SUMMARY
The main Neisseria meningitidis adhesion molecules, type IV pili (Tfp) and Neisseria adhesion A (NadA), play important roles in the pathogenesis of invasive meningococcal disease. PilE is the major Tfp subunit. In this study, the prevalence and genetic diversity of pilE and nadA were investigated in the prevalent serogroups and clonal complexes (CC) of N. meningitidis isolated in China. All serogroup A strains belonging to CC1 and CC5 and all CC11 serogroup W135 strains were clustered into class II PilE clades. All serogroup C and most of serogroup B isolates except CC8 and ST5642 were class I PilE clades. Class II pilE sequences were highly conserved. All isolates belonging to class I PilE isolates were nadA negative. However, nadA-positive strains were exclusively found in CC5 and CC11 isolates (class II PilE). This study showed that PilE and NadA may be related to epidemic or endemic meningococcal disease.
Key words: Infectious disease epidemiology, meningococcal disease, molecular epidemiology, Neisseria meningitidis
INTRODUCTION
Neisseria meningitidis is a leading cause of invasive bacterial infections globally, with devastating morbidity and mortality [1, 2]. The incidence rates of infections with meningococcal disease vary from 1 to 1000 cases per 100 000 in different regions worldwide with a constant fatality rate of about 10% [2, 3]. There are 12 serogroups of meningococci based on the biochemical composition of the polysaccharide capsule. Serogroups A, B, C, W135 and Y are responsible for more than 90% of the invasive meningococcal disease [4]. The epidemic of meningococcal disease shows regional characteristics. Serogroup A caused two global pandemics in the last century and was still associated with the highest morbidity in the meningitis belt of sub-Saharan Africa and Asia [2]. Serogroup B and C meningococci only caused regional epidemics of invasive meningococcal disease, in North American, Europe and China. Serogroup W135 strains caused an outbreak in 2000 and have subsequently caused sporadic disease and epidemics worldwide [2]. Multilocus sequence typing (MLST) is a commonly used tool for molecular characterization of N. meningitidis [5]. Based on MLST, hyperinvasive lineages can be recognized as different clonal complexes (CCs). Hyperinvasive CC8, CC11, CC32 and CC41/44 meningococci have been responsible for endemic invasive meningococcal disease in different regions. Serogroup A meningococci belonging to CC5 and CC1 were associated with two pandemics globally [6]. Serogroup W135 meningococci belonging to CC11 led to several outbreaks and were reported in different countries [7]. In China, CC1 and CC5 serogroup A were the main causes of meningococcal disease before outbreaks associated with CC4821 serogroup C occurred in 2003 [8, 9].
Colonization, which is indispensable for bacterial propagation and dissemination, is the first step in the pathogenesis of most infectious diseases [10]. Type IV pili (Tfp), the major adhesion molecules of N. meningitidis, are composed of a multimer of the major pilin subunit, PilE [11]. N. meningitidis expresses one of two structurally distinct types of pili according to their reaction (class I) or lack of reaction (class II) with the monoclonal antibody SM1 [12]. Not only do Tfp function as an important virulence factor involved in mediating bacteria adhesion to human cell receptors, they are also involved in twitching motility [13], biofilm formation [14] and natural competence for DNA uptake [15].
In addition to Tfp, the outer membrane protein and potential virulence factor Neisseria adhesion A (NadA) also plays an important role in adhesion and invasion of human cells. It is reported that nadA is present in about 50% of invasive meningococcal isolates [16] and is more frequently associated with strains belonging to hypervirulent lineages, such as CC32, CC11 and CC8. However, this gene is absent from CC41/44 [16, 17] and CC269 [18]. NadA is poorly represented in carriage isolates [16, 17], supporting the hypothesis that this is a disease-related gene essential for full meningococcal virulence. Currently, five NadA variants (NadA-1 to NadA-5) have been described, of which NadA-1, -2 and -3 are mainly represented in the CC32, CC11 and CC8 hypervirulent lineages and are highly cross-reactive. NadA-4 is mainly represented in carriage isolates [17]. NadA-5 is associated with sequence type (ST) 213 CC isolates [19, 20].
Adhesion is the first and most important step in disease generation, dissemination and epidemic [10]. Molecular and epidemiological characterization of the diverse serogroups and clonal complexes of adhesion-related proteins of N. meningitidis is crucial for the elucidation of the mechanism underlying the epidemiology of meningococcal disease. Therefore, in this study, the prevalence and sequence variation of the genes encoding the major pilin and NadA were investigated in disease-causing and carrier isolates of N. meningitidis.
METHODS
Meningococcal strains and DNA preparation
A total of 144 N. meningitidis strains, isolated from 25 provinces of China between 1963 and 2011, were selected for this study. This panel of strains was selected from our N. meningitidis strain database with 1433 N. meningitidis strains isolated from China with accurate data on serogroup and clonal complex, as well as epidemiological information such as isolation date, isolation province and source. We selected the panel of strains tested in this study based on both experimental data and epidemiological information. Almost 10% of strains of our database were used in this study. Details of these strains including year of isolation, sequence type (ST), clonal complex (CC) and serogroup are shown in the Supplementary online material (Supplementary Table S1). Isolates were propagated on single plates of Columbia agar in a 5% CO2 atmosphere at 37 °C for 18 h. Genomic DNA was extracted using a Qiagen DNA mini-kit (Qiagen, Germany) according to the manufacturer's instructions. The strains were isolated from 70 (48·6%) throat swab samples from healthy carriers and 74 (51·4%) blood or cerebrospinal fluid samples from patients. The following 10 clonal complexes were represented among these strains: ST1 (n = 11), ST103 (n = 1), ST11 (n = 10), ST174 (n = 3), ST198 (n = 3), ST32 (n = 2), ST41/44 (n = 2), ST4821 (n = 40), ST5 (n = 44) and ST8 (n = 1). Twenty-seven other strains had unassigned (UA) complex sequence types. The serogroups were A (n = 48), B (n = 35), C (n = 43), W135 (n = 10), X (n = 2) and non-groupable (NG, n = 6).
PCR amplification and sequencing
The major pilin genes pilE and nadA were amplified and sequenced using primers described previously [17, 20, 21]. The PCR conditions used were as described previously [20, 21]. Each locus sequence was analysed on an ABI Prism 3130 or 3730 DNA sequencer (Applied Biosystems, USA).
Nucleotide sequence determination, sequence alignment and analysis
The nucleotide sequence of each gene was analysed using NCBI nucleotide BLAST and by comparison with previously published data. Published whole-genome sequences were used in this study including 8013 [22], Z2491 [23] and MC58 [24], with pilE belonging to class I, and FAM18 [25], and WUE-2594 [26] belonging to class II pilE. The coding sequences of the pilE genes were identified by manual alignment with either 8013 or FAM18 pilE sequences using the DNASTAR program. The NadA amino-acid (aa) sequence was assigned to one of the five main protein forms; NadA-1 to NadA-5. The published NadA GenBank accession numbers used in this study for determination and alignment were FJ619641, FJ619642, AF452482, FJ619644 and FJ619645, corresponding to variants 1–5, respectively [16, 20]. The subvariants of NadA were classified in terms of their main variant group, i.e. variants 1, 2, 3, 4, or 5 [16, 17, 19]. Nucleotide and aa sequences were aligned using the Clustal W program of BioEdit software and manual adjustment, and presented using Boxshade (http://www.ch.embnet.org/software/BOX_form.html). Phylogenetic analyses of nucleotide and deduced aa sequences were performed with the MEGA 4 software package using the neighbour-joining method and Kimura's two-parameter and p-distance models. The positions containing alignment gaps and missing data were eliminated in pairwise sequence comparisons (pairwise deletion option).
RESULTS
Prevalence and distribution of pilE in Chinese strains
In all the strains (n = 144) analysed in this study, the pilE gene was detected in 91% (131/144) of strains with primers specific for either class I or class II alleles. We used PCR and dot blotting methods to detect the pilE gene in 144 strains. The pilE gene was not amplified from 13 strains, from CC4821 (n = 6), CC198 (n = 1) and UA (n = 6). Of the 13 strains, nine were carriage isolates and four were isolated from patients. Results from this primary analysis (Table 1) demonstrated that class I pilE alleles were present in 41·7% (60/144) of tested isolates in this study, containing strains belonging to seven different clonal complexes and five divergent serogroups. All 34 CC4821 pilE-positive strains contained class I pilE alleles. However, class II pilE alleles (49·3%, 71/144) were only present in isolates belonging to CC1, CC5, CC11 CC8 and ST5642 (UA). Of the 71 class II pilE isolates, 62% (44/71) of isolates belonged to CC5 and all of serogroup A were represented in class II. Of the 60 class I pilE isolates, 33 were carriage strains and 27 were disease strains. Carriage strains were from CC174, CC198, CC32, CC41/44, CC4821, UA, and disease strains were from CC103, CC32, CC41/44, CC4821, UA. In a total of 71 class II pilE isolates, 28 carriage strains were from CC1, CC5, CC11, ST5642, and 43 disease strains were from CC1, CC5, CC8, CC11, ST5642.
Table 1.
Prevalence and distribution of class I and class II pilE genes of N. meningitidis strains isolated in China
| pilE | No.of isolates | Clonal complex (CC) | Serogroup |
|---|---|---|---|
| Class I | 60 | CC4821(n = 34) | B (n = 6), C (n = 26), W135 (n = 1), X (n = 1) |
| UA (n = 16)* | B (n = 9), C (n = 5), NG (n = 2) | ||
| Five other clonal complexes (n = 10)† | B (n = 5), C (n = 1), W135 (n = 3), X (n = 1) | ||
| Class II | 71 | CC1 (n = 11) | A (n = 11) |
| CC5 (n = 44) | A (n = 37), C (n = 7)‡ | ||
| CC11 (n = 10) | B (n = 4), C (n = 1), W135 (n = 5) | ||
| CC8 (n = 1) and ST5642 (n = 5)§ | B (n = 6) |
UA, Unassigned to any currently known clonal complex.
Five other clonal complexes contain CC103 (n = 1), CC174 (n = 3), CC198 (n = 2), CC32 (n = 2) and CC41/44 (n = 2).
Seven ST7 serogroup C strains were confirmed to have undergone capsular switching from serogroup A ST7 strains [27].
Five ST5642 strains exhibited MLST allele profiles similar to CC8, and the sequences of class II pilE genes were identical to the strain of CC8.
Diversity of pilE in Chinese strains
The major pilin genes (n = 131) were sequenced, and the nucleotide and the deduced aa sequences of the corresponding mature proteins were aligned. The pilE gene from five sequenced meningococcal genomes was also included for comparison. The extent of sequence variation of class I and class II pilE genes was calculated as the overall mean distance between nucleotide sequences using Kimura's two-parameter model. As previously reported [21, 28], there was considerable variation in pilE between the strains harbouring class I pilins, as shown by the substantial overall mean distance between nucleotide sequences (0·122) (Table 2) and the alignment of pilE sequences (Fig. 1).
Table 2.
Genetic diversity of the major pilin (PilE) in N. meningitidis. Alignment length, number of isolates, alleles, amino-acid sequence types and overall mean distance between nucleotide sequences
| pilE class | Length (bp) | No. of isolates | No. of alleles | Mean distance between nucleotide sequences* | No. of peptide sequences |
|---|---|---|---|---|---|
| Class I | 498–528 | 63† | 55 | 0·122 | 55 |
| Class II (CC1) | 432 | 11 | 2 | 0·000 | 2 |
| Class II (CC5) | 444 | 45‡ | 4 | 0·000 | 4 |
| Class II (CC11) | 447–459 | 11§ | 6 | 0·094 | 6 |
| Class II (CC8, ST5642) | 447 | 6 | 1 | 0·000 | 1 |
The number of base differences per nucleotide site from averaging all sequences pairs using Kimura's two-parameter model.
Corresponds to 60 isolates described in Supplementary Table S1, and three sequenced N. meningitidis strains (8013, Z2491, MC58).
Corresponds to 44 isolates described in Supplementary Table S1, and one sequenced N. meningitidis strain (WUE-2594).
Corresponds to ten isolates described in Supplementary Table S1, and one sequenced N. meningitidis strain (FAM18).
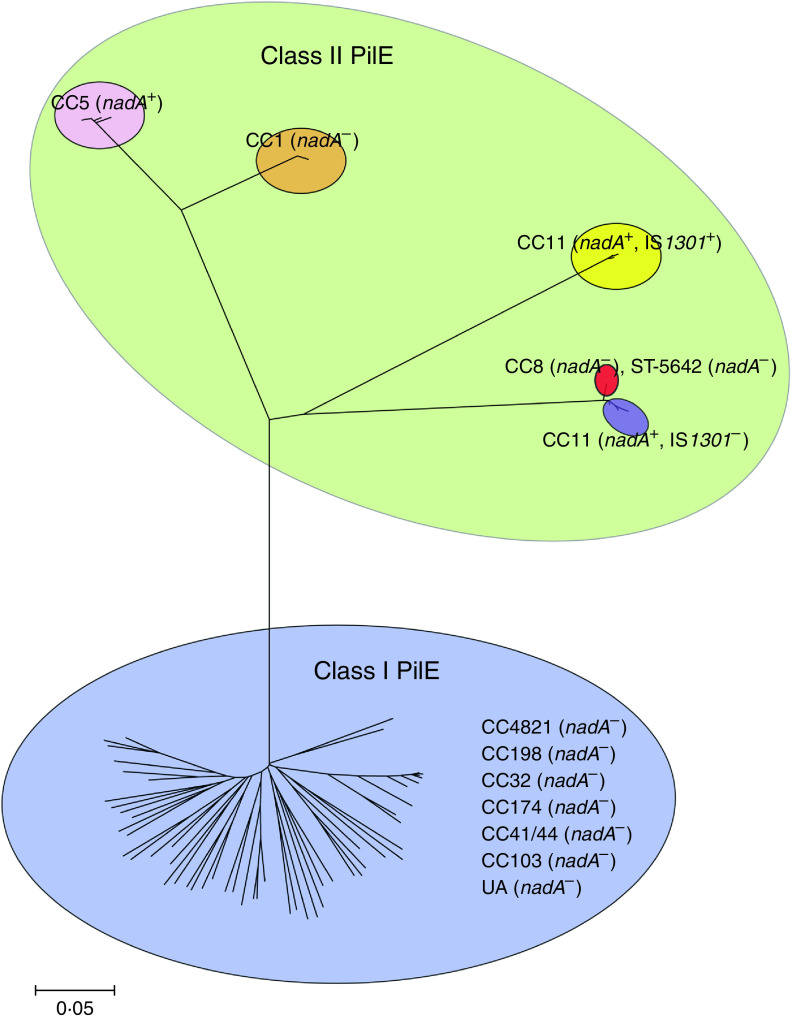
Fig. 1.

Alignment of class I pilins PilE sequences. Isolate identification number or name, clonal complex (CC) and serogroup are shown for each sequence. Conserved residues are white, non-identical residues are black on white background, similar residues are grey and gaps are indicated by hyphens. The mature full-length pilin sequences deduced from 60 N. meningitidis isolates and three sequenced strains Z2491, MC58 and 8013 were aligned using Clustal W. The sequenced strain 053442 (ST4821 CC) was isolate number 40 in our study.
In contrast, the class II pilE genes exhibited a high degree of sequence conservation. Pilins within the CC1, CC5, CC11, CC8 (and five of ST5642) were almost identical, as indicated by the very small mean distances between nucleotide sequences (0·000, 0·000, 0·094, 0·000, respectively) in these strains (Table 2). The mature pilins from the isolates belonging to CC1 differed only by a single aa at position seven (Supplementary Fig. S1). The majority (42/45) of CC5 strains were found to encode a PilE that was identical with the exception of one or two aa changes at positions 30, 102, 110 and 146 (Supplementary Fig. S2). Only one strain (ST5655) belonged to CC8, while five ST5642 strains contained three alleles shown by MLST to be identical to CC8 (ST8). Furthermore, the aligned aa sequences of class II PilE were identical to the CC8 strains (Supplementary Fig. S3). Of the CC11 isolates, six strains differed by only four aa residues (Supplementary Fig. S4). The remaining isolates, encoded a slightly different PilE compared to these six strains, although at least 99·5% identity was observed in the remaining five strains (Supplementary Fig. S5). The phylogenetic analysis of the major pilins revealed that only 13 aa sequence types were identified in all 73 isolates expressing class II pilins, whereas as many as 55 different aa sequence types were found for class I pilins (n = 63). The level of prevalence, distribution and diversity of pilE genes in Chinese strains was further highlighted by phylogenetic analysis of all the PilE aa sequences (Fig. 2).
Fig. 2.
Phylogenetic analysis of all the PilE amino acid (aa) sequences from the meningococcal strains used in this study (Supplementary Table S1) as well as five previously sequenced N. meningitidis isolates. All of the PilE aa sequences separated into two main parts: class I and class II PilE. Isolates (n = 63) of class I PilE belonging to seven different clonal complexes (CC) are presented in the blue ellipse. All class II PilE isolates, which split into five groups, are presented in the green ellipse. The strains of CC5 and CC1 are presented separately in pink and orange ellipses (nadA PCR+ and PCR−, respectively). CC11 strains are presented in yellow and purple ellipses (all nadA PCR+). However, the isolates in the yellow ellipse expressed nadA genes containing a whole insertion sequence IS1301. Isolates of CC8 (n = 1) and ST5642 strains (n = 5) are shown in the red ellipse. These strains harboured the same aa sequence as PilE and have no detectable nadA gene.
Prevalence and distribution of nadA in Chinese strains
The nadA gene was detected in 54/144 (37·5%) N. meningitidis isolates, and the distribution of clonal complexes in the 23 carriage and 31 disease strains was the same, with only CC5 and CC11 present in those isolates. The prevalence of nadA in different serogroups and its correlation with the pilE gene class is shown in Table 3. The nadA gene was more frequently detected in serogroup A strains (77·1%) than in other serogroups, with none detected in class I pilE isolates. All the strains (n = 44) belonged to CC5 and consisted of 37 serogroup A nadA PCR+ isolates and seven serogroup C strains, while the remaining isolates (n = 10) belonged to CC11 and consisted of four serogroup B nadA PCR+ isolates, one serogroup C and five W135 isolates. Of the nadA− isolates, 100% and 24% contained class I pilE and class II pilE genes, respectively.
Table 3.
nadA presence in different serogroups of N. meningitidis isolates based on correlation with the class of pilE gene
| Serogroup | Isolates tested (N) | nadA PCR+ (%) | nadA PCR+ in class I pilE (%) | nadA PCR+ in class II pilE (%) |
|---|---|---|---|---|
| A | 48* | 77·1 (37/48) | 0 (0/0) | 77·1 (37/48)† |
| B | 35 | 11·4 (4/35) | 0 (0/20) | 40 (4/10)‡§ |
| C | 43 | 18·6 (8/43) | 0 (0/32) | 100 (8/8)†‡§ |
| W135 | 10 | 50 (5/10) | 0 (0/4) | 100 (5/5)‡ |
| X | 2 | 0 (0/0) | 0 (0/2) | 0 (0/0) |
| NG | 6 | 0 (0/0) | 0 (0/2) | 0 (0/0) |
Consisted of 11 isolates of CC1 and 37 isolates of CC5 (Supplementary Table S1).
All of the serogroup A and C isolates, belonging only to the CC5 isolates conserved the nadA gene.
All of the serogroup B, C and W135 isolates, belonging only to the CC11 isolates conserved the nadA gene.
Four serogroup B and one serogroup C isolates belonging to CC11 contained one copy of the insertion sequence IS1301.
Diversity of nadA in Chinese strains
The nadA genes of these 54 isolates were sequenced. The nucleotide and deduced aa sequences of the corresponding mature proteins were aligned with the published data as described above. Six unique NadA subvariants were identified (Fig. 3), belonging to two main variants, NadA-3 (including five different subvariants) and NadA-4 (only one invasive isolate which belonged to CC5). The overall mean distance of five subvariants in NadA-3 was 0·003. However, only one isolate of NadA-4 was detected and therefore, it was not possible to determine mean distances for this subvariant. The level of conservation of NadA was shown by the result of phylogenetic analysis of all the NadA aa sequences (Supplementary Fig. S6). All the NadA-3 isolates (n = 53) were assigned to five subvariants belonging to CC5 and CC11. Compared to the published nadA sequence (GenBank accession number AF452482, NadA-3), 40 CC5 isolates were found to encode a completely identical NadA-3 variant. This was therefore designated subvariant NadA-3·1 in this study. The remaining three isolates of CC5 encoded three slightly different NadA-3 proteins (designated NadA-3·2 to NadA-3·4), which differed by a single aa at positions 115, 137 and 343, respectively (Fig. 3). Five out of ten isolates belonging to CC11 contained one copy of the insertion sequence IS1301. The insertion sites were identical and the whole sequence of IS1301 substituted part of the nadA sequence for 538 bp. The final 747 bp of the nadA sequence was consistent with AF452482 (NadA-3). The remaining five isolates of CC11 had four aa point mutations at the same positions (311, 312, 314, 322) in each sequence (designated NadA-3·5) (Fig. 3).
Fig. 3.
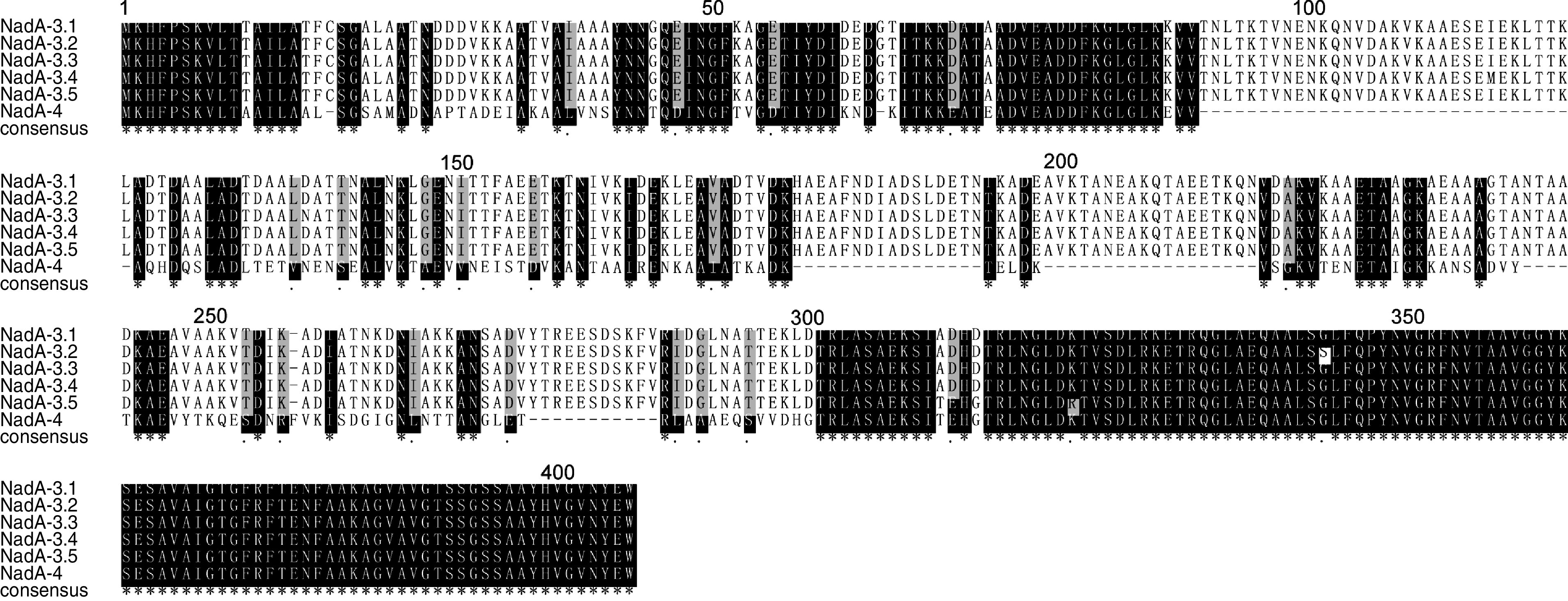
Alignment of six NadA subvariant sequences detected in this study. The designated identifications are shown for each sequence. Conserved residues are white, non-identical residues are black on white background, similar residues are grey and gaps are indicated by hyphens.
DISCUSSION
Adhesion of N. meningitidis to the mucosa of the human nasopharynx is the first step in host colonization and, consequently, the primary event leading to disease manifestation. Adhesion to the host is a dynamic process resulting from a balance of interactions between bacterial surface structures and host receptors. The success of such interactions is determined by the cooperation of multiple adhesins during the different stages of infection. Tfp is essential for the dynamic balance process of bacterial colonization and detachment, which is a prerequisite for the generation and dissemination of invasive disease [29]. At the same time, as a newly highlighted adhesin, NadA is regarded as an important co-factor in the pathogenesis of Neisseria and an encouraging candidate for the development of vaccines. In this study, the prevalence and genetic diversity of two adhesion-related genes, pilE and nadA, were determined. This formed the basis of a preliminary investigation into the potential relationship between the presence and distribution of the multiple adhesins detected in carriage and disease isolates with the presence of distinct epidemic characteristics of different clonal complexes.
This study aimed to elucidate the epidemiological characteristics of isolates in China and is complementary to similar analyses of adhesion-related proteins in N. meningitidis isolates conducted internationally. In this study, the majority of isolates belong to CC1, CC5, CC4821 and CC11 (73%, 105/144), which have been identified as the predominant epidemic isolates in China. Isolates belonging to CC32, CC8 and CC41/44 represent the majority of serogroup B and C meningococcal infections in the developed world but are rare in China. During the last century, over 95% of meningococcal disease and four national epidemics in China were due to serogroup A meningococci [30]. Disease due to CC1 was important in the 1970s, but few of these isolates have been detected since 1989. The ST5 clonal complex strains of serogroup A meningococci caused two epidemics in China, as well as a pandemic wave that spread in 1987 via the annual Hajj pilgrimage [30]. More recently, the number of cases of serogroup C disease has increased in China since 2003 due to the emergence of a new hyperinvasive lineage of CC4821 [8]. In addition to CC4821, CC11 also emerged in China during the years from 2003 to 2006, representing a significant proportion of serogroup C and W135 disease worldwide [31].
Analysis of the distribution of class I and class II pilE genes revealed that class II pilE alleles are highly conserved and present in hyperinvasive lineage isolates, which is consistent with previous reports [21]. Class II pilE genes have been reported to be present only in ST8 and ST11 clonal complexes [21]. However, in this study, class II pilE genes were detected in isolates in the related CC1 and CC5. Of note, according to our study and previous reports, all of the isolates belonging to class II pilE have caused epidemics (CC1, CC8) or pandemics (CC5, CC11) worldwide. In marked contrast, class I pilE genes were found in strains belonging to seven different clonal complexes, including some carriage isolates and some local epidemic isolates. For example CC4821 is present only in China while CC32 and CC41/44 are mainly detected in Europe and America.
Sequencing of the class I pilE genes confirmed the well-known diversity of the major pilin subunit genes. As many as 55 different aa sequence types were detected in 63 class I pilins. In contrast, class II pilE genes exhibited a high degree of sequence conservation. Only 13 different class II pilins were identified in a total of 73 sequences amplified from meningococci belonging to four hyperinvasive lineages, and expressing four different capsular groups. However, the reason for this degree of conservation in the major pilin subunits in these clonal complexes and the potential relationships between these clonal complexes and the disease remain to be elucidated. Some studies have confirmed that a vaccine based on pilus preparation is safe and induces the production of specific antibodies that effectively interfere with colonization by inhibiting bacterial attachment to host cells [32, 33]. However, because PilE undergoes frequent and extensive antigenic variation in Neisseria species, the development of a pilus-based vaccine has been halted [34]. The data obtained in this study suggest that a class II PilE-based vaccine (a previously abandoned idea) might be a suitable approach for these hypervirulent isolates.
Our data indicate that 54 of the 144 N. meningitidis isolates harbour the nadA gene, which was detected only in CC5 and CC11 of the corresponding class II PilE isolates. There was no difference of distribution of the pilE and nadA genes between the carriage and disease isolates. Hypervirulent lineages such as CC1, CC5, CC11, CC4821 were also found in carriage isolates. Further exploration of carriage and disease isolates from the same hypervirulent clonal complexes would be of interest. NadA-1, NadA-2 and NadA-5 have been reported from CC32 invasive disease strains from Europe and the USA, CC1 and CC8 invasive disease strains from Europe and USA and CC213 carriage and disease-causing strains from Europe, respectively [17, 19]. These three variants were not detected in this study. Phylogenetic analysis revealed six unique NadA subvariants belonged to two main variants, NadA-3 and NadA-4 (only one invasive isolate of which belonged to CC5), as identified in a previous study [16, 17]. In previous studies, all isolates of CC32 and CC8 harbour the nadA gene [16, 17, 19, 20]. However, in this study two isolates from CC32 and one from CC8 did not harbour the nadA gene. Furthermore, with the exception of one isolate of variant NadA-4, the isolates from CC5 (the predominant epidemic isolates in China) expressed nadA genes belonging to the NadA-3 variant, which forms the recombinant component of the investigational 4CMenB vaccine [35].
Purified surface-exposed capsular polysaccharide and polysaccharide protein conjugate vaccines are currently available to protect against meningococcal disease caused by serogroups A, C, Y and W135 [36]. However, an effective vaccine for prevention of infection with serogroup B meningococci has yet to be developed. The identification of surface-exposed, conserved and widely prevalent meningococcal antigens suitable for use as components of a broadly protective vaccine is a significant challenge [37]. Therefore, our results complement international studies of N. meningitidis adhesion-related proteins and vaccine research.
In conclusion, this analysis of the prevalence and genetic diversity of pilE and nadA in N. meningitidis in China provides further elucidation of the molecular characterization of these two adhesion-related genes. More importantly, it is interesting to note a highly significant correlation between clonal complexes and the two adhesion-related genes, although this correlation may not be complete. It can be hypothesized that the isolates from diverse clonal complexes may cluster with distinct epidemic features and virulence due to the different adhesion-related genes. For example, ST5 and ST11 clonal complexes both harbour pilE and nadA genes and these two hyperinvasive lineages have caused worldwide pandemics [2, 30]. Otherwise, all isolates expressing the class I pilE gene lack the nadA gene, suggesting that different adhesion-related genes play different roles in the pandemic of N. meningitidis disease; moreover, the epidemic of a N. meningitidis clone is the result of the combined effect of several adhesion-related genes. The correlation between pandemic and adhesion-related genes should be studied more in the future. Furthermore, knowledge of the molecular characteristics of adhesion-related genes is beneficial for the design of optimal vaccines. Finally, our results provide further understanding of the prevalence and genetic characterization of N. meningitidis adhesion-related genes and direct research into the potential epidemiological mechanism of meningococcal disease and the design of interventions for prevention of carriage, treatment of disease and eradication of infections.
Supplementary Material
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
ACKNOWLEDGEMENTS
This study was supported by the national 973 programme of China (2011CB504900) and the Priority Project on Infectious Disease Control and Prevention (grants 2011ZX10004-001 and 2012ZX10004215) from the Ministry of Health and the Ministry of Science and Technology and the National Natural Scientific Foundation of China (grant 81201332) from the Ministry of Science and Technology, People's Republic of China.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268812002944.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Moore PS. Meningococcal meningitis in sub-Saharan Africa: a model for the epidemic process. Clinical Infectious Diseases 1992; 14: 515–525. [DOI] [PubMed] [Google Scholar]
- 2.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27: B51–63. [DOI] [PubMed] [Google Scholar]
- 3.Caugant DA, Maiden MC. Meningococcal carriage and disease – population biology and evolution. Vaccine 2009; 27: B64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peltola H. Meningococcal disease: still with us. Reviews of Infectious Diseases 1983; 5: 71–91. [DOI] [PubMed] [Google Scholar]
- 5.Maiden MC, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proceedings of the National Academy of Sciences USA 1998; 95: 3140–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu P, et al. Fit genotypes and escape variants of subgroup III Neisseria meningitidis during three pandemics of epidemic meningitis. Proceedings of the National Academy of Sciences USA 2001; 98: 5234–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer LW, et al. Outbreak of W135 meningococcal disease in 2000: not emergence of a new W135 strain but clonal expansion within the electrophoretic type-37 complex. Journal of Infectious Diseases 2002; 185:1596–1605. [DOI] [PubMed] [Google Scholar]
- 8.Shao Z, et al. Identification of a new Neisseria meningitidis serogroup C clone from Anhui province, China. Lancet 2006; 367: 419–423. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, et al. Distribution of serogroups and sequence types in disease-associated and carrier strains of Neisseria meningitidis isolated in China between 2003 and 2008. Epidemiology and Infection 2012; 140: 1296–1303. [DOI] [PubMed] [Google Scholar]
- 10.Smith H. What happens to bacterial pathogens in vivo? Trends in Microbiology 1998; 6: 239–243. [DOI] [PubMed] [Google Scholar]
- 11.Coureuil M, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science 2009; 325: 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virji M, et al. Identification of epitopes recognized by monoclonal antibodies SM1 and SM2 which react with all pili of Neisseria gonorrhoeae but which differentiate between two structural classes of pili expressed by Neisseria meningitidis and the distribution of their encoding sequences in the genomes of Neisseria spp. Journal of General Microbiology 1989; 135: 3239–3251. [DOI] [PubMed] [Google Scholar]
- 13.Virji M, et al. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Molecular Microbiology 1991; 5: 1831–1841. [DOI] [PubMed] [Google Scholar]
- 14.Greiner LL, et al. Biofilm formation by Neisseria gonorrhoeae. Infection and Immunity 2005; 73: 1964–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seifert HS, et al. Shuttle mutagenesis of Neisseria gonorrhoeae: pilin null mutations lower DNA transformation competence. Journal of Bacteriology 1990; 172: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comanducci M, et al. NadA, a novel vaccine candidate of Neisseria meningitidis. Journal of Experimental Medicine 2002; 195: 1445–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comanducci M, et al. NadA diversity and carriage in Neisseria meningitidis. Infection and Immunity 2004; 72: 4217–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucidarme J, et al. Characterization of fHbp, nhba (gna2132), nadA, porA, sequence type (ST), and genomic presence of IS1301 in group B meningococcal ST269 clonal complex isolates from England and Wales. Journal of Clinical Microbiology 2009; 47: 3577–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bambini S, et al. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine 2009; 27: 2794–2803. [DOI] [PubMed] [Google Scholar]
- 20.Lucidarme J, et al. Characterisation of fHbp, nhba (gna2132), nadA, porA and sequence type in group B meningococcal case isolates collected in England and Wales during January 2008, and potential coverage of an investigational group B meningococcal vaccine. Clinical and Vaccine Immunology 2010; 17: 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cehovin A, et al. Sequence conservation of pilus subunits in Neisseria meningitidis. Vaccine 2010; 28: 4817–4826. [DOI] [PubMed] [Google Scholar]
- 22.Rusniok C, et al. NeMeSys: a biological resource for narrowing the gap between sequence and function in the human pathogen Neisseria meningitidis. Genome Biology 2009; 10: R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkhill J, et al. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 2000; 404: 502–506. [DOI] [PubMed] [Google Scholar]
- 24.Tettelin H, et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 2000; 287: 1809–1815. [DOI] [PubMed] [Google Scholar]
- 25.Bentley SD, et al. Meningococcal genetic variation mechanisms viewed through comparative analysis of serogroup C strain FAM18. PLoS Genetics 2007; 3: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoen C, et al. Whole-genome sequence of the transformable Neisseria meningitidis serogroup A strain WUE2594. Journal of Bacteriology 2011; 193: 2064–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Shao Z, Wang X. Genetic study of capsular switching between Neisseria meningitidis sequence type 7 serogroup A and C strains. Infection and Immunity 2010; 78: 3883–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuart AH, John KD. Pilin gene variation in Neisseria gonorrhoeae: reassessing the old paradigms. FEMS Microbiology Reviews 2009; 33: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. . Chamot-Rooke J, et al. Posttranslational modification of pili upon cell contact triggers N. meningitidis dissemination. Science 2011, 331: 778–782. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, et al. Genetic characteristics of serogroup A meningococci circulating in China, 1956–2005. Clinical Microbiology and Infection 2008; 14: 555–561. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, et al. Molecular characterization of serogroup C Neisseria meningitidis isolated in China. Journal of Medical Microbiology 2007; 56: 1224–1229. [DOI] [PubMed] [Google Scholar]
- 32.Siegel M, et al. Gonococcal pili: safety and immunogenicity in humans and antibody function in vitro. Journal of Infectious Diseases 1982; 145: 300–310. [DOI] [PubMed] [Google Scholar]
- 33.Tramont EC, et al. Gonococcal pilus vaccine. Studies of antigenicity and inhibition of attachment. Journal of Clinical Investigation 1981; 68: 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Criss AK, Kline KA, Seifert HS. The frequency and rate of pilin antigenic variation in Neisseria gonorrhoeae. Molecular Microbiology 2005; 58: 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai X, et al. Recombinant protein meningococcal serogroup B vaccine combined with outer membrane vesicles. Expert Opinion on Biological Therapy 2011; 11: 969–985. [DOI] [PubMed] [Google Scholar]
- 36.Snape MD, Pollard AJ. Meningococcal polysaccharide–protein conjugate vaccines. Lancet Infectious Diseases 2005; 5: 21–30. [DOI] [PubMed] [Google Scholar]
- 37.Lewis S, et al. Challenges and progress in the development of a serogroup B meningococcal vaccine. Expert Review of Vaccines 2009; 8: 729–745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
Supplementary information supplied by authors.
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268812002944.
click here to view supplementary material