Abstract
Schizophrenia is a chronic mental and behavioral disorder characterized by clusters of symptoms including hallucinations, delusions, disorganized thoughts and social withdrawal. It is mainly contributed by defects in dopamine, glutamate, cholinergic and serotonergic pathways, genetic and environmental factors, prenatal infections, oxidative stress, immune system activation and inflammation. Management of schizophrenia is usually carried out with typical and atypical antipsychotics, but it yields modest benefits with a diversity of side effects. Therefore, the current study was designed to determine the phytochemicals as new drug candidates for treatment and management of schizophrenia. These phytochemicals alter and affect neurotransmission, cell signaling pathways, endocannabinoid receptors, neuro-inflammation, activation of immune system and status of oxidative stress. Phytochemicals exhibiting anti-schizophrenic activity are mostly flavonoids, polyphenols, alkaloids, terpenoids, terpenes, polypropanoids, lactones and glycosides. However, well-designed clinical trials are consequently required to investigate potential protective effect and therapeutic benefits of these phytochemicals against schizophrenia.
Keywords: schizophrenia, phytochemicals, oxidative stress, flavonoids, dopamine
Introduction
Schizophrenia is a major debilitating disease of adults in every society, affecting about 1–1.5% of global population (Howes and Murray, 2014). The incidence of schizophrenia is higher among males than female at a ratio of 1.4 to1.0 (McGrath et al., 2008). Schizophrenia is the seventh most costly disorders in the world (Ross and Margolis, 2005; McGrath et al., 2008). It is a syndrome involving positive and negative symptoms, and cognitive problems (McCutcheon et al., 2019). Positive symptoms, including hallucinations and delusions are the foremost feature of this syndrome. Negative symptoms include the failure to express emotions and apathy. Cognitive problems arise before the appearance of psychosis and can act as better predictor of the disease (Dienel and Lewis, 2019). Unlike other degenerative diseases, its onset occurs during early adulthood or late adolescence (An der Heiden and Häfner, 2000). Schizophrenia predominantly occurs during second and third decade of the life, but it can also affect elderly individuals (Niemi et al., 2003). It increases the risk of other brain disorders such as Parkinson’s disease, autism, Alzheimer’s disease and multiple sclerosis (Brown, 2012).
A complex interaction of genetic, nutritional, microbial and environmental factors contribute to schizophrenia (Eyles, 2021). Several neurotransmitters such as Dopamine, gamma aminobutyric acid (GABA) and glutamate, serotonin and noradrenaline play significant role in the pathogenesis as well as progression of schizophrenia (Prestwood et al., 2021). Moreover, schizophrenia also results from interplay of neuro-inflammation, oxidative stress, cell signaling pathways and abnormal immune system activation with schizophrenia (Kokkinou et al., 2021).
Typical anti-psychotic drugs show higher affinity, stronger binding and more inhibition of dopamine receptors than the atypical anti-psychotic drugs. However, atypical anti-psychotic drugs are more effective than the typical antipsychotics due to their action at dopamine, serotonin and cholinergic receptors. Individual anti-schizophrenic drugs have variable efficacy in different patients (Bahta et al., 2021). Atypical antipsychotics are generally more effective, but have fewer side effects as compared to typical anti-psychotic drugs. General adverse effects of these synthetic drugs include but not limited to hormonal disturbances, vertigo, tardive dyskinesia, obesity, infertility, neuroleptic malignant syndrome, sedation and agitation. For avoiding these drug related problems, there is a great need of more efficacious and safer remedies (Prestwood et al., 2021).
Phytochemicals are of natural origin that offer cost effective, accessible and valuable source of drugs. Herbal therapies have played their beneficial role throughout human history. Humanity is turning towards herbal therapies due to questionable efficacy and toxic health implications of already used pharmacotherapy of schizophrenia (Datta et al., 2021). Moreover, the progress in developing synthetic anti-schizophrenic drugs is still glaringly slow because of diverse factors such as heterogeneity of schizophrenia phenotypes, ambiguous pathophysiology, pathological lesions, complex genetics changes and other risk factors (Yadav, 2021). Therefore, the phytochemicals offer potential and diverse alternatives to allopathic anti-schizophrenic medicines due to their wide array of biological activities such as anti-inflammatory activity, anti-oxidant potential, affecting neurotransmission, and modulating cell signaling pathways (Arnold et al., 2005; Ross and Margolis, 2005; Rapoport et al., 2005)
Pharmacotherapy of schizophrenia is usually carried out with typical and atypical antipsychotics, but these drugs yield only modest benefits with a diversity of side effects. Phytochemicals are diverse chemicals that offer themselves as useful alternative to conventional allopathic treatments. Therefore, the current review was designed to determine the potential of anti-schizophrenic phytochemicals as new drug candidates and, the pre-clinical and clinical progress regarding their antipsychotic action.
Risk Factors of Schizophrenia
Schizophrenia is a complex disease which remains rudimentary and has involvement of various genetic, nutritional, microbial and environmental factors (Caspi and Moffitt, 2006; Lewis and Sweet, 2009). A person can have several defective genes, but risk factors such as infections, drug abuse and obstetric complications may conclusively lead to illness (Craddock et al., 2005; Winterer et al., 2003). Infections like influenza, rubella, cytomegalovirus, Toxoplasma gondii, herpes simplex virus 1 and 2, and polio virus can predispose the vulnerable individuals to schizophrenia (Brown and Susser, 2002). Obstetric complications including low birth weight, premature birth, rhesus incompatibility, resuscitation at birth time, nutritional deficiency of fetus and emergency caesarean delivery have been strongly correlated to the disease (Kyle and Pichard, 2006; St Clair et al., 2005). After maternal infection, there is an increased production of cytokines that adversely affects the immune system culminating in brain damage (Brown and Derkits, 2010). Nutritional factors that can contribute to schizophrenia include continuous intake of high fat and high sugar diets, and deficiency of vitamin D, B9 and B12. Recent studies showed that a high level of maternal IL-8 had caused anatomical problems in fetus (Meyer 2011; Na et al., 2014).
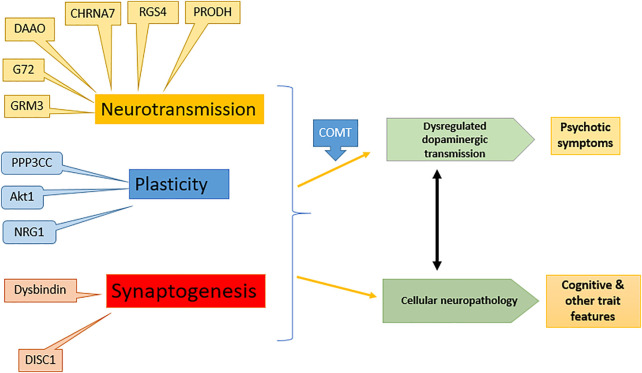
Schizophrenia has a strong hereditary tendency, showing 10% chance in close relatives of patient. A complex interaction of one or more of 20 genes is responsible for the disease (Harrison and Weinberger, 2005). The genes including neuregulin-1 (NRG1), dysbindin (DTNBP1), disrupted in schizophrenia (DISC1), d-amino acid oxidase (DAAO), regulator of G protein signaling-4 (RGSR), catechol-O-methyl transferase (COMT), proline dehydrogenase (PRODH) and G72 are schizophrenia susceptible while several genes affect the glutamatergic transmission pathway in the brain (Craddock et al., 2005; Ross and Margolis, 2005). The effect of various gene expressions on schizophrenia is shown in Figure 1.
FIGURE 1.
Effect of different genes on symptoms of schizophrenia: modified from (Harrison and Weinberger 2005). NRG1, Neuregulin-1; DTNBP1, Dystrobrevin-binding protein 1; DISC1, Disrupted in schizophrenia; DAAO, d-amino acid oxidase; RGSR, Regulator of G protein signaling-4; COMT, Catechol-O-methyl transferase; PRODH, Proline dehydrogenase, G72, d-amino acid oxidase activator.
There is another phenomenon called endo-phenotypes that is responsible for different clinical symptoms e.g. cognitive defects, neurological abnormalities, impaired emotions and eye movement abnormality (Cannon, 2005; Ross and Margolis, 2005). Different genes control different endo-phenotypes of specific characteristics, inherited in a Mendelian fashion and can cause full schizophrenia if all genes are inherited together (Rapoport et al., 2005; Khandaker et al., 2015).
Pathophysiology of Schizophrenia
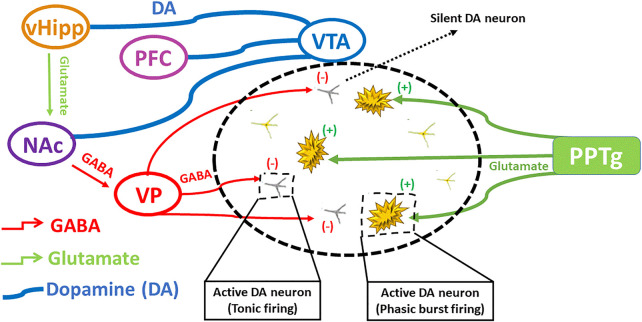
Etiopathogenicity of schizophrenia is mainly understood based on several hypotheses (Fendri et al., 2006). The dopamine hypothesis remains a mainstay in understanding schizophrenia and is based on the fact that antipsychotics produce their effect by blocking dopamine D2/D3 receptors. It was further validated by the action of those agents which enhance dopamine level (Abi-Dargham, 2004). Hypo-stimulation of D1 receptor in hippocampus causes negative and cognitive symptoms, while hyper-stimulation of dopamine D2 receptor causes positive symptoms in the subcortical regions (Davis et al., 1991). However, new approaches during recent times have demonstrated a complex interplay among different neurotransmitter circuits (Agren et al., 1991). Another hypothesis indicated that the reduced function of NMDA receptors could produce symptoms of schizophrenia (Moghaddam, 2003; Coyle, 2006). A controlled study showed that antipsychotic drugs positively affected negative symptoms and to lesser extent, cognitive and positive symptoms by increasing the function of NMDA receptors (Coyle, 2006). Moreover, the role of glutamate in schizophrenia was depicted by the discovery of phencyclidine (PCP angel dust) as it induces a psychotic condition by powerful antagonistic action on glutamate receptor i.e. NMDA receptor (Lodge, 1989). The action of dopaminergic neurons may either directly be enhanced by the glutamatergic neurons or indirectly inhibited through the involvement of GABAergic transmission. The interplay of different neuronal signals involved in schizophrenia is depicted in Figure 2.
FIGURE 2.
The interplay of different neuronal signals involved in schizophrenia. Ventral hippocampus regulates dopamine levels by excitatory projections towards ventral pallidum, that regulates GABAergic transmission producing silent DA neurons, influencing on DA rapid burst firing and tonic firing. Modified from (Grace and Gomes 2019).
Dopamine, GABA and glutamate are not the only neurotransmitters involved, but serotonin and noradrenaline also play a significant role in the onset of disease. Moreover, many atypical antipsychotics possess adrenergic blocking ability (Hayes and Schulz, 1983). Lysergic acid diethylamide acts as a serotonin agonist on limbic cortex affecting GABAergic neurons that causes a reduction in glutamatergic tone in corticostriatal area resulting in hallucinations (Gellman and Aghajanian, 1991). Newer atypical antipsychotics have better tolerability profile as compared to typical antipsychotics owing to higher affinity for 5HT2A and lower affinity for D2 receptors in comparison to typical antipsychotic drugs (Schotte et al., 1996). Other receptors usually involved are 5HT2C, 5HT6 or 5HT7 and their modulation shows fewer extra pyramidal symptoms (EPS) (Meltzer and Fatemi, 1996). In recent studies, it was found that atypical anti-psychotic action was partly mediated through their agonistic action at 5HT1A and 5HT2C, and antagonistic action at 5HT6 and 5HT7 (Meltzer, 1999). Some antipsychotics such as phenothiazines, induce less EPS, which shows their effects were partially muscarinic antagonistic in nature. In striatum, dopaminergic terminals have an affinity to affect cholinergic interneurons that eventually affect D2 inhibitory receptors (Pisani et al., 2007). When an antipsychotic agent blocks D2 receptors, it enhances acetylcholine release in striatum. Indeed, it is also now considered that 5HT has no direct involvement in pathophysiology of schizophrenia, but its manipulation with D2 antagonism can produce improved therapeutic effects (Meltzer and Nash, 1991).
Neurotransmitters and Brain Regions Involved in Schizophrenia
In addition to several neurotransmitters, there is also huge brain areas implicated in schizophrenia, including brainstem, striatum, limbic cortex, neocortex and basal ganglia (Grace and Gomes, 2019). Imaging studies have revealed the lateral and third ventricle enlargement, loss of some brain volume and, volume deficit in the prefrontal and temporal cortex, para-hippocampus, hippocampus and thalamus (Wright et al., 2000; Sullivan et al., 2003). Other cerebral lesions include cavum septi pellucid enlargement, and abnormalities in corpus collosum, cerebellar and basal ganglia (Niznikiewicz et al., 2003; Antonova et al., 2004; Honea et al., 2005). Moreover, cyto-architectural abnormalities in the grey matter of entorhinal area, corticolimbic portion, and aberrant neurons in the white matter of prefrontal cortex, para-hippocampus and temporal regions are evident (Arnold et al., 1997; Arnold et al., 2005).
The abnormal dopamine signaling in striatum is responsible for increase of positive and negative symptoms, and decline of cognition in schizophrenia. Striatum mainly associative striatum acts as an integrative hub that moderates communication between limbic and motor regions. In schizophrenia, anomalous dopamine signaling in associative striatum adversely affects integrative functions, connectivity between striatum and cortex disrupting the cortical input from emotional, cognition and motor regions. Dopamine receptors respond differently to dopamine in different regions of striatum. An increased level of D2 receptors was found in associative striatum of schizophrenic patients that was responsible for cognitive deficit and altered neuronal information arriving from various areas of prefrontal cortex (McCutcheon et al., 2019; Simpson et al., 2010). Summary of various brain regions involved in schizophrenia is shown in Figure 3.
FIGURE 3.
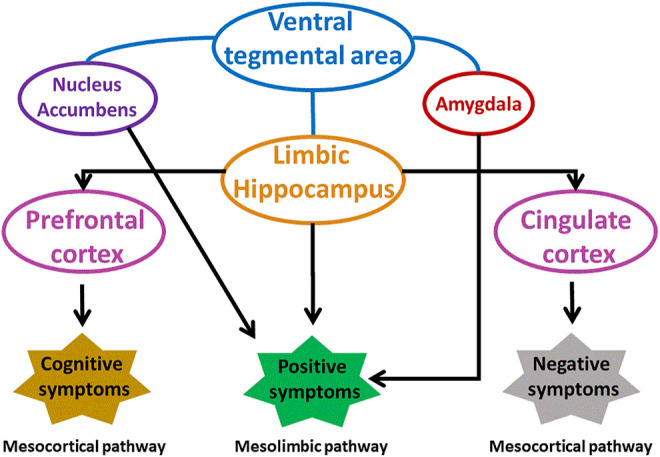
Involvement of different brain areas in pathophysiology of schizophrenia. Modified from (Grace and Gomes 2019). VP, ventral pallidum.
Oxidative Stress, Inflammation and Immune System Involved in the Schizophrenia
Recent investigations showed that the oxidative stress and neuro-inflammation played a critical role in the pathogenesis of schizophrenia. Inflammation, oxidative stress and altered expression of proteins collectively lead to schizophrenia. Mitochondrial damage in neurons results in the impairment of mitochondrial respiration and changes in morphology. It also causes a low pH in the brain leading to psychotic symptoms and cognitive defects (Manji et al., 2012; Morris and Maes, 2014). These effects in peripheral tissues work as biomarker of the disease. Oxidative stress is evident both in early onset and chronic schizophrenia (Boskovic et al., 2011). The immune system acts by producing ROS and RNS promoting the release of cytokines that causes neuro-inflammation (Halliwell, 2012). By manipulation of these oxidative stress responses, different new pharmacological treatments can be identified.
An increased amount of ROS and RNS saturates ability of antioxidants, such as glutathione, which neutralize them to cause oxidative stress (Sies, 2015). Inflamosomes, after formation, can stimulate the production of IL-18 and IL-1B that adversely affect the microglia, macrophages and astrocytes. Interleukins also interact with the cytokines (Calabrese, 2008). Moreover, there is variable response to anti-schizophrenic treatments in patients. An increase in IL-6 is associated with delayed response as resistance to treatment is associated with an elevated level of both IL-6 receptor and Tumor necrosis factor receptor (TNFR) (Lurie, 2018). Furthermore, stress can increase pro-inflammatory cytokines leading to schizophrenia (Müller and Bechter, 2013). There is evidence that schizophrenic patients have increased level of peripheral plasma cytokines, prostaglandin E2, IL-1, IL-8, and C reactive protein, indicating elevated immune response in peripheral plasma (Miller et al., 2011). It is now established that the immune changes in peripheral blood are indicative of brain function and behavior in different neuropsychiatric disorders (Dantzer, 2004). Understanding the causes and mechanism of neuro-inflammation associated with schizophrenia presents a potential target for the treatment of disease (Watkins and Andrews, 2016).
The involvement of abnormal immune response in pathogenesis of disease is evident (Feigenson et al., 2014). Traditionally, it is thought that the brain is protected immunologically by blood brain barrier, but recent studies demonstrated a complex interaction between brain, systemic inflammation and immune system, altering the mood and behavior (Khandaker et al., 2015). Moreover, alterations in immune system can profoundly affect neurotransmission involved in the pathogenesis of schizophrenia. It can activate the enzyme indoleamine 2,3-dioxygenase involved in tryptophan and kynurenic acid metabolism that influences glutamatergic and serotonergic neurotransmission through these neuroactive metabolites (Müller and Bechter, 2013).
Cholinergic System as Potential Target
Cholinergic system is a potential target for ameliorating the symptoms of schizophrenia, including negative and cognitive symptoms. This system affects working memory, attention and motivation (Berman et al., 2007). As, Nicotinic acetylcholine receptors (nAChR) belong to family of ligand gated ion channel and its homomeric subtype, nAChR-α7 is found in central and peripheral nervous system. It found to have pivotal role in the pathophysiology of several neurological disorders including psychosis. There is a direct role of nAChR-α7 and muscarinic M1 receptor in schizophrenia symptoms (Ochoa and Lasalde-Dominicci 2007; Raedler et al., 2007). Hence, agents acting on these targets are potential candidate for treating schizophrenia.
Phosphodiesterase Inhibitors for Schizophrenia
Phosphodiesterases (PDE) are the enzymes often targeted for their pharmacological inhibition because PDE inhibitors can potentiate the effect of different physiological processes which are mediated by cGMP or cAMP. These are identified as a new adjunctive therapy for different diseases including schizophrenia (Madeswaran et al., 2012).
Signaling Pathways as Treatment Target for Schizophrenia
It is found that the level of glycogen synthase kinase-3 (GSK-3) is increased while Akt (protein kinase) is reduced in schizophrenic patients (Sahin et al., 2014). Dopamine regulates lithium sensitive signaling cascade that involves GSK3β. Modulation in its increased activity can have impact on long lasting remission of the disease (Duda et al., 2018). A protein kinase, called Akt, is involved in a variety of functions such as neuronal cell size regulation, synaptic plasticity and cell survival, while Akt1 has the most important role in schizophrenia. It is activated by phosphorylation. The GSK-3, regulating the synaptic plasticity, is inactivated after phosphorylating with Akt1. The reduced level of Akt1 also decreases phosphorylation of GSK, thus the activity of GSK-isoform GSK-3 β is enhanced in the frontal cortex area of schizophrenic patients. Antipsychotic drugs are expected to increase Akt activity through blockade of D2 receptor activation (Karam et al., 2010). Other molecules such as Wnt, are lipoglycoproteins, which regulate embryonic development by acting as signaling molecules. Dysregulation in the Wnt signaling pathway contributes to various human diseases including schizophrenia. Moreover, reduction of β-catenin, increase in Wnt-1 expression and reduced GSK-3β contribute to multifaceted kinase present in Wnt signaling (Hoseth et al., 2018). Investigations are being carried out on natural and synthetic agents affecting these cascades in order to cope with schizophrenia.
Alteration in Hormonal Balance for Treatment Option of Schizophrenia
The protective role of estrogen against schizophrenia has been demonstrated previously. Recent clinical trials have validated the usefulness of estradiol in the treatment of disease (Gogos et al., 2015). Oxytocin and vasopressin have been implicated in the disease etiology and the antagonistic approach against vasopressin V1A receptors may provide an opportunity for treating schizophrenia (Park et al., 2020).
Current Pharmacological and Non-pharmacological Treatments
Antipsychotic drugs are also called neuroleptics (meaning, taking hold of one’s nerves) and are used to manage acute and chronic schizophrenia symptoms. Generally, first generation (typical) and second generation (atypical) anti-psychotic drugs are used in allopathy (Khandakeret al., 2015). Typical antipsychotics include chlorpromazine, thioridazine, fluphenazine, trifluperidol, flupenthixol, loxapine, triflupromazine, trifluperazine, haloperidol, penfluridol and pimozide. Risperidone, clozapine, aripiprazole, olanzapine, ziprasidone, quetiapine and sulpiride are a few examples of atypical antipsychotic drugs. Atypical antipsychotics are generally more effective, but have fewer side effects as compared to typical anti-psychotic drugs [27]. General adverse effects of these synthetic drugs include but not limited to diabetes, dizziness, tardive dyskinesia, weight gain, sexual dysfunction, neuroleptic malignant syndrome, sedation and agitation. For avoiding these drug related problems, more efficacious and safer remedies are needed [36].
Several non-pharmacological treatments are currently used for the management of schizophrenia. Aromatherapy and aroma massage are helpful in ameliorating depressive symptoms of schizophrenia [54]. Acupuncture activates different brain regions involved in controlling emotions of schizophrenic patients [42]. For improvement of constant negative symptoms, loving kindness mediation (LKM) has been proved useful [39]. Yoga and aerobic exercises are also very helpful for the psychiatric symptoms of schizophrenia [49].
Phytochemicals and Their Role in Schizophrenia
About 80% of the total population of Asia and Africa are dependent on natural therapeutics. Mainstream antipsychotic drugs are associated with several adverse effects. Therefore, several phytochemicals have been investigated for neuroprotection and anti-psychotic action in cell culture and animal models of CNS disorders. The vast majority of studies have demonstrated that the anti-psychotic and neuroprotective action of phytochemicals is due to their antioxidant action [19]. As pathophysiology of schizophrenia clearly depicts oxidative burden in brain, so the natural antioxidants in the form of extracts or individual phytochemicals are effective for treatment of schizophrenia. These phytochemicals have gained attention due to their therapeutic value, less adverse effects, better safety profile and high efficacy (Gao and Snyder 2013; Choudhury et al., 2014). Several phytochemicals investigated in pre-clinical and clinical studies are shown in Table 1 and 2.
TABLE 1.
Antipsychotic potential of phytochemicals in non-clinical studies.
| Chemical Class | Phytochemical | Source | Assay/Test | Animal/ Cell Type | Doses | Method | Mechanism | Result | References |
|---|---|---|---|---|---|---|---|---|---|
| Alkaloids | Arecoline | Areca catechu | Y-maze Behavioral test | cuprizone induced mouse model | 0, 2.5, or 5 mg/kg/Day | Recorded spontaneous alternation behavior | Preventing white matter injury, prevented memory impairment | Attenuated spatial working memory impairment, increased the expression of myelin basic protein in the frontal cortex | Xu, Adilijiang, Wang, You, Lin, Li and He (2019) |
| Stepholidine | Stephania intermedia | Paw test Pre-pulse inhibition | Male Wistar rats | 4–16 mg/kg | Determined limb retraction time and | D1 receptor agonist and D2 receptor antagonist | Increased hind limb retraction time Reverse apomorphine induced disruption | Ellenbroek, ZHANG and JIN (2006) | |
| Galantamine | Galanthus caucasicus | Dopamine receptor agonism by apomorphine, NMDA antagonism by MK-801, muscarinic receptor antagonism by scopolamine | Wistar rats | 0.3, 1.0 and 3.0 mg/kg | Apomorphine agonism, NMDA and Ach antagonism models | Increase in cholinergic activity | Pre-pulse inhibition was improved | Hohnadel et al. (2007) | |
| Corymine | Hunter zeylancia | 2 electrode voltage clamp technique | cDNA clones of NR1a and NR2b OF Xenopus | 100 µM | Potentiating effect of corymine was induced in presence of glycine | Potentiation on NMDA response | Potentiates the NMDA induced currents and can be used for schizophrenia | Leewanich et al. (2005) | |
| Reticuline | Ocotea duckei | Amphetamine induced hyper-motility | Swiss albino mice | 50–100 mg/kg | Number of steps recorded | Dopamine antagonist activity | Reduced hyper-motility | Morais, Barbosa-Filho and Almeida (1998) | |
| Geissoschizine methyl ether | Uncariae ramulus | Cell based Calcium imaging analysis | Human cell line and mouse brain tissue | Inhibited dopamine induced calcium response | Partial agonist/antagonist at D2, partial antagonist at 5HT receptors | Inhibited calcium induced serotonin current | Ueda et al. (2011) | ||
| Psychollatine | Psychotria umbellata | Male adult mice | a) Apomorphine induced climbing b)MK-801 induced hyperactivity | 100 mg/kg | Climbing behavior and locomotion determined | Interference with DA, 5HT and NMDA receptors | Attenuated the climbing and locomotion | Costa-Campos, Lara, Nunes and Elisabetsky (1998) | |
| Alstonine | Picralima nitida | Male albino mice Male Wistar rats | a)Apomorphine induced stereotypy, b)haloperidol induced catatonia | 0.5 to 2 mg/kg | Determined behavioral score and catatonic time | Modulating the DA uptake and serotonin receptors | Reduction in behavioral score, diminished catatonic time | Costa-Campos, Lara, Nunes and Elisabetsky (1998) | |
| Physostigmine | Physostigma venenosum | Conditioned emotional response | Male Wistar rats | 0.5 mg/kg | Pre-exposure and in conditioning response is measured | Induced Latent inhibition disruption | Reverse the cognitive impairment in schizophrenia | Barak and Weiner (2010) | |
| Amino acid and derivatives | Leucine | Cucurbeta pepo | Apomorphine induced stereotypy. Haloperidol induced catalepsy | Wistar rats | 0.7 mg/kg | Stereotypy, catalepsy | Anti-dopaminergic effect | Decreased stereotypy, potentiated catalepsy | Suresh and Raju (2013) |
| Betaine | Beta vulgaris | PPI, NOR | Male ICR mice | O, 30, 100 mg/kg | %PPI, recognition index % | Modulation of NMDA R glycine site | Attenuated ketamine induced disruption in PPI, improved novel recognition | Lin et al. (2016) | |
| Bioflavonoids/ Polyphenols | Quercetin-3- rutoside | Fagopyrum esculentum | PCR-RFLP method | Human brain | 10 µmol | Hetero-plasmic sequence variation determined | reduced oxidative stress | Quench the superoxide production | Marchbanks et al. (2003) |
| Scopoletin | Morinda citrifolia | a) Apomorphine induced Cage climbing, b) Amphetamine induced stereotypy | Male ICR mice | 0.1 mg/kg | Climbing and stereotypy determined | Anti-dopaminergic effect | Reduction in climbing and stereotypy | Pandy and VijeePallam (2017) | |
| Quercitin | Lonchocarpus cyanescens | Novel object recognition (NOR) | Balb-C mice | 25 and 50 mg/kg | Memory impairment model by ketamine used | Antioxidant potential | Improves cognitive deficit | Mert, Turgut, Arslanbas, Gungor and Kara (2019) | |
| Myricitrin | Eugenia uniflora | Apomorphine induced stereotypy, catalepsy and paw test | Swiss albino mice Wistar rats | 5,10 and 30 mg/kg | Stereotypy, climbing, limbs retraction and catalepsy noted | Nitric oxide and Protein kinase C inhibitor | Blocked stereotypy, climbing, impaired retraction time of limbs, increased catalepsy | Moore and Martin (1957); Pereira et al. (2011) | |
| Cannabinoids | Cannabidiol | Cannabis sativa L | Apomorphine induced stereotypy | Male Wistar rats | 15–480 mg/kg | Stereotypy and prolactin secretion were measured | Blockade of serotonin reuptake or increased GABAergic activity | Attenuated the stereotypy and increase in prolactin | Zuardi et al. (1991) |
| Carotenoids | Crocin | Crocus sativus L | MK-801 induced Rotarod test, open field test | Neonatal Sprague Dawley rats | 25,50 mg/kg | Balance, motor coordination and locomotion | regulations of SIRT1 and downstream BDNF expression in the hippocampus | Improved motor coordination, balance and locomotion deficit | Sun, Zhao, Xie and Wan (2020) |
| Cholesterols | Hydroxytyrosol | Olea europaea | Prenatal restraint stress model | Sprague dawley rats | 10 and 50 mg/kg/day | Spontaneous alteration performance, Morris water maze test performed | Antioxidant, anti-inflammatory and brain protecting | Improves cognitive functions and Might be used for schizophrenia | Young et al. (2008); Calabrese et al. (2017) |
| hydroxytyrosol | Olea europaea | Determination of DNA strand breakage (comet assay) | IMR-32 cell line; histiocytic lymphoma U937 cell line | extent of H2O2-induced DNA damage | Decrease DNA damage | Neuroprotective efficacy, might be useful for schizophrenia | |||
| Flavonoids/ Polyphenols | Naringin | Citrus paradisi | Locomotor activity, PPI | Male Wistar rats | 100 mg/kg | Counts per 5 min, %PPI | targeting Wnt/β-catenin together with Akt/GSK-3β pathways | Increased locomotor activity, increased %PPI | George et al. (2020) |
| Nobiletin | Citrus depressa | MK-801 induced learning impairment | ddY mice | 2–50 mg/kg | Step-through Passive-Avoidance Task | Improves hypo-function of NMDA receptor-ERK signaling | Improvement of cognitive symptoms, might be beneficial for schizophrenia | Naka et al. (2007) | |
| Glycosides | Bacosides A and B | Bacopa monnieri | Novel object recognition test | Rat | Discrimination ratio was obtained | Increasing VGLUT2 density to normal level | Increase in Discrimination Ratio score | Wetchateng and Piyabhan (2014) | |
| sulforaphane | Brassica oleracea | Locomotor activity, Pre-pulse inhibition | Male mice | 30 mg/kg | Hyper-locomotion and PPI deficits were examined | An antioxidant protects against dopaminergic neurotoxicity by increasing Nrf2 expression | Attenuated PCP-induced hyper locomotion and PPI deficits | Shirai et al. (2012) | |
| Hypericin | Hypericum perfolatum | Inhibit D3/D4 | Might be used for schizophrenia | Butterweck et al. (2002) | |||||
| Emodin | Rheum rhabarbarum | Acoustic startle response, Methamphetamine induced hyper-locomotion | Sprague dawley rats | 50 mg/kg | Startle response and locomotor activity | Targets ErbB signaling alters dopamine and serotonin metabolism | Suppressed acoustic startle response and hyper-locomotion | Mizuno et al. (2008); Mizuno et al. (2010) | |
| cardenolides | Nerium oleander | NMRI male albino mice | Might be used for schizophrenia | Zhang (2004) | |||||
| Polygalasaponins | Polygala tenuifolia | a) Female TO mice | 25–500mg/kg | Animal’s movements, behavioral patterns and hyperactivity measured | Dopamine and serotonin antagonist activity | Reduction in climbing, stereotypy and hyperactivity | Chung et al. (2002) | ||
| b) Male lister hooded rats | |||||||||
| Diosmin | Scrophularia nodosa | Apomorphine induced stereotypy, catalepsy | Swiss male mice | 25, 50, 100 mg/kg | Stereotypy scoring, cataleptic behavior | Enhancement of GABAergic neurotransmission | Attenuated stereotypy, devoid of cat | Eneni et al. (2020) | |
| Picroside II, wedelolactone, 7-o-methylwogonin and isoformononetin | Picrorhiza scrophulariiflora | In vitro studies | Docking studies | Interaction with NMDA receptor | Good docking score | Bagchi and Somashekhar (2014) | |||
| Polyphenols | Resveratrol | Vitis vinifera | a) Apomorphine induced stereotypy | Swiss albino mice | 200 and 400 mg/kg | Stereotypy and grooming determined | D1 receptor antagonistic effect | Decreased climbing and swim induced grooming | Magaji et al. (2017) |
| b) Swim induced grooming | |||||||||
| Kaempferol | Lonchocarpus cyanescens | a) Amphetamine induced-stereotype | Wistar rats albino mice | 50–400 mg/kg (i.p) | Stereotypy was measured, Spontaneous motor activity was measured | Suppressed stereotyped behavior. Reduction in spontaneous motor activity | Sonibare et al. (2012) | ||
| b) Open field test | |||||||||
| Rutin | Morinda citrifolia | a) Apomorphine induced Cage climbing | Male ICR mice | 0.1 mg/kg | Climbing and stereotypy determined | Inhibition of D2 receptors | Reduction in climbing and stereotypy | Pandy and VijeePallam, (2017) | |
| b) Amphetamine induced stereotypy | |||||||||
| Curcumin | Curcuma lona | Assay based on tietze method | Mice | 10 and 50 µM | Oxidized and reduced GSH level determined | Antioxidant action | Increased GSL and GSH level in astrocytes and neurons | Lavoie et al. (2009) | |
| Genistein | Genista tinctoria L | Locomotor activity, forced swim test, active avoidance | rats | 50 mg/kg | Hyperactivity, immobility, avoidance | Anti-dopaminergic activity due to increased estrogen | Hyperactivity, enhanced immobility and decreased avoidance | Kalpana, Raju and Merugu (2014) | |
| Gallic acid | Camellia sinensis | ketamine-induced psychosis | Swiss albino mice | 50, 100 and 200 mg/kg | Stereotypy, locomotor activity | enhancement of NMDA receptor function | Stereotypy improved and locomotor activity increased | Yadav et al. (2018a) | |
| Morin | Allium cepa | Open field, apomorphine-induced stereotypy, ketamine-induced stereotypy | Male Swiss mice | 50 and 100 mg/kg | Locomotor activity, stereotypy | Might be enhancement of GABA activity | reduced spontaneous locomotor activity. Also, morin suppressed apomorphine-induced stereotypy and ketamine induced stereotypy | Ben-Azu et al. (2018) | |
| Polypropanoid | Alpha (α)—asarone | Acorus calamus | Apomorphine-induced stereotypy | Swiss albino mice | 30 and 50 mg/kg | Climbing time and climbing behavior determined | Anti-dopaminergic property | Decrease in the cage climbing time and climbing behavior | Pandy and Vijeepallam (2016) |
| Sesquiterpene | Tutin | Coriaria ruscifolia | Ca2+ transients& CREB analysis | Mouse spinal cord neurons | 1, 3, 5 and 8 mg/kg | Inhibit GABA A receptor | Might be used or schizophrenia | Fuentealba et al. (2007) | |
| Steroids | Anaferine, Beta-Sitosterol, Withaferin A, Withanolide A, Withanolide B and Withanolide D | Withania somnifera | Molecular docking | Inhibition of GluN2B-containing NMDARs | Might be useful for schizophrenia | Kumar and Patnaik (2016) | |||
| Sterol | Stigmasterol | Akebia quinata | Ketamine induced stereotypy | Swiss albino mice | 50mg/kg | Stereotypy and hyperlocomotion measured | Antioxidant action and increase in GABA and decrease in dopamine and acetylcholine | Decrease in stereotypy and locomotion | Yadav et al. (2018b) |
| Terpenoid | 1,8-cineole | Hyptis martiusii | haloperidol-induced catalepsy, and ketamine-induced hyperkinesia | female Swiss mice | 50 mg/kg | Catalepsy and hyperkinesis | Possible modulation of dopaminergic and glutamatergic systems | potentiated haloperidol-induced catalepsy and reduced ketamine-induced hyperkinesia | Monteiro et al. (2019) |
| Xanthone/ Polyphenol | α-mangostin | Garcinia mangostana L | Pre-pulse inhibition (PPI) test, open field test (OFT), forced swim test (FST) | Sprague Dawley dams and offsprings | 20 mg/kg | Sensorimotor gating, locomotor activity and depressive behavior determine | antioxidant | %PPI, locomotor hyperactivity and depressive like behavior were reversed | Lotter (2018) |
| Magniferin | Magnifera indica | Open field test | Swiss mice, Wistar rat | 50 mg/k g | Locomotor behavioral changes | Antioxidant, anti-inflammatory effect | Overcome grooming and stereotypy | Rao et al. (2012) | |
TABLE 2.
Antipsychotic potential of phytochemicals in clinical studies.
| Chemical Class | Phytochemical | Source | Assessments | Dose | Study Design | Mechanism | Result | References |
|---|---|---|---|---|---|---|---|---|
| Alkaloids | Apomorphine | Nymphea caerulea | Interview using NHSI (new haven schizophrenia index) | 1.5 to 6 mg | Randomized double blind placebo study | Potent effect on presynaptic dopamine receptors in addition to its postsynaptic stimulation | Decrease in psychotic symptoms in chronic patients | Smith et al. (1977); Fletcher, Frith et al. (1996) |
| Reserpine | Rauolfia serpentina | Seven-point behavioral rating scale | 1–8 mg/day | Controlled study of 8 months | Depressor of hypothalamus and facilitator of synaptic transmission | Marked improvement in behavior occurred | Cowden et al. (1955) | |
| Nicotine | Nicotiana tabacum | Profile of mood states (POMS) and continuous performance test (CPT) | 7 mg/day | N/A | Alpha 7 nicotinic receptor agonist | Attentional function is increased | (Levin, Conners, Silva, Hinton, Meck, March and Rose 1998) | |
| Amino acid and derivatives | Glycine | Glycine max | PANSS and Scale for assessing Negative Symptoms (SANS) | 0.14 to 0.8 g/kg/day | Open label trial | Potentiate NMDA transmission | Improvement in negative symptoms | Leiderman et al. (1996) |
| Sarcosine | Arachis hypogaea | Positive and Negative Syndrome Scale total score | 1–2 g/day for six weeks add on therapy | Double blind randomized clinical trial | Glycine 1 transport inhibitor, increases N-methyl-d-aspartate transmission | Reduced positive and negative syndromes in anti-psychotic naïve patients | Lane et al. (2008) | |
| Cannabinoids | Tetrahydrocannabinol | Cannabis ruderalis | Clinical global impression, brief psychiatric rating scale | 2.5 to 10 mg twice a day | Clinical case study | Affecting endocannabinoid receptors | Refractory schizophrenia symptoms improved | Schwarcz et al. (2009) |
| Cannabidiol | Cannabis sativa | Positive and negative symptoms, Global assessment of functioning scale | 1000 mg/day for six weeks | Double blind randomized clinical trial | As adjunct therapy | Reduced positive symptoms and improved cognitive performance | Philip McGuirePsych. et al. (2018) | |
| Flavonoids/ Polyphenols | Luteolin | Salvia rosmarinus | N/A | N/A | N/A | Modifier of NMDA function | Schizophrenic symptoms decreased | Hannan et al. (2021) |
| Apigenin | Perilla fruitiscenscens | N/A | N/A | N/A | Restore function of NMDA receptor by modulating hSKCa3 channel | Schizophrenic symptoms decreased | Hannan et al. (2021) | |
| Phenolic acid and derivatives | Sodium benzoate | Styrax benzoin | Assessment of positive and negative symptoms, and clinical global impression in treatment refractory schizophrenia | 0.5 g twice a day for 12 weeks | Double blind randomized clinical trial | Adjunctive use | Lack of efficacy in patients with early psychosis | Scott et al. (2020) |
| Sodium benzoate | Styrax benzoin | Clinical Global Impression (CGI), assessment of negative symptoms | 1 g/day for six weeks add-on therapy | Double blind randomized clinical trial | d-amino acid oxidase inhibitor | Improvement of clinical symptoms and recognition | Lane et al. (2013) | |
| Polyphenols | Resveratrol | Vitis vinifera | positive and negative symptoms scale and extrapyramidal symptoms scale | 200 mg/day for eight weeks add on therapy | Double blind randomized clinical trial | Managed negative symptoms and increased efficacy of risperidone | Samaei et al. (2020) | |
| Sesquiterpenoids | Caryophyllene | Cannabis sativa | N/A | 25 to about 100 mg add-on therapy | Clinical trials (application N° EP13763464.8A) | CB2-selective phyto-cannabinoid | Might improve schizophrenia symptoms | Anavi-goffer and Gertsch (2015) |
Phytochemicals showing efficacy against schizophrenia belong to different phytochemical classes such as alkaloids, tannins, glycosides, phenolic acids, flavonoids, terpenes, terpenoids and essential oils. Theses phytochemical classes are summarized in Figure 4.
FIGURE 4.
Phytochemical classes effective against Schizophrenia.
Alkaloids
Alkaloids are present in all plant parts, especially in flowers (Girdhar et al., 2015). These are mainly useful in treating several neurodegenerative disorders. These phytochemicals are effective against schizophrenia via affecting acetylcholine concentration, increasing GABA, antagonizing NMDA receptors, anti-oxidant action, anti-amyloid activity and, preventing neuro-inflammation (Dey and Mukherjee, 2018). Several alkaloids have now been investigated for treatment of schizophrenia. Arecoline, a pyridine alkaloid, has shown a capacity for muscarinic receptors as cholinergic agonist and can improve cognitive symptoms in schizophrenic patients. It also exerts antioxidant action and prevents demyelination of the cerebral white matter to attenuate memory impairment (Xu et al., 2019). Stepholidine, a protoberberine alkaloid, has a special feature of combined D1 agonist and D2 antagonist effect, and is useful in improving memory deficit in schizophrenia (Ellenbroek et al., 2006). Aporphine alkaloids, including apomorphine, reportedly cause amelioration of schizophrenic symptoms in patients by potently antagonizing dopamine at its receptor site (Smith et al., 1977; Fletcher et al., 1996). Isoquinoline alkaloids have also been investigated against schizophrenia. Galantamine increases the NMDA current in the rat cortical neurons. It also enhanced the effects of Ach by positive modulation of nAchR that decreased the attentional impairment and, increased short term memory and attention (Moriguchi et al., 2005; Schubert et al., 2006). A combination of galantamine and memantine was effective to enhance cognition in schizophrenic patients (Koola et al., 2014). Reticuline has also demonstrated antipsychotic activity through anti-dopaminergic actions (Morais et al., 1998).
Nicotine, a pyridine alkaloid, was effective in schizophrenic patients to improve attention deficit through action as an alpha nicotinic receptor agonist (Levin et al., 1998). Some indole alkaloids, such as corymine, potentiated the NMDA current and showed efficacy against schizophrenia, while alstonine showed anti-schizophrenic effect by modulating the dopamine uptake and NMDA receptor. These alkaloids also reduced behavioral problems of schizophrenic patients (Costa-Campos et al., 1998). The ameliorating effect of geissoschinzine methyl ether against schizophrenia also occured through modulation of dopamine receptors as well as partial antagonistic effect against NMDA receptors.
Glycosides
In glycosides, a sugar moity is attached to non sugar molecule through glycosidic linkage. Glycosides are present in plants as secondary metabolites and are their “offense and defence” components (Shadkami and Jones, 2012; Wang et al., 2016). A study on use of bacoside A and B isolated from Bacopa monnieri has shown an improvement in cognitive defects in schizophrenic model by increasing vesicular glutamate transporter 2 in cingulate gyrus region (Wetchateng and Piyabhan, 2014). Isothiocyanates, such as sulforaphane, exhibited antipsychotic activity through activation of Nrf2 pathway, detoxification of phase 2 enzymes and, antioxidant action by enhancing electrophilic response elements (Shirai et al., 2012). Hypericin is nephthodianthrone and exhibits antioxidant properties. It inhibits D3/D4 receptors and is a candidate drug for the management of schizophrenia (Butterweck et al., 2002; Saranya et al., 2019). Emodin Targets ErbB signaling and alters dopamine and serotonin metabolism to exhibit ameliorating effects against schizophrenia symptoms (Mizuno et al., 2008; Mizuno et al., 2010). Polygalasaponin, a saponin glycoside, has anti-schizophrenic activity due to its dopamine and serotonin antagonist activities (Chung et al., 2002). It is also found that iridoid glycosides and cardenolides were effective in treating psychotic symptoms that required further investigation. Beta sitosterol also inhibited the GluN2B-containing NMDA receptors as demonstrated by docking studies. Picroside II also showed in vitro potential of antipsychotic activity (Bagchi and Somashekhar 2014; Kumar and Patnaik 2016).
Polyphenols
Polyphenols are plant secondary metabolites that have demonstrated neuroprotective and anti-schizophrenic activity. Several studies have indicated that these are useful against neurologic and psychotic disorders (Wu et al., 2009). Kaempferol has demonstrated neuroprotective effects against schizophrenia due to its anti-infammatory, antioxidant and anti-apoptotic effects (El-Kott et al., 2020). Baicalin is reported to ameliorate negative symptoms and cognitive dyfunction in psychosis. This psychotic effect may be attributed to its anti-prolyl-oligopeptidase, anti-inflammatory and antioxidant actions (Miao et al., 2020; Tarragó et al., 2008).
Quercitin, a bioflavonoid, has potential to improve symptoms of schizophrenia due to its free radical scavenging activity (Mert et al., 2019). Myricitrin is an inhibitor of nitric oxide and Protein Kinase C. Its anti-schizophrenic effect is attributed to antioxidant action (Amanzadeh et al., 2019). Scopoletin and rutin are useful for alleviation of positive symptoms of schizophrenia due to the inhibitory interaction with D2 receptor (Pandy and VijeePallam 2017). Xanthones, such as α-mangostin and magniferin, have also been studied for anti-schizophrenic activity. α-mangostin is an antioxidant and has anti-inflammatory properties. It also inhibited phosphodiesterases and 5HT2A receptors, and was shown to be effective in rodent models of schizophrenia. Magniferin improved cognition by its antioxidant mechanism, preserving mitochondrial functions, anti-inflammatory activity and, reduction of dopamine (Lotter 2018; Lum et al., 2020), Hydroxytyrosol is a cholesterol which showed neuroprotection for multiple neurological and psychological diseases. It decreased oxidative stress by activation of Nrf2 pathway and enhanced the mitochondrial functions. It restored the learning ability and memory in prenatal stressed animal and human off springs, when administered during pregnancy, showing its vitality for preserving neurogenesis and cognitive functions in off springs (Chen and Wei, 2021).
Curcumin is known for its several beneficial effects on the nervous system attributed to its ability to raise the level of reduced glutathione (Lavoie et al., 2009; Miodownik et al., 2019). Curcumin exerted add-on effects of regular anti-psychotic drugs in chronic schizophrenic patients. Such treatments have shown improvement in negative symptoms of schizophrenia. Curcumin regulates the gene expressions involved in inflammation and modulates NMDA activity, which are associated with symptoms of schizophrenia. Morin also exhibited anti-psychotic like effects, without exerting extrapyramidal side effect, by enhancing GABA activity (Ben-Azu et al., 2018). Gallic acid also plays protective role against psychotic like behaviour through enhancement of NMDA receptor (Yadav et al., 2018b). Nobiletin, a flavonoid, improves hypo-functioning of NMDA receptors by acting on extracellular signal-regulated kinases (ERK) signaling and ameliorates cognitive symptoms of schizophrenia (Nakajima et al., 2007).
Diosmin, a flavone, enhances GABA transmission to treat symptoms of schizophrenia (Eneni et al., 2020). Naringin is a flavonoid that acts on Wnt/β catenin and Akt/GSK-3β pathways to exert anti-schizophrenic effect (George et al., 2020). It is also found that genistein, an isoflvone, had exhibited therapeutic effects against different symptoms of schizophrenia by acting on estrogen receptor and affecting dopamine pathway (Kalpana et al., 2014). Furthermore, both apigenin and luteolin have demonstrated considerable potential to improve the symptoms of schizophrenia (Zhao and So, 2018).
Terpenes and Terpenoids
Tutin is a sesquiterpene that inhibited glycinergic activity and blocked GABA-A receptors. Moreover, 1,8 cineole is amonoterpenoid that acts on dopamine and glutamate pathways. Caryophylline is a sesquiterpene isolated from essential oils that acts as phytocannabinoid and is being effectively investigated in clinical research of schizophrenia (Kucerova et al., 2014).
Cannabinoids
Cannabinoids belong to terpenoid class and are helful in the treatment of neurodegenerative diseases. Results of a meta-analysis have concluded that the patients of schizophrenia have increased amount of endocannabinoid anandamide in their blood, cerebrospinal fluids and cannabinoid 1 receptors (CB1) present on immune cells (Davies and Bhattacharyya, 2019). Three randomized trials have reported the reduction in disease positive symptom and improved cognition by using cannabidiol (White, 2019).
Cannabidiol is a cannabinoid that blocks the serotonin uptake and increases GABAergic activity to exert anti-schizophrenic effect. This effect was also evident in schizophrenic patients who used cannabis (Morgan and Curran, 2008). Moreover, cannabidiol had also shown a clear advantage in clinical studies over other antipsychotics as it did not exhibit any movement like problems associated with the use of other antipsychotics (García-Gutiérrez et al., 2020).
Another cannabinoid, tetrahydrocannabinol, also improved the symptoms of schizophrenia due to its effect on the endocannabinoid receptors (Schwarcz et al., 2009). On the other hand in some reports suggested that the 9-tetrahydrocannabinol administration had increased the symptoms of psychosis. But researchers reported that tetrahydrocanabinol might have dose dependent effect. As at low doses, it improved the symptoms of psychosis while it inflicted disruption to brain circuits causing worsening of psychotic symptoms at large doses.
Phytosterols
Phytosterols and oxyphytosterols (oxidation products of phytosterol) are naturally synthesized by several plants. Exposure of these natural agents is growing due to increased intake of plant food enriched with phytosterol and oxyphytosterol (Jie et al., 2020).
Stigmasterol is a phytosterol present in vegetables, legumes, nuts, herbs and seeds. It is shown to inhibit ketamine induced biochemical, histopathological and behavioral alterations in mice to exhibit antipsychotic potential. It manages psychosis by ameliorating inflammation and oxidative stress, and by altering dopaminergic, acetylcholinergic and GABAergic neurotransmission (Yadav et al., 2018a).
Carotenoids
Saffron (Crocus sativus L.) and its active constituents such as crocins and safranal have shown high potential for treatment of various central nervous system disorders such as anxiety, depression and memory defficit (Pitsikas, 2015). Crocin is a carotenoid that showed effectiveness as antipsychotic drug by regulating Brain-derived neurotrophic factor (BDNF) in hippocampus (Sun et al., 2020). There are increasing preclinical evidences that crocins reversed the ketamine induced memory deficit, hypermotility and social isolation at 15–50 mg/kg dosage in rats (Georgiadou et al., 2014). It is also found that crocins had inhibited the apomorphine induced deficit in novel object recognition task associated with dopaminergic dysfunction in rats (Pitsikas and Tarantilis, 2017). Based on better safety profile and the preclinical evidences of efficacy against psychosis, there is strong need for controlled clinical studies of these agents agianst schizophrenia (Pitsikas, 2021).
Other Phytochemicals
Alpha asarone belongs to polypropanoid class of essential oils and exerts anti-schizophrenic activity due to antagonism of dopamine D2 and/or D1 receptors (Pandy and Vijeepallam, 2016). Glycine is an amino acid which improved the negative symptoms of schizophrenia in an open trial on human. This effect is attributed to its potentiating effect on NMDA receptors (Leiderman et al., 1996). It is found effective against treatment resistant schizophrenia, negative symptoms and cognitive problems when given as adjuvant to other medical therapies (Heresco-Levy et al., 2004). Leucine is also an amino acid that improved schizophrenic symptoms by acting on dopaminergic receptors (Suresh and Raju, 2013).
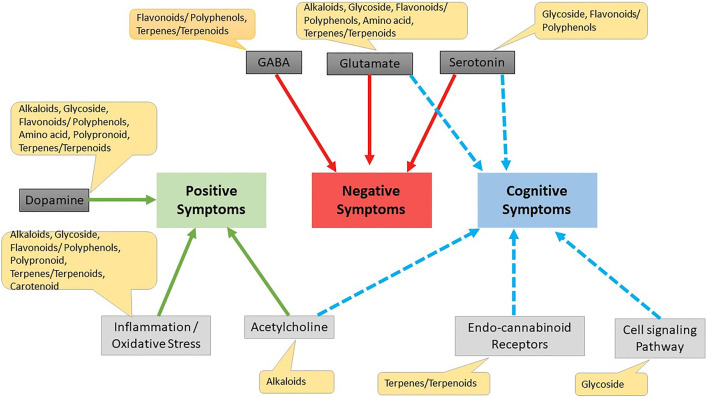
Kava is a known herb for several brain disorders and its activity was reported due to its constituent kavapyrone. Kavapyrone is a potential candidate for treating schizophrenia as it increases GABA-A receptor density and blocks glutamate release (Kumari et al., 2011). Withaferin A, Withanolide A, Withanolide B and Withanolide D are steroidal lactones which have shown positive effects on NMDA receptors through docking studies and can be useful in schizophrenia after further evaluation (Kumar and Patnaik, 2016). The effect of various phytochemicals on positive, negative and cognitive symptoms is summarized in Figure 5.
FIGURE 5.
Effect of different phytochemicals on different drug targets for schizophrenia. Cell signaling pathway Erbβ cell signaling pathway, wnt/β-catenin Akt/GsK3β pathway.
Conclusion
Schizophrenia is a multifactorial disease of complex etiology and pathogenesis that necessitates multiple targeted drug candidates for the improvement of positive and negative symptoms, and cognitive impairment. Natural drugs such as phytochemicals have demonstrated the therapeutic potential in the management of schizophrenia through modulation of oxidative stress, neuro-inflammation, immune system alterations and downstream signaling pathways, which are the hallmarks of disease. Alkaloids, glycosides, terpenes, terpenoids, polyphenols, flavonoids, poly-propanoids, steroidal lactones and amino acids are among the major classes of phytochemicals that have shown anti-schizophrenic activity in preclinical investigations. Apomorphine, luteolin, apigenin, caryophyllene, cannabinoids, baicalin and reserpine are among the phytochemicals that have demonstrated the anti-schizophrenic potential in human studies.
Therefore, it is reasonable to propose that the phytochemicals might be promising candidates for developing new agents with protective and therapeutic benefits against schizophrenia. Moreover, additional preclinical and clinical research is required for establishing pharmacokinetic and toxicity studies of phytochemicals, and their best possible combinations to minimize undesirable adverse effects. Unfortunately, in spite of abundant neuroprotective potential of the phytochemicals against schizophrenia, long-term studies of these agents against schizophrenia have not been carried out to address the effects of these agents to retard the progression of disease. Furthermore, the exact doses and combinations of phytochemicals should be investigated in clinical research to demonstrate the efficacy and safety in schizophrenic patients.
Author Contributions
QA, and AS collected the data. AS, QA, and MA all contributed in article writing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
5HT, 5 Hydroxy Tryptamine; Akt, Protein Kinase C; BDNF, Brain-derived neurotrophic factor; COMT, Catechol-O-methyl transferase; DAOAG72, d-amino acid oxidase; DISC1, Disrupted in schizophrenia; DNA, Deoxyribonucleic acid; DTNBP1, Dysbindin; EPS, Extra pyramidal symptoms; ERK, Extracellular signal-regulated kinases; GABA, Gamma amino butyric acid; GSK 3, Glycogen synthase kinase-3; IL, Interleukin; NRG1, Neuregulin 1; nAChR-Nicotinic acetylcholine receptors; NMDA, N-methyl-d-aspartate; PDE, Phosphodiesterase; PRODH, Proline dehydrogenase; RNS, Reactive nitrogen species; ROS, Reactive oxygen species; RGSR, Regulator of G protein signaling 4.
References
- Abi-Dargham A. (2004). Do We Still Believe in the Dopamine Hypothesis? New Data Bring New Evidence. Cambridge, UK: Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- Agren H., Reibring L., Hartvig P., Tedroff J., Bjurling P., Hörnfeldt K., et al. (1991). Low Brain Uptake of L-[11C]5-hydroxytryptophan in Major Depression: a Positron Emission Tomography Study on Patients and Healthy Volunteers. Acta Psychiatr. Scand. 83, 449–455. 10.1111/j.1600-0447.1991.tb05574.x [DOI] [PubMed] [Google Scholar]
- Ahmed S., Roth R. M., Stanciu C. N., Brunette M. F. (2021). The Impact of THC and CBD in Schizophrenia: a Systematic Review. Front. Psychiatry, 1225. 10.3389/fpsyt.2021.694394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanzadeh E., Esmaeili A., Rahgozar S., Nourbakhshnia M. (2019). Application of Quercetin in Neurological Disorders: from Nutrition to Nanomedicine. Rev. Neurosci. 30, 555–572. 10.1515/revneuro-2018-0080 [DOI] [PubMed] [Google Scholar]
- An der Heiden W., Häfner H. (2000). The Epidemiology of Onset and Course of Schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 250, 292–303. 10.1007/s004060070004 [DOI] [PubMed] [Google Scholar]
- Anavi-goffer S., Gertsch J. (2015). Treatment of Schizophrenia Using Beta-Caryophyllene and CB2 Receptor Agonists. US Patent App 14/385, 739. [Google Scholar]
- Antonova E., Sharma T., Morris R., Kumari V. (2004). The Relationship between Brain Structure and Neurocognition in Schizophrenia: a Selective Review. Schizophr Res. 70, 117–145. 10.1016/j.schres.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Arnold S. E., Ruscheinsky D. D., Han L. Y. (1997). Further Evidence of Abnormal Cytoarchitecture of the Entorhinal Cortex in Schizophrenia Using Spatial point Pattern Analyses. Biol. Psychiatry 42, 639–647. 10.1016/s0006-3223(97)00142-x [DOI] [PubMed] [Google Scholar]
- Arnold S. E., Talbot K., Hahn C. G. (2005). Neurodevelopment, Neuroplasticity, and New Genes for Schizophrenia. Prog. Brain Res. 147, 319–345. 10.1016/S0079-6123(04)47023-X [DOI] [PubMed] [Google Scholar]
- Bagchi P., Somashekhar R. (2014). “Identification of Novel Drug Leads for NMDA Receptor Implicated in Schizophrenia from Indian Traditional Herbs,” in Proceedings of the International conference on intelligent systems, data mining and information technology (ICIDIT’2014) (ISBN; ). [Google Scholar]
- Bahta M., Ogbaghebriel A., Russom M., Tesfamariam E. H., Berhe T. (2021). Impact of Adverse Reactions to First-Generation Antipsychotics on Treatment Adherence in Outpatients with Schizophrenia: a Cross-Sectional Study. Ann. Gen. Psychiatry 20, 27–7. 10.1186/s12991-021-00348-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S., Weiner I. (2010). Dissociating Scopolamine-Induced Disrupted and Persistent Latent Inhibition: Stage-dependent Effects of glycine and Physostigmine. Psychopharmacology (Berl) 209, 175–184. 10.1007/s00213-010-1785-z [DOI] [PubMed] [Google Scholar]
- Ben-Azu B., Aderibigbe A. O., Omogbiya I. A., Ajayi A. M., Iwalewa E. O. (2018). Morin Pretreatment Attenuates Schizophrenia-like Behaviors in Experimental Animal Models. Drug Res. (Stuttg) 68, 159–167. 10.1055/s-0043-119127 [DOI] [PubMed] [Google Scholar]
- Berman J. A., Talmage D. A., Role L. W. (2007). Cholinergic Circuits and Signaling in the Pathophysiology of Schizophrenia. Int. Rev. Neurobiol. 78, 193–223. 10.1016/S0074-7742(06)78007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskovic M., Vovk T., Kores Plesnicar B., Grabnar I. (2011). Oxidative Stress in Schizophrenia. Curr. neuropharmacology 9, 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. S., Derkits E. J. (2010). Prenatal Infection and Schizophrenia: a Review of Epidemiologic and Translational Studies. Am. J. Psychiatry 167, 261–280. 10.1176/appi.ajp.2009.09030361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. S. (2012). Epidemiologic Studies of Exposure to Prenatal Infection and Risk of Schizophrenia and Autism. Dev. Neurobiol. 72, 1272–1276. 10.1002/dneu.22024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. S., Susser E. S. (2002). In Utero infection and Adult Schizophrenia. Ment. Retard. Dev. Disabil. Res. Rev. 8, 51–57. 10.1002/mrdd.10004 [DOI] [PubMed] [Google Scholar]
- Butterweck V., Nahrstedt A., Evans J., Hufeisen S., Rauser L., Savage J., et al. (2002). In Vitro receptor Screening of Pure Constituents of St. John's Wort Reveals Novel Interactions with a Number of GPCRs. Psychopharmacology (Berl) 162, 193–202. 10.1007/s00213-002-1073-7 [DOI] [PubMed] [Google Scholar]
- Calabrese E. J. (2008). Alzheimer's Disease Drugs: an Application of the Hormetic Dose-Response Model. Crit. Rev. Toxicol. 38, 419–451. 10.1080/10408440802003991 [DOI] [PubMed] [Google Scholar]
- Calabrese V., Giordano J., Crupi R., Di Paola R., Ruggieri M., Bianchini R., et al. (2017). Hormesis, Cellular Stress Response and Neuroinflammation in Schizophrenia: Early Onset versus Late Onset State. J. Neurosci. Res. 95, 1182–1193. 10.1002/jnr.23967 [DOI] [PubMed] [Google Scholar]
- Cannon T. D. (2005). The Inheritance of Intermediate Phenotypes for Schizophrenia. Curr. Opin. Psychiatry 18, 135–140. 10.1097/00001504-200503000-00005 [DOI] [PubMed] [Google Scholar]
- Caspi A., Moffitt T. E. (2006). Gene-environment Interactions in Psychiatry: Joining Forces with Neuroscience. Nat. Rev. Neurosci. 7, 583–590. 10.1038/nrn1925 [DOI] [PubMed] [Google Scholar]
- Chen C., Ai Q.-d., Wei Y.-h. (2021). Potential Role of Hydroxytyrosol in Neuroprotection. J. Funct. Foods 82, 104506. 10.1016/j.jff.2021.104506 [DOI] [Google Scholar]
- Choudhury B., Saytode P., Shah V. (2014). Neurodegenrative Disorders: Past, Present and Future. [Google Scholar]
- Chung I. W., Moore N. A., Oh W. K., O'Neill M. F., Ahn J. S., Park J. B., et al. (2002). Behavioural Pharmacology of Polygalasaponins Indicates Potential Antipsychotic Efficacy. Pharmacol. Biochem. Behav. 71, 191–195. 10.1016/s0091-3057(01)00648-7 [DOI] [PubMed] [Google Scholar]
- Costa-Campos L., Lara D. R., Nunes D. S., Elisabetsky E. (1998). Antipsychotic-like Profile of Alstonine. Pharmacol. Biochem. Behav. 60, 133–141. 10.1016/s0091-3057(97)00594-7 [DOI] [PubMed] [Google Scholar]
- Cowden R. C., Zax M., Sproles J. A. (1955). Reserpine Alone and as an Adjunct to Psychotherapy in the Treatment of Schizophrenia. AMA Arch. Neurol. Psychiatry 74, 518–522. 10.1001/archneurpsyc.1955.02330170052009 [DOI] [PubMed] [Google Scholar]
- Coyle J. T. (2006). Glutamate and Schizophrenia: beyond the Dopamine Hypothesis. Cell Mol Neurobiol 26, 365–384. 10.1007/s10571-006-9062-8 [DOI] [PubMed] [Google Scholar]
- Craddock N., O'Donovan M. C., Owen M. J. (2005). The Genetics of Schizophrenia and Bipolar Disorder: Dissecting Psychosis. J. Med. Genet. 42, 193–204. 10.1136/jmg.2005.030718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. (2004). Cytokine-induced Sickness Behaviour: a Neuroimmune Response to Activation of Innate Immunity. Eur. J. Pharmacol. 500, 399–411. 10.1016/j.ejphar.2004.07.040 [DOI] [PubMed] [Google Scholar]
- Datta S., Ramamurthy P. C., Anand U., Singh S., Singh A., Dhanjal D. S., et al. (2021). Wonder or Evil?: Multifaceted Health Hazards and Health Benefits of Cannabis Sativa and its Phytochemicals. Saudi J. Biol. Sci. 28, 7290–7313. 10.1016/j.sjbs.2021.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C., Bhattacharyya S. (2019). Cannabidiol as a Potential Treatment for Psychosis. Ther. Adv. Psychopharmacol. 9, 2045125319881916. 10.1177/2045125319881916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. L., Kahn R. S., Ko G., Davidson M. (1991). Dopamine in Schizophrenia: a Review and Reconceptualization. Am. J. Psychiatry. [DOI] [PubMed] [Google Scholar]
- Dey A., Mukherjee A. (2018). “Plant-Derived Alkaloids,” in Discovery and Development of Neuroprotective Agents from Natural Products (Elsevier; ), 237–320. 10.1016/b978-0-12-809593-5.00006-9 [DOI] [Google Scholar]
- Dienel S. J., Lewis D. A. (2019). Alterations in Cortical Interneurons and Cognitive Function in Schizophrenia. Neurobiol. Dis. 131, 104208. 10.1016/j.nbd.2018.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kott A. F., Abd-Lateif A. M., Khalifa H. S., Morsy K., Ibrahim E. H., Bin-Jumah M., et al. (2020). Kaempferol Protects against Cadmium Chloride-Induced Hippocampal Damage and Memory Deficits by Activation of Silent Information Regulator 1 and Inhibition of Poly (ADP-Ribose) Polymerase-1. Sci. Total Environ. 728, 138832. 10.1016/j.scitotenv.2020.138832 [DOI] [PubMed] [Google Scholar]
- Ellenbroek B. A., Zhang Xx., Jin Gz. (2006). Effects of (-)stepholidine in Animal Models for Schizophrenia. Acta Pharmacol. Sin 27, 1111–1118. 10.1111/j.1745-7254.2006.00365.x [DOI] [PubMed] [Google Scholar]
- Eneni A-E. O., Ben-Azu B., Ajayi A. M., Aderibigbe A. O. (2020). Diosmin Attenuates Schizophrenia-like Behavior, Oxidative Stress, and Acetylcholinesterase Activity in Mice. Drug Metab. Personalized Ther. 10.1515/dmdi-2020-0119 [DOI] [PubMed] [Google Scholar]
- Eyles D. W. (2021). How Do Established Developmental Risk-Factors for Schizophrenia Change the Way the Brain Develops? Transl Psychiatry 11, 158–215. 10.1038/s41398-021-01273-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson K. A., Kusnecov A. W., Silverstein S. M. (2014). Inflammation and the Two-Hit Hypothesis of Schizophrenia. Neurosci. Biobehav Rev. 38, 72–93. 10.1016/j.neubiorev.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendri C., Mechri A., Khiari G., Othman A., Kerkeni A., Gaha L. (2006). Implication du stress oxydant dans la physiopathologie de la schizophrénie : revue de la literature. L'Encéphale 32, 244–252. 10.1016/s0013-7006(06)76151-6 [DOI] [PubMed] [Google Scholar]
- Fletcher P. C., Frith C. D., Grasby P. M., Friston K. J., Dolan R. J. (1996). Local and Distributed Effects of Apomorphine on Fronto-Temporal Function in Acute Unmedicated Schizophrenia. J. Neurosci. 16, 7055–7062. 10.1523/jneurosci.16-21-07055.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba J., Guzmán L., Manríquez-Navarro P., Pérez C., Silva M., Becerra J., et al. (2007). Inhibitory Effects of Tutin on glycine Receptors in Spinal Neurons. Eur. J. Pharmacol. 559, 61–64. 10.1016/j.ejphar.2006.12.018 [DOI] [PubMed] [Google Scholar]
- García-Gutiérrez M. S., Navarrete F., Gasparyan A., Austrich-Olivares A., Sala F., Manzanares J. (2020). Cannabidiol: a Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules 10 (11), 1575. 10.3390/biom10111575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellman R., Aghajanian G. (1991). IPSPs in Pyramidal Cells in Piriform Cortex Evoked by Monoamine Excitation of Interneurons Demonstrate a Convergence of Inputs. Proceedings of the Soc Neurosci Abstr. [Google Scholar]
- George M. Y., Menze E. T., Esmat A., Tadros M. G., El-Demerdash E. (2020). Potential Therapeutic Antipsychotic Effects of Naringin against Ketamine-Induced Deficits in Rats: Involvement of Akt/GSK-3β and Wnt/β-Catenin Signaling Pathways. Life Sci. 249, 117535. 10.1016/j.lfs.2020.117535 [DOI] [PubMed] [Google Scholar]
- Georgiadou G., Grivas V., Tarantilis P. A., Pitsikas N. (2014). Crocins, the Active Constituents of Crocus Sativus L., Counteracted Ketamine-Induced Behavioural Deficits in Rats. Psychopharmacology (Berl) 231, 717–726. 10.1007/s00213-013-3293-4 [DOI] [PubMed] [Google Scholar]
- Girdhar S., Girdhar A., Girdhar A., Verma S. K., Lather V., Pandita D. (2015). Plant Derived Alkaloids in Major Neurodegenerative Diseases: from Animal Models to Clinical Trials. J. Ayu. Her. Med. 1, 91–100. 10.31254/jahm.2015.1307 [DOI] [Google Scholar]
- Gogos A., Sbisa A. M., Sun J., Gibbons A., Udawela M., Dean B. (20152015). A Role for Estrogen in Schizophrenia: Clinical and Preclinical Findings. Int. J. Endocrinol. 10.1155/2015/615356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A. A., Gomes F. V. (2019). The Circuitry of Dopamine System Regulation and its Disruption in Schizophrenia: Insights into Treatment and Prevention. Schizophr Bull. 45, 148–157. 10.1093/schbul/sbx199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. (2012). Free Radicals and Antioxidants: Updating a Personal View. Nutr. Rev. 70, 257–265. 10.1111/j.1753-4887.2012.00476.x [DOI] [PubMed] [Google Scholar]
- Hannan M. A., Rahman M. A., Sohag A. A. M., Uddin M. J., Dash R., Sikder M. H., et al. (2021). Black Cumin (Nigella Sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 13, 1784. 10.3390/nu13061784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Weinberger D. R. (2005). Schizophrenia Genes, Gene Expression, and Neuropathology: on the Matter of Their Convergence. Mol. Psychiatry 10, 40–45. 10.1038/sj.mp.4001558 [DOI] [PubMed] [Google Scholar]
- Hayes P. E., Schulz S. C. (1983). The Use of Beta-Adrenergic Blocking Agents in Anxiety Disorders and Schizophrenia. Pharmacotherapy 3, 101–117. 10.1002/j.1875-9114.1983.tb03231.x [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U., Ermilov M., Lichtenberg P., Bar G., Javitt D. C. (2004). High-dose glycine Added to Olanzapine and Risperidone for the Treatment of Schizophrenia. Biol. Psychiatry 55, 165–171. 10.1016/s0006-3223(03)00707-8 [DOI] [PubMed] [Google Scholar]
- Hohnadel E., Bouchard K., Terry A. V., Jr (2007). Galantamine and Donepezil Attenuate Pharmacologically Induced Deficits in Prepulse Inhibition in Rats. Neuropharmacology 52, 542–551. 10.1016/j.neuropharm.2006.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R., Crow T. J., Passingham D., Mackay C. E. (2005). Regional Deficits in Brain Volume in Schizophrenia: a Meta-Analysis of Voxel-Based Morphometry Studies. Am. J. Psychiatry 162, 2233–2245. 10.1176/appi.ajp.162.12.2233 [DOI] [PubMed] [Google Scholar]
- Hoseth E. Z., Krull F., Dieset I., Mørch R. H., Hope S., Gardsjord E. S., et al. (2018). Exploring the Wnt Signaling Pathway in Schizophrenia and Bipolar Disorder. Transl Psychiatry 8, 55–10. 10.1038/s41398-018-0102-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O. D., Murray R. M. (2014). Schizophrenia: an Integrated Sociodevelopmental-Cognitive Model. Lancet 383, 1677–1687. 10.1016/S0140-6736(13)62036-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie F., Yang X., Wu L., Wang M., Lu B. (2020). Linking Phytosterols and Oxyphytosterols from Food to Brain Health: Origins, Effects, and Underlying Mechanisms. Crit. Rev. Food Sci. Nutr., 1–18. [DOI] [PubMed] [Google Scholar]
- Kalpana S., Raju A. B., Merugu M. S. (2014). Genestein, a Phytoestrogens for the Treatment of Schizophrenia. [Google Scholar]
- Karam C. S., Ballon J. S., Bivens N. M., Freyberg Z., Girgis R. R., Lizardi-Ortiz J. E., et al. (2010). Signaling Pathways in Schizophrenia: Emerging Targets and Therapeutic Strategies. Trends Pharmacol. Sci. 31, 381–390. 10.1016/j.tips.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G. M., Cousins L., Deakin J., Lennox B. R., Yolken R., Jones P. B. (2015). Inflammation and Immunity in Schizophrenia: Implications for Pathophysiology and Treatment. Lancet Psychiatry 2, 258–270. 10.1016/S2215-0366(14)00122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinou M., Irvine E. E., Bonsall D. R., Natesan S., Wells L. A., Smith M., et al. (2021). Reproducing the Dopamine Pathophysiology of Schizophrenia and Approaches to Ameliorate it: a Translational Imaging Study with Ketamine. Mol. Psychiatry 26, 2562–2576. 10.1038/s41380-020-0740-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koola M. M., Buchanan R. W., Pillai A., Aitchison K. J., Weinberger D. R., Aaronson S. T., et al. (2014). Potential Role of the Combination of Galantamine and Memantine to Improve Cognition in Schizophrenia. Schizophr Res. 157, 84–89. 10.1016/j.schres.2014.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucerova J., Tabiova K., Drago F., Micale V. (2014). Therapeutic Potential of Cannabinoids in Schizophrenia. Recent Pat CNS Drug Discov. 9, 13–25. 10.2174/1574889809666140307115532 [DOI] [PubMed] [Google Scholar]
- Kumar G., Patnaik R. (2016). Exploring Neuroprotective Potential of Withania Somnifera Phytochemicals by Inhibition of GluN2B-Containing NMDA Receptors: an In Silico Study. Med. Hypotheses 92, 35–43. 10.1016/j.mehy.2016.04.034 [DOI] [PubMed] [Google Scholar]
- Kumari R., Kaundal M., Ahmad Z., Ashwalayan V. (2011). Herbal and Dietary Supplements in Treatment of Schizophrenia: An Approach to Improve Therapeutics. Int. J. Pharm. Sci. Rev. Res. 10, 217–224. [Google Scholar]
- Kyle U. G., Pichard C. (2006). The Dutch Famine of 1944-1945: a Pathophysiological Model of Long-Term Consequences of Wasting Disease. Curr. Opin. Clin. Nutr. Metab. Care 9, 388–394. 10.1097/01.mco.0000232898.74415.42 [DOI] [PubMed] [Google Scholar]
- Lane H. Y., Lin C. H., Green M. F., Hellemann G., Huang C. C., Chen P. W., et al. (2013). Add-on Treatment of Benzoate for Schizophrenia: a Randomized, Double-Blind, Placebo-Controlled Trial of D-Amino Acid Oxidase Inhibitor. JAMA psychiatry 70, 1267–1275. 10.1001/jamapsychiatry.2013.2159 [DOI] [PubMed] [Google Scholar]
- Lane H. Y., Liu Y. C., Huang C. L., Chang Y. C., Liau C. H., Perng C. H., et al. (2008). Sarcosine (N-Methylglycine) Treatment for Acute Schizophrenia: a Randomized, Double-Blind Study. Biol. Psychiatry 63, 9–12. 10.1016/j.biopsych.2007.04.038 [DOI] [PubMed] [Google Scholar]
- Lavoie S., Chen Y., Dalton T. P., Gysin R., Cuénod M., Steullet P., et al. (2009). Curcumin, Quercetin, and tBHQ Modulate Glutathione Levels in Astrocytes and Neurons: Importance of the Glutamate Cysteine Ligase Modifier Subunit. J. Neurochem. 108, 1410–1422. 10.1111/j.1471-4159.2009.05908.x [DOI] [PubMed] [Google Scholar]
- Leewanich P., Tohda M., Takayama H., Sophasan S., Watanabe H., Matsumoto K. (2005). Corymine Potentiates NMDA-Induced Currents in Xenopus Oocytes Expressing NR1a/NR2B Glutamate Receptors. J. Pharmacol. Sci. 98, 58–65. 10.1254/jphs.fp0050023 [DOI] [PubMed] [Google Scholar]
- Leiderman E., Zylberman I., Zukin S. R., Cooper T. B., Javitt D. C. (1996). Preliminary Investigation of High-Dose Oral glycine on Serum Levels and Negative Symptoms in Schizophrenia: an Open-Label Trial. Biol. Psychiatry 39, 213–215. 10.1016/0006-3223(95)00585-4 [DOI] [PubMed] [Google Scholar]
- Levin E. D., Conners C. K., Silva D., Hinton S. C., Meck W. H., March J., et al. (1998). Transdermal Nicotine Effects on Attention. Psychopharmacology (Berl) 140, 135–141. 10.1007/s002130050750 [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Sweet R. A. (2009). Schizophrenia from a Neural Circuitry Perspective: Advancing toward Rational Pharmacological Therapies. J. Clin. Invest. 119, 706–716. 10.1172/JCI37335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Lee M. Y., Chan M. H., Chen Y. C., Chen H. H. (2016). Betaine Enhances Antidepressant-like, but Blocks Psychotomimetic Effects of Ketamine in Mice. Psychopharmacology (Berl) 233, 3223–3235. 10.1007/s00213-016-4359-x [DOI] [PubMed] [Google Scholar]
- Lodge D. (1989). Modulation of N-Methylaspartate Receptor Channel Complexes. Drugs Today 25, 395–411. [Google Scholar]
- Lotter J. (2018). Studies on Garcinia Mangostana Linn as a Therapeutic Intervention in an Immune-Inflammatory Model of Schizophrenia. North-West University. [Google Scholar]
- Lum P. T., Sekar M., Gan S. H., Pandy V., Bonam S. R. (2020). Protective Effect of Mangiferin on Memory Impairment: a Systematic Review. Saudi J. Biol. Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie D. I. (2018). An Integrative Approach to Neuroinflammation in Psychiatric Disorders and Neuropathic Pain. J. Exp. Neurosci. 12, 1179069518793639. 10.1177/1179069518793639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaji M. G., Iniaghe L. O., Abolarin M., Abdullahi O. I., Magaji R. A. (2017). Neurobehavioural Evaluation of Resveratrol in Murine Models of Anxiety and Schizophrenia. Metab. Brain Dis. 32, 437–442. 10.1007/s11011-016-9927-6 [DOI] [PubMed] [Google Scholar]
- Manji H., Kato T., Di Prospero N. A., Ness S., Beal M. F., Krams M., et al. (2012). Impaired Mitochondrial Function in Psychiatric Disorders. Nat. Rev. Neurosci. 13, 293–307. 10.1038/nrn3229 [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M., Ryan M., Day I. N., Owen M., McGuffin P., Whatley S. A. (2003). A Mitochondrial DNA Sequence Variant Associated with Schizophrenia and Oxidative Stress. Schizophr Res. 65, 33–38. 10.1016/s0920-9964(03)00011-2 [DOI] [PubMed] [Google Scholar]
- Martin E. A., Moore J. N. (1957). Trial of Reserpine in Treatment of Schizophrenia. Br. Med. J. 1, 8–14. 10.1136/bmj.1.5009.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon R. A., Abi-Dargham A., Howes O. D. (2019). Schizophrenia, Dopamine and the Striatum: from Biology to Symptoms. Trends Neurosci. 42, 205–220. 10.1016/j.tins.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J., Saha S., Chant D., Welham J. (2008). Schizophrenia: a Concise Overview of Incidence, Prevalence, and Mortality. Epidemiol. Rev. 30, 67–76. 10.1093/epirev/mxn001 [DOI] [PubMed] [Google Scholar]
- McGuirePsych. P., Robson P., Cubala W. J., Vasile D., Morrison P. D., Taylor A., et al. (2018). Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am. J. Psychiatry 175, 225–231. 10.1176/appi.ajp.2017.17030325 [DOI] [PubMed] [Google Scholar]
- Meltzer H. Y., Fatemi S. H. (1996). The Role of Serotonin in Schizophrenia and the Mechanism of Action of Antipsychotic Drugs. Serotonergic Mech. antipsychotic Treat., 77–107. [Google Scholar]
- Meltzer H. Y., Nash J. F. (1991). Effects of Antipsychotic Drugs on Serotonin Receptors. Pharmacol. Rev. 43, 587–604. [PubMed] [Google Scholar]
- Meltzer H. Y. (1999). The Role of Serotonin in Antipsychotic Drug Action. Neuropsychopharmacology 21, 106S–115S. 10.1016/S0893-133X(99)00046-9 [DOI] [PubMed] [Google Scholar]
- Mert D. G., Turgut N. H., Arslanbas E., Gungor H., Kara H. (2019). The Influence of Quercetin on Recognition Memory and Brain Oxidative Damage in a Ketamine Model of Schizophrenia. Psychiatry Clin. Psychopharmacol. 29, 1–7. 10.1080/24750573.2018.1442670 [DOI] [Google Scholar]
- Meyer U. (2011). Anti-inflammatory Signaling in Schizophrenia. Brain Behav. Immun. 25, 1507–1518. 10.1016/j.bbi.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Miao Y., Zhu Q., Kang Y., Yuan X., Li X., Wang S., et al. (2020). Efficacy and Safety of the Adjunctive Baicalin in Schizophrenia Patients with Negative Symptoms and Cognitive Impairment: A Randomized Pilot Study. [Google Scholar]
- Miller B. J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. (2011). Meta-analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects. Biol. Psychiatry 70, 663–671. 10.1016/j.biopsych.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodownik C., Lerner V., Kudkaeva N., Lerner P. P., Pashinian A., Bersudsky Y., et al. (2019). Curcumin as Add-On to Antipsychotic Treatment in Patients with Chronic Schizophrenia: a Randomized, Double-Blind, Placebo-Controlled Study. Clin. Neuropharmacol 42, 117–122. 10.1097/WNF.0000000000000344 [DOI] [PubMed] [Google Scholar]
- Mizuno M., Kawamura H., Ishizuka Y., Sotoyama H., Nawa H. (2010). The Anthraquinone Derivative Emodin Attenuates Methamphetamine-Induced Hyperlocomotion and Startle Response in Rats. Pharmacol. Biochem. Behav. 97, 392–398. 10.1016/j.pbb.2010.09.009 [DOI] [PubMed] [Google Scholar]
- Mizuno M., Kawamura H., Takei N., Nawa H. (2008). The Anthraquinone Derivative Emodin Ameliorates Neurobehavioral Deficits of a Rodent Model for Schizophrenia. J. Neural Transm. (Vienna) 115, 521–530. 10.1007/s00702-007-0867-5 [DOI] [PubMed] [Google Scholar]
- Moghaddam B. (2003). Bringing Order to the Glutamate Chaos in Schizophrenia. Neuron 40, 881–884. 10.1016/s0896-6273(03)00757-8 [DOI] [PubMed] [Google Scholar]
- Monteiro Á. B., de Menezes I. R. A., dos Santos Sales V., do Nascimento E. P., de Souza Rodrigues C. K., Primo A. J. B., et al. (2019). Effects of the Hyptis Martiusii Benth. Leaf Essential Oil and 1, 8-cineole (Eucalyptol) on the central Nervous System of Mice. Food Chem. Toxicol. 133, 110802. [DOI] [PubMed] [Google Scholar]
- Morais L. C., Barbosa-Filho J. M., Almeida R. N. (1998). Central Depressant Effects of Reticuline Extracted from Ocotea Duckei in Rats and Mice. J. Ethnopharmacol 62, 57–61. 10.1016/s0378-8741(98)00044-0 [DOI] [PubMed] [Google Scholar]
- Morgan C. J., Curran H. V. (2008). Effects of Cannabidiol on Schizophrenia-like Symptoms in People Who Use Cannabis. Br. J. Psychiatry 192, 306–307. 10.1192/bjp.bp.107.046649 [DOI] [PubMed] [Google Scholar]
- Moriguchi S., Zhao X., Marszalec W., Yeh J. Z., Narahashi T. (2005). Modulation of N-Methyl-D-Aspartate Receptors by Donepezil in Rat Cortical Neurons. J. Pharmacol. Exp. Ther. 315, 125–135. 10.1124/jpet.105.087908 [DOI] [PubMed] [Google Scholar]
- Morris G., Maes M. (2014). Oxidative and Nitrosative Stress and Immune-Inflammatory Pathways in Patients with Myalgic Encephalomyelitis (ME)/chronic Fatigue Syndrome (CFS). Curr. Neuropharmacol 12, 168–185. 10.2174/1570159X11666131120224653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N., Bechter K. (2013). The Mild Encephalitis Concept for Psychiatric Disorders Revisited in the Light of Current Psychoneuroimmunological Findings. Neurol. Psychiatry Brain Res. 19, 87–101. [Google Scholar]
- Na K. S., Jung H. Y., Kim Y. K. (2014). The Role of Pro-inflammatory Cytokines in the Neuroinflammation and Neurogenesis of Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 48, 277–286. 10.1016/j.pnpbp.2012.10.022 [DOI] [PubMed] [Google Scholar]
- Nakajima A., Yamakuni T., Matsuzaki K., Nakata N., Onozuka H., Yokosuka A., et al. (2007). Nobiletin, a Citrus Flavonoid, Reverses Learning Impairment Associated with N-Methyl-D-Aspartate Receptor Antagonism by Activation of Extracellular Signal-Regulated Kinase Signaling. J. Pharmacol. Exp. Ther. 321, 784–790. 10.1124/jpet.106.117010 [DOI] [PubMed] [Google Scholar]
- Niemi L. T., Suvisaari J. M., Tuulio-Henriksson A., Lönnqvist J. K. (2003). Childhood Developmental Abnormalities in Schizophrenia: Evidence from High-Risk Studies. Schizophr Res. 60, 239–258. 10.1016/s0920-9964(02)00234-7 [DOI] [PubMed] [Google Scholar]
- Niznikiewicz M. A., Kubicki M., Shenton M. E. (2003). Recent Structural and Functional Imaging Findings in Schizophrenia. Curr. Opin. Psychiatry 16, 123–147. 10.1097/00001504-200303000-00002 [DOI] [Google Scholar]
- Ochoa E. L., Lasalde-Dominicci J. (2007). Cognitive Deficits in Schizophrenia: Focus on Neuronal Nicotinic Acetylcholine Receptors and Smoking. Cel Mol Neurobiol 27, 609–639. 10.1007/s10571-007-9149-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandy V., VijeePallam K. (2017). Antipsychotic-like Activity of Scopoletin and Rutin against the Positive Symptoms of Schizophrenia in Mouse Models. Exp. Anim. 66, 417–423. 10.1538/expanim.17-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandy V., Vijeepallam K. (2016). Antipsychotic-Like Activity of α-Asarone in Mice: A Preliminary Report. Adv. Pharmacol. Clin. Trials 1, 000106. 10.23880/apct-16000106 [DOI] [Google Scholar]
- Park S. E., Paudel P., Wagle A., Seong S. H., Kim H. R., Fauzi F. M., et al. (2020). Luteolin, a Potent Human Monoamine Oxidase-A Inhibitor and Dopamine D4 and Vasopressin V1A Receptor Antagonist. J. Agric. Food Chem. 68, 10719–10729. 10.1021/acs.jafc.0c04502 [DOI] [PubMed] [Google Scholar]
- Pereira M., Siba I. P., Chioca L. R., Correia D., Vital M. A., Pizzolatti M. G., et al. (2011). Myricitrin, a Nitric Oxide and Protein Kinase C Inhibitor, Exerts Antipsychotic-like Effects in Animal Models. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1636–1644. 10.1016/j.pnpbp.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Pisani A., Bernardi G., Ding J., Surmeier D. J. (2007). Re-emergence of Striatal Cholinergic Interneurons in Movement Disorders. Trends Neurosci. 30, 545–553. 10.1016/j.tins.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Pitsikas N. (2021). Crocus Sativus L. Extracts and its Constituents Crocins and Safranal; Potential Candidates for Schizophrenia Treatment? Molecules 26, 1237. 10.3390/molecules26051237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsikas N., Tarantilis P. A. (2017). Crocins, the Active Constituents of Crocus Sativus L., Counteracted Apomorphine-Induced Performance Deficits in the Novel Object Recognition Task, but Not Novel Object Location Task, in Rats. Neurosci. Lett. 644, 37–42. 10.1016/j.neulet.2017.02.042 [DOI] [PubMed] [Google Scholar]
- Pitsikas N. (20152015). The Effect of Crocus Sativus L. And its Constituents on Memory: Basic Studies and Clinical Applications. Evid. Based Complement. Alternat Med. 2015, 926284. 2015/02/01. 10.1155/2015/926284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwood T. R., Asgariroozbehani R., Wu S., Agarwal S. M., Logan R. W., Ballon J. S., et al. (2021). Roles of Inflammation in Intrinsic Pathophysiology and Antipsychotic Drug-Induced Metabolic Disturbances of Schizophrenia. Behav. Brain Res. 402, 113101. 10.1016/j.bbr.2020.113101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raedler T. J., Bymaster F. P., Tandon R., Copolov D., Dean B. (2007). Towards a Muscarinic Hypothesis of Schizophrenia. Mol. Psychiatry 12, 232–246. 10.1038/sj.mp.4001924 [DOI] [PubMed] [Google Scholar]
- Rao V. S., Carvalho A. C., Trevisan M. T., Andrade G. M., Nobre-Júnior H. V., Moraes M. O., et al. (2012). Mangiferin Ameliorates 6-Hydroxydopamine-Induced Cytotoxicity and Oxidative Stress in Ketamine Model of Schizophrenia. Pharmacol. Rep. 64, 848–856. 10.1016/s1734-1140(12)70879-4 [DOI] [PubMed] [Google Scholar]
- Rapoport J. L., Addington A. M., Frangou S., Psych M. R. (2005). The Neurodevelopmental Model of Schizophrenia: Update 2005. Mol. Psychiatry 10, 434–449. 10.1038/sj.mp.4001642 [DOI] [PubMed] [Google Scholar]
- Ross C. A., Margolis R. L. (2005). Neurogenetics: Insights into Degenerative Diseases and Approaches to Schizophrenia. Clin. Neurosci. Res. 5, 3–14. 10.1016/j.cnr.2005.07.001 [DOI] [Google Scholar]
- Sahin C., Ünal G., Aricioglu F. (2014). The Involvement of Akt and GSK-3: Two Pathways, Two Pathology. Clin. Exp. Health Sci. 4, 51. 10.5455/musbed.20140321043920 [DOI] [Google Scholar]
- Samaei A., Moradi K., Bagheri S., Ashraf-Ganjouei A., Alikhani R., Mousavi S. B., et al. (2020). Resveratrol Adjunct Therapy for Negative Symptoms in Patients with Stable Schizophrenia: A Double-Blind, Randomized Placebo-Controlled Trial. Int. J. Neuropsychopharmacol. 23, 775–782. 10.1093/ijnp/pyaa006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saranya K., Aji A. M., Keerthana S., Babu N. R., Sivaraj C., Arumugam P. (2019). Isolation and Pharmacological Activities of Hypericin Fraction from the Leaves of Hypericum Hookerianum Wight & Arnott. Int. J. Herbal Med. 7, 36–42. [Google Scholar]
- Schotte A., Janssen P. F., Gommeren W., Luyten W. H., Van Gompel P., Lesage A. S., et al. (1996). Risperidone Compared with New and Reference Antipsychotic Drugs: In Vitro and In Vivo Receptor Binding. Psychopharmacology (Berl) 124, 57–73. 10.1007/BF02245606 [DOI] [PubMed] [Google Scholar]
- Schubert M. H., Young K. A., Hicks P. B. (2006). Galantamine Improves Cognition in Schizophrenic Patients Stabilized on Risperidone. Biol. Psychiatry 60, 530–533. 10.1016/j.biopsych.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Schwarcz G., Karajgi B., McCarthy R. (2009). Synthetic delta-9-tetrahydrocannabinol (Dronabinol) Can Improve the Symptoms of Schizophrenia. J. Clin. Psychopharmacol. 29, 255–258. 10.1097/JCP.0b013e3181a6bc3b [DOI] [PubMed] [Google Scholar]
- Scott J. G., Baker A., Lim C. C. W., Foley S., Dark F., Gordon A., et al. (2020). Effect of Sodium Benzoate vs Placebo Among Individuals with Early Psychosis: a Randomized Clinical Trial. JAMA Netw. open 3, e2024335. 10.1001/jamanetworkopen.2020.24335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadkami F., Jones A. D. (2012). “Nontargeted Profiling of Specialized Metabolites of Digitalis Purpurea with a Focus on Cardiac Glycosides,” in Emerging Trends in Dietary Components for Preventing and Combating Disease (ACS Publications; ), 185–205. 10.1021/bk-2012-1093.ch011 [DOI] [Google Scholar]
- Shirai Y., Fujita Y., Hashimoto K. (2012). Effects of the Antioxidant Sulforaphane on Hyperlocomotion and Prepulse Inhibition Deficits in Mice after Phencyclidine Administration. Clin. Psychopharmacol. Neurosci. 10, 94. 10.9758/cpn.2012.10.2.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. (2015). Oxidative Stress: a Concept in Redox Biology and Medicine. Redox Biol. 4, 180–183. 10.1016/j.redox.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. H., Kellendonk C., Kandel E. (2010). A Possible Role for the Striatum in the Pathogenesis of the Cognitive Symptoms of Schizophrenia. Neuron 65, 585–596. 10.1016/j.neuron.2010.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Tamminga C., Davis J. (1977). Effect of Apomorphine on Schizophrenic Symptoms. J. Neural Transm. 40, 171–176. 10.1007/BF01250567 [DOI] [PubMed] [Google Scholar]
- Snyder M. A., Gao W. J. (2013). NMDA Hypofunction as a Convergence point for Progression and Symptoms of Schizophrenia. Front Cel Neurosci 7, 31. 10.3389/fncel.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonibare M. A., Umukoro S., Shonibare E. T. (2012). Antipsychotic Property of Aqueous and Ethanolic Extracts of Lonchocarpus Cyanescens (Schumach and Thonn.) Benth.(Fabaceae) in Rodents. J. Nat. medicines 66, 127–132. 10.1007/s11418-011-0562-6 [DOI] [PubMed] [Google Scholar]
- St Clair D., Xu M., Wang P., Yu Y., Fang Y., Zhang F., et al. (2005). Rates of Adult Schizophrenia Following Prenatal Exposure to the Chinese Famine of 1959-1961. Jama 294, 557–562. 10.1001/jama.294.5.557 [DOI] [PubMed] [Google Scholar]
- Sullivan P. F., Kendler K. S., Neale M. C. (2003). Schizophrenia as a Complex Trait: Evidence from a Meta-Analysis of Twin Studies. Arch. Gen. Psychiatry 60, 1187–1192. 10.1001/archpsyc.60.12.1187 [DOI] [PubMed] [Google Scholar]
- Sun X-j., Zhao X., Xie J-n., Wan H. (2020). Crocin Alleviates Schizophrenia-like Symptoms in Rats by Upregulating Silent Information Regulator-1 and Brain Derived Neurotrophic Factor. Compr. Psychiatry 103, 152209. 10.1016/j.comppsych.2020.152209 [DOI] [PubMed] [Google Scholar]
- Suresh P., Raju A. B. (2013). Antidopam Inergic Effects of Leucine and Genistein on Shizophrenic Rat Models. Neurosciences J. 18, 235–241. [PubMed] [Google Scholar]
- Tarragó T., Kichik N., Claasen B., Prades R., Teixidó M., Giralt E. (2008). Baicalin, a Prodrug Able to Reach the CNS, Is a Prolyl Oligopeptidase Inhibitor. Bioorg. Med. Chem. 16, 7516–7524. 10.1016/j.bmc.2008.04.067 [DOI] [PubMed] [Google Scholar]
- Ueda T., Ugawa S., Ishida Y., Shimada S. (2011). Geissoschizine Methyl Ether Has Third-Generation Antipsychotic-like Actions at the Dopamine and Serotonin Receptors. Eur. J. Pharmacol. 671, 79–86. 10.1016/j.ejphar.2011.09.007 [DOI] [PubMed] [Google Scholar]
- Wang Y., Singh A. P., Nelson H. N., Kaiser A. J., Reker N. C., Hooks T. L., et al. (2016). Urinary Clearance of cranberry Flavonol Glycosides in Humans. J. Agric. Food Chem. 64, 7931–7939. 10.1021/acs.jafc.6b03611 [DOI] [PubMed] [Google Scholar]
- Watkins C. C., Andrews S. R. (2016). Clinical Studies of Neuroinflammatory Mechanisms in Schizophrenia. Schizophrenia Res. 176, 14–22. 10.1016/j.schres.2015.07.018 [DOI] [PubMed] [Google Scholar]
- Wetchateng T., Piyabhan P. (2014). EPA-0117–The Use of Bacosides a and B to Prevent a Cognitive Deficit in Schizophrenia Rat Models Resulting in Increased Vesicular Glutamate Transporter 2 (VGLUT2) in the Cingulate Gyrus. Eur. Psychiatry 29, 1. 10.1016/s0924-9338(14)77591-1 24119631 [DOI] [Google Scholar]
- White C. M. (2019). A Review of Human Studies Assessing Cannabidiol's (CBD) Therapeutic Actions and Potential. J. Clin. Pharmacol. 59 (7), 923–934. 10.1002/jcph.1387 [DOI] [PubMed] [Google Scholar]
- Winterer G., Egan M. F., Raedler T., Sanchez C., Jones D. W., Coppola R., et al. (2003). P300 and Genetic Risk for Schizophrenia. Arch. Gen. Psychiatry 60, 1158–1167. 10.1001/archpsyc.60.11.1158 [DOI] [PubMed] [Google Scholar]
- Wright I. C., Rabe-Hesketh S., Woodruff P. W., David A. S., Murray R. M., Bullmore E. T. (2000). Meta-analysis of Regional Brain Volumes in Schizophrenia. Am. J. Psychiatry 157, 16–25. 10.1176/ajp.157.1.16 [DOI] [PubMed] [Google Scholar]
- Wu D., Guo Z., Ren Z., Guo W., Meydani S. N. (2009). Green tea EGCG Suppresses T Cell Proliferation through Impairment of IL-2/IL-2 Receptor Signaling. Free Radic. Biol. Med. 47, 636–643. 10.1016/j.freeradbiomed.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Xu Z., Adilijiang A., Wang W., You P., Lin D., Li X., et al. (2019). Arecoline Attenuates Memory Impairment and Demyelination in a Cuprizone-Induced Mouse Model of Schizophrenia. Neuroreport 30, 134. 10.1097/WNR.0000000000001172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D. K. (2021). Potential Therapeutic Strategies of Phytochemicals in Neurodegenerative Disorders. Curr. Top. Med. Chem. 21, 2814–2838. 10.2174/1568026621666211201150217 [DOI] [PubMed] [Google Scholar]
- Yadav M., Jindal D. K., Dhingra M. S., Kumar A., Parle M., Dhingra S. (2018a). Protective Effect of Gallic Acid in Experimental Model of Ketamine-Induced Psychosis: Possible Behaviour, Biochemical, Neurochemical and Cellular Alterations. Inflammopharmacology 26, 413–424. 10.1007/s10787-017-0366-8 [DOI] [PubMed] [Google Scholar]
- Yadav M., Parle M., Jindal D. K., Dhingra S. (2018b). Protective Effects of Stigmasterol against Ketamine-Induced Psychotic Symptoms: Possible Behavioral, Biochemical and Histopathological Changes in Mice. Pharmacol. Rep. 70 (3), 591–599. 10.1016/j.pharep.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Young J., Wahle K. W., Boyle S. P. (2008). Cytoprotective Effects of Phenolic Antioxidants and Essential Fatty Acids in Human Blood Monocyte and Neuroblastoma Cell Lines: Surrogates for Neurological Damage In Vivo . Prostaglandins, Leukot. Essent. fatty Acids 78, 45–59. 10.1016/j.plefa.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Zhang Z-J. (2004). Therapeutic Effects of Herbal Extracts and Constituents in Animal Models of Psychiatric Disorders. Life Sci. 75, 1659–1699. 10.1016/j.lfs.2004.04.014 [DOI] [PubMed] [Google Scholar]
- Zhao K., So H-C. (2018). Drug Repositioning for Schizophrenia and Depression/anxiety Disorders: A Machine Learning Approach Leveraging Expression Data. IEEE J. Biomed. Health Inform. 23, 1304–1315. 10.1109/JBHI.2018.2856535 [DOI] [PubMed] [Google Scholar]
- Zuardi A. W., Rodrigues J. A., Cunha J. (1991). Effects of Cannabidiol in Animal Models Predictive of Antipsychotic Activity. Psychopharmacology 104, 260–264. 10.1007/BF02244189 [DOI] [PubMed] [Google Scholar]