Abstract
Acute kidney injury (AKI) is a complex condition which has an intricate pathology mostly involving hemodynamic, inflammatory, and direct toxic effects at the cellular level with high morbidity and mortality ratios. Renal ischemic reperfusion injury (RIRI) is the main factor responsible for AKI, most often observed in different types of shock, kidney transplantation, sepsis, and postoperative procedures. The RIRI-induced AKI is accompanied by increased reactive oxygen species generation together with the activation of various inflammatory pathways. In this context, plant-derived medicines have shown encouraging nephroprotective properties. Evidence provided in this systemic review leads to the conclusion that plant-derived extracts and compounds exhibit nephroprotective action against renal ischemic reperfusion induced-AKI by increasing endogenous antioxidants and decreasing anti-inflammatory cytokines. However, there is no defined biomarker or target which can be used for treating AKI completely. These plant-derived extracts and compounds are only tested in selected transgenic animal models. To develop the results obtained into a therapeutic entity, one should apply them in proper vertebrate multitransgenic animal models prior to further validation in humans.
1. Introduction
Acute kidney injury (AKI) is a widely spread and prospectively life-taking disease. Presently, AKI definition is based on the decline in the kidney function in more or less than a week [1]. Based on AKI genesis, this disease has been characterized into three categories, prerenal—a physiologic reaction of the normal structural kidney towards hypoperfusion; intrinsic or intrarenal—injury to the kidney parenchymal cells; and postrenal—a response towards a urinary tract obstruction [2]. The impact of AKI in long run on public health is enormous. AKI is associated with increased number of morbidity and mortality. The incidence of AKI has almost doubled in number over the past two decades [3, 4]. The inpatients are more likely to suffer from AKI as a result of secondary disease complications or due to an adverse reaction to therapy. The prevalence of AKI among seriously ill patients is 25–67%, while the mortality ratio is 30–60% even if the severity of the disease subsides [5–8].
Annually, about 2100 per million of the population suffer from AKI. In developed countries, the cases of AKI are expected to be more than 2 million per annum. Among these, 1.5 million patients survive of which many patients progress to advanced stages of chronic kidney disease (CKD) within a period of 24 months. To these numbers, one can add over 300,000 patients to the category of advanced stage of CKD per year. The episodes of AKI in CKD patients lead to upsurge in the development of end-stage renal disease (ESRD). These figures provide the fact that the major attributable risk in patients suffering from CKD is AKI [1].
AKI is a multifactorial condition, and the pathogenesis of which is based on hemodynamic, inflammatory, and direct toxic effects at the cellular level [9]. However, renal ischemic reperfusion injury (RIRI) is being considered as one of the foremost reasons of AKI which is accompanied by 50% mortality ratio in intensive care units [10–12]. Renal ischemic reperfusion injury may occur due to various reasons such as administration of vasoconstrictive drugs or radio-contrast agents. Hypotension also leads to RIRI which more commonly occurs in sepsis or when a large volume of fluid is lost in trauma. Similarly, it can be initiated by various clinical ailments like myocardial infarction, different types of strokes, or due to postsurgical operations such as organ transplant, cardiac surgery, extracorporeal lithotripsy, and adrenal aneurysm. In case of trauma or shock, the body compensates fluid losses by various mechanisms, but due to unmet need of high oxygen demand of cells and insufficient metabolic substrate availability, the cellular injury often leads to organ failure [13–15].
Literature data prove the hypothesis that acute kidney injury due to ischemic reperfusion is associated with changes in hemodynamics and dysfunction of endothelial cells because of a high level of reactive oxygen species (ROS) and reactive nitrogen species (RNS) leading to decreased production of nitrogen oxide and intracellular energy store exhaustion. Both ROS and RNS stresses cause lipid peroxidation, oxidative DNA damage, modification of inflammatory pathways, modification of leukocyte function, and microvascular reduction in blood flow to renal medulla because of vascular congestion [10]. Similarly, Malek and Nematbakhsh have mentioned that ROS is involved in kidney injury by lipid peroxidation, while oxidative damage of proteins and DNA contributes to apoptosis and cell necrosis. Downregulation of antioxidant enzymes such as catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPx) might be responsible for the pathophysiology of the ischemic reperfusion injury [16].
The most efficient and effective ways to prevent ischemic reperfusion (I/R) injury is thus to scavenge these ROS and free radicals. According to current scientific data, traditional natural medicines have been found very effective against ROS and oxidative stress. Moreover, natural therapies and mixtures have been shown to be very efficacious in various inflammatory conditions [17]. The purpose of this review is to summarize the treatments used for AKI induced by I/R injury via plant extracts and plant-derived natural compounds, thereby offering some clue for further in-depth research.
2. Materials and Methods
Relevant articles were retrieved from various database search engines such as Google Scholar, PubMed, and ScienceDirect by typing key terms “acute kidney injury,” “renal ischemic reperfusion in rats,” “renal ischemia reperfusion,” “renal ischemic reperfusion and plant extracts,” “nephroprotection against renal/kidney with renal. reperfusion,” and “plant extracts against kidney ischemia reperfusion.” Articles published from 2000 to 2019 were selected to write this review.
After careful reading of the articles, the central information was critically collected and discussed according to the following thematic sessions: kidney with renal. reperfusion injury, epidemiology, etiology of AKI, diagnosis, treatment of AKI, mechanisms by which plants ameliorated renal I/R injury induced-AKI, and effects on biomarkers and renal tissues. The final curatorship of the articles took place through the tabulation of relevant information such as substances, sources, experimental models, methodological aspects, mechanisms, and effects.
3. Results
3.1. Renal Ischemic Reperfusion Injury
Ischemic reperfusion (I/R) injury is a pathophysiological condition which is generated by preliminary impediment of blood flow to an organ followed by restoration or reperfusion. The duration and extent of ischemia govern the range of cell death or loss of organ function. The reperfusion phase is thought to reinstate oxygen and nutrient requirement of an organ though it also synergizes the oxidation and inflammatory stress both locally and systemically resulting in cell damage. This whole process is being entitled as I/R injury [18].
Renal I/R injury causes hypoxia as a result of which metabolism is altered leading to depletion of adenosine triphosphate (ATP) and increase in lactate concentration. This provokes an electrolyte imbalance such as an increased sodium level as well as a water influx and an intracellular calcium overload. As a result, an increase in apoptosis besides cell necrosis and intracellular acidosis is observed. Furthermore, reperfusion leads to generation of ROS decreasing SOD and catalase as well as GPx levels. Reperfusion inhibits the cytochrome c oxidase and increases NO levels. These parameters are responsible for protein and lipid peroxidation (which increases MDA levels) along with DNA damage, which further aggravates cell necrosis and apoptosis [19, 20]. Because of the redox imbalance, a local and systemic inflammatory process is activated. I/R injury is also responsible for the initiation of several inflammatory reactions within the renal parenchyma. In addition to infiltration of neutrophils, renal I/R injury is accountable for the generation of many inflammatory cytokines, for instance IL-6, IL-1, and TNF-α [21].
In experimental renal I/R injury models, the renal blood flow is completely blocked by clamping the renal artery. This blocking phase is called ischemia, the duration of which is usually from 20 to 60 minutes, and this phase is accompanied by hypoxia in addition to loss of the GFR function. Within the first 30 minutes of ischemia, the injury is still limited, but within 45 minutes, the injury becomes more regular and confluent with different levels of necrotic lesions. After 60 minutes, necrotic cells become infarcted [22–25]. A total blockage during ischemia results into endothelial cell injury with functional loss. It also results in substantial modifications of the transcription program of vasoactive cytokines and leukocyte function [26]. Different approaches are in practice for the induction of renal ischemia in experimental models, such as the simultaneous application of bilateral renal ischemia [27, 28], while others apply unilateral ischemia in one kidney and perform nephrectomy of the other [29, 30].
Removal of clamps and restoration of blood supply and nutrients is called reperfusion. This rapid resupply of blood is essential for the survival of cells, which are being damaged by ischemia. However, reperfusion, which is accompanied by a return of oxygen, itself is the major step responsible for further cell injury [20]. The foremost damaging effect of reperfusion is the generation of ROS via mitochondria, which are responsible for acute injury to cells, persisting for weeks without any intervention. ROS causes a disturbance in adenosine triphosphate (ATP) production and calcium regulation leading to mitochondrial permeability transition pore (MPTP) opening. This dysregulation initiates cell necrosis and apoptosis as well as cell death [31].
Various sources have been linked with ROS generation following I/R injury such as enzymatic sources including xanthine oxidase, NADPH oxidase, the mitochondrial electron transport chain, and nitric oxide synthase [32]. The ROS generated from these sources by I/R injury highly target proteins, cell membrane lipids, and nucleic acids [33]. Besides the direct cytotoxic effect of renal I/R injury, ROS also prompts an inflammatory response in renal endothelial and parenchymal cells. As a result, many proinflammatory cytokines such as the tissue necrosis factor-α (TNF-α), interleukins (IL) 1, IL-6, and many chemokines like the monocyte chemoattractant protein (MCP) and IL8 are released. The ROS and cytokines generated as a result of kidney injury induce an upsurge in the expression of adhesion biomarkers, for instance, the intercellular adhesion molecule (ICAM), the vascular cell adhesion molecule (VCAM), and the P-selectin [21, 34]. Assembling of cytokines, chemokines, ROS, and adhesion molecules further aggravate the renal injury.
3.2. Clinical Presentation of AKI
3.2.1. Epidemiology
Accurate elucidation of AKI epidemiology is modulated by many factors such as various definition of AKI and variations in case mingling. For instance, patients of non-ICU cases are different from ICU patients. Similarly, there is a difference between cases in general hospital ICU compared to rural hospital ICU; postoperative cardiac patients are different from those displaying liver cirrhosis [35]. Many factors are involved in the variation of AKI figures, in developed as well as in developing countries [36]. Aged populations are more affected by AKI in developed countries [37], while in developing countries, mostly adults and children suffer from AKI due to socioeconomic and environmental influences [38]. Studies involving adult population have revealed that AKI is accompanied by an increased rate of mortality, hospital ICU stay, and dependency on mechanical ventilation [39–41]. AKI commonly occurs in hospitalized patients and more frequently in those who are admitted in ICU [39, 42]. The prevalence of AKI in ICU admitted patients is mentioned in various epidemiological studies in accordance with KDIGO criteria [39, 43, 44]. A study performed by Kaddourah et al. included children and young adults having an age range from 3 months to 25 years. The total number of patients registered were 4683, admitted in 32 ICUs. During the first week of admission at the hospital, the reported AKI incidence was 27%, of whom 12% developed severe AKI [45].
The ratio of AKI in hospitalized pediatric patients is about 5%, while in seriously ill pediatric patients, it ranges between 20 and 70% accompanied by high morbidity and mortality rates [46, 47]. A systematic review based on 312 cohort studies from high-income developed countries comprising 49 million patients of AKI has shown that one in five adults suffered from AKI. Likewise, one in three children were displaying AKI. Another cohort study (n = 5, 23,390) performed in Scotland and based on the RIFLE classification system reported that the incidence of AKI was 1,811 per million residents [48]. The prevalence of AKI was also studied in 120,123 patients from January 2000 to December 2005 in 57 ICUs across Australia [49], the number of AKI cases mentioned being 36%. According to the RIFLE classification of AKI, most of the cases (16%) belonged to the “R” category (stage I injury), 13.6% were of “I” (stage II injury), and 6.3% cases were related to the “F” category (stage III injury). The AKI was associated with an increased mortality ratio [49]. Concerning AKI prevalence in ICUs, a study was conducted in Thailand including 5,377 patients from February 2013 till July 2015. Among them, 2471 (53%) patients were diagnosed with AKI during hospital admission [50]. Another study from Scotland has shown a prevalence of 2147 AKI cases per one million residents [48]. Most of the aforementioned studies have thus reported a sovereign link of AKI with a greater risk of mortality. The worldwide projected mortality rate of AKI is 24% in the adult population and 13.8% in children. Among aged population, the risk of mortality from AKI is even higher [51].
3.2.2. Etiology of AKI
There is a difference in the susceptibility of each individual to develop AKI caused by exposure to numerous factors depending, for instance, on the duration of the exposure, its type, heterogeneity, and severity [52]. As stated before, AKI is more common in critically ill patients and its cause is frequently multifactorial. Sepsis is one of the serious conditions that is increasing in hospitalized patients. For example, a 22-year analysis of hospitalized patients carried out in the U.S has revealed a yearly growth of 8.7% in sepsis diagnosis [53]. Sepsis has been found as the foremost cause of AKI accounting for 45 to 70% of the cases of AKI being linked to sepsis. A large prospective observational study performed by Bagshaw et al. has mentioned that, among 29,000 patients, 5.7% developed AKI of which 47.5% cases were due to sepsis [54]. Likewise, pediatric studies showed that sepsis was the major risk factor in 18 to 58% of the patients acquiring AKI [55].
Major surgery is another factor leading to AKI, as revealed by Grams et al. (2016) according to a large cohort study involving 161,185 participants. Among the participants, 11.8% suffered from AKI after a major surgery, though other risk factors in these patients were old age, male gender, overweight, and African American race. There were differences in the prevalence of AKI with respect to the type of surgery, but the most affected ones were patients with cardiac surgery [56].
Another major cause of AKI is cardiogenic shock, an ailment characterized by insufficient cardiac output causing low blood pressure with symptoms of end organ hypoperfusion such as oliguria [57]. A study performed by Van den Akker et al. mentioned that, out of 39 cardiogenic shock patients admitted in ICU, 24 developed AKI within the first 48 hours of their admission [58]. The prevalence of AKI in cardiogenic shock patients is very frequent and associated with high mortality during the first 90 days of cardiogenic shock [57]. Burns can likewise lead to AKI with a very high incidence rate of 30% and with 80% mortality. A large amount of fluids loss from a burn injury causes hypovolemia and a substantial decline in cardiac output resulting in decreased renal flow and leading to ischemia and cellular death. The ischemia further aggravates free radical formation and cellular structure damage ultimately causing additional kidney injury [59]. Drugs are notorious for causing nephrotoxicity with 20 to 40% AKI cases due to medications, this ratio reaching 60% among the elderly population. Aminoglycosides and other antimicrobials (antivirals and antifungals) are the most common drug classes responsible for AKI [60]. Other potential classes of drugs include nonsteroidal anti-inflammatory drugs (NSAID's), angiotensin-converting enzyme inhibitors (ACE), and calcineurin inhibitors. In case of appearance of any AKI symptoms in patients during therapy, drug administration should be stopped [61]. Besides, numerous infectious diseases are responsible for AKI such as malaria, leptospirosis, dengue, yellow fever, and scrub typhus. Similarly, animal venoms such as snakes and various arthropods also cause AKI [62].
3.2.3. Diagnosis
The diagnosis of AKI has been evolved over the time. Conventionally, AKI diagnosis is based on the measurement of serum creatinine (SCr) and the reduction in urine yield. The definition of AKI by Acute Dialysis Quality Initiative (ADQI) group based on Risk, Injury, Failure, Loss, End-stage (RIFLE) criteria has been modified by the AKI-network (AKIN) with slight amendments [63, 64]. These two definitions and classification benchmarks of AKI were merged in 2012, and consequently, Kidney Disease Improving Global Outcomes (KDIGO) criteria came into existence. In view of the KDIGO criteria, AKI is diagnosed if SCr ≥0.3 mg/dl within 48 hours or escalating by 1.5 times from the baseline level within a week or less. AKI stages were classified by determining changes in SCr or urine output [65].
Current criteria of AKI diagnosis are widely used and accepted but have, regrettably, limitations. Both SCr and urine output are imperfect biomarkers as compared to other biomarkers which may characterize AKI in its earlier stages [66, 67]. For the subclinical diagnosis of AKI, two types of novel biomarkers known as damage and stress biomarkers are used. The novel damage biomarkers are the neutrophil gelatinase-associated lipocalin (NGAL) and the kidney injury molecule 1 (KIM-1), whereas the insulin-like growth factor binding protein 7 (IGFBP-7) and tissue inhibitor of metalloproteinase-2 (TIMP-2) belong to the novel stress biomarkers. These biomarkers may predict the detection of AKI, but their clinical application is still uncertain [68, 69]. A combo of urinary TIMP-2 and IGFBP-7 called as nephron-check has been recognized by the FDA for the diagnosis of AKI [70]. Other tools that are used for AKI diagnosis are clinical imaging techniques involving ultrasonography, contrast-enhanced ultrasonography, computerized tomography, conventional B-mode imaging, and magnetic resonance imaging [66]. AKI is a severe disease, progressive in nature, meaning that continued insult will lead to increased injury and loss of organ function with serious consequences such as death. Timely diagnosis of AKI will lead the clinician to intervene and make a proper treatment plan [71].
3.2.4. Treatment of AKI Using Drugs and Plant-Based Therapies
Treatment and management plans for AKI are based on its causative agent. Those patients in whom AKI is not developed but display a risk factor or been exposed to a risk factor must undertake clinical assessment and investigation [72]. As AKI is a multifactorial and heterogeneous disease often accompanied by comorbidities, and identification of an appropriate pharmacological approach, which can assist in full cure, is quite challenging. Moreover, at the time of the diagnosis, the disease is almost established in most of the cases. AKI patients frequently suffer from increased potassium levels, metabolic acidosis, fluid overload, or increased level of blood urea due to decreased GFR. The pharmacological therapy is commonly based on treating these symptoms rather than the disease itself [73].
Various drug classes are used for the treatment of AKI according to its triggering factors. For instance, vasopressors, diuretics, as well as intravenous (I/V) fluids are administered for management of the oliguria which is related with decreased GFR in addition to an increase in salt and water retention. The purpose of this therapy is to resume a normal cardiac output, a systemic hypotension, and a neuroendocrine response. However, as observed in many studies, this therapy leads to shoddier organ function loss with worse consequences in routine surgery cases. Maintaining this therapy for a long time is also challenging and leads to many adverse responses such as interstitial edema and organ dysfunction [74].
Loop diuretics are being used based on a well-known notion that it transforms oliguric AKI patients into nonoliguric ones providing an electrolyte balance [75]. Regardless of its extensive prescription in AKI patients, improvement of the clinical picture is still missing. Moreover, some data suggest more damage compared to benefits in selected cases [76]. The results of the study carried out by Mehta et al. have shown that diuretic administration in critically ill patients suffering from kidney diseases is accompanied with increased rate of mortality and irreversible renal function loss [77]. A meta-analysis by Ho and Power has mentioned that furosemide, a loop diuretic, when used in AKI patients, has no impact on mortality ratio and risk of renal replacement therapy (RRT) reduction [78].
Among the renal vasodilators, dopamine has shown inability to protect or change the progression of ischemic AKI. Likewise, fenoldopam induces a dose-dependent hypotension which may aggravate AKI. However, according to another review based on 13 studies regarding fenoldopam role in patients enduring cardiovascular surgery, it was reported that it may decrease the RRT and in-hospital mortality rate [79]. Another pharmacological intervention in AKI is statin therapy. A meta-analysis by He et al. showed that pretreatment and posttreatment with statins in patients undergoing cardiac surgery increases the risk of cardiac surgery associated with AKI, the risk being higher with rosuvastatin compared to atorvastatin [80]. Many drugs display encouraging effects in specific stages of AKI, but none of them have shown any assured evidence in the protection of AKI. Many factors may be responsible for such a failure, e.g., most pharmacological therapies are targeting only a single pathway. Similarly, there is vagueness on initiation, discontinuation, and exact dosing for a given pharmacological therapy [81]. Obstacles in clinical trials is another accountable factor, for instance, secondary diseases in enrolled patients for clinical trials are mainly responsible for increased mortality ratio. Likewise, lack of agreement on a common definition of AKI, and its complex etiology are additional factors responsible for insufficient pharmacological options to treat this ailment [82].
With the expansion of research, new cellular and subcellular information has become available regarding the pathophysiology of AKI. As a result, more emphasis has been laid on inflammation, oxidative stress, and immune response modulation [83]. One essential condition of AKI is a kidney I/R injury, which is accompanied by inflammatory responses such as macrophage and neutrophil infiltration. Similarly, mitochondria are also affected due to ROS generation, causing changes both at cellular and vascular levels [27, 84]. Renal I/R injury leads to the reduction of antioxidant molecules such as glutathione and an increase in lipid peroxidation which can be identified by an enhanced level of malondialdehyde [84]. The generation of ROS, inflammatory molecules, and activation of apoptotic pathways and the caspase pathway leads to renal cytotoxicity and initiate a vicious circle of cell injury [85].
Another emerging pharmacological therapy is the use of plant-derived extracts and natural compounds with antioxidant and anti-inflammatory properties. Moreover, these latter ones act via multiple mechanisms to protect cell injury from ROS and inflammatory cytokines [86]. Since ancient times, many plants are used for treatment purposes and are still in practice all over the world [87, 88]. The use of medicinal plants for treatment purposes is based on hundred-year-old beliefs and innumerable experiences [89–91].
There is a growing interest in developing medicinal plant-derived products as treatments all over the world. The modern pharmaceutical industry is also capitalizing in research based on new chemical entities (NCEs) from medicinal plants. Currently, among the approved NCEs from natural sources, 25% are derived from plants. Moreover, in some therapeutic areas such as oncology, there are 60% approved plant-derived medicines [92–94]. According to the World Health Organization (WHO), 65–80% population are using plant-derived medicines in developing countries [95].
As stated previously, oxidative and inflammatory stresses are the main factors contributing to the pathophysiology of I/R injury [96–99]. In this context, easy availability and accessibility to plant-derived therapies are of prime importance. Plants represent a rich source of phytochemicals which have a high potential to act both as exogenous antioxidant and anti-inflammatory agents, as evidenced by many studies [27, 100–104]. Therefore, treating I/R-induced AKI with plant-derived extracts and compounds is the most practical approach. The aim of this review is to enlist such plant-based therapies which are tested in experimental models of renal I/R injury, thus providing an insight for future research.
Plant-based therapies provide nephroprotection against I/R-induced AKI mainly by increasing the levels of antioxidant enzymes such as superoxide dismutase (SOD) and catalase as well as glutathione levels, thereby producing antioxidant effects against ROS [105–108]. Similarly, they provide an anti-inflammatory effect mostly by inhibiting inflammatory cytokines like the tissue necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), and interleukin-10 (IL-10) [100, 109, 110].
The nephroprotective role provided by various plants against I/R injury can be attributed to their rich contents in phytochemicals such as flavonoids, phenols, polyphenolics, alkaloids, tannins, terpenes, and saponins. Many plants contain more than one of these highly antioxidant and anti-inflammatory compounds. A summary of the various effects of plants against I/R induced-AKI is presented in Table 1.
Table 1.
Plant-derived extracts and compounds having nephroprotective effects against renal ischemic reperfusion injury-induced acute kidney injury.
| Substance used | Source | Experimental model | Ischemia | Mechanism | Route of Administration and order | Effects | Reference |
|---|---|---|---|---|---|---|---|
| (−)-α-Bisabolol | Matricaria chamomilla, M. crassifolium, Salvia runcinata, Vanillosmopsis sp. | Male Wistar albino rat (in vivo) LLC-MK 2 cells (in vitro) | Right renal nephrectomy and left kidney ischemia for 60 min followed by 48 h reperfusion | Antioxidant, anti-inflammatory, antiapoptotic | Oral route posttreatment | ↓Urine osmolality ↓SCr, urea, uric acid, MDA ↓FENa+, FEK+, FECl− ↓uKim-1, ↓Proteinuria, Albuminuria ↓Renal histopathological score ↑Creatinine clearance, GSH ↑Water consumption, diuresis ↑Cell viability |
[26] |
| Various plants and herbs, e.g., in essential oil of Matricaria chamomilla | HK2 cells | In vitro ischemic reperfusion model by the anaerobic chamber method. Firstly, ischemia was induced for 24 h followed by 3 h reperfusion. | Antioxidant, antiapoptotic | Posttreatment | ↓Cell apoptosis ↓TBARS ↓KIM-1 ↑Cell viability ↑GSH Inhibit NADPH oxidase 4 |
[111] | |
|
| |||||||
| Acai fruit extract | Euterpe oleracea | Male Wistar albino rat | Bilateral ischemia for 45 min followed by 6 h reperfusion | Antioxidant, antiinflammatory | Oral route pretreatment | ↓SCr, BUN, renal KIM-1 ↓MDA, MPO, IFN-γ, caspase-3 ↓Collagen IV, endothelin-1 ↑IL-10 |
[112] |
| Aloperine | Sophora alopecuroides | C57BL/6 mice RAW264.7 and HK2 cells | Bilateral ischemia for 45 min followed by 24 h reperfusion | By regulating PI3K/Akt/mTOR signaling and NF-κB transcriptional activity | ↓IFN-ϒ, IL-1β, caspase-3 ↓NF-κB expression (in-vitro & in-vivo) ↓PI3K/AkT/mTOR pathway signaling ↑IL-10, SOD |
[113] | |
|
| |||||||
| Apigenin | Celery parsley wheat sprouts | Male Sprague Dawley rat NRK-52E cells | Bilateral ischemia for 45 min followed by 24 h reperfusion | Activation of the JAK2/STAT3 pathway | Intraperitoneal route pretreatment | ↓SCr, BUN, MDA ↑SOD and GPx levels in vivo and in vitro ↑JAK2 and STAT3 phosphorylation in vivo and in vitro ↑Bcl-2 and procaspase-3 expression ↓Bax and caspase-3 expression |
[114] |
|
| |||||||
| Aqueous extract | Cuscuta chinensis (seeds) | Male Sprague Dawley rat | Bilateral ischemia for 45 min followed by 4 days reperfusion | By upregulation of water channels and Na+K+ ATPase | Oral route Posttreatment | ↓Urine volume ↑Urine osmolality ↑Urinary Na+, K+, Cl− ↑Creatinine clearance ↑AQP-2, AQP-3 expression ↑Na+K+ ATPase |
[115] |
|
| |||||||
| Aqueous extract | Murraya koenigii (leaves) | Male Wistar albino rat | Unilateral ischemia in the left kidney for 60 minutes followed by reperfusion | Antioxidant | Oral route pretreatment and posttreatment | ↓SCr, BUN, MDA, MPO ↓Proteinuria ↑SOD, CAT, GSH |
[116] |
|
| |||||||
| Arctigenin | Arctium lappa (fruit) | Male C57BL/6 mice | Bilateral ischemia for 30 min followed by 24 h reperfusion | Anti-inflammatory effect | Oral route pretreatment | ↓IL-1β, IL-6, TNF-α ↓IL-10 ↓TLR4/Myd88 protein expression ↓NF-κB expression |
[117] |
|
| |||||||
| Ascorbic acid | Potatoes, green leafy vegetables, root vegetables, citrus fruits etc. | Male Sprague Dawley rat | Right nephrectomy with left renal ischemia for 45 min followed by 3 h reperfusion | Inhibiting oxidative stress | Intraperitoneal route pretreatment | ↓SCr, BUN, LDH, MDA ↓Renal histopathological score ↑GSH |
[118, 119] |
| Berberine | Berberis vulgaris, Hydrastis canadensis, Coptis chinensis, Arcangelisia flava, B. aquifolium, B. aristata | Male Wistar albino rat | Right renal nephrectomy and left kidney ischemia for 45 min followed by 4 weeks reperfusion | Nephroprotection by caspase mitochondria dependent pathway | Oral route posttreatment | ↓SCr, BUN, MDA, MPO ↓Na-K-ATPase and Ca-ATPase ↓KIM-1 and TNF-α mRNA expression ↓Bax, ↓Caspase-3 mRNA expression ↑Renal SOD and GSH ↑Bcl2 mRNA expression |
[30] |
| Betulinic acid | Betula alba (bark) | Right nephrectomy with left renal ischemia for 45 min followed by 6 h reperfusion | Inhibit leukocyte apoptosis and upregulation of Na+K+ ATPase | Intraperitoneal route pretreatment | ↓SCr, BUN, LDH, MDA ↓TNF-α, MPO ↓Leukocyte apoptosis ↑Na+K+ ATPase, GSH |
[120] | |
| Caffeic acid | Commonly found in grains, fruits, and dietary add-ons as simple esters with quinic acid or saccharides | Bilateral ischemia reperfusion for 90 minutes followed by reperfusion for 24 hours | Inhibit 5-lipoxygenase pathway. Anti-inflammatory and antioxidant effect | Oral route pretreatment | ↓SCr, BUN, MDA, ↓TNF α ↑Catalase, SOD |
[121] | |
|
| |||||||
| Cannabidiol | Cannabis sativa | Male Sprague Dawley rat | Bilateral ischemia for 30 min followed by 24 h reperfusion | Antioxidant, anti-inflammatory, antiapoptotic activity | Intravenous pre- and posttreatment | ↓SC, MDA, NO ↓iNOS, TNF-α, COX-2 ↓ NF-κB, FasL caspase-3 ↑GSH |
[122] |
|
| |||||||
| Curcumin | Curcuma longa | Male Wistar albino rat | Bilateral ischemia for 45 min followed by 24 h days reperfusion | Antioxidant, free radical scavenging | Oral route pretreatment | ↓SCr, BUN, cystatin C ↓MDA, NO ↓Total oxidant status ↑SOD, GPx (serum and renal) ↑Catalase (renal) ↑Total antioxidant capacity |
[123] |
| Dioscin | Dioscorea nipponica | Male Sprague Dawley rat NRK-52E and the HK-2 cells | Bilateral kidney ischemia for 45 min followed by 24 h reperfusion | Inhibiting theTLR4/MyD88 signaling pathway via upregulation of HSP70 | ↓SCr, BUN ↓TLR4, MyD88, TRAF6 in vitro and in vivo ↓IL-1, IL-6, TNF-α, ICAM-1 ↓IFN-ϒ in vitro and in vivo ↑HSP 70 in vitro and in vivo ↑Cell viability |
[124] | |
|
| |||||||
| Epigallocatechin gallate | Camellia sinensis | Male Sprague Dawley rat | Right nephrectomy and left kidney ischemia for 45 min followed by 24 h reperfusion | Anti-inflammatory suppressing NF-κB decreasing apoptosis | Intraperitoneal route pretreatment | ↓SCr, BUN ↓TNF-α, IL-6, IL-1β ↓Cleavage caspase-3 and Bax ↑Caspase-3 and BCL-2 Suppressing NF-κB |
[125] |
| Ethanolic extract | Apium graveolens (leaves and stem) | Bilateral ischemia for 45 min followed by reperfusion | Antioxidant and anti-inflammatory | Oral route pretreatment and posttreatment | ↓SCr, urea ↑SOD and nitrogen oxide level |
[126] | |
| Crateva nurvala (leaves and bark) | Male Wistar albino rat | Unilateral ischemia for 1 hour followed by 24 h reperfusion | Antioxidant and anti-inflammatory | Oral route pretreatment | ↓SCr, BUN, LDH, MDA ↑NR-F2, GSH ↓TNF-α, IL-6, caspase-3 |
[27] | |
| Sonchus oleraceus | Bilateral ischemia for 45 min followed by 15 h reperfusion | Antioxidant and anti-inflammatory | Pretreatment | ↓SCr, BUN, MDA ↑SOD ↓IL-6, IL-1β, TNF-α |
[110] | ||
| Brassica rapa (roots) | Bilateral ischemia for 60 min followed by 24 h reperfusion | Anti-inflammatory antioxidant | ↓SCr, urea, uric acid ↓Renal tissue hemorrhage ↓Necrosis and tubular distention |
[127] | |||
| Hypericum perforatum (flowering herb) | Male Sprague Dawley rat | Left renal ischemia for 45 min with right renal nephrectomy followed by 3 h reperfusion | Antioxidant and anti-inflammatory | Intraperitoneal route pretreatment | ↓SCr, BUN, MDA ↑SOD, catalase, GPx |
[105] | |
| Petroselinum cripum | Male Wistar albino rat | Bilateral renal ischemia for 30 min followed by reperfusion for 24 hours | Attenuating oxidative stress and inflammation | ↓SCr, BUN, MDA, ↓NO, ICAM-1, TNF-α ↓Leukocyte infiltration rate ↑Glomerulus dimeter, FRAP level |
[128] | ||
| Salvia miltiorrhiza | Bilateral ischemia for 60 min followed by 30 min reperfusion | Antioxidant anti-inflammatory | Oral route pretreatment | ↓SCr, BUN, MDA ↓IL-6, IL-8, TNF-α ↑GSH, SOD, catalase, GPx |
[129] | ||
| Tribulus terrestris | Male Sprague Dawley rat | Bilateral ischemia for 30 min followed by 24 h reperfusion | Antioxidant | ↓SCr, BUN, MDA ↓Na+, K+ excretion, FRAP ↑Urine osmolality, ↑Creatinine clearance |
[130] | ||
| Dalbergia ecastaphyllum | Male Wistar albino rats | Right nephrectomy and left kidney ischemia for 60 min followed by 48 h reperfusion | By reducing oxidative stress, eNOS, and heme-oxygenase upregulation | ↓SCr, serum urea ↓Renal and urine MDA, GSH Restores eNOS expression ↑Heme-oxygenase expression |
[131] | ||
|
| |||||||
| Ferulic acid | Commelinid plants, grasses, grains, vegetables, flowers, fruits, leaves, beans, seeds of coffee, artichoke, peanut, and nuts | Male C57/BL6 mice | Bilateral ischemia for 35 min followed by 24 h reperfusion | Increasing adenosine generation via HIF-1α | Oral route pretreatment | ↓SCr, BUN, caspase-3 ↓IL-1β, TNF-α, MPO activity ↑Adnesine activity ↑CD 73, CD 39 ↑HIF-1α mRNA |
[132] |
| Garlic juice | Allium sativum (bulbs) | Male Wistar albino rats | Right nephrectomy with left renal ischemia for 45 min followed by 24 h reperfusion | Antioxidant, antiapoptotic | ↓SC, urea, FENa+, FEK+ ↓Renal histopathological score ↑Urinary creatinine |
[133] | |
| Garlic oil | Bilateral ischemia for 45 min followed by 6 h days reperfusion | Antioxidant, anti-inflammatory | ↓SC, Urea, Cystatin C ↓Oxidative stress index ↓MPO, NO |
[134] | |||
| Ginger | Zingiber officinale | Right nephrectomy with left renal ischemia for 45 min followed by 24 h reperfusion | Antioxidant, anti-inflammatory | ↓SC, urea, FENa+ ↑Urinary creatinine and urea ↑Urinary K+ excretion ↓Renal histopathological score |
[135] | ||
| Ginkgo biloba EGb761 extract | Ginkgo biloba | Unilateral ischemia in left kidney for 60 minutes followed by 60 minutes of reperfusion | Antioxidant | ↓MDA ↓Necrosis and cast formation ↑SOD, CAT |
[136] | ||
| Hydroalcoholic extract | Rosa canina (fruits) | Bilateral ischemia for 45 min followed by 24 h reperfusion | Antioxidant, anti-inflammatory | ↓SCr, BUN ↓Renal tissue histopathological score |
[137] | ||
| Crocus sativus | Bilateral ischemia for 30 min followed by 24 h reperfusion | Antioxidant and anti-inflammatory | ↓SCr, BUN, MDA ↓Lymphocytes infiltration ↓TNF-α, ICAM-1 mRNA expression ↑FRAP |
[138] | |||
| Juglans mollis (bark) | Bilateral renal ischemia for 45 min followed by 15-hour reperfusion | Antioxidant and anti-inflammatory. | ↓SCr, BUN, MDA ↑SOD ↓TNF-α, IL-1β, IL-6 |
[109] | |||
| Lavender oil | Lavandula angustifolia | Right nephrectomy followed by renal ischemia for 45 min followed by reperfusion for 24 hours | Attenuating oxidative stress and inflammation | Intraperitoneal posttreatment | ↓SCr, BUN, MDA ↓TNFα, IL1β, IL10 ↓Apoptotic cells ↓Total histopathological score ↑SOD, GPx, catalase |
[100] | |
|
| |||||||
| Liposomes containing curcumin | Curcuma longa | Male C57BL/6 mice | Bilateral ischemia for 30 min followed by 24 h reperfusion | Targeted cellular delivery to renal tubular epithelial cells and antigen presenting cells conferring protection from IR injury, mediated by NF-kB | Intravenous pretreatment | Suppress NF-кB activity ↓SC, urea, ↓Renal histopathological score ↓TNF-α, CCL5, CCL2, CXCL2 ↓iNOS ↑SOD |
[139] |
|
| |||||||
| Luteolin | Carrots, peppers, celery, olive oil, peppermint, thyme, rosemary, and oregano | Male Swiss albino mice | Bilateral ischemia | Antioxidant and anti-inflammatory | Pretreatment | ↓SCr, BUN, MDA ↑SOD, CAT, GSH ↓TNF-α, IL-1β, IL-6 ↓Bax and caspase-3 expression ↑Bcl-2 expression |
[106] |
| Lycopene | Tomato, apricots, papaya, pink grapefruit, guava, and watermelon | Bilateral renal ischemia for 30 min followed by reperfusion for 2 hours | Antioxidant and anti-inflammatory | Intraperitoneal pretreatment | ↓ Scr, blood urea, plasma NGAL ↓Tissue Bax concentration ↓F21sop, ↓Notch2/HeS1 ↓Renal TLR 2, renal IL-6 |
[140] | |
|
| |||||||
| Mangiferin | Mangifera indica also present in 16 other plant families including Anacardiacae, Gentianaceae, and Iridaceae | Male C57/BL6-mice | Left nephrectomy and right kidney ischemia for 30 min followed by 24 h reperfusion | Anti-inflammation and inducing adenosine production | Oral route pretreatment | ↓SCr, BUN, ↓Plasma potassium (K+) ↓ Caspase-3 mRNA expression ↓TNF-α, IL-1β ↓NO, MPO ↑Adenosine and CD73 expression |
[141] |
|
| |||||||
| Methanolic extract | Aruncus dioicus (whole plant) | Male Sprague Dawley rat | Right nephrectomy with left renal ischemia for 40 min followed by 24 h reperfusion | Antioxidant, antiapoptotic | Intraperitoneal route pretreatment | ↓SC ↓Renal tissue histopathological score ↓Bcl-2/Bax ratio |
[142] |
| Cassia mimosoides var. Nomame | Right nephrectomy with left renal ischemia for 40 min followed by 24 h reperfusion | Antioxidant | ↓SC ↓Caspase-3 ↓Bcl-2/Bax ratio |
[143] | |||
| Stevia rebaudiana | Left renal ischemia for 45 min with right renal nephrectomy | Antioxidant and anti-inflammatory | Oral pretreatment | ↓SCr, FENa+, Creatinine clearance ↓MDA ↑GSH, Catalase |
[144] | ||
| Benincasa cerifera (fruits) | Female Wistar albino rat | Bilateral ischemia for 60 min followed by 6 h reperfusion | Free radical scavenging activity | ↓SCr, BUN, uric acid, MDA ↑SOD, catalase, GSH |
[145] | ||
| Nigella sativa oil | Nigella sativa (seeds) | Male Wistar albino rat | Bilateral ischemia for 60 min followed by 24 h reperfusion | Antioxidant, free radical scavenging. | ↓SCr, BUN, cystatin c. ↓MDA, NO ↓Renal histopathological score ↓Total oxidant status ↑SOD, GPx (serum and renal) ↑Catalase (renal) ↑Total antioxidant capacity |
[146] | |
| Oleanolic acid | Olea europaea, Viscum album, Aralia chinensis, >120 other plant species | Bilateral renal ischemia for 45 min followed by 6 h reperfusion | Antioxidant, anti-inflammatory reductions in Nrf-2 | Intraperitoneal route pretreatment | ↓SCr, BUN, renal KIM-1 ↓LDH, MDA, caspase-3 expression ↓IFN-γ, IL-6, MPO ↑SOD, GST, GPx and CAT ↑IL-10 ↑Nrf-2 |
[85] | |
| Osajin | Maclura pomifera | Unilateral ischemia in left renal artery for 60 min followed by 10 min reperfusion | Antioxidant | Oral route pretreatment | ↑SOD, GSH-Px (serum) ↑Total antioxidant capacity ↓Renal histopathological score effectively at a dose of 10 mg |
[147] | |
| Osthole | Cnidium monnieri, Angelica pubescens, Peucedanum ostruthium | Right nephrectomy and unilateral ischemia in left kidney for 45 min followed by 1, 6, and 24 h reperfusion | Antioxidant, antiapoptotic | ↓SCr, BUN ↓Caspase-3 ↑SOD, CAT ↑Bcl-2/Bax ratio |
[148] | ||
| Picroliv | Picrorhiza kurrooa (roots and rhizome) | Male Sprague Dawley rat | Unilateral ischemia in left renal artery for 60 min followed by 5, 60, 120, and 240 min reperfusion | Antioxidant, antiapoptotic | ↓MDA (maximal at 120 min) ↑GSH (maximal at 240 min) ↑GPx, SOD ↓NO ↓Renal ICAM-1 ↓Apoptosis |
[149] | |
| Piperine | Piper nigrum (seeds) | Male Wistar albino rat | Bilateral renal ischemia for 30 min followed by reperfusion for 24 hours | Attenuating oxidative stress and inflammation | ↓SCr, BUN, MDA ↓TNF-a and ICAM-1 mRNA expression ↓Total histopathological score ↑Total FRAP level |
[104] | |
| Polysaccharide extract | Dipsacus asperoides (roots) | Bilateral ischemia for 45 min followed by 24 h reperfusion | Antioxidant | ↓SCr, BUN, LDH, MDA ↑SOD, CAT, GPx |
[150] | ||
|
| |||||||
| Polydatin | Polygonum cuspidatum (roots) | Male BALB/c mice primary renal tubular epithelial cells (RTECs) | Unilateral ischemia in left kidney for 30 min followed by reperfusion | Antioxidative stress and anti-inflammation by activating the sonic Hedgehog (SHH) signaling pathway | Intraperitoneal route pre and posttreatment | ↓Caspase-3 expression ↑Shh mRNA expression (in vivo and in vitro) ↑SOD, GST, GPx, and CAT (in vivo) |
[107] |
|
| |||||||
| Polyphenols | Camellia sinensis | White male rabbit | Bilateral ischemia for 30, 60, 90, and 120 min, followed by 24 h reperfusion | Antioxidant, antinecrotic | Intravenous route pretreatment | ↓SCr, BUN ↓Renal histopathological score ↓Immune peroxidase labeling of CD8+T cells in kidney tissues All the results were found significant at 90 min of ischemia |
[151] |
|
| |||||||
| Polysaccharide peptide | Ganoderma lucidum (fruits) | Male C57BL/6J mice NRK-52E cells | Left nephrectomy and right kidney ischemia for 35 min followed by 24 h reperfusion | Counteracting oxidative stress | Intraperitoneal route pretreatment | ↓SCr, BUN ↓MPO, MDA ↓Bax/Bcl-2 ratio, ↑SOD, CAT, GSH and GPx ↑Cell viability |
[152] |
|
| |||||||
| Polysaccharides | Lycium barbarum | Wistar albino rat | Bilateral renal ischemia for 45 min followed by 24 h reperfusion | Antioxidant and anti-inflammatory inhibiting apoptosis | Oral route pretreatment | ↓SCr, Serum urea, MDA ↑SOD, ↓Serum IL-1β, TNF-α Enhanced renal expression of Bcl-2 mRNA |
[28] |
| Proanthocyanidin | Grape seed | Male Sprague Dawley rat | Bilateral ischemia for 60 min followed by 6 h reperfusion | Decreasing oxidative and nitrosative stress | ↓SCr, BUN, AST ↓MDA, NOx ↓Renal histopathological score ↑SOD, GPx |
[153] | |
|
| |||||||
| Pycnogenol | Pinus maritima (fresh bark) | Male Wistar albino rat | Right nephrectomy with left renal ischemia for 45 min followed by 3 h reperfusion | Antioxidant, anti-inflammatory; inhibit neutrophil infiltration | Intraperitoneal route pretreatment | ↓SCr, BUN, MDA ↓TNF-a, IL-1β, and IL-6 ↓MPO ↓Renal histopathological score ↑GSH, Na+K+ ATPase |
[154] |
| Quercetin | Apples, berries, Brassica vegetables, capers, grapes, onions, shallots, tea, tomatoes seeds, nuts, flowers, barks, and leaves | Male Swiss albino mice | Bilateral renal ischemia for 30 min followed by reperfusion for 24 hours | Attenuating oxidative stress and inflammation | ↓Blood urea, SCr, plasma NGAL ↑BCl-2 level ↓Tissue Bax concentration ↓F2-isoprostane ↓Renal notch-1 jagged-1 level |
[155] | |
|
| |||||||
| Resveratrol | Grapes, wine, peanuts, soy | Male Wistar albino rat | Bilateral ischemia for 40 min followed by 24 h reperfusion | Antioxidant, free radical scavenging | Intravenous route pretreatment | ↓Mortality rate ↓SCr ↓TBARS ↓Renal histopathological score ↑NO |
[156, 157] |
| Rhizome extract | Coptidis japonica (rhizome) | Bilateral ischemia for 60 min followed by 6 and 24 h reperfusion | Antioxidant | Oral route pretreatment | ↓SCr, BUN, MDA ↑SOD, catalase ↑GSH (renal) ↓DNA fragmentation rate |
[17] | |
|
| |||||||
| Rosmarinic acid | Rosmarinus officinalis, Melissa officinalis | Male Sprague Dawley rat | Right nephrectomy and ischemia in the left kidney for 60 min followed by 60 min reperfusion | By decreasing oxidative stress | Intraperitoneal route pretreatment | ↓MDA, MPO ↑SOD, GPx |
[158, 159] |
| Rutin | Buckwheat and many vegetables, fruits, beverages such as tea and wine | Male Wistar albino rat | Right nephrectomy with left renal ischemia for 45 min followed by 3 h reperfusion | Decreasing oxidative stress, anti-inflammatory | ↓SCr, BUN, LDH, MDA ↓Renal histopathological score ↑GSH, MnSOD |
[160] | |
|
| |||||||
| Sesamin | Sesamum indicum (seed and oil) | Male C57/BL6 mice | Left renal nephrectomy and right kidney ischemia for 30 min followed by 24 h reperfusion | Inhibiting tubular cell death and inflammatory response, upregulating CD39-adenosine-A2AR signals | Oral Pre-treatment | ↓SCr, BUN, MDA ↓Caspase-3 expression ↓Infiltration of Ly6G+ neutrophils ↓MPO activity ↓TNF-α, IL-1β ↑Adenosine level |
[161] |
| Silymarin | Silybum marianum | Male Sprague Dawley rat | Right nephrectomy with left renal ischemia for 45 min followed by 6 h reperfusion | Anti-inflammatory, antinecrosis, free radical scavenging | ↓Tubular dilatation ↓Tubular vacuolization ↓Inflammation ↓Tubular and glomerular necrosis |
[162, 163] | |
| Total flavonoids | Rosa laevigata | NRK-52E cells male Sprague Dawley rat | Bilateral renal ischemia for 45 min followed by reperfusion for 24 h | Attenuating oxidative stress and inflammation | ↓SCr, BUN, MDA, ↓Levels of Keap 1 and NF-KBP65 ↓IL-1β, IL-6, TNF-α ↑Sirt 1, Nrf 2, and HO 1 ↑GSH, GPx, SOD |
[108] | |
|
| |||||||
| Ursolic acid | Crataegus sp., Arctostaphylos uva-ursi, Chinese elder herb, Actinidia deliciosa, Prunella vulgaris | Male Sprague Dawley rat | Right renal nephrectomy and left kidney ischemia for 45–90 minutes | Decrease in oxidative stress. Suppressing STAT3, NF-κB and caspase-3 activities. | Posttreatment | ↓SCr, Angiotensin II ↓STAT3 protein phosphorylation ↓NF-κB expression Inhibit caspase-3 activity |
[164] |
4. Mechanisms by Which Plants Ameliorated Renal I/R Injury-Induced AKI
4.1. Increasing Antioxidant Levels
Plant-derived extracts and natural compounds ameliorated kidney I/R injury-induced AKI by increasing the levels of antioxidant enzymes and antioxidants (Table 1). These enzymes include superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx). The SOD provides natural defense against oxidative stress as it converts O2 into H2O2 (equation (1)). H2O2 does not contain any unpaired electrons and as such is not a free chemical radical. However, H2O2 can penetrate easily into cells and act as a poor oxidizing agent. The catalase and GPx then detoxify H2O2 into H2O, O2, and H2O, respectively (equation (2) and (3)) [165, 166].
| (1) |
| (2) |
| (3) |
A number of plant-derived extracts and natural compounds (Table 1) are able to increase the levels of these antioxidant enzymes, thereby providing protection against RIRI-induced AKI. There are numerous plants, which increase the levels of SOD as shown in example (Table 1) [28, 30, 109, 110, 126]. Similarly, some plant extracts were reported to increase both SOD and catalase levels [106, 116, 121, 136, 148], while others were reported to increase all the three antioxidant enzymes SOD, catalase, and GPx [85, 105, 107, 123, 129, 146, 150, 152]. It is therefore concluded that different plants exhibited their own mechanism of protection (Table 1).
Antioxidant compounds such as glutathione (GSH) modify the cell response against ROS generation in I/R injury-induced AKI. GSH plays an important role in both the detoxification of drug metabolites and the regulation of gene expression and apoptosis [167]. Depletion of GSH levels lead to an increase in oxidative stress and is directly associated with I/R injury [168]. A number of plants as shown in Table 1 were reported to increase the levels of GSH, thereby protecting kidney from ROS generated by I/R injury [27, 111, 118, 120, 122] (Table 1), and were reported to increase the levels of GSH, SOD, and catalase [116, 145], while some others have been found to increase GSH, GSH-Px, SOD, and catalase [152]. Thus, plants exhibited versatile mechanisms of protection.
4.2. Decreasing Anti-Inflammatory Cytokines
The dying and injured cells as a consequence of renal I/R injury release proinflammatory cytokines such as interleukins and tumor necrosis factor (TNF) and chemotactic cytokines (CCL5, CCL2, and CXCL2). There is also an activation of some transcription factors such as heat shock proteins (HSP), high mobility group box-1 (HMGB1), and hypoxia-inducible factor-1 (HIF1) [169, 170]. These factors are responsible for the stimulation of cell surface receptors, which in turn triggers inflammatory and cytotoxic reactions [171].
Plant-derived extracts and natural compounds target proinflammatory cytokines to halt inflammation and ameliorate renal I/R injury-induced AKI. The main inflammatory cytokines and chemokines which are decreased by plant-derived extracts include tissue necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), interleukin-10 (IL-10), interleukin-6 (IL-6), interleukin-8 (IL-8), and interferon gamma (IFN-γ) [172]. Natural compounds that decrease the levels of TNF-α, IL-1β, and IL-6 include arctigenin [117], luteolin [106], epigallocatechin gallate [125], pycnogenol [154], and ferulic acid [132] as well as plant-derived extracts such as Juglans mollis [109] and Sonchus oleraceus [110] (Table 1).
The TNF-α induces proinflammatory effects by activating transmembrane TNF-α receptors, causing the stimulation of the nuclear factor-κB (NF-κB). NF-κB is responsible for the expression of over 400 genes including cyclooxygenase-2, lipoxygenase-2 (LOX-2), inducible nitric oxide synthase (iNOS), and the transcription of inflammatory cytokines and chemokines [173, 174]. The peak levels of NF-κB in rat models of renal I/R injury AKI were found after 15 minutes of reperfusion [173]. Therefore, inhibiting the NF-κB-mediated inflammatory pathway is another approach to ameliorate I/R injury induced-AKI inflammation [175]. Many plant-derived compounds were found to inhibit or decrease the levels of NF-κB expression such as ursolic acid [164], epigallocatechin gallate [125], aloperine [113], arctigenin [117], and cannabidiol [122] (Table 1). The effects of renal ischemic reperfusion (I/R) induced acute kidney injury (AKI) and administration of plant-derived extracts and compounds on kidney have been illustrated in Figure 1.
Figure 1.
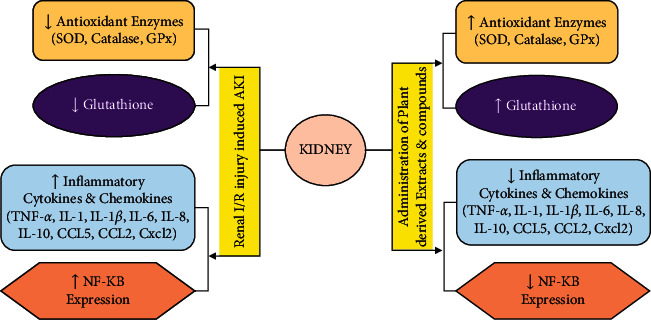
Effect of renal ischemic reperfusion (I/R) induced acute kidney injury (AKI) and administration of plant-derived extracts and compounds on kidney. The upward arrow shows “increase,” and downward arrow direction shows “decrease.”
4.3. Increasing Adenosine Levels
Adenosine is an endogenous nucleotide composed of adenine and ribose. Adenosine plays an important role against hypoxia as a result of renal I/R injury [176, 177]. Adenosine regulates essential kidney functions such as the glomerular filtration rate (GFR), renin release, and tubular glomerular feedback mechanisms [178]. Adenosine produces its effects on kidney via the stimulation of adenosine receptors (AR), which has four subtypes (A1AR, A2AAR, A2BAR, and A3AR). Necrosis, apoptosis, and inflammation due to renal I/R injury induced-AKI are reduced by adenosine via stimulation of the A1AR receptor [179]. Adenosine has 100 times more affinity to bind with A1AR and A2AR receptors compared to the other two subtypes of AR receptors. Stimulation of both A1AR and A2AR receptors play a role in controlling inflammation after renal I/R injury-induced AKI [180].
Various plant-derived extracts and compounds (Table 1) were reported to increase the levels of adenosine activity. Pretreatment with sesamin [161], mangiferin [141], and ferulic acid [132] remarkedly increased adenosine levels in the I/R treatment group compared to the renal I/R injury AKI group. Increases in adenosine levels were accompanied by a decrease in caspase-3 expression and inflammatory cytokine levels, which shows a reduction in apoptosis, necrosis, and inflammation, respectively.
4.4. Other Mechanisms
The phosphatidylinositol-3-kinase (PI3K)/Akt/mTOR pathways play a significant role in cell survival processes such as apoptosis, metabolism, and angiogenesis. These pathways are so interrelated that, in many circumstances, they are regarded as a single pathway [181, 182]. The PI3K/Akt/mTOR signaling is assumed to be involved in renal I/R injury-induced inflammation [183, 184]. Among the compounds listed in Table 1, only aloperine was found to regulate the PI3K/Akt/mTOR pathway and have markedly reduced its signaling compared to renal I/R injury AKI group [113].
Another pathway known as Janus kinase/signal transducers and activators of transcription (JAK/STAT) is essential for growth hormone and other cytokine signaling. The cytokines bind to their receptors and activates JAK, which then causes the phosphorylation of STAT. The STATs are then transferred into the nucleus where they initiate target gene expression [185, 186]. The JAK/STAT pathway is supposed to be involved in renal I/R injury [187]. Among the JAK/STAT pathways, the subtype JAK2 signaling via STAT1 and STAT3 is the best studied in diseases affecting kidney [188]. Among the natural compounds enlisted in Table 1, apigenin [114] and ursolic acid [164] have been found to ameliorate renal I/R injury induced-AKI via action on the JAK2/STAT3 pathway.
The sonic Hedgehog (Shh) is a glycoprotein and a key ligand of the Hedgehog pathway. It has an important role in cell differentiation and apoptosis and governs the embryonic development. The Shh signaling has diffused effects on various organ systems [189, 190]. The Shh signaling is also known to have a role in kidney development and tissue repair after injury. Its release is often induced in postischemia of a tissue and regulates important biological processes, for instance, antiapoptosis and antioxidant effects, thereby promoting tissue repairing [191, 192]. Polydatin (Table 1) ameliorates renal I/R injury induced-AKI by activating the Shh signaling pathway [107]. This latter study suggests that the Shh pathway can constitute a new target to treat renal I/R injury induced-AKI in the future.
Heat shock proteins (HSPs) regulate normal cell functions in kidney after I/R injury. Among HSPs, HSP70 is the most studied molecule as it has cytoprotective characteristics and is often chosen as a therapeutic target. The HSP70 is known to be involved in anti-inflammatory and antiapoptotic effects and in the stimulation of regulatory T-cells in renal I/R injury [193]. Among the plant-derived compounds (Table 1), dioscin has been found to ameliorate renal I/R injury-induced AKI via the upregulation of HSP70. Upregulation of HSP70 results in the inhibition of TLR4/MyD88 signaling and cyclooxygenase-2 (COX-2) pathways [124].
5. Effects on Biomarkers and Renal Tissues
Renal I/R injury or other diseases such as hypertension, genetic disorders, infections or toxins leads to AKI, which ultimately causes a decrease of the renal function. Biomarkers of the renal function are assessed to evaluate the severity of kidney injury, identify risk factors, and more importantly to analyze responses towards the applied therapies [194]. Serum creatinine (SCr) is still the gold standard biomarkers to diagnose the AKI according to recent KDIGO classification system [65]. The majority of the plant-derived extracts and compounds listed in Table 1 decrease the levels of SCr and blood urea nitrogen (BUN). However, there is accumulative evidence in the literature which shows that SCr and BUN have many limitations and are therefore considered as suboptimal biomarkers [195–197]. Cystatin C, a renal biomarker, has many advantages over creatinine. It is a small size protein (13 kDa) and is generated by all nucleated cells, as compared to SCr which is reliant on muscle mass [198]. For patients displaying muscular complications, cystatin C offers a clinical edge over creatinine [199]. Cystatin C has also limitations as its level increases in conditions like hyperthyroidism, during corticosteroid use and when there is a high cell turnover [200]. Plant extracts or compounds showing an effect on cystatin C levels include Nigella sativa oil [146] and curcumin [123] (Table 1). The biomarkers NGAL and KIM-1 are considered to diagnose AKI in its early stages compared to other surrogate biomarkers [201, 202]. Among the plant-derived extracts and compounds which lowered NGAL and KIM-1 levels are quercetin [155], lycopene [140] and α-bisabolol [29], oleanolic acid [85], as well as acai fruit extracts [112], respectively (Table 1).
The renal I/R injury induced-AKI causes necrosis, apoptosis, hemorrhages, vascular congestion, inflammatory cell infiltration, cellular edema, and other degenerative changes in kidney tissues [27, 104, 131, 148]. Plant-derived extracts and compounds have shown marked effects on kidney tissues, almost all the plants effectively lowered the total renal histopathological scores (Table 1).
6. Discussion
Plants displaying a nephroprotective capability against I/R-induced-AKI have been summarized (Table 1). According to Amin and Khan (2016), natural products represent an incredible source for the development of new molecules for drug discovery and development. Moreover, since the last three decades, 35% of the newly developed molecules are derived from natural sources. Plants having therapeutic potential are being used for numerous pathological conditions for many centuries [203, 204].
I/R injury is considered as one of the major clinical problems for clinicians, specifically during hospital surgeries often leading to loss of function in tubular epithelial cells and causing AKI accompanied by other complications. AKI induced by I/R injury includes oxidative stress in addition to activation of immune responses and upregulation of cytokines and chemokines [205]. Kidneys are very vulnerable to the I/R effects, progressive injury, and unmet oxygen requirements resulting in dehydration as well as electrolytic imbalance with increased morbidity and mortality [14]. Renal I/R injury induced-AKI causes a series of biochemical and pathophysiological changes that are reflected as alterations in the levels of biomarkers used clinically for the diagnosis and the monitoring of the general health status of the patients. Research involving the use of pharmacologically active molecules in the prevention and treatment of ischemic pathologies uses biomarkers as assessment tools. However, it is important to recognize their applications, limitations, and their proper interpretation before using them [206].
Almost all the plants included in this review possess activity against I/R-induced-AKI by reducing serum creatinine in addition to blood urea nitrogen (BUN), while many others decrease the cystatin C levels, for instance, garlic oil, Nigella sativa oil, and curcumin (Table 1). Similarly, NGAL and KIM-1 are lowered by quercetin, lycopene and α-bisabolol, oleanolic acid, as well as acai fruit extracts, respectively (Table 1). The RIRI-induced AKI causes severe renal histopathological changes. The major renal histopathological changes that were observed in most of the studies (Table 1) were cell vacuolization, interstitial hemorrhage foci, glomerular congestion, inflammatory infiltrate, cellular damage in loop of Henle, moderate to severe necrosis, hyaline cast, and loss of brush border [26, 118, 119, 133, 135]. In agreement with the biochemical results, almost all the plants effectively lowered the total renal histopathological score as well. The oral route is the most desired route of drug administration because it has many benefits, e.g., ease of administration, patient compliance, and flexibility in dosage form. Majority of the plant extracts and compounds (Table 1) were administered via oral route. Moreover, most of the active pharmaceutical products are being used orally. All these plant extracts and derivative compounds have the potential to be developed as a therapeutic entity that can be administered orally for the treatment of RIRI-induced AKI.
The plant-derived extracts and compounds (Table 1) ameliorate renal I/R injury via different mechanisms, but the antioxidant and anti-inflammatory mechanisms were found as the most prominent ones. The oxidative stress levels were reduced mostly via increasing the levels of the endogenous antioxidants such as the total flavonoids from Rosa lavigata Michx fruit, which reduced in vivo oxidative stress by increasing SOD, GSH, and GSH-Px and decreasing MDA levels. Similar effects have been observed with caffeic acid which also increases catalase levels. Moreover, polysaccharides in Lycium barbarum, the bark of Juglans mollis, luteolin, ethanolic extracts of Hypericum perforatum, apigenin, oleanolic acid, berberine, and many others, as listed in Table 1, are some of the examples of plant-derived extracts and compounds having an effect against one or more of these oxidative stress parameters.
Because of the redox imbalance, a local and systemic inflammatory process is activated. Renal I/R injury initiates several inflammatory reactions within the renal parenchyma. Besides infiltration of neutrophils, renal I/R injury is accountable for the generation of many inflammatory cytokines, for instance, IL-6, IL-1, and TNF-α (Thurman, 2007). An anti-inflammatory activity was displayed by various plant extracts (Table 1) against inflammatory cytokines, e.g., the ethanolic extracts of Sonchus oleraceus decreased the IL-6, IL-1β, and TNF-α levels compared to I/R groups. Similar effects were also observed with leaf and bark extracts of Crateva nurvala, ferulic acid, arctigenin, luteolin, seasamin, mangiferin, epigallocatechin gallate, and ethanolic extracts of Salvia miltiorrhiza, while others display activity against one or more of these inflammatory parameters (Table 1).
This review demonstrated that I/R process causes a series of intracellular events altering homeostasis and renal function. These events are related to the production and the accumulation of ROS, which cause oxidative stress, thus altering important functions of energy metabolism such as mitochondrial transmembrane potential. These phenomena interrupt oxidative phosphorylation, inhibiting the production of ATP, and causing energy deficit and consequent cell death. This can lead to tissue damage that, if not reversed quickly, can cause chronic disease [111]. Natural antioxidant substances are shown to be nephroprotective and the mechanisms of their action are well described. The experimental data endorse the role of antioxidant therapy as observed from the studies summarized in Table 1. Further toxicological, pharmacological, and human studies are warranted to develop these entities into lead drugs or final therapeutic molecules.
7. Conclusion
Renal I/R injury is the leading cause of AKI, which is associated with high ratios of mortality and morbidity. Renal I/R injury is responsible for the generation of ROS and RNS leading to increase in oxidative and inflammatory stress. These stresses induce proinflammatory cytokines and depletion of antioxidant enzymes as well as antioxidant compounds, activating several pathways and leading to cell necrosis and apoptosis. Recently, several clinical biomarkers and molecular targets of AKI have been discovered. However, there is no defined biomarkers or targets, which can be used for the treatment of AKI. Various natural plant-derived therapies have shown best outcomes in animal studies. These plant-derived extracts and compounds have only been tested in selected transgenic animal models. To develop them into an efficacious therapeutic entity, they should be further tested in proper vertebrate multitransgenic animal models so that their human use can be validated. Many plant-derived extracts and compounds presented in this study have shown nephroprotective effects against renal I/R injury induced-AKI via elevating antioxidant activities, decreasing inflammatory cytokines, and increasing adenosine levels and activating other pathways. Future research should be focused on toxicity, pharmacokinetic profiling, and effective dose concentration for the design of clinical trials for the treatment of renal I/R induced-AKI.
Acknowledgments
The authors are thankful to the laboratory of “Pharmaceutical Bioprospecting and Clinical Biochemistry” in the department of pharmacology at Universidade Federal do Ceará (UFC), Brazil, for providing favorable environment for this study.
Abbreviations
- AKI:
Acute kidney injury
- AQP:
Aquaporin
- AST:
Aspartate amino transferase
- ATP:
Adenosine triphosphate
- BUN:
Blood urea nitrogen
- CKD:
Chronic kidney disease
- COX-2:
Cyclooxygenase-2
- eNOS:
Endothelial nitric oxide synthase
- ESRD:
End-stage renal disease
- FasL:
Fas ligand
- FENa+:
Fraction sodium excretion
- FRAP:
Tissue ferric-reducing antioxidant power
- GFR:
Glomerulus filtration rate
- GPx:
Glutathione peroxidase
- GSH:
Glutathione
- HIF-1α:
Hypoxia inducible factor-1 alpha
- HMGB1:
High mobility group box-1
- HO 1:
Heme-oxygenase
- HSP 70:
Heat shock protein 70
- I/R:
Ischemic reperfusion
- ICAM-1:
Intercellular adhesion molecule-1
- ICU:
Intensive care unit
- IFN-ɣ:
Interferon gamma
- IGFBP-7:
Insulin-like growth factor binding protein 7
- IL:
Interleukin
- iNOS:
Inducible nitric oxide synthase
- JAK2/STAT3:
Janus kinase 2/signal transducer and activator of transcription 3
- Keap 1:
Kelch-like ECH-associated protein-1
- KIM-1:
Kidney injury molecule 1
- LDH:
Lactate dehydrogenase
- MCP:
Monocyte chemoattractant protein
- MDA:
Malondialdehyde
- MPO:
Myeloperoxidase
- MPTP:
Mitochondrial permeability transition pore
- mRNA:
Messenger ribonucleic acid
- MyD88:
Myeloid differentiation primary response gene
- NADPH:
Nicotinamide adenine dinucleotide phosphate
- NF-KBP65:
Nuclear translocation of nuclear factor-Bp65
- NGAL:
Neutrophil gelatinase associated lipocalin
- NO:
Nitric oxide
- Nrf-2:
Nuclear factor erythroid 2-related factor-2
- NR-F2:
Nuclear factor erythroid 2-related factor 2
- PI3K/Akt:
Phosphatidylinositol-3-kinase
- RIRI:
Renal ischemic reperfusion injury
- RNS:
Reactive nitrogen species
- ROS:
Reactive oxygen species
- ROS:
Reactive oxygen species
- RRT:
Risk of renal replacement therapy
- SCr:
Serum creatinine
- Shh:
Sonic Hedgehog
- Sirt 1:
Silent information regulator factor 2-related enzyme 1
- SOD:
Superoxide dismutase
- TBARS:
Thiobarbituric acid reactive substances
- TIMP-2:
Tissue inhibitor of metalloproteinase-2
- TLR:
Toll-like receptors
- TNF:
Tissue necrosis factor
- TRAP 6:
Tumor necrosis factor receptor associated factor 6
- VCAM:
Vascular cell adhesion molecule.
Contributor Information
Haroon Khan, Email: haroonkhan@awkum.edu.pk.
Esra Küpeli Akkol, Email: esrak@gazi.edu.tr.
Data Availability
The data used to support the findings of this study are all included and available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.James M. T., Chawla L. S., Kimmel P. L. Acute kidney injury and chronic kidney disease. Chronic Renal Disease . 2020;12:397–409. doi: 10.1016/b978-0-12-815876-0.00026-7. [DOI] [Google Scholar]
- 2.Parameswaran P., Devarajan P. Critical Care Nephrology . Amsterdam, Netherlands: Elsevier; 2019. Cellular and molecular mechanisms of acute kidney injury; pp. 1194–1204. [Google Scholar]
- 3.Hsu R. K., McCulloch C. E., Dudley R. A., Lo L. J., Hsu C.-Y. Temporal changes in incidence of dialysis-requiring AKI. Journal of the American Society of Nephrology . 2013;24(1):37–42. doi: 10.1681/asn.2012080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue J. L., Daniels F., Star R. A., et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. Journal of the American Society of Nephrology . 2006;17(4):1135–1142. doi: 10.1681/asn.2005060668. [DOI] [PubMed] [Google Scholar]
- 5.Case J., Khan S., Khalid R., Khan A. Epidemiology of acute kidney injury in the intensive care unit. Critical Care Research and Practice . 2013;2013:9. doi: 10.1155/2013/479730.479730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang C.-H., Fan P.-C., Chang M.-Y., et al. Acute kidney injury enhances outcome prediction ability of sequential organ failure assessment score in critically ill patients. PLoS One . 2014;9(10) doi: 10.1371/journal.pone.0109649.e109649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medeiros P., Nga H. S., Menezes P., Bridi R., Balbi A., Ponce D. Acute kidney injury in septic patients admitted to emergency clinical room: risk factors and outcome. Clinical and Experimental Nephrology . 2015;19(5):859–866. doi: 10.1007/s10157-014-1076-9. [DOI] [PubMed] [Google Scholar]
- 8.White L. E., Hassoun H. T., Bihorac A., et al. Acute kidney injury is surprisingly common and a powerful predictor of mortality in surgical sepsis. Journal of Trauma and Acute Care Surgery . 2013;75(3):432–438. doi: 10.1097/ta.0b013e31829de6cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macdougall I. C., Richards T. Restricting red-cell transfusions in cardiac surgery: No increase in AKI. Journal of the American Society of Nephrology . 2019;30(7):1143–1144. doi: 10.1681/asn.2019050509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez F., Bonacasa B., Fenoy F. J., Salom M. G. Reactive oxygen and nitrogen species in the renal ischemia/reperfusion injury. Current Pharmaceutical Design . 2013;19(15):2776–2794. doi: 10.2174/1381612811319150014. [DOI] [PubMed] [Google Scholar]
- 11.Samimagham H. R., Kheirkhah S., Haghighi A., Najmi Z. Acute kidney injury in intensive care unit: incidence, risk factors and mortality rate. Saudi journal of kidney diseases and transplantation . 2011;22:464–470. [PubMed] [Google Scholar]
- 12.Yokota L. G., Sampaio B., Rocha E. P., Balbi A., Sousa Prado I., Ponce D. Acute kidney injury in elderly patients: narrative review on incidence, risk factors, and mortality. International Journal of Nephrology and Renovascular Disease . 2018;11:217–224. doi: 10.2147/ijnrd.s170203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nativ O., Bahouth Z., Sabo E., et al. Method used for tumor bed closure (suture vs. Sealant), ischemia time and duration of surgery are independent predictors of post-nephron sparing surgery acute kidney injury. Urologia Internationalis . 2018;101(2):184–189. doi: 10.1159/000490107. [DOI] [PubMed] [Google Scholar]
- 14.Pefanis A., Ierino F. L., Murphy J. M., Cowan P. J. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney International . 2019;96(2):291–301. doi: 10.1016/j.kint.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Sharfuddin A. A., Molitoris B. A. Pathophysiology of ischemic acute kidney injury. Nature Reviews Nephrology . 2011;7(4):189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 16.Malek M., Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. Journal of Renal Injury Prevention . 2015;4:p. 20. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho E. J., Yokozawa T., Rhee S. H., Park K. Y. The role of Coptidis Rhizoma extract in a renal ischemia-reperfusion model. Phytomedicine . 2004;11(7-8):576–584. doi: 10.1016/j.phymed.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Kalogeris T., Baines C. P., Krenz M., Korthuis R. J. Cell biology of ischemia/reperfusion injury. International Review of Cell and Molecular Biology . 2012;298:229–317. doi: 10.1016/b978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ávila C., Líbano L., Rojas I., Rodrigo R. Role of ischemia-reperfusion in oxidative stress-mediated injury during kidney transplantation. Clinical Research . 2019;5:1–4. [Google Scholar]
- 20.Chatterjee P. K. Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn-Schmiedeberg’s Archives of Pharmacology . 2007;376(1-2):1–43. doi: 10.1007/s00210-007-0183-5. [DOI] [PubMed] [Google Scholar]
- 21.Thurman J. M. Triggers of inflammation after renal ischemia/reperfusion. Clinical Immunology . 2007;123(1):7–13. doi: 10.1016/j.clim.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brezis M. Forefronts in nephrology: summary of the newer aspects of renal cell injury. Kidney International . 1992;42(3):523–539. doi: 10.1038/ki.1992.317. [DOI] [PubMed] [Google Scholar]
- 23.Ferencz A., Nedvig K., László E., Magyarlaki T., Lőrinczy D. DSC examination of kidney tissue following warm ischemia and reperfusion injury. Thermochimica Acta . 2011;525(1-2):161–166. doi: 10.1016/j.tca.2011.08.005. [DOI] [Google Scholar]
- 24.Heyman S. N., Rosenberger C., Rosen S. Experimental ischemia-reperfusion: biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney International . 2010;77(1):9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- 25.Schumer M., Colombel M. C., Sawczuk I. S., et al. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. American Journal Of Pathology . 1992;140:831–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J., Wei X., Tang Z., et al. Elucidating the molecular pathways and immune system transcriptome during ischemia-reperfusion injury in renal transplantation. International Immunopharmacology . 2020;81 doi: 10.1016/j.intimp.2020.106246.106246 [DOI] [PubMed] [Google Scholar]
- 27.Choucry M. A., Khalil M. N. A., El Awdan S. A. Protective action of Crateva nurvala Buch. Ham extracts against renal ischaemia reperfusion injury in rats via antioxidant and anti-inflammatory activities. Journal of Ethnopharmacology . 2018;214:47–57. doi: 10.1016/j.jep.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 28.Li Z., Shan T., Zhang S., et al. Protective effects of Lycium barbarum polysaccharide (LBP) on rats with renal ischemia-reperfusion injury (IRI) International Journal of Clinical and Experimental Medicine . 2019;12:12186–12191. [Google Scholar]
- 29.Sampaio T. L., Menezes R. R. P. P. B. D., da Costa M. F. B., et al. Nephroprotective effects of (−)-α-bisabolol against ischemic-reperfusion acute kidney injury. Phytomedicine . 2016;23(14):1843–1852. doi: 10.1016/j.phymed.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Visnagri A., Kandhare A. D., Bodhankar S. L. Renoprotective effect of berberine via intonation on apoptosis and mitochondrial-dependent pathway in renal ischemia reperfusion-induced mutilation. Renal Failure . 2015;37(3):482–493. doi: 10.3109/0886022x.2014.996843. [DOI] [PubMed] [Google Scholar]
- 31.Chouchani E. T., Pell V. R., James A. M., et al. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metabolism . 2016;23(2):254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Granger D. N., Kvietys P. R. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biology . 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loor G., Kondapalli J., Iwase H., et al. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research . 2011;1813(7):1382–1394. doi: 10.1016/j.bbamcr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kher A., Meldrum K., Wang M., Tsai B., Pitcher J., Meldrum D. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovascular Research . 2005;67(4):594–603. doi: 10.1016/j.cardiores.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Hoste E. A. J. Epidemiology of acute kidney injury in critically ill patients. In: Ronco C., Bellomo R., Kellum J. A., Ricci Z., editors. Critical Care Nephrology . 3rd. Philadelphia, PA, USA: Content Repository Only!; 2019. pp. 81–85. [DOI] [Google Scholar]
- 36.Adhikari N. K. J., Rubenfeld G. D. Worldwide demand for critical care. Current Opinion in Critical Care . 2011;17(6):620–625. doi: 10.1097/mcc.0b013e32834cd39c. [DOI] [PubMed] [Google Scholar]
- 37.Rosner M. H., La Manna G., Ronco C. Acute Kidney Injury-Basic Research and Clinical Practice . Basel, Switzerland: Karger Publishers; 2018. Acute kidney injury in the geriatric population; pp. 149–160. [DOI] [PubMed] [Google Scholar]
- 38.Cerdá J., Bagga A., Kher V., Chakravarthi R. M. The contrasting characteristics of acute kidney injury in developed and developing countries. Nature Clinical Practice Nephrology . 2008;4:138–153. doi: 10.1038/ncpneph0722. [DOI] [PubMed] [Google Scholar]
- 39.Hoste E. A. J., Bagshaw S. M., Bellomo R., et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Medicine . 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 40.Kaddourah A., Basu R. K., Bagshaw S. M., Goldstein S. L. Epidemiology of acute kidney injury in critically ill children and young adults. New England Journal of Medicine . 2016;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uber A. M., Sutherland S. M. Acute kidney injury in hospitalized children: consequences and outcomes. Pediatric Nephrology . 2020;35(2):213–220. doi: 10.1007/s00467-018-4128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H. E., Muntner P., Chertow G. M., Warnock D. G. Acute kidney injury and mortality in hospitalized patients. American Journal of Nephrology . 2012;35(4):349–355. doi: 10.1159/000337487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouchard J., Acharya A., Cerda J., et al. A prospective international multicenter study of AKI in the intensive care unit. Clinical Journal of the American Society of Nephrology . 2015;10(8):1324–1331. doi: 10.2215/cjn.04360514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nisula S., Kaukonen K.-M., Kaukonen K.-M., et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Medicine . 2013;39(3):420–428. doi: 10.1007/s00134-012-2796-5. [DOI] [PubMed] [Google Scholar]
- 45.Kaddourah A., Basu R. K., Bagshaw S. M., Goldstein S. L. Epidemiology of acute kidney injury in critically ill children and young adults. New England Journal of Medicine . 2017;376(1):11–20. doi: 10.1056/nejmoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alobaidi R., Morgan C., Goldstein S. L., Bagshaw S. M. Population-based epidemiology and outcomes of acute kidney injury in critically ill children∗. Pediatric Critical Care Medicine . 2020;21(1):82–91. doi: 10.1097/pcc.0000000000002128. [DOI] [PubMed] [Google Scholar]
- 47.Macedo E., Cerdá J., Hingorani S., et al. Recognition and management of acute kidney injury in children: the ISN 0by25 Global Snapshot study. PLoS One . 2018;13(5) doi: 10.1371/journal.pone.0196586.e0196586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ali T., Khan I., Simpson W., et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. Journal of the American Society of Nephrology . 2007;18(4):1292–1298. doi: 10.1681/asn.2006070756. [DOI] [PubMed] [Google Scholar]
- 49.Bagshaw S. M., George C., Bellomo R. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrology Dialysis Transplantation . 2008;23(5):1569–1574. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 50.Srisawat N., Kulvichit W., Mahamitra N., et al. The epidemiology and characteristics of acute kidney injury in the Southeast Asia intensive care unit: a prospective multicentre study. Nephrology Dialysis Transplantation . 2019;35(10):1729–1738. doi: 10.1093/ndt/gfz087. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y., Fang Y., Teng J., Ding X. Acute Kidney Injury-From Diagnosis to Care . Basel, Switzerland: Karger Publishers; 2016. Acute kidney injury epidemiology: from recognition to intervention; pp. 1–8. [DOI] [PubMed] [Google Scholar]
- 52.Kellum J. A., Lameire N., Group K. A. G. W. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Critical Care . 2013;17(1):p. 204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alobaidi R., Bagshaw S. M. Core Concepts in Acute Kidney Injury . Berlin, Germany: Springer; 2018. Sepsis and acute kidney injury: epidemiology, pathophysiology, diagnosis, and management; pp. 165–180. [DOI] [Google Scholar]
- 54.Bagshaw S. M., Uchino S., Bellomo R., et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clinical Journal of the American Society of Nephrology . 2007;2(3):431–439. doi: 10.2215/cjn.03681106. [DOI] [PubMed] [Google Scholar]
- 55.Bailey D., Phan V. R., Litalien C., et al. Risk factors of acute renal failure in critically ill children: a prospective descriptive epidemiological study∗. Pediatric Critical Care Medicine . 2007;8(1):29–35. doi: 10.1097/01.pcc.0000256612.40265.67. [DOI] [PubMed] [Google Scholar]
- 56.Grams M. E., Sang Y., Coresh J., et al. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. American Journal of Kidney Diseases . 2016;67(6):872–880. doi: 10.1053/j.ajkd.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarvasmäki T., Haapio M., Mebazaa A., et al. Acute kidney injury in cardiogenic shock: definitions, incidence, haemodynamic alterations, and mortality. European Journal of Heart Failure . 2018;20:572–581. doi: 10.1002/ejhf.958. [DOI] [PubMed] [Google Scholar]
- 58.van den Akker J. P. C., Bakker J., Groeneveld A. B. J., den Uil C. A. Risk indicators for acute kidney injury in cardiogenic shock. Journal of Critical Care . 2019;50:11–16. doi: 10.1016/j.jcrc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Clark A., Neyra J. A., Madni T., et al. Acute kidney injury after burn. Burns . 2017;43(5):898–908. doi: 10.1016/j.burns.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 60.Morales-Alvarez M. C. Nephrotoxicity of antimicrobials and antibiotics. Advances in Chronic Kidney Disease . 2020;27(1):31–37. doi: 10.1053/j.ackd.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Bellomo R., Kellum J. A., Ronco C. Acute kidney injury. The Lancet . 2012;380(9843):756–766. doi: 10.1016/s0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 62.Burdmann E. A., Jha V. Acute kidney injury due to tropical infectious diseases and animal venoms: a tale of 2 continents. Kidney International . 2017;91(5):1033–1046. doi: 10.1016/j.kint.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 63.Bagga A., Bakkaloglu A., Bakkaloglu A., et al. Improving outcomes from acute kidney injury: report of an initiative. Pediatric Nephrology . 2007;22(10):1655–1658. doi: 10.1007/s00467-007-0565-4. [DOI] [PubMed] [Google Scholar]
- 64.Bellomo R., Ronco C., Kellum J. A., Mehta R. L., Palevsky P. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Critical Care . 2004;8:p. R204. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kellum J. A., Lameire N., Aspelin P., et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney International Supplements . 2012;2:1–138. [Google Scholar]
- 66.Darmon M., Ostermann M., Cerda J., et al. Diagnostic work-up and specific causes of acute kidney injury. Intensive Care Medicine . 2017;43(6):829–840. doi: 10.1007/s00134-017-4799-8. [DOI] [PubMed] [Google Scholar]
- 67.Ostermann M., Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Critical Care . 2016;20(1):p. 299. doi: 10.1186/s13054-016-1478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tohme F. A., Kellum J. A. Evidence-Based Critical Care . Berlin, Germany: Springer; 2020. Traditional and novel tools for diagnosis of acute kidney injury; pp. 361–365. [DOI] [Google Scholar]
- 69.Vandenberghe W., De Loor J., Hoste E. A. J. Diagnosis of cardiac surgery-associated acute kidney injury from functional to damage biomarkers. Current Opinion in Anaesthesiology . 2017;30(1):66–75. doi: 10.1097/aco.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 70.Edelstein C. L. Biomarkers of Kidney Disease . Amsterdam, Netherlands: Elsevier; 2017. Biomarkers in acute kidney injury; pp. 241–315. [DOI] [Google Scholar]
- 71.Porter C. J., Juurlink I., Bisset L. H., Bavakunji R., Mehta R. L., Devonald M. A. J. A real-time electronic alert to improve detection of acute kidney injury in a large teaching hospital. Nephrology Dialysis Transplantation . 2014;29(10):1888–1893. doi: 10.1093/ndt/gfu082. [DOI] [PubMed] [Google Scholar]
- 72.Kanagasundaram N. S. Assessment and management of acute kidney injury. Medicine . 2019;47 [Google Scholar]
- 73.Moore P. K., Hsu R. K., Liu K. D. Management of acute kidney injury: core curriculum 2018. American Journal of Kidney Diseases . 2018;72(1):136–148. doi: 10.1053/j.ajkd.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 74.Prowle J. R., Echeverri J. E., Ligabo E. V., Ronco C., Bellomo R. Fluid balance and acute kidney injury. Nature Reviews Nephrology . 2010;6(2):107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 75.Nigwekar S. U., Waikar S. S. Seminars in Nephrology . 6. Vol. 31. Amsterdam, Netherlands: Elsevier; 2011. Diuretics in acute kidney injury; pp. 523–534. [DOI] [PubMed] [Google Scholar]
- 76.Lachance P., Bagshaw S. M. Loop and thiazide diuretics. Critical Care Nephrology . 2019:358–364. doi: 10.1016/b978-0-323-44942-7.00061-3. [DOI] [Google Scholar]
- 77.Mehta R. L., Pascual M. T., Soroko S., Chertow G. M., Group P. S. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA . 2002;288(20):2547–2553. doi: 10.1001/jama.288.20.2547. [DOI] [PubMed] [Google Scholar]
- 78.Ho K. M., Power B. M. Benefits and risks of furosemide in acute kidney injury. Anaesthesia . 2010;65:283–293. doi: 10.1111/j.1365-2044.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 79.Lameire N., Van Biesen W., Hoste E., Vanholder R. The prevention of acute kidney injury an in-depth narrative review: Part 2: drugs in the prevention of acute kidney injury. Clinical Kidney Journal . 2009;2(1):1–10. doi: 10.1093/ndtplus/sfn199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He S.-J., Liu Q., Li H.-Q., Tian F., Chen S.-Y., Weng J.-X. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Therapeutics and Clinical Risk Management . 2018;14:475–482. doi: 10.2147/tcrm.s160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kunzendorf U., Haase M., Rölver L., Haase-Fielitz A. Novel aspects of pharmacological therapies for acute renal failure. Drugs . 2010;70(9):1099–1114. doi: 10.2165/11535890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 82.Jo S. K., Rosner M. H., Okusa M. D. Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clinical Journal of the American Society of Nephrology . 2007;2(2):356–365. doi: 10.2215/cjn.03280906. [DOI] [PubMed] [Google Scholar]
- 83.Busse L. W., Chawla L. S. Novel drugs for acute kidney injury. Critical Care Nephrology . 2019:307–314. doi: 10.1016/b978-0-323-44942-7.00052-2. [DOI] [Google Scholar]
- 84.Gonsalez S. R., Cortês A. L., Silva R. C. D., Lowe J., Prieto M. C., Silva Lara L. D. Acute kidney injury overview: from basic findings to new prevention and therapy strategies. Pharmacology & Therapeutics . 2019;200:1–12. doi: 10.1016/j.pharmthera.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Long C., Yang J., Yang H., Li X., Wang G. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, anti-inflammatory, and anti-apoptotic activities. Molecular Medicine Reports . 2016;13(6):4697–4704. doi: 10.3892/mmr.2016.5128. [DOI] [PubMed] [Google Scholar]
- 86.Boozari M., Hosseinzadeh H. Natural medicines for acute renal failure: a review. Phytotherapy Research . 2017;31(12):1824–1835. doi: 10.1002/ptr.5943. [DOI] [PubMed] [Google Scholar]
- 87.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Molecular Aspects of Medicine . 2006;27(1):1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 88.Schippmann U., Leaman D. J., Cunningham A. Biodiversity and the Ecosystem Approach in Agriculture, Forestry and Fisheries . Rome Italy: FAO; 2002. Impact of cultivation and gathering of medicinal plants on biodiversity: global trends and issues. [Google Scholar]
- 89.Aboelsoud N. H. Herbal medicine in ancient Egypt. Journal of Medicinal Plants Research . 2010;4:082–086. [Google Scholar]
- 90.Efferth T. Biotechnology applications of plant callus cultures. Engineering . 2019;5(1):50–59. doi: 10.1016/j.eng.2018.11.006. [DOI] [Google Scholar]
- 91.Vaidya A. D. B., Devasagayam T. P. A. Current status of herbal drugs in India: an overview. Journal of Clinical Biochemistry & Nutrition . 2007;41(1):1–11. doi: 10.3164/jcbn.2007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mishra B. B., Tiwari V. K. Natural products: an evolving role in future drug discovery. European Journal of Medicinal Chemistry . 2011;46(10):4769–4807. doi: 10.1016/j.ejmech.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 93.Newman D. J., Cragg G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Journal of Natural Products . 2012;75(3):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patridge E., Gareiss P., Kinch M. S., Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discovery Today . 2016;21(2):204–207. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 95.Calixto J. B. Twenty-five years of research on medicinal plants in Latin America. Journal of Ethnopharmacology . 2005;100(1-2):131–134. doi: 10.1016/j.jep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Amani H., Habibey R., Hajmiresmail S. J., Latifi S., Pazoki-Toroudi H., Akhavan O. Antioxidant nanomaterials in advanced diagnoses and treatments of ischemia reperfusion injuries. Journal of Materials Chemistry B . 2017;5(48):9452–9476. doi: 10.1039/c7tb01689a. [DOI] [PubMed] [Google Scholar]
- 97.Dominguez J. H., Liu Y., Gao H., Dominguez J. M., Xie D., Kelly K. J. Renal tubular cell-derived extracellular vesicles accelerate the recovery of established renal ischemia reperfusion injury. Journal of the American Society of Nephrology . 2017;28(12):3533–3544. doi: 10.1681/asn.2016121278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Emma F., Montini G., Parikh S. M., Salviati L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nature Reviews Nephrology . 2016;12(5):267–280. doi: 10.1038/nrneph.2015.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sheashaa H., Lotfy A., Elhusseini F., et al. Protective effect of adipose-derived mesenchymal stem cells against acute kidney injury induced by ischemia-reperfusion in Sprague-Dawley rats. Experimental and Therapeutic Medicine . 2016;11(5):1573–1580. doi: 10.3892/etm.2016.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aboutaleb N., Jamali H., Abolhasani M., Pazoki Toroudi H. Lavender oil (Lavandula angustifolia) attenuates renal ischemia/reperfusion injury in rats through suppression of inflammation, oxidative stress and apoptosis. Biomedicine & Pharmacotherapy . 2019;110:9–19. doi: 10.1016/j.biopha.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 101.Baiano A., Del Nobile M. A. Antioxidant compounds from vegetable matrices: biosynthesis, occurrence, and extraction systems. Critical Reviews in Food Science and Nutrition . 2016;56(12):2053–2068. doi: 10.1080/10408398.2013.812059. [DOI] [PubMed] [Google Scholar]
- 102.Li S., Tan H.-Y., Wang N., et al. The role of oxidative stress and antioxidants in liver diseases. International Journal of Molecular Sciences . 2015;16(11):26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food and Bioproducts Processing . 2011;89(3):217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- 104.Mohammadi M., Najafi H., Yarijani Z. M., Vaezi G., Hojati V. Protective effect of piperine in ischemia-reperfusion induced acute kidney injury through inhibition of inflammation and oxidative stress. Journal of Traditional and Complementary Medicine . 2019;10:570–576. doi: 10.1016/j.jtcme.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cakir M., Duzova H., Baysal I., et al. The effect of Hypericum perforatum on kidney ischemia/reperfusion damage. Renal Failure . 2017;39(1):385–391. doi: 10.1080/0886022x.2017.1287734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y., Shi B., Li Y., Zhang H. Protective effect of luteolin against renal ischemia/reperfusion injury via modulation of pro-inflammatory cytokines, oxidative stress and apoptosis for possible benefit in kidney transplant. Medical Science Monitor . 2017;23:5720–5727. doi: 10.12659/msm.903253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meng Q.-H., Liu H.-B., Wang J.-B. Polydatin ameliorates renal ischemia/reperfusion injury by decreasing apoptosis and oxidative stress through activating sonic hedgehog signaling pathway. Food and Chemical Toxicology . 2016;96:215–225. doi: 10.1016/j.fct.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 108.Zhao L., Xu L., Tao X., et al. Protective effect of the total flavonoids from Rosa laevigata michx fruit on renal ischemia-reperfusion injury through suppression of oxidative stress and inflammation. Molecules . 2016;21(7):p. 952. doi: 10.3390/molecules21070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perez-Meseguer J., Torres-González L., Gutiérrez-González J. A., et al. Anti-inflammatory and nephroprotective activity of Juglans mollis against renal ischemia-reperfusion damage in a Wistar rat model. BMC Complementary and Alternative Medicine . 2019;19(1):p. 186. doi: 10.1186/s12906-019-2604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Torres-González L., Cienfuegos-Pecina E., Perales-Quintana M. M., et al. Nephroprotective effect of sonchus oleraceus extract against kidney injury induced by ischemia-reperfusion in wistar rats. Oxidative Medicine and Cellular Longevity . 2018;2018:7. doi: 10.1155/2018/9572803.9572803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sampaio T. L., Menezes R. R. P. P. B. D., Lima D. B., et al. Involvement of NADPH-oxidase enzyme in the nephroprotective effect of (−)-α-bisabolol on HK2 cells exposed to ischemia—Reoxygenation. European Journal of Pharmacology . 2019;855:1–9. doi: 10.1016/j.ejphar.2019.04.044. [DOI] [PubMed] [Google Scholar]
- 112.El Morsy E. M., Ahmed M. A., Ahmed A. A. Attenuation of renal ischemia/reperfusion injury by açaí extract preconditioning in a rat model. Life Sciences . 2015;123:35–42. doi: 10.1016/j.lfs.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 113.Hu S., Zhang Y., Zhang M., et al. Aloperine protects mice against ischemia-reperfusion (IR)-induced renal injury by regulating PI3K/AKT/mTOR signaling and AP-1 Activity. Molecular Medicine . 2015;21(1):912–923. doi: 10.2119/molmed.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu Y., Wang L., Du Y., et al. Effects of apigenin pretreatment against renal ischemia/reperfusion injury via activation of the JAK2/STAT3 pathway. Biomedicine & Pharmacotherapy . 2017;95:1799–1808. doi: 10.1016/j.biopha.2017.09.091. [DOI] [PubMed] [Google Scholar]
- 115.Shin S., Lee Y. J., Kim E. J., Lee A. S., Kang D. G., Lee H. S. Effect ofCuscuta chinensison renal function in ischemia/reperfusion-induced acute renal failure rats. The American Journal of Chinese Medicine . 2011;39(05):889–902. doi: 10.1142/s0192415x11009287. [DOI] [PubMed] [Google Scholar]
- 116.Punuru P., Sujatha D., Kumari B., Charisma V. V. L. Evaluation of aqueous extract of Murraya koenigii in unilateral renal ischemia reperfusion injury in rats. Indian Journal of Pharmacology . 2014;46(2):p. 171. doi: 10.4103/0253-7613.129310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Han F., Xia X.-X., dou M., et al. Arctigenin: a two-edged sword in ischemia/reperfusion induced acute kidney injury. Biomedicine & Pharmacotherapy . 2018;103:1127–1136. doi: 10.1016/j.biopha.2018.04.169. [DOI] [PubMed] [Google Scholar]
- 118.Korkmaz A., Kolankaya D. The protective effects of ascorbic acid against renal ischemia-reperfusion injury in male rats. Renal Failure . 2009;31(1):36–43. doi: 10.1080/08860220802546271. [DOI] [PubMed] [Google Scholar]
- 119.García-Closas R., Berenguer A., Tormo M. J., et al. Dietary sources of vitamin C, vitamin E and specific carotenoids in Spain. British Journal of Nutrition . 2004;91:1005–1011. doi: 10.1079/bjn20041130. [DOI] [PubMed] [Google Scholar]
- 120.Ekşioğlu-Demiralp E., Kardaş E. R., Özgül S., et al. Betulinic acid protects against ischemia/reperfusion-induced renal damage and inhibits leukocyte apoptosis. Phytotherapy Research . 2010;24:325–332. doi: 10.1002/ptr.2929. [DOI] [PubMed] [Google Scholar]
- 121.Kinra M., Arora D., Mudgal J., Pai K. S. R., Mallikarjuna Rao C., Nampoothiri M. Effect of caffeic acid on ischemia-reperfusion-induced acute renal failure in rats. Pharmacology . 2019;103(5-6):315–319. doi: 10.1159/000497474. [DOI] [PubMed] [Google Scholar]
- 122.Fouad A. A., Al-Mulhim A. S., Jresat I. Cannabidiol treatment ameliorates ischemia/reperfusion renal injury in rats. Life Sciences . 2012;91(7-8):284–292. doi: 10.1016/j.lfs.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 123.Bayrak O., Uz E., Bayrak R., et al. Curcumin protects against ischemia/reperfusion injury in rat kidneys. World Journal of Urology . 2008;26(3):285–291. doi: 10.1007/s00345-008-0253-4. [DOI] [PubMed] [Google Scholar]
- 124.Qi M., Zheng L., Qi Y., et al. Dioscin attenuates renal ischemia/reperfusion injury by inhibiting the TLR4/MyD88 signaling pathway via up-regulation of HSP70. Pharmacological Research . 2015;100:341–352. doi: 10.1016/j.phrs.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 125.Lv J., Feng M., Zhang L., et al. Protective effect of epigallocatechin gallate, a major constituent of green tea, against renal ischemia-reperfusion injury in rats. International Urology and Nephrology . 2015;47(8):1429–1435. doi: 10.1007/s11255-015-1030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Afifah A., Muflikhah K., Ati V. R. B., Tsani R. M., Khasanah D., Maulana W. Protective effect of ethanol extract of celery (Apium graveolens L) on kidney damage in ischemia/reperfusion injury rats model. Molekul . 2019;14(1):11–17. doi: 10.20884/1.jm.2019.14.1.448. [DOI] [Google Scholar]
- 127.Mohajeri D., Gharamaleki M. N., Hejazi S. S., Nazeri M. Preventive effects of turnip (Brassica rapa L.) on renal ischemia-reperfusion injury in rats. Life Science Journal . 2013;10:1165–1170. [Google Scholar]
- 128.Roshankhah S., Jalili C., Salahshoor M. R. Protective effects of Petroselinum crispum on ischemia/reperfusion-induced acute kidney injury in rats. Physiol Pharmacol . 2019;23:129–139. [Google Scholar]
- 129.Chen G., Fu Y., Wu X. Protective effect of Salvia miltiorrhiza extract against renal ischemia-reperfusion-induced injury in rats. Molecules . 2012;17(2):1191–1202. doi: 10.3390/molecules17021191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Najafi H., Firouzifar M. R., Shafaat O., Changizi Ashtiyani S., Hosseini N. Protective effects of Tribulus terrestris L extract against acute kidney injury induced by reperfusion injury in rats. Iranian journal of kidney diseases . 2014;8:292–8. [PubMed] [Google Scholar]
- 131.da Costa M. F. B., Libório A. B., Teles F., et al. Red propolis ameliorates ischemic-reperfusion acute kidney injury. Phytomedicine . 2015;22(9):787–795. doi: 10.1016/j.phymed.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 132.Zhou Q., Gong X., Kuang G., et al. Ferulic acid protected from kidney ischemia reperfusion injury in mice: possible mechanism through increasing adenosine generation via HIF-1α. Inflammation . 2018;41(6):2068–2078. doi: 10.1007/s10753-018-0850-3. [DOI] [PubMed] [Google Scholar]
- 133.Bagheri F., Gol A., Dabiri S., Javadi A. Preventive effect of garlic juice on renal reperfusion injury. Iranian journal of kidney diseases . 2011;5(3):194–200. [PubMed] [Google Scholar]
- 134.Savas M., Yeni E., Ciftci H., et al. The antioxidant role of oral administration of garlic oil on renal ischemia-reperfusion injury. Renal Failure . 2010;32(3):362–367. doi: 10.3109/08860221003611711. [DOI] [PubMed] [Google Scholar]
- 135.Maghsoudi S., Gol A., Dabiri S., Javadi A. Preventive effect of ginger (Zingiber officinale) pretreatment on renal ischemia-reperfusion in rats. European Surgical Research . 2011;46(1):45–51. doi: 10.1159/000321704. [DOI] [PubMed] [Google Scholar]
- 136.Akdere H., Tastekin E., Mericliler M., Burgazli K. M. The protective effects of Ginkgo biloba EGb761 extract against renal ischemia-reperfusion injury in rats. European Review for Medical and Pharmacological Sciences . 2014;18:2936–2941. [PubMed] [Google Scholar]
- 137.Gholampour F., Javadifar T. S., Karimi S., Eslam-Zadeh T., Owji S. M. The effects of the hydroalcohol extract of Rosa canina L. fruit on ischemic acute renal failure in Wistar rats. Comparative Clinical Pathology . 2012;21(6):1433–1438. doi: 10.1007/s00580-012-1533-3. [DOI] [Google Scholar]
- 138.Mahmoudzadeh L., Najafi H., Ashtiyani S. C., Yarijani Z. M. Anti-inflammatory and protective effects of saffron extract in ischaemia/reperfusion-induced acute kidney injury. Nephrology . 2017;22(10):748–754. doi: 10.1111/nep.12849. [DOI] [PubMed] [Google Scholar]
- 139.Rogers N., Stephenson M., Kitching A., Horowitz J., Coates P. Amelioration of renal ischaemia-reperfusion injury by liposomal delivery of curcumin to renal tubular epithelial and antigen-presenting cells. British Journal of Pharmacology . 2012;166(1):194–209. doi: 10.1111/j.1476-5381.2011.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hussien Y. A., Abdalkadim H., Mahbuba W., Hadi N. R., Jamil D. A., Al-Aubaidy H. A. The nephroprotective effect of lycopene on renal ischemic reperfusion injury: a mouse model. Indian Journal of Clinical Biochemistry . 2020;35(4):474–481. doi: 10.1007/s12291-019-00848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang B., Wan J., Gong X., Kuang G., Cheng X., Min S. Mangiferin attenuates renal ischemia-reperfusion injury by inhibiting inflammation and inducing adenosine production. International Immunopharmacology . 2015;25(1):148–154. doi: 10.1016/j.intimp.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 142.Baek H.-S., Lim S.-H., Ahn K.-S., Lee J.-W. Methanol extract of goat’s-beard (aruncus dioicus) reduces renal injury by inhibiting apoptosis in a rat model of ischemia-reperfusion. Preventive Nutrition and Food Science . 2012;17(2):101–108. doi: 10.3746/pnf.2012.17.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baek H. S., Lim S. H., Ahn K. S., Lee J. W. Effect of methanol extract from Cassia mimosoides var. nomame on ischemia/reperfusion-induced renal injury in rats. The Korea Journal of Herbology . 2013;28(6):135–143. doi: 10.6116/kjh.2013.28.6.135. [DOI] [Google Scholar]
- 144.Elsaid F. H., Khalil A. A., Khalil A. A., Ibrahim E. M., Mansour A., Hussein A. M. Effects of exercise and stevia on renal ischemia/reperfusion injury in rats. Acta Scientiarum Polonorum Technologia Alimentaria . 2019;18(3):317–332. doi: 10.17306/j.afs.2019.0652. [DOI] [PubMed] [Google Scholar]
- 145.Bhalodia Y., Kanzariya N., Patel R., et al. Renoprotective activity of benincasa cerifera fruit extract on ischemia/reperfusion-induced renal damage in rat. Iran J Kidney Dis . 2009;3(2):80–85. doi: 10.4103/0973-8258.59738. [DOI] [PubMed] [Google Scholar]
- 146.Bayrak O., Bavbek N., Karatas O. F., et al. Nigella sativa protects against ischaemia/reperfusion injury in rat kidneys. Nephrology Dialysis Transplantation . 2008;23(7):2206–2212. doi: 10.1093/ndt/gfm953. [DOI] [PubMed] [Google Scholar]
- 147.Bartošíková L., Nečas J., Suchý V., et al. Protective effects of osajin in ischemia-reperfusion of laboratory rat kidney. Die Pharmazie . 2006;61:552–555. [PubMed] [Google Scholar]
- 148.Zheng Y., Lu M., Ma L., Zhang S., Qiu M., Wang Y. Osthole ameliorates renal ischemia-reperfusion injury in rats. Journal of Surgical Research . 2013;183(1):347–354. doi: 10.1016/j.jss.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 149.Seth P., Kumari R., Madhavan S., et al. Prevention of renal ischemia-reperfusion-induced injury in rats by picroliv. Biochemical Pharmacology . 2000;59(10):1315–1322. doi: 10.1016/s0006-2952(00)00268-9. [DOI] [PubMed] [Google Scholar]
- 150.Cong G., Cui L., Zang M., Hao L. Attenuation of renal ischemia/reperfusion injury by a polysaccharide from the roots of Dipsacus asperoides. International Journal of Biological Macromolecules . 2013;56:14–19. doi: 10.1016/j.ijbiomac.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 151.Rah D. K., Han D.-W., Baek H. S., Hyon S.-H., Park B. Y., Park J.-C. Protection of rabbit kidney from ischemia/reperfusion injury by green tea polyphenol pretreatment. Archives of Pharmacal Research . 2007;30(11):1447–1454. doi: 10.1007/bf02977370. [DOI] [PubMed] [Google Scholar]
- 152.Zhong D., Wang H., Liu M., et al. Ganoderma lucidum polysaccharide peptide prevents renal ischemia reperfusion injury via counteracting oxidative stress. Scientific Reports . 2015;5(1) doi: 10.1038/srep16910.16910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yanarates O., Guven A., Sizlan A., et al. Ameliorative effects of proanthocyanidin on renal ischemia/reperfusion injury. Renal Failure . 2008;30(9):931–938. doi: 10.1080/08860220802359410. [DOI] [PubMed] [Google Scholar]
- 154.Ozer Şehirli A., Şener G., Ercan F. Protective effects of pycnogenol against ischemia reperfusion-induced oxidative renal injury in rats. Renal Failure . 2009;31:690–697. doi: 10.3109/08860220903085971. [DOI] [PubMed] [Google Scholar]
- 155.Al-Jabbar W. A., Hadi N. R., Ghafil F. A. A., et al. Nephroprotective effects of quercetin in renal ischemia reperfusion injury in mice. Systematic Reviews in Pharmacy . 2019;10:184–193. [Google Scholar]
- 156.Giovannini L., Migliori M., Longoni B. M., et al. Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. Journal of Cardiovascular Pharmacology . 2001;37(3):262–270. doi: 10.1097/00005344-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 157.Burns J., Yokota T., Ashihara H., Lean M. E. J., Crozier A. Plant foods and herbal sources of resveratrol. Journal of Agricultural and Food Chemistry . 2002;50(11):3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 158.Ozturk H., Ozturk H., Terzi E. H., Ozgen U., Duran A., Uygun I. Protective effects of rosmarinic acid against renal ischaemia/reperfusion injury in rats. JPMA. The Journal of the Pakistan Medical Association . 2014;64:260–265. [PubMed] [Google Scholar]
- 159.Park S. U., Uddin R., Xu H., Kyoung Kim Y., Sook-Young L. Biotechnological applications for rosmarinic acid production in plant. African Journal of Biotechnology . 2008;7:4959–4965. [Google Scholar]
- 160.Korkmaz A., Kolankaya D. Protective effect of rutin on the ischemia/reperfusion induced damage in rat kidney. Journal of Surgical Research . 2010;164(2):309–315. doi: 10.1016/j.jss.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 161.Li K., Gong X., Kuang G., Jiang R., Wan J., Wang B. Sesamin protects against renal ischemia reperfusion injury by promoting CD39-adenosine-A2AR signal pathway in mice. American Journal of Tourism Research . 2016;8:2245–54. [PMC free article] [PubMed] [Google Scholar]
- 162.Senturk H., Kabay S., Bayramoglu G., et al. Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World Journal of Urology . 2008;26(4):401–407. doi: 10.1007/s00345-008-0256-1. [DOI] [PubMed] [Google Scholar]
- 163.Corchete P. Bioactive Molecules and Medicinal Plants . Berlin, Germany: Springer; 2008. Silybum marianum (L.) gaertn: the source of silymarin; pp. 123–148. [DOI] [Google Scholar]
- 164.Peng J., Ren X., Lan T., Chen Y., Shao Z., Yang C. Renoprotective effects of ursolic acid on ischemia/reperfusion-induced acute kidney injury through oxidative stress, inflammation and the inhibition of STAT3 and NF-κB activities. Molecular Medicine Reports . 2016;14(4):3397–3402. doi: 10.3892/mmr.2016.5654. [DOI] [PubMed] [Google Scholar]
- 165.Bayr H. Reactive oxygen species. Critical Care Medicine . 2005;33:S498–S501. doi: 10.1097/01.ccm.0000186787.64500.12. [DOI] [PubMed] [Google Scholar]
- 166.Schneider M. P., Sullivan J. C., Wach P. F., et al. Protective role of extracellular superoxide dismutase in renal ischemia/reperfusion injury. Kidney International . 2010;78(4):374–381. doi: 10.1038/ki.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jefferies H., Coster J., Khalil A., Bot J., McCauley R. D., Hall J. C. Glutathione. ANZ Journal of Surgery . 2003;73(7):517–522. doi: 10.1046/j.1445-1433.2003.02682.x. [DOI] [PubMed] [Google Scholar]
- 168.Shang Y., Siow Y. L., Isaak C. K. Downregulation of glutathione biosynthesis contributes to oxidative stress and liver dysfunction in acute kidney injury. Oxidative Medicine and Cellular Longevity . 2016;2016:13. doi: 10.1155/2016/9707292.9707292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bianchi M. E., Manfredi A. A. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunological Reviews . 2007;220(1):35–46. doi: 10.1111/j.1600-065x.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 170.Villanueva S., Céspedes C., Vio C. P. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology . 2006;290(4):R861–R870. doi: 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- 171.Chadha R., Heidt S., Jones N. D., Wood K. J. Th17: contributors to allograft rejection and a barrier to the induction of transplantation tolerance? Transplantation . 2011;91(9):939–945. doi: 10.1097/tp.0b013e3182126eeb. [DOI] [PubMed] [Google Scholar]
- 172.Kany S., Vollrath J. T., Relja B. Cytokines in inflammatory disease. International Journal of Molecular Sciences . 2019;20:p. 6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cao C. C., Ding X. Q., Ou Z. L., et al. In vivo transfection of NF-κB decoy oligodeoxynucleotides attenuate renal ischemia/reperfusion injury in rats. Kidney International . 2004;65(3):834–845. doi: 10.1111/j.1523-1755.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 174.Sethi G., Sung B., Aggarwal B. B. TNF: a master switch for inflammation to cancer. Frontiers in Bioscience . 2008;13:5094–5107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 175.Liu T., Zhang L., Joo D., Sun S. C. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy . 2017;2:1–9. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bauerle J. D., Grenz A., Kim J.-H., Lee H. T., Eltzschig H. K. Adenosine generation and signaling during acute kidney injury. Journal of the American Society of Nephrology . 2011;22(1):14–20. doi: 10.1681/asn.2009121217. [DOI] [PubMed] [Google Scholar]
- 177.Eltzschig H. K., Warner D. S., Warner M. A. Adenosine: an old drug newly discovered. Anesthesiology . 2009;111(4):904–915. doi: 10.1097/aln.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vallon V., Osswald H. Adenosine receptors and the kidney. Adenosine Receptors in Health and Disease . 2009;193:443–470. doi: 10.1007/978-3-540-89615-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yap S. C., Lee H. T. Adenosine and protection from acute kidney injury. Current Opinion in Nephrology and Hypertension . 2012;21(1):24–32. doi: 10.1097/mnh.0b013e32834d2ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vincent I. S., Okusa M. D. Adenosine 2A receptors in acute kidney injury. Acta Physiologica . 2015;214(3):303–310. doi: 10.1111/apha.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Porta C., Paglino C., Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Frontiers in Oncology . 2014;4:p. 64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xu F., Na L., Li Y., Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell & Bioscience . 2020;10:p. 54. doi: 10.1186/s13578-020-00416-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 183.Wei Q., Zhao J., Zhou X., Yu L., Liu Z., Chang Y. Propofol can suppress renal ischemia-reperfusion injury through the activation of PI3K/AKT/mTOR signal pathway. Gene . 2019;708:14–20. doi: 10.1016/j.gene.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 184.Zhang J., Yao Y., Xiao F., et al. Administration of dexamethasone protects mice against ischemia/reperfusion induced renal injury by suppressing PI3K/AKT signaling. International Journal of Clinical and Experimental Pathology . 2013;6:2366–75. [PMC free article] [PubMed] [Google Scholar]
- 185.Chuang P. Y., He J. C. JAK/STAT signaling in renal diseases. Kidney International . 2010;78:231–234. doi: 10.1038/ki.2010.158. [DOI] [PubMed] [Google Scholar]
- 186.Das A., Salloum F. N., Durrant D., Ockaili R., Kukreja R. C. Rapamycin protects against myocardial ischemia–reperfusion injury through JAK2–STAT3 signaling pathway. Journal of Molecular and Cellular Cardiology . 2012;53:858–869. doi: 10.1016/j.yjmcc.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang N., Luo M., Li R., et al. Blockage of JAK/STAT signalling attenuates renal ischaemia-reperfusion injury in rats. Nephrology Dialysis Transplantation . 2008;23:91–100. doi: 10.1093/ndt/gfm509. [DOI] [PubMed] [Google Scholar]
- 188.Si Y., Bao H., Han L., et al. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. Journal of Translational Medicine . 2013;11:p. 141. doi: 10.1186/1479-5876-11-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bambakidis N. C., Onwuzulike K. Chapter seventeen—sonic hedgehog signaling and potential therapeutic indications. In: Litwack G., editor. Vitam. Horm . Cambridge, MA, USA: Academic Press; 2012. pp. 379–394. [DOI] [PubMed] [Google Scholar]
- 190.Choudhry Z., Rikani A. A., Choudhry A. M., et al. Sonic hedgehog signalling pathway: a complex network. Annals of Neurosciences . 2014;21:28–31. doi: 10.5214/ans.0972.7531.210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou D., Li Y., Zhou L., et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. Journal of the American Society of Nephrology . 2014;25:2187–2200. doi: 10.1681/asn.2013080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou D., Tan R. J., Liu Y. Sonic hedgehog signaling in kidney fibrosis: a master communicator. Science China Life Sciences . 2016;59:920–929. doi: 10.1007/s11427-016-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.O’Neill S., Harrison E. M., Ross J. A., Wigmore S. J., Hughes J. Heat-shock proteins and acute ischaemic kidney injury. Nephron Experimental Nephrology . 2014;126:167–174. doi: 10.1159/000363323. [DOI] [PubMed] [Google Scholar]
- 194.Krstic D., Tomic N., Radosavljevic B., et al. Biochemical markers of renal function. Current Medicinal Chemistry . 2016;23:2018–2040. doi: 10.2174/0929867323666160115130241. [DOI] [PubMed] [Google Scholar]
- 195.Bellomo R., Kellum J. A., Ronco C. Defining acute renal failure: physiological principles. Intensive Care Medicine . 2004;30:33–37. doi: 10.1007/s00134-003-2078-3. [DOI] [PubMed] [Google Scholar]
- 196.Lin J., Fernandez H., Shashaty M. G., et al. False-positive rate of AKI using consensus creatinine–based criteria. Clinical Journal of the American Society of Nephrology . 2015;10:1723–1731. doi: 10.2215/cjn.02430315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Waikar S. S., Betensky R. A., Bonventre J. V. Creatinine as the Gold Standard for Kidney Injury Biomarker Studies? Oxford, UK: Oxford University Press; 2009. [DOI] [PubMed] [Google Scholar]
- 198.Filler G., Bökenkamp A., Hofmann W., Bricon T. L., Martínez-Brú C., Grubb A. Cystatin C as a marker of GFR-history, indications, and future research. Clinical Biochemistry . 2005;38:1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 199.Frazee E., Rule A. D., Lieske J. C., et al. Cystatin C-guided vancomycin dosing in critically ill patients: a quality improvement project. American Journal of Kidney Diseases . 2017;69:658–666. doi: 10.1053/j.ajkd.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 200.Madureira e Silva M. V., Moscoso Solorzano G., Nishida S. K., Kirsztajn G. M. Are serum cystatin C levels influenced by steroid doses in lupus nephritis patients? Brazilian Journal of Nephrology . 2011;33:306–312. doi: 10.1590/s0101-28002011000300006. [DOI] [PubMed] [Google Scholar]
- 201.Nguyen M. T., Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatric Nephrology . 2008;23:2151–2157. doi: 10.1007/s00467-007-0470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Treacy O., Brown N. N., Dimeski G. Biochemical evaluation of kidney disease. Translational Andrology and Urology . 2019;8:S214–S223. doi: 10.21037/tau.2018.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Amin S., Khan H. Revival of natural products: utilization of modern technologies. Current Bioactive Compounds . 2016;12:103–106. doi: 10.2174/1573407212666160314195845. [DOI] [Google Scholar]
- 204.Thomford N. E., Senthebane D. A., Rowe A., et al. Natural products for drug discovery in the 21st century: innovations for novel drug discovery. International Journal of Molecular Sciences . 2018;19:p. 1578. doi: 10.3390/ijms19061578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Smith S. F., Hosgood S. A., Nicholson M. L. Ischemia-reperfusion injury in renal transplantation: 3 key signaling pathways in tubular epithelial cells. Kidney International . 2019;95:50–56. doi: 10.1016/j.kint.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 206.Shao Y., Wu B., Jia W., Zhang Z., Chen Q., Wang D. Prognostic value of pretreatment neutrophil-to-lymphocyte ratio in renal cell carcinoma: a systematic review and meta-analysis. BMC Urology . 2020;20:1–14. doi: 10.1186/s12894-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are all included and available within the article.


