Abstract
Objective
According to consensus guidelines, eosinophilic esophagitis (EoE) is defined as a clinicopathological entity whose symptoms and histology must always be considered together. However, endoscopic findings typical of EoE are often seen in asymptomatic esophageal eosinophilia (aEE). We aimed to clarify the clinicopathological features of aEE.
Methods
We retrospectively compared cases of aEE and those of symptomatic EoE.
Materials
We reviewed 146 patients who underwent upper gastrointestinal endoscopy and were confirmed histopathologically to have esophageal eosinophil infiltration of at least 15 eosinophils per high-power field. They were divided into the aEE group (n=75) and the EoE group (n=71). Patients' clinicopathological findings were then collected and examined.
Results
The EoE group experienced dysphagia (47.9%), heartburn (40.8%), food impaction (40.8%), chest pain (16.9%), and other symptoms (8.5%). There was no significant difference between the two groups with regard to age, sex, current smoking status, or alcohol consumption. The aEE group had a significantly higher body mass index (p<0.01) and significantly lower frequency of concurrent allergic diseases (p<0.01) than the EoE group. No significant differences were found between the two groups with regard to the mean peripheral blood eosinophil count, non-specific immunoglobulin E concentration, peak eosinophil infiltration in the biopsy specimens, EoE histology scoring system, phenotype and location of typical endoscopic findings of EoE, or thickness of the esophagus wall or the mucosal and submucosal layer as measured by endoscopic ultrasonography. Two patients in the aEE group who were followed up without treatment subsequently developed esophageal symptoms.
Conclusion
aEE and EoE may have the same clinicopathological features.
Keywords: eosinophilia, eosinophilic esophagitis, endoscopy, endosonography
Introduction
Eosinophilic esophagitis (EoE) was first reported in the 1970s and defined as an entity with its own clinical and histological characteristics by Attwood et al. in 1993 (1-3). Although it is a relatively rare disease, its incidence and prevalence have been increasing recently (4,5).
EoE is defined as a chronic, local immune-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil predominant inflammation (6,7). Thus, EoE is understood to be a clinicopathological entity whose symptoms and histology must always be considered together (6,8).
However, asymptomatic cases of esophageal eosinophilia (EE) are often encountered that show esophageal eosinophil infiltration and typical endoscopic findings of EoE, such as longitudinal furrows/ridges, esophageal rings, and white exudate. Such asymptomatic EE (aEE) may have the same etiology as symptomatic EoE, but few studies have investigated this.
Therefore, in the present study, we clarified the clinical and histological features of EE by classifying patients by the presence of symptoms.
Materials and Methods
Patients and design
This study reviewed patients who underwent upper gastrointestinal endoscopy screening during a comprehensive medical checkup at Toranomon Hospital, Tokyo, Japan, between January 1, 2010, and December 12, 2019, and were histopathologically confirmed to have esophageal infiltration of at least 15 eosinophils per high-power field (eos/hpf), as stated in the guidelines for EE (6,7). Biopsies were performed for at least two different proximal and distal esophageal sites with or without typical endoscopic findings of EoE.
We retrospectively reviewed those patients who were diagnosed with EE and excluded those who had a history of esophageal surgery, chemoradiation therapy that included the esophagus in the radiation field, and secondary causes of EE, such as eosinophilic gastroenteritis, gastroesophageal candida esophagitis, achalasia, graft-versus-host disease, and eosinophilic granulomatosis with polyangiitis. Patients with reflux esophagitis only were also excluded, but concurrent cases were included.
The 146 total consecutive patients who consented to this study were then divided into the aEE group (n=75) and the EoE group who met the diagnostic criteria for EoE (n=71). In this study, we defined “asymptomatic” as the absence of any of the aforementioned symptoms related to esophageal dysfunction within the last three months and occurring less than once every six months. We defined “symptomatic” as the presence of one or more symptoms related to esophageal dysfunction, such as dysphagia, heartburn, chest pain, food impaction, and other digestive symptoms.
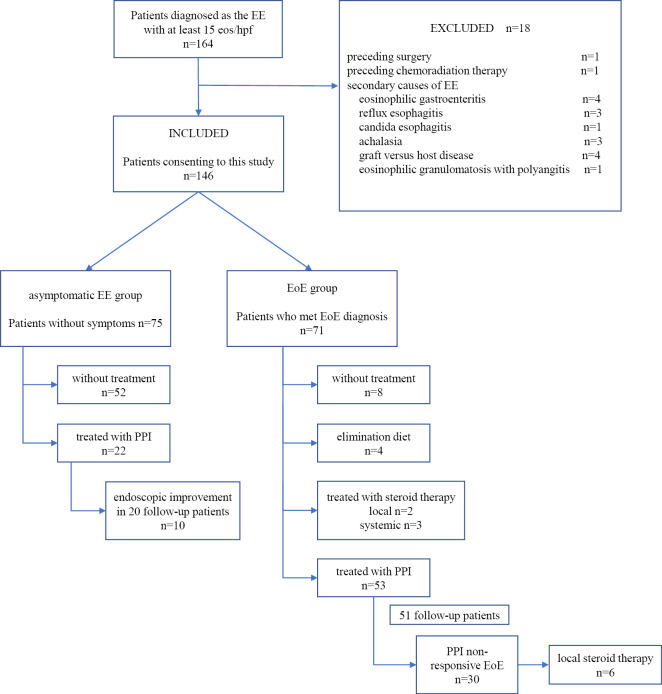
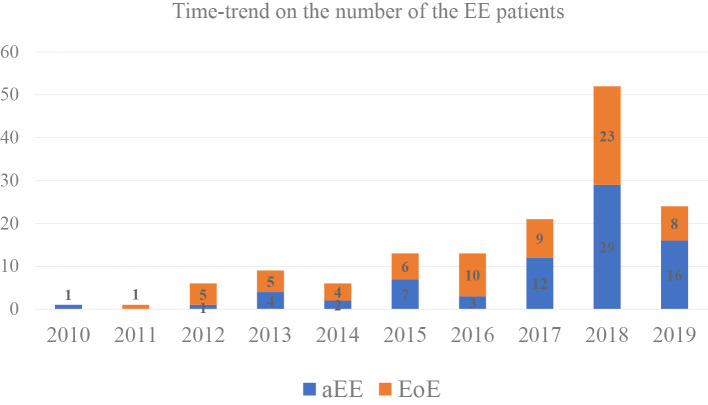
A study flowchart is shown in Fig. 1. Fig. 2 shows the time-trend in the number of subjects in this study. The numbers of patients diagnosed with EE have been increasing over the past three years.
Figure 1.
Patient flowchart showing the 146 patients who consented to the study divided into the aEE group (n=75) and EoE group (n=71).
Figure 2.
Time-trend in the number of EE patients in this study. The numbers of patients diagnosed with EE have been increasing over the past three years.
Clinical information, endoscopic findings, and histopathological findings were collected and compared between the two groups. The blood count and serum biochemical findings were obtained at the time of the initial diagnosis. Patients in the EoE group were started on a proton pump inhibitor (PPI) or an elimination diet. The oral dose of PPI was lansoprazole 15-30 mg or esomeprazole 10-20 mg or rabeprazole 10 mg. PPIs were administered for at least eight weeks. The PPI response was determined based on the eosinophilic infiltration decreasing to <15 eos/hpf or the improvement in endoscopic findings after 2 months of PPI treatment, and patients who failed to respond were treated with topical corticosteroids. In the aEE group, only patients who gave their consent were treated with PPI. The oral dose of PPI was lansoprazole 15 mg or esomeprazole 10-20 mg, administered for at least 8 weeks. Patients underwent follow-up endoscopy once a year until December 31, 2020, and the clinicopathological findings obtained at the follow-up were analyzed.
This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendment, and the protocol was approved by the ethics committee of our hospital (Approval number: 1783). As this was a retrospective observational study, written informed consent was not necessarily required because it used information recorded in the medical records without using samples. By disclosing information related to clinical research on our website, we offered patients the opportunity to withdraw consent for their information to be used anonymously in research. The need for informed consent to participate was waived by the ethics committee (Federation of National Public Service Personnel Mutual Aid Associations Toranomon Hospital Certified Review Board).
Endoscopic findings
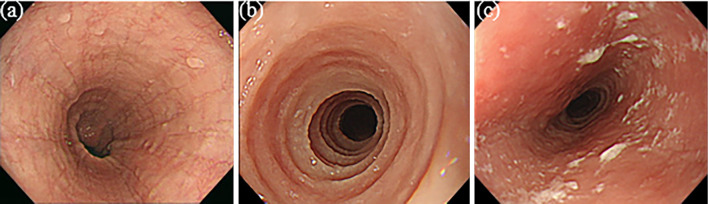
The location of each finding considered to be typical of EoE, such as longitudinal furrows/ridges, esophageal rings, and white exudate, was determined from images obtained by white-light endoscopy and narrow-band imaging endoscopy. The typical findings observed in this study are shown in Fig. 3. Endoscopic findings were evaluated based on the EoE endoscopic reference score (EREFS) (9). The inflammatory and fibrostenotic scores were calculated (10). Regarding the location of endoscopic findings, the phenotype was divided into diffuse and localized EE in the previous study (11). The localized type was defined as a small area of EE localized within 1 to 2 cm. The diffuse type was defined as a widespread area of EE involving one or more of three locations: upper, middle, and lower esophagus. Other endoscopic findings that could induce digestive symptoms were also assessed, such as pharyngeal findings, atrophic gastritis, hiatus hernia, and esophageal mucosal breaks based on the Los Angeles Classification (12). Diagnoses were obtained retrospectively by two board-certified fellows of the Japan Gastroenterological Endoscopy Society (JGES).
Figure 3.
Typical endoscopic findings obtained by white-light imaging: (a) longitudinal furrows/ridges; (b) esophageal rings; and (c) white exudates.
Helicobacter pylori infection
The diagnosis of H. pylori infection was based on the presence of H. pylori antibody, stool antigen, or urea breath tests. Current infection was defined as gastric atrophy with a positive H. pylori test, past infection as gastric atrophy with a negative H. pylori test, and negative infection as no gastric atrophy with a negative H. pylori test.
Endoscopic ultrasonography (EUS)
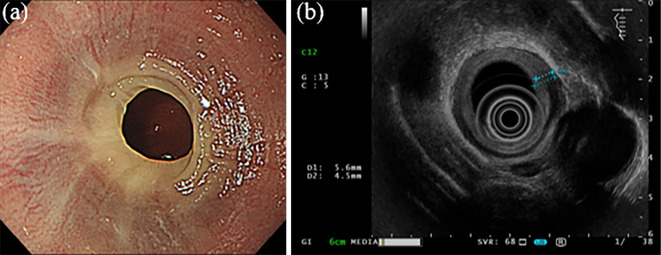
The details of EUS were explained to all enrolled patients, and only those patients who gave their consent were subjected to the examination. The thickness of the esophageal wall and mucosa and submucosal layer was determined with the GF-UM2000 mechanical radial scope (Olympus Medical Systems, Tokyo, Japan). Measurements were made in areas where the thickness was most noticeable. The frequencies used for EUS were 20 and 12 MHz. A typical measurement taken on EUS is shown in Fig. 4.
Figure 4.
Typical measurements obtained by EUS. The esophageal wall thickness and combined mucosa and submucosa layer thickness were measured at 20 and 12 MHz using the GF-UM2000: (a) white-light image; (b) EUS image.
The histopathological assessment of the specimens
All biopsy specimens were fixed in 10% formalin, stained with hematoxylin and eosin, and assessed pathologically based on the EoE histology scoring system (EoEHSS) (13). The EoEHSS evaluates eosinophilic inflammation and other features, including epithelial basal zone hyperplasia, eosinophilic abscesses, eosinophil surface layering, dilated intercellular spaces, surface epithelial alteration, dyskeratotic epithelial cells, and lamina propria fibrosis. The grade and stage of abnormalities are scored using a 4-point scale [0 (normal) to 3 (maximum change)]. The peak value for eos/hpf was determined by specialist pathologists from the Japanese Society of Pathology.
Statistical analyses
Data are presented as the mean±standard deviation. Statistical analyses were performed using an unpaired t-test, chi-squared test, Fisher's exact test, or the Mann-Whitney U test as appropriate. A p value <0.05 was considered significant. All statistical analyses were performed using SPSS version 25 (SPSS IBM statistics; IBM, Armonk, USA).
Results
Patient characteristics
Table 1 shows the patients' clinicopathological characteristics. The diagnosis was made based on endoscopy screening during a comprehensive medical checkup in 56 of the 75 patients (74.7%) with aEE vs. 32 of the 71 patients (45.1%) with EoE. The diagnosis was made based on endoscopy performed for other reasons in the remaining 19 aEE patients (25.3%) and 39 EoE patients (54.9%). The man:woman ratio was 65:10 in the aEE group and 55:16 in the EoE group (p=0.146). The mean age was not significantly different between the aEE and EoE groups (49.4±10.9 vs. 49.4±11.5 years, respectively; p=0.807). The symptoms noted in the EoE group were dysphagia in 34 patients (47.9%), heartburn in 29 (40.8%), food impaction in 29 (40.8%), chest pain in 12 (16.9%), and other symptoms, such as nausea and abdominal discomfort, in 6 (8.5%).
Table 1.
Patients’ Clinicopathological Characteristics.
| aEE (n=75) | EoE (n=71) | p value | ||||
|---|---|---|---|---|---|---|
| Age, years, mean±SD | 49.4±10.9 | 49.4±11.5 | 0.807 | |||
| Gender, male:female, (%) | 65:10, (86.7) | 55:16, (77.4) | 0.146 | |||
| Symptoms, n (%) (duplicates counted) | ||||||
| Dysphagia | - | 34 (47.9) | ||||
| Heartburn | - | 29 (40.8) | ||||
| Chest pain | - | 12 (16.9) | ||||
| Food impaction | - | 29 (40.8) | ||||
| Others | - | 6 (8.5) | ||||
| BMI, kg/m2, mean±SD | 25.1±3.24 | 23.6±3.35 | 0.009* | |||
| Current smoking, n (%) | 12/59 (20.3) | 17/51 (33.3) | 0.123 | |||
| Brinkman Index | 57.4±167.9 | 124±233.4 | 0.021* | |||
| Daily alcohol use, n (%) | 26/59 (44.1) | 24/51 (47.1) | 0.353 | |||
| Concurrent allergic disease, n (%) (duplicates counted) | 38/65 (37.4) | 55/66 (83.3) | 0.002* | |||
| Allergic rhinitis | 19 (29.2) | 25 (38.5) | 0.295 | |||
| Bronchial asthma | 10 (15.4) | 16 (24.6) | 0.204 | |||
| Atopic dermatitis | 8 (12.3) | 14 (21.5) | 0.173 | |||
| Food or other allergy | 10 (15.4) | 14 (21.5) | 0.389 | |||
| Peripheral blood eosinophil, /μL, mean±SD | 30.3±15.92 | 30.7±20.73 | 0.139 | |||
| Non-specific IgE, IU/mL, mean±SD | 297.4±649.9 | 437.6±596.1 | 0.720 | |||
| Histological findings, EoEHSS grade score, mean±SD | ||||||
| EI | 3.36±0.056 | 3.44±0.060 | 0.294 | |||
| BZH | 2.49±0.083 | 2.56±0.078 | 0.576 | |||
| DIS | 2.64±0.060 | 2.69±0.066 | 0.639 | |||
| LPF | 1.23±0.152 | 1.31±0.160 | 0.723 | |||
| EA | 0.41±0.080 | 0.41±0.075 | 0.976 | |||
| SL | 1.27±0.076 | 0.39±0.094 | 0.357 | |||
| SEA | 0.84±0.092 | 0.81±0.110 | 0.882 | |||
| DEC | 0.21±0.078 | 0.30±0.092 | 0.785 | |||
| PEC, eos/hpf, mean±SD | 61.2±44.1 | 68.4±52.0 | 0.094 | |||
| Observation period, months, mean±SD | 25.5±24.8 | 31.0±26.9 | 0.201 |
*p<0.05
aEE: asymptomatic esophageal eosinophilia, BMI: body mass index, EE: esophageal eosinophilia, EoE: eosinophilic esophagitis, EoEHSS: eosinophilic esophagitis histologic scoring system, EI: eosinophilic inflammation, BZH: basal cell hyperplasia, DIS: dilated intercellular spaces, LPF: lamina propria fibrosis, EA: eosinophilic abscess, SL: eosinophil surface layering, SEA: surface epithelial alteration, DEC: dyskeratotic epithelial cells, PEC: peak eosinophil count
There was no significant difference between the aEE and EoE groups with regard to current smoking status [20.3% (12/75) vs. 33.3% (17/71); p=0.123] or daily alcohol consumption [44.1% (26/75) vs. 47.1% (24/71); p=0.353]. However, the aEE group had a significantly higher body mass index (BMI) (25.1±3.24 vs. 23.6±3.35 kg/m2, respectively; p<0.01) and significantly lower frequency of concurrent allergic disease, such as allergic rhinitis, bronchial asthma, atopic dermatitis, and food or drug allergy (37.4% vs. 83.3%, respectively; p<0.01), than the EoE group.
There was no significant difference between the aEE and EoE groups with regard to the blood count or serum biochemical findings: the mean peripheral blood eosinophil count was 30.3±15.9/μL and 30.7±20.7/μL, respectively (p=0.139), and the mean non-specific immunoglobulin E (IgE) was 297.4±649.9 IU/mL and 437.6±596.1 IU/mL, respectively (p=0.720).
Histologically, the peak eos/hpf value tended to be lower in the aEE group than in the EoE group, but this was not a significant difference (61.2±44.1 vs. 68.4±52.0, respectively; p=0.094). Regarding EoEHSS, there was no significant difference between the two groups in any of the evaluation items.
Endoscopic findings
Table 2 shows the endoscopic findings for the two groups. The prevalence of typical endoscopic findings of EoE did not significantly differ between the aEE and EoE groups: 90.7% vs. 91.5% for longitudinal furrows/ridges, 44.0% vs. 52.1% for esophageal rings, and 61.3% vs. 62.0% for white exudate, respectively. The total inflammatory and fibrostenotic score of EREFS was not significantly different between two groups. Furthermore, the location of such endoscopic findings and the phenotype did not differ significantly between the groups, nor did pharyngeal or abdominal findings, such as papilloma and dysplasias, hiatus hernia, esophageal mucosal break, and atrophic gastritis.
Table 2.
Endoscopic Findings and Helicobacter Pylori infection Status.
| aEE(n=75) | EoE(n=71) | p value | ||||
|---|---|---|---|---|---|---|
| Endoscopic findings, EREFSa | ||||||
| Longitudinal furrows/ridges. n (%) | 1.000 | |||||
| 0 | 7 (9.3) | 6 (8.5) | ||||
| 1 | 68 (90.7) | 65 (91.5) | ||||
| Esophageal rings. n (%) | 33 (44) | 37 (52.1) | 0.573 | |||
| 0 | 42 (56) | 34 (47.9) | ||||
| 1 | 31 (41.3) | 33 (46.5) | ||||
| 2 | 2 (2.7) | 3 (4.2) | ||||
| 3 | 0 (0) | 1 (1.4) | ||||
| White exudates. n (%) | 46 (61.3) | 44 (62.0) | 0.943 | |||
| 0 | 29 (38.7) | 27 (38.0) | ||||
| 1 | 42 (56) | 41 (57.7) | ||||
| 2 | 4 (5.3) | 3 (4.2) | ||||
| Stricture. n (%) | 0.433 | |||||
| 0 | 73 (97.3) | 67 (94.4) | ||||
| 1 | 2 (2.7) | 4 (5.6) | ||||
| Edema. n (%) | 1.000 | |||||
| 0 | 8 (10.7) | 7 (9.9) | ||||
| 1 | 67 (89.3) | 64 (90.1) | ||||
| Inflammatory scoreb, median (min, max) | 3 (1-4) | 3 (1-4) | 0.382 | |||
| Fibrostenotic scorec, median (min, max) | 0 (0-3) | 1 (0-4) | 0.875 | |||
| Total scored, median (min, max) | 3 (1-6) | 3 (1-8) | 0.920 | |||
|
Phenotype (Localized)
Location, n (%) |
26 (34.7) | 18 (25.4) | 0.704 | |||
| Ut-Lt | 17 (65.4) | 13 (72.2) | ||||
| Mt-Lt | 7 (26.9) | 3 (16.7) | ||||
| Lt | 2 (7.7) | 2 (11.1) | ||||
|
Phenotype (Diffuse)
Location, n (%) |
49 (65.3) | 53 (74.6) | 0.512 | |||
| Ut-Lt | 7 (14.3) | 5 (9.4) | ||||
| Mt-Lt | 24 (49.0) | 23 (43.4) | ||||
| Lt | 18 (36.7) | 25 (47.2) | ||||
| Other endoscopic findings, n (%) | ||||||
| atrophic gastritis | 12 (16.0) | 11 (15.5) | 0.933 | |||
| reflux esophagitis (LA classification; N-M/A/B) | 72/1/2 | 64/5/2 | 0.222 | |||
| hiatus hernia | 45 (60.0) | 41 (57.7) | 0.782 | |||
| pharyngeal abnormal findings | 2 (2.7) | 2 (2.8) | 0.963 | |||
| EUS measurement | ||||||
| esophageal wall thickness, mm, mean±SD | 3.78±1.11 | 3.90±1.36 | 0.857 | |||
| combined mucosa and submucosa layer, mm, mean±SD | 2.50±0.733 | 2.67±0.987 | 0.599 | |||
| Helicobacter pylori infection, n (%) | 0.412 | |||||
| current infection | 5 (7.9) | 5 (9.6) | ||||
| past infection | 11 (17.5) | 14 (26.9) | ||||
| negative infection | 47 (74.6) | 33 (63.5) |
*p<0.05
a Maximum score per participant over the three sites.
b The inflammatory score is the sum of the exudate, edema, and furrows scores.
c The fibrostenotic score is the sum of the rings and stricture scores.
d The total score is calculated from the sum of the exudate, edema, furrows, rings, and stricture scores.
aEE: asymptomatic esophageal eosinophilia, EE: esophageal eosinophilia, EoE: eosinophilic esophagitis, LA: Los Angeles, Lt: lower part of the thoracic esophagus, Mt: middle part of the thoracic esophagus, Ut: upper part of the thoracic esophagus
The low number of patients with atrophic gastritis in both groups (12 in the aEE group and 11 in the EoE group) reflects the low prevalence of H. pylori infection in the groups, as 74.6% in the aEE group and 63.5% in the EoE group had no infection.
EUS findings
Six patients in the aEE group and 12 in the EoE group were examined by EUS. There was no significant difference between the aEE and EoE groups in mean thickness of the esophageal wall (3.78 mm vs. 3.90 mm, respectively) or in the mucosal and submucosal layer (2.64 mm vs. 2.39 mm, respectively).
Treatment progression
Fifty-two patients in the aEE group and eight in the EoE group did not agree to treatment and requested follow-up. After the initial esophageal biopsy, the aEE group was observed for a mean period of 25.5 months, and the PPI response was not significantly different between the aEE and EoE groups [50.0% (10/20) vs. 41.2% (21/51)]. In the aEE group, patients treated with PPI had a median total EREFS of 2.5 after treatment and 3.0 before treatment. In contrast, the median total EREFS of aEE patients without PPI treatment was 3.0 at the time of the initial diagnosis and 4.0 after follow-up. Two patients in the same group who were followed without treatment developed esophageal symptoms 43 and 59 months after the diagnosis (2.7%). One of these patients was treated with a PPI and showed clinical improvement.
Six of the 30 PPI-nonresponsive EoE patients received additional treatment with topical corticosteroids, and all showed endoscopic and histological improvements.
Discussion
Some patients with EoE have persistent symptoms, whereas others have intermittent symptoms, remaining asymptomatic between periods of exacerbation (14). Symptoms can persist for a long time (mean, three to five years) in both children and adults before a diagnosis of EoE is reached, especially if the disease appears progressively (14,15). The severity of symptoms does not necessarily correlate with the extent of eosinophilic inflammation, and symptoms alone are insufficient for a diagnosis or assessment of the response to treatment (16,17). It is also suggested that EoEs and aEE display similar immunohistological profiles (18). Thus, aEE and symptomatic EoE may have the same pathogenesis and lie on the same spectrum of disease pathology (19). A younger age and diffuse disease distribution at the first detection in endoscopic findings are considered to be risk factors for progression to symptomatic EE in aEE patients (10,20). Recent review articles have suggested the utility of a symptom-based diagnostic and therapeutic approach for EoEs (21,22).
In the present study, we examined the differences in the clinicopathological features of aEE by comparing symptomatic patients with EoE and asymptomatic patients who had been incidentally diagnosed with EE. Endoscopic findings were assessed based on the EREFS, while pathological findings were assessed based on the EoEHSS, and this study was conducted among a relatively large number of aEE patients compared with previous studies.
The patient characteristics, including the age, sex, current smoking status, and alcohol consumption, were not significantly different between the two groups.
Of note, the BMI was significantly lower in the EoE group than in the aEE group in our study. Obesity is associated with gastroesophageal reflux disease (GERD), which may cause esophageal symptoms but not nonerosive GERD (23-26), and obesity and hiatal hernia have been suggested to be risk factors for EE in Japanese adults (27). However, the BMI tends to be lower in patients with EoE than in healthy controls, and weight loss or a low BMI is considered to be associated with a risk of esophageal remodeling (28). This may suggest that esophageal remodeling, which causes esophageal narrowing and generates symptoms, had occurred in the EoE group but had not yet occurred in the aEE group in this study.
EoE is regarded as an esophageal inflammatory disease associated with atopic diseases (29). Susceptibility to EoE is mediated by multiple genes, which have synergistic effects and include genes of general atopic disease and genes specific to EoE (30). Patients with EoE usually have other concurrent allergic diseases, such as rhinitis, asthma, and atopic dermatitis. Furthermore, IgE-mediated food allergies are common in EoE patients (31). In the present study, there were significantly fewer cases of concurrent allergic disease or food allergy in the aEE group than in the EoE group. In contrast, the peak eos/hpf value in the biopsy specimens from the aEE group tended to be lower than in the EoE group, although not to a significant degree. In the present study, the histological assessment by the EoEHSS grade score showed no significant difference between the two groups. The presence rate of lamina propria fibrosis was 59.6% in the aEE group and 58.2% in the EoE group. There was also no significant difference between the two groups with regard to the mean peripheral blood eosinophil count or mean non-specific IgE concentration, nor was there a significant difference in either the EREFS or location of endoscopic findings, which were considered to reflect the allergic background of both groups. These findings suggest that the endoscopic findings seen in aEE are caused by the same adaptive immune response and pathogenesis as in EoE. Because the signs of fibrosis, such as strictures, rings, esophageal wall thickness, and lamina propria fibrosis, are considered influential on subsequent esophageal stenosis, aEE patients with these signs require careful continuous follow-up observation (21).
Genetic disposition, Th2 lymphocytes, cytokines, and chemokines are considered to cause deterioration of the esophageal epithelial barrier (30-32), so an examination is required to compare the gene expression profile and immunochemical qualitative and quantitative data in aEE and EoE.
When the esophagus is dilating, the average esophageal wall thickness in the esophagus without esophageal diseases is approximately 2 mm, and a wall thickness greater than 5 mm is considered abnormal (33,34). High-resolution EUS has shown that untreated EoE patients have a significantly thicker esophageal wall than healthy controls, and the thickening processes involves individual tissue layers, such as the mucosa, submucosa, and muscularis propria (35). This finding is attributable to the eosinophil infiltration that is present throughout the esophagus, but mainly in the submucosa and muscularis propria (36,37). In our study, EUS revealed no significant difference in the thickness of either the esophageal wall thickness or the mucosal and submucosal layers. This finding may reflect the same level of eosinophil infiltration - and therefore the same pathogenesis - in both groups.
A recent systematic review showed that nearly half of patients with EE responded to PPI administration (38,39). In this study, the remission rate by PPI administration in both patients with aEE and those with EoE was lower than in previous studies. The lower dose of PPI may therefore affect the low remission rate in comparison to the findings published in previous studies. However, the fact that there was no significant difference in responsiveness to PPI treatment between the two groups suggests that aEE may have the same pathogenic mechanism underlying the PPI response as EoE.
In the present study, only 2 patients in the aEE group developed symptoms (2.7%), with an average of 51 months passing until the symptom onset. This finding was likely due to most of the asymptomatic patients not wishing to be treated and the inability to observe them over the long term. To elucidate the natural history of aEE, long-term follow-up of asymptomatic patients is necessary.
Several limitations associated with the present study warrant mention. First, it was a single-center study with a limited sample size and retrospective design. Second, the biopsy sites were not the same among patients. A histological examination of EoE showed that EE is irregular and can vary between the distal and proximal esophagus, and the current recommendation is to perform at least six biopsies of two different sites, typically from the distal and proximal esophagus (14,40). Our variation from this recommendation may have affected the histopathological findings. Third, we did not consider the long-term outcomes and subsequent course of treatment. A larger scale prospective randomized controlled trial is needed to verify the natural history of aEE and EoE.
In conclusion, the results of this study suggest that aEE with typical endoscopic findings of EoE may have the same pathogenesis as EoE. Confirmation of these results is warranted in a prospective study.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology 72: 1312-1316, 1977. [PubMed] [Google Scholar]
- 2. Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology 74: 1298-1301, 1978. [PubMed] [Google Scholar]
- 3. Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 38: 109-116, 1993. [DOI] [PubMed] [Google Scholar]
- 4. Furuta K, Adachi K, Kowari K, et al. A Japanese case of eosinophilic esophagitis. J Gastroenterol 41: 706-710, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol 7: 1055-1061, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE Conference. Gastroenterology 155: 1022-1033, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lucendo AJ, Molina-Infante J, Arias Á, et al. Guidelines on eosinophilic esophagitis: evidencebased statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J 5: 335-358, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molina-Infante J, Lucendo J. Eosinophilic esophagitis: a practical approach to diagnosis and management. Expert Rev Gastroenterol Hepatol 8: 925-934, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 62: 489-495, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Dellon ES, Cotton CC, Gebhart JH, et al. Accuracy of the eosinophilic esophagitis endoscopic reference score in diagnosis and determining response to treatment. Clin Gastroenterol Hepatol 14: 31-39, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kon T, Abe Y, Sasaki Y, et al. Clinical features of esophageal eosinophilia according to endoscopic phenotypes. Intern Med 59: 2971-2979, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 111: 85-92, 1996. [DOI] [PubMed] [Google Scholar]
- 13. Collins MH, Martin LJ, Alexander ES, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus 30: 1-8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 128: 3-20, 2011. [DOI] [PubMed] [Google Scholar]
- 15. Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 145: 1230-1236, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Pentiuk S, Putnam PE, Collins MH, Rothenberg ME. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 48: 152-160, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alexander JA, Jung KW, Arora AS, et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 10: 742-749, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Kitamura H, Tanaka F, Nadatani Y, et al. Eosinophilic esophagitis and asymptomatic esophageal eosinophilia display similar immunohistological profiles. J Clin Biochem Nutr 68: 246-252, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishimura N, Sumi S, Okada M, et al. Is Asymptomatic esophageal eosinophilia the same disease entity as eosinophilic esophagitis? Clin Gastroenterol Hepatol 17: 1405-1407, 2019. [DOI] [PubMed] [Google Scholar]
- 20. Ishibashi F, Fukushima K, Onizuka R, Tanaka R. Risk of progression to eosinophilic esophagitis in patients with asymptomatic esophageal eosinophilia: a retrospective pilot study. JGH Open 4: 422-428, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schreiner P, Biedermann L, Greuter T, Wright BL, Straumann A. How to approach adult patients with asymptomatic esophageal eosinophilia. Dis Esophagus 34: 1-7, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujiwara Y. Symptom-based diagnostic approach for eosinophilic esophagitis. J Gastroenterol 55: 833-845, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moki F, Kusano M, Mizuide M, et al. Association between reflux oesophagitis and features of the metabolic syndrome in Japan. Aliment Pharmacol Ther 26: 1069-1075, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Iwakiri K, Kinoshita Y, Habu Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol 51: 751-767, 2016. [DOI] [PubMed] [Google Scholar]
- 25. Nilsson M, Lagergren J. The relation between body mass and gastro-oesophageal reflux. Best Pract Res Clin Gastroenterol 18: 1117-1123, 2004. [DOI] [PubMed] [Google Scholar]
- 26. Chua CS, Lin YM, Yu FC, et al. Metabolic risk factors associated with erosive esophagitis. J Gastroenterol Hepatol 24: 1375-1379, 2009. [DOI] [PubMed] [Google Scholar]
- 27. Tanaka F, Fukumoto S, Morisaki T, et al. Obesity and hiatal hernia may be non-allergic risk factors for esophageal eosinophilia in Japanese adults. Esophagus 16: 309-315, 2019. [DOI] [PubMed] [Google Scholar]
- 28. Wolf WA, Piazza NA, Gebhart JH, et al. Association between body mass index and clinical and endoscopic features of eosinophilic esophagitis. Dig Dis Sci 62: 143-149, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirano I. 2015 David Y. Graham lecture: the first two decades of eosinophilic esophagitis-from acid reflux to food allergy. Am J Gastroenterol 111: 770-776, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin LJ, He H, Collins MH, Abonia JP, Biagini Myers J, Ebyet M, et al. Eosinophilic esophagitis (EoE) genetic susceptibility is mediated by synergistic interactions between EoE-specific and general atopic disease loci. J Allergy Clin Immunol 141: 1690-1698, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gomez Torrijos E, Gonzalez-Mendiola R, Alvarado M, et al. Eosinophilic esophagitis: review and update. Front Med (Lausanne) 5: 247, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clayton F, Peterson K. Eosinophilic esophagitis: pathophysiology and definition. Gastrointest Endosc Clin N Am 28: 1-14, 2018. [DOI] [PubMed] [Google Scholar]
- 33. Xia F, Mao J, Ding J, Yang H. Observation of normal appearance and wall thickness of esophagus on CT images. Eur J Radiol 72: 406-411, 2009. [DOI] [PubMed] [Google Scholar]
- 34. Desai RK, Tagliabue JR, Wegryn SA, Einstein DM. CT evaluation of wall thickening in the alimentary tract. Radiographics 11: 771-783, 1991. [DOI] [PubMed] [Google Scholar]
- 35. Fox VL, Nurko S, Teitelbaum JE, Furuta GT. High-resolution EUS in children with eosinophilic “allergic” esophagitis. Gastrointest Endosc 57: 30-36, 2003. [DOI] [PubMed] [Google Scholar]
- 36. Stevoff C, Rao S, Parsons W, Kahrilas PJ, Hirano I. EUS and histopathologic correlates in eosinophilic esophagitis. Gastrointest Endosc 54: 373-377, 2001. [DOI] [PubMed] [Google Scholar]
- 37. Yamabe A, Irisawa A, Shibukawa G, et al. Clinical effects of eosinophilic esophagitis observed using endoscopic ultrasound. Clin J Gastroenterol 7: 305-309, 2014. [DOI] [PubMed] [Google Scholar]
- 38. Lucendo AJ, Arias A, Molina-Infante J. Efficacy of proton pumpinhibitor drugs for inducing clinical and histologic remission in patientswith symptomatic esophageal eosinophilia: a systematic review andmeta-analysis. Clin Gastroenterol Hepatol 14: 13-22, 2016. [DOI] [PubMed] [Google Scholar]
- 39. Ishimura N, Kinoshita Y. Eosinophilic esophagitis in Japan: focus on response to acid suppressive therapy. J Gastroenterol Hepatol 33: 1016-1022, 2018. [DOI] [PubMed] [Google Scholar]
- 40. Kim HP, Vance RB, Shaheen NJ, Dellon ES. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol 10: 988-996, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]