Abstract
From Bradyrhizobium japonicum highly reiterated sequence-possessing (HRS) strains indigenous to Niigata and Tokachi in Japan with high copy numbers of the repeated sequences RSα and RSβ (K. Minamisawa, T. Isawa, Y. Nakatsuka, and N. Ichikawa, Appl. Environ. Microbiol. 64:1845–1851, 1998), several insertion sequence (IS)-like elements were isolated by using the formation of DNA duplexes by denaturation and renaturation of total DNA, followed by treatment with S1 nuclease. Most of these sequences showed structural features of bacterial IS elements, terminal inverted repeats, and homology with known IS elements and transposase genes. HRS and non-HRS strains of B. japonicum differed markedly in the profiles obtained after hybridization with all the elements tested. In particular, HRS strains of B. japonicum contained many copies of IS1631, whereas non-HRS strains completely lacked this element. This association remained true even when many field isolates of B. japonicum were examined. Consequently, IS1631 occurrence was well correlated with B. japonicum HRS strains possessing high copy numbers of the repeated sequence RSα or RSβ. DNA sequence analysis indicated that IS1631 is 2,712 bp long. In addition, IS1631 belongs to the IS21 family, as evidenced by its two open reading frames, which encode putative proteins homologous to IstA and IstB of IS21, and its terminal inverted repeat sequences with multiple short repeats.
Bradyrhizobium japonicum is an agronomically important gram-negative bacterium that has the ability to form root nodules on soybeans and to fix atmospheric nitrogen. As described in a previous study (17), some isolates of B. japonicum indigenous to Niigata and Tokachi in Japan had much higher copy numbers of the repeated sequences RSα and RSβ (highly reiterated sequence-possessing [HRS] strains) than other B. japonicum isolates and strains. RSα has structural properties similar to those of a prokaryotic insertion sequence (IS) element (11). B. japonicum HRS strains exhibited slower growth than non-HRS strains, although no difference in symbiotic properties was detected (17). HRS strains were more sensitive to antibiotics, such as chloramphenicol, than non-HRS strains (26a). Several lines of evidence suggested that in individual fields, HRS strains are generated from non-HRS B. japonicum strains by DNA rearrangements, which may be mediated by IS elements (17).
IS elements have been identified as mobile DNA elements in the genomes, plasmids, and bacteriophages of a wide range of bacterial genera and species. They have been postulated to play an important role in the evolution and adaptation of bacteria (3, 33). A single species of bacteria may contain many different IS elements. Although the distribution of an IS element is often restricted to related hosts, the multiplicity of each IS element is variable and independent at the level of the strain. For example, six distinct IS elements, including IS1, IS2, IS3, IS4, IS5, and IS30, commonly exist in Escherichia coli. Most strains of Rhizobium meliloti have at least three types of IS elements: ISRm1, ISRm2, and ISRm3. In B. japonicum, several repeated sequences (RSγ, RSδ, RSɛ, and RSζ) other than RSα and RSβ have been found but have not been characterized as IS elements (7). In addition, Judd and Sadowsky identified a hyperreiterated DNA region, HRS1, as an IS element in B. japonicum serocluster 123 strains (10). These facts prompted us to survey IS elements other than RSα and RSβ in B. japonicum HRS strains in order to gain some understanding of the involvement of IS elements in DNA rearrangement in B. japonicum.
To detect and isolate IS elements, various entrapment plasmids for positive selection have been devised (4, 23, 28). However, we could not use these entrapment plasmids because the associated selection systems have not worked well in very slow growing B. japonicum HRS strains. An alternative method is based on the formation of duplex DNA by denaturation and renaturation of total DNA, followed by treatment with S1 nuclease (15). This procedure is considered suitable for the isolation of IS elements from HRS strains because they carry high copy numbers of IS elements (17). We isolated several IS-like elements, including RSα and RSβ, from HRS strains of B. japonicum by method and investigated the distribution of these IS-like elements in many strains of B. japonicum.
MATERIALS AND METHODS
Bacterial strains, growth media, and growth conditions.
The major Bradyrhizobium strains and plasmids are listed in Table 1. The other strains of B. japonicum were isolated from the soils of the Tokachi field at the Tokachi Agricultural Station (Memuro, Tokachi, Hokkaido, Japan), the Nakazawa and Nagakura fields at the Niigata Agricultural Experiment Station (Nagaoka, Niigata, Japan), the Ami field at the experimental farm of Ibaraki University (Ami, Ibaraki, Japan), the Fukuyama field at the experimental farm of Hiroshima University (Fukuyama, Hiroshima, Japan), and the Ishigaki field at the experimental field of the Ishigaki Island Branch of the Tropical Agriculture Research Center (Ishigaki, Okinawa, Japan) as described previously (17). Bradyrhizobium strains were grown aerobically at 30°C in HM salt medium (19) supplemented with 0.1% arabinose and 0.025% yeast extract (Difco, Detroit Mich.). E. coli strains were grown on Luria-Bertani medium (14) at 37°C and supplemented with ampicillin (100 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Bradyrhizobium strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| B. japonicum HRS isolates | ||
| NC3a, NC32a | Field isolate from Nakazawa, Niigata, Japan | 18 |
| NK5, NK6, NK34 | Field isolate from Nagakura, Niigata, Japan | 17 |
| T2, T15, T22 | Field isolate from Tokachi, Hokkaido, Japan | 17 |
| USDA123 | Keysera | |
| B. japonicum non-HRS strains or isolates | ||
| NC4a, NC6a, NC41a | Field isolate from Nakazawa, Niigata, Japan | 18 |
| NK2, NK8, NK23 | Field isolate from Nagakura, Niigata, Japan | 17 |
| T7, T9, T12 | Field isolate from Tokachi, Hokkaido, Japan | 17 |
| USDA110, USDA122 | Keyser | |
| B. elkanii | ||
| USDA31, USDA76, USDA94 | Keyser | |
| USDA83 | Triplettb | |
| B. liaoningense 2281T | Type strain of B. liaoningense | Fanc |
| Plasmids | ||
| pRJ676 | pBR322 clone of nif region (9.2-kb HindIII fragment) in B. japonicum USDA110 | 8 |
| pCNTR | Ampr | 5 Prime→3 Prime, Inc. |
| pαHD7 | pCNTR containing ISB12 (RSα) (1.2 kb) in B. japonicum HRS isolate NK5 | This study |
| pβHD6 | pCNTR containing IS1632 (1.4 kb) in B. japonicum HRS isolate NK5 | This study |
| pT14HD4 | pCNTR containing ISB14B (RSβ) (1.4 kb) in B. japonicum HRS isolate T2 | This study |
| pT20HD4 | pCNTR containing ISB20 (2.0 kb) in B. japonicum HRS isolate T2 | This study |
| pT27HD5 | pCNTR containing IS1631 (2.7 kb) in B. japonicum HRS isolate T2 | This study |
| pC27HD8 | pCNTR containing ISB27B (2.7 kb) in B. japonicum HRS isolate NC3a | This study |
| pK09HD1 | pCNTR containing FK1 (0.8 kb) in B. japonicum HRS isolate NK5 | This study |
H. H. Keyser, U.S. Department of Agriculture, Beltsville, Md.
E. W. Triplett, University of Wisconsin, Madison.
H. Fan, Chinese Academy of Agriculture Sciences, Beijing, China.
Isolation of IS-like elements.
Total DNA was isolated as described previously (16). Isolation of repetitive sequences was performed by a method modified from that of Ohtsubo (15, 22). The concentration of total DNA from Bradyrhizobium strains was adjusted to 0.65 μg/μl with TE buffer (10 mM Tris–1 mM EDTA [pH 8.0]) (14). A 70-μl aliquot of the DNA solution was transferred to an Eppendorf tube (1.5 ml), denatured at 100°C for 5 min in boiling water, and immediately chilled on ice. Then 30 μl of 1 M NaCl was added to the denatured DNA solution in order to adjust the sodium concentration to 0.3 M. The solution (100 μl) was kept at 65°C for 40 s to enable renaturation at repetitive sequences, then chilled on ice quickly. Single-stranded DNA was digested with S1 nuclease as follows. The reaction mixture (111 to 112 μl), containing 68 to 137 U of S1 nuclease (Takara Shuzo Co., Ltd, Shiga, Japan) (1.5 to 3.0 U of S1 nuclease/μg of total input DNA), the denatured DNA solution (100 μl), 30 mM CH3COONa, 280 mM NaCl, and 1 mM ZnSO4, was incubated at 28°C for 5 h. S1 nuclease-resistant duplex DNAs were separated by electrophoresis on a 1.5% (wt/vol) agarose gel.
Cloning of duplex DNA.
Bands of S1 nuclease-resistant duplex DNA were visualized by using ethidium bromide and excised from the gel. The duplex DNA fragments (0.9, 1.2, 1.4, 2.0, and 2.7 kb) from HRS strains NC3a, NK5, and T2 were purified from the gel bands by using glass filters (16) and cloned with the General Contractor DNA Cloning System with the pCNTR vector (5 Prime→3 Prime, Inc., Boulder, Colo.), which enabled us to clone DNA fragments with irregular ends. The DNA fragments were ligated into the pCNTR vector, and the resulting constructs were used to transform competent E. coli cells [F− φ80d lacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdR17 (rK− mK+) deoR thi-1 sup44λ− gyrA96 relA1]. For each band of duplex DNA, at least five independent clones were examined by using DNA sequencing and several of the methods described below.
DNA hybridization.
Two types of hybridization were carried out. First, the S1 nuclease-resistant duplex DNAs were electrophoresed through a 0.8% agarose gel in TAE buffer. Second, total DNAs (3 μg/lane) from B. japonicum were digested with BamHI, XhoI, or HindIII and then electrophoresed under the same conditions. DNA from both gels was transferred onto nylon membranes (Hybond-N; Amersham, Tokyo, Japan). Hybridization was performed as described previously (16). For hybridization of the S1 nuclease-resistant DNA duplex, a 0.2-kb HindIII-ClaI fragment from RSα and a 0.25-kb XhoI-BglII fragment from RSβ were used as probes (18). For Southern blot hybridization of total DNA, the insert DNA fragments excised from pαHD7, pβHD6, pT14HD4, pT20HD4, pT27HD5, pC27HD8, and pK09HD1 (Table 1) were used as probes.
Estimation of copy numbers of RSα and RSβ.
The numbers of copies of RSα and RSβ were estimated by comparing the intensities and numbers of bands after hybridization with RSα- and RSβ-specific probes to those of USDA110, which contains 12 copies of RSα and 6 copies of RSβ (17).
DNA sequencing.
The DNA sequences of at least five clones from each band of duplex DNA were determined by using the dideoxy chain termination method (27) with an A.L.F. DNA sequencer II (Pharmacia Biotech, Uppsala, Sweden). DNA sequencing was carried out from both strands with the AutoRead Sequencing Kit (Pharmacia Biotech) and M13 universal and M13 reverse primers. When the resultant DNA sequences were aligned, a few base pairs of nucleotide sequences sometimes differed in length at their terminal ends, probably because of S1 nuclease attack at the blunt ends of the DNA duplex. Therefore, we selected the clone showing the longest DNA sequence that formed terminal inverted repeats (TIRs). Plasmid designations of several representative clones (pαHD7, pβHD6, pT14HD4, pT20HD4, pT27HD5, pC27HD8, and pK09HD1) are shown in Table 1. Further DNA sequencing of pT27HD5 was performed by using synthesized primers, the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit, and a model 373S DNA sequencer (Perkin-Elmer Applied BioSystems, Warrington, United Kingdom). Series of deletion clones from pT14HD4 and pT20HD4 were constructed with the Kilo-sequence Deletion Kit (Takara Shuzo Co. Ltd).
Nucleotide sequence accession number.
Novel DNA sequences determined in the present study have been submitted to the DDBJ/EMBL/GenBank database and can be found under accession no. AB011021 (IS1631), AB003134 (IS1632), AB003296 [ISB14B (RSβ)-L], AB003297 [ISB14B (RSβ)-R], AB003294 (ISB20-L), AB003295 (ISB20-R), AB003302 (ISB27B-L), AB003196 (ISB27B-R), and AB003299 (FK1).
RESULTS
Detection of repetitive sequences in B. japonicum HRS strains by an S1 nuclease-resistant DNA duplex technique.
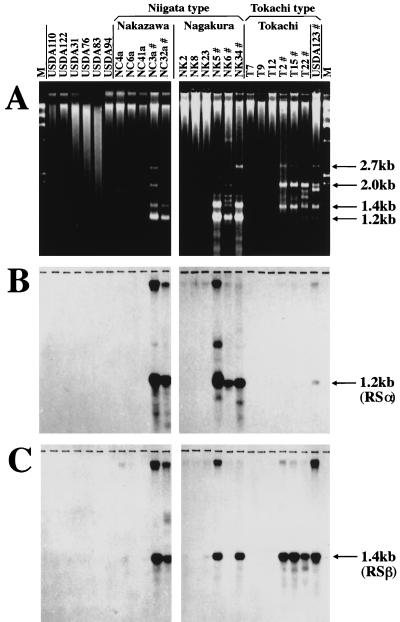
When total DNAs of soybean bradyrhizobia were analyzed by the Ohtsubo technique, using the formation of DNA duplexes by denaturation and renaturation of total DNA and by treatment with S1 nuclease (15, 22), several bands of S1 nuclease-resistant double-stranded DNA appeared exclusively in B. japonicum HRS strains (Fig. 1A). In contrast, we could not detect any clear bands in non-HRS B. japonicum and Bradyrhizobium elkanii strains (Fig. 1A). When RSα and RSβ were hybridized to blots of the S1 nuclease-resistant DNA duplexes, distinctive bands of 1.2 kb (RSα) and 1.4 kb (RSβ) were identified (Fig. 1B and C). RSα duplexes (1.2 kb) occurred exclusively in Niigata-type HRS strains with extremely high copy numbers of RSα (17), whereas RSβ duplexes were generally common in Niigata- and Tokachi-type HRS strains. In addition, other S1 nuclease-resistant duplex DNAs (for example, 2.0- and 2.7-kb bands) were observed in HRS strains.
FIG. 1.
Analysis of S1 nuclease-resistant double-stranded DNA in HRS (#) and non-HRS strains of B. japonicum and B. elkanii. S1 nuclease-resistant double-stranded DNA was electrophoresed on a 0.8% agarose gel (A), transferred onto nylon membranes, and hybridized with RSα (B) and RSβ (C). Strains with the prefix NC, NK, or T are B. japonicum (17, 18). Strains USDA110, USDA122, and USDA123 are B. japonicum, whereas strains USDA31, USDA76, USDA83, and USDA94 are B. elkanii. HRS strains have been categorized into Niigata and Tokachi types according to the copy numbers of RSα and RSβ (17).
Isolation of S1 nuclease-resistant duplex DNA from B. japonicum HRS strains.
When the S1 nuclease-resistant duplex DNAs from HRS strains were cloned and sequenced, five IS-like elements other than RSα and RSβ were found in NC3a, NK5, and T2 (Table 2). We have generally named these elements by using the prefix ISB (IS of Bradyrhizobium) when TIRs were found at both ends; otherwise the prefix FK was used. IS numbers IS1631 and IS1632 were assigned to two novel IS-like elements from the Plasmid Reference Center (E. Lederberg, Stanford University).
TABLE 2.
IS-like elements isolated from B. japonicum HRS strains
| IS element | Length (kb) | TIRa | Source | Sequence with highest level of homologyb (accession no.) | % of DNA homologyb | IS familyc |
|---|---|---|---|---|---|---|
| IS1631 | 2.7 | 41/53 | T2 | Alcaligenes eutrophus DR2 (D64144) | 55.0 | IS21 (IS3) |
| IS1632 | 1.4 | 34/44 | NK5 | Burkholderia cepacia IS1413 (U58191) | 60.0 | IS256 |
| RSα (ISB12) | 1.2 | 5/5 | NK5 | Bradyrhizobium japonicum RSRjα9 (M10925) | 97.8 | IS630-Tc1 |
| RSβ (ISB14B) | 1.4 | 17/22 | T2 | Shigella dysenteria IS911 (X17613) | 57.5 | IS3 |
| ISB20 | 2.0 | 22/26 | T2 | Bradyrhizobium japonicum 123 HRS1 (L09226) | 95.0 | |
| ISB27B | 2.7 | 17/23 | NC3a | Agrobacterium tumefaciens IS866 (M25805) | 63.1 | |
| FK1 | 0.8 | NK5 | Pseudomonas cepacia IS401 (U84154) | 58.3 | IS21 (IS3) |
Number of identical residues/total number of residues.
The DDBJ/EMBL/GenBank database was searched for homologies to the IS sequences; the maximum percentage of homology is shown. The entire sequences of IS1631, IS1632, and FK1 were available in the database. We have not determined the complete sequences of ISB12 (RSα), ISB14B (RSβ), ISB20, and ISB27B. Therefore, when the percentage of homology differed between the left and right sides of the IS element, the higher value is shown.
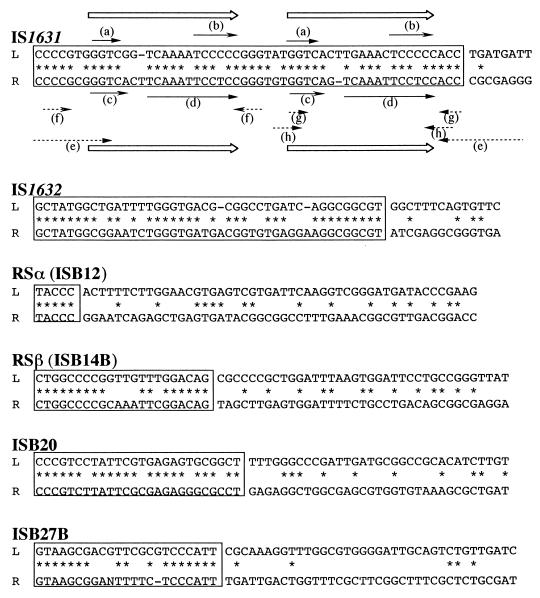
IS1631, IS1632, ISB20, and ISB27B possessed unique TIRs (Table 2 and Fig. 2), a characteristic of prokaryotic IS elements, and were homologous to other known bacterial IS elements (Table 2). ISB12 and ISB14B corresponded to RSα and RSβ, respectively; these were verified by hybridization using pRJ676 from B. japonicum USDA110 (8), pαHD7, and pT14HD4 (Table 1). RSβ was 1.4 kb in length and contained a 22-bp TIR (Table 2; Fig. 2), although the size of RSβ previously had been estimated as 0.95 kb (11). FK1 (787 bp) was shorter than the homologous IS element Pseudomonas cepacia IS401 (1.3 kb) (2) and did not contain TIRs (Table 2). Hence, this sequence may not represent a full-length copy; S1 nuclease might have attacked mismatched regions of the duplex DNA, leading to its truncation. Indeed, FK1 has a region homologous to the left part of IS401 (data not shown).
FIG. 2.
Comparison of sequences of putative TIRs of IS elements isolated from B. japonicum HRS strains. “L” denotes sequences at the 5′ (left) end, and “R” denotes complementary sequences at the 3′ (right) end, of the elements. Boxed and asterisked nucleotides are identical in and around the L and R sequences of the putative TIRs. For IS1631, peculiar structural features in and around the TIR are emphasized by solid arrows (short direct repeats) and dashed arrows (inverted repeats). The left TIR (nucleotides 1 to 53) contained 5′-GGTC (a) and 5′-TCCCCC (b) sequences repeated in a direct orientation. The right TIR (nucleotides 2660 to 2712) contained 5′-TGACC (c) and 5′-TCAAATTCCTCC (d) sequences repeated in a direct orientation. Only the right TIR contained four pairs of short inverted repeats that could form various hairpin structures: a 10-bp sequence with a 1-base mismatch (e), two 4-bp sequences (f and h), and a 3-bp sequence (g). IS1631 TIRs seem to be composed of consensus repeats (18 or 19 bp) of 5′-GGTCNN(N)TNAAANTCCNCC-3′ (open arrows), which is a common feature of the IS21 family (13).
Southern blot hybridization of IS-like elements.
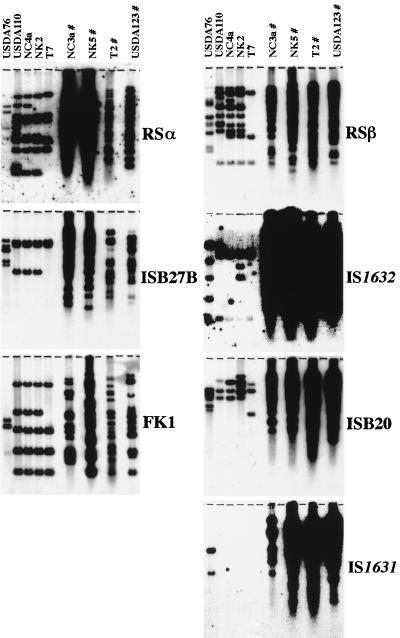
To examine the distribution and multiplicity of the new IS-like elements, total DNAs from B. japonicum HRS and non-HRS strains and a B. elkanii strain were digested with appropriate restriction enzymes and hybridized with the five new IS-like elements, RSα, and RSβ (Fig. 3). Hybridization profiles of HRS strains, revealed a smear of bands (Fig. 3). This result is not due to overloading of DNA on the agarose gel or to partial digestion of total DNA. More likely, the smearing in these lanes is due to high copy numbers of these elements as described previously (17, 18). The profiles from the ISB27B- and FK1-specific hybridization were similar to those from RSα-specific hybridization in that the copy numbers of the elements appeared to be highest in Niigata-type HRS strains. Similarly, intense signals were observed in all HRS strains after hybridization with IS1632, ISB20, and IS1631; the profiles were similar to those obtained with RSβ. Interestingly, no IS1631-specific hybridization was detected in B. japonicum non-HRS strains, although these strains showed several bands of hybridization with the other six elements (RSα ISB27B, FK1, RSβ IS1632, and ISB20). B. elkanii USDA76 showed a few bands of hybridization with all IS-like elements tested, including IS1631.
FIG. 3.
Southern hybridization with seven different IS-like elements. DNA probes RSα, ISB27B, FK1, RSβ, IS1632, ISB20, and IS1631 were prepared from plasmids pαHD7, pC27HD8, pK09HD1, pT14HD4, pβHD6, pT20HD4, and pT27HD5, respectively. Total DNAs from B. elkanii USDA76 and B. japonicum USDA110, NC4a, NK2, T7, NC3a, NK5, T2, and USDA123 were digested with BamHI for RSα-, ISB27B-, FK1-, IS1632-, and IS1631-specific hybridization, with XhoI for RSβ-specific hybridization, and with HindIII for ISB20-specific hybridization. The digested DNAs from each strain (3 μg/lane) were electrophoresed in 0.8% agarose-TAE (14), blotted onto a nylon filter, and hybridized with the radioactive probes. B. japonicum HRS strains (#) generally had numerous hybridization bands. This result is not due to overloading on the agarose gel or to partial digestion of total DNA as described previously (17, 18).
Distribution of IS1631 among more B. japonicum field isolates and Bradyrhizobium liaoningense.
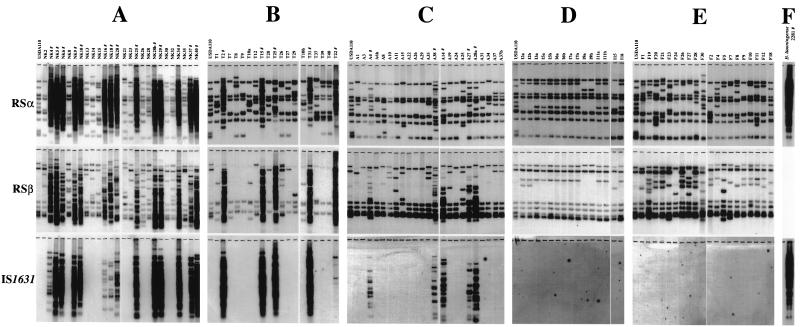
To assess whether the distribution of IS1631 is specific to HRS strains of B. japonicum, many field isolates from various sites in Japan were tested (Fig. 4A through E). Niigata- and Tokachi-type HRS strains were previously characterized by their higher copy numbers of RSα and RSβ (17). HRS strains from the Nagakura (Fig. 4A) and Tokachi (Fig. 4B) sites that had high copy numbers of RSα and RSβ hybridized with IS1631. In contrast, non-HRS strains of B. japonicum from these sites did not have the element (Fig. 4A and B).
FIG. 4.
Distribution of IS1631 among B. japonicum field isolates and B. liaoningense. Total DNAs from B. japonicum field isolates from five field sites in Nagakura (A), Tokachi (B), Ami (C), Ishigaki (D), and Fukuyama (E) and from the type strain, of B. liaoningense, 2281 (F), were digested with XhoI. USDA110, a non-HRS strain of B. japonicum, was used as a control. Field isolates were designated by numbers prefixed with A, T, NC, NK, F, or I. #, HRS strains (determined according to the copy numbers of RSα and RSβ [17]). Niigata-type HRS strains from the Nagakura site (A) had a markedly higher number of RSα copies than non-HRS strains, whereas Tokachi-type HRS strains showed abundant copies of RSβ (B) (17). HRS strains from the Ami site (C) had a significantly higher copy number of RSβ than non-HRS strains from this site and were defined as Ami-type HRS strains in the present study (see the text). B. liaoningense 2281T fell into the category of HRS strains in terms of hybridization with RSα and IS1631.
Five strains isolated from the Ami site (A4, A38, A14, A27, and A28a) hybridized with IS1631, although these strains had seemed to be intermediate between HRS and non-HRS strains on the basis of copy numbers of RSα (Fig. 4C). Nevertheless, these five Ami strains possessed significantly more copies of RSβ than other strains from this site (Fig. 4C). These five IS1631-carrying strains had an estimated 9 to 17 (mean ± standard deviation, 12.8 ± 2.9) copies of RSβ, whereas other strains from Ami had 5 to 7 (6.4 ± 0.8) copies of RSβ. On the basis of copy numbers of RSα and RSβ, we previously categorized HRS strains into two types, the Niigata-type strains (Nagakura site [Fig. 4A]) and the Tokachi-type bacteria (Tokachi site [Fig. 4B]). In light of the occurrence of IS1631 and the increased numbers of RSβ copies in Ami strains, we have assigned these five strains to the newly designated category of Ami-type HRS strains (Fig. 4C). No IS1631-carrying strains were collected from the Ishigaki (Fig. 4D) and Fukuyama (Fig. 4E) sites, and apparently no HRS strains were isolated from these sites.
Xu et al. (31) proposed the name Bradyrhizobium liaoningense sp. nov. in light of the phenotypic features of the very slow growing soybean bradyrhizobia that are indigenous to Chinese soils. HRS strains of B. japonicum resemble B. liaoningense in their extremely slow growth and sensitivity to antibiotics (17). To evaluate whether the distribution of IS elements is similar to that in B. japonicum HRS strains, total DNA from the type strain of B. liaoningense was hybridized with RSα and IS1631. Like B. japonicum HRS strains, B. liaoningense 2281T carried many copies of both RSα and IS1631 (Fig. 4F).
Nucleotide sequence and structural features of IS1631.
Because IS1631 occurred only in HRS strains of B. japonicum and not in non-HRS strains, we determined the entire nucleotide sequence of IS1631 (as cloned in pT27HD5) from the B. japonicum HRS strain T2. The nucleotide sequence of IS1631 showed similarity to those of IS21 (24), IS1162 (29), Alcaligenes eutrophus DR2 (20), and other members of the IS21 family (Table 2). IS1631 was 2,712 bp in length and had an imperfect TIR of 53 bp with 12 mismatches. In and around the putative TIR of IS1631, there were peculiar structural features: short direct and inverted repeats that were composed of two 18- or 19-bp repeats of 5′-GGTCN2–3TNAAANTCCNCC-3′ (Fig. 2). Several members of the IS21 family have multiple repeated sequences (17 to 23 bp) at their ends; these sequences include part of the TIR and may represent transposase binding sites (13).
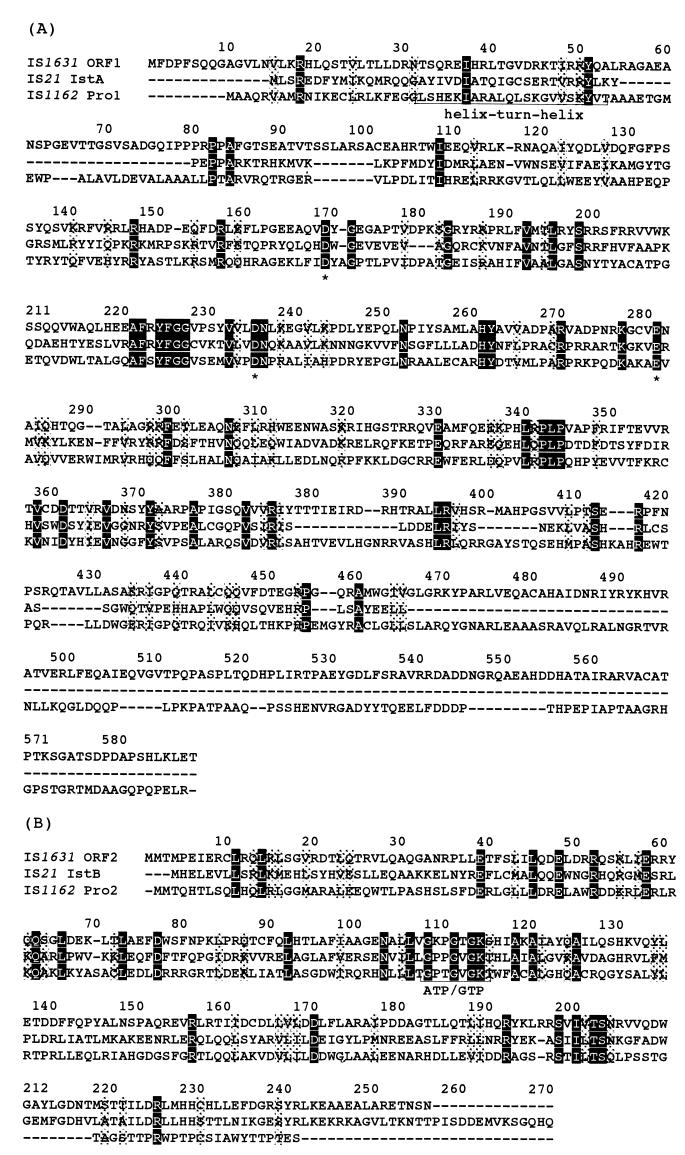
Sequence analysis revealed the presence of two open reading frames (ORFs) on the same DNA strand. Shine-Dalgarno sequences were located upstream of the two ORFs (data not shown). The −35 and −10 promoter regions were found upstream of ORF1. ORF1 (nucleotides 108 to 1865) encodes a putative protein of 585 amino acids (66,026 Da). The amino acid sequence of this protein resembles those of transposases IstA from IS21 and Pro1 from IS1162. In addition, ORF1 contained two motifs of transposases and integrases that are common in the IS21 family (Fig. 5A): (i) in the N-terminal region, a helix-turn-helix motif capable of DNA binding and (ii) a DDE triad motif, which is a catalytic domain for bacterial transposase and retroviral integrase (6, 13).
FIG. 5.
Alignment of the proteins deduced from the nucleotide sequences of IS1631, IS21, and IS1162. Heavy shading indicates identical amino acid residues in all elements; light shading denotes similar or partially conserved residues. Residues within the following groups were considered similar: G, A, S, T, and P; L, I, V, and M; F, Y, and W; D, E, N, and Q; and K, R, and H. (A) IS1631 ORF1 protein and related proteins. ∗, DDE motif. (B) IS1631 ORF2 protein and related proteins. ATP/GTP, ATP-GTP binding site motif.
The putative protein encoded by ORF2 of IS1631 (nucleotides 1870 to 2637) contains 255 amino acids (29,266 Da). It is similar to IstB from IS21 and Pro2 from IS1162, which are helper proteins for transposition and cointegration. ORF2 contained an ATP-GTP binding motif that is conserved in all members of the IS21 family (6, 13, 21) (Fig. 5B).
Nucleotide sequences and structural features of other IS-like elements from B. japonicum HRS strains.
IS1632, isolated from the B. japonicum HRS strain NK5, was 1,395 bp long and contained a 44-bp imperfect TIR with 10 mismatches (Table 2). IS1632 resembled Burkholderia cepacia IS1413 (9), Mycobacterium smegmatis IS6120 (5), Sinorhizobium meliloti ISRm3 (30), Staphylococcus aureus IS256 (12), and Thiobacillus ferrooxidans IST2 (32) in the nucleotide sequence, total length, and ORF size and in the amino acid sequence of the putative transposase. These IS elements are members of the IS256 family (13, 21).
ISB20 (2.0 kb) from the B. japonicum HRS strain T2 was highly homologous (95% identity) to HRS1 (2.1 kb) from B. japonicum USDA424, which has DNA and amino acid sequence homology to the Acetobacter pasteurianus insertion sequence IS1380 (10) (Table 2). When DNA sequences of ISB20 and HRS1 were compared, it was found that the 3′ end of HRS1 has a direct repeat (at positions 1780 to 1891 and 1892 to 2003) of a 112-bp sequence (accession no. L09226) (10), whereas ISB20 contained only one copy of this sequence. ISB20 had imperfect TIRs (Fig. 2), but HRS1 had no TIR because of substitution of a few base pairs. These results suggest that ISB20 and HRS1 have the same origin. ISB27B was homologous to IS866, which is distributed among Ti plasmids and chromosomes of Agrobacterium tumefaciens octopine biotypes (1).
DISCUSSION
We successfully purified IS-like elements from B. japonicum HRS strains as double-stranded DNA fragments by denaturation and renaturation of total DNA followed by treatment with S1 nuclease. This technique is based on the rapid formation of duplexes from the inverted repeat DNA sequences during renaturation. Ohtsubo and Ohtsubo (22) detected two copies of IS1 in an inverted orientation with a spacer region of about 34 kb in plasmid R100-25 by forming the duplex of IS1 by this technique. The fact that S1 nuclease-resistant double-stranded IS-like elements appeared exclusively in Niigata- and Tokachi-type HRS strains of B. japonicum (Fig. 1) suggests that IS-like elements are distributed throughout the genome and plasmids of B. japonicum HRS strains in pairs, with the members of each pair adjacent to one another and in an inverted orientation. However, the possibility remains that IS elements that lie distant from each other generate duplexes because of the high numbers of copies of the element. Nevertheless, HRS and non-HRS strains of B. japonicum differ markedly in the distribution and abundance of the IS elements examined.
The unique distribution of IS1631 in B. japonicum HRS strains (Fig. 3 and 4) suggests that the increase in IS-like elements in B. japonicum HRS strains may involve the presence of IS1631. IS1631 is a typical member of the IS21 family (Table 2; Fig. 5). Among several pathways of transposition mediated by IS21, the formation of cointegrates between a plasmid containing tandem repeats of IS21 and a target replicon is most active; this rearrangement presumably proceeds via a nonreplicative cut-and-paste mechanism (24–26). Cointegration of an IS21–IS21 plasmid is very similar to linear retroviral insertion (6). One possible explanation for HRS strain-specific distribution of IS1631 among B. japonicum isolates is that a conjugative plasmid or a retrovirus containing at least two copies of the element and derived from a soil microbial community might be integrated into a replicon in IS1631-free non-HRS strains of B. japonicum by the above pathway.
Southern hybridization with the seven different IS-like elements (Fig. 3) suggested that HRS strains harbor higher copy numbers of putative IS elements other than RSα and RSβ than do non-HRS strains of B. japonicum. To assess the mechanisms by which the copy numbers of IS-like elements have increased simultaneously and by which genome rearrangement may have occurred in HRS strains (17), genetic and physical analyses in symbiotic regions would be a possible approach. Shifts and duplications of nifDK- and hupLS-specific hybridization profiles were observed in Niigata-type HRS strains containing many copies of RSα (17).
B. japonicum HRS strains resembled B. liaoningense 2281T in their hybridization profile with RSα and IS1631 (Fig. 4F) and their extremely slow growth. If HRS strains are identical to B. liaoningense, HRS strains of B. japonicum might be indigenous to soils in China as well as to soils in Japan and the United States (serogroup 123) (17). The technique of IS1631-specific hybridization may be used to efficiently survey and identify B. japonicum HRS strains and, when combined with phenotypic tests, such as production of indole-3-acetic acid, to distinguish B. japonicum from B. elkanii (18). To clarify the phylogenic relationships between B. japonicum HRS strains and B. liaoningense, classification including analysis of 16S rRNA sequences will be required.
ACKNOWLEDGMENTS
This work was supported in part by grants to K.M. from the Ministry of Education, Science, and Culture of Japan (07660077 and 10460028) and the Joint Research Program of the Institute of Genetic Ecology, Tohoku University (942206, 953009, and 981002), and by a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists (to T.I.).
We thank T. Hattori (Tohoku University) for valuable discussions.
REFERENCES
- 1.Bonnard G, Vincent F, Otten L. Sequence and distribution of IS866, a novel T region-associated insertion sequence from Agrobacterium tumefaciens. Plasmid. 1989;22:70–81. doi: 10.1016/0147-619x(89)90037-1. [DOI] [PubMed] [Google Scholar]
- 2.Byrne A M, Lessie T G. Characteristics of IS401, a new member of the IS3 family implicated in plasmid rearrangements in Pseudomonas cepacia. Plasmid. 1994;34:138–147. doi: 10.1006/plas.1994.1015. [DOI] [PubMed] [Google Scholar]
- 3.Galas D J, Chandler M. Bacterial insertion sequences. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C: American Society for Microbiology; 1989. pp. 110–162. [Google Scholar]
- 4.Gay P, Le Coq D, Steinmetz M, Berkelman T, Kado C I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol. 1985;164:918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guilhot C, Gicquel B, Davies J, Martin C. Isolation and analysis of IS6120, a new insertion sequence from Mycobacterium smegmatis. Mol Microbiol. 1992;6:107–114. doi: 10.1111/j.1365-2958.1992.tb00842.x. [DOI] [PubMed] [Google Scholar]
- 6.Haas D, Berger B, Schmid S, Seitz T, Reimmann C. Insertion sequence IS21: related insertion sequence elements, transpositional mechanisms, and application to linker insertion mutagenesis. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular biology of Pseudomonads. Washington, D.C: American Society for Microbiology; 1996. pp. 238–249. [Google Scholar]
- 7.Hahn M, Hennecke H. Mapping of Bradyrhizobium japonicum DNA region carrying genes for symbiosis and an asymmetric accumulation of reiterated sequences. Appl Environ Microbiol. 1987;53:2247–2252. doi: 10.1128/aem.53.9.2247-2252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennecke H. Recombinant plasmids carrying nitrogen fixation genes from Rhizobium japonicum. Nature (London) 1981;291:354–355. [Google Scholar]
- 9.Hubner A, Hendrickson W. A fusion promoter created by a new insertion sequence, IS1490, activates transcription of 2,4,5-trichlorophenoxyacetic acid catabolic genes in Burkholderia cepacia AC1100. J Bacteriol. 1997;179:2717–2723. doi: 10.1128/jb.179.8.2717-2723.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Judd A K, Sadowsky M J. The Bradyrhizobium japonicum serocluster 123 hyperreiterated DNA region, HRS1, has DNA and amino acid sequence homology to IS1380, an insertion sequence from Acetobacter pasteurianus. Appl Environ Microbiol. 1993;59:1656–1661. doi: 10.1128/aem.59.5.1656-1661.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaluza K, Hahn M, Hennecke H. Repeated sequences similar to insertion elements clustered around the nif region of the Rhizobium japonicum genome. J Bacteriol. 1985;162:535–542. doi: 10.1128/jb.162.2.535-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyon B R, Gillespie M T, Skurray R A. Detection and characterization of IS256, an insertion sequence in Staphylococcus aureus. J Gen Microbiol. 1987;133:3031–3038. doi: 10.1099/00221287-133-11-3031. [DOI] [PubMed] [Google Scholar]
- 13.Mahillon J, Chandler M. Insertion sequences. Microbiol Mol Biol Rev. 1998;62:725–774. doi: 10.1128/mmbr.62.3.725-774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 15.Matsutani S, Ohtsubo H, Maeda Y, Ohtsubo E. Isolation and characterization of IS elements repeated in the bacterial chromosome. J Mol Biol. 1987;196:445–455. doi: 10.1016/0022-2836(87)90023-4. [DOI] [PubMed] [Google Scholar]
- 16.Minamisawa K. Division of rhizobitoxine-producing and hydrogen-uptake positive strains of Bradyrhizobium japonicum by nifDKE sequence divergence. Plant Cell Physiol. 1990;31:81–89. [Google Scholar]
- 17.Minamisawa K, Isawa T, Nakatsuka Y, Ichikawa N. New Bradyrhizobium japonicum strains that possess high copy numbers of the repeated sequence RSα. Appl Environ Microbiol. 1998;64:1845–1851. doi: 10.1128/aem.64.5.1845-1851.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minamisawa K, Seki T, Onodera S, Kubota M, Asami T. Genetic relatedness of Bradyrhizobium japonicum field isolates as revealed by repeated sequences and various other characteristics. Appl Environ Microbiol. 1992;58:2832–2839. doi: 10.1128/aem.58.9.2832-2839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieukoop A J, Banfalvi Z, Deshmane N, Garhold D, Schell M G, Sirotkin K M, Stacey G. A locus encoding host range is linked to the common nodulation genes of Bradyrhizobium japonicum. J Bacteriol. 1987;169:2631–2638. doi: 10.1128/jb.169.6.2631-2638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa N, Miyashita K. Recombination of a 3-chlorobenzoate catabolic plasmid from Alcaligenes eutrophus NH9 mediated by direct repeat elements. Appl Environ Microbiol. 1995;61:3788–3795. doi: 10.1128/aem.61.11.3788-3795.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtsubo E, Sekine Y. Bacterial insertion sequences. Curr Top Microbiol Immunol. 1996;204:1–26. doi: 10.1007/978-3-642-79795-8_1. [DOI] [PubMed] [Google Scholar]
- 22.Ohtsubo H, Ohtsubo E. Isolation of inverted repeat sequences, including IS1, IS2, and IS3, in Escherichia coli plasmids. Proc Natl Acad Sci USA. 1976;73:2316–2320. doi: 10.1073/pnas.73.7.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raabe T, Jenny E, Meyer J. A selection cartridge for rapid detection and analysis of spontaneous mutations including insertions of transposable elements in Enterobacteriaceae. Mol Gen Genet. 1988;215:176–180. doi: 10.1007/BF00331322. [DOI] [PubMed] [Google Scholar]
- 24.Reimmann C, Haas D. Mode of replicon fusion mediated by the duplicated insertion sequence IS21 in Escherichia coli. Genetics. 1987;155:619–625. doi: 10.1093/genetics/115.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reimmann C, Haas D. The istA gene of insertion sequence IS21 is essential for cleavage at the inner 3′ ends of tandemly repeated IS21 elements in vitro. EMBO J. 1990;9:4055–4063. doi: 10.1002/j.1460-2075.1990.tb07627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reimmann C, Rella M, Haas D. Integration of replication-defective R68.45-like plasmids into the Pseudomonas aeruginosa chromosome. J Gen Microbiol. 1988;134:1515–1523. doi: 10.1099/00221287-134-6-1515. [DOI] [PubMed] [Google Scholar]
- 26a.Sameshima, R., et al. Unpublished data.
- 27.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon R, Hötte B, Klauke B, Kosier B. Isolation and characterization of insertion sequence elements from gram-negative bacteria by using new broad-host-range, positive selection vectors. J Bacteriol. 1991;173:1502–1508. doi: 10.1128/jb.173.4.1502-1508.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solinas F, Marconi A M, Ruzzi M, Zennaro E. Characterization and sequence of a novel insertion sequence, IS1162, from Pseudomonas fluorescens. Gene. 1995;155:77–82. doi: 10.1016/0378-1119(94)00922-f. [DOI] [PubMed] [Google Scholar]
- 30.Wheatcroft R, Laberge S. Identification and nucleotide sequence of Rhizobium meliloti insertion sequence ISRm3: similarity between the putative transposase encoded by ISRm3 and those encoded by Staphylococcus aureus IS256 and Thiobacillus ferrooxidans IST2. J Bacteriol. 1991;173:2530–2538. doi: 10.1128/jb.173.8.2530-2538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu L M, Ge C, Cui Z, Li J, Fan H. Bradyrhizobium liaoningense sp. nov., isolated from the root nodules of soybeans. Int J Syst Bacteriol. 1995;45:706–711. doi: 10.1099/00207713-45-4-706. [DOI] [PubMed] [Google Scholar]
- 32.Yates J R, Cunningham R P, Holmes D S. IST2: an insertion sequence from Thiobacillus ferrooxidans. Proc Natl Acad Sci USA. 1988;85:7284–7287. doi: 10.1073/pnas.85.19.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zekri S, Soto M J, Toro N. ISRm4-1 and ISRm9, two novel insertion sequences from Sinorhizobium meliloti. Gene. 1998;207:93–96. doi: 10.1016/s0378-1119(97)00625-2. [DOI] [PubMed] [Google Scholar]