SUMMARY
The purpose of this study was to understand the seasonal, geographical and clinical characteristics of Taiwanese patients hospitalized for non-typhoidal Salmonella (NTS) infections and their economic burden. Hospital data obtained from the Taiwan National Health Insurance (NHI) database between 2006 and 2008 were analysed. Infants had the highest annual incidence of 525 cases/100 000 person-years. Elderly patients aged >70 years had the highest in-hospital mortality rate (2·6%). Most (82·6%) gastroenteritis occurred in children aged <10 years. Septicaemia, pneumonia, arthritis and osteomyelitis occurred mainly in patients aged >50 years. A median medical cost for NTS-associated hospitalizations was higher for patients with septicaemia than for those with gastroenteritis. Seasonal variation of NTS-associated hospitalizations was correlated with temperature in different areas of Taiwan. In summary, infants had a high incidence of NTS-associated hospitalizations. However, the elderly had a higher in-hospital mortality rate and more invasive NTS infections than children.
Key words: Economic burden, epidemiology, non-typhoidal Salmonella, seasonality, Taiwan
INTRODUCTION
Non-typhoidal Salmonella (NTS) is one of the major bacterial pathogens of foodborne infections in developed and developing countries [1]. NTS infections often occur in infants and elderly persons, and cause morbidity and mortality particularly in elderly patients [2, 3]. In an American study, the chance of hospitalization was 47–80% for infected elderly persons aged ⩾65 years [4]. Moreover, the NTS-associated economic burden to the healthcare system is estimated to be enormous. In addition to compromised quality of life due to illness, the treatment for those infections is expensive in developed countries. The average cost for Salmonella-related hospitalization is about US$7400, according to data obtained between 1990 and 1999 in USA [3], and €2411 (US$3375) in a cohort study between 1997 and 2006 in Spain [5].
Previous studies indicated that Salmonella infections often peaked during warm months [5, 6]. However, few nationwide studies of NTS infections in tropical or subtropical regions have been reported. Taiwan is a subtropical area with a humid and rainy climate, but there is no information about the incidence and economic burden of NTS infections in Taiwan. Of note, the emergence of resistance to ampicillin, trimethoprim-sulfamethoxazole, chloramphenicol, and particularly fluoroquinolone in NTS has been reported not only in Taiwan but also globally [7, 8]. Infections with such drug-resistant NTS isolates can be linked to clinical failure of antimicrobial therapy, and increasing morbidity and mortality in affected individuals [9].
In order to delineate the healthcare and financial impact of NTS infections in Taiwan, we analysed the hospitalization data of NTS infections from the Taiwan National Health Insurance (NHI) database between 2006 and 2008.
MATERIALS AND METHODS
At the end of 2008, the NHI covered 22 918 144 persons in Taiwan, reaching a coverage rate of 99·5% of Taiwanese people [10]. To identify hospitalized patients with NTS infections from 2006 to 2008, hospitalization records of the NHI database were included. There is one major diagnosis and at most four secondary diagnoses. The hospitalization data were included for analysis only if the major diagnosis fitted NTS infection criteria. NTS infections were diagnosed according to the attending physicians' discretion, and coded on the basis of the International Classification of Diseases, 9th revision – Clinical modification (ICD-9-CM). The ICD-9-CM coding for NTS infections included gastroenteritis (0030), septicaemia (0031), other specified Salmonella infections (0038), unspecified Salmonella infections (0039), pneumonia (00322), arthritis (00323), osteomyelitis (00324), meningitis (00321), other localized Salmonella infections (00329), and unspecified localized Salmonella infection (00320). Only the latest hospitalization data was analysed if multiple admissions for NTS infections in the same person were recorded. If an individual had more than one NTS infection during the same hospitalization, only one primary site of NTS infection was included based on the principal diagnosis. The medical cost for each person represented the aggregate of all itemized costs for services and disposables billed to the NHI. Medical costs in different countries were compared with US$ based on the current exchange rate (1 US$ = €0.7; 1 US$ = 30 New Taiwan Dollars).
To calculate the incidence of NTS-associated hospitalizations, patients who had admission records related to NTS infections in 2005 were excluded. To analyse seasonal variations of NTS-associated hospitalizations, the relationship between the average monthly incidence of NTS-associated hospitalizations and average monthly ambient temperature of six representative cities were measured. Data of average monthly temperature were obtained from the Central Weather Bureau, Taiwan (http://www.cwb.gov.tw; accessed 31 October 2011).
In different areas of Taiwan, the association between the incidence of NTS-associated hospitalizations for children (aged <10 years old) and child population density was studied. The child population density was calculated as total number of children in counties or cities where the hospitals were located. Data of population density at the end of 2008 were retrieved from published data in the website of the Directorate-General of Budget, Accounting, and Statistics, Executive Yuan, Taiwan (http://www.dgbas.gov.tw/ct.asp?xItem=19882&CtNode=3365&mp=1; accessed 31 October 2011).
Statistical analysis
Analysis was conducted using SAS version 9.2 (SAS Institute Inc., USA) and SPSS for Windows version 14.0 (SPSS Inc., USA). The significance of differences in proportions was determined by two-tailed χ2 test. Continuous variables of age, length of stay and medical cost for hospitalization were expressed as medians (interquartile ranges) and were compared by the Mann–Whitney U or Kruskal–Wallis H tests. The trend test for ordered Poisson rates was performed by the method proposed by Breslow & Day [11] to assess whether the incidence of NTS-associated hospitalizations for children was linearly associated with local child population density. The correlations between the monthly incidence of NTS-associated hospitalizations and temperature were analysed by simple linear regression.
RESULTS
During the 3-year study period, a total of 13 264 patients admitted for NTS infections were recorded. The average incidence was 19·3 NTS-associated hospitalizations/100 000 person-years, and the average in-hospital mortality rate was 0·26% (34 patients). There was a slight male predominance (54·6%) in hospitalized patients. Most patients were at the extremes of age (Table 1). Infants (aged <1 year) and children aged <10 years accounted for 78·7%, and the elderly aged ⩾70 years accounted for 5·3%. In the different age groups, the average incidence of NTS-associated hospitalizations in the general population was the highest in infants (524·7/100 000 person-years), followed by children aged 1–9 years (110·8/100 000 person-years). In contrast, the highest (2·6%) in-hospital mortality rate was noted in the elderly (aged ⩾70 years). Notably, all patients aged between 30 and 50 years survived after discharge from hospital.
Table 1.
The incidence, in-hospital mortality, associated medical cost, and admission duration of hospitalization due to non-typhoidal Salmonella infections, according to different age groups and various infection sites, 2006 and 2008
| Variables | Age group (years) | |||||
|---|---|---|---|---|---|---|
| <1 | 1–9 | 10–29 | 30–49 | 50–69 | ⩾70 | |
| Total case numbers | 3004 | 7441 | 703 | 672 | 737 | 707 |
| Annual incidence (per 100 000 person-years) | 524·7 | 110·8 | 3·4 | 3·0 | 5·4 | 14·8 |
| In-hospital mortality | 0 (0·00) | 1 (0·01) | 3 (0·43) | 0 (0·00) | 12 (1·63) | 18 (2·55) |
| Medical cost (US$) | 481 (346–682) | 455 (330–623) | 455 (308–739) | 619 (375–1210) | 853 (487–1789) | 1038 (598–2220) |
| Admission duration (days) | 5 (4–7) | 5 (4–7) | 5 (3–6) | 5 (4–8) | 7 (4–11·5) | 8 (5–14) |
| Infection sites, case numbers | ||||||
| Gastroenteritis (n = 12 315) | 2902 (99·6) | 7265 (97·6) | 650 (92·5) | 542 (80·7) | 513 (69·6) | 443 (62·7) |
| Septicaemia (n = 838) | 88 (2·9) | 161 (2·2) | 47 (6·7) | 107 (15·9) | 193 (26·2) | 242 (34·2) |
| Osteomyelitis (n = 25) | 0 (0·0) | 0 (0·0) | 2 (0·28) | 8 (1·2) | 12 (1·6) | 3 (0·42) |
| Pneumonia (n = 15) | 0 (0·0) | 1 (0·01) | 2 (0·28) | 4 (0·60) | 2 (0·27) | 6 (0·85) |
| Arthritis (n = 14) | 0 (0·0) | 1 (0·01) | 2 (0·28) | 3 (0·45) | 6 (0·81) | 2 (0·28) |
| Meningitis (n = 5) | 3 (0·1) | 1 (0·01) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 1 (0·14) |
| Other sites of infection (n = 52) | 11 (0·37) | 12 (0·16) | 0 (0·0) | 8 (1·2) | 11 (1·5) | 10 (1·41) |
Data are expressed as case number (%) or median value (interquartile range).
Gastroenteritis was the most common NTS infection (12 315 patients, 92·8%), followed by septicaemia (838, 6·3%). Most patients with NTS gastroenteritis survived in hospital, with an in-hospital mortality rate of 0·04%. Of patients with NTS septicemia, the in-hospital mortality rate was 3·3% (28/838 patients).
The age distribution of different NTS infections is given in Table 1. The majority (82·6%) of gastroenteritis occurred in children aged <10 years. In contrast, meningitis occurred exclusively in those of extreme ages (four children aged <10 years and one person aged ⩾70 years). Over half the hospitalizations due to NTS septicaemia, osteomyelitis, pneumonia, or arthritis, were observed in patients aged >50 years. The medical cost and length of stay increased with age for different types of NTS infections.
Annual incidences of hospitalized children (aged <10 years) with NTS infections within the areas of different child population density are given in Table 2. We further categorized the areas with varied child population densities into four groups: areas with ⩽54, >54 to ⩽180, >180 to ⩽692, and >692 children/km2). Our data showed a significant increasing trend (χ2 = 18·78, P < 0·001) in the annual incidence of NTS infections in children in areas with a higher child population density.
Table 2.
Annual incidence of hospitalized children (<10 years old) with non-typhoidal Salmonella infections in areas with varied child population density in Taiwan during 2006 and 2008
| Population density group (children/km2) | ||||
|---|---|---|---|---|
| ⩽54 | >54 to ⩽180 | >180 to ⩽692 | >692 | |
| Total case number | 2445 | 3748 | 1465 | 2787 |
| Population size of children aged <10 years | 608 547 | 950 069 | 179 331 | 481 537 |
| Incidence (per 100 000 children-years) | 133·9 | 131·5 | 272·3 | 192·9 |
Overall, the patients with NTS gastroenteritis were significantly younger with a median age of 1 year, and had a lower in-hospital mortality rate (0·04%) than those with septicaemia (aged 52 years, 3·3%) and those with other sites of infection (aged 48 years, 0·9%) (Table 3). The median medical cost per hospitalization for NTS gastroenteritis (US$468) was significantly lower than for septicaemia (US$1465) and other sites of infection (US$1918). Similarly, the median of the hospital stay for NTS gastroenteritis (5 days) was shorter than that for septicaemia (11 days) and other sites of NTS infection (12 days).
Table 3.
Characteristics, hospital cost and length of hospital stay of patients with non-typhoidal Salmonella infections
| Variables | Gastroenteritis (G) (n = 12 315) | Septicaemia (S) (n = 838) | Other infections (OI) (n = 111) | P value | P value | ||
|---|---|---|---|---|---|---|---|
| G vs. S | G vs. OI | S vs. OI | |||||
| Age (years) | 1 (1–5) | 52 (2–72) | 48 (8–64) | <0·0001 | <0·0001 | <0·0001 | 0·265 |
| Length of stay (days) | 5 (4–7) | 11 (7–16) | 12 (7–29) | <0·0001 | <0·0001 | <0·0001 | 0·094 |
| Medical cost (US$) | 468 (336–659) | 1465 (864–2906) | 1918 (666–5152) | <0·0001 | <0·0001 | <0·0001 | 0·342 |
| Male | 6713 (54·5) | 461 (55) | 70 (63·1) | 0·192 | — | — | — |
| Death | 5 (0·04) | 28 (3·3) | 1 (0·9) | <0·0001 | <0·0001 | 0·052 | 0·239 |
Data are expressed as median value (interquartile range) or case number (%).
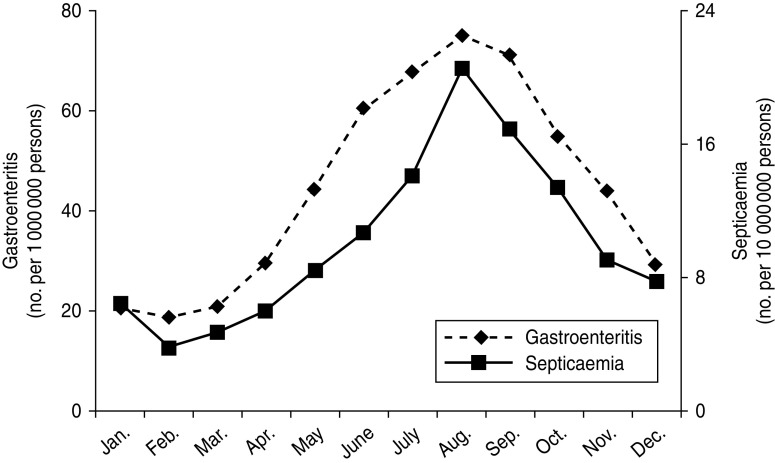
There are six branches of the Bureau of National Health Insurance, which are located in different areas of Taiwan (Fig. 1 a). The incidences of NTS-associated hospitalizations were steady over the three study years, but varied in different areas. The incidence of NTS-associated hospitalizations was correlated with temperature, and such a correlation was constantly present in six cities in Taiwan (Fig. 1 b). The incidence of NTS-associated hospitalizations increased from May, reaching a plateau in August, and a similar pattern was seen for hospitalizations due to NTS gastroenteritis or septicaemia (Fig. 2).
Fig. 1.
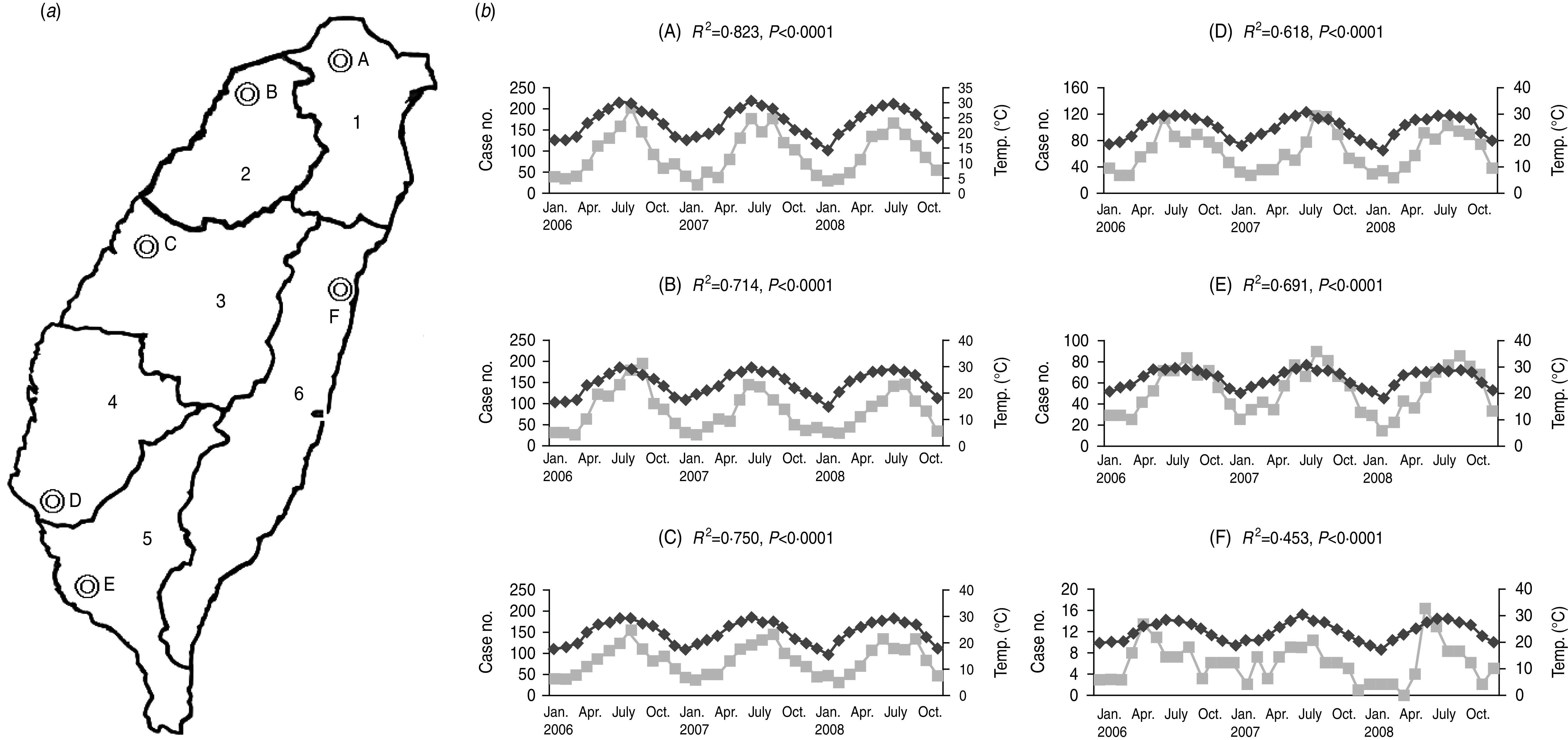
(a) Six areas, which were administrative regions of six branches of the Bureau of National Health Insurance of Taiwan, including Taipei (1), Northern (2), Central (3), Southern (4), Kao-Ping (5), and Eastern (6). (b) Significant correlation between case numbers ( ) of hospitalized patients with non-typhoidal Salmonella infections in the six areas and average temperature (°C) (◆) of six representative cities: (A) Taipei city, (B) Hsinchu city, (C) Taichung city, (D) Tainan city, (E) Kaohsiung city, and (F) Hualien city.
) of hospitalized patients with non-typhoidal Salmonella infections in the six areas and average temperature (°C) (◆) of six representative cities: (A) Taipei city, (B) Hsinchu city, (C) Taichung city, (D) Tainan city, (E) Kaohsiung city, and (F) Hualien city.
Fig. 2.
Seasonal variation of average monthly incidence of hospitalized patients with non-typhoidal Salmonella (NTS) gastroenteritis (n = 12 315) and septicaemia (n = 838) between 2006 and 2008 in Taiwan. The data were abstracted from the Taiwan National Health Insurance database.
DISCUSSION
Although NTS infection is not highly lethal for infected individuals, our data showed that NTS-associated hospitalizations accounted for more than 4400 admissions each year (i.e. annual incidence rate of 19·3/100 000 person-years) in Taiwan, and indeed created a heavy burden for the healthcare system. The annual incidence of NTS-associated hospitalizations in Taiwan was similar to those observed in Denmark (22·9/100 000 person-years) [12], Spain (16·2/100 000 person-years) [5], and France (9·5–19·2/100 000 person-years) [13]. The epidemiological data of human NTS in eastern Asia is rarely reported. In a study from Miyagi Prefecture, Japan, the estimated incidence of human salmonellosis, including laboratory-confirmed typhoid and NTS infections, was 32 cases/100 000 person-years [14], which was not higher than that reported in European countries [5, 12, 13, 15].
The incidence of NTS-associated hospitalizations was expected to be different geographically since the risk of infection is influenced by epidemiological and host factors in different countries. The difference could be partially explained by variable methods of case ascertainment in different studies. Some countries have established programmes to precisely estimate the burden of foodborne diseases by active surveillance, such as the Foodborne Diseases Active Surveillance Network (FoodNet) in the USA [16, 17] and a similar reporting system in Finland [18]. Of note, the incidence would be 520 cases/100 000 people in the USA, based on an estimate of 38·6 cases of Salmonella infections for each culture-proven case in the FoodNet data [16]. Our study utilized the NHI hospital database to estimate the epidemiology and medical expense of NTS-associated hospitalizations in Taiwan, and such data provided an epidemiological picture of the complicated form of NTS infectious diseases. It is obvious that without the NHI ambulatory visit data of NTS infection, the incidence of NTS infections will be underestimated.
Unsurprisingly, the highest annual incidence rate of NTS-associated hospitalizations was present in infants (524·7/100 000 person-years) and children aged 1–9 years (110·8/100 000 person-years). The reported hospitalization rate for children aged ⩽4 years was 89·12/100 000 children in Spain, and 444·8/100 000 children aged ⩽2 years in Poland [3, 5]. Therefore, NTS infection in Taiwan is a great threat to the health of infants and children, and results in a huge economic burden for social and healthcare resources.
In the present study, an important demographic factor, population density, which has been associated with Salmonella infections [19, 20], was documented to be correlated with the incidence of NTS infections in children. Risk factors of Salmonella infections in children, such as exposure to reptiles and amphibians, consumption of concentrated liquid infant formula, eggs or meat, international travel, and attending day care with children suffering from diarrhoea, have been well recognized [19, 20]. However, specific factors predisposing children to NTS infections in Taiwan could not be revealed in the present study due to a limitation of ecological analysis.
Although NTS infections occur commonly in children aged <10 years and elderly persons (⩾70 years), the in-hospital mortality rate was higher in the latter group (0·009% vs. 2·6%, P < 0·0001). In addition, invasive NTS infections, such as septicaemia, pneumonia, or osteomyelitis, occurred mainly in the elderly. One exception was NTS meningitis, which is a rare complication of NTS infection and usually leads to severe consequences and mortality in infants [21]. Unfavourable outcome in the elderly with NTS infections may be related to concurrent chronic underlying diseases and impaired host immunity in this population group [22, 23]. Gut immaturity, gastric hypoacidity and intra-family transmission may explain the fact that children or infants are more susceptible to NTS infections [24–26]. However, the reason for a low mortality rate of NTS infections in infants is still not clearly understood.
In the present study, annual medical expenditure of NTS-associated hospitalizations in Taiwan was estimated to be about US$3.2 million. The annual cost for salmonellosis-related hospitalization was estimated to be US$22.7 million in Spain, and US$11.4–20 million in California [3, 5]. Although Salmonella infections, especially gastroenteritis, cause little mortality, their cost is great. NTS gastroenteritis accounts for major resource consumption in both developing and developed countries [1]. NTS extra-intestinal infections, such as septicaemia or pneumonia, although less common, might lead to longer hospital stays, and more deaths and medical costs than gastroenteritis. Similar findings have been reported in two studies in Spain and California, USA [3, 5]. However, the medical costs for NTS infections from the NHI data are conservative estimates, since such estimates did not include the expense of outpatients or post-hospital care, and the productivity loss of affected patients or those taking care of the patients.
The study demonstrated a seasonal variation of NTS-associated hospitalizations in Taiwan. A peak of NTS infection during summer seasons is similar to that observed in countries with warm climates [5, 6]. Seasonality has been related to multiple factors, such as seasonal human behaviours, environmental changes influencing the virulence and infectivity of pathogens, and variations of host susceptibility [6]. A Canadian study found that common activities, e.g. gardening or having a barbeque in the summer were potential risk factors for salmonellosis [6]. Since the variations of seasonal human activities in different areas may influence the risk of NTS infections, further surveillance is necessary to identify preventable risk factors or human behaviours. Moreover, our study provides useful public health information to plan rational distribution of healthcare resources during the summer months.
There were several limitations to the present study. First, NTS infections in this study were likely to be the severe form requiring hospitalization, not the mild, self-limiting form of gastroenteritis. However, such summation results from the NHI system could provide a comprehensive picture of moderate-to-severe NTS infectious disease, because the NHI covers over 99% of the general population in Taiwan. Second, the diagnosis of NTS infections was based on ICD-9-CM coding and the chance of overestimation of NTS-associated hospitalizations is minimal because the clinical diagnosis of NTS infections is frequently made on the basis of microbiological reports. In addition, regular audits of hospital records are essential for reimbursement in the NHI system, and thus the accuracy of diagnostic coding can be expected. Third, individuals with NTS infections, especially the elderly, had underlying diseases, and therefore it is difficult to analyse the cause of mortality among such cases even with the diagnosis of NTS infections. Only the in-hospital mortality rate was evaluated in the present study. Similarly, medical cost of hospitalization directly associated with NTS infections can not be obtained from the NHI data. However, our data still delineated the crude mortality rate and relevant medical costs for patients hospitalized with NTS infections.
CONCLUSIONS
In conclusion, NTS infections are a great burden to healthcare systems, as they occur mainly in infants and children and the mortality related to NTS infections is significant in the elderly.
ACKNOWLEDGEMENTS
We are grateful to Miss Jia-Ling Wu for providing the statistical consulting services from the Biostatistics Consulting Center, National Cheng Kung University Hospital.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Majowicz SE, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clinical Infectious Diseases 2010; 50: 882–889. [DOI] [PubMed] [Google Scholar]
- 2.Olsen SJ, et al. The changing epidemiology of salmonella: trends in serotypes isolated from humans in the United States, 1987–1997. Journal of Infectious Diseases 2001; 183: 753–761. [DOI] [PubMed] [Google Scholar]
- 3.Trevejo RT, et al. Epidemiology of salmonellosis in California, 1990–1999: morbidity, mortality, and hospitalization costs. American Journal of Epidemiology 2003; 157: 48–57. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy M, et al. Hospitalizations and deaths due to Salmonella infections, FoodNet, 1996–1999. Clinical Infectious Diseases 2004; 38 (Suppl. 3): S142–S148. [DOI] [PubMed] [Google Scholar]
- 5.Gil Prieto R, et al. Epidemiology of hospital-treated Salmonella infection; data from a national cohort over a ten-year period. Journal of Infection 2009; 58: 175–181. [DOI] [PubMed] [Google Scholar]
- 6.Ravel A, et al. Seasonality in human salmonellosis: assessment of human activities and chicken contamination as driving factors. Foodborne Pathogens and Disease 2010; 7: 785–794. [DOI] [PubMed] [Google Scholar]
- 7.Chiu CH, et al. The emergence in Taiwan of fluoroquinolone resistance in Salmonella enterica serotype choleraesuis. New England Journal of Medicine 2002; 346: 413–419. [DOI] [PubMed] [Google Scholar]
- 8.Su LH, et al. Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clinical Infectious Diseases 2004; 39: 546–551. [DOI] [PubMed] [Google Scholar]
- 9.Chen PL, et al. Extraintestinal focal infections in adults with Salmonella enterica serotype Choleraesuis bacteremia. Journal of Microbiology, Immunology and Infection 2007; 40: 240–247. [PubMed] [Google Scholar]
- 10.Department of Health Executive Yuan TR, 2010 (http://www.doh.gov.tw/statistic/%A5%FE%A5%C1%B0%B7%ABO/95.htm). Accessed 30 October 2011.
- 11.Breslow NE, Day NE. Statistical methods in cancer research. Volume II – The design and analysis of cohort studies. IARC Science Publications 1987; 82: 114. [PubMed] [Google Scholar]
- 12.Fisker N, et al. Clinical review of nontyphoid Salmonella infections from 1991 to 1999 in a Danish county. Clinical Infectious Diseases 2003; 37: 47–52. [DOI] [PubMed] [Google Scholar]
- 13.Vaillant V, et al. Foodborne infections in France. Foodborne Pathogens and Disease 2005; 2: 221–232. [DOI] [PubMed] [Google Scholar]
- 14.Kubota K, et al. The human health burden of foodborne infections caused by Campylobacter, Salmonella, and Vibrio parahaemolyticus in Miyagi Prefecture, Japan. Foodborne Pathogens and Disease 2008; 5: 641–648. [DOI] [PubMed] [Google Scholar]
- 15.Weinberger M, Keller N. Recent trends in the epidemiology of non-typhoid Salmonella and antimicrobial resistance: the Israeli experience and worldwide review. Current Opinion in Infectious Diseases 2005; 18: 513–521. [DOI] [PubMed] [Google Scholar]
- 16.Voetsch AC, et al. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clinical Infectious Diseases 2004; 38 (Suppl. 3): S127–S134. [DOI] [PubMed] [Google Scholar]
- 17.Mermin J, et al. Reptiles, amphibians, and human Salmonella infection: a population-based, case-control study. Clinical Infectious Diseases 2004; 38 (Suppl. 3): S253–S261. [DOI] [PubMed] [Google Scholar]
- 18.Laupland KB, et al. Salmonella enterica bacteraemia: a multi-national population-based cohort study. BMC Infectious Diseases 2010; 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones TF, et al. A case-control study of the epidemiology of sporadic Salmonella infection in infants. Pediatrics 2006; 118: 2380–2387. [DOI] [PubMed] [Google Scholar]
- 20.Lee WS, Puthucheary SD, Omar A. Salmonella meningitis and its complications in infants. Journal of Paediatrics and Child Health 1999; 35: 379–382. [DOI] [PubMed] [Google Scholar]
- 21.Morris JG Jr., Potter M. Emergence of new pathogens as a function of changes in host susceptibility. Emerging Infectious Diseases 1997; 3: 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna KV, Markham RB. A perspective on cellular immunity in the elderly. Clinical Infectious Diseases 1999; 28: 710–713. [DOI] [PubMed] [Google Scholar]
- 23.Hohmann EL. Nontyphoidal salmonellosis. Clinical Infectious Diseases 2001; 32: 263–269. [DOI] [PubMed] [Google Scholar]
- 24.Shimoni Z, et al. Nontyphoid Salmonella bacteremia: age-related differences in clinical presentation, bacteriology, and outcome. Clinical Infectious Diseases 1999; 28: 822–827. [DOI] [PubMed] [Google Scholar]
- 25.Schutze GE, et al. The home environment and salmonellosis in children. Pediatrics 1999; 103: E1. [DOI] [PubMed] [Google Scholar]