Abstract
Although many people prefer fecal immunochemical test (FIT) over colonoscopy due to its noninvasive nature, it is unclear whether FIT would still be preferred for colorectal cancer (CRC) screening if they were explicitly informed that + FIT requires follow-up colonoscopy. To address this gap, we administered two conjoint analysis surveys—one that explained the need for a follow-up colonoscopy after + FIT while the other did not—to a US nationwide sample of Americans and then assessed whether there were differences in colonoscopy/FIT preferences between cohorts. We recruited adults ≥ 40yo who had not undergone CRC screening via an online survey research firm. We deployed two surveys that used conjoint analysis to assess decision making surrounding CRC screening tests: Survey 1 (4/2–4/15/2021)—did not inform participants that they need a colonoscopy following a + FIT; Survey 2 (4/29–6/2/2021)—informed respondents of the potential need. Using the conjoint analysis data, we determined the proportion of those who preferred FIT or colonoscopy and then used logistic regression to assess for differences in colonoscopy/FIT preferences between the cohorts. Overall, 501 and 1,000 individuals completed Survey 1 (without description of need for colonoscopy after + FIT) and Survey 2 (with description), respectively. There was no difference in test preference between cohorts after adjusting for covariates in the logistic regression (adjusted p = 0.09): Survey 1 (without description)—colonoscopy 28.5%, FIT 71.5%; Survey 2 (with description)—colonoscopy 26.7%, FIT 73.3%. Thus, knowledge that a + FIT requires a follow-up colonoscopy does not alter people’s strong preference for non-invasive stool testing with FIT.
1. Introduction
In the US, fecal immunochemical test (FIT) and colonoscopy are the most common screening modalities for colorectal cancer (CRC), and they are tier 1 tests according to the US Multi-Society Task Force (MSTF) on CRC (Rex et al., 2017, Shaukat et al., 2021). Although many people prefer FIT over colonoscopy due to its noninvasive nature (von Wagner et al., 2020, Hyams et al., 2021, Zhu et al., 2021), it is unclear whether FIT would still be preferred if people were explicitly informed that a + FIT requires a follow-up colonoscopy. To address this gap, we administered two conjoint analysis surveys—one that explained the need for a follow-up colonoscopy after + FIT while the other did not. We then assessed whether there were differences in colonoscopy/FIT preferences between the cohorts.
2. Methods
To determine how people make tradeoffs when deciding among CRC screening tests, we developed a survey that employed conjoint analysis—a technique used to determine how respondents make complex decisions. We collaborated with a global online survey research firm (Cint; Stockholm, Sweden) to recruit Americans ≥ 40 years old with no prior history of CRC screening. We excluded those who had been diagnosed with colon polyps, Crohn’s disease, or ulcerative colitis, as well as those with a first-degree relative with CRC. This study was approved by the Cedars-Sinai Institutional Review Board (STUDY599).
The Supplemental File contains the full survey instruments, and we describe the survey development process and conjoint analysis elsewhere (Makaroff et al., 2021, Gale et al., 2021). In brief, we employed a choice-based conjoint with alternative-specific design, and participants viewed a random set of nine side-by-side profiles drawn from 300 potential sets generated through a balanced overlap design. Fig. 1 shows a sample conjoint exercise and participants were instructed to “choose which [screening test], if any, you would be most likely to do [for CRC screening]” and to “assume that medical insurance will cover each one and that you will not have any out-of-pocket costs.” Prior to completing the conjoint exercises, respondents received information on each of the different testing modalities and their attributes (see Supplemental File for these descriptions). The presentation order for information regarding testing options was randomized among participants to reduce order bias. Of note, while the survey modeled five CRC tests, this study focused on comparing preferences for FIT versus colonoscopy.
Fig. 1.
Panel A: Sample conjoint exercise where participants consider three hypothetical colorectal cancer screening tests side by side and decide which one, if any, they would be most likely to do. Participants were shown a total of nine vignettes. Panel B: Surveys 1 and 2 were the same except for Survey 2 including a statement that those with a positive stool test need to do a follow-up colonoscopy.
To assess whether knowing that a + FIT requires follow-up colonoscopy affects decision making when considering FIT and colonoscopy at the outset, we created two versions of the survey (Supplemental File). Survey 1 (April 2 to April 15, 2021) did not inform participants of the need for a colonoscopy following a + FIT, while Survey 2 (April 29 to June 2, 2021) explicitly informed respondents of the need for follow-up colonoscopy in the setting of a positive result (Fig. 1).
Bivariate comparisons of demographics, CRC screening knowledge, attitudes, and beliefs, and comorbidities between those who completed Survey 1 and Survey 2 were performed using Student’s t-test, Pearson’s chi-squared test, or Fisher’s exact test where appropriate. We then performed simulations using the conjoint analysis data to determine the proportion of individuals who preferred FIT or colonoscopy in each cohort. Afterwards, multivariable logistic regression was used to assess for differences in colonoscopy/FIT preferences between the cohorts. The model was adjusted for all variables in Table 1 to account for confounding and results were reported as adjusted odds ratios (aOR) with 95% confidence intervals (CI). All statistical analyses were performed using SAS version 9.4 (Cary, NC) and a two-tailed p < 0.05 was considered statistically significant.
Table 1.
Demographics, comorbidities, and CRC screening knowledge, attitudes, and beliefs of respondents who completed Surveys 1 (without description of need for colonoscopy after + FIT) and 2 (with description).
| Variable |
Survey 1: without description (n = 501) |
Survey 2: with description (n = 1,000) |
P-value |
|---|---|---|---|
| Age: | 0.04 | ||
| 40–49 yo | 194 (38.7%) | 456 (45.6%) | |
| 50–59 yo | 146 (29.1%) | 267 (26.7%) | |
| ≥60 yo | 161 (32.1%) | 277 (27.7%) | |
| Sex: | 0.45 | ||
| Male | 256 (51.1%) | 487 (48.7%) | |
| Female | 244 (48.7%) | 512 (51.2%) | |
| Prefer not to say | 1 (0.2%) | 1 (0.1%) | |
| Race/ethnicity: | 0.33 | ||
| Non-Hispanic White | 389 (77.6%) | 806 (80.6%) | |
| Non-Hispanic Black | 27 (5.4%) | 64 (6.4%) | |
| Hispanic | 37 (7.4%) | 57 (5.7%) | |
| Non-Hispanic Asian | 26 (5.2%) | 41 (4.1%) | |
| Other | 22 (4.4%) | 32 (3.2%) | |
| Educational attainment: | 0.94 | ||
| High school degree or less | 140 (27.9%) | 281 (28.1%) | |
| Some college education | 128 (25.5%) | 248 (24.8%) | |
| College degree | 167 (33.3%) | 347 (34.7%) | |
| Graduate degree | 66 (13.2%) | 124 (12.4%) | |
| Marital status: | 0.94 | ||
| Married or living with a partner | 266 (53.1%) | 529 (52.9%) | |
| Not married | 235 (46.9%) | 471 (47.1%) | |
| Total household income, $: | 0.006 | ||
| <50,000 | 244 (48.7%) | 517 (51.7%) | |
| 50,000–100,000 | 146 (29.1%) | 298 (29.8%) | |
| ≥100,001 | 81 (16.2%) | 161 (16.1%) | |
| Prefer not to say | 30 (6.0%) | 24 (2.4%) | |
| Employment status: | 0.34 | ||
| Unemployed, on disability, on leave of absence from work, retired, or a homemaker | 249 (49.7%) | 471 (47.1%) | |
| Employed or student | 252 (50.3%) | 529 (52.9%) | |
| Has health insurance | 418 (83.4%) | 845 (84.5%) | 0.59 |
| Has usual source of care | 379 (75.6%) | 800 (80.0%) | 0.05 |
| Self-reported health status: | 0.34 | ||
| Excellent | 57 (11.4%) | 88 (8.8%) | |
| Very good | 130 (25.9%) | 280 (28.0%) | |
| Good | 210 (41.9%) | 407 (40.7%) | |
| Fair/Poor | 104 (20.8%) | 225 (22.5%) | |
| Number of medical comorbidities a: | 0.008 | ||
| 0 | 156 (31.1%) | 252 (25.2%) | |
| 1 | 124 (24.8%) | 225 (22.5%) | |
| ≥2 | 221 (44.1%) | 523 (52.3%) | |
| Number of GI comorbidities b: | 0.02 | ||
| 0 | 412 (82.2%) | 760 (76.0%) | |
| 1 | 62 (12.4%) | 170 (17.0%) | |
| ≥2 | 27 (5.4%) | 70 (7.0%) | |
| Number of GI symptoms experienced in past 3 months c: | 0.02 | ||
| 0 | 279 (55.7%) | 479 (47.9%) | |
| 1 | 87 (17.4%) | 202 (20.2%) | |
| ≥2 | 135 (26.9%) | 319 (31.9%) | |
| US region: | 0.15 | ||
| Northeast | 89 (17.8%) | 225 (22.5%) | |
| South | 174 (34.7%) | 344 (34.4%) | |
| Midwest | 119 (23.8%) | 207 (20.7%) | |
| West | 119 (23.8%) | 224 (22.4%) | |
| Plans to get screened for CRC | 243 (48.5%) | 519 (51.9%) | 0.21 |
| Has non-first degree relative or friend diagnosed with CRC | 59 (11.8%) | 138 (13.8%) | 0.27 |
| Self-perceived CRC susceptibility (1–5 scale; higher = more susceptible) |
2.6 [1.8, 3.0] | 2.6 [2.0, 3.0] | 0.91 |
| Self-perceived impact of CRC diagnosis (1–5 scale; higher = more severe impact) |
3.1 [2.8, 3.6] | 3.2 [2.8, 3.7] | 0.06 |
| Self-perceived benefits of CRC screening (1–5 scale; higher = more beneficial) |
4.0 [3.6, 4.4] | 4.0 [3.6, 4.4] | 0.55 |
| Self-perceived barriers to CRC screening (1–5 scale; higher = more barriers) |
2.7 [2.3, 3.1] | 2.7 [2.3, 3.1] | 0.60 |
| Data are presented as n (% of column) or median [interquartile range]. P-values were computed using Pearson’s chi-squared test, Fisher’s exact test, or Student’s t-test. CRC, colorectal cancer; FIT, fecal immunochemical test; GI, gastrointestinal. a: Includes anemia or other blood disease, back pain, cancer, depression, diabetes, heart disease, high blood pressure, kidney disease, lung disease, migraines, osteoarthritis or degenerative arthritis, rheumatoid arthritis, or other medical problems. b: Includes celiac disease, cirrhosis, diverticulitis, gallstones, gastroenteritis, gastroesophageal reflux disease, gastroparesis, irritable bowel syndrome, liver disease, pancreatitis, or ulcer or stomach disease. c: Includes abdominal pain or discomfort, anal or rectal pain, bloating, bowel incontinence, constipation, diarrhea, dysphagia, heartburn, nausea/vomiting, or regurgitation. | |||
3.
Overall, 501 and 1,000 individuals completed Survey 1 (without description of need for colonoscopy after + FIT) and Survey 2 (with description), respectively. Characteristics for the two cohorts are shown in Table 1, which were largely comparable save for a few exceptions.
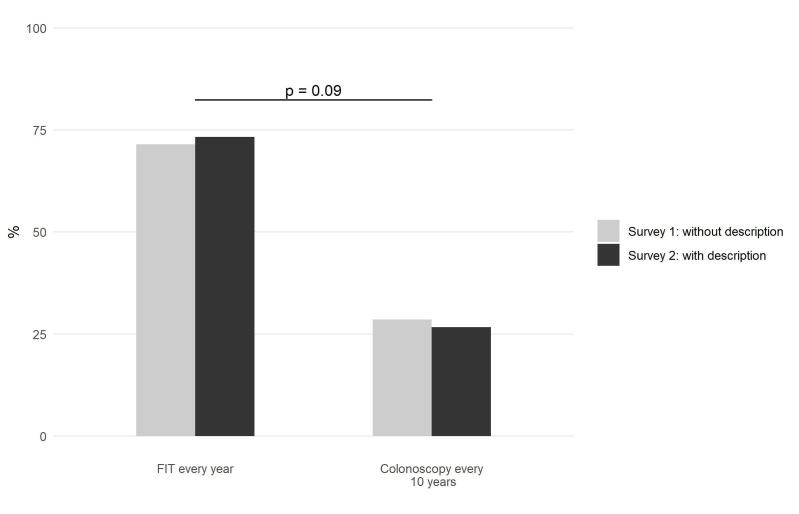
Fig. 2 shows the conjoint analysis-derived colonoscopy/FIT preferences for the two cohorts. For Survey 1 (without description; n = 501), 143 (28.5%) individuals preferred colonoscopy while 358 (71.5%) preferred FIT. As for Survey 2 (with description; n = 1,000), 267 (26.7%) and 733 (73.3%) respondents preferred colonoscopy and FIT, respectively. After using logistic regression to adjust for the covariates shown in Table 1, no statistical difference was seen in colonoscopy vs. FIT preferences between the cohorts (adjusted p = 0.09).
Fig. 2.
Proportion of respondents who preferred FIT and colonoscopy among those who completed Surveys 1 (n = 501) and 2 (n = 1,000). Each participant’s preferred test was determined through simulations using their conjoint analysis data. Note: the adjusted p-value is from the logistic regression model that included all variables in Table 1. FIT, fecal immunochemical test.
Supplemental Table 1 presents data from the logistic regression for the remaining covariates in the model. When compared to those aged 40–49 years, people in the 50–59 (aOR 1.45, 95% CI 1.09–1.93) and ≥ 60 year-old (aOR 2.16, 95% CI 1.54–3.03) age groups were more likely to prefer FIT over colonoscopy. Individuals with higher self-perceived barriers to CRC screening were also more likely to prefer FIT (aOR 1.33, 95% CI 1.08–1.63). Conversely, people who plan to get screened for CRC (aOR 0.62, 95% CI 0.47–0.82) and those with higher self-perceived CRC susceptibility (aOR 0.74, 95% CI 0.63–0.87) were less likely to prefer FIT over colonoscopy. The remaining variables were not statistically associated with decision making.
4. Discussion
Although many people strongly prefer noninvasive stool testing over colonoscopy for initial CRC screening (von Wagner et al., 2020, Hyams et al., 2021, Zhu et al., 2021), it is possible this preference would change if people explicitly understood that a positive stool test must be followed up with a colonoscopy. However, in the case of FIT testing, our study indicates that explicitly instructing people that + FIT requires a colonoscopy does not alter decision making when choosing between FIT and colonoscopy for initial CRC screening. This finding is consistent with a prior stated-choice survey by Marshall et al. that revealed that need for follow-up confirmatory testing was the least important attribute when respondents considered different CRC test options (Marshall et al., 2009). While Marshall et al. did not specifically describe the follow-up test as a colonoscopy, our study highlighted this key fact yet still found no change in the proportion of individuals preferring noninvasive FIT over colonoscopy. Conversely, our findings contrast with those from a discrete choice experiment by Benning et al. in The Netherlands; they found that providing people with information about invasive follow-up testing would decrease CRC screening participation by 4.8% (Benning et al., 2014). Notably, The Netherlands employs a screening strategy where non-invasive screening options are performed first and only followed by colonoscopy after a positive test (Benning et al., 2014). It is unclear whether this finding would extend to the US where many patients have initial access to both FIT and colonoscopy; this is worthy of further study.
We also observed that nearly three-quarters of people prefer FIT over colonoscopy for CRC screening. While informing people that + FIT requires a follow-up colonoscopy does not affect initial decision making, it remains vital to inform people of the potential need and ensure that those with a + FIT undergo timely colonoscopy (Forbes et al., 2021). Moreover, we found that CRC screening decision making is highly individualized; save for a few exceptions, demographics and comorbidity status largely do not predict whether one prefers FIT or colonoscopy. To increase screening uptake, providers should provide patients with multiple options for CRC screening. This is supported by randomized controlled trial data that found that offering patients a choice between colonoscopy or FIT (26.5%) improves screening uptake when compared to only recommending colonoscopy (17.5%; p < 0.001) (Pilonis et al., 2021). However, studies investigating CRC screening discussion patterns found that providers only discuss multiple options with patients up to half the time (Zapka et al., 2011, Wolf et al., 2007, Laiyemo et al., 2014, Lafata et al., 2011). For example, a direct observation study noted that colonoscopy was the only modality offered in 70% of cases (Lafata et al., 2011).
There are limitations to our study. First, our study was conducted solely in the US; there may be cultural factors leading to differential preferences for FIT vs. colonoscopy among countries. Further research examining CRC screening test preferences in other countries is warranted, particularly those with choice-based screening programs as well as those that have or will lower their CRC screening age to 45 years (Ebell et al., 2018). Second, while we recruited over 1,500 people to complete the survey, our findings may not extend to groups not as well represented in our study sample; for example, only 20.4% of respondents were from racial/ethnic minority groups. Additional research examining CRC screening test preferences among larger, more diverse cohorts is needed.
5. Conclusion
Our data emphasizes that people have a strong preference for noninvasive stool testing over colonoscopy, even when they know that a colonoscopy is required after a + FIT. As we noted nearly a 3:1 preference of FIT over colonoscopy, systematic and organized approaches in clinical practice for discussing and offering patients a choice between the two tests may significantly improve CRC screening rates.
6. Disclosure of funding and conflicts of interest
Grant SUPPORT: This study was supported by a National Cancer Institute K08 CA245033 grant. The study sponsor was not involved in the design or conduct of the study, collection, management, analysis or interpretation of the data, preparation, review, or approval of the manuscript, or in the decision to submit the manuscript for publication. Dr. Almario was supported by a National Institutes of Health Loan Repayment Program Award (L30 CA265419). This study was approved by the Cedars-Sinai Institutional Review Board (STUDY599).
Prior PRESENTATION: The contents of this manuscript have not been copyrighted or published previously.
CRediT authorship contribution statement
Jaspreet Shergill: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. Katherine E. Makaroff: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. Marie Lauzon: Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Brennan M.R. Spiegel: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. Christopher V. Almario: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2022.101825.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Rex D.K., Boland R.C., Dominitz J.A., Giardiello F.M., Johnson D.A., Kaltenbach T., Levin T.R., Lieberman D., Robertson D.J. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017;112(7):1016–1030. doi: 10.1038/ajg.2017.174. [DOI] [PubMed] [Google Scholar]
- Shaukat A., Kahi C.J., Burke C.A., Rabeneck L., Sauer B.G., Rex D.K. ACG clinical guidelines: colorectal cancer screening 2021. Am. J. Gastroenterol. 2021;116(3):458–479. doi: 10.14309/ajg.0000000000001122. [DOI] [PubMed] [Google Scholar]
- von Wagner C, Verstraete W, Hirst Y, et al. Public preferences for using quantitative faecal immunochemical test versus colonoscopy as diagnostic test for colorectal cancer: evidence from an online survey. BJGP Open 2020;4. [DOI] [PMC free article] [PubMed]
- Hyams T., Golden B., Sammarco J., Sultan S., King-Marshall E., Wang M.Q., Curbow B. Evaluating preferences for colorectal cancer screening in individuals under age 50 using the Analytic Hierarchy Process. BMC Health Serv. Res. 2021;21(1) doi: 10.1186/s12913-021-06705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Parks P.D., Weiser E., Fischer K., Griffin J.M., Limburg P.J., Finney Rutten L.J. National survey of patient factors associated with colorectal cancer screening preferences. Cancer Prev. Res. (Phila) 2021;14(5):603–614. doi: 10.1158/1940-6207.CAPR-20-0524. [DOI] [PubMed] [Google Scholar]
- Makaroff K., Shergill J., Lauzon M., Spiegel B., Almario C.V. American College of Gastroenterology Annual Scientific Meeting. Las Vegas; NV: 2021. Determining patient preferences for colorectal cancer screening tests using conjoint analysis. [Google Scholar]
- Gale R, Eberlein S, Fuller G, et al. Public perspectives on decisions about emergency care seeking for care unrelated to COVID-19 during the COVID-19 pandemic. JAMA Netw Open 2021;4:e2120940. [DOI] [PMC free article] [PubMed]
- Marshall D.A., Johnson F.R., Kulin N.A., Özdemir S., Walsh J.M.E., Marshall J.K., Van Bebber S., Phillips K.A. How do physician assessments of patient preferences for colorectal cancer screening tests differ from actual preferences? A comparison in Canada and the United States using a stated-choice survey. Health Econ. 2009;18(12):1420–1439. doi: 10.1002/hec.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning T.M., Dellaert B.G.C., Severens J.L., Dirksen C.D. The effect of presenting information about invasive follow-up testing on individuals' noninvasive colorectal cancer screening participation decision: results from a discrete choice experiment. Value Health. 2014;17(5):578–587. doi: 10.1016/j.jval.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Forbes N., Hilsden R.J., Martel M., Ruan Y., Dube C., Rostom A., Shorr R., Menard C., Brenner D.R., Barkun A.N., Heitman S.J. Association between time to colonoscopy after positive fecal testing and colorectal cancer outcomes: a systematic review. Clin. Gastroenterol. Hepatol. 2021;19(7):1344–1354.e8. doi: 10.1016/j.cgh.2020.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilonis N.D., Bugajski M., Wieszczy P., Rupinski M., Pisera M., Pawlak E., Regula J., Kaminski M.F. Participation in competing strategies for colorectal cancer screening: a randomized health services study (PICCOLINO Study) Gastroenterology. 2021;160(4):1097–1105. doi: 10.1053/j.gastro.2020.11.049. [DOI] [PubMed] [Google Scholar]
- Zapka J.M., Klabunde C.N., Arora N.K., Yuan G., Smith J.L., Kobrin S.C. Physicians' colorectal cancer screening discussion and recommendation patterns. Cancer Epidemiol. Biomarkers Prev. 2011;20(3):509–521. doi: 10.1158/1055-9965.EPI-10-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M.S., Baker D.W., Makoul G. Physician-patient communication about colorectal cancer screening. J. Gen. Intern. Med. 2007;22(11):1493–1499. doi: 10.1007/s11606-007-0289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiyemo A.O., Adebogun A.O., Doubeni C.A., Ricks-Santi L., McDonald-Pinkett S., Young P.E., Cash B.D., Klabunde C.N. Influence of provider discussion and specific recommendation on colorectal cancer screening uptake among U.S. adults. Prev. Med. 2014;67:1–5. doi: 10.1016/j.ypmed.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafata J.E., Cooper G.S., Divine G., Flocke S.A., Oja-Tebbe N., Stange K.C., Wunderlich T. Patient-physician colorectal cancer screening discussions: delivery of the 5A's in practice. Am. J. Prev. Med. 2011;41(5):480–486. doi: 10.1016/j.amepre.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebell M.H., Thai T.N., Royalty K.J. Cancer screening recommendations: an international comparison of high income countries. Public Health Rev. 2018;39:7. doi: 10.1186/s40985-018-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.