Abstract
Background
Our case explored the spectrum of autoimmune and infectious neurological complications of Coronavirus Disease 2019. In addition, we also reviewed and discussed clinical features, neuroimaging, CSF findings, and outcomes in patients with COVID-19-associated Myelin Oligodendrocyte Glycoprotein Antibody Disorder (MOGAD) CNS inflammatory disorder.
Case presentation
Here we presented a case of post-Coronavirus Disease 2019 infection Myelin Oligodendrocyte Glycoprotein Antibody Disorder in a 41-year-old male who presented with gait instability, urinary retention, and confusion. Workup done in hospital showed transverse myelitis in cervical spine region and left optic neuritis. Laboratory findings showed Myelin Oligodendrocyte Glycoprotein-IgG antibodies were positive in serum (1:100), suggestive of post-COVID Myelin Oligodendrocyte Glycoprotein Antibody Disorder.
Conclusion
To our knowledge, this is the first comprehensive case report and the literature review that includes the clinical features, neuroimaging, CSF findings, and outcomes in COVID-19-associated Myelin Oligodendrocyte Glycoprotein Antibody Disorder.
Keywords: COVID-19, SARS-CoV-2, MOG, Optic neuritis, Myelitis, CSF, MRI in COVID-19
Introduction
The novel coronavirus has led to a myriad of complications, including central (CNS) and peripheral (PNS) nervous system dysfunction [1–5]. Coronavirus (SARS-CoV-2) neuropathogenesis is characterized by its hematogenous affinity for Angiotensin-converting enzyme 2 (ACE2) receptors localized to brain endothelial cells and retrograde spread via the glossopharyngeal, vagus, and olfactory nerves [6, 7]. Furthermore, the presence of ACE2 receptors in neurons and glial cells suggests a detrimental role of SARS-CoV-2 on CNS function [7].
Reports of Coronavirus Disease 2019 presenting as Acute Disseminated Encephalomyelitis (ADEM) [8], Myelin Oligodendrocyte Glycoprotein Antibody Disorder (MOGAD) [9], Miller Fisher syndrome [10], and Guillain–Barré syndrome [5] are critical examples of how SARS-CoV-2 may affect proper CNS function due to an aberrant immune response. MOG-IgG's target MOG expressed in oligodendrocytes, which serve as a cellular receptor, adhesion molecule, or regulator of microtubule stability. The pathological effect of MOG-IgG relies on the ability of antibodies to enter the CNS. Thereafter, MOG-IgG-associated neuroinflammation is mediated by T cells and complement-fixing antibodies, ultimately presenting as optic neuritis, transverse myelitis, and (ADEM) [11, 12].
We reported a Myelin Oligodendrocyte Glycoprotein Antibody Disorder case in a 41-year-old male who previously had mild Coronavirus Disease 2019 symptoms based on the ATS/IDSA guidelines [13]. The patient initially presented with transverse myelitis followed by left optic neuritis. Furthermore, we retrospectively discuss the various manifestations, relevant neuroimaging, and cerebrospinal fluid markers of Coronavirus infectious disease-2019 (nCov) associated with MOG-IgG reported to date.
Case presentation
A 41-year-old male tested positive for the Coronavirus Disease 2019 virus in early November of 2020. Initially, he recovered at home; however, he began to deteriorate, reporting whole-body shivering, confusion, paresthesia, gait instability, and urinary retention 16 days post-COVID diagnosis. His medical comorbidities included hypertension, ethanol, and methamphetamine substance abuse. On examination, the patient appeared diaphoretic, uncomfortable, and was slow to respond to questioning. Initial workup revealed elevated BUN/Creatinine ratio of 24 (range 10:1–20:1), prothrombin time 14.2 secs (range 9.1–13.9), INR 1.23 secs (range 0.80–1.20), venous pH 7.44 (range 7.31–7.41), lactate 1.4 mmol/L (range 0.0–1.3), venous bicarbonate 27 mmol/L (range 22–26), WBC count 18.3 × 103/µL (range 3.7–11.0 × 103), CRP inflammatory maker 9 mg/L (range > 8), neutrophil 14.15 × 103 (range 1.50–7.70 × 103) and PO2 30 mm/Hg (range 35–50), and creatine kinase 23 U/L (range 45–225).
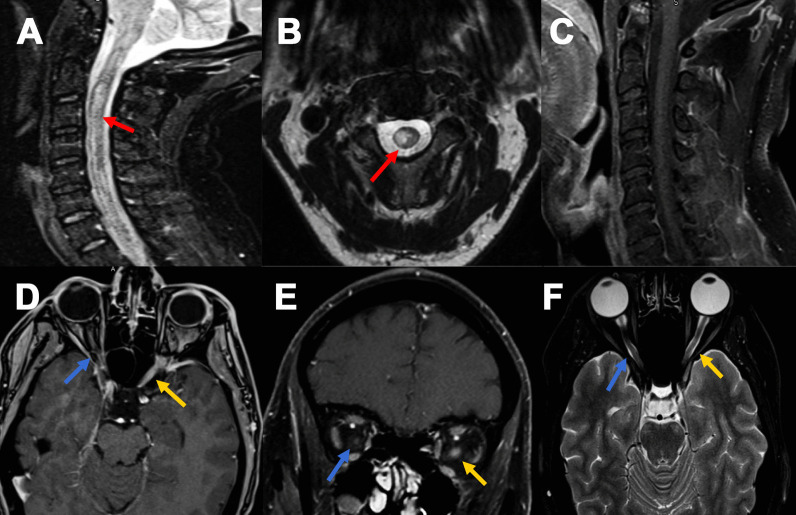
Upon admission, the patient underwent magnetic resonance imaging of the spine and brain with and without (w/wo) contrast. MRI of the spine revealed abnormal T2 hyperintensity from C2–C4 without post-contrast enhancement. These results were suggestive of longitudinally extensive transverse myelitis and post-infectious transverse myelitis (Fig. 1). Subsequent lumbar puncture revealed polymorphonuclear leukocytes with an absence of organisms and neutrophilic predominance. CSF revealed 45/µL nucleated cells (range 0–5), 90% lymphocytic predominance, 116 mg/dL protein (range 15–50), 37 mg/dL glucose (range 50–80), 0 oligoclonal bands, and IgG index 0.48. CSF and serum NMO Anti-AQP4 AB were negative, and an MOG test was not performed. For possible meningoencephalitis, the patient was treated with IV ceftriaxone, vancomycin, ampicillin, and acyclovir. On day 6 of admission, a repeat brain MRI suggested a new T2-FLAIR lesion of the left corona radiata and right parietal subcortical white matter. Antibiotics were discontinued as CSF was negative for viral PCR; however, the patient remained confused and lethargic. IV Solumedrol 1 g/day for 5 days was administered, and possible post-COVID CNS inflammatory disorder was suspected.
Fig. 1.
MRI sagittal STIR images (A) of the cervical spine reveal ill-defined long segment hyperintensity with prominent cord expansion C2–C4 (red arrow); B shows axial cut with cord signal alteration at C3 (red arrow), and C post-contrast sagittal image showed no abnormal enhancement. MRI orbit Axial T1-weighted fat suppression post-contrast (D) and coronal images (E) reveals abnormal enhancement of left optic nerve pre-chiasmatic (intracanicular); (yellow arrow) with corresponding hyperintense on axial T2 weighted images (F) (yellow arrow) with no abnormality of the right optic nerve (blue arrow)
On day 7 of admission, a follow-up lumbar puncture revealed several polymorphonuclear leukocytes with an absence of organisms and a lymphocytic predominance. CSF analysis yielded 23/µL nucleated cells, protein 62 mg/dL and glucose 44 mg/dL. The patient's mental status improved significantly with the administration of Solumedrol. The diagnosis of post-infectious COVID-19 encephalomyelitis was made, and the patient was discharged 22 days after admission and instructed to follow-up with a neurologist in the next 3 months. At this time, the patient was stable without deficits.
Six months later, the patient returned with complaints of a mild left-sided headache and left-sided retro-orbital pain with visual blurriness occurring over 1 week. The patient was initially seen in the ED, and a workup for possible demyelinating lesions was recommended. Examination of visual acuity in both eyes showed impaired left visual acuity (20/70), right 20/25, and left afferent pupillary defect (APD). Intraocular pressure and extraocular muscles (EOM) were within normal limits and intact. Ophthalmology recommended the patient for orbital MRI with/without contrast for evaluation of potential optic nerve lesions. Brain and orbital MRI w/wo contrast revealed pre-chiasmatic (intracanicular) enhancement of the left optic nerve, suggestive of optic neuritis (Fig. 1). The patient’s concerning encephalopathy, optic neuritis, spinal, and brain lesions raised concern for neurological inflammatory post-COVID vaccination sequala. Hence, CNS inflammatory marker MOG-IgG antibody levels were sent out and tested commercially at the Mayo Clinic via live-cell fluorescent-activated cell sorting assay. MOG-IgG antibodies were positive in serum (1:100), suggestive of post-COVID MOGAD syndrome. Neurology was then consulted for further workup based on the patient’s history of prior CNS lesions and current complaint of left-sided headache and left eye pain. Per neurology recommendations, a repeat MRI of the cervical and thoracic spine w/wo contrast was ordered to rule out multiple sclerosis (MS). MRI imaging was unremarkable and showed no new or enhancing lesion, with an absence of demyelinating lesions in the thoracic spinal cord. Brain MRI revealed decreased conspicuity of previously visualized white matter lesions. Although the patient was admitted, he was started on IV solumedrol (1 g/day for 3 days) and prescribed oral prednisone 1250 mg daily for an additional 2 days to be taken at home following discharge. Although admitted, the patient was scheduled to receive five cycles of PLEX therapy; however, on cycle 3/5, the patient became septic, and PLEX was held in the setting of bacteremia and thrombophlebitis due to a port infection. The remaining PLEX cycles were deferred due to sepsis. The patient stated he had significant improvement of his optic neuritis and visual acuity after receiving 3/5 PLEX cycles and concurrent solumedrol. Following resolution of the patient’s acute bacteremia, he was instructed to return in 1 month for a follow-up visit.
At the patient’s follow-up visit with neurology, the patient reported that his vision had significantly improved, and he is currently stable since his last admission. He no longer has pain and stated his visual acuity had returned near to baseline. Per neurology recommendations and infectious disease clearance, the patient will continue monthly IVIG therapy for suspected post-COVID MOGAD optic neuritis.
Discussion
Myelin Oligodendrocyte Glycoprotein Antibody Disorder is an inflammatory demyelinating condition of the central nervous system characterized by a monophasic or relapsing neurological dysfunction (optic neuritis, myelitis, and brainstem encephalitis). The findings specific to MOGAD include seropositive MOG-IgG antibodies, and frequently, CNS demyelination via MRI. These findings are MOGAD specific and do not meet diagnostic criteria for MS or other known neuroinflammatory conditions [11, 12, 14].
According to the literature, almost 50% of the patients diagnosed with Myelin Oligodendrocyte Glycoprotein Antibody Disorder present with myalgia, fever, respiratory symptoms, and gastrointestinal symptoms [15, 16]. The post-infectious state of MOGAD may be due to herpes simplex virus, cytomegalovirus, Borrelia, and Epstein–Barr virus infections [16–19]. Several cytokines and inflammatory markers have been implicated to play a role in the post-infectious state and development of MOG-IgG antibodies, including CRP, d-dimer, IL-6, 7, 19, GCSF, IP 10, MCP1.MIP1A and TNF alpha [20]. However, the mechanism contributing to the MOG-IgG antibody is unclear [11].
Interestingly, the recent data suggest a role for Coronavirus Disease 2019 in Myelin Oligodendrocyte Glycoprotein Antibody Disorder relapse [21]. COVID-19-associated MOGAD relapse is likely from the host reaching a threshold, leading to production of MOG-IgG1B-cell [22–24]. More importantly, these findings demonstrate that the SARS-CoV-2 virus may impact disease exacerbation in other relapsing CNS inflammatory disorders [21].
Among initial and relapsing presentations of Myelin Oligodendrocyte Glycoprotein Antibody Disorder, optic neuritis is the most common in adults, ranging between 54 and 60%. A unilateral lesion is seen in 22–40% and bilateral lesion seen in 22–42% [15, 16, 25]. Other clinical manifestations include myelitis, seen in 22–36% of cases, and symptoms of brainstem and encephalopathy syndrome. However, symptomatic presentation of MOGAD appears to be age dependent, as a child will most commonly present with ADEM [15, 26].
Myelin Oligodendrocyte Glycoprotein Antibody Disorder can be further differentiated from other neurological inflammatory conditions based on cerebrospinal fluid findings. MOGAD patients have been shown to yield pleocytosis in 33–66%, and Cobo-Calvo and his colleagues demonstrate it to be more common among children [27]. CSF protein elevation is only found in 27–37% and 11–22% cases can have presence of oligoclonal bands. Differences in CSF composition were further explored in our literature review. Fisher’s exact test was used to assess alterations in CSF composition, including protein, cell count, lymphocytes in post-COVID MOGAD patients. We showed no significant differences in protein, cell count, and lymphocytes were found by age (50+ versus 50−), gender, COVID-19 severity, and fatality, respectively (refer to Table 1).
Table 1.
CSF findings in patients with post-COVID MOGAD disease
| Variables | CSF protein | CSF cell count | CSF lymphocyte % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (≤ 45) | High (> 45) | N (%) | p | No (≤ 5) | Yes (> 5) | N (%) | p | ≤ 50% | > 50% | N (%) | p | |
| Age | ||||||||||||
| 50+ | 0 (0) | 1 (100) | 1 (14.3) | 0.4 | 0 (0) | 1 (100) | 1 (20) | 1 | No CSF | No CSF | NA | |
| < 50 | 4 (67) | 2 (33) | 6 (85.7) | 2 (50) | 2 (50) | 4 (80) | 3(75) | 1(25) | 4(100) | |||
| Gender | ||||||||||||
| Female | 1 (50) | 1 (50) | 2 (28.6) | 1 | 1 (100) | 0 (0) | 1 (20) | 0.4 | 1 (100) | 0 (0) | 1 (25) | 1 |
| Male | 3 (60) | 2 (40) | 5 (71.4) | 1 (25) | 3 (75) | 4 (80) | 2 (67) | 1 (33) | 3 (75) | |||
| COVID-19 severitya | ||||||||||||
| Non-severe | 3 (50) | 3 (50) | 6 (85.7) | 1 | 1 (25) | 3 (75) | 4 (80) | 0.4 | 2 (67) | 1 (33) | 3 (75) | 1 |
| Severe | 1 (100) | 0 (0) | 1 (14.3) | 1 (100) | 0 (0) | 1 (20) | 1 (100) | 0 (0) | 1 (25) | |||
| Fatality | ||||||||||||
| Non-fatal | 4 (57.1) | 3 (42.9) | 7 (100) | NA | 2 (40) | 3 (60) | 5 (100) | NA | 3 (75) | 1 (25) | 4 (100) | NA |
| Fatal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
CSF cerebrospinal fluid, NA not applicable
P values were calculated from Fisher’s exact test. All observations were non-fatal and thus a Fisher’s exact test was not available between fatality and the outcomes of interest
aSeverity based on the IDSA/ATS guidelines
Shared similarities were noted in our case report and published literature. For instance, the patient described in this report initially presented with paresthesia and gait disturbances that improved with Solumedrol. 6 months later, the patient presented with deteriorating symptoms and complaints of optic neuritis. This progression is consistent with MOGAD’s relapsing symptomatic clinical presentation [15, 26]. Furthermore, MRI imaging studies in our patient were consistent with post-infectious transverse myelitis and optic neuritis. Likewise, MOG-IgG antibody titers were elevated (1:100). Taken together, the clinical presentation, imaging studies, and serological analysis seen in our patient strongly supported findings consistent with typical MOGAD presentation [11, 12, 14]. Literature also suggests pleocytosis is a unique and consistent finding in the CSF, as shown in our patient presentation [28].
In addition to our described case report, we conducted a literature review to examine findings from published Myelin Oligodendrocyte Glycoprotein Antibody Disorder data. Table 2 describes MOGAD cases that showed a correlation between COVID-19 and MOGAD. After a comprehensive literature review, 12 cases were identified, with 11 cases fulfilling the diagnostic criteria for MOGAD based on MOG encephalomyelitis: international recommendations on diagnosis and antibody testing [14]. Regarding MOG-IgG Ab, all 11 cases of MOGAD were found to be MOG-IgG Ab serum positive, while only a single case by Khan and his colleagues tested CSF for MOG-IgG Ab and was found to be negative [28] (refer to Table 2). Symptomatic presentation of MOGAD was consistent. Among the 12 reviewed cases, eight presented with symptoms of optic neuritis, as reported by Sawalha and his colleagues, Zhou and his colleagues, Khan and his colleagues, Sardar and his colleagues, Kogure and his colleagues, and de Ruijter and his colleagues, most commonly involving the pre-chiasmal optic nerve [9, 28–31]. Other patient presentations included altered mental status and seizure disorder by Vraka and his colleagues. and Peters and his colleagues, whereas Jumah and his colleagues reported a case of paraplegia [26, 32, 33].
Table 2.
Study origin, demographics, CSF, MRI findings, severity and outcomes in COVID-19 and MOG-associated disease
| Author/country | Patient age/gender | Time duration from COVID-19 to neurological symptom onset | Time duration from COVID-19 to MOG AB positive | Co-morbidity | Neurological presentation | CSF findings | Serum/CSF AQP4, and ANTI-MOG Ab | MRI findings | Diagnosis | Management | Outcomes | Severity based on IDSA/ATSa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sawalha et al./USA | 44/M | 2 weeks | 3 weeks | None | Bilateral eye pain and vision loss |
CSF WBC 3/mm3, protein 50 mg/dL, glucose 88 mg/dLb OCB negative |
Serum AQP4 negative, CSF AQP4 NA, serum MOG Ab positive, CSF MOG Ab NA | Brain MRI showed enhancement in the right more than the left optic nerve suggestive of optic neuritis although no other abnormalities were noted in brain, cervical, or thoracic spine | MOG antibody-associated optic neuritis |
IVMP * 5 days Tapering Prednisolone |
Not fatal | Not sever |
| Zhou et al./USA | 26/M | < 1 week | 1 day | None | Sequential vision loss first affecting the left eye, then the right eye 3 days later |
CSF WBC 55/mm3, protein 31 mg/dL, glucose-57 mg/dLb OCB mirror |
Serum AQP4 negative, CSF AQP4 NA, serum, MOG Ab positive, CSF MOG Ab NA | MRI of the brain and orbits uniform enhancement and thickening of both optic nerves with non-specific focal lesion in left occipital area. MRI spine focal patchy lesion in C and T spine with enhancement | MOG associated optic neuritis and myelitis |
IVMP * 5 days Tapering Prednisolone |
Not fatal | Not sever |
| Khan et al./India | 11/M | < 1 week | 2 weeks | None | Rapidly progressive loss of vision |
CSF WBC 55/mm3, protein normal, glucose normalb OCB negative |
Serum AQP4 negative, CSF AQP4 NA Serum MOG Ab positive, CSF MOG Ab negative |
MRI orbit bilateral asymmetrical optic neuritis, with enhancement of the optic nerve sheath in the right orbit MRI brain and spine normal |
MOG associated optic neuritis |
IVMP * 5 days Tapering Prednisolone |
Not fatal | Not sever |
| Sardar et al./Qatar | 38/F | 2 weeks | NA |
Diabetes Migraine Obesity Obstructive sleep apnea Gastritis |
Headache Diminution of vision With painful eye movement |
CSF WBC normal, protein normal, glucose-normalb OCB-NA |
Serum AQP4 NA, CSF AQP4 NA, Serum MOG Ab NA, CSF MOG Ab NA | MRI orbit T2 signal bilateral optic nerve and enhancement | Seronegative NMOSD and IIH |
IVMP for 5 d, IVIG * 5 days PLEX Acetazolamide |
Not fatal | Not sever |
| Zorić et al./Serbia | 63/M | 4 weeks | 11 weeks | Diabetes |
Headache Left eye visual loss |
CSF NA | Serum AQP4 positive, CSF AQP4 NA, serum MOG Ab positive, CSF MOG Ab NA | MRI brain microangiopathic and a neuroglial cyst on the right temporal side, and normal appearing orbits and optic nerves. MRI C and T spine no abnormal cord signal | MOG associated optic neuritis |
IVMP * 5 days Tapering Prednisolone |
Not fatal | Not sever |
| Pinto et al./UK | 44/F | 1 week | 30 days | None | Mild expressive and receptive dysphasia, visual and sensory inattention |
CSF WBC 13/mm3, protein 507 mg/dL, glucose-52 mg/dLb OCB negative |
Serum AQP4 negative, CSF AQP4 NA, serum MOG Ab positive, CSF MOG Ab NA | MRI brain hyperintensity within bilateral periventricular area extending left temporal and occipital horns and into the subcortical deep white matter bilateral. There was perivascular enhancement within the lesions, and MRA was normal. MRI the spine normal. MRI orbit not reported | CNS inflammatory vasculopathy with anti-MOG |
IVMP * 5 days Tapering Prednisolone PLEX * 5 Sessions |
Not fatal | Not sever |
| Vraka et al./UK | 13m/F | 1 week | 4 days | None | Altered consciousness, seizures |
CSF WBC 10/mm3, protein 31 mg/dL, glucose-84 mg/dLb OCB negative |
Serum AQP4 negative, CSF AQP4 NA, serum MOG Ab positive, CSF MOG Ab NA |
MRI brain hyperintensity in the splenium of the corpus callosum with associated diffusion restriction and high signal in the thalami and pons MRI spine normal |
ADEM with anti-MOG | IVMP, acyclovir, levetiracetam | Not fatal | Severe |
| Kogure et al./Japan | 47/M | NA | 2 days |
Recurrent Paranasal sinusitis Adrenal resection |
Left eye pain Visual loss |
CSF WBC normal, protein normal, glucose normalb OCB-NA |
Serum AQP4 negative, CSF AQP4 NA, serum MOG Ab positive, CSF MOG Ab negative | MRI orbit revealed the bilateral uniform enhancement along with optic nerve sheaths | MOG associated optic neuritis |
IVMP * 3 days Tapering Prednisolone |
Not fatal | Not sever |
| Jumah et al./USA | 61/M | 1 week | 1 day | None |
Retention sensory Level at T7, paraplegia |
CSF WBC 279/mm3, protein 106 mg/dL, glucose NAb OCB negative |
Serum AQP4 negative, CSF AQP4 NA, serum MOG Ab positive, CSF MOG Ab NA | MRI showed T2 hyperintense lesions of variable length in the mid-thoracic spinal cord, while brain MRI was unremarkable | MOG-antibody myelitis/HHV myelitis | IVMP, PLEX, ganciclovir | Not fatal | Not sever |
| de Ruijter et al./Netherland | 15/M | > 1 week | NA | None | Subacute bilateral visual loss over the course of 7 day |
CSF WBC normal, protein normal, glucose normalb OCB-NA |
Serum AQP4 negative, CSF AQP4 NA, Serum MOG Ab positive, CSF MOG Ab NA | MRI orbit revealed a bilateral edematous optic nerve lesion | MOG associated optic neuritis | IVMP * 3 days | Not fatal | Not sever |
| Woodhall et al./UK | 39/F | < 1 week | 6 days | MOGAD | Painful progressive right visual loss consistent with optic neuritis | CSF NA | Serum AQP4 negative, CSF AQP4 NA, serum MOG Ab positive, CSF MOG Ab NA | MRI progression of right optic nerve atrophy and subtle T2 signal hyperintensity | MOG associated relapse optic neuritis |
IVMP * 5 days Mycopheno-late, PLEX * 5 Sessions |
Not fatal | Not sever |
| Peters et al./USA | 23/M | 2 weeks | 2 weeks | Childhood non-febrile seizures |
Generalized tonic Clonic seizure, slowing Fever |
CSF WBC 57/mm3, protein 40 mg/dL, glucose-60 mg/dLb OCB negative |
Serum AQP4 negative, CSF AQP4 NA, serum MOG Ab positive, CSF MOG Ab NA |
MRI brain diffuse left-hemispheric cortical hyperintensity, most pronounced in the left occipital and posterior temporal lobe with leptomeningeal enhancement Spine MRI not reported |
MOG-associated encephalitis | IVMP * 5 days | Not fatal | Not sever |
MOGAD myelin oligodendrocytes glycoprotein antibody disease, AQP4 aquaporin antibody, IVIG intravenous immunoglobulin, PLEX plasmapheresis, IVMP intravenous methylprednisolone, MRI magnetic resonance imaging, CSF cerebrospinal fluid, OCB oligoclonal bands
aSeverity based on the Infectious Diseases Society of America IDSA and American Thoracic Society ATS guidelines
bSerum glucose not reported or available
In all cases, Myelin Oligodendrocyte Glycoprotein Antibody Disorder-IgG antibody levels were measured to diagnose Myelin Oligodendrocyte Glycoprotein Antibody Disorder related to Coronavirus Disease 2019 sequela. In 4 cases, MOG antibody tested positive between 2 and 4 weeks [28, 30, 32, 34] whereas Žorić and his colleagues. reported a case where MOG-IgG was positive more than 4 weeks after SARS-CoV-2 RT-PCR positive test [35]. The remaining cases of MOG-IgG positivity were reported within 1 week of the initial COVID-19 positive test (refer to Table 2). Based on this data, MOGAD should not be dismissed solely of the amount of time passed between the initial COVID-19 positive test and subsequent MOG-IgG testing.
Cerebrospinal fluid was also analyzed to distinguish Myelin Oligodendrocyte Glycoprotein Antibody Disorder from other neuroinflammatory diseases further. More than half of the CSF in the studied cases (6 out of 10) revealed pleocytosis. In contrast, only 3 cases by Sawalha and his colleagues, Pinto and his colleagues, and Jumah and his colleagues. reported high protein levels in the CSF [30, 33, 34]. Oligoclonal bands in CSF were assessed in 8 cases; however, no cases had unique isolated bands in CSF (refer to Table 2).
Upon further analysis of the cases, two-third of cases (7/12) showed T2 hyperintensity and post-contrast enhancement in the prechiasmal optic nerves with sparing of chiasma and optic tract. Woodhall and his colleagues. was the only case to report unilateral optic nerve lesions, with all others showing bilateral optic nerve involvement [21]. In addition, Zhou and his colleagues reported a case of MOGAD with findings of optic neuritis and myelitis. Subsequent MRI showed non-enhancing, non-specific periventricular T2 hyperintensity adjacent to the occipital horn of the right lateral ventricle with patchy T2 hyperintensities in the lower cervical and upper thoracic spinal cord. Mild central thickening and gadolinium enhancement were also observed [9]. However, Jumah and his colleagues. reported a case of MOGAD with subsequent spinal MRI imaging revealing the spine revealing non-enhancing T2 hyperintense lesions of variable length in the mid-thoracic spinal cord [33]. Peters and his colleagues. reported encephalitis with MRI finding of T2 hyperintensity in left occipital and posterior temporal lobe with leptomeningeal enhancement [32]. Moreover, Vraka and his colleagues reported MRI findings of bilateral widespread white matter high-signal abnormalities, including the splenium of the corpus callosum with associated diffusion restriction and high signal in the thalami and pons in a diagnosed MOGAD 13-month-old female [26] (refer Table 2).
The studied cases used similar treatment for Myelin Oligodendrocyte Glycoprotein Antibody Disorder. All cases recovered after initiation of intravenous methylprednisolone. Only 4 cases by Sardar and his colleagues, Pinto and his colleagues, Jumah and his colleagues, and Woodhall and his colleagues were treated with plasma therapy due to the slow and partial improvement in symptoms [21, 29, 33, 34]. The above therapies proved to yield a favorable outcome with the treatment of MOG antibody disorder.
One limitation of this review study was the small sample size used. A future study should be considered with a larger sample size when there is a greater prevalence of concurrent COVID-19 related MOGAD. This will help further explore similarities and differences among patient symptom presentations, imaging similarities, CSF analysis, and treatment outcomes.
Conclusion
Our case explored the spectrum of autoimmune and infectious neurological complications of Coronavirus Disease 2019. In addition, we also reviewed and discussed clinical features, neuroimaging, CSF findings, and outcomes in patients with COVID-19-associated MOGAD CNS inflammatory disorder. Our cases provided insight regarding the need to test specific demyelinating antibodies, such as MOG-IgG in the setting of a suspicious clinical picture, such as longitudinally extensive myelitis or severe optic neuritis, especially in the setting of a concurrent or previous COVID-19 infection. Future research should focus on early diagnosis of MOGAD and testing modalities, such as MRI imaging, serum, and CSF analyses. In light of this, we believe this review will be an essential aid to future studies on CNS inflammatory disorders, such as MOGAD in relation to COVID-19 and provide helpful information for researchers and registries to collect future data on MOGAD and COVID-19.
Acknowledgements
West Virginia Clinical and Translational Science Institute, Morgantown, WV; SW and SS supported in part by WVCTSI via US National Institute of General Medical Sciences of National Institute of Health under award under 5U54GM104942-05
Abbreviations
- COVID-19
Coronavirus infectious disease-2019
- nCov
Novel Coronavirus
- SARS-CoV-2
Severe Acute Respiratory Distress Syndrome coronavirus 2
- CSF
Cerebrospinal fluid
- CNS
Central nervous system
- PNS
Peripheral nervous system
- ACE2
Angiotensin-converting enzyme 2
- RT-PCR
Reverse transcription polymerase chain reaction
- IDSA/ATS
Infectious Diseases Society of America/American Thoracic Society
- AHNE
Acute hemorrhagic necrotizing encephalitis
- AHLE
Acute hemorrhagic leukoencephalitis
- ADEM
Acute Disseminated Encephalomyelitis
- MRI
Magnetic resonance imaging
- CLOCC
Cytotoxic lesion of the Corpus Callosum
- MOG
Myelin Oligodendrocyte Glycoprotein
- MERS
Mild encephalopathy reversible splenium lesion
- MOGAD
Myelin Oligodendrocyte Glycoprotein Antibody Disorder
- GBS
Guillain–Barre syndrome
- WHO
World Health Organization
- IVIG
Intravenous immunoglobulin
- IVMP
Intravenous methylprednisolone
- PLEX
Plasma exchange/plasmapheresis
Author contributions
Conceptualization: SS. Drafting manuscript: DN, AC, AS, ME, SJ, SS. Data abstraction and data analysis: SR, SW. Final edit: SS. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data was extracted from the articles published in PUBMED, Google Scholar, Scopus. This will be provided on request.
Declarations
Ethics approval and consent to participate
This is under compliance with institutional review board guidelines and approval IRB protocol number 2004958561 from WVU (date 4-6-2020).
Informed consent
Informed written consent from the patient was obtained.
Consent for publication
Available and IRB approved; and written consent obtained from patient.
Competing interests
All authors declared no potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munhoz RP, Pedroso JL, Nascimento FA, Almeida SMD, Barsottini OGP, Cardoso FEC, et al. Neurological complications in patients with SARS-CoV-2 infection: a systematic review. Arq Neuropsiquiatr. 2020;78(5):290–300. doi: 10.1590/0004-282x20200051. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhary A, Subedi R, Tandon M, Wen S, Patel J, Kataria S, et al. Relevance and clinical significance of magnetic resonance imaging of neurological manifestations in COVID-19: a systematic review of case reports and case series. Brain Sci. 2020;10(12):1017. doi: 10.3390/brainsci10121017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger JR. COVID-19 and the nervous system. J Neurovirol. 2020;26(2):143–148. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sriwastava S, Kataria S, Tandon M, Patel J, Patel R, Jowkar A, et al. Guillain Barré syndrome and its variants as a manifestation of COVID-19: a systemic review of case report and case series. J Neurol Sci. 2020;420:117263. doi: 10.1016/j.jns.2020.117263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Liu T, Yang N, Han D, Mi X, Li Y, et al. Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front Med. 2020;14(5):533–541. doi: 10.1007/s11684-020-0786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 8.Novi G, Rossi T, Pedemonte E, Saitta L, Rolla C, Roccatagliata L, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020 doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J Neuro-Ophthalmol. 2020 doi: 10.1097/WNO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 11.Wynford-Thomas R, Jacob A, Tomassini V. Neurological update: MOG antibody disease. J Neurol. 2019;266(5):1280–1286. doi: 10.1007/s00415-018-9122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lana-Peixoto MA, Talim N. Neuromyelitis optica spectrum disorder and anti-MOG syndromes. Biomedicines. 2019;7(2):42. doi: 10.3390/biomedicines7020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflamm. 2018;15(1):134. doi: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramanathan S, Mohammad S, Tantsis E, Nguyen TK, Merheb V, Fung VSC, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89(2):127–137. doi: 10.1136/jnnp-2017-316880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariotto S, Ferrari S, Monaco S, Benedetti MD, Schanda K, Alberti D, et al. Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study. J Neurol. 2017;264(12):2420–2430. doi: 10.1007/s00415-017-8635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura M, Iwasaki Y, Takahashi T, Kaneko K, Nakashima I, Kunieda T, et al. A case of MOG antibody-positive bilateral optic neuritis and meningoganglionitis following a genital herpes simplex virus infection. Mult Scler Relat Disord. 2017;17:148–150. doi: 10.1016/j.msard.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Vieira JP, Sequeira J, Brito MJ. Postinfectious anti-myelin oligodendrocyte glycoprotein antibody positive optic neuritis and myelitis. J Child Neurol. 2017;32(12):996–999. doi: 10.1177/0883073817724927. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura Y, Nakajima H, Tani H, Hosokawa T, Ishida S, Kimura F, et al. Anti-MOG antibody-positive ADEM following infectious mononucleosis due to a primary EBV infection: a case report. BMC Neurol. 2017;17(1):76. doi: 10.1186/s12883-017-0858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodhall M, Mitchell JW, Gibbons E, Healy S, Waters P, Huda S. Case Report: myelin oligodendrocyte glycoprotein antibody-associated relapse with COVID-19. Front Neurol. 2020;11:598531. doi: 10.3389/fneur.2020.598531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piddlesden SJ, Lassmann H, Zimprich F, Morgan BP, Linington C. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol. 1993;143(2):555–564. [PMC free article] [PubMed] [Google Scholar]
- 23.Bettelli E, Baeten D, Jäger A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116(9):2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reindl M, Di Pauli F, Rostásy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol. 2013;9(8):455–461. doi: 10.1038/nrneurol.2013.118. [DOI] [PubMed] [Google Scholar]
- 25.Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. 2018;90(21):e1858–e1869. doi: 10.1212/WNL.0000000000005560. [DOI] [PubMed] [Google Scholar]
- 26.Vraka K, Ram D, West S, Chia WYE, Kurup P, Subramanian G, et al. Two paediatric patients with encephalopathy and concurrent COVID-19 infection: two sides of the same coin? Case Rep Neurol Med. 2021;2021:6658000. doi: 10.1155/2021/6658000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cobo-Calvo Á, Ruiz A, D'Indy H, Poulat AL, Carneiro M, Philippe N, et al. MOG antibody-related disorders: common features and uncommon presentations. J Neurol. 2017;264(9):1945–1955. doi: 10.1007/s00415-017-8583-z. [DOI] [PubMed] [Google Scholar]
- 28.Khan A, Panwala H, Ramadoss D, Khubchandani R. Myelin oligodendrocyte glycoprotein (MOG) antibody disease in a 11 year old with COVID-19 infection. Indian J Pediatr. 2021;88:488–489. doi: 10.1007/s12098-020-03656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sardar S, Safan A, Okar L, Sadik N, Adeli G. The diagnostic dilemma of bilateral optic neuritis and idiopathic intracranial hypertension coexistence in a patient with recent COVID-19 infection. Clin Case Rep. 2021;9:e04347. doi: 10.1002/ccr3.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawalha K, Adeodokun S, Kamoga GR. COVID-19-induced acute bilateral optic neuritis. J Investig Med High Impact Case Rep. 2020;8:2324709620976018. doi: 10.1177/2324709620976018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Ruijter NS, Kramer G, Gons RAR, Hengstman GJD. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: a case report. Mult Scler Relat Disord. 2020;46:102474. doi: 10.1016/j.msard.2020.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters J, Alhasan S, Vogels CBF, Grubaugh ND, Farhadian S, Longbrake EE. MOG-associated encephalitis following SARS-COV-2 infection. Mult Scler Relat Disord. 2021;50:102857. doi: 10.1016/j.msard.2021.102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jumah M, Rahman F, Figgie M, Prasad A, Zampino A, Fadhil A, et al. COVID-19, HHV6 and MOG antibody: a perfect storm. J Neuroimmunol. 2021;353:577521. doi: 10.1016/j.jneuroim.2021.577521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto AA, Carroll LS, Nar V, Varatharaj A, Galea I. CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurol Neuroimmunol Neuroinflamm. 2020 doi: 10.1212/NXI.0000000000000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Žorić L, Rajović-Mrkić I, Čolak E, Mirić D, Kisić B. Optic neuritis in a patient with seropositive myelin oligodendrocyte glycoprotein antibody during the post-COVID-19 period. Int Med Case Rep J. 2021;14:349–355. doi: 10.2147/IMCRJ.S315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data was extracted from the articles published in PUBMED, Google Scholar, Scopus. This will be provided on request.