Abstract
A deep comprehension of the vaginal ecosystem may hold promise for unraveling the pathophysiology of pregnancy and may provide novel biomarkers to identify subjects at risk of maternal-fetal complications. In this prospective study, we assessed the characteristics of the vaginal environment in a cohort of pregnant women throughout their different gestational ages and puerperium. Both the vaginal bacterial composition and the vaginal metabolic profiles were analyzed. A total of 63 Caucasian women with a successful pregnancy and 9 subjects who had a first trimester miscarriage were enrolled. For the study, obstetric examinations were scheduled along the three trimester phases (9-13, 20-24, 32-34 gestation weeks) and puerperium (40-55 days after delivery). Two vaginal swabs were collected at each time point, to assess the vaginal microbiome profiling (by Nugent score and 16S rRNA gene sequencing) and the vaginal metabolic composition (1H-NMR spectroscopy). During pregnancy, the vaginal microbiome underwent marked changes, with a significant decrease in overall diversity, and increased stability. Over time, we found a significant increase of Lactobacillus and a decrease of several genera related to bacterial vaginosis (BV), such as Prevotella, Atopobium and Sneathia. It is worth noting that the levels of Bifidobacterium spp. tended to decrease at the end of pregnancy. At the puerperium, a significantly lower content of Lactobacillus and higher levels of Gardnerella, Prevotella, Atopobium, and Streptococcus were observed. Women receiving an intrapartum antibiotic prophylaxis for Group B Streptococcus (GBS) were characterized by a vaginal abundance of Prevotella compared to untreated women. Analysis of bacterial relative abundances highlighted an increased abundance of Fusobacterium in women suffering a first trimester abortion, at all taxonomic levels. Lactobacillus abundance was strongly correlated with higher levels of lactate, sarcosine, and many amino acids (i.e., isoleucine, leucine, phenylalanine, valine, threonine, tryptophan). Conversely, BV-associated genera, such as Gardnerella, Atopobium, and Sneathia, were related to amines (e.g., putrescine, methylamine), formate, acetate, alcohols, and short-chain fatty-acids (i.e., butyrate, propionate).
Keywords: vaginal microbiome, vaginal metabolome, pregnancy, puerperium, miscarriage, women’s health
Introduction
In healthy reproductive-aged women, the vaginal microbiome is generally dominated by members of the Lactobacillus genus (van der Wijgert et al., 2014; Smith and Ravel, 2017). Lactobacilli promote the maintenance of the vaginal health, preventing the colonization and growth of adverse microorganisms through various mechanisms, such as vaginal pH lowering, bioactive compounds production, competition for adhesion, and modulation of immune response (Parolin et al., 2015; Petrova et al., 2015; Foschi et al., 2017). On the other hand, the reduction of lactobacilli combined with the increase of different species of anaerobic bacteria (e.g., Gardnerella, Atopobium, Prevotella, Mobiluncus) results in the switch from a normal vaginal ecosystem to a polymicrobial dysbiosis, namely bacterial vaginosis (BV) (Parolin et al., 2018; Ceccarani et al., 2019). This condition is accompanied by marked alterations in the composition of vaginal metabolites, being higher concentrations of various biogenic amines and short chain fatty acids (SCFAs) common fingerprints of BV condition (Yeoman et al., 2013).
On a regular basis, the composition of the vaginal microbiome can vary throughout a woman’s life in response to various factors, such as hormonal status, diet, sexual habits, pharmaceutical treatments, and urogenital infections (Kroon et al., 2018; Noyes et al., 2018; Dall'Asta et al., 2021). Specifically, during pregnancy, the vaginal microbiome undergoes marked changes, with a significant decrease in overall bacterial diversity, an increased stability, and an enrichment of Lactobacillus spp. (Aagaard et al., 2012; DiGiulio et al., 2015; Gupta et al., 2020; Marangoni et al., 2021). Contrariwise, in the postpartum period (i.e., puerperium), the vaginal microbiome becomes less Lactobacillus spp. dominated, with increased biodiversity (MacIntyre et al., 2015).
It is well known that the composition of the vaginal bacterial communities and related metabolites play a crucial role in maternal-fetal health (Fox and Eichelberger, 2015; Nelson et al., 2016; Laghi et al., 2021). As for healthy vaginal environments, healthy pregnancies are usually characterized by a lactobacilli-dominated ecosystem, whereas reduced lactobacilli abundances, increased bacterial diversity, and higher levels of specific vaginal metabolites (e.g., acetone, formate, isopropanol, methanol) are associated with preterm birth and other complications (Prince et al., 2014; Ansari et al., 2020; Di Simone et al., 2020). For example, reduced prevalence of Lactobacillus spp. and higher levels of selected vaginal metabolites (inosine, fumarate, xanthine, benzoate, ascorbate) (Al-Memar et al., 2020; Xu et al., 2020; Marangoni et al., 2021) seem to be predictors of a higher risk of spontaneous miscarriage.
In this context, only few studies have investigated the association between the structure of the vaginal ecosystem and the first trimester miscarriage (Zhang et al., 2019; Al-Memar et al., 2020; Fan et al., 2020; Xu et al., 2020), while many aspects about the dynamic interactions between the inhabitants of the vaginal ecosystem, their metabolites, and the host remain to be fully elucidated despite the recent advances in the study of the human microbiome during pregnancy and puerperium (Vinturache et al., 2016; Gupta et al., 2020).
Therefore, the aim of this study was to deepen the characteristics of the vaginal environment in a cohort of Caucasian women with a normal pregnancy throughout their different gestational ages (i.e., first, second, third trimester) and puerperium. A group of women suffering a spontaneous first trimester miscarriage was also included for a wider characterization. For each subject and each time point, both the vaginal bacterial composition (16S rRNA sequencing) and the vaginal metabolic profiles (Proton nuclear magnetic resonance spectroscopy-1H-NMR) were analyzed.
Materials and Methods
Study Cohort and Samples Collection
Subjects were enrolled among all the Caucasian pregnant women presenting to the Family Advisory Health Centers of Ravenna (Italy) for prenatal care starting from April 2019.
Exclusion criteria were the following: (i) age < 18 years; (ii) HIV positivity; (iii) body mass index (BMI) > 33; (iv) medically assisted procreation; (v) use of any antibiotics in the month preceding the sampling; (vi) use of vaginal douches or topical agents in the two weeks before sampling; (vii) presence of uncontrolled chronic diseases (e.g., diabetes, autoimmune disorders, malignancies); (viii) drug addiction or heavy smokers (> 15 cigarettes/day). Moreover, women with urogenital infections due to sexually transmitted pathogens (i.e., Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Mycoplasma genitalium), aerobic vaginitis (AV) or symptomatic vulvo-vaginal candidiasis (VVC) were further excluded after the laboratory testing.
At gestational age of 9-13 weeks (first trimester), 20-24 weeks (second trimester), 32-34 weeks (third trimester), and puerperium (40-55 days after delivery) women underwent an obstetric examination. For all patients, demographic data and information about urogenital symptoms were recorded.
Women colonized with Group B Streptococcus (GBS) at the third trimester of pregnancy received intrapartum antibiotic prophylaxis (IAP) (i.e., penicillin G or ampicillin), following international guidelines (Kolkman et al., 2020).
Two vaginal swabs were collected at each time point. The first one (E-swab, Copan, Brescia, Italy) was used for microbiological diagnostic tests and Nugent score assessment. The second was collected with a sterile cotton bud, re-suspended in 1 mL of sterile saline, and stored at -80°C until use. Frozen vaginal swabs were thawed, vortexed for 1 min and removed from the liquid. After centrifugation (10000 × g for 15 min), the cell-free supernatants were used for metabolomic analysis, whereas bacterial pellets were employed for vaginal microbiome profiling.
A written informed consent was obtained from all subjects and the study protocol was approved by the Ethics Committee of Romagna (CEROM) (n° 2032 of 21st February 2018). This study was carried out in accordance with the Declaration of Helsinki, following the recommendations of the Ethics Committee.
Microbiological Investigations
A commercial nucleic acid amplification technique (NAAT) was used for C. trachomatis, N. gonorrhoeae, T. vaginalis and M. genitalium detection (Seeplex STI Master Panel 1; Seegene, Seoul, KR). VVC was excluded by the microscopic presence of fungal buds and a significant growth of Candida colonies by culture (Yano et al., 2019). AV were diagnosed by means of a microscopic examination (i.e., diminished/absent lactobacilli, presence of leukocytes, parabasal cells, small coliform bacilli, cocci, or chains), combined with the growth of aerobic microorganisms, mainly of intestinal origin, by culture (Donders et al., 2011).
A Gram stain scoring system (Nugent score) was used for a preliminary assessment of the vaginal flora composition (Nugent et al., 1991). Based on this score, women were grouped as follows: ‘H’ group (normal lactobacilli-dominated microbiota, score 0-3), ‘I’ group (intermediate microbiota/flora, score 4-6), ‘BV’ group (bacterial vaginosis, score 7-10) (Zozaya-Hinchliffe et al., 2010).
Vaginal Microbiome Profiling
Nucleic acids were extracted from vaginal swabs by means of the Versant molecular system (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) equipped with a sample preparation module designed for automated sample preparation (Marangoni et al., 2015).
The V3-V4 hypervariable regions of the bacterial 16S rRNA gene were amplified, according to the 16S metagenomic sequencing library preparation protocol (Illumina, San Diego, CA, USA). Final indexed libraries were prepared by equimolar (4 nmol/L) pooling, denaturation, and dilution to 6 pmol/L before loading onto the MiSeq flow cell (Illumina). A 2 × 300 bp paired-end run was used.
Raw reads were analyzed according to a previously described procedure (Severgnini et al., 2021). Briefly, a single fragment from two overlapping pairs was generated using PandaSeq software (v2.5, Masella et al., 2012). Downstream analyses, such as filtering, zero-radius Operational Taxonomic Units (zOTUs) creation, taxonomy assignments, and diversity analyses were performed using the QIIME suite (release 1.9.0, Caporaso et al., 2010), unoise3 algorithm (Edgar, 2016), RDP classifier (Wang et al., 2007), and SILVA 16S rRNA database (release 132, https://www.arb-silva.de/fileadmin/silva_databases/qiime/Silva_132_release.zip).
Characterization of Lactobacillus spp. was performed by BLAST-aligning all reads belonging to that genus to a custom reference database made up collecting all available reference sequences in NIH-NCBI database ftp://ftp.ncbi.nlm.nih.gov/genomes/GENOME_REPORTS/prokaryotes.txt of 17 Lactobacillus species commonly found in the vaginal environment, with finishing status of “complete genome”, “chromosome” or “scaffold”. Potential matches were filtered in order to retrieve an unequivocal classification for each read, according to the procedures already described (Ceccarani et al., 2019, Supplementary Material ). Since 2020, Lactobacillus taxonomy underwent major update, with the re-classification of the genus in 25 different genera (23 of which are novel) (Zheng J. et al., 2020). Old and new species names used in the present article are available as Supplementary Table S1 .
Alpha-diversity evaluation was estimated according to several microbial diversity metrics (i.e., chao1, Shannon index, observed species, Good’s coverage, and Faith’s phylogenetic distance). Beta-diversity analysis was conducted using both weighted and unweighted Unifrac metrics (Lozupone et al., 2011), and through the Principal Coordinates Analysis (PCoA).
Metabolomic Analysis
Metabolomic analysis was performed by means of a 1H-NMR spectroscopy starting from 700 µL of the cell-free supernatants of the vaginal swabs, added to 100 μL of a D2O solution of 3-(trimethylsilyl)-propionic-2,2,3,3-d4 acid sodium salt (TSP) 10 mM set to pH 7.0 (Foschi et al., 2018)
1H-NMR spectra were recorded at 298 K with an AVANCE III spectrometer (Bruker, Milan, Italy) operating at a frequency of 600.13 MHz, equipped with Topspin software (Ver. 3.5) according to previously described procedures (Foschi et al., 2018). The signals originating from large molecules were suppressed by a CPMG filter of 400 spin-echo periods, generated by 180° pulses of 24 μs separated by 400 μs (Ventrella et al., 2016).
To each spectrum, line broadening (0.3 Hz) and phase adjustment was applied by Topspin software, while any further spectra processing, molecules quantification and data mining step was performed in R computational language (version 4.1.2., R Core Team, 2021) by means of in-house developed scripts.
The spectra were aligned towards the right peak of alanine doublet, set to 1.473 ppm. The spectra were then baseline-adjusted by means of peak detection according to the “rolling ball” principle (Kneen and Annegarn, 1996) implemented in the “baseline” R package (Liland et al., 2016). A linear correction was then applied to each spectrum, to make the points pertaining to the baseline randomly spread around zero.
The signals were assigned by comparing their multiplicity and chemical shift with Chenomx software data bank (ver 8.3, Chenomx Inc., Edmonton, Alberta, Canada). Quantification of the molecules was performed in the first sample acquired by employing the added TSP as an internal standard. To compensate for differences in sample amount, any other sample was then normalized to such sample by means of probabilistic quotient normalization (Dieterle et al., 2006). Integration of the signals was performed for each molecule by means of rectangular integration.
Data Analysis and Statistics
Statistical evaluation of α-diversity indices was performed by non-parametric Monte Carlo-based tests through the QIIME pipeline. Beta-diversity differences were assessed by a permutation test with pseudo F-ratios using the “adonis” function from R package “vegan” (version 2.0-10, Oksanen et al., 2013). Pairwise relative abundance analysis was performed using a non-parametric Mann–Whitney U test. For comparing relative abundances across multiple categories, we applied a Kruskal-Wallis test, followed by Dunn’s post-hoc test for pairwise comparisons.
Metabolite concentrations were correlated to bacterial composition by calculating Spearman’s correlation coefficient between metabolites and bacterial genera present ≥1% in at least 1 sample (n=51). In this analysis, we considered all data points at T1, T2, T3, and T4. We performed a Spearman’s rank-based correlation between genus relative abundances and metabolite quantities, selecting only those with p-value< 0.05 (i.e.: correlation significantly different from 0). To better visualize patterns of positively correlated bacteria and metabolites, a heatmap was drawn, clustering correlation coefficients for metabolites and bacteria (using Pearson’s correlation as clustering metric and average linkage).
For each statistical analysis, unless otherwise stated, p-values < 0.05 were considered as significant. Statistical analyses were performed using MATLAB software (Natick, MA, USA).
Data Availability
Raw sequencing data of 16S rRNA gene are available at NCBI Short-reads Archive (SRA) with BioProject accession number PRJNA766806 (https://www.ncbi.nlm.nih.gov/sra/PRJNA766806). Raw metabolomic data are available as a Supplementary material (Data Sheet S1) .
Results
Study Population
A total of 63 Caucasian pregnant women with a mean age of 30.8 ± 5.1 years (min: 21, max: 44) and a mean BMI of 23.5 ± 3.5 (min: 16.0, max: 32.5) were enrolled and sampled during all gestational ages; for 30 of them, clinical and microbiological data were also available for the puerperium.
In addition, 9 women (mean age: 33.8 ± 6.4 years; mean BMI: 24.5 ± 4.7) who had a spontaneous miscarriage at the first trimester of pregnancy (gestational age: 11-13 weeks) during the study were included. From the first to the third trimester of pregnancy, we noticed a significant decrease of BV cases, together with an increase of samples characterized by a normal microbiota (p=0.001; Table 1 ). During the puerperium, only one third of the women (33.3%) showed a lactobacilli-dominated flora, being most of them characterized by an alteration of the vaginal bacterial composition (26.6% intermediate flora, 40.0% BV-condition).
Table 1.
Characteristics of the vaginal environment (Nugent score), stratified by the different gestation periods and conditions.
| First trimester miscarriage (n=9) | Normal pregnancies (n=63) | Puerperium (n=30**) | |||
|---|---|---|---|---|---|
| 1st trimester | 2nd trimester | 3rd trimester | |||
|
Nugent score
0-3 (H) 4-6 (I) 7-10 (BV) |
22.2% (2/9) 55.5% (5/9) 22.2% (2/9) |
49.2% (31/63) 33.3% (21/63) 17.5% (11/63) |
74.6% (47/63) 15.9% (10/63) 9.5% (6/63) |
82.5% (52/63) 11.1% (7/63) 6.4% (4/63) |
33.3% (10/30) 26.6% (8/30) 40.0% (12/30) |
| p=0.001* | |||||
H, lactobacilli-dominated microbiome; I, intermediate flora; BV, bacterial vaginosis.
*statistical significance is referred to the differences in microbial composition (Nugent score) during the three trimesters of pregnancy, excluding women suffering a first trimester miscarriage.
**clinical and microbiological data during the puerperium were available only for 30 women.
Ten out of the 30 (33.3%) women with puerperium data available received an intrapartum antibiotic prophylaxis to prevent GBS neonatal infection.
Most cases of spontaneous abortion were associated with an altered vaginal microbiome (55.5% intermediate status (I); 22.2% BV condition).
Vaginal Microbiome Structure Characterization
Overall, microbiota composition assessed through 16S rRNA sequencing was in accordance with what expected for the vaginal environment, with the Lactobacillus genus having an average relative abundance of 77.9%, followed by Gardnerella (8.9% on average), Bifidobacterium (3.5%), Atopobium (2.1%), Prevotella (1.8%), and Megasphaera (1.3%). Other genera, such as Sneathia, Ureaplasma, Aerococcus, and Dialister had <1% abundance. The first 12 genera accounted for 97.7% of the overall relative abundance, confirming the relatively low biodiversity of the vaginal samples ( Supplementary Figure S1 ).
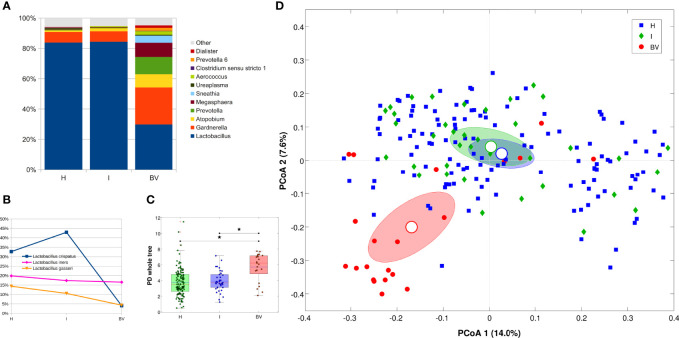
Microbiota structure was evaluated according to the vaginal status derived from the Nugent score, for a total of 189 samples (63 women at 3 time points each), comparing healthy (H) with intermediate (I) and bacterial vaginosis (BV) status. As expected, BV condition was characterized by a profound alteration of the microbiota, with a dramatic reduction of Lactobacillus spp. (83.8% vs 29.8% for H and BV, respectively) and an increase of opportunistic bacteria, (i.e., Gardnerella, Atopobium, Prevotella, Megasphaera, Sneathia, Aerococcus). The I condition seemed to be composed by nearly the same bacterial members of the H samples, with little exceptions in low-abundant members of the community (i.e., Dietzia, Actinomyces, Enterococcus, Cutibacterium, and ‘Eubacterium eligens group’), all contributing with <0.2% of average abundance ( Figure 1A ). Among the lactobacilli species, a significant reduction of L. crispatus was highlighted in BV samples as compared to both H and I (avg. abundance: 4.1% BV vs 32.7% H, 42.9% I); on the other hand, L. iners (avg. abundance: 19.9% H, 17.4% I, 16.5% BV), as well as other Lactobacillus species, was not affected ( Figure 1B ). Differences in microbial composition were reflected in α-diversity analysis, which highlighted a significant increase in biodiversity in BV samples as compared to H and I ones (p=0.003 for all metrics) ( Figure 1C ). Moreover, we recorded a significant separation of microbial profiles (β-diversity) among BV, I, and H (p<0.039 for all pairwise group comparisons, unweighted Unifrac); at the same time, BV samples differed from the other two groups in major contributors of the microbiota (p=0.001 against both H and I conditions, weighted Unifrac) ( Figure 1D ). Similarly, distances between H and BV samples were higher than H vs I ones and I vs BV distances were higher than H vs I (both for weighted and unweighted Unifrac); at the same time, weighted Unifrac distances among BV samples resulted higher than that among H or I samples, confirming that BV status was characterized by a deeper alteration of the microbial composition with respect to other conditions.
Figure 1.
Microbiota characterization according to the vaginal status (H, I or BV). (A) Barplot of average relative abundances at genus level. Genera with rel. ab. ≤1% were grouped in “Others” category; (B) Line plot of average Lactobacillus species abundance per vaginal status; only the 3 most abundant species are represented; (C) Boxplot of Faith’s phylogenetic diversity of the samples (estimated at endpoint) for each vaginal status. Stars above the plots represent statistical significance (p<0.05); (D) Principal Coordinate Analysis (PCoA) based on unweighted Unifrac distance among samples. Each point represents a sample; ellipses are 95% SEM-based confidence intervals; point and ellipses are grouped according to vaginal status; the first and the second coordinate are represented.
Taxonomic Composition of the Vaginal Bacterial Communities During Pregnancy
We evaluated the vaginal microbiota dynamics along the three trimesters of pregnancy for a total of 63 women (189 total samples). The proportion of samples with the same vaginal status at each trimester was found to be statistically different for the H subjects (p<0.001, two-sided proportion test without continuity correction; increasing from 49.2% to 74.6% and 82.5% respectively at T1, T2, and T3) and the I subjects (p=0.005; decreasing from 33.3% at T1 to 15.9% at T2 and 11.1% at T3). On the other hand, no differences were highlighted for BV status (proportion of 17.5%, 9.5%, and 6.3% respectively at T1, T2, and T3).
There were no significant or noticeable differences in biodiversity over time, other than T1 vs T2 in chao1 (p=0.039) and T1 vs T3 for the Faith’s phylogenetic diversity metric (p=0.015). With regards to microbial composition, T3 points were statistically separated from T1 and T2 sets (p ≤ 0.015, unweighted Unifrac), which were indistinguishable from each other; no differences on the weighted Unifrac distance matrix were highlighted ( Supplementary Figures S2A, B ).
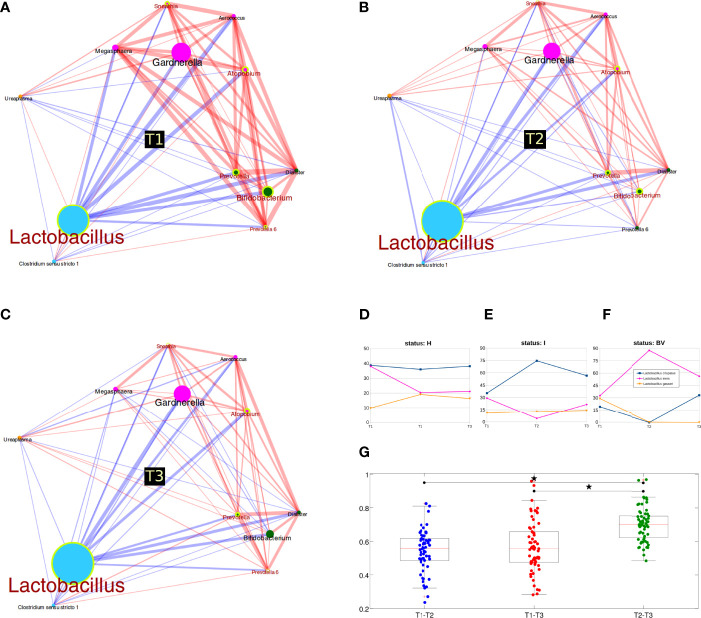
Analyzing bacterial genera co-abundance patterns, we were able to identify four co-abundance groups (CAGs) ( Supplementary Figure S3 ): (i) Ureaplasma alone; (ii) Lactobacillus CAG (also including Clostridium); (iii) ‘opportunistic’ bacteria CAG (including Bifidobacterium, Prevotella and Dialister); (iv) BV-associated bacteria CAG (i.e.: Gardnerella, Atopobium, Megasphaera, Sneathia and Aerococcus). At all three time points, Lactobacillus CAG was inversely correlated to other CAGs, whereas opportunistic and BV-related CAGs were directly associated to one another, although with a different strength of correlation.
Many genera were statistically different over time, suggesting a deep reshaping of the microbiota between the first two trimesters: all groups were differential in both T1 vs T2 and T1 vs T3 comparisons, but not for T2 vs T3. In particular, we revealed increased Lactobacillus abundances and reduced levels of opportunistic (such as Bifidobacterium and Prevotella) and BV-related bacteria (Atopobium and Sneathia) ( Figures 2A–C ). Stratification by the vaginal status allowed a deeper evaluation of changes over time: in BV samples, we highlighted a shift between T1 and T2 among the phyla Actinobacteria (increased) and Fusobacteria (decreased), while at genus level ‘Prevotella (group 6)’ was found decreased; for the I condition, no major members of the microbiota were statistically different among pregnancy trimesters (we observed only a total of 6 differential genera over time, all with average relative abundance <0.3%); finally, in the H samples, we observed a reduction of the phylum Actinobacteria and of its related genus Bifidobacterium (avg. rel. ab. of 7.5%, 2.1%, and 3.2% respectively at T1, T2, T3); the Bifidobacterium reduction was also confirmed when limiting the analysis to the 22 women with a “healthy” microbiota (H group) at each pregnancy time-point (avg. rel. ab. 8.4%, 1.6%, and 1.0% respectively at T1, T2, T3). The Streptococcus genus was also decreased in the H group (entirely taken), but with a consistently lower abundance (avg. rel. ab. 0.5%, 0.1%, 0.2% respectively for T1, T2, and T3) ( Supplementary Figures S2C ).
Figure 2.
Microbiota evolution during three trimesters of pregnancy. (A–C) Co-abundance networks of bacterial genera over time. Circle size is proportional to genus relative abundance for each time and colors are according to co-abundance groups (see also Suppl_Figure3_heatmap_CAGnetwork); edge size is proportional to the strength of correlation; red lines mean positive correlation, while blue lines indicate negative correlation. Genera resulting statistically different over time points are highlighted with a yellow circle and a red label; (D–F) Lactobacillus species abundance over time, stratified for vaginal status. Only the three most abundant species are represented; (G) Boxplot of unweighted Unifrac distances between samples over time. Distances were calculated for each pair of samples belonging to the same women, sampled at T1, T2 or T3; stars above the plots represent statistical significance (p<0.05).
As for evaluations within the Lactobacillus species, stratifying for the vaginal status, we observed several variations. In the H group, we highlighted the slight (non-significant) reduction of L. iners and the increase of L. gasseri in T1 vs T2, whereas abundances were nearly identical for T2 and T3; on the other hand, L. crispatus abundances were fundamentally unaltered. Considering the I group, a significant reduction of proportion of L. iners and a significant increase of L. crispatus was observed between T1 and T2. BV samples had the opposite trend, with a reduction of L. crispatus and an increase of L. iners between T1 and T2 ( Figures 2D–F ). No differences in Lactobacillus species were highlighted considering all samples together, regardless of their vaginal status.
Lastly, we analyzed the Unifrac distances among samples. The first interesting evidence was that the microbial profiles of all samples collected from one woman were more similar to each other than to those collected from the other women (p<0.001, intra- vs inter-distance for both weighted and unweighted Unifrac). When evaluating distances over time, we recorded that T2 vs T3 distance (unweighted Unifrac) was significantly higher than both T1 vs T2 and T1 vs T3 (comparisons not significantly different), indicating that between the second and third trimester of pregnancy the microbiota develops in a more independent way ( Figure 2G ). Considering the H samples alone, the evolution between T2 and T3 was confirmed; furthermore, our result suggests that T3 represents an evolution of the microbiota from T2 (as T1 had lower distance values to T2 than to T3), although average distances were very similar (T1-T2: 0.68, T1-T3: 0.71, T2-T3: 0.71).
Vaginal Microbiome at the Puerperium
In addition, we sought to evaluate the vaginal microbiome characteristics during the puerperium period in a group of 30 women, sampled a fourth time during the study (total subset time-points: T1, T2, T3 during pregnancy; T4 at puerperium, 40-55 days after delivery).
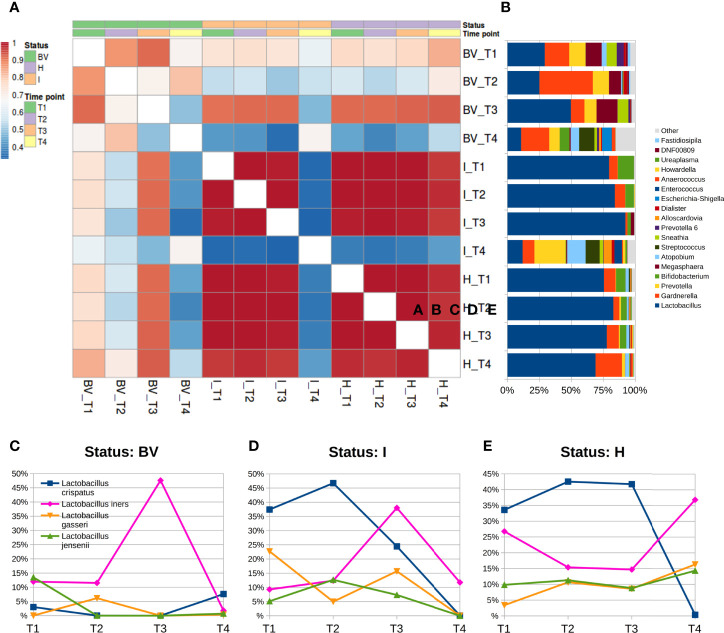
We did not observe a different biodiversity over time (p>0.05 for all α-diversity metrics tested); on the other hand, there seemed to be some separation in microbial composition within the β-diversity analysis, as T4 points were statistically different from T1, T2, and T3 (both unweighted and weighted Unifrac) ( Supplementary Figure S4A ). Over time, the analysis of microbial relative abundances at genus level suggested a composition variation at T4, with a lower content of Lactobacillus and a consistent presence of Gardnerella, Prevotella, Atopobium, and Streptococcus; those changes were significant (p<0.05) when compared to T1 (all except Atopobium and Gardnerella), to T2 (all except Gardnerella), and to T3 (all genera) ( Supplementary Figure S4B ). At species level, this was reflected in a significant decrease of all Lactobacillus species, L. crispatus and L. jensenii in particular (p<0.05), while L. gasseri was also found decreased but not significantly; contrariwise, L. iners was observed to be unchanged during the puerperium.
The stratification by vaginal status highlighted how these differences were mainly due to a change in bacterial members for the BV and I groups, whereas microbial profiles of H women resulted more stable, as evidenced by analyzing the correlation coefficients among average microbial profiles at genus level over time. Within the H group, microbial composition did not vary consistently, with an average Pearson correlation between T4 and all of the other three time-points of r=0.977, similar to the average r=0.998 between the paired comparisons of T1, T2, and T3; on the other hand, correlation coefficients for BV women were lower and slightly different over time (r=0.659 between T4 and the other three time points; r=0.826 among T1, T2, and T3); finally, the most substantial differences were observed for the I group, as the correlation coefficient dropped from r=0.994 to r=0.340 when comparing T4 to the other three time points ( Figures 3A, B ). BV samples at T4 were characterized by a significant reduction of the genera Lactobacillus, Megasphaera, and Prevotella, and by an increase of Streptococcus and Finegoldia with respect to T1; we observed a significant reduction of Lactobacillus and an increase of Prevotella, Streptococcus, and Dialister for the I condition in the comparison of T4 to all other gestational time-points; despite a slightly increase in the Gardnerella abundance, microbial profiles of the H group resulted very similar over all four time-points.
Figure 3.
Microbiota evolution during puerperium (T4). (A) Heatmap of Pearson’s correlation coefficients calculated between average relative abundances at genus level over time and stratified for vaginal status; (B) barplots of average relative abundances at genus level over time and stratified for vaginal status; genera with rel. ab. ≤1% were grouped in “Others” category; (C–E) Line plots of average abundances of Lactobacillus species over time. Only the four most abundant species are represented.
The Lactobacillus species analysis stratified by the vaginal status suggested that BV samples at puerperium had a switch compared to both the first and second trimester: L. crispatus showed a higher abundance (T4 7.6% vs T1 3.0% and T2 <0.1%; p<0.05 for T2 vs T4) while L. iners a lowered one (T4 1.8% vs T1 12.0% and T2 11.5%; p<0.05 for T1 vs T4). We could not evaluate the BV composition during the third trimesters (T3) since we had only 1 sample for this time-status combination. In the I samples, T4 microbiota displayed a dramatic decrease of L. crispatus (0.1% vs 36.2% of the gestational time-points average; p<0.05 forT2 vs T4). A similar decrease of L. crispatus was observed for H women as well (0.4% vs 39.3% on average of the gestational time points), together with a somewhat higher abundance of L. iners (36.8% vs 19.0% of the gestational time-points average). Due to the extreme variability among individuals, between T3 and T4 the sole L. crispatus reduction was statistically significant ( Supplementary Figures S3C–E ).
Among the women evaluated at T4, 10 out 30 (33.3%) received an intrapartum antibiotic prophylaxis for GBS. Microbial profiles of these women did not result significantly different from the untreated group (n=20) neither by alpha- (p>0.05 for all metrics tested) nor beta-diversity (p=0.937 and p=0.112 for unweighted and weighted Unifrac distances, respectively). Only one taxon, the Prevotella genus, was significantly altered, showing an increase in antibiotic-treated women, when compared to the untreated ones (rel. ab. of 20.0% with antibiotics vs 6.0% without antibiotics) ( Supplementary Figure S5 ). At the same time, this difference was reflected in higher-level taxonomies as well (Bacteroides, Bacteroidia, Bacteroidales: 22.9% vs 8.0%; Prevotellaceae: 22.2% vs 7.3%, with vs without antibiotics). No differences were highlighted by the species-level characterization of the Lactobacillus genus.
Association Between Microbiome Composition and First Trimester Miscarriage
We compared the microbiome profiles at T1 of the 63 women with successful pregnancies to the ones of 9 women who suffered a first trimester miscarriage. No significant differences were found on both α- (p>0.05 for chao1, Shannon, Good’s coverage, Observed species, Faith’s phylogenetic diversity metrics) and β-diversity (p=0.412 and p=0.110 for unweighted and weighted Unifrac distances, respectively) analyses. Nevertheless, we observed an overgrowth of Fusobacterium (rel. ab. 1.1%, p=0.02) in the miscarriage group compared to successful pregnancies (0.1%). No significant differences were highlighted for the Lactobacillus species.
Vaginal Metabolites Composition and Metabolite-Microbiome Correlation
In the supernatants of the vaginal swabs, a total of 63 metabolites were detected and quantified by 1H-NMR spectroscopy. Molecules mainly belonged to the groups of SCFAs, organic acids, amino acids, and biogenic amines (Data sheet S1).
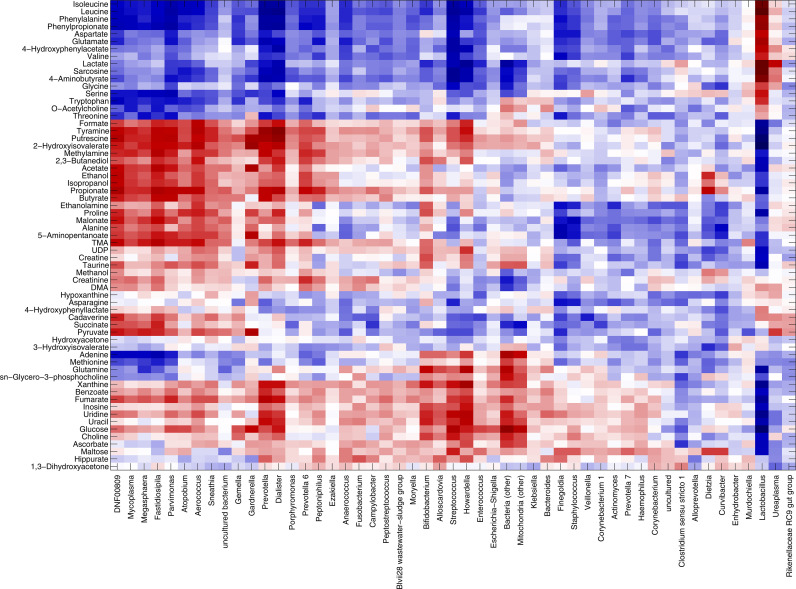
We performed a correlation analysis aimed at relating microbial composition to metabolite concentrations, using Spearman’s rank correlation to determine monotonically increasing or decreasing relationships. All samples collected over the four time-points were considered (n=219); miscarriage samples were analyzed separately.
We could define three main clusters of correlations: (i) Lactobacillus stood by itself, separated from all other bacteria, strongly positively correlated to lactate and sarcosine (r=0.62 and r=0.61, respectively). Moreover, positive correlations were evidenced for many amino acids (i.e., isoleucine, leucine, phenylalanine, aspartate, glutamate, valine, glycin, serine, threonine, tryptophan, with correlation values ranging from 0.26 to 0.65); (ii) BV-associated genera, such as Gardnerella, Prevotella, Atopobium, Dialister, Aerococcus, and Sneathia, were positively correlated to putrescine, methylamine, tyramine, formate, trimethylamine (TMA), alcohols (i.e., ethanol, isopropanol), and SCFAs (i.e., acetate, butyrate, propionate); (iii) other lower-abundance bacteria, such as Bifidobacterium, Streptococcus, and Alloscardovia correlated with nucleotides (i.e., adenine, glutamine, inosine, uracil), glucose, choline, benzoate, and fumarate ( Figure 4 ).
Figure 4.
Correlation between metabolome and microbiota. Heatmap showing the Spearman’s correlation coefficient between metabolites concentration and the relative abundances of the main bacterial genera over all samples collected, excluding miscarriages (n=219). Only groups present at >1% of relative abundance in at least one sample were considered. Metabolite and microbial data were clustered using Pearson’s correlation metric and average linkage.
Microbiome-metabolites correlation patterns were further refined by looking at the possible relationships with spontaneous miscarriages (n=9). Overall, only few correlations were significant (p-value of the linear model <0.05). Correlation patterns for Lactobacillus and BV-associated genera were in accordance with those described above for the samples of women with a successful pregnancy. Interestingly, Fusobacterium was found positively correlated to the nucleotides and their components (i.e., uracil, adenine, UDP, tyramine, r range 0.36-0.56), as well as to methionine (r=0.65), formate (r=0.54), choline, xanthine, and maltose (r=0.43-0.49), putrescine (r=0.38), and methylamine (r=0.31) ( Supplementary Figure S6 ).
Discussion
A deep comprehension of the vaginal ecosystem may hold promise for unraveling the pathophysiology of pregnancy and may provide novel markers to identify women at risk of complications, such as miscarriage and preterm births. Moreover, considering that microbial communities can be transferred from the mother’s vaginal niche to the newborn gut, the study of the vaginal microbiome during pregnancy and puerperium can open new perspectives for infant’s microbiome development and future health (Dominguez-Bello et al., 2010).
That is why in this study we characterized the vaginal environment in the situations of both a normal pregnancy, at the three gestational trimesters and the puerperium period, and a spontaneous first trimester miscarriage. In particular, we assessed the vaginal bacterial composition and the vaginal metabolic profiles.
At first, we confirmed that, irrespective of the period and type of pregnancy, BV cases were characterized by a dramatic reduction of Lactobacillus and an increase of anaerobic bacteria, such as Gardnerella, Atopobium, Prevotella, Megasphaera, Sneathia, Aerococcus (Deka et al., 2021).
In line with previous findings, the relative and absolute proportion of L. crispatus, a hallmark of vaginal eubiosis, inclined to decrease in the transition from H to BV conditions (Ceccarani et al., 2019). As for L. iners, the abundance of this species did not differ between H and BV groups in our cohort, even though it has been considered a transitional species typically associated with dysbiotic conditions (Yoshimura et al., 2020); on the other hand, L. iners has also been reported to be the dominating taxon in a large subset of women worldwide, being its presence associated with young age and unprotected sexual practices (France et al., 2020; Novak et al., 2021).
When considering the changes of the vaginal microbiome during the three trimesters of pregnancy, we observed that several bacterial genera were statistically different between the first and second trimester, suggesting a deep reshaping of the microbiome profiling towards a ‘healthier condition’: moving from the first to the third trimester, in line with the higher proportion of H cases, we found an increase of Lactobacillus genus and a decrease of BV-related genera (e.g., Prevotella, Atopobium, Sneathia), with no differences in Lactobacillus species.
Taken together, these data confirmed that the vaginal microbiome becomes more stable throughout the entire pregnancy, being less diverse and mainly dominated by lactobacilli (Li et al., 2020; Rasmussen et al., 2020; Marangoni et al., 2021; Pace et al., 2021).
It is worth noting that bifidobacteria, typical beneficial commensals inhabiting the human intestine, have tended to decrease their vaginal ecosystem abundances at the end of pregnancy. It has been shown that Bifidobacterium is the dominant genus of some vaginal microbiomes and that overall bifidobacteria have the potential to be as protective as lactobacilli, according to the current understanding of a healthy vaginal microbiome (Freitas and Hill, 2017). Nevertheless, Lee and colleagues recently observed that the relative abundance of Bifidobacterium spp. significantly increased during pregnancy in women with an intermediate and BV status compared to normal vaginal microbiota, and that some dysbiotic conditions were dominated by Bifidobacterium breve (Lee et al., 2020). In line with these observations, our data highlighted a co-abundant vaginal pattern, characterized by several BV-associated genera, such as Prevotella and Dialister, and Bifidobacterium spp. Since the role of this vaginal microbial group is yet to be understood, further studies are needed to investigate the clinical significance of the bifidobacteria reduction at the end of pregnancy, as well as to assess the potential impact on newborn’s health (Bozzi et al., 2018).
Other interesting data emerged when looking at the vaginal environment after delivery. In agreement with previous reports (Nunn et al., 2021), at the puerperium we found a significantly lower content of Lactobacillus, and higher levels of Gardnerella, Prevotella, Atopobium, and Streptococcus compared to the third trimester of pregnancy. These variations are consequences of after-delivery vaginal alterations that profoundly altered the host environment and, thus, led to changes in different bacterial species survival and proliferation capabilities (Nunn et al., 2021).
Moreover, we observed a significant increase in Prevotella abundance in women who received an intrapartum antibiotic prophylaxis (IAP) for GBS compared to untreated ones. This aspect deserves attention considering that members of Prevotella genera have been associated with negative ‘outcomes’ of the cervicovaginal environment, being responsible for strong inflammatory conditions, cytotoxicity, and alterations of the reproductive tract (Campisciano et al., 2020; Salliss et al., 2021). It is well known that IAP can negatively affect the gut microbiome of infants vaginally delivered, specifically in relation to microbial composition and occurrence of antibiotic resistance genes (Garcia, 2021). However, the effect of antibiotic prophylaxis on the vaginal microbiome after delivery is still little explored. Even if further studies are needed to clarify the reasons behind the increase in Prevotella levels in women receiving IAP, we can speculate that beta-lactam antibiotics could have selected this bacterial genus, as it is potentially able to produce β-lactamase enzymes (Toprak et al., 2020).
Moving to the analysis of bacterial relative abundances in women suffering a first trimester miscarriage, we highlighted a significant vaginal overgrowth of Fusobacterium in abortions compared to successful pregnancies, at all taxonomic levels. This microbial genus has been strongly associated with genital inflammation and dysbiosis, being Fusobacterium able to cooperate with other taxa to disrupt the normal vaginal bacterial composition, leading to microbial imbalance (Lennard et al., 2017; Agarwal et al., 2020). It has been shown that Fusobacterium has a mutualistic relationship with the BV-correlated bacteria: as they are major sialidase-producers, they enable Fusobacterium to consume sialic acids from the host-produced mucus. At the same time, F. nucleatum exposure to vaginal communities may encourage features of dysbiosis (e.g., increased sialidase activity and G. vaginalis abundance) in susceptible vaginal communities (Agarwal et al., 2020). In addition, F. nucleatum has been previously associated with preterm labor, since it was found in greater abundance in preterm placental membranes than at term (Doyle et al., 2014). To the best of our knowledge, this is the first time that Fusobacterium got linked to the risk of first trimester miscarriage, considering that previous investigations highlighted the potential role of other microorganisms, such as Finegoldia, Coprococcus, Roseburia, Atopobium, and Prevotella (Al-Memar et al., 2020; Xu et al., 2020; Jiao et al., 2021; Liu et al., 2021).
The vaginal bacterial community profiles found during pregnancy were accompanied by peculiar fingerprints in the composition of the vaginal metabolites. In agreement with recent observations, Lactobacillus abundance was strongly correlated with higher levels of lactate, sarcosine, and many amino acids, whereas BV-associated genera, such as Gardnerella, Atopobium, Sneathia, were correlated to amines (putrescine, methylamine, TMA), formate, alcohols (ethanol, isopropanol), and short-chain fatty-acids (SCFAs, as butyrate, acetate, propionate) (Ceccarani et al., 2019; Laghi et al., 2021). On the one hand, the lactate production by Lactobacillus species reduces the vaginal pH, contributing to the homeostasis against potentially endogenous or exogenous pathogens. These microorganisms are also known producers of branched-chain amino acids, thus the higher concentration of some of them, such as valine, leucine, and isoleucine, is another fingerprint of the prevalence of lactobacilli in ‘healthy’ women (Vitali et al., 2015). Conversely, during dysbiotic conditions, the proliferation of diverse bacterial genera, some of which typical of the gut microbiota, and the imbalance between lactobacilli and BV-related bacteria lead to higher levels of amines, organic acids, and SCFAs (Vitali et al., 2015). In this context, higher levels of Fusobacterium, associated with the higher risk of spontaneous abortion, were positively correlated to several vaginal molecules, including methionine, formate, putrescine, and methylamine. Considering the low number of data points (n=9), the exact role of the vaginal metabolome in first trimester miscarriages, as well as the causative relationship between microbiota and immune responses, remain to be further elucidated, to enable the best possible diagnosis and therapeutics of early pregnancy loss.
In conclusion, we deepened the existing literature knowledge about the composition of the vaginal ecosystem during pregnancy and puerperium, highlighting peculiar microbial/metabolic fingerprints.
Our data could help implement ‘prognostic’ criteria (e.g., evaluation of the risk of spontaneous miscarriage based on the microbiome/metabolome profiles), as well as strategies for the prevention of early pregnancy loss, based on the ‘manipulation’ of the vaginal bacterial inhabitants (e.g., use of probiotics and prebiotics). Moreover, the microbial changes induced by GBS prophylaxis (i.e., increase in Prevotella levels) deserve attention, leading to the idea of new approaches able to reduce the impact of antibiotics in maternal/neonatal health.
As a strength of our work, we excluded from the enrollment all the women harboring conditions able to perturb per se the vaginal microbiome composition (e.g., VVC, AV, presence of STIs) and we combined multiple ‘omic’ sciences (i.e., genomic and metabolomic) to decipher the vaginal environment in pregnancy and puerperium. On the other hand, we are fully aware of some limitations of the study, as the potential loss of low concentration molecules, due to the reduced sensitivity of 1H-NMR compared to other metabolomic techniques (e.g., high resolution chromatographic separation techniques coupled to accurate tandem mass spectrometry).
To further understand the interactions between vaginal microbes and the host, future studies perspectives will include (i) the increase in number of women suffering a spontaneous first trimester miscarriage, to strengthen the conclusions regarding this group (ii) the evaluation of several inflammatory markers, (iii) the assessment of vaginal proteomic profile, and (iv) the evaluation of bacterial subspecies/clades.
Data Availability Statement
The original contributions presented in the study are publicly available. This data can be found here: National Center for Biotechnology Information (NCBI) BioProject database under accession number PRJNA766806.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Romagna (CEROM) (n° 2032 of 21st February 2018). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AM and CF conceived and designed the study. SZ and GP recruited the patients. LL, MP, SM, MS, CCo, CCe, and TC performed the experiments. CF, LL, MS, CCo, CCe, and TC analyzed the data. AM and VS contributed reagents/materials and analysis tools. CF, AM, and MS wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by ‘Fondazione del Monte di Bologna e Ravenna’ (Prot. N°329bis/2017). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We wish to thank all the enrolled volunteers and all staff involved in this study. Special thanks to Mrs. Oriana Gasperoni of the Family Advisory Health Centre in Ravenna for her skillful support during the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.838405/full#supplementary-material
References
- Aagaard K., Riehle K., Ma J., Segata N., Mistretta T. A., Coarfa C., et al. (2012). A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLos One 7, e36466. doi: 10.1371/journal.pone.0036466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal K., Robinson L. S., Aggarwal S., Foster L. R., Hernandez-Leyva A., Lin H., et al. (2020). Glycan Cross-Feeding Supports Mutualism Between Fusobacterium and the Vaginal Microbiota. PLos Biol. 18, e3000788. doi: 10.1371/journal.pbio.3000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Memar M., Bobdiwala S., Fourie H., Mannino R., Lee Y. S., Smith A., et al. (2020). The Association Between Vaginal Bacterial Composition and Miscarriage: A Nested Case-Control Study. BJOG 127, 264–274. doi: 10.1111/1471-0528.15972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Lee H., You Y. A., Jung Y., Park S., Kim S. M., et al. (2020). Identification of Potential Biomarkers in the Cervicovaginal Fluid by Metabolic Profiling for Preterm Birth. Metabolites 10, 349. doi: 10.3390/metabo10090349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzi C. N., Baffoni L., Gaggìa F., Di Gioia D. (2018). Therapeutic Microbiology: The Role of Bifidobacterium Breve as Food Supplement for the Prevention/Treatment of Paediatric Diseases. Nutrients 10, e1723. doi: 10.3390/nu10111723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisciano G., Iebba V., Zito G., Luppi S., Martinelli M., Fischer L., et al. (2020). Lactobacillus Iners and Gasseri, Prevotella Bivia and HPV Belong to the Microbiological Signature Negatively Affecting Human Reproduction. Microorganisms 9, 39. doi: 10.3390/microorganisms9010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., et al. (2010). Correspondence QIIME Allows Analysis of High Throughput Community Sequencing Data Intensity Normalization Improves Color Calling in SOLiD Sequencing. Nat. Methods 7, 335–6. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarani C., Foschi C., Parolin C., D'Antuono A., Gaspari V., Consolandi C., et al. (2019). Diversity of Vaginal Microbiome and Metabolome During Genital Infections. Sci. Rep. 9, 14095. doi: 10.1038/s41598-019-50410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Asta M., Laghi L., Morselli S., Re M. C., Zagonari S., Patuelli G., et al. (2021). Pre-Pregnancy Diet and Vaginal Environment in Caucasian Pregnant Women: An Exploratory Study. Front. Mol. Biosci. 8. doi: 10.3389/fmolb.2021.702370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka N., Hassan S., Seghal Kiran G., Selvin J. (2021). Insights Into the Role of Vaginal Microbiome in Women's Health. J. Basic. Microbiol. 61, 1071–1084. doi: 10.1002/jobm.202100421 [DOI] [PubMed] [Google Scholar]
- Dieterle F., Ross A., Schlotterbeck G., Senn H. (2006). Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 78, 4281–4290. doi: 10.1021/ac051632c [DOI] [PubMed] [Google Scholar]
- DiGiulio D. B., Callahan B. J., McMurdie P. J., Costello E. K., Lyell D. J., Robaczewska A., et al. (2015). Temporal and Spatial Variation of the Human Microbiota During Pregnancy. Proc. Natl. Acad. Sci. U. S. A. 112, 11060–11065. doi: 10.1073/pnas.1502875112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simone N., Santamaria Ortiz A., Specchia M., Tersigni C., Villa P., Gasbarrini A., et al. (2020). Recent Insights on the Maternal Microbiota: Impact on Pregnancy Outcomes. Front. Immunol. 11. doi: 10.3389/fimmu.2020.528202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello M. G., Costello E. K., Contreras M., Magris M., Hidalgo G., Fierer N., et al. (2010). Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota Across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. U. S. A. 107, 11971–11975. doi: 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders G., Bellen G., Rezeberga D. (2011). Aerobic Vaginitis in Pregnancy. BJOG 118, 1163–1170. doi: 10.1111/j.1471-0528.2011.03020.x [DOI] [PubMed] [Google Scholar]
- Doyle R. M., Alber D. G., Jones H. E., Harris K., Fitzgerald F., Peebles D., et al. (2014). Term and Preterm Labour are Associated With Distinct Microbial Community Structures in Placental Membranes Which are Independent of Mode of Delivery. Placenta 35, 1099–1101. doi: 10.1016/j.placenta.2014.10.007 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2016). UNOISE2: Improved Error-Correction for Illumina 16S and ITS Amplicon Sequencing. bioRxiv, 081257. doi: 10.1101/081257 [DOI] [Google Scholar]
- Fan T., Zhong X. M., Wei X. C., Miao Z. L., Luo S. Y., Cheng H., et al. (2020). The Alteration and Potential Relationship of Vaginal Microbiota and Chemokines for Unexplained Recurrent Spontaneous Abortion. Med. (Baltimore) 99, e23558. doi: 10.1097/MD.0000000000023558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foschi C., Laghi L., D'Antuono A., Gaspari V., Zhu C., Dellarosa N., et al. (2018). Urine Metabolome in Women With Chlamydia Trachomatis Infection. PLos One 13, e0194827. doi: 10.1371/journal.pone.0194827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foschi C., Laghi L., Parolin C., Giordani B., Compri M., Cevenini R., et al. (2017). Novel Approaches for the Taxonomic and Metabolic Characterization of Lactobacilli: Integration of 16S rRNA Gene Sequencing With MALDI-TOF MS and 1H-NMR. PLos One 12, e0172483. doi: 10.1371/journal.pone.0172483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C., Eichelberger K. (2015). Maternal Microbiome and Pregnancy Outcomes. Fertil. Steril. 104, 1358–1363. doi: 10.1016/j.fertnstert.2015.09.037 [DOI] [PubMed] [Google Scholar]
- France M. T., Ma B., Gajer P., Brown S., Humphrys M. S., Holm J. B., et al. (2020). VALENCIA: A Nearest Centroid Classification Method for Vaginal Microbial Communities Based on Composition. Microbiome 8, 166. doi: 10.1186/s40168-020-00934-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas A. C., Hill J. E. (2017). Quantification, Isolation and Characterization of Bifidobacterium From the Vaginal Microbiomes of Reproductive Aged Women. Anaerobe 47, 145–156. doi: 10.1016/j.anaerobe.2017.05.012 [DOI] [PubMed] [Google Scholar]
- Garcia V. R. (2021). Impact of Intrapartum Antibiotic Prophylaxis for Group B Streptococcus on the Term Infant Gut Microbiome: A State of the Science Review. J. Midwifery. Womens. Health 66, 351–359. doi: 10.1111/jmwh.13245 [DOI] [PubMed] [Google Scholar]
- Gupta P., Singh M. P., Goyal K. (2020). Diversity of Vaginal Microbiome in Pregnancy: Deciphering the Obscurity. Front. Public. Health 8. doi: 10.3389/fpubh.2020.00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X., Zhang L., Du D., Wang L., Song Q., Liu S. (2021). Alteration of Vaginal Microbiota in Patients With Recurrent Miscarriage. J. Obstet. Gynaecol. 21, 1–8. doi: 10.1080/01443615.2021.1904851 [DOI] [PubMed] [Google Scholar]
- Kneen M. A., Annegarn H. J. (1996). Algorithm for Fitting XRF, SEM and PIXE X-Ray Spectra Backgrounds. Nucl. Instrum. Methods Phys. Res. B 109, 209–213. doi: 10.1016/0168-583X(95)00908-6 [DOI] [Google Scholar]
- Kolkman D. G. E., Rijnders M. E. B., Wouters M. G. A. J., Dommelen P. V., de Groot C. J. M., Fleuren M. A. H. (2020). Adherence to Three Different Strategies to Prevent Early Onset GBS Infection in Newborns. Women Birth. 33, e527–e534. doi: 10.1016/j.wombi.2019.12.004 [DOI] [PubMed] [Google Scholar]
- Kroon S. J., Ravel J., Huston W. M. (2018). Cervicovaginal Microbiota, Women's Health, and Reproductive Outcomes. Fertil. Steril. 110, 327–336. doi: 10.1016/j.fertnstert.2018.06.036 [DOI] [PubMed] [Google Scholar]
- Laghi L., Zagonari S., Patuelli G., Zhu C., Foschi C., Morselli S., et al. (2021). Vaginal Metabolic Profiles During Pregnancy: Changes Between First and Second Trimester. PLos One 16, e0249925. doi: 10.1371/journal.pone.0249925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Oh K. Y., Hong H., Jin C. H., Shim E., Kim S. H., et al. (2020). Community State Types of Vaginal Microbiota and Four Types of Abnormal Vaginal Microbiota in Pregnant Korean Women. Front. Public Health 8. doi: 10.3389/fpubh.2020.507024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard K., Dabee S., Barnabas S. L., Havyarimana E., Blakney A., Jaumdally S. Z., et al. (2017). Microbial Composition Predicts Genital Tract Inflammation and Persistent Bacterial Vaginosis in South African Adolescent Females. Infect. Immun. 86, e00410–e00417. doi: 10.1128/IAI.00410-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Chi X. Z., Zhang L., Chen R., Cao J. R., Sun X. Y., et al. (2020). Vaginal Microbiome Analysis of Healthy Women During Different Periods of Gestation. Biosci. Rep. 40, BSR20201766. doi: 10.1042/BSR20201766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liland K. H., Almøy T., Mevik B. H. (2010). Optimal Choice of Baseline Correction for Multivariate Calibration of Spectra. Appl. Spectrosc. 64, 1007–1016. doi: 10.1366/000370210792434350 [DOI] [PubMed] [Google Scholar]
- Liu X., Cao Y., Xie X., Qin X., He X., Shi C., et al. (2021). Association Between Vaginal Microbiota and Risk of Early Pregnancy Miscarriage. Comp. Immunol. Microbiol. Infect. Dis. 77, 101669. doi: 10.1016/j.cimid.2021.101669 [DOI] [PubMed] [Google Scholar]
- Lozupone C., Lladser M. E., Knights D., Stombaugh J., Knight R. (2011). UniFrac: An Effective Distance Metric for Microbial Community Comparison. ISME. J. 5, 169–172. doi: 10.1038/ismej.2010.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre D. A., Chandiramani M., Lee Y. S., Kindinger L., Smith A., Angelopoulos N., et al. (2015). The Vaginal Microbiome During Pregnancy and the Postpartum Period in a European Population. Sci. Rep. 5, 8988. doi: 10.1038/srep08988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni A., Foschi C., Nardini P., Compri M., Cevenini R. (2015). Evaluation of the Versant CT/GC DNA 1.0 Assay (kPCR) for the Detection of Extra-Genital Chlamydia Trachomatis and Neisseria Gonorrhoeae Infections. PLos One 10, e0120979. doi: 10.1371/journal.pone.0120979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni A., Laghi L., Zagonari S., Patuelli G., Zhu C., Foschi C., et al. (2021). New Insights Into Vaginal Environment During Pregnancy. Front. Mol. Biosci. 8. doi: 10.3389/fmolb.2021.656844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masella A. P., Bartram A. K., Truszkowski J. M., Brown D. G., Neufeld J. D. (2012). PANDAseq: Paired-End Assembler for Illumina Sequences. BMC Bioinform. 13, 31. doi: 10.1186/1471-2105-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. B., Rockwell L. C., Prioleau M. D., Goetzl L. (2016). The Role of the Bacterial Microbiota on Reproductive and Pregnancy Health. Anaerobe 42, 67–73. doi: 10.1016/j.anaerobe.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Novak J., Ravel J., Ma B., Ferreira C. S. T., Tristão A. D. R., Silva M. G., et al. (2021). Characteristics Associated With Lactobacillus Iners-Dominated Vaginal Microbiota. Sex Transm. Infect., sextrans–2020-054824. doi: 10.1136/sextrans-2020-054824. online ahead of print. [DOI] [PubMed] [Google Scholar]
- Noyes N., Cho K. C., Ravel J., Forney L. J., Abdo Z. (2018). Associations Between Sexual Habits, Menstrual Hygiene Practices, Demographics and the Vaginal Microbiome as Revealed by Bayesian Network Analysis. PLos One 13, e0191625. doi: 10.1371/journal.pone.0191625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent R. P., Krohn M. A., Hillier S. L. (1991). Reliability of Diagnosing Bacterial Vaginosis is Improved by a Standardized Method of Gram Stain Interpretation. J. Clin. Microbiol. 29, 297–301. doi: 10.1128/JCM.29.2.297-301.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn K. L., Witkin S. S., Schneider G. M., Boester A., Nasioudis D., Minis E., et al. (2021). Changes in the Vaginal Microbiome During the Pregnancy to Postpartum Transition. Reprod. Sci. 28, 1996–2005. doi: 10.1007/s43032-020-00438-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J., Blanchet F. G., Kindt R., Legendre P., Minchin P. R., O'Hara R. B., et al. (2013). “Package “Vegan”, in R Package Version 2.0–10. Available at: https://cran.r-project.org/src/contrib/Archive/vegan/vegan_2.0-10.tar.gz. [Google Scholar]
- Pace R. M., Chu D. M., Prince A. L., Ma J., Seferovic M. D., Aagaard K. M. (2021). Complex Species and Strain Ecology of the Vaginal Microbiome From Pregnancy to Postpartum and Association With Preterm Birth. Med. (N. Y.) 2, 1027–1049. doi: 10.1016/j.medj.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin C., Foschi C., Laghi L., Zhu C., Banzola N., Gaspari V., et al. (2018). Insights Into Vaginal Bacterial Communities and Metabolic Profiles of Chlamydia Trachomatis Infection: Positioning Between Eubiosis and Dysbiosis. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.00600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin C., Marangoni A., Laghi L., Foschi C., Ñahui Palomino R. A., Calonghi N., et al. (2015). Isolation of Vaginal Lactobacilli and Characterization of Anti-Candida Activity. PLos One 10, e0131220. doi: 10.1371/journal.pone.0131220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova M. I., Lievens E., Malik S., Imholz N., Lebeer S. (2015). Lactobacillus Species as Biomarkers and Agents That can Promote Various Aspects of Vaginal Health. Front. Physiol. 6. doi: 10.3389/fphys.2015.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A. L., Antony K. M., Chu D. M., Aagaard K. M. (2014). The Microbiome, Parturition, and Timing of Birth: More Questions Than Answers. J. Reprod. Immunol. 104-105, 12–19. doi: 10.1016/j.jri.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M. A., Thorsen J., Dominguez-Bello M. G., Blaser M. J., Mortensen M. S., Brejnrod A. D., et al. (2020). Ecological Succession in the Vaginal Microbiota During Pregnancy and Birth. ISME. J. 14, 2325–2335. doi: 10.1038/s41396-020-0686-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salliss M. E., Maarsingh J. D., Garza C., Łaniewski P., Herbst-Kralovetz M. M. (2021). Veillonellaceae Family Members Uniquely Alter the Cervical Metabolic Microenvironment in a Human Three-Dimensional Epithelial Model. NPJ Biofilms. Microbiome. 7, 57. doi: 10.1038/s41522-021-00229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severgnini M., Camboni T., Ceccarani C., Morselli S., Cantiani A., Zagonari S., et al. (2021). Distribution of Ermb, Ermf, Tet(W), and Tet(M) Resistance Genes in the Vaginal Ecosystem of Women During Pregnancy and Puerperium. Pathogens 10, 1546. doi: 10.3390/pathogens10121546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. B., Ravel J. (2017). The Vaginal Microbiota, Host Defence and Reproductive Physiology. J. Physiol. 595, 451–463. doi: 10.1113/JP271694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toprak N. U., Akgul O., Sóki J., Soyletir G., Nagy E., ESCMID Study Group for Anaerobic Infections (ESGAI) (2020). Detection of Beta-Lactamase Production in Clinical Prevotella Species by MALDI-TOF MS Method. Anaerobe 65, 102240. doi: 10.1016/j.anaerobe.2020.102240 [DOI] [PubMed] [Google Scholar]
- van de Wijgert J. H., Borgdorff H., Verhelst R., Crucitti T., Francis S., Verstraelen H., et al. (2014). The Vaginal Microbiota: What Have We Learned After a Decade of Molecular Characterization? PLos One 9, e105998. doi: 10.1371/journal.pone.0105998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventrella D., Laghi L., Barone F., Elmi A., Romagnoli N., Bacci M. L. (2016). Age-Related 1h NMR Characterization of Cerebrospinal Fluid in Newborn and Young Healthy Piglets. PLos One 11, e0157623. doi: 10.1371/journal.pone.0157623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinturache A. E., Gyamfi-Bannerman C., Hwang J., Mysorekar I. U., Jacobsson B., Preterm Birth International Collaborative (PREBIC) (2016). Maternal Microbiome - A Pathway to Preterm Birth. Semin. Fetal. Neonatal. Med. 21, 94–99. doi: 10.1016/j.siny.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Vitali B., Cruciani F., Picone G., Parolin C., Donders G., Laghi L. (2015). Vaginal Microbiome and Metabolome Highlight Specific Signatures of Bacterial Vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 34, 2367–2376. doi: 10.1007/s10096-015-2490-y [DOI] [PubMed] [Google Scholar]
- Wang Q., Garrity G. M., Tiedje J. M., Cole J. R. (2007). Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences Into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Huang L., Lian C., Xue H., Lu Y., Chen X., et al. (2020). Vaginal Microbiota Diversity of Patients With Embryonic Miscarriage by Using 16s rDNA High-Throughput Sequencing. Int. J. Genomics 2020, 1764959. doi: 10.1155/2020/1764959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J., Sobel J. D., Nyirjesy P., Sobel R., Williams V. L., Yu Q., et al. (2019). Current Patient Perspectives of Vulvovaginal Candidiasis: Incidence, Symptoms, Management and Post-Treatment Outcomes. BMC Women's. Health 19, 48. doi: 10.1186/s12905-019-0748-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoman C. J., Thomas S. M., Miller M. E., Ulanov A. V., Torralba M., Lucas S., et al. (2013). A Multi-Omic Systems-Based Approach Reveals Metabolic Markers of Bacterial Vaginosis and Insight Into the Disease. PLos One 8, e56111. doi: 10.1371/journal.pone.0056111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Ogawa M., Saito M. (2020). In Vitro Characteristics of Intravaginal Lactobacilli; Why is L. Iners Detected in Abnormal Vaginal Microbial Flora? Arch. Gynecol. Obstet. 302, 671–677. doi: 10.1007/s00404-020-05634-y [DOI] [PubMed] [Google Scholar]
- Zhang F., Zhang T., Ma Y., Huang Z., He Y., Pan H., et al. (2019). Alteration of Vaginal Microbiota in Patients With Unexplained Recurrent Miscarriage. Exp. Ther. Med. 17, 3307–3316. doi: 10.3892/etm.2019.7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Wittouck S., Salvetti E., Franz C. M. A. P., Harris H. M. B., Mattarelli P., et al. (2020). A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae . Int. J. Syst. Evol. Microbiol. 70, 2782–2858. doi: 10.1099/ijsem.0.004107 [DOI] [PubMed] [Google Scholar]
- Zozaya-Hinchliffe M., Lillis R., Martin D. H., Ferris M. J. (2010). Quantitative PCR Assessments of Bacterial Species in Women With and Without Bacterial Vaginosis. J. Clin. Microbiol. 48, 1812–1819. doi: 10.1128/JCM.00851-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing data of 16S rRNA gene are available at NCBI Short-reads Archive (SRA) with BioProject accession number PRJNA766806 (https://www.ncbi.nlm.nih.gov/sra/PRJNA766806). Raw metabolomic data are available as a Supplementary material (Data Sheet S1) .
The original contributions presented in the study are publicly available. This data can be found here: National Center for Biotechnology Information (NCBI) BioProject database under accession number PRJNA766806.