SUMMARY
Toxoplasmosis seroprevalence varies considerably between countries. We studied the seoprevalence of Toxoplasma gondii IgG antibodies in a national sample of the Israeli population; 2794 sera were tested. The highest age-adjusted seroprevalence rate was in Arabs (non-Bedouins) (60·4%), significantly higher compared to the rate in Jews (19·9%) and Bedouins (27·5%) (P < 0·01). There were no significant gender differences. Seropositivity increased with age in all population groups. For Jews, seropositivity was associated with place of birth and socioeconomic status. A finding of low seroprevalence rate in Bedouins despite their poor living conditions and close contact with livestock is surprising, and might be attributed to the dry and hot climate conditions in their area of residence. In women of reproductive age the seroprevalence was 15·1% in Jews, 25·4% in Bedouins and 72·3% in Arabs (non-Bedouins). Thus, the majority of pregnant women are susceptible to primary infection with T. gondii, and the risk for congenital toxoplasmosis remains high.
Key words: Arabs, Bedouins, Israel, Jews, sera bank, seroprevalence, Toxoplasma gondii
INTRODUCTION
Toxoplasmosis is a systemic protozoal disease caused by Toxoplasma gondii. The disease can often be asymptomatic, but it can also be manifested as an acute disease characterized by lymphadenopathy, fever and malaise. In immunocompromised patients, such as HIV-positive individuals, it can cause encephalitis, chorioretinitis, cerebritis and myocarditis, often leading to death [1]. A primary infection during early pregnancy can result in a wide range of clinical pathologies in the foetus, the most severe being brain damage and hydrocephaly [1, 2].
The definitive hosts of T. gondii are domestic cats and other felidae, and the intermediate hosts are humans and warm-blooded animals. Sporulated oocysts survive for a long time in the environment, mainly in moist climates [1]. Humans are infected through ingestion of foods, including muscle cysts from beef, mutton and chicken meat, unwashed vegetables and fruits, as well as soil and water contaminated with oocysts. Infection can also be intrauterine [1, 3].
Data on population seroprevalence were reported from several countries and showed considerable variation. The overall age-adjusted seroprevalence rate in the USA was 22·5% in sera obtained during 1988–1994 [3] and 10·8% in sera obtained during 1999–2004 [4]. A decrease in prevalence for T. gondii antibodies was also observed in The Netherlands between 1995–1996 and 2006–2007 [5] and in pregnant women from France between 1995 and 2003 [6]. Studies conducted in Scandinavian countries showed a wide variation of seropositivity, ranging from 9·8% to 54·9% [7]. Studies from Middle Eastern countries revealed a 29·8% seropositivity rate in Qatar and 62·2% in Lebanon [8, 9].
Toxoplasmosis is not a reportable disease in Israel therefore there is sparse data on its epidemiology. Seroprevalence studies conducted around two decades ago on limited population groups from specific geographical regions, showed lower rates in Jews compared to Arabs (13·6–40% and 49–74%, respectively) [10–13]. Table 1 summarizes the main findings of these studies. The aim of the present study was to evaluate the prevalence of T. gondii IgG antibodies in a national sample of the Israeli population with an emphasis on Jews, Arabs and Bedouins.
Table 1.
Studies on Toxoplasma gondii IgG prevalence in Israel
| Year of study | Population (N) | Test (cut-off) | Prevalence (%) |
|---|---|---|---|
| 1970–1973 [11] | Patients referred for serological examination for toxoplasmosis (15012) | Sabin–Feldman dye test (1:16) | 34·6 |
| Healthy women (554) | 29·0 | ||
| 1988–1989 [10] | Pregnant women of northern Israel (185) | Immunofluorescent antibody test (1:16) and Sabin–Feldman dye test | 21 |
| Early 1990s [13] | Rural population of northern Israel | Indirect immunofluorescent test (1:16) and Sabin–Feldman dye test | |
| Jews (487) | 22·2 | ||
| Arabs (399) | 55·8 | ||
| Early 1990s [12] | Rural population of central Israel (1315) | Immunofluorescent antibody test (1:16) and Sabin–Feldman dye test | 29·3 |
METHODS
Study population
A cross-sectional study was performed using a random sample of stored sera that had been collected for the Israeli sera bank at the Israeli Center for Disease Control (established in 1997). Sera from both genders are collected from all regions of Israel throughout the entire year. Sera of persons aged ⩽18 years and ⩾55 years are residual sera from diagnostic laboratories, while sera of persons aged 19–55 years are collected from both healthy blood donors and residual sera from diagnostic laboratories. Sera from subjects with confirmed or suspected immunological disorders are discarded, and the rest of the sera are stored at −80°C. Information collected for each serum sample includes age, gender, place of residence and country of birth of the individual.
The Israeli population is comprised of two major population groups: the Jewish population (80%) and Arab population (20%). Within the Arab population there is a distinct subpopulation of semi-nomadic tribes, the Bedouins, who live mostly in the desert area in the southern part of Israel. Representative samples for the Jewish population were randomly selected from samples collected during 2006–2007 using an age- and sex-stratified sampling design. Since representative samples for the whole Arab populations are available in the sera bank from 2011 onwards, samples for the Arab population were randomly selected from samples collected during 2011.
A rank was assigned to each residential area according to the Israel Central Bureau of Statistics (CBS) definitions [14]. The ranks ranged from 1 (low) to 10 (high). This definition is based on education, housing density, financial resources and dependencies, working power and employment, motorization and standard of living. Rank was used as an indicator for socioeconomic status (SES). We considered ranks 1–5 as ‘low’ and 6–10 as ‘high’ SES. Ninety-seven percent of the Bedouin residential areas are ranked as 1. Residential areas of < 10 000 inhabitants were considered ‘rural’ and those with ⩾10 000 inhabitants as ‘urban’.
Laboratory testing
Laboratory analysis was performed at the National Toxoplasmosis Reference Laboratory which is part of the National Public Health Laboratory in Tel Aviv, Israel. The presence of IgG antibodies against T. gondii was evaluated by enzyme immunoassay using alkaline phosphatase-linked monoclonal antibodies against human IgG. The tests were performed with the automated Vitek Diagnostic Assay System (VIDAS, bioMérieux, France), according to the manufacturer's instructions. This test is used in our laboratory, as well as in other diagnostic laboratories throughout the world, as a routine primary screening test for exposure to T. gondii [15]. The sensitivity of the test is > 97% and the specificity is > 99% [16, 17]. We considered IgG values of 4 IU/ml as the cut-off titre, IgG ⩾4 IU/ml were considered positive while lower values were considered negative results.
Statistical analysis
Prevalence rates of T. gondii IgG antibodies and 95% confidence intervals (CIs) were calculated by age and population group [Jews, Arabs (non-Bedouins), Bedouins]. Age-adjusted rates were calculated using the Israeli census for 2007 as reference. Comparisons between the prevalence rates by age, sex, population group, place of birth (for the Jewish population), SES, and type of residence (urban or rural) were done by χ2 test and multivariate analysis. Crude and adjusted prevalence ratios (PRs) and 95% CIs were calculated. A two-tailed P < 0·05 value was considered statistically significant. All analyses were performed using SPSS software v. 19.0.0 (SPSS Inc., USA) and Excel software.
RESULTS
There were 1813 samples of Jews, 597 of Arabs (non-Bedouins) and 384 of Bedouins available for analysis. Table 2 shows the number of samples that were analysed by age and population group.
Table 2.
Number of samples analysed by age and population groups
| Age group (yr) | Jews | Arabs | Bedouins |
|---|---|---|---|
| 0–9 | 182 | 128 | 63 |
| 10–19 | 353 | 156 | 59 |
| 19–29 | 366 | 62 | 44 |
| 30–39 | 366 | 59 | 82 |
| 40–49 | 195 | 68 | 75 |
| 50–59 | 191 | 74 | 39 |
| ⩾60 | 160 | 50 | 22 |
| Total | 1813 | 597 | 384 |
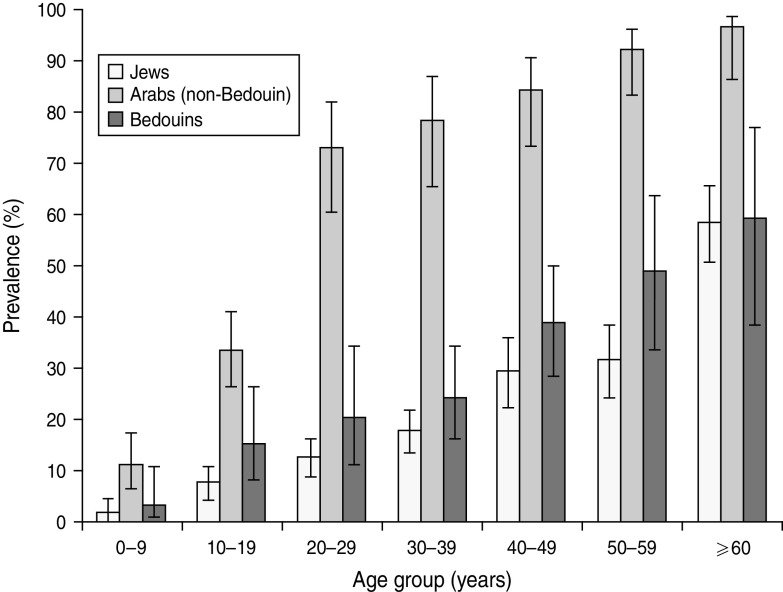
For the Jewish population, 351 (19·4%) samples were positive, and the age-adjusted prevalence rate was 19·9% (95% CI 18·2–21·6). Of these, 179 (19·4%) males and 172 (19·3%) females were positive (P = n.s.). Seropositivity increased significantly with age: it was 1·6% in the 0–9 years age group compared to 7·6% in the 10–19 years age group (P = 0·01). There was a gradual increase in the seroprevalence rate from 7·6% in the 10–19 years age group to 31·4% in the 50–59 years age group, followed by a sharp increase to 58·1% in the ⩾60 years age group (Fig. 1). Seroprevalence was 15·1% (95% CI 12·0–18·8) in women of childbearing age (18–44 years).
Fig. 1.
The prevalence of Toxoplasma gondii IgG antibodies in Israeli Jews and Arabs by age group.
The prevalence of antibodies varied significantly with place of birth. The highest rates were for Jews born in Africa, Europe and Asia (41·9%, 39·0% and 37·1%, respectively), and the lowest rates were in those born in North America and Australia (6·6%) (Table 3). Significantly higher rates were found in individuals living in urban areas compared to rural areas (21·6% and 14·3%, respectively, P < 0·001). Those of lower SES had significantly higher rates compared to those of higher SES (24·2% and 16·8%, respectively, P < 0·001) (Table 3). In the multivariate analysis that included age, place of birth, area of residence and SES, older age, being born in Europe, Asia or Africa and lower SES were significantly associated with higher seropositivity rates (Table 4).
Table 3.
The prevalence of Toxoplasma gondii IgG antibodies in the Jewish population by sociodemographic parameters
| Variable | Prevalence (%) | OR (95% CI) | P |
|---|---|---|---|
| Age group (yr) | |||
| <10 | 1·6 | Ref. | |
| 10–19 | 7·6 | 4·9 (1·5–16·5) | 0·009 |
| 20–29 | 12·6 | 8·6 (2·6–28·0) | <0·001 |
| 30–39 | 17·8 | 12·9 (4·0–41·6) | <0·001 |
| 40–49 | 29·2 | 24·7 (7·6–80·4) | <0·001 |
| 50–59 | 31·4 | 27·3 (8·4–89·0) | <0·001 |
| ⩾60 | 58·1 | 82·8 (25·4–27·5) | <0·001 |
| Gender | |||
| Male | 19·4 | Ref. | |
| Female | 19·3 | 1·0 (0·8–1·3) | 0·95 |
| Place of birth | |||
| Israel | 13·0 | Ref. | |
| Europe | 39·0 | 4·2 (3·2–5·6) | <0·001 |
| Asia | 37·1 | 4·2 (2·2–8·1) | <0·001 |
| Africa | 41·9 | 4·8 (3·1–7·5) | <0·001 |
| Central & South America | 22·5 | 1·9 (0·9–4·2) | 0·09 |
| North America & Australia | 6·6 | 0·5 (0·2–1·3) | 0·15 |
| Area of residence | |||
| Rural | 14·3 | Ref. | |
| Urban | 21·6 | 1·6 (1·3–2·2) | <0·001 |
| Socioeconomic status | |||
| High | 16·8 | Ref. | |
| Low | 24·2 | 1·6 (1·2–2·0) | <0·001 |
OR, Odds ratio; CI, confidence interval.
Table 4.
Multivariate analysis of factors associated with Toxoplasma gondii IgG seroprevalence in the Jewish population
| Variable | Adjusted OR (95% CI) | P |
|---|---|---|
| Age group (years) | ||
| <10 | Ref. | |
| 10–19 | 4·8 (1·4–16·2) | 0·01 |
| 20–29 | 8·4 (2·6–27·6) | <0·001 |
| 30–39 | 12·4 (3·8–40·1) | <0·001 |
| 40–49 | 21·8 (6·6–72·0) | <0·001 |
| 50–59 | 21·8 (6·6–72·1) | <0·001 |
| ⩾60 | 55·5 (16·5–186·9) | <0·001 |
| Place of birth | ||
| Israel | Ref. | |
| North America & Australia | 0·3 (0·1–0·7) | 0·009 |
| Europe, Asia, Africa and Latin America | 1·6 (1·2–2·1) | 0·004 |
| Area of residence | ||
| Rural | Ref. | |
| Urban | 1·2 (0·9–1·6) | 0·33 |
| Socioeconomic status | ||
| High | Ref. | |
| Low | 1·4 (1·0–1·8) | 0·03 |
OR, Odds ratio; CI, confidence interval.
For Arabs (non-Bedouins), 330 (55·3%) samples were positive. The overall age-adjusted rate was 60·4% (95% CI 57·2–63·5). Prevalence rates increased threefold from the 0–9 years to 10–19 years (10·9% and 33·3%, respectively) age groups, doubled at the 20–29 years (72·6%) age group and increased gradually with age, peaking in the ⩾60 years (96·0%) age group (Fig. 1). Seropositivity rates for Arabs were markedly and significantly higher than for Jews for all age groups. Those of lower SES had higher rates compared to those of higher SES (P = 0·09). Seroprevalence was 72·3% (95% CI 63·6–79·5) in women of childbearing age (18–44 years).
Bedouins had significantly lower rates compared to Arabs (non- Bedouins) (26·3% vs. 55·3%, P < 0·001), for all age groups. The age-adjusted rate for Bedouins was 27·5% (95% CI 22·7–32·2) compared to 60·4% for Arabs (non-Bedouins), and 19·9% for Jews. Prevalence rate increased sharply from the 0–9 years to 10–19 years (3·2% and 15·3%, respectively) age groups and thereafter increased gradually to reach 59·1% in the ⩾60 years age group. Seroprevalence was 25·4% (95% CI 18·8–33·2) in women of childbearing age (18–44 years). For the Bedouins higher rates were in urban compared to rural populations.
Both in Arabs (non-Bedouins) and in Bedouins there were no significant differences between males and females (Table 5).
Table 5.
The prevalence of Toxoplasma gondii IgG antibodies in Arabs (non-Bedouins) and Bedouins by sociodemographic parameters
| Variable | Arabs (non-Bedouins) | Bedouins | ||||
|---|---|---|---|---|---|---|
| Prevalence (%) | OR (95% CI) | P | Prevalence (%) | OR (95% CI) | P | |
| Age group (yr) | ||||||
| <10 | 10·9 | Ref. | 3·2 | Ref. | ||
| 10–19 | 33·3 | 4·1 (2·1–7·8) | <0·001 | 15·3 | 5·5 (1·1–26·6) | 0·03 |
| 20–29 | 72·6 | 21·6 (9·8–47·4) | <0·001 | 20·5 | 7·8 (4·6–38·4) | 0·01 |
| 30–39 | 78·0 | 28·8 (12·6–66·0) | <0·001 | 24·4 | 9·8 (2·2–43·9) | 0·003 |
| 40–49 | 83·8 | 42·2 (18·0–98·9) | <0·001 | 38·7 | 19·2 (4·4–84·7) | <0·001 |
| 50–59 | 91·9 | 92·3 (33·9–251·5) | <0·001 | 48·7 | 29·0 (6·2–135·4) | <0·001 |
| ⩾60 | 96·0 | 195·4 (42·8–893·1) | <0·001 | 59·1 | 44·1 (8·5–228·3) | <0·001 |
| Gender | ||||||
| Male | 51·4 | Ref. | 25·0 | Ref. | ||
| Female | 58·3 | 1·3 (1·0–1·8) | 0·09 | 27·2 | 1·1 (0·7–1·8) | 0·64 |
| Area of residence | ||||||
| Rural | 52·8 | Ref. | 21·8 | Ref. | ||
| Urban | 56·8 | 1·2 (0·8–1·6) | 0·35 | 30·6 | 1·6 (1·0–2·5) | 0·05 |
| Socioeconomic status | ||||||
| High | 44·2 | Ref. | − | − | ||
| Low | 56·3 | 1·6 (0·9–2·9) | 0·09 | − | − | |
OR, Odds ratio; CI, confidence interval.
DISCUSSION
We studied the seroprevalence of T. gondii IgG antibodies in the Israeli population. The age-adjusted prevalence of T. gondii IgG antibodies in Jews was 19·9%. This rate is higher than rates reported from the USA (10·8%) [4], and lower than those reported from The Netherlands (26·0%) [5], Italy (24·4%) [18], Slovakia (24·2%) [19] and other Middle Eastern countries, such as Iran (41·4%) and Qatar (29·8%) [8, 20]. The age-adjusted prevalence rates in Arabs (non-Bedouins) was 60·4%, significantly higher than in Jews, and in the same range as that reported in Lebanon (62·2%) [9]. Differences in seroprevalence between Jews and Arabs have been reported previously in Israel. Raz et al. [13] reported significantly higher prevalence rates of T. gondii antibodies in Arabs living in rural areas compared to Jews in similar areas (55·8% and 22·2%, respectively). Population differences were described in the USA, where a higher seroprevalence rate was found in non-Hispanic blacks and Mexicans than in non-Hispanic whites [4].
Low SES was associated in the present study with higher prevalence rates in Jews and Arabs (non-Bedouins). Jones et al. [3, 4] found that poverty and living in crowded conditions increased the risk of seropositivity, and Rosso et al. [21] found that lower SES was a risk factor for seropositivity to toxoplasmosis in pregnant women in Colombia. Although the SES of the Arab population in Israel is lower compared to Jews, this could not fully explain the difference in seroprevalence between Jews and Arabs. While the seroprevalence in Jews of low SES was 24·2%, it was much higher (56·3%) in Arabs of the same SES. Other explanations for the population group differences could be related to dietary habits, such as the consumption of undercooked or raw meat, unpasteurized goat and cow milk, and variations in the amount of mutton in the diet. It is also possible that exposure to cats and infectivity of cats is different in Jews and Arabs. Salant & Spira [22] found a higher seroprevalence of T. gondii IgG antibodies in cats in Arab areas of Jerusalem than in cats of Jewish neighbourhoods of the city, possibly indicating higher environmental contamination in Arab neighbourhoods. However, data regarding the distribution of feral cats in other parts of the country is lacking.
A significantly lower rate was found in Bedouins compared to the rest of Arab population. To the best of our knowledge, this is the first study to report on seroprevalence of T. gondii in Bedouins. This finding is of special interest. The Bedouin community is traditional and conservative. Their livelihood is from rearing livestock in the deserts of southern Israel. Many of them are settled, semi-nomadic, and engage in agriculture, and about half are now urban dwellers who nevertheless continue to keep goats and sheep. They have poor living conditions and are considered as having the lowest SES. Therefore the findings regarding the Bedouins are surprising and warrant an explanation. One possibility is the climate conditions. The mean maximum temperature in the area where the Bedouins live is >30°C from May to November and the average precipitation is < 75 mm, even during the country's rainy period (November–March) [23]. Previous studies have demonstrated a lower seroprevalence in hot and dry [3, 5] or extremely cold [24] climates. Jones et al. [4] reported a lower seroprevalence in US-born Mexican Americans compared to US-born non-Hispanic whites and blacks, a finding which those authors explained by the reduced ability of the parasite to survive in the environments where Mexican Americans usually live. Another explanation that should be investigated is the Bedouins' dietary habits and the sources of the foods they consume. Information on the feline population in the South area is not readily available, and it is possible that the lack of a clear-cut host is the reason for the lower seroprevalence. The finding of a low prevalence rate in Bedouins despite their poor living conditions certainly warrants further study.
We found that seropositivity increased with age in all population groups. This finding corresponds well with those of other studies that demonstrated a linear increase in seroprevalence with age [4, 9, 25].
Place of birth remained a significant risk factor in Jews in the multivariate analysis. Those born in Europe, Asia or Africa rather than in Israel, North America or Australia, had higher rates. Nishri et al. [12] reported high rates in 20-year-old Israelis originating from Africa and Asia (48·9%), followed by those from Europe/America/Australia (40·0%) and native Israelis (25·6%). Differences in seropositivity rates in immigrants from different countries have also reported elsewhere [8]. Our data lacks information regarding age at immigration and the duration since immigration, which could have helped to assume infection prior or after arrival in Israel.
The seroprevalence rate in Jewish and Bedouin women of childbearing age (15·1% and 25·4%, respectively) is lower than that reported from many Western countries [6, 18, 26, 27], but slightly higher than the overall US figure (11·0%) [4]. The rate for Arab (non-Bedouin) women (72·3%) is higher than for most European countries (8·2–63·2%) and resembles reports from countries from South America (6·1–77·5%) [26].
It is difficult to comment on time trends of T. gondii seroprevalence in Israel based on the findings of the present study since the earlier studies that had been conducted were on a limited number of individuals from defined geographical areas (Table 1). One study conducted in the early 1990s in a rural area of central Israel found a seropositivity rate of 29·3% [12]. It is possible that the results of the present study indicate a decreasing trend. Indeed, most studies in other countries revealed declines in seropositivity with time [4, 5, 26] and only a few showed increasing seropositivity [21, 26]. Sera of the Arab population were collected 5 years after collection of sera from the Jewish population. We did not consider this as a limitation to the study, it is reasonable to believe that this would have reduced the differences in prevalence rates between the Arab and the Jewish populations.
In order to understand the differences in seroprevalence rates between Jews, Arabs (non-Bedouins) and Bedouins further studies with detailed data on dietary habits and access to cats in all populations would be beneficial
In conclusion, we found a low toxoplasmosis seroprevalence rate in Jews and Bedouins and a high rate in Arabs (non-Bedouins) living in Israel. These differences are only partially explained by differences in SES and should be further explored.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Hill D, Dubey J. Toxoplasma gondii: transmission, diagnosis and prevention. Clinical Microbiology and Infection 2002; 8: 634–640. [DOI] [PubMed] [Google Scholar]
- 2.Rorman E, et al. Congenital toxoplasmosis – prenatal aspects of Toxoplasma gondii infection. Reproductive Toxicology 2006; 21: 458–472. [DOI] [PubMed] [Google Scholar]
- 3.Jones JL, et al. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. American Journal of Epidemiology 2001; 154: 357. [DOI] [PubMed] [Google Scholar]
- 4.Jones JL, et al. Toxoplasma gondii infection in the United States, 1999, 2004, decline from the prior decade. American Journal of Tropical Medicine and Hygiene 2007; 77: 405. [PubMed] [Google Scholar]
- 5.Hofhuis A, et al. Decreased prevalence and age-specific risk factors for Toxoplasma gondii IgG antibodies in The Netherlands between 1995/1996 and 2006/2007. Epidemiology and Infection 2010; 24: 9. [DOI] [PubMed] [Google Scholar]
- 6.Berger F, et al. Toxoplasmosis among pregnant women in France: risk factors and change of prevalence between 1995 and 2003. Revue d'Épidémiologie et de Santé Publique 2009; 57: 241–248. [DOI] [PubMed] [Google Scholar]
- 7.Birgisdóttir A, et al. Seroprevalence of Toxoplasma gondii in Sweden, Estonia and Iceland. Scandinavian Journal of Infectious Diseases 2006; 38: 625–631. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Madi MA, Al-Molawi N, Behnke JM. Seroprevalence and epidemiological correlates of Toxoplasma gondii infections among patients referred for hospital-based serological testing in Doha, Qatar. Parasites & Vectors 2008; 1: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouhamdan SF, et al. Seroprevalence of Toxoplasma antibodies among individuals tested at hospitals and private laboratories in Beirut. Lebanese Medical Journal 2010; 58: 8–11. [PubMed] [Google Scholar]
- 10.Franklin DM, Dror Z, Nishri Z. The prevalence and incidence of Toxoplasma antibodies in pregnant women. Israel Journal of Medical Sciences 1993; 29: 285–286. [PubMed] [Google Scholar]
- 11.Nishri Z, et al. Toxoplasmosis in Israel, 1970–73: evaluation of laboratory data. Israel Journal of Medical Sciences 1978; 14: 1039–1047. [PubMed] [Google Scholar]
- 12.Nishri Z, et al. Prevalence of antibodies to Toxoplasma gondii in the Tel-Mond area. Israel Journal of Medical Sciences 1993; 29: 30–32. [PubMed] [Google Scholar]
- 13.Raz R, et al. Seroprevalence of antibodies against Toxoplasma gondii among two rural populations in northern Israel. Israel Journal of Medical Sciences 1993; 29: 636. [PubMed] [Google Scholar]
- 14.Anon. Statistical Abstract of Israel, 2008, 59th edn. Jerusalem: Central Bureau of Statistics, 2008. [Google Scholar]
- 15.Calderaro A, et al. Laboratory diagnosis of Toxoplasma gondii infection. International Journal of Medical Sciences 2009; 6: 135–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calderaro A, et al. Evaluation of Toxoplasma gondii immunoglobulin G (IgG) and IgM assays incorporating the new Vidia analyzer system. Clinical and Vaccine Immunology 2008; 15: 1076–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofgärtner WT, et al. Detection of immunoglobulin G (IgG) and IgM antibodies to Toxoplasma gondii: evaluation of four commercial immunoassay systems. Journal of Clinical Microbiology 1997; 35: 3313–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosti M, et al. A 4-year evaluation of toxoplasmosis seroprevalence in the general population and in women of reproductive age in central Italy. Epidemiology and Infection. Published online: 11 December 2012. doi: 10.1017/S0950268812002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Studenicova C, Bencaiova G, Holková R. Seroprevalence of Toxoplasma gondii antibodies in a healthy population from Slovakia. European Journal of Internal Medicine 2006; 17: 470–473. [DOI] [PubMed] [Google Scholar]
- 20.Mostafavi SN, et al. Seroepidemiology of Toxoplasma gondii infection in Isfahan province, central Iran: a population based study. Journal of Research in Medical Sciences 2011; 16: 496. [PMC free article] [PubMed] [Google Scholar]
- 21.Rosso F, et al. Prevalence of infection with Toxoplasma gondii among pregnant women in Cali, Colombia, South America. American Journal of Tropical Medicine and Hygiene 2008; 78: 504. [PubMed] [Google Scholar]
- 22.Salant H, Spira DT. A cross-sectional survey of anti-Toxoplasma gondii antibodies in Jerusalem cats. Veterinary Parasitology 2004; 124: 167–177. [DOI] [PubMed] [Google Scholar]
- 23.Anon. Statistical Abstract of Israel, 2011, 62nd edn. Jerusalem: Central Bureau of Statistics, 2011. [Google Scholar]
- 24.Jenum P, et al. Prevalence of Toxoplasma gondii specific immunoglobulin G antibodies among pregnant women in Norway. Epidemiology and Infection 1998; 120: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakikawa M, et al. Anti-Toxoplasma antibody prevalence, primary infection rate, and risk factors in a study of toxoplasmosis in 4,466 pregnant women in Japan. Clinical and Vaccine Immunology 2012; 19: 365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. International Journal for Parasitology 2009; 39: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 27.Lopes AP, et al. Seroepidemiology of Toxoplasma gondii infection in women from the North of Portugal in their childbearing years. Epidemiology and Infection 2012; 140: 872–877. [DOI] [PubMed] [Google Scholar]