Abstract
Objectives:
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is often reported to be caused by an infectious agent. However, it is unclear whether one infectious agent might be the cause or whether there might be many different infectious agents. The objective of this study was to identify self-reported infectious illnesses associated with the onset of ME/CFS.
Methods:
The present study involved data from multiple sites in several countries. 1773 individuals diagnosed with either ME, CFS or ME/CFS provided qualitative data concerning infectious triggers which were coded and classified for analysis.
Results:
60.3% of patients report a variety of infectious illnesses some time before onset of ME/CFS. The most frequently reported infectious illness was Mononucleosis, which occurred in 30% of infections. However, over 100 other infectious illnesses were mentioned.
Discussion:
The findings suggest that many infectious agents might be associated with the onset of ME/CFS.
Keywords: Chronic fatigue syndrome, myalgic encephalomyelitis, ME/CFS, infectious illness, mononucleosis
Patient perceptions of infectious illnesses preceding Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) has many debilitating symptoms including post-exertional malaise (PEM), sleep disturbances, and cognitive impairment.1–3 Functional impairment can range from being minor (able to do light physical work/work part-time) to being completely bedbound.4 Compared to patients with cardiovascular and other neurological disorders, patients with ME/CFS are significantly worse on virtually every subscale of the Medical Outcome Study Short-Form 36.5
For many patients, the onset of ME/CFS occurs following an infectious illness.6 An out-break of ME/CFS in 1984 in Incline Village, Nevada, US, was thought to be due Chronic Epstein-Barr virus.7 Infectious pathogens may precipitate or chronically reactivate ME/CFS by either modulating immune system functions or activating a neural response.2,8 A “hit and run” effect has been proposed,9 whereby an infectious illness disrupts immune functions, giving rise to ME/CFS with disease symptoms persisting even after the initial infectious illness are resolved.
Early research concerning the etiology of ME/CFS suggested infectious agents were precipitating suspects in up to 72% of cases.10 Similarly, endorsement of infectious etiology has been reported in 73.5% of participants with sudden onset ME/CFS and 66.3% of participants with gradual onset ME/CFS.11 Patient narratives have further supported these figures, with many indicating that they believe their illness to have been caused by an acute viral illness.11–13 This has led to the attribution of a variety of infectious illnesses causing ME/CFS, including Epstein-Barr virus (EBV), cytomegalovirus (CMV), enteroviruses, human parvovirus B19 (B19V) and many others.14,15 An estimated 64% of individuals with ME/CFS have been reported to have infection-related onset episodes.16 Generally, it is believed that common respiratory infections do not cause ME/CFS, whereas other infectious illnesses, such as Epstein-Barr virus, Q fever, and viral meningitis can be a cause of the illness.17 Despite years of researching these potential pathogenic factors, a single infectious etiology for ME/CFS has not been identified.6,18 This is further complicated by suggestions that an unknown or undiscovered novel infectious pathogen that may be responsible for ME/CFS.4
The current study investigated infectious illnesses experienced by patients with ME/CFS prior to their illness onset. The method involved analyzing patient self-report responses in a large, internationally aggregated dataset. This was a descriptive, exploratory study that attempted to chronicle the types of infectious illness reported at illness onset by a large sample of patients with ME/CFS.
Methods
Participants
Nine patient samples were aggregated for the purposes of this study. In descending order of sample size, these samples included the BioBank sample, the Chronic Illness sample, the DePaul sample, the Spain sample, the Norway I sample, the Norway III sample, the Japan sample, the Newcastle sample (United Kingdom), and the Norway II sample (Table 1). For the purposes of this study, only participants who were at least 18 years-old and had a current diagnosis of ME, CFS, or ME/CFS were included. 1773 participants were included in the study, and 1069 (60.3%) indicated they experienced an infectious illness prior to their ME/CFS onset (Table 1). Endorsement of an infectious trigger ranged from 24% (Spain sample) to 74% (Norway III sample). Further descriptions of the data sources are provided below.
Table 1.
Data sources and endorsement of infectious triggers.
| Data source | % Total sample (n) | % Infectious trigger (n) |
|---|---|---|
| BioBank | 26.34 (467) | 61.67 (288) |
| Chronic illness | 19.80 (351) | 63.82 (224) |
| DePaul | 11.56 (205) | 70.24 (144) |
| Spain | 10.04 (178) | 23.60 (42) |
| Norway I | 9.76 (173) | 68.21 (118) |
| Norway III | 9.42 (167) | 74.25 (124) |
| Japan | 5.47 (97) | 48.45 (47) |
| Newcastle | 4.91 (87) | 56.32 (49) |
| Norway II | 2.71 (48) | 68.75 (33) |
| Total | 100.00 (1773) | 60.29 (1069) |
Biobank sample.
A sample collected by the U.S.-based Solve ME/CFS Initiative was made available to the DePaul University research team; the sample included patient data with clinical information and blood samples on individuals diagnosed by a licensed physician specializing in ME/CFS. Participants were recruited by the Solve ME/CFS Initiative through physician referral. Participants completed the study using electronic or hard-copy measures.
Chronic illness study sample.
A cross-sectional sample of adults with chronic illnesses were recruited as part of a larger study.19 Participants were recruited via email requests to ME/CFS national foundations, postings on patient support groups, research forums, and social media platforms. The larger study consisted of individuals with various chronic illnesses, but the present study includes only individuals with a diagnosis of ME, CFS or ME/CFS.
Depaul sample.
An international convenience sample of adults self-identifying as having ME, CFS or ME/CFS was recruited. Participants were required to be at least 18 years-old and have a current diagnosis of ME/CFS. Participants had to be capable of reading and writing English. Study measures were completed either via a electronic/paper survey or verbal survey over the phone.
Spain sample.
Participants were recruited by a specialist physician with experience in diagnosing ME/CFS from a public tertiary referral center in Barcelona, Spain. Participants completed study measures via Research Electronic Data Capture (REDCap), an online data collection tool. Patients were required to be at least 18 years-old and meet the CDC-1994/Fukuda criteria.1
Norway I sample.
Patients with CFS were recruited via a randomized controlled trial of a CFS self-management program. Participants were recruited from four mid-sized towns in southern Norway, two suburbs of Oslo, and some surrounding communities. Participants were required to be at least 18 years-old and diagnosed with ME, CFS, or ME/CFS by a physical or medical specialist. Additionally, to quality participants could not be pregnant, needed to be physically able enough to attend the self-management program, and provide permission to confirm their ME, CFS, or ME/CFS diagnosis with their physician or medical specialist.
Norway III sample.
Patients diagnosed with ME/CFS by the Canadian Consensus criteria (CCC)2 between ages 18–65 were recruited while attending a tertiary ME/CFS center for evaluation of their diagnoses. Participants were diagnosed by physicians experienced with the field of ME/CFS.
Japan sample.
Participants were recruited from the ME Japan association and affiliated physician clinics specializing in ME/CFS. Patients with profound fatigue completed study measures in-person.
Newcastle sample.
Participants were referred for a medical assessment at the Newcastle-upon-Tyne Royal Victoria Infirmary clinic due to a suspected diagnosis of ME, CFS, or ME/CFS. An experienced physician performed a comprehensive medical history and examination. Individuals who met eligibility criteria completed a written informed consent process. Study measures were completed via hard-copy surveys.
Norway II sample.
Participants were recruited from an inpatient medical ward for severely ill patients, as well as from the outpatient clinic at a multidisciplinary ME/CFS center. Patients with a suspected diagnosis of ME, CFS, or ME/CFS were referred for evaluation. Participants underwent a comprehensive medical history interview and a detailed medical examination by experienced consultant physicians and a psychologist Participants were required to be between 18 to 65 years-old, and capable of reading and writing Norwegian. Participants completed study measures via hard-copy.
Measures
Participants completed the DePaul Symptom Questionnaire (DSQ-1), which comprises 54 items measuring ME/CFS symptomatology. This survey includes demographical, educational, medical, and social history.20 As part of the DSQ-1, participants were asked “Did your fatigue/energy related illness start after you experienced an infectious illness?” If a participant answered yes, they could then write-in what infectious illness they experienced. The DSQ-2 was also utilized, as it was released after the study had already begun. All items in the DSQ-1 are included in the DSQ-2. Both the DSQ-1 and DSQ-2 have excellent reliability and validity.20
Categorizing infectious agents
Each write-in response was reviewed and transcribed into categories of various infectious illnesses. Write-in responses that were unrelated to the question (i.e. listing health conditions like diabetes), too vague (i.e. only describing general symptoms), left blank, or could not be translated to English were not included. Although unrelated health conditions were excluded, conditions that could be caused by bacterial, viral, or other pathogens were included as is (i.e. jaundice caused by liver infection). Participants could also report multiple infectious illnesses in their responses.
Categories of infectious illness were created on a case by case basis. For example, Human Simplex virus (HSV) is usually parsed out as Human Simplex virus 1 (HSV-1) and Human Simplex virus 2 (HSV-2). In the present study, no participants reported HSV-1 or HSV-2 but 6 participants reported the more general category of HSV. Participants who reported “flu” or “flu-like symptoms” or “flu-like illness” were coded under the Flu/Flu-Like category. Due to the expected usage of general and non-clinical language of some reports, some reports were categorized separately to avoid making excessive erroneous assumptions (e.g. “trachea infection” and “throat infection” or “thrush” and “yeast infection”). Additionally, 83 participants left the relevant item blank, 4 reported illnesses that were not infectious illnesses (unrelated health conditions), 130 reported incidences that were either too vague or symptoms were described without clarifying an infectious illness that induced them, and 16 reported incidences could not be translated into English due to spelling errors.
Results
The mean age of the aggregate sample was 47.9 (SD = 13.39). 83.1% (n = 877) were female and 16.8% (n = 177) were male (See Table 2). A total of 1109 reported incidences of various infectious illnesses were compiled. Figure 1 shows the most common infectious illness categories. The three most frequently reported infectious illnesses were “mononucleosis” (225 incidences; 20.29% of total incidences), “flu/flu-like” (204; 18.39%), and “Epstein-Barr virus” (77; 6.94%). These categories were followed by “Cold” (66; 5.95%), “Sinus Infection” (49; 4.42%), “Pneumonia” (36; 3.25%), “Varicella Zoster virus” (27; 2.43%), “Bronchitis” (27; 2.43%), “Borreliosis” (25; 2.25%), “Gastroenteritis” (24; 2.16%), “Upper respiratory infection” (23; 2.07%), “Cytomegalovirus” (22; 1.98%), “Strep throat” (20; 1.80%), “Ear infection” (15; 1.35%), “Respiratory infection” (15; 1.35%), “Mycoplasma pneumonia” (15; 1.35%), “Throat infection” (14; 1.26%), and “Dental infection” (14; 1.26%). Two hundred and eleven (19.03%) other reported incidences that did not have rates >1% are not in this figure, but all infectious agents and their prevalence rates can be found in Table 3.
Table 2.
Demographic characteristics of those endorsing an infectious trigger (N = 1069).
| Age |
M 47.9 % |
(SD) (13.39) n |
|---|---|---|
| Gender | ||
| Male | 16.8 | (177) |
| Female | 83.1 | (877) |
| Race | ||
| White/Caucasian | 93.8 | (989) |
| Black/African-American | 0.1 | (1) |
| Asian/Pacific Islander | 4.9 | (52) |
| Other | 1.2 | (13) |
| Education | ||
| Less than high school | 3.8 | (40) |
| Some high school | 2.6 | (27) |
| High school degree or GED | 16.1 | (169) |
| Partial college or specialized training | 14.5 | (153) |
| Standard college degree or higher | 63.0 | (663) |
| Marital Status | ||
| Married or living with partner | 54.3 | (569) |
| Separated | 1.8 | (19) |
| Widowed | 1.3 | (14) |
| Divorced | 14.1 | (148) |
| Never married | 28.4 | (297) |
Figure 1.
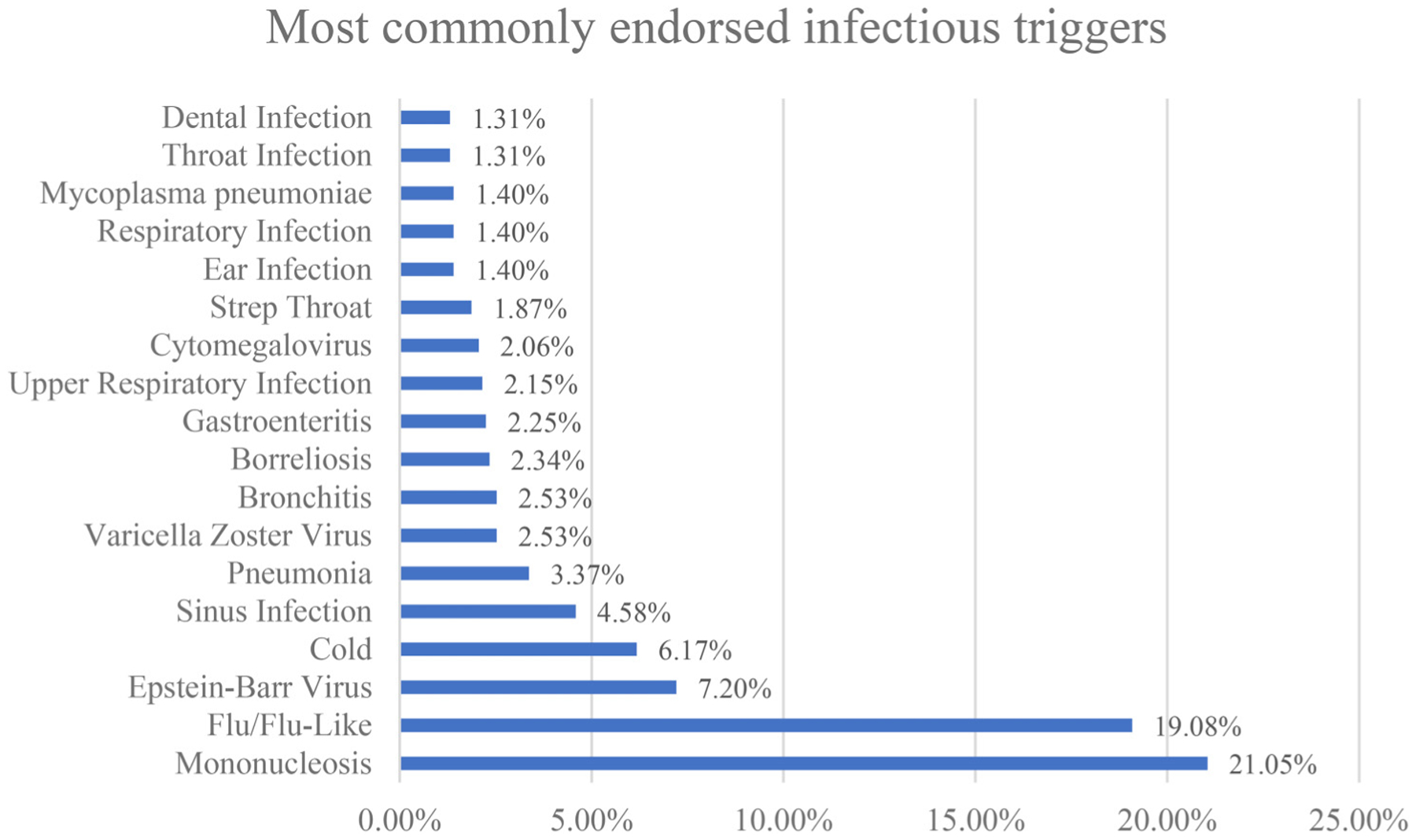
Most commonly endorsed infectious triggers.
Note: Infectious triggers reported by >1% of participants (N = 1069).
Table 3.
Prevalence of infectious triggers among those with infectious trigger endorsement (N = 1069).
| Infectious Illness | Prevalence | (n) |
|---|---|---|
| Mononucleosis | 21.05% | (225) |
| Flu/Flu-Like | 19.08% | (204) |
| Epstein-Barr Virus | 7.20% | (77) |
| Cold | 6.17% | (66) |
| Sinus Infection | 4.58% | (49) |
| Pneumonia | 3.37% | (36) |
| Varicella Zoster Virus | 2.53% | (27) |
| Bronchitis | 2.53% | (27) |
| Borreliosis | 2.34% | (25) |
| Gastroenteritis | 2.25% | (24) |
| Upper Respiratory Infection | 2.15% | (23) |
| Cytomegalovirus | 2.06% | (22) |
| Strep Throat | 1.87% | (20) |
| Ear Infection | 1.40% | (15) |
| Respiratory Infection | 1.40% | (15) |
| Mycoplasma pneumoniae | 1.40% | (15) |
| Throat Infection | 1.31% | (14) |
| Dental Infection | 1.31% | (14) |
| Urinary Tract Infection | 0.84% | (9) |
| Tick-related Illness | 0.84% | (9) |
| Swine Flu | 0.75% | (8) |
| Parvovirus | 0.65% | (7) |
| Kidney Infection | 0.65% | (7) |
| HHV-6 | 0.65% | (7) |
| Human Simplex Virus | 0.56% | (6) |
| Coxsackievirus | 0.56% | (6) |
| Hepatitis | 0.56% | (6) |
| Giardia | 0.56% | (6) |
| Chest Infection | 0.47% | (5) |
| Coxsackie B Virus | 0.47% | (5) |
| Mold (General) | 0.47% | (5) |
| Encephalitis | 0.47% | (5) |
| Measles | 0.47% | (5) |
| Pertusis | 0.47% | (5) |
| Meningitis | 0.37% | (4) |
| Malaria | 0.37% | (4) |
| Closridium difficile | 0.37% | (4) |
| Chlamydia | 0.37% | (4) |
| Laryngitis | 0.28% | (3) |
| Scarlet Fever | 0.28% | (3) |
| Rubella | 0.28% | (3) |
| Rickettsia conorii | 0.28% | (3) |
| Staph Infection | 0.28% | (3) |
| Avian Flus | 0.28% | (3) |
| Labyrinthitis | 0.28% | (3) |
| Septicemia | 0.28% | (3) |
| Salmonella | 0.19% | (2) |
| E. coli | 0.19% | (2) |
| Bartonella | 0.19% | (2) |
| Esophagitis | 0.19% | (2) |
| MRSA | 0.19% | (2) |
| Vestibular Neuritis | 0.19% | (2) |
| Hepatitis C | 0.19% | (2) |
| Toxoplasmosis | 0.19% | (2) |
| Infectious Rhinitis | 0.19% | (2) |
| Pseudomonas aeruginosa | 0.19% | (2) |
| Fungal Infection | 0.19% | (2) |
| Bowel Infection | 0.19% | (2) |
| Hepatitis A | 0.19% | (2) |
| Jaundice | 0.19% | (2) |
| Yeast Infection | 0.19% | (2) |
| Peritonitis | 0.19% | (2) |
| Jaw Infection | 0.19% | (2) |
| Trachea Infection | 0.09% | (1) |
| Thrush | 0.09% | (1) |
| Scrofula | 0.09% | (1) |
| Dengue Fever | 0.09% | (1) |
| Haemophilus influenzae | 0.09% | (1) |
| Typhoid Fever | 0.09% | (1) |
| HIV | 0.09% | (1) |
| Intraday Uterine Infection | 0.09% | (1) |
| Mumps | 0.09% | (1) |
| Japanese Encephalitis Virus | 0.09% | (1) |
| Tapeworm | 0.09% | (1) |
| Phayngitis | 0.09% | (1) |
| Helicobacter pylori | 0.09% | (1) |
| Amoebic Dysentery | 0.09% | (1) |
| Liver Infection | 0.09% | (1) |
| Breast Infection | 0.09% | (1) |
| Amoeba Infection | 0.09% | (1) |
| Leptospirosis | 0.09% | (1) |
| Infectious Thyroiditis | 0.09% | (1) |
| Rheumatic Fever | 0.09% | (1) |
| Tuberculosis | 0.09% | (1) |
| Non-specific Urethritis | 0.09% | (1) |
| Bladder Infection | 0.09% | (1) |
| Intra-abdominal Abscess | 0.09% | (1) |
| Hepatitis B | 0.09% | (1) |
| Genital Tuberculosis | 0.09% | (1) |
| Aquarium Granuloma | 0.09% | (1) |
| Stenotrophomonas | 0.09% | (1) |
| Barmah Forest Virus | 0.09% | (1) |
| Pericarditis | 0.09% | (1) |
| Cellulitis | 0.09% | (1) |
| Iritis | 0.09% | (1) |
| Diverticulitis | 0.09% | (1) |
| Gingivitis | 0.09% | (1) |
| Gastroparesis | 0.09% | (1) |
| Osteomyelitis | 0.09% | (1) |
Note: Participants were able to document multiple infectious triggers.
Discussion
Within this large international sample, there were many infectious agents reported. The infectious illness with the highest frequency of being mentioned was mononucleosis. As there are a number of self-reported infections or words that can signify this illness, by adding together mononucleosis, Epstein Barr virus, and Cytomegalovirus, the overall rate of mononucleosis increases to 30.3%. Although EBV is the cause of 90% of mononucleosis cases, it could potentially be underreported here.
Previous research concerning rate of precipitating mononucleosis in ME/CFS have had small sample sizes which made it more difficult to provide reliable rate estimates. In a recent study using plasma from a sample of 58 participants with ME/CFS, reported rates of serum-positive infectious DNA for Epstein-Barr virus (24.1%) and Cytomegalovirus (3.4%) were comparable to the current study.21 Salit reported that among individuals with ME/CFS with a precipitating infectious illness, 7% (7/96) were reported to have “probable” or “definite” infectious mononucleosis.10 However, for 77% of this sample, a definite infectious trigger could not be ascertained. Similar sampling challenges were found in a qualitative study by Evans and Jason, where 43% of participants (6/14) reported that mononucleosis/Epstein-Barr virus was a precursor to their illness onset.11 It is the hope that the robust, large-sample approach taken in the current study will serve as a fitting extension of the aforementioned works.
In our sample, 60.3% of individuals with ME/CFS reported experiencing an infectious illness prior to their ME/CFS onset, slightly lower than figures reported from previous research where rates were in the range of 66–74%.10,11 It is noteworthy that with excluding the Spanish sample, where the prevalence of a precipitating infectious illness was found to be only 23.6%, the rate in the current study increases to a more comparable estimate (64.4%). Compared to other samples, the sample from Spain tended towards greater ME/CFS symptomatology, more severe impairment, and suspected undiagnosed or unreported comorbidities.22 These sample traits could have potentially led to participants’ beliefs in a wide array of illness etiologies, thus minimizing the self-reported causal role of infectious agents in the development of ME/CFS in this sample.
Many different infectious diseases were identified within the patient experience relating to onset of ME/CFS. The lack of clear etio-logical source contributes to illness uncertainly, which has important implications for patients with ME/CFS. Illness uncertainty has three primary factors: pain association is unpredictable, treatments varies widely in effectiveness, and etiology is unknown.23 Illness uncertainty can lead to varying professional opinions on fundamental aspects of their illness, including etiology.24 This has contributed to poor health care experiences as 77% of patients with ME/CFS report negative experiences with health care providers.25 Furthermore, an estimated 95% of those seeking treatment have reported feelings of estrangement.26 Poor understanding of the illness and patient reception has also led to high levels of controversy over treatments such as graded exercise therapy and cognitive behavioral therapy, with the majority of patients considering them inappropriate.27
There are a number of limitations in the current study. Several samples, while asking participants if they were diagnosed by a licensed physician, did not require a confirmation of any reported answers. Due to the self-report methodology, general terms used by many participants were difficult to categorize. Terms like “bowel infection” were inadequate to attribute to an infectious agent. Other limitations of the self-report measures include the usage of vague or colloquial language when participants described their past infectious illnesses. Terms like “cold” or “flu” are often used as general descriptors for common symptoms like runny nose, sneezing, and coughing. Additionally, Eurocentric countries are over-represented in the study sample, which contained only the comparatively small and singular Japanese sample. Besides geographic underrepresentation, because the samples are predominantly middle-aged women of Caucasian descent with at least a college degree, our findings may not apply to groups that do not fall into this demographic profile (i.e. men, less educated people, etc.). As such, we hope future work in this area may focus on etiologic factors of ME/CFS in more diverse samples. Future work may also attempt to elucidate the relationship between infectious illness and ME/CFS by examining differences of diagnosis (i.e. self-reported vs. clinical diagnosis) and case definition. Though the current study utilized a large, worldwide sample of individuals with ME/CFS, differences in sampling methodology made such comparisons challenging.
Finally, interpretations of the results presented herein should be made with caution. In the current study, 60.3% of patients reported an infectious event that preceded symptomatic onset of ME/CFS. This does not imply that the other 39.7% of patients did not have an infectious illness prior to ME/CFS onset. For example, the fact that people can have asymptomatic cases of mononucleosis and infect others makes it evident that asymptomatic cases of serious illnesses can occur. Alternatively, patients may not be inclined to report an initial, non-serious infection as a potential trigger for ME/CFS onset. For instance, if an individual reports occurrence of pneumonia prior to ME/CFS onset, it is possible the pneumonia only occurred because of a patient’s increased susceptibility following a non-serious infection.
The current study represents an exploratory overview of infectious illnesses which precede ME/CFS, as identified through patient self-report. Using a large-sample approach, we hope this study serves as a point of departure in future work seeking to explicate the etiologies of ME/CFS, perhaps achieved through longitudinal and quasi-experimental study designs among diverse cohorts.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Neurological Disorders and Stroke (grant number 5R01NS111105).
Guarantor
Leonard A Jason
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
The present study was reviewed by the DePaul University Institutional Review Board, which judged that it was not subject to review because it was limited to secondary analysis of de-identified data.
Informed consent
All participants in this study provided written informed consent.
References
- 1.Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med 1994; 121: 953–959. [DOI] [PubMed] [Google Scholar]
- 2.Carruthers BM, Jain AK, De Meirleir KL, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr 2003; 11: 7–115. [Google Scholar]
- 3.Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington, D.C.: The National Academies Press, 2015. [PubMed] [Google Scholar]
- 4.Underhill RA. Myalgic encephalomyelitis, chronic fatigue syndrome: an infectious disease. Med Hypotheses 2015; 85: 765–773. [DOI] [PubMed] [Google Scholar]
- 5.Komaroff AL, Fagioli LR, Doolittle TH, et al. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. Am J Med 1996; 101: 281–290. [DOI] [PubMed] [Google Scholar]
- 6.Rasa S, Nora-Krukle Z, Henning N, et al. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med 2018; 16: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedberg F and Jason LA. Understanding chronic fatigue syndrome: an empirical guide to assessment and treatment. Washington, D.C.: American Psychological Association, 1998. [Google Scholar]
- 8.Glaser R and Kiecolt-Glaser JK. Stress-associated immune modulation: relevance to viral infections and chronic fatigue syndrome. Am J Med 1998; 105: 35S–42S. [DOI] [PubMed] [Google Scholar]
- 9.Levy JA. Viral studies of chronic fatigue syndrome. Clin Infect Dis 1994; 18: S117–S120. [DOI] [PubMed] [Google Scholar]
- 10.Salit IE. Precipitating factors for the chronic fatigue syndrome. J Psychiatr Res 1997; 31: 59–65. [DOI] [PubMed] [Google Scholar]
- 11.Evans MA and Jason LA. Onset patterns of chronic fatigue syndrome and myalgic encephalomyelitis. Res Chronic Dis 2018; 2: 1–30. [Google Scholar]
- 12.Loades ME, Coetzee B, Du Toit S, et al. ‘… But i’m still tired’: the experience of fatigue among South African adolescents receiving antiretroviral therapy. AIDS Care 2018; 30: 746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitehead L Toward a trajectory of identity reconstruction in chronic fatigue syndrome/myalgic encephalomyelitis: a longitudinal qualitative study. Int J Nurs Stud 2006; 43: 1023–1031. [DOI] [PubMed] [Google Scholar]
- 14.Dowsett EG, Ramsay AM, McCartney RA, et al. Myalgic encephalomyelitis–a persistent enteroviral infection? Postgrad Med J 1990; 66: 526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straus SE, Tosato G, Armstrong G, et al. Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann Intern Med 1985; 102: 7–16. [DOI] [PubMed] [Google Scholar]
- 16.Chu L, Valencia IJ, Garvert DW, et al. Onset patterns and course of myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatr 2019; 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White PD. What causes chronic fatigue syndrome? Br Med J 2004; 329: 928–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorusso L, Mikhaylova SV, Capelli E, et al. Immunological aspects of chronic fatigue syndrome. Autoimmun Rev 2009; 8: 287–291. [DOI] [PubMed] [Google Scholar]
- 19.Jason LA, Ohanian D, Brown A, et al. Differentiating multiple sclerosis from myalgic encephalomyelitis and chronic fatigue syndrome. Insights Biomed 2017; 2(2): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jason LA and Sunnquist M. The development of the depaul symptom questionnaire: original, expanded, brief, and pediatric versions. Front Pediatr 2018; 6: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shikova E, Reshkova V, Kumanova A, et al. Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic еncephalomyelitis/chronic fatigue syndrome. J Med Virol 2020; 92: 3682–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatia S, Olczyk N, Jason LA, et al. A cross-national comparison of myalgic encephalomyelitis and chronic fatigue syndrome at tertiary care settings from the US and Spain. Am J Soc Sci Humanit 2020; 5: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright LJ, Afari N, and Zautra A. The illness uncertainty concept: a review. Curr Pain Headache Rep 2009; 13: 133. [DOI] [PubMed] [Google Scholar]
- 24.Jason LA, Sorenson M, Porter N, et al. An etiological model for myalgic encephalomyelitis/chronic fatigue syndrome. Neurosci Med 2011; 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson JS and Ferrans CE. The quality of life of persons with chronic fatigue syndrome. J Nerv Ment Dis 1997; 185: 359–367. [DOI] [PubMed] [Google Scholar]
- 26.Green J, Romei J, and Natelson BH. Stigma and chronic fatigue syndrome. J Chronic Fatigue Syndr 1999; 5: 63–75. [Google Scholar]
- 27.Twisk FN and Maes M. A review on cognitive behavorial therapy (CBT) and graded exercise therapy (GET) in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS): CBT/GET is not only ineffective and not evidence-based, but also potentially harmful for many patients with ME/CFS. Neuro Endocrinol Lett 2009; 30: 284–299. [PubMed] [Google Scholar]


