Abstract
A young woman with microscopic polyangiitis (MPA) requiring hemodialysis showed repeated posterior reversible encephalopathy syndrome (PRES) with spatiotemporal multiple lesions over a period of two months. The first PRES episode with confusion and the second PRES episode with vertigo and nausea were caused by MPA, hypertension and renal failure. These symptoms were improved by the reinforcement of MPA treatment and blood pressure management. The third PRES episode with nausea, headache, seizure and visual changes was induced by rituximab infusion and hypertension. The PRES was improved with blood pressure and convulsant management. These conditions are challenging to diagnose and treat.
Keywords: antineutrophil cytoplasmic antibody-associated vasculitis, cerebral aneurysm, dialysis, microscopic polyangiitis, posterior reversible encephalopathy syndrome, rituximab
Introduction
Microscopic polyangiitis (MPA) is a small- to medium-vessel vasculitis and part of a spectrum of disorders called antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) (1). Although neurological involvement, typically mononeuritis multiplex, is not uncommon in MPA, central nervous system (CNS) involvement is relatively uncommon and presents a variety of imaging findings (2,3).
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological syndrome with magnetic resonance imaging (MRI) findings characterized by acute, reversible cerebral endotheliopathy with consecutive disruption of the blood-brain barrier and vasogenic edema (4). Vasogenic edema predominantly occurs in the bilateral parieto-occipital region, along with acute neurological deficits, but lesions affecting the frontal lobe, cerebellum, or basal ganglia are common (4). The thalamus and brainstem can also be affected (4). Clinical manifestations of PRES include headache, nausea, vomiting, seizures, visual disturbances, and altered mental states (4). A wide variety of medical conditions and medications, such as renal failure, hypertensive crises, sepsis, cytotoxic drugs, preeclampsia, eclampsia, or autoimmune disorders, including AAV, have been implicated as causes of PRES (5,6).
We herein report a young woman with ANCA-associated MPA requiring hemodialysis. She showed repeated episodes of PRES with spatiotemporal multiple cranial lesions, probably due to different etiologies in the short periods. She also had a rare cerebral aneurysm as a complication. These conditions are rare and challenging for physicians to diagnose and treat.
Case Report
A 19-year-old woman with renal failure on hemodialysis was transferred to our hospital for further treatment. She visited the outside hospital complaining of general fatigue, nausea, vomiting, appetite loss and edema two months prior to admission to our hospital. Hemodialysis was introduced because of uremic symptoms, with a serum creatinine level of 18.78 mg/dL and C-reactive protein (CRP) level of 0.11 mg/dL. She was subsequently treated with steroid pulse therapy with oral prednisolone at 50 mg/day for 3 weeks and 2 cycles of low-density lipoprotein (LDL) apheresis for severe renal failure with massive proteinuria and hematuria.
At admission, she was taking prednisolone at 40 mg/day and cilnidipine at 10 mg/day. A physical examination revealed a blood pressure of 140/68 mmHg, a heart rate of 93/min, a body temperature of 37.0℃ and an oxygen saturation of 98%. She was oriented, was not in acute distress and had no significant neurological abnormalities; however, she had leg edema. The laboratory data (Table 1) showed renal failure with massive proteinuria and hematuria. CRP and proteinase 3-anti-neutrophil cytoplasmic antibody (PR3-ANCA) were negative, but myeloperoxidase (MPO)-ANCA was positive at 7.0 U/mL, and perinuclear (P)-ANCA was also positive. The MPO-ANCA titer was 11.8 U/mL after corticosteroid therapy at another hospital. She showed hypocomplementemia, and slight bilateral atrophy of the kidneys was observed, with a hyperechoic cortex, suggesting chronic damage.
Table 1.
Laboratory Data on Admission.
| Urine | ||
| Protein | 3+ | |
| Occult blood | 3+ | |
| Red blood cell | 10-19 | /high power field |
| White blood cell | 5-9 | /high power field |
| Protein | 5.9 | g/g creatinine |
| α1-microglobulin | 55.8 | mg/L (<11.9) |
| Complete blood count | ||
| WBC | 10,700 | /µL |
| Hb | 12.0 | g/dL |
| Platelet | 19.2×104 | /µL |
| Blood chemistry | ||
| Total protein | 5.5 | g/dL |
| Albumin | 3.4 | g/dL |
| Urea nitrogen | 62.8 | mg/dL |
| Creatinine | 8.51 | mg/dL |
| Aspartate aminotransferase | 14 | IU/L |
| Alanine aminotransferase | 14 | IU/L |
| Alkaline phosphatase | 148 | IU/L |
| γ glutamyltransferase | 12 | IU/L |
| Lactate dehydrogenase | 239 | IU/L |
| Na | 137 | mEq/L |
| K | 4.1 | mEq/L |
| Cl | 100 | mEq/L |
| Mg | 1.8 | mg/dL |
| Triglyceride | 128 | mg/dL |
| LDL cholesterol | 16 | mg/dL |
| Fe | 24 | μg/dL |
| TIBC | 209 | μg/dL |
| Ferritin | 139.5 | ng/mL |
| estimated GFR | 5.9 | mL/min/1.73 m2 |
| Immunologic test | ||
| IgG | 887 | mg/dL |
| IgA | 101 | mg/dL |
| IgM | 66 | mg/dL |
| CH50 | 17 | U/mL |
| C3 | 21 | mg/dL |
| C4 | 5 | mg/dL |
| C-reactive protein | 0.01 | mg/dL |
| Antinuclear antibody | ×80 | |
| Anti-dsDNA antibody | 0.9 | IU/mL (<9.0) |
| MPO-ANCA | 7.0 | U/mL (<3.4) |
| PR3-ANCA | 1.0 | U/mL (<3.4) |
| P-ANCA | positive | |
| Anti-GBM antibody | 2.0 | U/mL (<2.9) |
| Cryoglobulin | negative | |
| ASO | 24.2 | U/mL |
| HBs antigen | negative | |
| HCV antibody | negative |
TIBC: total iron binding capacity, GFR: glomerular filtration rate, MPO-ANCA: myeloperoxidase-anti-neutrophil cytoplasmic antibody, PR3-ANCA: proteinase 3-anti-neutrophil cytoplasmic antibody, P-ANCA: perinuclear-anti-neutrophil cytoplasmic antibody, GBM: glomerular basement membrane, HBs: hepatitis B surface antigen, HCV: hepatitis C virus
The values in the parentheses show the normal range.
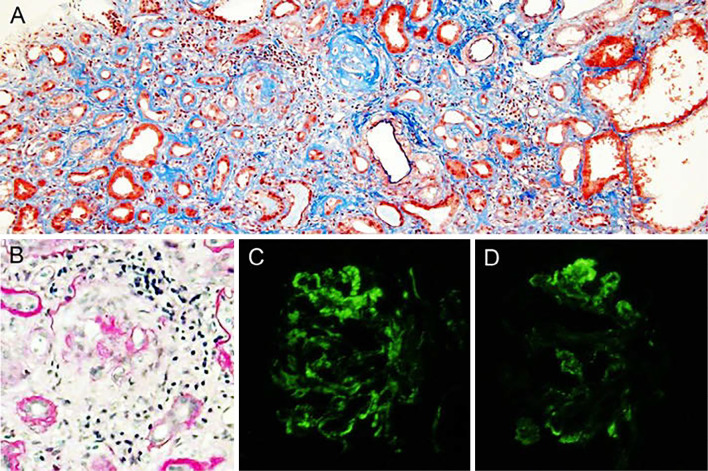
A kidney biopsy revealed that 9 out of 12 glomeruli had global sclerosis, with the disappearance of Bowman's capsule and capillary loop disruption, and the 3 remaining glomeruli showed circumferential fibrocellular crescents, with the disappearance of Bowman's capsule (Fig. 1A, B). Tubulointerstitial inflammatory cell infiltration and tubular atrophy or degeneration were found in large areas of the cortex (Fig. 1A). Immunofluorescence showed depositions of IgM and C3 in a peripheral glomerulus (Fig. 1C, D), but electron microscopy did not show significant electron-dense deposits. Therefore, the depositions of IgM and C3 were considered nonspecific, and C3 glomerulopathy was unlikely, although she had hypocomplementemia upon admission (7). The advanced phase of MPO-ANCA-associated crescentic glomerulonephritis due to MPA was diagnosed. Maintenance hemodialysis was considered inevitable. Tapering of prednisolone was planned because the MPO-ANCA titer was low and CRP was negative.
Figure 1.
Light microscopic findings of the kidney biopsy. A: Tubulointerstitial inflammatory cell infiltration and tubular atrophy, tubular dilatation or degeneration are found in large areas of the cortex. There are two globally sclerotic glomeruli associated with the disappearance of Bowman’s capsule and the disruption of capillary loops. No vasculitis of the small arteries is seen. Elastica-Masson staining. Original magnification ×200. B: Glomeruli show circumferential fibrocellular crescents with the disappearance of Bowman’s capsule. Periodic acid-Schiff staining. Original magnification ×200. An immunofluorescent study shows depositions of IgM (C) and C3 (D) in a peripheral glomerulus.
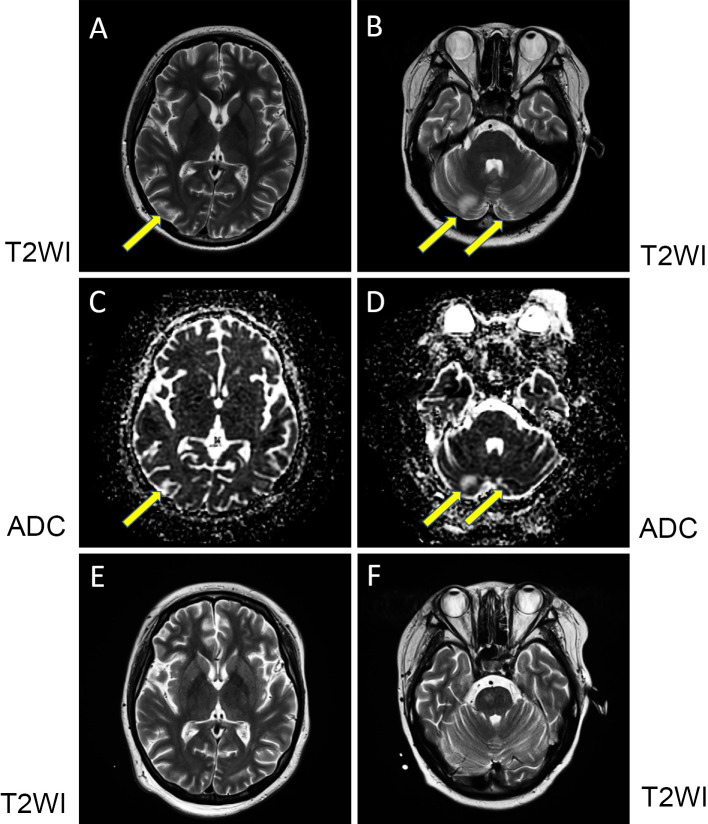
On prednisolone at 30 mg/day, she suddenly presented with nausea, vertigo and right ear pain, followed by right-sided hearing loss, right-sided partial facial palsy and blisters of the right auricle with pain over 7 days. She was diagnosed with Ramsay Hunt syndrome. At the occurrence of facial palsy, she also showed confusion with restlessness and irritability. She began to take valaciclovir hydrochloride and prednisolone at 60 mg/day with planned short-term tapering for Ramsay Hunt syndrome and clotiazepam for confusion. Cranial MRI revealed bilateral occipital and bilateral cerebellar high-intensity lesions on T2-weighted imaging (T2WI) (Fig. 2A, B) and fluid-attenuated inversion recovery (FLAIR). The lesions showed a high intensity on apparent diffusion coefficient (ADC) mapping (Fig. 2C, D), indicative of vasogenic edema (8). Magnetic resonance angiography (MRA) did not show any abnormalities. Her condition was considered to be indicative of PRES. At the onset of her confusion, her blood pressure was 150/100 mmHg, which was reduced to 140/80 mmHg by nifedipine CR at 80 mg/day. Her nausea, vertigo and confusion improved within three days. Although her blisters improved, hearing loss and facial palsy remained.
Figure 2.
Cranial MRI. At the first cranial MRI procedure, T2-weighted imaging (T2WI) in the coronal view showed a high intensity in the right occipital lobe (arrow) (A) and bilateral cerebellum (arrows) (B). Increased apparent diffusion coefficient (ADC) mapping was found in the same lesions (arrows) (C and D). At the second cranial MRI procedure, the corresponding lesions showed a reduced intensity on T2WI (E, occipital lobe and F, cerebellum).
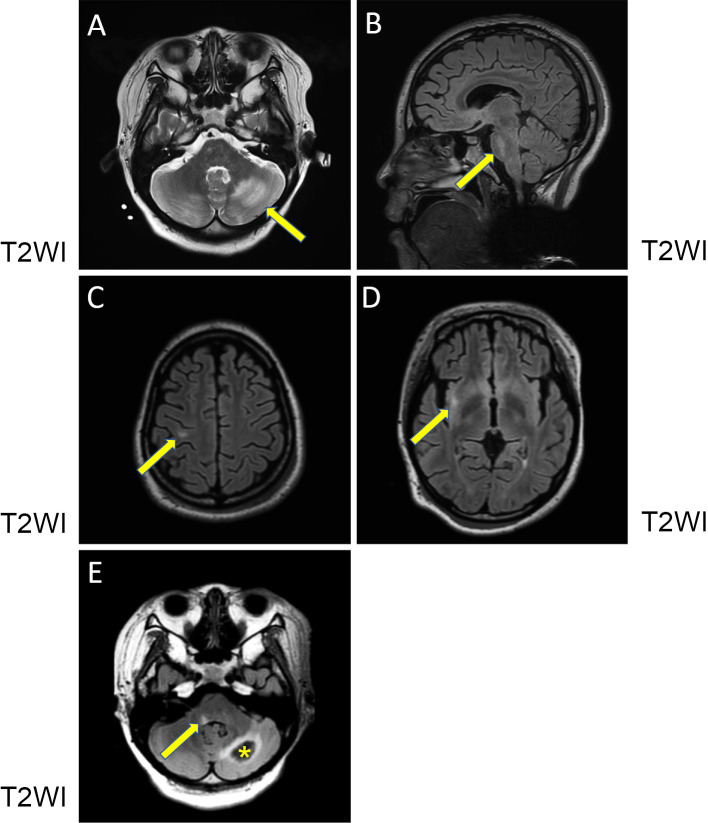
On prednisolone at 20 mg/day, 14 days after the improvement of her confused state, she suddenly complained of nausea, vomiting and vertigo. Second cranial MRI performed 17 days after the first procedure showed a reduction in the previously observed lesions (Fig. 2E, F), but new lesions appeared in the left cerebellar white matter, the right side of basilar part of pons, the right precentral gyrus subcortical white matter and the right insular gyrus white matter (Fig. 3A-D). All of the lesions showed a high intensity on T2WI and FLAIR. The lesions in the cerebellum and pons showed a high intensity on ADC mapping, suggestive of PRES. However, ADC mapping in the other sporadic small lesions was equivocal. Complement fixing antibody titers against varicella zoster virus (VZV) did not increase in paired cerebrospinal fluid samples (both samples <1×). We considered her CNS manifestations to be related to active MPA. Therefore, steroid pulse therapy and subsequently oral prednisolone at 60 mg/day was administered.
Figure 3.
Cranial MRI. At the second cranial MRI procedure, T2WI in the coronal view (A, C and D) and sagittal view (B) showed high-intensity lesions in the left cerebellar white matter (arrow) (A), the right side of the basilar part of the pons (arrow) (B), the right precentral gyrus subcortical white matter (arrow) (C) and the right insular gyrus white matter (arrow) (D). At the third cranial MRI procedure, T2WI in the coronal view (E) showed subacute hemorrhaging within the previous left cerebellar white matter lesion (asterisk) and a high-intensity lesion at the dorsal surface of the right medulla oblongata (arrow).
Her nausea and vertigo disappeared soon after steroid pulse therapy. She received plasma exchange for two cycles. Although CRP was continuously negative, MPO-ANCA was later reported to be positive (Table 2). Third cranial MRI performed 16 days after the second procedure showed the resolution of the previous lesions but subacute hemorrhaging within the area of the previous left cerebellar white matter lesion and a new lesion at the dorsal surface of the right medulla oblongata (Fig. 3E). Subsequent plasma exchange using anticoagulants was stopped to avoid further brain hemorrhaging. Fourth cranial MRI performed 12 days after the third procedure showed that the brain lesions had improved, except for the subacute hematoma of the left cerebellar white matter, with depositions of blood products detected by susceptibility-weighted imaging (SWI) (not shown).
Table 2.
Findings of MRI/MRA and Laboratory Data of MPO-ANCA, P-ANCA and CRP.
| Number of MRI procedures | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days after admission | 32 | 49 | 65 | 77 | 88 | 98 | 112 | 126 | 147 | |||||||||
| MRI new findings | ||||||||||||||||||
| Occipital lobe | R | L | H†(L) | |||||||||||||||
| Cerebellum | Bil | L | H(L) | H*(L) | Bil | H*(R), H*(L) | ||||||||||||
| Pons | R | |||||||||||||||||
| Precentral gyrus | R | |||||||||||||||||
| Insular gyrus | R | |||||||||||||||||
| Medulla oblongata | R | |||||||||||||||||
| MRA new findings | LPCA | |||||||||||||||||
| aneurysm | ||||||||||||||||||
| MPO-ANCA (U/mL) | 5.3 | 3.0 | 1.0> | 1.0> | 1.0> | 1.0> | ||||||||||||
| P-ANCA | ×80 | negative | ||||||||||||||||
| CRP (mg/dL) | 0.01 | 0.04 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 |
MRI: magnetic resonance imaging, MRA: magnetic resonance angiography, MPO-ANCA: myeloperoxidase-antineutrophil cytoplasmic antibody, P-ANCA: perinuclear-anti-neutrophil cytoplasmic antibody, CRP: C-reactive protein, Bil: bilateral lesions, L: left lesion, R: right lesion, H: hemorrhaging, H*: deposition of blood products, H†: subarachnoid hemorrhaging, LPCA: left posterior cerebral artery
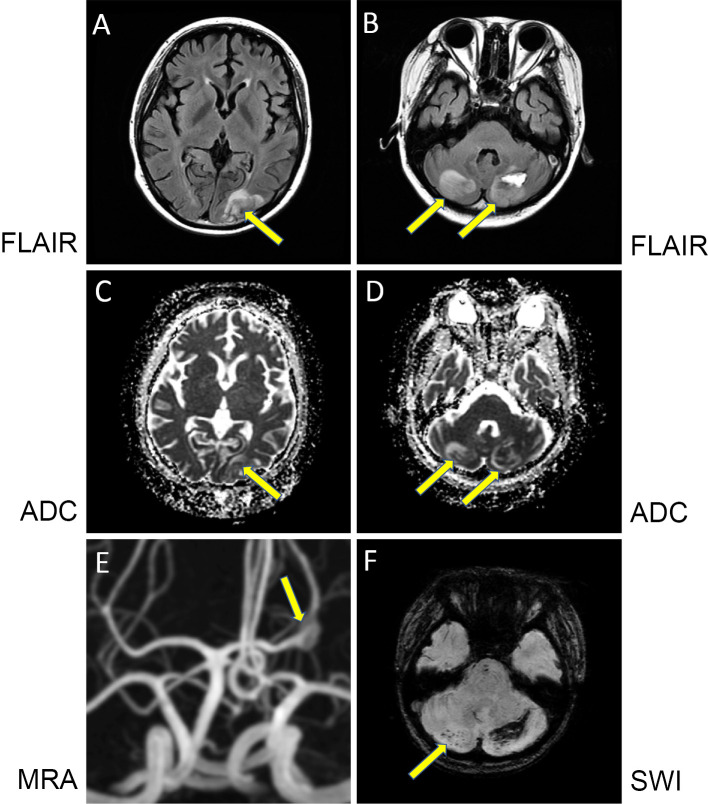
For MPA treatment, rituximab at 500 mg/day was infused. Six days after rituximab administration, she complained of nausea and vomiting on prednisolone at 50 mg/day. Her blood pressure was 172/120 mmHg on amlodipine besylate at 2.5 mg/day, which was used after obstruction of the arteriovenous fistula for hemodialysis access occurred due to a drop in her blood pressure. Her blood pressure was soon managed with nicardipine hydrochloride intravenously. Just after an emergency fifth cranial MRI procedure, she complained of headache and had a generalized tonic-clonic seizure. The seizure was controlled by phenytoin sodium and then valproate sodium. She also complained of visual changes. Fifth cranial MRI showed new high-intensity lesions in the left occipital lobe and bilateral cerebellar lesions on FLAIR and ADC mapping (Fig. 4A-D), indicating PRES. MRA further revealed a P2-segment fusiform aneurysm in the left posterior cerebral artery (Fig. 4E). Her blood pressure was finally controlled at approximately 140/80 mmHg by oral antihypertensive drugs. All of her symptoms disappeared within seven days. As MPO-ANCA had become negative (Table 2), reinforcement of treatment for MPA, including steroid pulse therapy, was not chosen, and rituximab was withdrawn, as these therapies are risk factors for PRES.
Figure 4.
Cranial MRI and MRA. At the fourth cranial MRI procedure, fluid-attenuated inversion recovery (FLAIR) imaging in the coronal view showed high-intensity lesions in the left occipital lobe (arrow) (A) and bilateral cerebellum (arrows) (B). Increased ADC imaging was found in the corresponding lesions (arrows) (C and D). MRA revealed the left posterior cerebral artery fusiform aneurysm (arrow) (E). Susceptibility-weighted imaging (SWI) showed the loss of signal within the right cerebellar lesion (arrow) and at the periphery of the left cerebellar lesion, suggesting microhemorrhaging or deposition of blood products (F).
Follow-up cranial MRI performed 10 days after the fifth procedure showed a reduction in the previous PRES lesions, but microhemorrhaging or blood products in the right and left cerebellum was found by SWI (Fig. 4F) as possible complications of PRES. Seventh cranial MRI performed 14 days after the sixth procedure showed the disappearance of PRES lesions and the existence of a subacute hematoma in the left cerebellum as well as isolated cortical subarachnoid hemorrhaging in the sulcus of the left occipital lobe (not shown). MRA revealed no marked change in the aneurysm.
We considered her ANCA-associated MPA to be in remission, so prednisolone was gradually tapered. Eighth cranial MRI (14 days after the seventh procedure) and ninth MRI (21 days after the eighth procedure) showed no new lesions except for a chronic hematoma with gradual regression. She was discharged on prednisolone at 25 mg/day. Her complement levels of C3 and C4 had increased and were near the lower end of the normal range throughout her clinical course. Regular cranial MRA showed the presence of the same-sized aneurysm. She had no vasculitic symptoms or signs at the outpatient hemodialysis unit on prednisolone at 3 mg/day at the last follow-up 45 months post-discharge.
Discussion
We encountered a young woman with advanced crescentic glomerulonephritis requiring hemodialysis due to ANCA-associated MPA who experienced repeated PRES episodes, probably due to different etiologies, over a period of two months.
The first CNS manifestations in our patient on prednisolone at 30 mg/day developed with the onset of Ramsay Hunt syndrome. Cranial MRI (Fig. 2) suggested PRES, and all her symptoms were included in the clinical manifestations of PRES (4,9). She had multiple risk factors for PRES, including prednisolone use, elevated blood pressure, renal failure on hemodialysis and ANCA-associated MPA (5). With increased doses of prednisolone for Ramsay Hunt syndrome and blood pressure management, her symptoms were cured within three days, and reversible brain lesions were confirmed. Thus, she was diagnosed with PRES associated with active MPA. Interestingly, there has been one reported case of herpes zoster ophthalmics (Ramsay Hunt syndrome) presenting with PRES (10).
At the second CNS manifestation, she presented with nausea and vomiting again approximately seven days after the disappearance of previous symptoms. Second MRI showed multiple newly developed white matter lesions. The lesions in cerebellum and pons showed a high intensity on ADC mapping, indicating reversible vasogenic edema (8). However, the other sporadic small lesions did not show a high intensity on ADC mapping (8). Therefore, these lesions might have been ischemic lesions caused by vasculitis due to MPA (6). Her symptoms improved soon after steroid pulse therapy. Her brain lesions on MRI were reversible; thus, the series of brain lesions could be regarded as PRES. MPO-ANCA and P-ANCA were positive at the time of the second MRI procedure (Table 2); therefore, it is likely that the CNS manifestations were associated with MPA. VZV vasculopathy is caused by productive VZV infection of cerebral arteries, most often leading to transient ischemic attacks or stroke (11); however, the complement fixing antibody titers against VZV did not demonstrate any increase in the paired cerebrospinal fluid samples.
A very recent analysis demonstrated a strong relationship between PRES and autoimmune diseases, including AAV, using a large population-based data set as the reference (12). Autoimmune disease is considered to cause PRES by mechanisms of both vasogenic edema and endothelial dysfunction (13). Interestingly, patients with autoimmune disease were reported to be more likely to have cerebellar involvement (14), as in our case, than others. There was a small number of cases of AAV complicated by PRES. Seven cases with P-ANCA- or MPO-ANCA-associated MPA [four from the English literature (15-18) and three from Japanese literature (19,20)] were reported in detail (Table 3). At the onset of PRES, six patients were using corticosteroids. Four patients showed hypertension, and four had renal failure. Corticosteroid use with hypertension and renal failure, as in our case, was found in three patients. The cause of PRES in six patients was considered to be active MPA. Intravenous cyclophosphamide was suspected to be the cause of PRES in one patient.
Table 3.
Reported Cases of P- or MPO-ANCA-associated MPA Complicated with PRES.
| Reported year (ref) |
Age (years)/ Sex |
ANCA | Medication at the onset | Cr (mg/ dL) |
BP (mmHg) at the onset | Neurological symptoms | MRI/MRA | Treatment for PRES | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 2002 (19) |
73/F | P- | mPSL+PSL | 0.97 | 200/100 | H, S, AM, FMD | O, thalamus/NS | mPSL, antihypertensive | clinical resolution in 13 days MRI normal in 47 days |
| 2002 (19) |
77/F | P- | mPSL+PSL | 3.08 | 186/123 | AM, S | O, thalamus, C, brainstem/NS | mPSL, antihypertensive | MRI normal in 29 days death probably due to pulmonary hemorrhaging |
| 2006 (15) |
76/F | P- | PSL | 0.8 | 136/86 | H, AM | P, O, F/ arterial dilatation | mPSL | death due to pulmonary hemorrhaging |
| 2007 (20) |
20/F | MPO | PSL | 0.6 | 120/80 | H, S | O, T, F, P, pons/ NS | anticonvulsant, PSL+IVCYC | clinical resolution on the day MRI normal in 2 months |
| 2012 (16) |
18/F | P- | Non | 4.5< | 1st: norm 2nd: hyper |
1st: S 2nd: S |
1st: F, P, O, H/normal 2nd: NS |
1st: HD,PSL+CYC+PE 2nd: NS |
1st: on HD MRI normal in 3 weeks 2nd: NS |
| 2014 (17) |
40/M | P- | 1st: mPSL+PSL 2nd: missed HD sessions, tapered PSL |
24.48 | 1st: 160/90 | 1st: VS, S, FMD 2nd: S |
1st: T, O, B/normal | 1st: HD, mPSL+PSL antihypertensive 2nd: intensive HD, dose-up PSL |
1st: partial improvement of vision, on HD 2nd: clinical resolution in 5 months after the initial event |
| 2015 (18) |
10/F | P- MPO | mPSL+PSL IVCYC+PE | 6.3 HD | 1st: 170/100 2nd: 163/104 |
1st: N, S, FMD 2nd: S |
1st: P/normal 2nd: F, P, T, O/normal |
1st: HD, anticonvulsant, antihypertensive 2nd: antihypertensive, withdrawal of IVCYC, rituximab |
1st: clinical resolution in 2 days, on HD 2nd: clinical resolution in 3 days, on HD |
| Our case | 19/F | MPO P- | 1st: PSL 2nd: PSL 3rd: PSL, rituximab |
HD | 1st: 150/100 2nd: 140/80 3rd: 172/120 |
1st: N, AM 2nd: N 3rd: N, H, S, VS |
1st: O, C/normal 2nd: C, pons, gyrus, H, (medulla oblongata)/normal 3rd: O, C, H/ aneurysm |
1st: anticonvulsant, antihypertensive, PSL 2nd: mPSL+PSL+PE 3rd: anticonvulsant, antihypertensive |
1st: clinical resolution in 3 days, on HD MRI reduction in 17 days 2nd: clinical resolution in 3 days, on HD MRI resolution in 28 days 3rd: clinical resolution in 7 days, on HD MRI normal in 24 days except hematoma |
ref: reference, MRI: magnetic resonance imaging, MRA: magnetic resonance angiography, ANCA: antineutrophil cytoplasmic antibody, P-: perinuclear-ANCA, MPO: myeloperoxidase-ANCA, mPSL: methylprednisolone pulse, PSL: prednisolone, IVCYC: intravenous cyclophosphamide, CYC: cyclophosphamide, PE: plasma exchange, Cr: serum creatinine, HD: hemodialysis, BP: blood pressure, H: headache, S: seizure, AM: altered mental state, FMD: focal motor deficits, VS: visual symptoms, N: nausea (or vomiting), NS: not stated, O: occipital lobe, C: cerebellum, P: parietal lobe, F: frontal lobe, T: temporal lobe, B: basal ganglia, H: hemorrhaging
Third cranial MRI showed a new lesion in the right medulla oblongata and hemorrhaging within the area of the previous left cerebellar lesion. The medulla oblongata lesion was considered to be included in the same series of previous brain lesions as PRES or vasculitis. Intracranial hemorrhaging is a frequent complication in PRES and occurs in 10-20% of reported cases (4), and imaging findings in hemorrhaging may include focal hematomas, petechial gyral hemorrhaging and/or subarachnoid hemorrhaging (21). Our patient also showed the deposition of blood products and subarachnoid hemorrhaging near the lesions at the second or third PRES episodes. A high rate of microhemorrhaging and deposition of blood products in PRES have been found using SWI, but their clinical significance is unclear (22). Several studies have found that intracranial hemorrhaging is associated with incomplete recovery (5), but our patient showed a full clinical recovery.
The third PRES episode included the typical symptoms of headache, visual disturbance and a generalized tonic-clonic seizure along with new left occipital and both cerebellar lesions at the fifth cranial MRI procedure. Unexpectedly, a left posterior cerebral artery fusiform aneurysm was found. To our knowledge, only four cases of AAV [two cases of MPA and two cases of granulomatosis with polyangiitis (GPA)] complicated by cerebral aneurysms have been reported in the English literature (23-26). One case of GPA showed an unruptured fusiform aneurysm in the internal carotid artery (24). Severe headache or unconsciousness due to subarachnoid hemorrhaging from ruptured cerebral aneurysms was reported in the other three cases. Necrotizing changes in the affected vessels may occur, followed by weakening of the vessel wall and aneurysm formation (27). Since MPO-ANCA or P-ANCA and CRP had become negative (Table 2) and all of her symptoms disappeared quickly, we assumed that her MPA was under control. However, the newly appearing occipital lobe and cerebellar lesions showed a high intensity on ADC mapping only at the periphery of the lesions (Fig. 4C, D). Therefore, given the concomitant appearance of the cerebral aneurysm, vasculitis by MPA might have contributed to the lesions.
CNS symptoms appeared six days after rituximab infusion. Given the expression of CD20 in activated endothelial cells, rituximab may cause direct cell damage, endothelin-mediated vasospasm and dysfunction, which may lead to PRES (28,29). PRES was reported to occur soon after the infusion of rituximab or five to six days after the last weekly infusion of rituximab on a uremic background with or without severe hypertension in patients with systemic lupus erythematosus or systemic vasculitis (28-30). Therefore, we suspected that her third PRES episode might have been due to rituximab infusion associated with hypertension rather than MPA. Cyclosporin (31), azathioprine (32), cyclophosphamide (33) and mycophenolate mofetil have been reported to induce PRES in patients with AAV.
In summary, physicians should pay attention to CNS manifestations, including PRES and cerebral aneurysm, in patients with MPA. Patients with AAV tend to have multiple risk factors for PRES, which includes varied neurological symptoms and many atypical imaging features. An evaluation of the underlying causes of PRES is crucial, especially when patients are treated with agents for AAV. Physicians are faced with the challenging decision to discontinue the agents or reinforce treatment. Therefore, causal or prognostic biomarkers or imaging techniques for PRES are needed to facilitate prompt and adequate management of PRES.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1-11, 2013. [DOI] [PubMed] [Google Scholar]
- 2. Graf J. Central nervous system disease in antineutrophil cytoplasmic antibodies-associated vasculitis. Rheum Dis Clin North Am 43: 573-578, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Abdel Razek AA, Alvarez H, Bagg S, Refaat S, Castillo M. Imaging spectrum of CNS vasculitis. Radiographics 34: 873-894, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Liman TG, Siebert E, Endres M. Posterior reversible encephalopathy syndrome. Curr Opin Neurol 32: 25-35, 2019. [DOI] [PubMed] [Google Scholar]
- 5. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 14: 914-925, 2015. [DOI] [PubMed] [Google Scholar]
- 6. Zheng Y, Zhang Y, Cai M, Lai N, Chen Z, Ding M. Central nervous system involvement in ANCA-associated vasculitis: what neurologists need to know. Front Neurol 9: 1166, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fakhouri F, Le Quintrec M, Frémeaux-Bacchi V. Practical management of C3 glomerulopathy and Ig-mediated MPGN: facts and uncertainties. Kidney Int 98: 1135-1148, 2020. [DOI] [PubMed] [Google Scholar]
- 8. Lamy C, Oppenheim C, Mas JL. Posterior reversible encephalopathy syndrome. Handb Clin Neurol 121: 1687-1701, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Granata G, Greco A, Iannella G, et al. Posterior reversible encephalopathy syndrome - Insight into pathogenesis, clinical variants and treatment approaches. Autoimmun Rev 14: 830-836, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Kuczynski AM, Wallace CJ, Wada R, Tyler KL, Kapadia RK. Crossed zoster syndrome: a rare clinical presentation following herpes zoster ophthalmicus. Can J Neurol Sci 47: 711-713, 2020. [DOI] [PubMed] [Google Scholar]
- 11. Nagel MA, Gilden D. Developments in varicella zoster virus vasculopathy. Curr Neurol Neurosci Rep 16: 12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manadan A, Kambhatla S, Gauto-Mariotti E, Okoli C, Block JA. Rheumatic diseases associated with posterior reversible encephalopathy syndrome. J Clin Rheumatol. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 13. Fischer M, Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol 264: 1608-1616, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc 85: 427-432, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tajima Y, Matsumoto A. Reversible posterior leukoencephalopathy syndrome in p-ANCA-associated vasculitis. Intern Med 45: 1169-1171, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Nasseri F, Hunter JV, Elenberg E, Muscal E. A unique case of intraventricular hemorrhage associated with posterior reversible encephalopathy syndrome in an adolescent. J Child Neurol 27: 1048-1051, 2012. [DOI] [PubMed] [Google Scholar]
- 17. Patel UV, Patel NJ. Posterior reversible leukoencephalopathy syndrome as a presenting manifestation of p-ANCA-associated vasculitis. BMJ Case Rep bcr2013202022, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang S, Habib S, Umer S, Reisman L, Raman V. Recurrent posterior reversible encephalopathy syndrome in a child with microscopic polyangiitis. J Clin Rheumatol 21: 113-114, 2015. [DOI] [PubMed] [Google Scholar]
- 19. Kawano H, Kimura E, Ishizaki M, et al. [Reversible posterior leukoencephalopathy syndrome in two patients with microscopic polyarteritis nodosa]. Rinsho Shinkeigaku (Clin Neurol) 42: 949-953, 2002(in Japanese). [PubMed] [Google Scholar]
- 20. Kamimura H, Hirose S, Tazaki K, Suzuki Y, Satou M. [Reversible posterior leukoencephalopathy syndrome with microscopic polyangiitis]. Nihon Naika Gakkai Zasshi (J Jpn Soc Intern Med) 96: 2532-2535, 2007(in Japanese). [DOI] [PubMed] [Google Scholar]
- 21. Donmez FY, Basaran C, Kayahan Ulu EM, Yildirim M, Coskun M. MRI features of posterior reversible encephalopathy syndrome in 33 patients. J Neuroimaging 20: 22-28, 2010. [DOI] [PubMed] [Google Scholar]
- 22. McKinney AM, Sarikaya B, Gustafson C, Truwit CL. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. AJNR Am J Neuroradiol 33: 896-903, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takei H, Komaba Y, Kitamura H, et al. Aneurysmal subarachnoid hemorrhage in a patient with Wegener's granulomatosis. Clin Exp Nephrol 8: 274-278, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Onodera H, Hiramoto J, Morishima H, Tanaka Y, Hashimoto T. Treatment of an unruptured fusiform aneurysm of the internal carotid artery associated with Wegener's granulomatosis by endovascular balloon occlusion. Case report. Neurol Med Chir (Tokyo) 52: 216-218, 2012. [DOI] [PubMed] [Google Scholar]
- 25. Kimura H, Akutsu N, Shiomi R, Kohmura E. Subarachnoid hemorrhage caused by ruptured intracranial fusiform aneurysm associated with microscopic polyangiitis. Neurol Med Chir (Tokyo) 52: 495-498, 2012. [DOI] [PubMed] [Google Scholar]
- 26. Ihara K, Kimura M, Yamamuro M, Inoshita S. Microscopic polyangiitis associated with subarachnoid hemorrhage. J Rural Med 14: 125-131, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mavrogeni S, Manoussakis MN, Karagiorga TC, et al. Detection of coronary artery lesions and myocardial necrosis by magnetic resonance in systemic necrotizing vasculitides. Arthritis Rheum 61: 1121-1129, 2009. [DOI] [PubMed] [Google Scholar]
- 28. Verdesca SVS, Villani C, Rossini M, Manno C, Gesualdo L, Montinaro V. [Posterior Reversible Encephalopathy Syndrome (PRES) induced by Rituximab in two patients with vasculitis, and treated by hemodialysis]. G Ital Nefrol 35: 2018. (in Italian). [PubMed] [Google Scholar]
- 29. Pathireddy S, Bose S, Baradhi K, Aeddula NR. Rare but not beyond care: a young female with altered mental status and seizures. Oxf Med Case Reports 2019: omz072, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mavragani CP, Vlachoyiannopoulos PG, Kosmas N, Boletis I, Tzioufas AG, Voulgarelis M. A case of reversible posterior leucoencephalopathy syndrome after rituximab infusion. Rheumatology (Oxford) 43: 1450-1451, 2004. [DOI] [PubMed] [Google Scholar]
- 31. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 334: 494-500, 1996. [DOI] [PubMed] [Google Scholar]
- 32. Vilas-Boas S, Corte-Real A. Posterior reversible encephalopathy syndrome and azathioprine. Eur J Case Rep Intern Med 6: 001032, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. How J, Blattner M, Fowler S, Wang-Gillam A, Schindler SE. Chemotherapy-associated posterior reversible encephalopathy syndrome: a case report and review of the literature. Neurologist 21: 112-117, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]