Abstract
Background and Objective
Coronavirus disease (COVID-19) is milder with favorable outcomes in children than in adults. However, detailed data regarding COVID-19 in children from Saudi Arabia are scarce. This study aimed to describe COVID-19 among children in Al-Madinah, Saudi Arabia.
Methods
This retrospective observational study included children <14 years old hospitalized with COVID-19 between May 1, 2020 and July 31, 2020. Clinical data, COVID-19 disease severity, and outcomes were collected. The total number of presenting symptoms and signs were computed by counting those recorded upon presentation. The Kruskal-Wallis non-parametric test was used to compare the number of symptoms and signs across all levels of COVID-19 severity.
Result
Overall, 106 patients met the inclusion criteria; their ages ranged from 2 weeks to 13 years. Most patients were ≤12 months of age (43.4%). Bronchial asthma was the most common comorbidity (9.4%). Among 99 symptomatic patients, fever was the most common symptom (84.8%); seven patients (7%) were diagnosed with febrile seizure. Most COVID-19 cases were mild (84%); one patient (0.94%) was in critical condition and one patient (0.94%) met the Multisystem Inflammatory Syndrome in children criteria. The mean number of symptoms and signs in children with severe or critical COVID-19 was significantly higher than that in children with mild cases or non-severe pneumonia (P < .001). One patient died owing to COVID-19 (0.94%).
Conclusions
COVID-19 mortality in children is rare; however, while most children exhibit mild disease with favorable outcomes, children with chronic lung disease may be at higher risk for severe disease.
Keywords: Children, Coronavirus, Febrile seizure, SARS-CoV-2, Saudi Arabia
1. Introduction
Coronaviruses are RNA viruses that cause diseases in their host species, including humans. Few have crossed species to infect humans and cause major public health threats [1]. These include severe acute respiratory syndrome coronavirus 1 in 2003; Middle East respiratory syndrome coronavirus causing Middle East respiratory syndrome since 2012; and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing the ongoing coronavirus disease (COVID-19) pandemic since December 2019 [[2], [3], [4]].
Data regarding COVID-19 in children are evolving. According to a systematic literature review of 45 scientific papers, children represent 1%–5% of all confirmed COVID-19 cases [5]. An early report from the Centers for Disease Control and Prevention in the United States indicated that 10% of children with COVID-19 required hospitalization compared to about 25% of adults, and less than 2% of children required intensive care unit (ICU) admission [6]. Infants under 1 year of age were found to be at a higher risk for hospitalization, while children with underlying chronic diseases, especially chronic lung diseases, were at a higher risk for ICU admission [5,6].
The Kingdom of Saudi Arabia (KSA) has had 385,834 confirmed COVID-19 cases, with 6618 deaths as of March 24, 2021. As in most cities, Al-Madinah has been an endemic area for COVID-19 since March 2020, with 23,040 confirmed COVID-19 cases (5.9% of the KSA cases) and 214 deaths as of March 24, 2021 [7]. Detailed data regarding COVID-19 among children from Saudi Arabia are limited; therefore, this study aimed to describe the epidemiology, clinical features, and outcomes of children infected with SARS-CoV-2 in Al-Madinah City, Saudi Arabia.
2. Materials and methods
2.1. Study design and patient selection
This was a retrospective observational study including children who were hospitalized with laboratory-confirmed COVID-19 infection at either of two pediatric centers in Al-Madinah City, Saudi Arabia between May 1 and July 31, 2020. Inclusion criteria were as follows: age ≤14 years; positive SARS-CoV-2 real-time reverse-transcriptase polymerase chain reaction (RT-PCR) results; and complete demographic, clinical, basic laboratory (i.e., complete blood count, basic chemistry, and radiographic reports), and outcome data. A positive SARS-CoV-2 RT-PCR was defined as two positive target genes (i.e., envelope protein and spike surface glycoprotein genes) according to the World Health Organization (WHO) guidelines [8]. Testing of nasopharyngeal swabs was performed at the Regional Laboratory of the Ministry of Health, Al-Madinah, Saudi Arabia. Asymptomatic patients admitted for isolation were included if their complete demographic, clinical, laboratory, and outcome data were available. Hospitalized patients with incomplete data were excluded, as well as outpatients, as they did not undergo laboratory or radiological investigation. The study was approved by the Research Ethics Board at the General Directorate of Health Affairs of Al-Madinah, Ministry of Health, Saudi Arabia, and the requirement for informed consent was waived due to the retrospective nature of the study.
2.2. Data collection
Demographic data, exposure history, symptoms and signs at time of presentation, preexisting comorbid conditions, COVID-19 disease severity, laboratory and radiological investigations, treatment received, and outcomes were collected from patients’ files using data collection forms.
Demographic data included age, sex, and weight per age percentile. All symptoms and signs at the time of presentation were recorded, and the total number of symptoms and signs were calculated for each patient. Preexisting comorbid conditions (cardiorespiratory, neurodevelopmental, metabolic, prematurity, renal, hematological, immunodeficiency, and gastrointestinal disorders) were reviewed based on past medical histories recorded in the patients’ files.
COVID-19 severity was divided into five categories based on the WHO classification [9]: 1) asymptomatic, 2) mild illness, 3) non-severe pneumonia, 4) severe pneumonia, and 5) critical disease. Mild illness was defined as upper respiratory tract symptoms, such as cough, or non-specific symptoms, such as fever or fatigue, without pneumonia. Non-severe pneumonia was defined as difficulty breathing plus tachypnea, without signs of severe pneumonia; tachypnea was defined based on age (in breaths/min): <2 months, ≥ 60; 2–11 months, ≥ 50; 1–5 years, ≥ 40. Severe pneumonia was defined by presence of at least one of the following: central cyanosis or SpO2 <90%; severe respiratory distress (i.e., grunting or severe chest retraction); >50% lung involvement on imaging within 24–48 h; and/or signs of pneumonia with a general danger sign (inability to breastfeed or drink, lethargy, unconsciousness, or convulsions). Critical diseases were respiratory failure, shock, or multiorgan dysfunction. COVID-19 severity was based on the maximum medical support required during hospitalization and children who met the Multisystem Inflammatory Syndrome in Children (MIS-C) criteria were described in a separate category [10].
Laboratory test results, including those of complete blood count (leucocytes, neutrophils, lymphocytes, hemoglobin, and platelets) and blood chemistry (sodium, potassium, urea, creatinine, alanine aminotransferase, aspartate aminotransferase, total bilirubin), were collected. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were calculated. Serum ferritin, lactate dehydrogenase, D-dimer, and C-reactive protein levels were collected when available.
The chest radiographs and computed tomography (CT) reports of patients who underwent these tests were reviewed and results were recorded as either normal or abnormal based on the radiologists’ reports; specific pathological patterns were recorded if available (i.e., consolidation, infiltration, or ground glass opacity [GGO]).
Medications received for COVID-19 were recorded with the outcome classified as a full recovery, recovery with sequelae (defined as the requirement for medical support after discharge), or death.
2.3. Statistical analysis
The mean with standard deviation was used to describe continuous variables, while frequencies and percentages were used to describe categorical variables. Histograms and the Kolmogorov-Smirnov statistical test of normality were used to assess the statistical normality assumption of the continuous variables. The total number of presenting symptoms and signs were computed by counting those recorded upon presentation to the hospital. The Kruskal-Wallis non-parametric test was used to compare the mean number of presenting symptoms and signs, NLR, and PLR across the COVID-19 severity levels. SPSS version 21 statistical software (IBM Corp, Armonk, New York, USA) was used for data analysis, and statistical significance was set at 0.05.
3. Results
There were 106 patients who met the inclusion criteria. Demographic data, exposure history, preexisting comorbid conditions, and COVID-19 disease severity are shown in Table 1. Twenty-nine patients (27%) had at least one pre-existing condition, with bronchial asthma being the most common (9.4%). Regarding COVID-19 disease severity, most cases were mild (84%); and one patient (0.94%) met the MIS-C criteria.
Table 1.
Demographic characteristics, exposure history, COVID-19 severity, and preexisting conditions of COVID-19 patients (n = 106).
| Frequency (n) | Percentage (%) | |
|---|---|---|
| Sex | ||
| Female | 54 | 50.9 |
| Male | 52 | 49.1 |
| Age (mo), mean (SD) | 38.89 (45.66) | |
| Age | ||
| ≤12 mo | 46 | 43.4 |
| 1–3 y | 24 | 22.6 |
| 3–6 y | 15 | 14.2 |
| ≥7 y | 21 | 19.8 |
| Weight (kg), mean (SD) | 14.86 (15.18) | |
| Sex and age standardized weight percentile, mean (SD) | 46.05 (33.79) | |
| History of recent contact with COVID-19 infected person | ||
| No/unknown | 60 | 56.6 |
| Yes | 46 | 43.4 |
| COVID-19 severity | ||
| Asymptomatic | 7 | 6.6 |
| Mild | 89 | 84 |
| Non-severe pneumonia | 4 | 3.8 |
| Severe pneumonia | 4 | 3.8 |
| Critical | 1 | 0.94 |
| Multisystem inflammatory syndrome in children | 1 | 0.94 |
| Has one or more preexisting conditions | ||
| No | 77 | 72.6 |
| Yes | 29 | 27.4 |
| Preexisting conditions | ||
| Premature birth | 5 | 4.7 |
| Asthma | 10 | 9.4 |
| Chronic respiratory disease | 4 | 3.8 |
| Heart disease | 6 | 5.7 |
| Diabetes | 1 | 0.9 |
| Chronic kidney disease | 1 | 0.9 |
| Neuromuscular/metabolic disease | 5 | 4.7 |
| Immunodeficiency | 1 | 0.9 |
| Malignancy | 2 | 1.9 |
| Hematological disorder | 2 | 1.9 |
| Gastrointestinal disease | 2 | 1.9 |
COVID-19, coronavirus disease; SD, standard deviation.
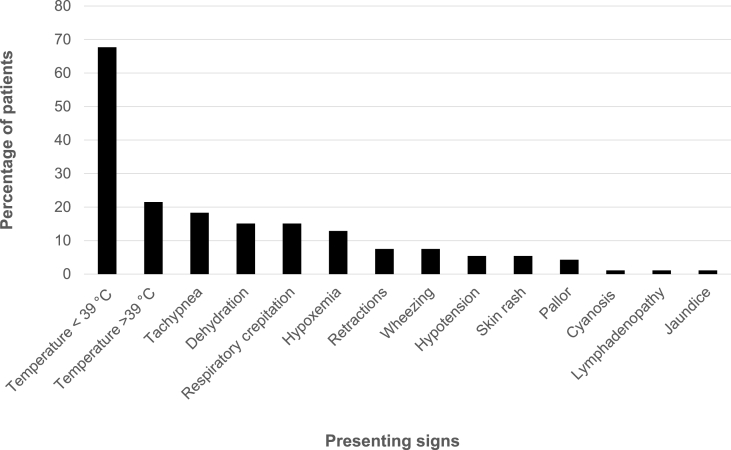
Table 2 shows the descriptive analysis of symptoms documented at hospital admission among the 99 symptomatic patients; fever (temperature >38 °C) was the most common symptom and sign at the time of presentation (84.8%). The mean duration of symptoms before hospital admission was 2.8 days. Physical examination at presentation indicated that most patients (67.7%) exhibited fever (37.7–39 °C). The frequency of physical signs is shown in Fig. 1. The mean overall number of symptoms and signs at the time of presentation was 4.63; 25% of patients exhibited six or more symptoms and/or signs. The mean number of symptoms and signs at the time of presentation in children with severe pneumonia and critical COVID-19 were 9.0 and 9.25, respectively, which was significantly higher than in children with mild cases (4.61) and non-severe pneumonia (5.75) (P < .001).
Table 2.
Descriptive analysis of COVID-19 symptoms at time of hospital admission symptomatic patients (n = 99).
| Frequency (n) | Percentage (%) | |
|---|---|---|
| Duration of symptoms before presenting to the hospital (d), mean (SD) | 2.86 (2.40) | |
| Total number of symptoms and signs, mean (SD) | 4.63 (2.5) | |
| Fever | 84 | 84.84 |
| Runny nose | 18 | 18.18 |
| Sore throat | 6 | 6.06 |
| Myalgia | 4 | 4.04 |
| Diarrhea | 26 | 26.26 |
| Decreased appetite | 31 | 31.31 |
| Abdominal pain | 6 | 6.06 |
| Vomiting | 28 | 28.28 |
| Cough | 32 | 32.32 |
| Shortness of breath | 22 | 22.22 |
| Headache | 6 | 6.06 |
| Decreased activity | 30 | 30.3 |
| Seizure | 10 | 10.1 |
| Diminished level of consciousness/mental level | 3 | 3.03 |
| Skin rash | 4 | 4.04 |
SD, standard deviation.
Fig. 1.
Percentage of children with different COVID-19 signs at presentation to the hospital.
Ten patients (10.1%) presented with seizure; two had a history of epilepsy, while eight were previously healthy. Two of these patients exhibited respiratory symptoms without fever, and all except one had mild COVID-19. Six patients underwent enhanced CT of the brain; all had normal brain imaging results, except one who exhibited cortical sulci and leptomeningeal enhancement. Four patients, including the patient with an abnormal CT scan, underwent cerebrospinal fluid (CSF) examination. All had normal protein, glucose, and cell counts with negative cultures; COVID-19 RT-PCR was not performed on the CSF samples because it had not been validated for non-respiratory samples.
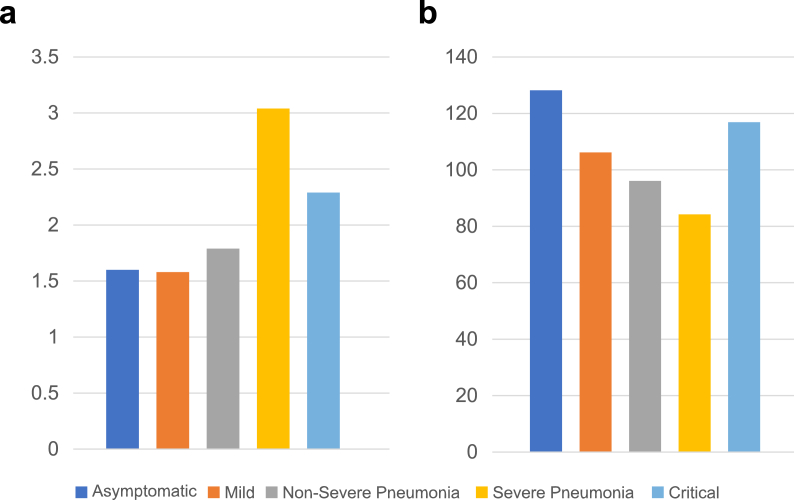
Investigation results are shown in Table 3. Among the 58 patients who underwent chest radiographs, 19 (32.8%) exhibited abnormal findings; bilateral consolidation with GGOs was seen in nine of these patients (47.3%). Regarding hematological abnormalities, 16% had a neutrophil count less than 1 × 103/μL, and 22% had a lymphocyte count less than 2 × 103/μL. The mean NLR and PLR were 1.66 and 106.61, respectively. Children with severe disease had higher NLRs than those with milder disease (Fig. 2A and B); however, this difference was not statistically significant (P = .221).
Table 3.
Investigation results of patients admitted with COVID-19 (n = 106).
| Frequency | Percentage | |
|---|---|---|
| Chest radiography findings | ||
| Abnormal | 19 | 17.9 |
| Normal | 39 | 36.8 |
| Not done | 48 | 45.3 |
| White blood cell count ( × 103μL), mean (SD) | 10.01 (5.76) | |
| Blood neutrophil count ( × 103μL), mean (SD) | 4.42 (4.10) | |
| Normal >1.5 | 82 | 77.4 |
| Mild neutropenia 1–1.5 | 7 | 6.6 |
| Moderate neutropenia 0.50–1.00 | 14 | 13.2 |
| Severe neutropenia <0.50 | 3 | 2.8 |
| Blood lymphocytes (103/μL), mean (SD) | 4.12 (2.79) | |
| ≥2 | 82 | 77.4 |
| <2 | 24 | 22.6 |
| Serum hemoglobin level (g/dL), mean (SD) | 12.47 (2.11) | |
| Blood platelet count (109/L), mean (SD) | 317.43 (115.10) | |
| Low <150 | 4 | 3.8 |
| Normal 150 − 450 | 90 | 84.9 |
| High >450 | 12 | 11.3 |
| Neutrophil-to-lymphocyte ratio, mean (SD) | 1.66 (2.25) | |
| Platelet-to-lymphocyte ratio, mean (SD) | 106.61 (67.23) | |
| Serum alanine aminotransferase (U/L), mean (SD) | 33.99 (56.82) | |
| Serum aspartate aminotransferase (U/L), mean (SD) | 36.06 (20.90) | |
| Serum lactic dehydrogenase (U/L), mean (SD) (n = 32) | 305.56 (108.16) | |
| Blood ferritin (ng/mL), mean (SD) (n = 35) | 424.35 (763.85) | |
| Normal <500 | 30 | 85.7 |
| High ≥500 | 5 | 14.3 |
| Blood creatinine (μmol/L), mean (SD) | 37.40 (25.68) | |
| Serum sodium (mmol/L), mean (SD) | 137.15 (3.79) | |
| Serum C-reactive protein (mg/L), mean (SD) (n = 36) | 8.59 (30.02) | |
| Normal ≤10 | 32 | 88.9 |
| High >10 | 4 | 11.1 |
COVID-19, coronavirus disease; SD, standard deviation.
Fig. 2.
Ratios according to COVID-19 severity. a) Mean neutrophil-to-lymphocyte ratio according to COVID-19 severity. b) Mean platelet-to-lymphocyte ratio according to COVID-19 severity.
Patients were treated according to the guidelines of the Ministry of Health, Saudi Arabia [11]; the outcomes and frequencies of the different medications used for treatment of symptomatic COVID-19 patients are shown in Table 4. Nine (9.09%) patients required pediatric ICU admission: five had severe or critical COVID-19, without preexisting conditions; one had MIS-C; two had metabolic disorders; and one was ventilator-dependent due to chronic lung disease. Six patients (6.06%) required mechanical ventilation, with a mean duration of 7 days. Antibiotics were prescribed in 19 (19.1%) patients for suspected or confirmed secondary bacterial infection; however, only two (1.88%) positive bacterial cultures were obtained from the endotracheal aspirates of two intubated patients within 7 days of admission (Klebsiella pneumoniae and Staphylococcus aureus).
Table 4.
Frequency of different medications used for treating symptomatic COVID-19 and their outcomes (n = 99).
| Frequency (n) | Percentage (%) | |
|---|---|---|
| Required medical therapy administered to the patient in-hospital | ||
| Supplemental oxygen | 17 | 17.17 |
| Steroids/NSAIDs | 11 | 11.11 |
| Bronchodilators | 10 | 10.1 |
| Hydroxychloroquine | 2 | 2.02 |
| Oseltamivir | 1 | 1.01 |
| Lopinavir/ritonavir | 2 | 2.01 |
| Antibiotics | 19 | 19.19 |
| Duration on antibiotics (d), mean (SD) (n = 15) | 5.9 (3.13) | |
| Pediatric intensive care admission | 9 | 9.09 |
| Pediatric intensive care length of stay (d), median (IQR), (n = 9) | 9.5 (17.75) | |
| Required endotracheal intubation | 6 | 6.06 |
| Duration on mechanical ventilator (d), median (IQR) (n = 6) | 7 (11.5) | |
| Outcomes | ||
| Fully recovered | 96 | 96.96 |
| Recovered with sequelae | 1 | 1.01 |
| Death | 2 | 2.02 |
COVID-19, coronavirus disease; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drug; SD, standard deviation.
Most patients (96.9%) made a full recovery, while one patient with cerebral palsy, asthma, and chronic lung disease was discharged on home oxygen; two patients (2%) died. The first mortality was observed in a premature 3-year-old boy with chronic lung disease and cerebral palsy; he died 16 days after admission. The second mortality was in a 3-week-old girl with a suspected metabolic disorder and severe metabolic crisis who died 3 days after admission.
4. Discussion
This is an observational study that describes children hospitalized with COVID-19 at two of the largest pediatric centers in Al-Madinah, Saudi Arabia. The age distribution in our study is consistent with both national and international studies in children. In a recent study from Saudi Arabia, about 35% of hospitalized patients were under one year of age [12]. Data from the United States showed that the cumulative rate for hospitalization was highest among children aged <2 years (24.8 per 100,000 population), compared with the overall rate among children aged <18 years [13].
In most published studies in children, the rate of contact with infected family members ranges from 50 to 96% [6,12,14]: however, this rate was lower in our study (43.4%). This may be explained by transmission via asymptomatic household member contact [15].
Most patients (89%) in our study exhibited mild COVID-19 infection, and less than 2% were in critical condition, like most previous pediatric studies. An early study from China describing a group of 74 hospitalized pediatric patients showed that 73% had mild COVID-19, with no critical cases [14]. Similarly, a systematic review of 65 studies regarding disease severity in 7480 children aged 0–18 years showed that 42.5% and 39.6% were mild and moderate in severity, respectively, while 2% and 0.6% were severe and critical, respectively. However, most studies included in this review were from China [16].
The symptom frequencies were like most published pediatric studies, with fever and cough being the most common [16]. The association of weight-for-age percentile with disease severity could not be determined due to the small number of patients with severe or critical COVID-19, however a recent large cross-sectional study including 43,465 patients with COVID-19 aged 18 years or younger showed that children with asthma and obesity had increased risks of hospitalization and severe diseases (RR, 3.07; 95% CI, 2.66, 3.54 and RR, 1.09; 95% CI, 0.98, 1.21, respectively) [17].
Ten patients in our study presented with seizure, seven of which were diagnosed with febrile seizure; however, two patients had seizure without fever, and one had a history of epilepsy. Seizure is a rare symptom of COVID-19 in children, with only a few cases reported [[18], [19], [20]]. Chegondi et al. reported a case of febrile seizure and status epilepticus in a previously healthy 2-year-old girl with COVID-19. She exhibited mild COVID-19 pneumonia, had normal brain CT and CSF analysis results and recovered without complications [18].
Abnormal chest radiographs were documented in about one-third of children who underwent chest radiography in our study; GGOs were seen in about half, consistent with most pediatric studies [16]. Although no patients in our study underwent chest CT, this may be more sensitive than chest radiography. In a systematic review of 29 studies that evaluated CT scan findings among children with COVID-19, about 65% of children had abnormal chest CT scans; GGOs and consolidation/infiltration were observed in 37.2% and 22.3% of these children, respectively [21].
Like most studies, most patients in our study had a normal complete blood count, blood chemistry, and inflammatory markers [16]. However, NLR and PLR have not been reported in most published pediatric studies [12,14,16]. In our study, children with severe disease had higher NLRs than those with milder disease; however, this difference was not statistically significant (P = .221). Data regarding NLR and PLR in children with COVID-19 are scarce; however, Wang et al. published a study in adult that showed an association between NLR and mortality based on the receiver operating characteristic curve; an NLR of 2.306 was associated with all-cause mortality, with a sensitivity and specificity of 100% and 56.7.%, respectively [22].
Nine patients (9%) in our study required pediatric ICU admission, with disease severity ranging from moderate pneumonia to critical COVID-19; six patients required mechanical ventilation. This was consistent with two recent studies in hospitalized children with COVID-19 from Saudi Arabia, which reported that the rates of pediatric ICU admission were 9.8% (7/71) and 8% (7/88), respectively [12,23]. Compared with the two preceding studies, our study showed a lower rate of MIS-C cases of 0.94% versus 7% and 3.4%, respectively [12,23]. However, the rate of pediatric ICU admission in most pediatric studies based on a large systematic review of 65 studies in children was lower (2%) than in our study [16].
Majority of patients received only supportive therapy, as most were mild cases. Steroids were used in patients with pneumonia and critical disease. Data regarding use of dexamethasone for COVID-19 were evolving; however, evidence now supports the use of dexamethasone in moderate to severe cases based on multiple randomized clinical trials and meta-analyses [24].
Less than one-quarter (19.1%) of patients in our study received antibiotics for suspected secondary bacterial infections, with only two documented positive bacterial cultures from endotracheal aspirates in intubated patients. Data are scarce and still evolving regarding bacterial co-infection or secondary infection in children with COVID-19. A recent systematic review and meta-analysis of 24 studies reported respiratory bacterial co-infection or secondary infection among 3338 COVID-19 patients, 55 of which were children. Bacterial co-infection (defined as co-infection at presentation) and secondary bacterial infection (defined as emerging infection during hospital stay) were identified in 3.5% and 14.3% of patients, respectively; the overall proportion of patients with bacterial infection was 6.9% [25].
Like most pediatric COVID-19 studies, most children recovered without complications [16]. There were two mortalities in our study, and both patients had comorbid conditions. If we exclude the second case, as it is not clear whether the cause of death was due to COVID-19 or the suspected coexisting metabolic disorder, our study has a case fatality rate of 0.94%. Children with chronic lung disease might be at a higher risk of severe COVID-19 as reported in a multicenter study from 176 centers across Europe that included 185 pediatric patients with underlying chronic conditions [26]. In that study, two of nine patients with bronchopulmonary dysplasia required pediatric ICU admission; additionally, seven of another 13 patients who required pediatric ICU admission had other chronic respiratory conditions [26]. The case fatality rate due COVID-19 in children ranges from 0 to less than 1% in most pediatric studies, with an overall estimated mortality of 0.08% [16].
Limitations of our study include the retrospective design and the small number of moderate and severe cases; however, it represents one of the largest sample sizes from a single city in Saudi Arabia. Future, prospective multicenter studies with larger sample sizes are recommended to clarify risk factors and outcomes of COVID-19 among children in Saudi Arabia.
5. Conclusions
Our study showed that most children with COVID-19 have mild disease with favorable outcomes; however, it is possible that the disease may present with febrile seizures. The NLR might help with stratification of patients at presentation for closer monitoring. Mortality is rare in children; however, children with chronic conditions, especially chronic lung disease, may be at higher risk of severe disease.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participate
The study was approved by the Research Ethics Board at the General Directorate of Health Affairs of Al-Madinah, Ministry of Health, Saudi Arabia (IRB No. H-03-M-084) and the requirement for informed consent was waived.
Availability of data and material
All data are included in the manuscript.
Consent to participate
The requirement for informed consent was waived by IRB as the study is a retrospective study.
Authors' contributions
Amer Alshengeti prepared the study protocol and data for analysis, as well as drafted the manuscript. Hatem Alahmadi, Ashwaq Barnawi, Nouf Alfuraydi, and Abdulsalam Alawfi completed the data entries and analysis. Arwa Al-Ahmadi, Mohammad Sheikh, Amani Almaghthawi, Zahera Alnakhli, and Raghad Rasheed completed the data collection. Amany Ibrahim and Ahmed Sobhi participated in data collection and revised the analysis. Dayel Al Shahrani prepared the patient data collection forms. Faisal Kordy supervised the entire project. All authors read and approved the manuscript.
Declaration of competing interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. All authors declare that no conflict-of-interest present.
Acknowledgements
The authors would like to acknowledge the Medical Records Departments at Al-Madinah Maternity and Children Hospital and The Saudi German Hospital, Al-Madinah, Saudi Arabia, for their contribution in preparing patients' file for data collection.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpam.2021.11.001.
Visual abstract
The following is the supplementary data related to this article:
References
- 1.Shi Z., Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008;133:74–87. doi: 10.1016/j.virusres.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly C.A., Ghani A.C., Leung G.M., Hedley A.J., Fraser C., Riley S., et al. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cauchemez S., Fraser C., Van Kerkhove M.D., Donnelly C.A., Riley S., Rambaut A., et al. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect Dis. 2014;14:50–56. doi: 10.1016/S1473-3099(13)70304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu P., Hao X., Lau E.H.Y., Wong J.Y., Leung K.S.M., Wu J.T., et al. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25:2000044. doi: 10.2807/1560-7917.ES.2020.25.3.2000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC COVID-19 Response Team Coronavirus disease 2019 in children-United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID-19 dashboard: Saudi Arabia. https://covid19.moh.gov.sa/. Accessed 25 March 2021.
- 8.World Health Organization . World Health Organization; 2020. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: interim guidance, 19 March 2020. [Google Scholar]
- 9.World Health Organization . World Health Organization; 2020. Clinical management of COVID-19: interim guidance, 27 May 2020. [DOI] [Google Scholar]
- 10.World Health Organization . World Health Organization; 2020. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. [Google Scholar]
- 11.Ministry of Health . Ministry of Health; 2021. Saudi MoH protocol for patients suspected of/confirmed with COVID-19: supportive care and antiviral treatment of suspected or confirmed COVID-19 infection.https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/MOH-therapeutic-protocol-for-COVID-19.pdf Version 1.1, February 2020. Accessed 19 February 2021. [Google Scholar]
- 12.Alharbi M., Kazzaz Y.M., Hameed T., Alqanatish J., Alkhalaf H., Alsadoon A., et al. SARS-CoV-2 infection in children, clinical characteristics, diagnostic findings and therapeutic interventions at a tertiary care center in Riyadh, Saudi Arabia. J Infect Public Health. 2021;14:446–453. doi: 10.1016/j.jiph.2020.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim L., Whitaker M., O'Halloran A., Kambhampati A., Chai S.J., Reingold A., et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19-COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1081–1088. doi: 10.15585/mmwr.mm6932e3. COVID-NET Surveillance Team. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Q., Xing Y., Shi L., Li W., Gao Y., Pan S., et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;146 doi: 10.1542/peds.2020-0961. [DOI] [PubMed] [Google Scholar]
- 15.Madewell Z.J., Yang Y., Longini I.M., Jr., Halloran M.E., Dean N.E. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liguoro I., Pilotto C., Bonanni M., Ferrari M.E., Pusiol A., Nocerino A., et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179:1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kompaniyets L., Agathis N.T., Nelson J.M., Preston L.E., Ko J.Y., Belay B., et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chegondi M., Kothari H., Chacham S., Badheka A. Coronavirus disease 2019 (COVID-19) associated with febrile status epilepticus in a child. Cureus. 2020;12 doi: 10.7759/cureus.9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatta S., Sayed A., Ranabhat B., Bhatta R.K., Acharya Y. New-onset seizure as the only presentation in a child with COVID-19. Cureus. 2020;12 doi: 10.7759/cureus.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musolino A.M., Supino M.C., Buonsenso D., Ferro V., Valentini P., Magistrelli A., et al. Roman lung ultrasound study team for pediatric COVID-19 (ROMULUS COVID team). Lung ultrasound in children with COVID-19: preliminary findings. Ultrasound Med Biol. 2020;46:2094–2098. doi: 10.1016/j.ultrasmedbio.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nino G., Zember J., Sanchez-Jacob R., Gutierrez M.J., Sharma K., Linguraru M.G. Pediatric lung imaging features of COVID-19: a systematic review and meta-analysis. Pediatr Pulmonol. 2021;56:252–263. doi: 10.1002/ppul.25070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., Li X., Shang Y., Wang J., Zhang X., Su D., et al. Ratios of neutrophil-to-lymphocyte and platelet-to-lymphocyte predict all-cause mortality in inpatients with coronavirus disease 2019 (COVID-19): a retrospective cohort study in a single medical centre. Epidemiol Infect. 2020;148:e211. doi: 10.1017/S0950268820002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kari J.A., Shalaby M.A., Albanna A.S., Alahmadi T.S., Sukkar S.A., Mohamed Nur H.A.H., et al. Coronavirus disease in children: a multicenter study from the Kingdom of Saudi Arabia. J Infect Public Health. 2021;14:543–549. doi: 10.1016/j.jiph.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C.C., et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa478. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moeller A., Thanikkel L., Duijts L., Gaillard E.A., Garcia-Marcos L., Kantar A., et al. COVID-19 in children with underlying chronic respiratory diseases: survey results from 174 centres. ERJ Open Res. 2020;6:409–2020. doi: 10.1183/23120541.00409-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript.