Abstract
Background:
Choline supplementation (+Ch) improves cognitive function in impaired animals and humans. Chemotherapy-related cognitive deficits (CRCDs) occur in cancer patients, and these deficits persist following treatment, adversely impacting quality of life. To date, there are no approved treatments for this condition.
Aim:
Because +Ch improves impaired memory, it was of interest to determine whether +Ch can attenuate spatial memory deficits induced by the chemotherapeutic agents doxorubicin (DOX) and cyclophosphamide (CYP).
Methods:
Female BALB/C mice, 64 days of age, were trained in the Morris water maze and baseline performance determined on day 15. Following baseline assessment, mice were placed on +Ch diet (2.0% Ch) or remained on standard diet (SD; 0.12% Ch). Mice received intravenous injections of DOX (2.5 mg/kg) and CYP (25 mg/kg), or equivalent volumes of saline (0.9% NaCl), on days 16, 23, 30 and 37, and spatial memory was assessed weekly, from day 22 to 71.
Results:
DOX and CYP produced a prolonged impairment in spatial memory as indicated by an increased latency to the correct zone (p<0.05), and a decrease in time in the correct zone (p<0.05), % of total swim distance in the correct zone (p<0.05) and % entries to the correct zone (p<0.05). These effects were attenuated by +Ch.
Conclusions:
Although it remains to be determined whether this effect extends to other cognitive domains and whether +Ch is prophylactic or therapeutic, these findings suggest that +Ch may be an effective intervention for CRCDs.
Keywords: Chemotherapy, Choline, Cognitive Deficit, Female, Mice, Spatial Memory
Introduction
Choline (Ch) is an essential nutrient for cell membrane biosynthesis, lipid metabolism, and muscle, liver and brain function. Ch serves as a methyl donor for many metabolic processes and is necessary for the synthesis of phosphatidylcholine and sphingomyelin, as well as the production of acetylcholine (ACh) (Jope and Jenden, 1980), a neurotransmitter critical for attention, learning and memory (Drachman and Leavitt, 1974; Bartus and Johnson, 1976; Baxter and Chiba, 1999; Schliebs and Arendt, 2006; Pepeu and Giovannini, 2010; Everitt and Robbins, 1997). Although Ch can be synthesized by the body, the amount is insufficient to meet the functional demand for this nutrient, and thus, Ch must also be obtained from dietary sources (Wallace et al., 2018; Wallace, 2018).
It is well established that insufficient Ch during pregnancy leads to neural tube defects and cleft palates in newborns and that choline depletion in adults leads to nonalcoholic fatty liver disease and skeletal muscle damage (Zeisel et al., 1991; da Costa et al., 2004; Cole et al., 2012; Buchman et al., 2001; Schwarzenberg et al., 2018; Shaw et al., 2006; Shaw et al., 2004; Carmichael et al., 2010; Mills et al., 2014). Additionally, studies indicate that small to moderate reductions in Ch availability may negatively impact cognition in adults (Poly et al., 2011; Nurk et al., 2013). The average daily intake of Ch by adults (19–70 y) in the US ranges from 271 – 280 mg/day for females, and 391 – 421 mg/day for males (Wallace et al., 2018), yielding plasma Ch concentrations from 7 – 20 umol/L in healthy adults. Lower than average dietary choline intake is associated with deficits of verbal and visual memory in adults (36–83 y) and low plasma Ch concentrations (<8.36 umol/l) in the elderly (70–74 y) are associated with reduced perceptual processing speed, executive function and global measures of cognition (Poly et al., 2011; Nurk et al., 2013).
One reason that low choline intake and low circulating choline levels are associated with poor cognitive function in adults is that reduced choline availability impairs ACh synthesis. The rate limiting factor for ACh synthesis is the amount of Ch transported into cholinergic neurons from extracellular sources via high-affinity choline uptake (HACU) (Jope and Jenden, 1980; Collier, 1988; Bussiere et al., 2001). Because extracellular Ch concentrations are proportional to blood Ch concentrations, the amount of Ch available for HACU and ACh synthesis is dependent on the dietary intake of Ch (Klein et al., 1992; Michel et al., 2006). Insufficient dietary Ch will reduce the neuronal supply of Ch and attenuate ACh synthesis (Wecker et al., 1989; Wecker, 1991; Wecker, 1986; Wecker et al., 1978; Wecker and Schmidt, 1980; Schmidt and Wecker, 1981; Trommer et al., 1982; Jope, 1982; Dolezal and Tucek, 1982), while dietary choline supplementation (+Ch) will support HACU, even when the demand for the precursor is high (Wecker et al., 1989; Wecker, 1991; Wecker, 1986; Wecker et al., 1978; Wecker and Schmidt, 1980; Schmidt and Wecker, 1981; Trommer et al., 1982; Jope, 1982; Dolezal and Tucek, 1982).
Impaired HACU may occur when circulating hormone concentrations are reduced by chemotherapy-induced damage to gonadal tissue (Jarrell et al., 1987; Ahles and Saykin, 2007; Petrek et al., 2006; Minisini et al., 2009; Swain et al., 2009; Swain et al., 2010). Animal studies have demonstrated that HACU is impaired by ovariectomy and that this effect can be reversed by the administration of 17β-estradiol (O’Malley et al., 1987; Singh et al., 1994), indicating that reductions in circulating estrogens restrict the neural supply of Ch. Therefore, chemotherapy-associated ovarian suppression or ablation could result in cognitive deficits by restricting the neuronal supply of Ch, reducing ACh synthesis and impairing cholinergic function. Indeed, cognitive deficits are a common occurrence for patients receiving chemotherapeutic agents and are most commonly reported by women (Aluise et al., 2010; Tannock et al., 2004). Further, women experiencing amenorrhea following chemotherapy exhibit significant cognitive deficits when compared to chemotherapy patients who do not experience amenorrhea, implicating reductions of circulating estrogen in the manifestation of chemotherapy-related cognitive deficits (CRCDs) (Vearncombe et al., 2011; Jenkins et al., 2006). Men may also experience CRCDs resulting from a reduction in the neuronal supply of Ch as a consequence of low circulating estrogen. For example, men undergoing androgen ablation therapy for prostate cancer exhibit a significant decline in cognitive ability (Salminen et al., 2004; Green et al., 2002; Beer et al., 2006) and these deficits can be reversed with estradiol therapy (Beer et al., 2006; Hess, 2003).
Although preclinical and clinical studies have examined several treatment approaches for CRCDs, very few have tried to prevent the development of this condition. Dietary Ch supplementation (+Ch) using choline chloride, choline bitartrate, citicholine or choline alfoscerate (alpha-GPC) attenuates cognitive deficits in animal models and humans. In laboratory animals, +Ch attenuates scopolamine-induced cognitive impairments, protects against the development of memory deficits in aging rats or rats raised in impoverished environments, facilitates the retention of learned behaviors in animals experiencing hypoxia, and attenuates cognitive deficits resulting from traumatic brain injury (Teather and Wurtman, 2005; Teather and Wurtman, 2003; Sigala et al., 1992; Guseva et al., 2008; Secades and Frontera, 1995). In humans, +Ch improves cognitive functioning following stroke and improves attention, verbal memory and memory efficiency in elderly individuals with poor memory, vascular dementia or Alzheimer’s disease (Alvarez et al., 1997; Moreno, 2003; Garcia-Cobos et al., 2010; Clark et al., 1997).
The present study used an established mouse model of CRCDs to determine whether +Ch could attenuate chemotherapy-induced deficits in cognitive function when provided during and following chemotherapy. Because studies have demonstrated that chemotherapeutic agents impair performance in tasks mediated by the hippocampus (Hcc) (Macleod et al., 2007; Yang et al., 2010a; Christie et al., 2012; Kitamura et al., 2015; Mustafa et al., 2008; Lyons et al., 2011), the Morris water maze was used to assess spatial memory, a well-established method sensitive to changes in Hcc function (Morris et al., 1982). Additionally, because the majority of patients who report CRCDs are women (Aluise et al., 2010; Tannock et al., 2004; Kohli et al., 2007) and the majority of CRCD research has been conducted in breast cancer patients, the present study used female BALB/C mice injected with doxorubicin (DOX) and cyclophosphamide (CYP), two chemotherapeutic agents commonly used for the treatment of breast cancer.
Methods
Adult female BALB/C mice (N=50; Envigo, Indianapolis, IN), 8 weeks of age upon arrival, were used for these studies. Females were chosen specifically because the vast majority of CRCD self-reports are from women and we have previously demonstrated that female, but not male, mice are vulnerable to spatial memory deficits using the paradigm herein described (Philpot et al., 2016). Animals were housed 2–4 per cage (with ‘enrichment’ consisting of a small, translucent ‘igloo’) in a temperature and humidity-controlled vivarium on a 12–12 hour light-dark cycle (6am-6pm) with food and water available ad libitum. Animals were acclimated for 1 week prior to study and handled daily. All behavioral assessments took place between 9am and 3pm. The care and use of animals were approved in accordance with guidelines set by the University of South Florida Institutional Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (IACUC #: R IS00003840).
Apparatus
Morris water maze (MWM) assessments were conducted in a circular pool (130cm diameter) filled with opaque water. The apparatus has been described in detail (Philpot et al., 2016). Noldus Ethovision software was used for the analysis of behavior in the pool and in each of 6 equal sized “virtual” zones. For each trial, the average velocity while swimming, frequency of zone entries, total distance moved and time in the platform zone were determined. Additionally, the % of entries into and % of distance moved in the platform zone, were calculated.
Behavioral Training and Baseline Assessment
An experimental timeline is provided for reference (Fig. 1A). As described (Philpot et al., 2016), one week following arrival, on day 8, mice received orientation training consisting of 5 trials/day for a period of 2 days. Spatial memory training began on day 10 and consisted of 5 trials/day on days 10–14. During each training trial, the platform was placed below the surface of the water in a consistent location for each animal. On day 15, trials 1, 2, 4 and 5 occurred as described, with trial 3 serving as a probe trial to assess spatial memory. During the probe trial, the platform was removed and mice were allowed to swim for a total of 60sec before being removed from the apparatus. Following the probe trial, 2 additional platform trials were performed (trials 4 and 5) to prevent possible extinction of spatial memory.
Fig 1.
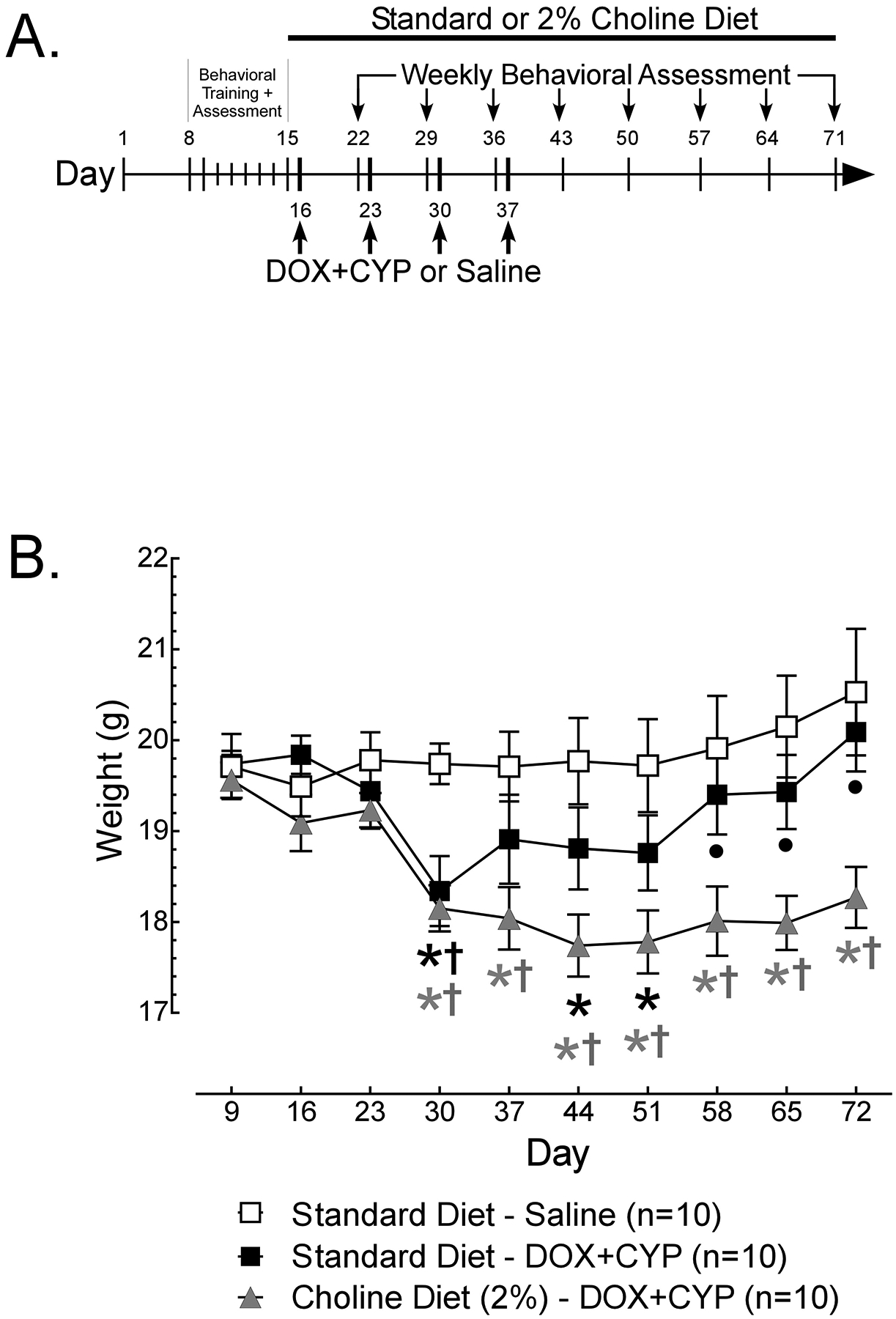
(A) Timeline of Morris water maze (MWM) training and assesment, diet manipulations and administration of cyclophosphamide (CYP), doxorubicin (DOX) or 0.9% saline. Female BALB/C mice (N=30) were trained in the MWM for 5 days (days 10–14). Weekly assessments were performed on days 15–71, with trials 1, 2, 4 and 5 occurring with the platform present, and trial 3 serving as a probe trial (platform removed) to assess spatial memory. Following baseline assessment on day 15, mice either remained on standard diet or were switched to a 2% choline diet. CYP (25mg/kg) and DOX (2.5mg/kg) were administered (i.v.) on days 16, 23, 30 and 37 to mice on standard (n=10) or 2% choline (n=10) diet. A separate group of mice on standard diet (n=10) received injections (i.v.) of saline (0.9%) on days 16, 23, 30 and 37 and were assessed cuncurrently. (B) The weights (g) of female BALB/C mice (N=30) were assessed weekly throughout the study. Data are presented as group means +/− S.E.M. Using Dunnett’s test, signifcant (p<0.05) differences from baseline (day 9) weights are indicated with a *. Using Tukey’s HSD, signifcant (p<0.05) differences from the Standard Diet – Saline group are indicated with † and differences from Choline Diet (2%) – DOX+CYP group are indicated with •.
Some mice do not actively explore the MWM and therefore are unlikely to learn to locate the platform and/or are unable to demonstrate spatial memory during the probe trial. Thus, only data from mice that swam for a majority (>50%) of the baseline (day 15) probe trial were considered valid assessment of spatial memory. Data obtained from mice that did not swim for >50% of the baseline probe trial were excluded from all analyses. Among the mice that did swim for the majority of the baseline probe trial, those that produced at least 20% of their total swim distance in the correct zone during baseline probe trial were considered to have demonstrated learning and memory of the platform location. Data obtained from mice that did not meet this ≥20% criteria were excluded from all analyses.
Dietary Choline Supplementation
On day 15, following baseline assessment, mice matched for spatial memory (% Total Swim Distance in Correct Zone) were randomly assigned to cages with either standard diet (Teklad Global Rodent Diet; 0.12% Choline, 18.6% Protein, 6.2% Fat, 44.2% Carbohydrate) or a 2% choline chloride diet (Teklad TD.150428; 2.0% Choline, 17.8% Protein, 5.7% Fat, 46.9% Carbohydrate) for the remainder of the study. A diet of 2% choline was chosen because Hcc and striatal sections derived from animals fed this diet in vivo exhibit elevated concentrations of free choline (Wecker et al., 1989; Wecker, 1988) and elevated free choline following in vivo choline supplementation increases HACU and ACh synthesis in Hcc sections (Wecker et al., 1989; Wecker, 1988). Unfortunately, the typical daily intake of 2% choline diet by our mice was indeterminable because: 1) we were unable to measure consumption for individual mice because they were housed in groups (2–4/cage); and 2) the commercial diet pellets tended to disintegrate when handled/manipulated/masticated, falling into the bedding below, making measurements of the amount consumed (g/day/cage) unreliable.
Administration of Chemotherapeutic Agents
On day 16, mice began receiving tail vein injections of DOX (2.5 mg/kg) followed by CYP (25 mg/kg) once per week for 4 weeks (days 16, 23, 30 and 37). This dose combination has been demonstrated to impair spatial memory in female BALB/C mice (Philpot et al., 2016; Philpot et al., 2019). To verify that the MWM performance of chemotherapy-naïve controls was stable across weeks of assessment, a separate group of mice received tail vein injections of equivalent volumes of 0.9% saline once per week for 4 weeks on days 16, 23, 30 and 37.
Behavioral Assessment During and Following Chemotherapy
Six days following each administration of DOX+CYP or 0.9% saline, and for an additional 4 weeks following the final injection, mice were assessed for spatial memory performance in the MWM. Each assessment day included 4 platform trials (trials 1, 2, 4 and 5) and a probe trial (trial 3).
Statistical Analysis
Analyses were conducted using Prism (GraphPad Software; San Diego, CA). All analyses were conducted using a significance criterion of α = 0.05 and family-wise error was reduced using appropriate post hoc tests. Data from each measure on training days 10–14 were analyzed using repeated measures analysis of variance (ANOVA) with subsequent multiple comparisons (Dunnett’s) to assess changes from day 10. To verify an established spatial memory, single sample t-tests were performed comparing the % of correct zone entries, time in the correct zone and % of distance moved in the correct zone to chance performance (1/6 zones= 16.67% for % correct entries and % distance moved in correct zone; 1/6 zones × 60sec = 10sec for time in correct zone). Data from platform and probe trials on days 15–71 were assessed using 3 × 9 ANOVA with subsequent multiple comparisons (Dunnett’s) to assess changes from baseline performance (day 15) and differences between groups (Tukey’s HSD).
Results
BALB/C mice frequently respond to being placed in the water by floating, rather than swimming to search for an escape (Philpot et al., 2016; Francis et al., 1995; Wahlsten et al., 2005), thus these mice cannot demonstrate spatial memory of the platform location. Of the 50 mice that began training, 16 would not swim during behavioral training (days 8–14) or testing (day 15) and/or did not meet a minimum criteria for spatial memory at baseline assessment (≥20% of distance moved in the correct zone during the day 15 baseline probe trial). These mice were eliminated from the study on day 15, undergoing no further behavioral assessments and receiving no dietary manipulation or injections of chemotherapeutic agents. An additional 4 mice developed DOX-induced necrosis at the injection site during the 4 weeks of chemotherapy and were sacrificed prior to completion of the study, per IACUC guidelines.
Weights
The weights (Fig. 1B) of the remaining 30 mice differed significantly across weeks depending on treatment group [Treatment × Week interaction, F(18, 243)=3.542, p<0.05]. Beginning average weights were between 19 and 20g and did not differ significantly between groups on days 9–23. The weights of mice who remained on standard diet and received injections of saline did not change significantly across weeks of assessment relative to day 9. For mice remaining on standard diet and administered DOX+CYP, weights were significantly lower on days 30, 44 and 51, relative to day 9 (*), and were significantly lower on day 30 than weights of mice on standard diet administered saline (†). For mice on 2% choline diet and administered DOX+CYP, weights were significantly lower on days 30–72 relative to day 9 (*). These weights were also significantly lower than mice on standard diet administered saline (†) on days 30–72 and significantly lower than weights of mice on standard diet administered DOX+CYP (•) on days 58–72.
Training and Baseline Assessment
Performance of mice during training days (days 10–14) improved significantly over days (Fig 2A – C), exhibiting a 24–54% reduction in total swim distance [Main Effect of Day, F(4,108)=14.22, p<0.05], a 36–48% reduction in incorrect zone entries [Main Effect of Day, F(4,108)=10.01, p<0.05] and a 34–40% decrease in latency to the platform [Main Effect of Day, F(4,108)=10.54, p<0.05]. During the baseline probe trial (day 15) mice performed significantly better than chance (Fig. 2D – E): 31% (vs. 16.67%) of zone entries were to the correct zone [t(29)=9.72, p<0.05]; mice spent 27sec (vs. 10sec) in the correct zone [t(29)=10.13, p<0.05]; and 44% (vs. 16.67%) of movement occurred in the correct zone [t(29)=10.84, p<0.05]. Taken together, data indicate effective training and a spatial memory of the platform location at baseline (day 15).
Fig 2.
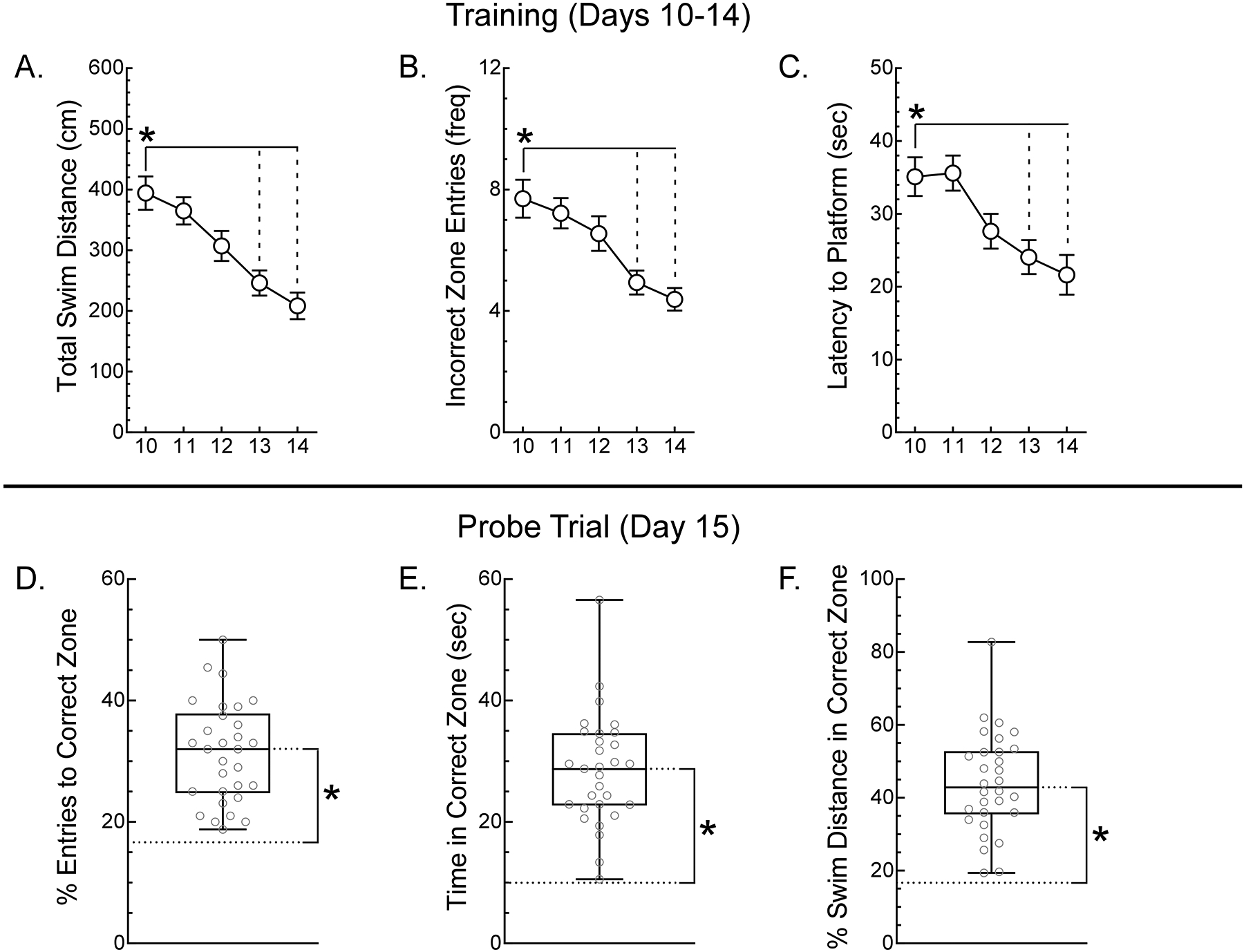
Female BALB/C mice (N=30) were trained in the MWM for 5 days (days 10–14) and (A) the mean total swim distance, (B) mean incorrect zone entries and (C) latency to the platform of mice were calculated. Data are presented as group means +/− S.E.M. Significant (p<0.05) differences from day 10 were identified using Dunnett’s test are indicated with a *. To assess the spatial memory of mice following training, on day 15, the (D) % entires to the correct zone, (E) time in the correct zone and (F) % swim distance in the correct zone were determined with the platform removed from the MWM. Data are presented as min:max box and whisker plots with individual values in grey, the box representing the middle 50% and the overall mean indicated by the horizontal line inside the box. Significant (p<0.05) differences of the overall mean from chance performance (dashed line), determined using single sample tests, are indicated with a *.
Although mice were randomly assigned to groups following training and baseline assessment, a retrospective analysis of the training data was performed to insure group similarity. For Latency to Platform, on Day 11, mice assigned to the saline-injected standard diet group (mean = 29.22 +/− 4.16) did find the platform significantly faster than mice assigned to the DOX+CYP-injected choline diet group (mean = 43.48 +/− 3.50) [F(8,108)=2.40, p<0.05]. Differences were not observed at any other time point for this measure and no significant group differences were observed for Total Swim Distance or Incorrect Zone Entries across days of training. A retrospective analysis of probe trial performance found no significant group differences at baseline for % Entries to Correct Zone, Time in correct Zone or % Swim Distance in Correct Zone. Therefore, groups behaved similarly during training and presented similar baseline performance.
Weekly Assessments
There were few significant changes in the performance of mice across weeks of assessment or between groups during the platform trials (data not shown). During probe trials, the total swim distance (Fig 3A) of mice relative to baseline (day 15), differed significantly between groups [Treatment × Week interaction, F(16, 216)=3.97, p<0.05]. The total swim distance of saline-injected mice on standard diet was stable across days of assessment, ranging from 99–112% of baseline. For mice injected with DOX+CYP, regardless of diet, total swim distance increased 9–33% on days 22–36 relative to day 15 (*). Subsequently, DOX+CYP-injected mice on standard diet exhibited a statistically significant 19–31% decrease in total swim distance on days 50–71 relative to day 15, while the total swim distance of DOX+CYP-injected +Ch diet mice did not differ from baseline values on days 43–71 and was significantly greater than DOX+CYP-injected standard diet mice on days 64 and 71 (•). Thus, DOX+CYP administration increased activity of mice during weeks of administration, and led to an eventual reduction in activity in mice on standard diet.
Fig. 3.
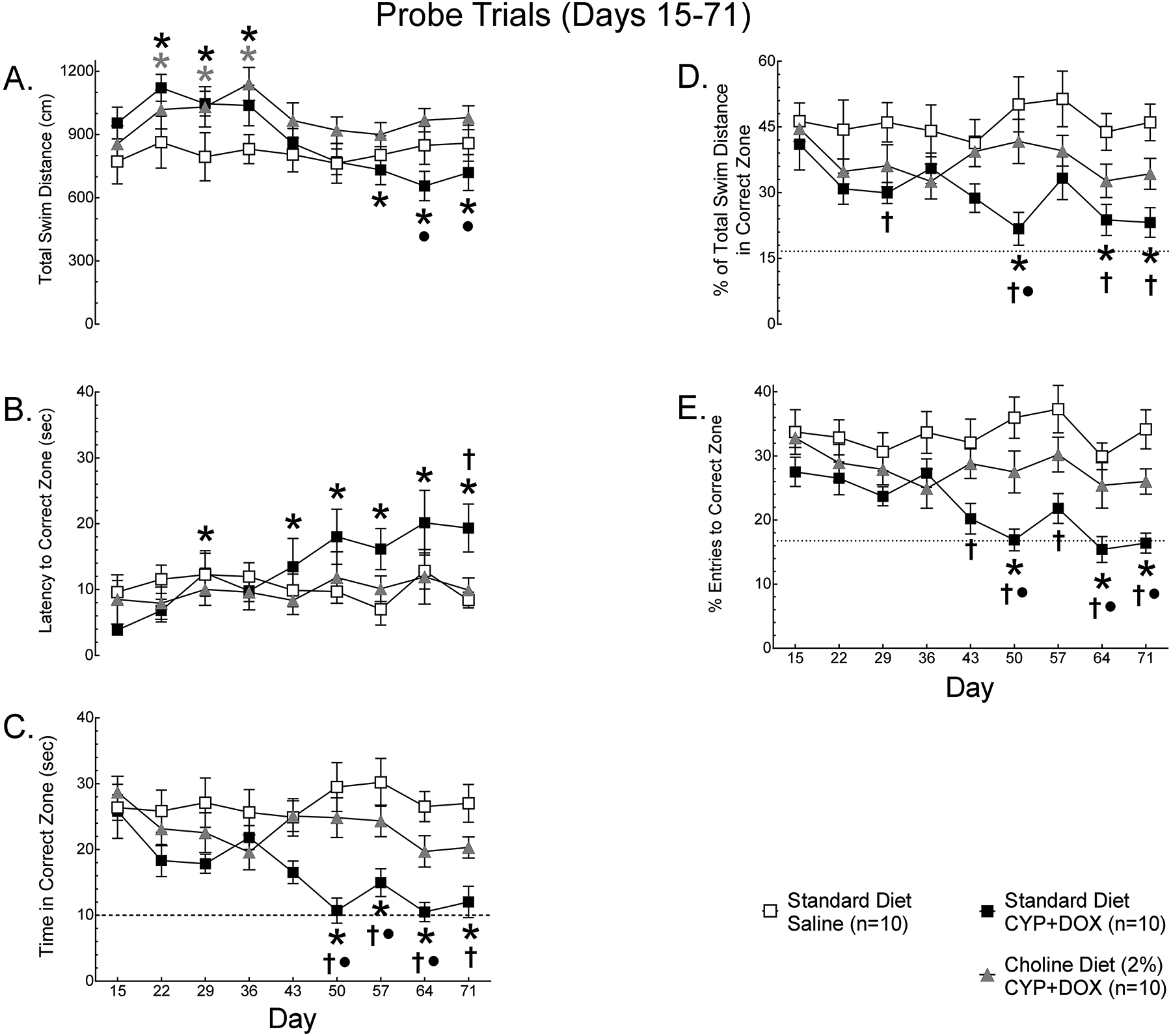
The (A) mean total swim distance, (B) latency to the correct zone, (C) time in the correct zone, (D) % of total swim distance in the correct zone and (E) % entries to the correct zone of female BALB/C mice (N=30) on standard diet administered 0.9% saline (n=10), standard diet administered cyclophosphamide (CYP) and doxorubicin (DOX) or 2% choline diet administered DOX+CYP was determined weekly during probe trials. Data are presented as group means +/− S.E.M. Using Dunnett’s test, signifcant (p<0.05) differences from baseline (day 15) performance are indicated with a *. Using Tukey’s HSD, signifcant (p<0.05) differences from the Standard Diet – Saline group are indicated with † and differences from Choline Diet (2%) – DOX+CYP group are indicated with •.
The latency to the correct zone (Fig. 3B) differed significantly between groups across weeks of assessment [Treatment × Week interaction, F(16, 216)=2.52, p<0.05]. The latency of saline-injected mice on standard diet was stable across weeks of assessment, relative to baseline (day 15). In contrast, DOX+CYP-injected mice exhibited significant increases in latency relative to baseline (*) on days 29 and 43–71 and took significantly longer to find the correct zone than saline-injected mice on standard diet (†) on day 71, indicating an impairment. When placed on +Ch diet, DOX+CYP-injected mice did not exhibit a significant change in latency relative to baseline and did not differ from saline-injected mice or DOX+CYP-injected mice on standard diet across weeks of assessment. Thus, when mice are on standard diet, DOX+CYP increases the time it takes mice to find the platform zone, however this effect may be due to reduced activity.
The time spent in the correct zone (Fig. 3C) also differed significantly between groups across weeks of assessment [Treatment × Week interaction, F(16, 216)=1.98, p<0.05]. Saline-injected mice on standard diet did not exhibit significant changes across weeks of assessment, relative to baseline, but DOX+CYP-injected mice on standard diet spent significantly less time in the correct zone on days 50–71 than on day 15 (*) and performed significantly worse than saline-injected mice (†) on standard diet on days 29 and 50–71, indicating impairment. DOX+CYP-injected mice on +Ch diet did not exhibit a reduction in time in the correct zone relative to baseline, did not differ from saline-injected mice on standard diet at any point of assessment, and performed significantly better than DOX+CYP-injected mice on standard diet (•) on days 50–64. Thus, when mice are on standard diet, DOX+CYP decreases the time mice spent in the platform zone, however this effect may be because these mice swim for a longer period before they enter the correct zone than other groups.
Because differences in latency to the correct zone and time in the correct zone could be the result of differences in general activity, we calculated the % of total swim distance in the correct zone and the % of total entries to the correct zone, to reduce the impact of differences in activity on measures of spatial memory. For % of total swim distance in the correct zone (Fig. 3D) there were significant main effects of weeks of assessment [Main Effect of Week, F(8,216)=2.00, p<0.05] and treatment [Main Effect of Treatment, F(2,27)=11.71, p<0.05]. Saline-injected mice on standard diet did not exhibit a change in probe trial performance across weeks of assessment, when compared to baseline (day 15). However, DOX+CYP-injected mice on standard diet exhibited a significant decline relative to baseline performance (*), on days 50 and 64–71, and performed significantly worse than saline-injected mice on standard diet (†) on days 29, 50, 64 and 71. In contrast, DOX+CYP-injected mice on +Ch diet did not exhibit a significant decline relative to baseline (day 15), performed significantly better than DOX+CYP-injected mice on standard diet (•) on day 50 and did not differ from saline-injected mice on standard diet during any assessment. Similarly, for % entries to the correct zone (Fig. 3E), there were significant main effects of weeks of assessment [Main Effect of Week, F(8,216)=2.65, p<0.05] and treatment [Main Effect of Treatment, F(2,27)=21.03, p<0.05]. Saline-injected mice on standard diet did not exhibit a change in probe trial performance across weeks of assessment, when compared to baseline (day 15). However, the % of entries in the correct zone decreased relative to baseline (*) for DOX+CYP-injected standard diet mice on days 50 and 64–71, and was significantly lower than saline-injected standard diet mice (†) on days 43–71. DOX+CYP-injected mice on +Ch diet did not exhibit a significant reduction in % entries to the correct zone relative to baseline (day 15) during any assessment, performed significantly better than DOX+CYP-injected mice on standard diet (•) on days 50, 64 and 71, and did not differ from saline-injected mice on standard diet during any assessment. Taken together, these data indicate that DOX+CYP-injected mice on standard diet had worse spatial memory than saline-injected mice on standard diet, indicating impairment, and DOX+CYP-injected mice placed on +Ch diet had better spatial memory following chemotherapy than DOX+CYP-injected mice remaining on standard diet, suggesting that +Ch protected these mice from the spatial memory impairing effects of DOX+CYP.
Discussion
The present study demonstrates that dietary supplementation with 2% choline can attenuate the decline in spatial memory observed when female mice are exposed to a 4-week regimen of DOX+CYP. Although this study focused on the domain of spatial memory, the finding suggests that +Ch may be an effective intervention for the prevention or treatment of CRCDs.
The behavior of our BALB/C mice in the Morris water maze agrees with reports that a subset of this strain are inactive when placed in water (Philpot et al., 2016; Francis et al., 1995; Wahlsten et al., 2005). In the present study, 32% of mice floated in the water, remaining stationary during the majority of training and/or baseline testing, rather than searching for an escape by swimming. The data from these ‘non-performing’ mice was removed from all analyses because these mice did not provide a valid baseline for subsequent statistical comparisons and their data would have confounded interpretation of the activity and spatial memory data. Training, baseline assessment and exclusion of ‘non-performing’ mice occurred before assignment to experimental conditions, and the remaining mice were subsequently balanced across groups based on baseline probe trial performance. Therefore, the removal of data obtained from ‘non-performing’ mice was not disproportional across groups and concerns that the effects reported here resulted from selective exclusion of this subset should be alleviated.
Similar to other reports (Flanigan et al., 2018), the repeated administration of DOX+CYP to mice resulted in a ≈5–10% reduction in weight that was not observed in saline-injected mice. The weight of mice on standard diet returned to baseline 2 weeks after the final administration of DOX+CYP, but the weight of mice on 2% choline diet remained low for the remainder of the study. Rodents can quickly develop aversions to novel flavors when ingestion is followed by illness (Welzl et al., 2001). The persistent reduction in weight observed in +Ch mice following DOX+CYP could reflect the development of a mild conditioned aversion to the 2% choline diet since mice were placed on the new diet less than 24hrs prior to the first injection of DOX+CYP. Future studies should allow mice to develop familiarity with new diets for several days before introducing treatments that induce illness.
Multiple studies have demonstrated that chemotherapeutic agents impair performance in behavioral tasks mediated by the Hcc (Macleod et al., 2007; Yang et al., 2010b; Christie et al., 2012; Kitamura et al., 2015; Mustafa et al., 2008; Lyons et al., 2011). The present finding that repeated DOX+CYP administration impairs Hcc-mediated spatial memory is in agreement with studies conducted in male Wistar rats (Kitamura et al., 2015; Kitamura et al., 2017) but is at odds with another study examining DOX+CYP-induced cognitive impairment in female C57Bl/6J mice (Flanigan et al., 2018). However, this latter study used ovariectomized mice in order to prevent alterations in cognitive function caused by changes in circulating estrogen, while our study and the studies of Kitamura et al. (2015; 2017) used animals with intact gonads. Because both DOX and CYP damage gonadal tissue (Fouad et al., 2019; Onaolapo et al., 2018; Overbeek et al., 2017; Gadducci et al., 2007) it may be that the spatial memory deficits observed in our study and others (Kitamura et al., 2015; Kitamura et al., 2017) involve hormonal changes following chemotherapy-induced damage to the gonads (Fouad et al., 2019; Onaolapo et al., 2018; Jarrell et al., 1987; Plowchalk and Mattison, 1992). A potential mechanism underlying this hypothesis is described later in the discussion.
Mice on standard diet that were injected with DOX+CYP exhibited a reduction in total swim distance on days 57–71. This reduction in total swim distance corresponded with an increase in the latency to the correct zone and a decrease in time spent, and distance moved, in the correct zone. Importantly, the velocity of mice while swimming did not differ across groups (data not shown), indicating that, when they were swimming, mice in each group moved at similar speeds. Therefore, the most plausible explanation of the reduced swimming distance of the mice in the Standard Diet – DOX+CYP group is that these mice would pause their movement while searching the environment for spatial cues to locate the correct zone, dropping the movement of the mouse below the threshold to be considered swimming.
The present findings agree with previous studies indicating that increasing cholinergic function during chemotherapy can attenuate CRCDs. Winocur et al. (Winocur et al., 2011) demonstrated that chemotherapy-induced deficits in spatial memory can be attenuated by the acetylcholinesterase (AChE) -inhibitor donepezil and we have demonstrated that the AChE-inhibitors donepezil and galantamine can prevent the development of DOX+CYP-induced deficits in spatial memory (Philpot et al., 2019). Additionally, Kitamura et al. (Kitamura et al., 2015; Kitamura et al., 2017) have demonstrated that nicotine administration prevents DOX+CYP-induced deficits in spatial memory and that these effects are mediated by action at α7 and α4β2 nicotinic receptors. Since +Ch has been shown to: 1) increase the Ch available for ACh synthesis in the central nervous system (Klein et al., 1992; Michel et al., 2006); 2) increase the density of high affinity neuronal nicotinic receptors in the brain (Coutcher et al., 1992); and 3) increase the activity of cholinergic neurons that express α7 nAChRs, where Ch acts as a full agonist (Mike et al., 2000; Uteshev et al., 2003); it seems likely that the 2% choline diet enhanced cholinergic function in our mice during and following DOX+CYP exposure. Although we did not measure free choline in our mice, it is established that 2% Ch diet increases free Ch in the circulation by >50% and increases free Ch concentrations in Hcc and striatal sections derived from animals on this diet (Wecker, 1988), Therefore, it is possible that +Ch-mediated increases in free choline compensate for an alteration in the function of cholinergic projections to the Hcc following the administration of chemotherapeutic agents.
The mechanisms by which chemotherapeutic agents produce CRCDs and how +Ch attenuates these effects remain to be determined. However, one plausible explanation involves the reduction of circulating estrogen caused by many chemotherapeutic agents (Jarrell et al., 1987; Petrek et al., 2006; Minisini et al., 2009; Swain et al., 2009; Swain et al., 2010) and the consequences to cholinergic systems important for neurogenesis, synaptic plasticity and performance on a variety of cognitive tasks, including spatial navigation (Mohapel et al., 2005; Masuoka et al., 2019). Studies in animals have shown that reducing circulating estrogen via ovariectomy significantly reduces high-affinity choline uptake (HACU), an effect reversed by the administration of 17β-estradiol (O’Malley et al., 1987; Singh et al., 1994). Because HACU is rate-limiting for ACh synthesis (Collier, 1988), and estrogen levels regulate HACU, reductions in circulating estrogen will reduce Ch uptake and ACh synthesis, adversely impacting cholinergic activity. Indeed, we have published in abstract and presented (Johns et al., 2019; Johns et al., 2020) data from female C57Bl/6J and tumor-bearing female MMTV-PyVT mice demonstrating that DOX+CYP impairs HACU in the Hcc and striatum. Since +Ch prevented the manifestation of spatial memory deficits during and following exposure to DOX+CYP, and +Ch promotes ACh synthesis when the demand for Ch exceeds the neuronal supply (Wecker and Schmidt, 1980; Jope, 1982; Dolezal and Tucek, 1982), the present findings support the idea that CRCDs can result from impaired HACU limiting the supply of Ch to cholinergic neurons and decreasing cholinergic function.
Conclusions
The present findings support the use of +Ch as an intervention for CRCDs and suggest that impairments of hippocampal-mediated cognitive function following exposure to DOX+CYP may be the result of altered cholinergic function. Further studies are necessary to determine if +Ch provides similar benefits in individuals with tumors, and importantly, whether +Ch contributes to tumor development and growth or adversely impacts the effectiveness of chemotherapy. However, these results suggest that increasing the intake of Ch during cancer treatment could reduce vulnerability to the cognitive impairing aspects of cancer treatment.
Acknowledgements
The authors thank April Lindon for her technical assistance during this study and Dr. Heather Jim for her valuable suggestions and feedback.
Support
National Institutes of Health, NCCIH 1R21AT009734
University of South Florida, Bridge Grant
University of South Florida, Proposal Development Award
Abbreviations
- +Ch
Choline supplementation
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- Ch
Choline
- CRCD
Chemotherapy Related Cognitive Deficit
- CYP
Cyclophosphamide
- DOX
Doxorubicin
- Hcc
Hippocampus
- MWM
Morris water maze
- nAChR
nicotinic acetylcholine receptor
Footnotes
Declarations of Interest
Dr. Rex Philpot and Ms. Bethany Johns have filed provisional patent applications on the use of dietary choline for the prevention of CRCDs and on the use of dietary choline for the suppression of tumor growth. Neither Rex Philpot nor Bethany Johns have other interests to declare.
Dr. Lynn Wecker, Ms. Melissa Ficken and Ms. Melanie Engberg have no interests to declare.
References
- Ahles TA and Saykin AJ (2007) Candidate mechanisms for chemotherapy-induced cognitive changes. Nature reviews. Cancer 7(3): 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluise CD, Sultana R, Tangpong J, et al. (2010) Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Advances in experimental medicine and biology 678: 147–156. [DOI] [PubMed] [Google Scholar]
- Alvarez XA, Laredo M, Corzo D, et al. (1997) Citicoline improves memory performance in elderly subjects. Methods and findings in experimental and clinical pharmacology 19(3): 201–210. [PubMed] [Google Scholar]
- Bartus RT and Johnson HR (1976) Short-term memory in the rhesus monkey: disruption from the anti-cholinergic scopolamine. Pharmacology, biochemistry, and behavior 5(1): 39–46. [DOI] [PubMed] [Google Scholar]
- Baxter MG and Chiba AA (1999) Cognitive functions of the basal forebrain. Current opinion in neurobiology 9(2): 178–183. [DOI] [PubMed] [Google Scholar]
- Beer TM, Bland LB, Bussiere JR, et al. (2006) Testosterone loss and estradiol administration modify memory in men. The Journal of urology 175(1): 130–135. [DOI] [PubMed] [Google Scholar]
- Buchman AL, Ament ME, Sohel M, et al. (2001) Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: proof of a human choline requirement: a placebo-controlled trial. JPEN. Journal of parenteral and enteral nutrition 25(5): 260–268. [DOI] [PubMed] [Google Scholar]
- Bussiere M, Vance JE, Campenot RB, et al. (2001) Compartmentalization of choline and acetylcholine metabolism in cultured sympathetic neurons. Journal of biochemistry 130(4): 561–568. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Yang W and Shaw GM (2010) Periconceptional nutrient intakes and risks of neural tube defects in California. Birth defects research. Part A, Clinical and molecular teratology 88(8): 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie LA, Acharya MM, Parihar VK, et al. (2012) Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research 18(7): 1954–1965. [DOI] [PubMed] [Google Scholar]
- Clark WM, Warach SJ, Pettigrew LC, et al. (1997) A randomized dose-response trial of citicoline in acute ischemic stroke patients. Citicoline Stroke Study Group. Neurology 49(3): 671–678. [DOI] [PubMed] [Google Scholar]
- Cole LK, Vance JE and Vance DE (2012) Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochimica et biophysica acta 1821(5): 754–761. [DOI] [PubMed] [Google Scholar]
- Collier B (1988) About the coupling of acetylcholine hydrolysis and choline uptake at cholinergic nerve terminals. Journal of neurochemistry 50(1): 323–324. [DOI] [PubMed] [Google Scholar]
- Coutcher JB, Cawley G and Wecker L (1992) Dietary choline supplementation increases the density of nicotine binding sites in rat brain. The Journal of pharmacology and experimental therapeutics 262(3): 1128–1132. [PubMed] [Google Scholar]
- da Costa KA, Badea M, Fischer LM, et al. (2004) Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. The American journal of clinical nutrition 80(1): 163–170. [DOI] [PubMed] [Google Scholar]
- Dolezal V and Tucek S (1982) Effects of choline and glucose on atropine-induced alterations of acetylcholine synthesis and content in the caudate nuclei of rats. Brain research 240(2): 285–293. [DOI] [PubMed] [Google Scholar]
- Drachman DA and Leavitt J (1974) Human memory and the cholinergic system. A relationship to aging? Archives of neurology 30(2): 113–121. [DOI] [PubMed] [Google Scholar]
- Everitt BJ and Robbins TW (1997) Central cholinergic systems and cognition. Annual review of psychology 48: 649–684. [DOI] [PubMed] [Google Scholar]
- Flanigan TJ, Anderson JE, Elayan I, et al. (2018) Effects of Cyclophosphamide and/or Doxorubicin in a Murine Model of Postchemotherapy Cognitive Impairment. Toxicological sciences : an official journal of the Society of Toxicology 162(2): 462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad AA, Refaie MMM and Abdelghany MI (2019) Naringenin palliates cisplatin and doxorubicin gonadal toxicity in male rats. Toxicol Mech Methods 29(1): 67–73. [DOI] [PubMed] [Google Scholar]
- Francis DD, Zaharia MD, Shanks N, et al. (1995) Stress-induced disturbances in Morris water-maze performance: interstrain variability. Physiology & behavior 58(1): 57–65. [DOI] [PubMed] [Google Scholar]
- Gadducci A, Cosio S and Genazzani AR (2007) Ovarian function and childbearing issues in breast cancer survivors. Gynecol Endocrinol 23(11): 625–631. [DOI] [PubMed] [Google Scholar]
- Garcia-Cobos R, Frank-Garcia A, Gutierrez-Fernandez M, et al. (2010) Citicoline, use in cognitive decline: vascular and degenerative. Journal of the neurological sciences 299(1–2): 188–192. [DOI] [PubMed] [Google Scholar]
- Green HJ, Pakenham KI, Headley BC, et al. (2002) Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: a randomized controlled trial. BJU international 90(4): 427–432. [DOI] [PubMed] [Google Scholar]
- Guseva MV, Hopkins DM, Scheff SW, et al. (2008) Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. Journal of neurotrauma 25(8): 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA (2003) Estrogen in the adult male reproductive tract: a review. Reproductive biology and endocrinology : RB&E 1: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell J, Lai EV, Barr R, et al. (1987) Ovarian toxicity of cyclophosphamide alone and in combination with ovarian irradiation in the rat. Cancer research 47(9): 2340–2343. [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Deutsch G, et al. (2006) A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. British journal of cancer 94(6): 828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns BE, Bothello R, Wecker L, et al. (2020) Increasing dietary choline to prevent chemotherapy related cognitive deficits and attenuate tumor growth in MMTV-PyVT mice. In: Experimental Biology, San Diego, CA. [Google Scholar]
- Johns BE, Wecker L and Philpot RM (2019) High choline diet prevents cyclophosphamide- and doxorubicin-induced reductions in high affinity choline uptake in the striatum and hippocampus of C57Bl/6J and MMTV-PyVT mice and attenuates tumor growth. Society for Neuroscience. Chicago, Il. [Google Scholar]
- Jope RS (1982) Effects of phosphatidylcholine administration to rats on choline in blood and choline and acetylcholine in brain. The Journal of pharmacology and experimental therapeutics 220(2): 322–328. [PubMed] [Google Scholar]
- Jope RS and Jenden DJ (1980) The utilization of choline and acetyl coenzyme A for the synthesis of acetylcholine. Journal of neurochemistry 35(2): 318–325. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Hattori S, Yoneda S, et al. (2015) Doxorubicin and cyclophosphamide treatment produces anxiety-like behavior and spatial cognition impairment in rats: Possible involvement of hippocampal neurogenesis via brain-derived neurotrophic factor and cyclin D1 regulation. Behavioural brain research 292: 184–193. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Kanemoto E, Sugimoto M, et al. (2017) Influence of nicotine on doxorubicin and cyclophosphamide combination treatment-induced spatial cognitive impairment and anxiety-like behavior in rats. Naunyn-Schmiedeberg’s archives of pharmacology 390(4): 369–378. [DOI] [PubMed] [Google Scholar]
- Klein J, Koppen A, Loffelholz K, et al. (1992) Uptake and metabolism of choline by rat brain after acute choline administration. Journal of neurochemistry 58(3): 870–876. [DOI] [PubMed] [Google Scholar]
- Kohli S, Griggs JJ, Roscoe JA, et al. (2007) Self-reported cognitive impairment in patients with cancer. Journal of oncology practice / American Society of Clinical Oncology 3(2): 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons L, ElBeltagy M, Umka J, et al. (2011) Fluoxetine reverses the memory impairment and reduction in proliferation and survival of hippocampal cells caused by methotrexate chemotherapy. Psychopharmacology 215(1): 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod JE, DeLeo JA, Hickey WF, et al. (2007) Cancer chemotherapy impairs contextual but not cue-specific fear memory. Behavioural brain research 181(1): 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka T, Uwada J, Kudo M, et al. (2019) Augmentation of Endogenous Acetylcholine Uptake and Cholinergic Facilitation of Hippocampal Long-Term Potentiation by Acetylcholinesterase Inhibition. Neuroscience 404: 39–47. [DOI] [PubMed] [Google Scholar]
- Michel V, Yuan Z, Ramsubir S, et al. (2006) Choline transport for phospholipid synthesis. Experimental biology and medicine 231(5): 490–504. [DOI] [PubMed] [Google Scholar]
- Mike A, Castro NG and Albuquerque EX (2000) Choline and acetylcholine have similar kinetic properties of activation and desensitization on the alpha7 nicotinic receptors in rat hippocampal neurons. Brain research 882(1–2): 155–168. [DOI] [PubMed] [Google Scholar]
- Mills JL, Fan R, Brody LC, et al. (2014) Maternal choline concentrations during pregnancy and choline-related genetic variants as risk factors for neural tube defects. The American journal of clinical nutrition 100(4): 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minisini AM, Menis J, Valent F, et al. (2009) Determinants of recovery from amenorrhea in premenopausal breast cancer patients receiving adjuvant chemotherapy in the taxane era. Anti-cancer drugs 20(6): 503–507. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Leanza G, Kokaia M, et al. (2005) Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiology of aging 26(6): 939–946. [DOI] [PubMed] [Google Scholar]
- Moreno M (2003) Cognitive improvement in mild to moderate Alzheimer’s dementia after treatment with the acetylcholine precursor choline alfoscerate: A multicenter, double-blind, randomized, placebo-controlled trial. Clinical therapeutics 25(1): 178–193. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, et al. (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297(5868): 681–683. [DOI] [PubMed] [Google Scholar]
- Mustafa S, Walker A, Bennett G, et al. (2008) 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. The European journal of neuroscience 28(2): 323–330. [DOI] [PubMed] [Google Scholar]
- Nurk E, Refsum H, Bjelland I, et al. (2013) Plasma free choline, betaine and cognitive performance: the Hordaland Health Study. The British journal of nutrition 109(3): 511–519. [DOI] [PubMed] [Google Scholar]
- O’Malley CA, Hautamaki RD, Kelley M, et al. (1987) Effects of ovariectomy and estradiol benzoate on high affinity choline uptake, ACh synthesis, and release from rat cerebral cortical synaptosomes. Brain research 403(2): 389–392. [DOI] [PubMed] [Google Scholar]
- Onaolapo AY, Oladipo BP and Onaolapo OJ (2018) Cyclophosphamide-induced male subfertility in mice: An assessment of the potential benefits of Maca supplement. Andrologia 50(3). [DOI] [PubMed] [Google Scholar]
- Overbeek A, van den Berg MH, van Leeuwen FE, et al. (2017) Chemotherapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: A systematic review. Cancer treatment reviews 53: 10–24. [DOI] [PubMed] [Google Scholar]
- Pepeu G and Giovannini MG (2010) Cholinesterase inhibitors and memory. Chemico-biological interactions 187(1–3): 403–408. [DOI] [PubMed] [Google Scholar]
- Petrek JA, Naughton MJ, Case LD, et al. (2006) Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 24(7): 1045–1051. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Ficken M, Johns BE, et al. (2019) Spatial memory deficits in mice induced by chemotherapeutic agents are prevented by acetylcholinesterase inhibitors. Cancer chemotherapy and pharmacology 84(3): 579–589. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Ficken M and Wecker L (2016) Doxorubicin and cyclophosphamide lead to long-lasting impairment of spatial memory in female, but not male mice. Behavioural brain research 307: 165–175. [DOI] [PubMed] [Google Scholar]
- Plowchalk DR and Mattison DR (1992) Reproductive toxicity of cyclophosphamide in the C57BL/6N mouse: 1. Effects on ovarian structure and function. Reproductive toxicology 6(5): 411–421. [DOI] [PubMed] [Google Scholar]
- Poly C, Massaro JM, Seshadri S, et al. (2011) The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. The American journal of clinical nutrition 94(6): 1584–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen EK, Portin RI, Koskinen A, et al. (2004) Associations between serum testosterone fall and cognitive function in prostate cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research 10(22): 7575–7582. [DOI] [PubMed] [Google Scholar]
- Schliebs R and Arendt T (2006) The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. Journal of neural transmission 113(11): 1625–1644. [DOI] [PubMed] [Google Scholar]
- Schmidt DE and Wecker L (1981) CNS effects of choline administration: evidence for temporal dependence. Neuropharmacology 20(6): 535–539. [DOI] [PubMed] [Google Scholar]
- Schwarzenberg SJ, Georgieff MK and Committee On N (2018) Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 141(2). [DOI] [PubMed] [Google Scholar]
- Secades JJ and Frontera G (1995) CDP-choline: pharmacological and clinical review. Methods and findings in experimental and clinical pharmacology 17 Suppl B: 1–54. [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Laurent C, et al. (2006) Maternal nutrient intakes and risk of orofacial clefts. Epidemiology 17(3): 285–291. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Yang W, et al. (2004) Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. American journal of epidemiology 160(2): 102–109. [DOI] [PubMed] [Google Scholar]
- Sigala S, Imperato A, Rizzonelli P, et al. (1992) L-alpha-glycerylphosphorylcholine antagonizes scopolamine-induced amnesia and enhances hippocampal cholinergic transmission in the rat. European journal of pharmacology 211(3): 351–358. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, et al. (1994) Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain research 644(2): 305–312. [DOI] [PubMed] [Google Scholar]
- Swain SM, Jeong JH and Wolmark N (2010) Amenorrhea from breast cancer therapy--not a matter of dose. The New England journal of medicine 363(23): 2268–2270. [DOI] [PubMed] [Google Scholar]
- Swain SM, Land SR, Ritter MW, et al. (2009) Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast cancer research and treatment 113(2): 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock IF, Ahles TA, Ganz PA, et al. (2004) Cognitive impairment associated with chemotherapy for cancer: report of a workshop. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 22(11): 2233–2239. [DOI] [PubMed] [Google Scholar]
- Teather LA and Wurtman RJ (2003) Dietary cytidine (5’)-diphosphocholine supplementation protects against development of memory deficits in aging rats. Progress in neuro-psychopharmacology & biological psychiatry 27(4): 711–717. [DOI] [PubMed] [Google Scholar]
- Teather LA and Wurtman RJ (2005) Dietary CDP-choline supplementation prevents memory impairment caused by impoverished environmental conditions in rats. Learning & memory 12(1): 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommer BA, Schmidt DE and Wecker L (1982) Exogenous choline enhances the synthesis of acetylcholine only under conditions of increased cholinergic neuronal activity. Journal of neurochemistry 39(6): 1704–1709. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM and Papke RL (2003) Regulation of neuronal function by choline and 4OH-GTS-21 through alpha 7 nicotinic receptors. Journal of neurophysiology 89(4): 1797–1806. [DOI] [PubMed] [Google Scholar]
- Vearncombe KJ, Rolfe M, Andrew B, et al. (2011) Cognitive effects of chemotherapy-induced menopause in breast cancer. The Clinical neuropsychologist 25(8): 1295–1313. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Cooper SF and Crabbe JC (2005) Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behavioural brain research 165(1): 36–51. [DOI] [PubMed] [Google Scholar]
- Wallace TC (2018) A Comprehensive Review of Eggs, Choline, and Lutein on Cognition Across the Life-span. Journal of the American College of Nutrition 37(4): 269–285. [DOI] [PubMed] [Google Scholar]
- Wallace TC, Blusztajn JK, Caudill MA, et al. (2018) Choline: The Underconsumed and Underappreciated Essential Nutrient. Nutrition today 53(6): 240–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecker L (1986) Neuochemical effects of choline supplementation. Can. J. Physiol. Pharmacol 64: 329–333. [DOI] [PubMed] [Google Scholar]
- Wecker L (1988) Influence of dietary choline availability and neuronal demand on acetylcholine synthesis by rat brain. Journal of neurochemistry 51(2): 497–504. [DOI] [PubMed] [Google Scholar]
- Wecker L (1991) The synthesis and release of acetylcholine by depolarized hippocampal slices is increased by increased choline available in vitro prior to stimulation. Journal of neurochemistry 57(4): 1119–1127. [DOI] [PubMed] [Google Scholar]
- Wecker L, Cawley G and Rothermel S (1989) Acute choline supplementation in vivo enhances acetylcholine synthesis in vitro when neurotransmitter release is increased by potassium. Journal of neurochemistry 52(2): 568–575. [DOI] [PubMed] [Google Scholar]
- Wecker L, Dettbarn WD and Schmidt DE (1978) Choline administration: modification of the central actions of atropine. Science 199(4324): 86–87. [DOI] [PubMed] [Google Scholar]
- Wecker L and Schmidt DE (1980) Neuropharmacological consequences of choline administration. Brain research 184(1): 234–238. [DOI] [PubMed] [Google Scholar]
- Welzl H, D’Adamo P and Lipp HP (2001) Conditioned taste aversion as a learning and memory paradigm. Behavioural brain research 125(1–2): 205–213. [DOI] [PubMed] [Google Scholar]
- Winocur G, Binns MA and Tannock I (2011) Donepezil reduces cognitive impairment associated with anti-cancer drugs in a mouse model. Neuropharmacology 61(8): 1222–1228. [DOI] [PubMed] [Google Scholar]
- Yang M, Kim JS, Song MS, et al. (2010a) Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiology of learning and memory 93(4): 487–494. [DOI] [PubMed] [Google Scholar]
- Yang M, Kim JS, Song MS, et al. (2010b) Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiology of learning and memory 93(4): 487–494. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, Da Costa KA, Franklin PD, et al. (1991) Choline, an essential nutrient for humans. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 5(7): 2093–2098. [PubMed] [Google Scholar]


