inferiority of LAAO to VKAs. The results of this meta-analysis were statistically insignificant for differences in all-cause mortality, stroke, postoperative AF, postoperative complications, and reoperation for bleeding between LAAO and no LAAO. Furthermore, there was no statistically significant difference in all-cause mortality, embolic events, major bleeding, stroke, or MACE between patients who underwent LAAO and those who were treated with NOACs/DOACs. These outcomes further confirm the non-inferiority of LAAO to no LAAO and DOAC/NOAC therapy.
Previous meta-analyses confirmed the potential of LAAO to minimize all-cause mortality, stroke, thromboembolic episodes, and hemorrhagic events in patients undergoing cardiac surgery [21], [22], [23], [24]. The findings of the present meta-analysis expand these outcomes in favor of LAAO in the setting of AF. The added benefits of LAAO in patients with AF include a reduced risk of embolic events and MACE. The pooled results further affirm the non-inferiority of LAAO to DOAC/warfarin or no LAA measures for embolic events, all-cause mortality, MACE, postoperative AF, stroke, or reoperation for bleeding.
LAAO effectively improves the reservoir expansion index, volume, and function of the LA [47]. Enhancement of the contractile function of the LA after LAAO helps to improve peak atrial contraction strain of the LA and left ventricular ejection fraction in patients with sinus rhythm. LAAO further impacts the Frank-Starling effect, which enhances the mechanical function of the LA. The LAA is a major risk site for development of thrombus in patients with non-valvular AF. Accordingly, exclusion of the LAA reduces the risk of thromboembolic events, particularly in patients who are poorly tolerant of oral anticoagulation [48]. Percutaneous or surgical LAAO minimizes the contractility of the LAA, which eventually reduces the risk of clot formation. Patients with non-valvular AF also have a 10% risk of intramural thrombus, which increases the need for modification of the LAA [49]. Patients with AF and a known history of atrial fibrosis have a high propensity for ischemic/hemorrhagic stroke and transient ischemic attacks. The thromboembolic risk in patients with AF is due to the complex interplay between anatomical factors involving the LAA, left atrial fibrosis, and atrial myopathy. The protective role of LAAO in preventing cardiovascular events can be attributed to a reduction in atrial myocardial stretch, left atrial volume, and left atrial filling pressures. Closure of the LAA requires either an epicardial suture via surgery or a self-expanding nitinol implant through a transseptal puncture across the base/orifice of the LAA [50]. However, the current guidelines recommend continuation of oral anticoagulation therapy despite LAAO during cardiac surgery.
4.1. Limitations
First, the high percentage of studies with selection and performance bias limits the generalizability of the findings of this study. Second, the limited availability of randomized controlled studies and lack of allocation concealment (i.e., blinding of participants) further limit the reliability of our findings concerning the benefits of LAAO in cardiac surgery. Third, our results were not stratified according to whether LAAO was surgery-based or percutaneously administered. Fourth, the limited number of studies available for evaluation of LAAO vs DOACs and VKAs might have produced weak results concerning the potential to replace DOAC/VKA therapy with LAAO. Finally, the findings of this meta-analysis hold limited validity for young adults.
4.2. Conclusions
LAAO is potentially superior to no LAAO in terms of reducing the incidence of embolic events and MACE in patients with AF. The therapeutic safety and efficacy of LAAO is equivalent to NOACs/DOACs in reducing the incidence of all-cause mortality, embolic events, MACE, major bleeding, and stroke in AF scenarios. In addition, LAAO is non-inferior to VKAs in reducing the incidence of all-cause mortality, stroke, and embolic events. It is also equivalent to no-LAAO in reducing the incidence of all-cause mortality, postoperative AF, postoperative complications, reoperation for bleeding, and stroke in patients with AF. However, it remains debatable whether it is possible to completely replace DOAC/NOAC/warfarin therapy with LAAO in patients with AF.
Funding
The study was not supported by any funding sources
Disclosures
None
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Author contributions: NN conceived the study hypothesis. NN, MN, SL TS, LG, AF,SB, MT, YB and RB designed the study and performed the systematic search, study selection, and data extraction. NN analyzed the data. All authors contributed to the interpretation of the data, writing and critical editing of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2022.100998.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Alsagheir A., Koziarz A., Belley-Côté E.P., Whitlock R.P. Left Atrial Appendage Occlusion: A Narrative Review. J. Cardiothorac. Vasc. Anesth. 2019;33(6):1753–1765. doi: 10.1053/j.jvca.2019.01.054. [DOI] [PubMed] [Google Scholar]
- 2.Berti S., Pastormerlo L.E., Santoro G., Brscic E., Montorfano M., Vignali L., Danna P., Tondo C., Rezzaghi M., D’Amico G., Stabile A., Saccà S., Patti G., Rapacciuolo A., Poli A., Golino P., Magnavacchi P., Meucci F., Pezzulich B., Stolcova M., Tarantini G. Intracardiac Versus Transesophageal Echocardiographic Guidance for Left Atrial Appendage Occlusion. JACC: Cardiovasc. Interventions. 2018;11(11):1086–1092. doi: 10.1016/j.jcin.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann K.J., Arshi A., Yakubov S.J. An Overview of Left Atrial Appendage Occlusion Devices. Curr. Cardiol. Rep. 2015;17(4) doi: 10.1007/s11886-015-0573-0. [DOI] [PubMed] [Google Scholar]
- 4.Akin I., Nienaber C.A. Left atrial appendage occlusion: A better alternative to anticoagulation? World J. Cardiol. 2017;9(2):139. doi: 10.4330/wjc.v9.i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccini J.P., Sievert H., Patel M.R. Left atrial appendage occlusion: rationale, evidence, devices, and patient selection. Eur. Heart J. 2016 doi: 10.1093/eurheartj/ehw330. [DOI] [PubMed] [Google Scholar]
- 6.Dar T., Turagam M.K., Yarlagadda B., Tantary M., Sheldon S.H., Lakkireddy D. Indication, Patient Selection, and Referral Pathways for Left Atrial Appendage Closure. Interventional Cardiol. Clinics. 2018;7(2):169–183. doi: 10.1016/j.iccl.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Zweiker D., Sieghartsleitner R., Fiedler L., Toth G.G., Luha O., Stix G., Gabriel H., Vock P., Lileg B., Strouhal A., Delle-Karth G., Pfeffer M., Aichinger J., Tkalec W., Steinwender C., Sihorsch K., Binder R.K., Rammer M., Barbieri F., Mueller S., Verheyen N., Ablasser K., Zirlik A., Scherr D. Indications and Outcome in Patients Undergoing Left Atrial Appendage Closure—The Austrian LAAC Registry. J. Clin. Med. 2020;9(10):3274. doi: 10.3390/jcm9103274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahiner L., et al. Left Atrial Appendage Occlusion in Patients With Thrombus in Left Atrial Appendage. J. Invasive Cardiol. 2020;32(6):222–227. [PubMed] [Google Scholar]
- 9.P. Agasthi, R. Arsanjani, Catheter Management Of Left Atrial Appendage Closure Devices, in: StatPearls, Treasure Island (FL), 2021. [PubMed]
- 10.Whitlock R.P., Belley-Cote E.P., Paparella D., Healey J.S., Brady K., Sharma M., Reents W., Budera P., Baddour A.J., Fila P., Devereaux P.J., Bogachev-Prokophiev A., Boening A., Teoh K.H.T., Tagarakis G.I., Slaughter M.S., Royse A.G., McGuinness S., Alings M., Punjabi P.P., Mazer C.D., Folkeringa R.J., Colli A., Avezum Á., Nakamya J., Balasubramanian K., Vincent J., Voisine P., Lamy A., Yusuf S., Connolly S.J. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N. Engl. J. Med. 2021;384(22):2081–2091. doi: 10.1056/NEJMoa2101897. [DOI] [PubMed] [Google Scholar]
- 11.Reddy V.Y., Sievert H., Halperin J., Doshi S.K., Buchbinder M., Neuzil P., Huber K., Whisenant B., Kar S., Swarup V., Gordon N., Holmes D. Percutaneous Left Atrial Appendage Closure vs Warfarin for Atrial Fibrillation. JAMA. 2014;312(19):1988. doi: 10.1001/jama.2014.15192. [DOI] [PubMed] [Google Scholar]
- 12.Marroquin L., et al. Management and outcomes of patients with left atrial appendage thrombus prior to percutaneous closure. Heart. 2021 doi: 10.1136/heartjnl-2021-319811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alkhouli M., Busu T., Shah K., Osman M., Alqahtani F., Raybuck B. Incidence and Clinical Impact of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion. JACC: Clin. Electrophysiol. 2018;4(12):1629–1637. doi: 10.1016/j.jacep.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Reddy V.Y., Holmes D., Doshi S.K., Neuzil P., Kar S. Safety of Percutaneous Left Atrial Appendage Closure. Circulation. 2011;123(4):417–424. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 15.Kemi Y., Yamashita E., Fujiwara T., Kario K., Sasaki T., Minami K., Miki Y., Goto K., Take Y., Nakamura K., Naito S. The prevalence and characteristics of candidates for percutaneous left atrial appendage occlusion using a WATCHMAN device among patients who underwent atrial fibrillation ablation in a Japanese population. J. Echocardiography. 2021;19(4):243–249. doi: 10.1007/s12574-021-00538-5. [DOI] [PubMed] [Google Scholar]
- 16.Agudelo V.H., Millán X., Li C.-H., Moustafa A.-H., Asmarats L., Serra A., Arzamendi D. Prevalence, Mechanisms and Impact of Residual Patency and Device-Related Thrombosis Following Left Atrial Appendage Occlusion: a Computed Tomography Analysis. EuroIntervention. 2021;17(11):e944–e952. doi: 10.4244/EIJ-D-21-00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morillo C.A., et al. Atrial fibrillation: the current epidemic. J. Geriatr Cardiol. 2017;14(3):195–203. doi: 10.11909/j.issn.1671-5411.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertog S., Sievert H. Left atrial appendage closure: prevalence and risk of device-associated thrombus formation. Cardiovasc. Diagn Ther. 2019;9(1):104–109. doi: 10.21037/cdt.2018.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thakkar J., et al. Incidence, Prevention, and Management of Periprocedural Complications of Left Atrial Appendage Occlusion. Interv. Cardiol. Clin. 2018;7(2):243–252. doi: 10.1016/j.iccl.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Deegan R., Ellis C.R., Bennett J.M. The Left Atrial Appendage, Including LAA Occlusion Devices Line Watchman, Amulet, and Lariat. Seminars Cardiothorac. Vasc. Anesthesia. 2019;23(1):70–87. doi: 10.1177/1089253218789159. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim A.M., Tandan N., Koester C., Al-Akchar M., Bhandari B., Botchway A., Abdelkarim J., Maini R., Labedi M. Meta-Analysis Evaluating Outcomes of Surgical Left Atrial Appendage Occlusion During Cardiac Surgery. Am. J. Cardiol. 2019;124(8):1218–1225. doi: 10.1016/j.amjcard.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 22.Atti V., Anantha-Narayanan M., Turagam M.K., Koerber S., Rao S., Viles-Gonzalez J.F., Suri R.M., Velagapudi P., Lakkireddy D., Benditt D.G. Surgical left atrial appendage occlusion during cardiac surgery: A systematic review and meta-analysis. World J. Cardiol. 2018;10(11):242–249. doi: 10.4330/wjc.v10.i11.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanif H., Belley-Cote E.P., Alotaibi A., Dvirnik N., Neupane B., Beyene J., Eikelboom J.W., Holmes D., Whitlock R.P. Left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation: a systematic review and network meta-analysis of randomized controlled trials. J. Cardiovasc. Surgery. 2018;59(1) doi: 10.23736/S0021-9509.17.09824-X. [DOI] [PubMed] [Google Scholar]
- 24.Li X., Wen S.-N., Li S.-N., Bai R., Liu N., Feng L.i., Ruan Y.-F., Du X., Dong J.-Z., Ma C.-S. Over 1-year efficacy and safety of left atrial appendage occlusion versus novel oral anticoagulants for stroke prevention in atrial fibrillation: A systematic review and meta-analysis of randomized controlled trials and observational studies. Heart Rhythm. 2016;13(6):1203–1214. doi: 10.1016/j.hrthm.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 25.Bax L., et al. A systematic comparison of software dedicated to meta-analysis of causal studies. BMC Med. Res. Methodol. 2007;7:40. doi: 10.1186/1471-2288-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazra A. Using the confidence interval confidently. J. Thoracic Disease. 2017;9(10):4124–4129. doi: 10.21037/jtd.2017.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins J.P.T., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343(oct18 2):d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao X., Gersh B.J., Holmes D.R., Melduni R.M., Johnsrud D.O., Sangaralingham L.R., Shah N.D., Noseworthy P.A. Association of Surgical Left Atrial Appendage Occlusion With Subsequent Stroke and Mortality Among Patients Undergoing Cardiac Surgery. JAMA. 2018;319(20):2116. doi: 10.1001/jama.2018.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Healey J.S., Crystal E., Lamy A., Teoh K., Semelhago L., Hohnloser S.H., Cybulsky I., Abouzahr L., Sawchuck C., Carroll S., Morillo C., Kleine P., Chu V., Lonn E., Connolly S.J. Left Atrial Appendage Occlusion Study (LAAOS): Results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am. Heart J. 2005;150(2):288–293. doi: 10.1016/j.ahj.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 30.García-Fernández M.N., Pérez-David E., Quiles J., Peralta J., García-Rojas I., Bermejo J., Moreno M., Silva J. Role of left atrial appendageobliteration in stroke reductionin patients with mitral valve prosthesis. J. Am. Coll. Cardiol. 2003;42(7):1253–1258. doi: 10.1016/s0735-1097(03)00954-9. [DOI] [PubMed] [Google Scholar]
- 31.Kim R., Baumgartner N., Clements J. Routine left atrial appendage ligation during cardiac surgery may prevent postoperative atrial fibrillation–related cerebrovascular accident. J. Thorac. Cardiovasc. Surgery. 2013;145(2):582–589. doi: 10.1016/j.jtcvs.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Nagpal A.D., Torracca L., Fumero A., Denti P., Cioni M., Alfieri O. Concurrent prophylactic left atrial appendage exclusion: results from a randomized controlled trial pilot study☆. Eur. J. Cardiothorac. Surg. 2009;36(3):553–557. doi: 10.1016/j.ejcts.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Whitlock R.P., Vincent J., Blackall M.H., Hirsh J., Fremes S., Novick R., Devereaux P.J., Teoh K., Lamy A., Connolly S.J., Yusuf S., Carrier M., Healey J.S. Left Atrial Appendage Occlusion Study II (LAAOS II) Can. J. Cardiol. 2013;29(11):1443–1447. doi: 10.1016/j.cjca.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Lee C.-H., Kim J.B., Jung S.-H., Choo S.J., Chung C.H., Lee J.W. Left Atrial Appendage Resection Versus Preservation During the Surgical Ablation of Atrial Fibrillation. Ann. Thoracic Surgery. 2014;97(1):124–132. doi: 10.1016/j.athoracsur.2013.07.073. [DOI] [PubMed] [Google Scholar]
- 35.A. Zapolanski et al., Epicardial Surgical Ligation of the Left Atrial Appendage is Safe, Reproducible, and Effective by Transesophageal Echocardiographic Follow-up. Innovations: Technology and Techniques in Cardiothoracic and Vascular Surgery 8(5) (2013) 371-375. [DOI] [PubMed]
- 36.Melduni R.M., Schaff H.V., Lee H.-C., Gersh B.J., Noseworthy P.A., Bailey K.R., Ammash N.M., Cha S.S., Fatema K., Wysokinski W.E., Seward J.B., Packer D.L., Rihal C.S., Asirvatham S.J. Impact of Left Atrial Appendage Closure During Cardiac Surgery on the Occurrence of Early Postoperative Atrial Fibrillation, Stroke, and Mortality. Circulation. 2017;135(4):366–378. doi: 10.1161/CIRCULATIONAHA.116.021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park-Hansen J., Holme S.J.V., Irmukhamedov A., Carranza C.L., Greve A.M., Al-Farra G., Riis R.G.C., Nilsson B., Clausen J.S.R., Nørskov A.S., Kruuse C.R., Rostrup E., Dominguez H. Adding left atrial appendage closure to open heart surgery provides protection from ischemic brain injury six years after surgery independently of atrial fibrillation history: the LAACS randomized study. J. Cardiothoracic Surgery. 2018;13(1) doi: 10.1186/s13019-018-0740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elbadawi A., Ogunbayo G.O., Elgendy I.Y., Olorunfemi O., Saad M., Ha L.D., Alotaki E., Baig B., Abuzaid A.S., Shahin H.I., Shah A., Rao M. Impact of Left Atrial Appendage Exclusion on Cardiovascular Outcomes in Patients With Atrial Fibrillation Undergoing Coronary Artery Bypass Grafting (From the National Inpatient Sample Database) Am. J. Cardiol. 2017;120(6):953–958. doi: 10.1016/j.amjcard.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Elbadawi A., Olorunfemi O., Ogunbayo G.O., Saad M., Elgendy I.Y., Arif Z., Badran H., Saheed D., Ahmed H.M.A., Rao M. Cardiovascular Outcomes With Surgical Left Atrial Appendage Exclusion in Patients With Atrial Fibrillation Who Underwent Valvular Heart Surgery (from the National Inpatient Sample Database) Am. J. Cardiol. 2017;119(12):2056–2060. doi: 10.1016/j.amjcard.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 40.Friedman D.J., Piccini J.P., Wang T., Zheng J., Malaisrie S.C., Holmes D.R., Suri R.M., Mack M.J., Badhwar V., Jacobs J.P., Gaca J.G., Chow S.-C., Peterson E.D., Brennan J.M. Association Between Left Atrial Appendage Occlusion and Readmission for Thromboembolism Among Patients With Atrial Fibrillation Undergoing Concomitant Cardiac Surgery. JAMA. 2018;319(4):365. doi: 10.1001/jama.2017.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deshmukh A., Bhatia A., Sayer G.T., Kim G., Raikhelkar J., Imamura T., Ozcan C., Ota T., Jeevanandam V., Uriel N. Left Atrial Appendage Occlusion With Left Ventricular Assist Device Decreases Thromboembolic Events. Ann. Thoracic Surgery. 2019;107(4):1181–1186. doi: 10.1016/j.athoracsur.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Godino C., Melillo F., Bellini B., Mazzucca M., Pivato C.A., Rubino F., Figini F., Mazzone P., Della Bella P., Margonato A., Colombo A., Montorfano M. Percutaneous left atrial appendage closure versus non-vitamin K oral anticoagulants in patients with non-valvular atrial fibrillation and high bleeding risk. EuroIntervention. 2020;15(17):1548–1554. doi: 10.4244/EIJ-D-19-00507. [DOI] [PubMed] [Google Scholar]
- 43.Osmancik P., Herman D., Neuzil P., Hala P., Taborsky M., Kala P., Poloczek M., Stasek J., Haman L., Branny M., Chovancik J., Cervinka P., Holy J., Kovarnik T., Zemanek D., Havranek S., Vancura V., Opatrny J., Peichl P., Tousek P., Lekesova V., Jarkovsky J., Novackova M., Benesova K., Widimsky P., Reddy V.Y. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2020;75(25):3122–3135. doi: 10.1016/j.jacc.2020.04.067. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen-Kudsk J.E., Korsholm K., Damgaard D., Valentin J.B., Diener H.-C., Camm A.J., Johnsen S.P. Clinical Outcomes Associated With Left Atrial Appendage Occlusion Versus Direct Oral Anticoagulation in Atrial Fibrillation. JACC: Cardiovasc. Interventions. 2021;14(1):69–78. doi: 10.1016/j.jcin.2020.09.051. [DOI] [PubMed] [Google Scholar]
- 45.Paiva L., Coelho J., Barra S., Costa M., Sargento-Freitas J., Cunha L., Gonçalves L. Non-vitamin K antagonist oral anticoagulation versus left atrial appendage occlusion for primary and secondary stroke prevention after cardioembolic stroke. Rev. Port. Cardiol. 2021;40(5):357–365. doi: 10.1016/j.repce.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 46.Holmes D.R., Kar S., Price M.J., Whisenant B., Sievert H., Doshi S.K., Huber K., Reddy V.Y. Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients With Atrial Fibrillation Versus Long-Term Warfarin Therapy. J. Am. Coll. Cardiol. 2014;64(1):1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 47.Coisne A., Pilato R., Brigadeau F., Klug D., Marquie C., Souissi Z., Richardson M., Mouton S., Polge A.-S., Lancellotti P., Lacroix D., Montaigne D. Percutaneous left atrial appendage closure improves left atrial mechanical function through Frank-Starling mechanism. Heart Rhythm. 2017;14(5):710–716. doi: 10.1016/j.hrthm.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 48.Akinapelli A., et al. Left Atrial Appendage Closure –The WATCHMAN Device. Curr. Cardiol. Reviews. 2015;11(4):334–340. doi: 10.2174/1573403X11666150805115822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rashid H.N., Layland J. Modification of the left atrial appendage and its role in stroke risk reduction with non-valvular atrial fibrillation. IJC Heart Vasculature. 2021;32:100688. doi: 10.1016/j.ijcha.2020.100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakellaridis T., et al. Left atrial appendage exclusion-Where do we stand? J. Thorac. Dis. 2014;6(Suppl 1):S70–S77. doi: 10.3978/j.issn.2072-1439.2013.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.