Abstract
Comprehensive estimates of vaccination coverage and timeliness of vaccine receipt among American Indian/Alaska Native (AI/AN) children in the United States are lacking. This study’s objectives were to quantify vaccination coverage and timeliness, as well as the proportion of children with specific undervaccination patterns, among AI/AN and non-Hispanic White (NHW) children ages 0–24 months in Montana, a large and primarily rural U.S. state. Data from Montana’s immunization information system (IIS) for children born 2015–2017 were used to calculate days undervaccinated for all doses of seven recommended vaccine series. After stratifying by race/ethnicity, up-to-date coverage at key milestone ages and the proportion of children demonstrating specific patterns of undervaccination were reported. Among n = 3,630 AI/AN children, only 23.1% received all recommended vaccine doses on-time (i.e., zero days undervaccinated), compared to 40.4% of n = 18,022 NHW children (chi-square p < 0.001). A greater proportion of AI/AN children were delayed at each milestone age, resulting in lower overall combined 7-vaccine series completion, by age 24 months (AI/AN: 56.6%, NHW: 64.3%, chi-square p < 0.001). As compared with NHW children, a higher proportion of AI/AN children had undervaccination patterns suggestive of structural barriers to accessing immunization services and delayed starts to vaccination. More than three out of four AI/AN children experienced delays in vaccination or were missing doses needed to complete recommended vaccine series. Interventions to ensure on-time initiation of vaccine series at age 2 months, as well initiatives to encourage completion of multi-dose vaccine series, are needed to reduce immunization disparities and increase vaccination coverage among AI/AN children in Montana.
Keywords: American Indian/Alaska Native, Childhood immunization, Undervaccination, Timeliness, Schedule, Health equity, Immunization information system (IIS)
Abbreviations: ACIP, Advisory Committee on Immunization Practices; AI/AN, American Indian/Alaska Native; CI, Confidence interval; DTaP, Diphtheria, tetanus, acellular pertussis; HepB, Hepatitis B; Hib, Haemophilus influenzae type b; IHS, Indian Health Service; IIS, Immunization Information System; IPV, Inactivated poliovirus vaccine; I/T/U, Indian Health Service/Tribal/Urban Indian health services; MMR, Measles, mumps, and rubella; MSA, Metropolitan statistical area; NHW, non-Hispanic White; NIS, National Immunization Survey; NVAC, National Vaccine Advisory Committee; PCV, Pneumococcal conjugate vaccine; UTD, Up-to-date; VAR, Varicella; VPD, Vaccine Preventable Disease
1. Introduction
Early childhood vaccinations are among the greatest public health achievements and are a cornerstone of preventive medicine (CDC, 1999, CDC, 2011). As recently highlighted by the COVID-19 pandemic, and throughout history, American Indian and Alaska Native (AI/AN) populations have been disproportionately affected by infectious disease outbreaks compared to other U.S. racial and ethnic groups (Hatcher et al., 2020, Singleton et al., 2006, Singleton et al., 2009, Williamson et al., 2021). AI/AN children have experienced higher rates of hospitalizations and severe sequelae associated with infections of vaccine preventable diseases (VPDs) (Holman et al., 2003, Holman et al., 2011, Murphy et al., 2008, Wenger et al., 2010). Health disparities have persisted for five centuries due to social, economic, and political conditions perpetuated by European colonization (Jones, 2006). Historical, environmental, and social determinants among AI/AN populations have led to a higher prevalence of well-documented risk factors for undervaccination, such as lower socioeconomic status, lower education levels, and household crowding (Favin et al., 2012, Rainey et al., 2011, Strine et al., 2003). For these reasons, ensuring immunization equity among AI/AN populations is of critical importance to decrease risk of VPDs and to better promote the health and sustain the strengths of AI/AN communities.
AI/AN pediatric health is an understudied research area, and immunization coverage estimates are varied and conflicting. The U.S Centers for Disease Control and Prevention (CDC) monitors vaccination coverage through the annual National Immunization Survey-Child (NIS-Child), a nationally representative annual telephone survey of caregivers of age-eligible children, followed by provider verification of a child’s immunization history (CDC, 2020, Hill et al., 2021, Smith et al., 2001, Wolter et al., 2017). Previous studies analyzing NIS-Child data show that vaccination coverage was slightly lower among AI/AN children nationally compared to non-AI/AN children between 1998 and 2004, though not all differences were statistically significant (Groom et al., 2008, Strine et al., 2003). Between 2005 and 2010 the coverage gap narrowed due to increased vaccination rates among AI/AN children and decreasing rates among comparison populations, including non-Hispanic White (NHW) children (Groom et al., 2012, Groom et al., 2008). However, when NIS-Child data were stratified by geographic regions with the greatest population percentages of AI/AN children for the years 1998–2000 and 2006–2010, national vaccination coverage estimates indicated vaccine uptake was higher among AI/AN children compared to non-AI/AN children (CDC, 2003, Groom et al., 2012, Strine et al., 2003). In contrast, a more recent study analyzing detailed state immunization registry data found racial disparities in vaccination coverage in the U.S. state of North Dakota, with AI/AN children being significantly less up-to-date at key milestone ages compared to NHW children (Woinarowicz and Howell, 2020).
Immunization data sources have different strengths and limitations. While NIS-Child data are valuable for monitoring trends in national- and state-level vaccine uptake, small survey sample sizes of AI/AN children have resulted in large variances in coverage estimates. Studies analyzing NIS-Child data have been unable to describe the true magnitude of disparities due to this uncertainty, because the data collection methodology was not designed to provide precise estimates for population subgroups within geographic regions (Smith et al., 2001). Additionally, Indian Health Service (IHS), Tribal, and Urban Indian (I/T/U) immunization programs’ performance reports do not include AI/AN children accessing some or all health services outside of I/T/U facilities. The majority of AI/AN peoples reside in urban areas, and may not be near an I/T/U facility (Castor et al., 2006). However, there are new opportunities to realize the benefit of centralized state immunization information systems (IIS) to evaluate vaccination coverage by race/ethnicity at the state level. IIS are confidential, population-based databases that collect and consolidate immunization records from participating providers (CDC, 2013, Murthy et al., 2017). IIS facilitate coordination of care between multiple immunization providers and can be used to identify sub-populations with low vaccine coverage (Groom et al., 2015).
To inform initiatives to improve immunization equity and increase vaccine coverage for racial and ethnic minority populations, we evaluated Montana IIS data for children born 2015–2017. American Indians and Alaska Natives are the second largest racial group in Montana, comprising 6.7% of the state’s population (Census, 2021). This study’s objectives were to quantify and compare vaccine timeliness, recommended immunization schedule adherence, and the prevalence of specific undervaccination patterns among AI/AN and NHW children ages 0–24 months.
2. Methods
2.1. Data source
Our data source was a limited dataset from the Montana state IIS, ImMTrax, which compiles records of administered vaccines from immunization providers, including I/T/U facilities, in one secure database. While provider participation is not required by state law, over 90% of clinics that provide immunization services to young children in Montana use ImMTrax.
This current study builds from previous research by Newcomer et al., which described vaccine timeliness and undervaccination patterns overall among Montana children ages 0–24 months (Newcomer et al., 2021). While this current study used the same dataset, it is distinct in at least two ways. First, in this current study we investigated immunization uptake stratified by race/ethnicity to determine if immunization disparities were present. Given the limited previous work on early childhood vaccination coverage in AI/AN populations in the U.S., this study’s specific emphasis on racial/ethnic immunization disparities offers a unique contribution to the literature. Second, in this study, we conducted new analyses of up-to-date vaccination status by milestone ages to determine when children fall behind schedule, and for which vaccines.
The University of Montana Institutional Review Board approved this study under the exempt category of review.
2.2. Study cohort
The study population included Montana children born in 2015–2017 with at least one vaccine recorded in ImMTrax on or after their 1st birthday. While ImMTrax includes all administered vaccines reported by immunization providers, we excluded children who only had vaccine records in their first year of life. This exclusion criterion was applied because children are not age-eligible to receive two vaccines (measles, mumps, rubella (MMR) and varicella) until their 1st birthday. Since ImMTrax does not have a mechanism for tracking children who move out of state, requiring at least one immunization visit after the 1st birthday ensured that all children in the study cohort had the opportunity to receive MMR and varicella vaccines. Children with only influenza vaccinations or other data abnormalities, such as records of vaccines not approved for children by the Advisory Committee on Immunization Practices (ACIP), were excluded from analyses (Newcomer et al., 2021).
2.3. Race variable definitions
Race and ethnicity were self-reported by the parent or guardian and documented in ImMTrax by health care providers. Races and ethnicities of Montana children were classified into four groups: (1) AI/AN, all ethnicities, (2) non-Hispanic, White, (3) other known race/ethnicity and, (4) missing race/ethnicity. Due to small sample sizes, children who identified as Black, Asian, Native Hawaiian or Pacific Islander or Hispanic ethnicity (non-AI/AN) were combined into one collapsed category of other known race/ethnicity. Some children were missing race/ethnicity data. Therefore, to determine if coverage estimates differed for children with missing race/ethnicity, we included children with missing race/ethnicity data as a distinct category in some analyses. Additionally, missing race/ethnicity was evaluated by clinic type at which vaccines were administered.
2.4. Vaccination coverage and series completion
We evaluated coverage for all vaccines in the combined 7-vaccine series: four doses of diphtheria, tetanus, and acellular pertussis (DTaP), three doses of poliovirus (IPV), one dose of MMR, three doses of hepatitis B (HepB), three or four doses of Haemophilus influenzae type b (Hib) depending on brand, one dose of varicella (VAR), and four doses of pneumococcal conjugate vaccine (PCV). The ACIP recommends all doses in the combined 7-vaccine series be completed by age 19 months; the CDC monitors combined 7-vaccine series completion by age 24 months at national, state, and some local levels (Hill et al., 2020, Hill et al., 2021). We reported the number and percentage of children who completed the combined 7-vacccine series by age 24 months by race/ethnicity. Chi-square tests were conducted to determine statistically significant differences between AI/AN and NHW children.
2.5. Timeliness of vaccine receipt
Timeliness of vaccine receipt was assessed using two metrics: days undervaccinated and average days undervaccinated. Days undervaccinated is defined as the difference between the child’s age in days when the vaccine dose should have been administered per the ACIP schedule and the age in days when the dose was actually received (Luman et al., 2005). For each vaccine dose, the days undervaccinated count was initiated one month after the dose was recommended. Average days undervaccinated was calculated by summing all days undervaccinated across all recommended doses in the combined 7-vaccine series and dividing by seven vaccines, thus representing the average number of days a child was undervaccinated across series (Glanz et al., 2013b). Based on these calculations, children were categorized as having received all doses on-time (zero days undervaccinated); received all doses, but not all on-time (≥1 day undervaccinated but completed all series); or missing some or all doses by age 24 months (≥1 day undervaccinated but did not complete all series). The number and percentage of children within each of these three categories were reported for each individual vaccine series as well as for the combined 7-vaccine series overall, and compared using chi-square tests.
Up-to-date vaccination coverage was evaluated at six milestone ages: 3, 5, 7, 16, 19, and 25 months (Table 1). These milestones represent one month after doses were recommended by the ACIP, as the recommended schedule includes multiple doses at 2, 4, 6, 12–15, and 15–18 months. CDC monitors series completion by age 24 months, so up-to-date coverage was measured through and including 24 months.
Table 1.
Number of total doses administered in each vaccine series by key milestone ages.
|
Age |
||||||
|---|---|---|---|---|---|---|
| Vaccine | 3 monthsa | 5 monthsb | 7 monthsc | 16 monthsd | 19 monthse | 25 monthsf |
| HepB | 1 dose | 2 doses | 2 doses | 2 doses | 3 doses | 3 doses |
| DTaP | 1 dose | 2 doses | 3 doses | 3 doses | 4 doses | 4 doses |
| Hib | 1 dose | 2 doses | ≥ 2 doses | ≥ 3 doses | ≥3 doses | ≥3 doses |
| PCV | 1 dose | 2 doses | 3 doses | 4 doses | 4 doses | 4 doses |
| IPV | 1 dose | 2 doses | 2 doses | 2 doses | 3 doses | 3 doses |
| MMR | — | — | — | 1 dose | 1 dose | 1 dose |
| VAR | — | — | — | 1 dose | 1 dose | 1 dose |
| All series up-to-date | 5 doses | 10 doses | ≥12 doses | ≥16 doses | ≥19 doses | ≥19 doses |
ACIP, Advisory Committee on Immunization Practices; HepB, hepatitis B; DTaP, diphtheria-tetanus-acellular pertussis; Hib, Haemophilus influenzae type b; PCV, pneumococcal conjugate; IPV, poliovirus; MMR, measles-mumps-rubella; VAR, varicella.
3 months: one dose each of HepB, DTaP, Hib, PCV, and IPV. Per ACIP recommendations, two doses of HepB are recommended by 3 months. Similar to previous studies, we assessed timeliness of HepB vaccination similar to DTaP and IPV, since HepB is frequently administered as part of a combination DTaP-IPV-HepB combination vaccine (Pediarix, GlaxoSmithKline) (Daley et al., 2021; Glanz et al., 2013b; Newcomer et al., 2021).
5 months: two doses each of HepB, DTaP, Hib, PCV, and IPV.
7 months: three doses each of DTaP and PCV; two or three doses of Hib (depending on brand); two doses of HepB and IPV are considered up to date at this milestone.
16 months: four doses of PCV; three or four doses of Hib (depending on brand); three doses of DTaP; two doses of HepB and IPV; one dose each of MMR and VAR.
19 months: four doses each of PCV and DTaP; three or four doses of Hib (depending on brand); three doses of HepB and IPV; one dose each of MMR and VAR.
25 months: four doses each of PCV and DTaP; three or four doses of Hib (depending on brand); three doses of HepB and IPV; one dose each of MMR and VAR.
2.6. Undervaccination patterns
Seven patterns of undervaccination were evaluated hierarchically such that children were only reported within one category (Newcomer et al., 2021). First, children with (1) no days undervaccinated (received all vaccine doses in the combined 7-vaccine series on-time) were identified and considered to be vaccinated per the ACIP schedule. Recent studies have shown the rate of parental vaccine refusals, choices to delay, and decisions to follow alternative schedules have increased, and vaccine confidence is associated with schedule compliance (Daley et al., 2021, Freeman et al., 2022, Glanz et al., 2013b, Glanz et al., 2016, Hargreaves et al., 2020, Hough-Telford et al., 2016, Nadeau et al., 2015, Robison et al., 2012, Smith et al., 2010). Therefore, previously validated undervaccination patterns indicative of parental hesitancy were identified next and classified as: (2) restrictive shot-limiting (≥6 visits with ≤ 3 vaccines at each visit), (3) episodic shot-limiting (≥1 visit(s) with ≤ 2 vaccines before age 15 months and ≤ 2 visits with ≥ 3 vaccines administered), or (4) selective vaccination (did not start ≥ 1 vaccine series). For evaluating the restrictive and episodic shot-limiting patterns, all vaccines, including oral rotavirus vaccines, influenza, Hepatitis A, and the vaccines in the combined 7-vaccine series were considered. Combination vaccines were considered as one “shot.” Next, children who received no vaccinations before age three months were identified as a (5) delayed start to vaccination; this undervaccination pattern could be indicative of either parental choice or other barriers limiting timely access to immunization services (Glanz et al., 2013b, Newcomer et al., 2021). Finally, patterns indicative of structural barriers to accessing immunization services included: (6) initiated all series, but missing doses (≥1 dose missing by age 24 months) and (7) completed all doses, but some or all late (≥1 day undervaccinated, but all series were completed by age 24 months).
3. Statistical analyses
Demographics and characteristics of the study cohort were described as well as vaccination coverage measures by age 24 months. In addition to race/ethnicity, demographic variables included sex, birth year, and whether the child accessed immunization clinics within a metropolitan statistical area (MSA) or outside an MSA, which is considered rural (HHS, 2020). The number and percentage of children receiving vaccines were reported by race/ethnicity for each vaccine type as well as for the combined 7-vaccine series. Chi-square tests were conducted to evaluate statistical significance.
Vaccine timeliness and patterns of undervaccination were evaluated by race/ethnicity. The number and percentage of children who received all vaccine doses on-time, received all doses by age 24 months but not all on-time, and those who did not complete all series by 24 months were calculated. Up-to-date vaccination coverage was calculated at key milestone ages for AI/AN and NHW children. The number and percentage of children in each undervaccination pattern were reported. A two-sided significance level of 0.05 was used for all statistical tests. All analyses were conducted using SAS 9.4 (Cary, NC).
4. Results
Among the study population of N = 31,422 children, 11.6% were AI/AN (n = 3,630), 57.4% were NHW (n = 18,022), 3.8% were identified as other known race/ethnicity (n = 1,178), and 27.3% had missing race/ethnicity data (n = 8,592) (Table 2). The distribution of sex was balanced across racial groups. The majority of AI/AN children (65.9%) as well as NHW children (57.8%) accessed immunization services outside of MSAs. Among all vaccines administered at I/T/U clinics, 2.7% were missing race/ethnicity data. In comparison, 27.8% of all vaccine doses administered at non-I/T/U clinics were missing race/ethnicity data.
Table 2.
Characteristics of study cohort and vaccination coverage by age 24 months for Montana children born 2015–2017, by race/ethnicity.
|
Race/Ethnicitya |
|||||
|---|---|---|---|---|---|
| Characteristic |
Total Study Cohort N = 31,422 (column %) |
American Indian/Alaska Native n = 3,630 (column %) |
Non-Hispanic White n = 18,022 (column %) |
Other Known Race/Ethnicity n = 1,178 (column %) |
Missing Race/Ethnicity n = 8,592 (column %) |
| Sex | |||||
| Female | 15,201 (48.8) | 1800 (49.6) | 8714 (48.4) | 572 (48.6) | 4115 (49.3) |
| Male | 15,946 (51.2) | 1830 (50.4) | 9292 (51.6) | 605 (51.4) | 4219 (50.6) |
| Year of Birth | |||||
| 2015 | 11,371 (36.2) | 1302 (35.9) | 6104 (33.9) | 426 (36.2) | 3539 (41.2) |
| 2016 | 10,944 (34.8) | 1293 (35.6) | 6304 (35.0) | 391 (33.2) | 2956 (34.4) |
| 2017 | 9107 (29.0) | 1035 (28.5) | 5614 (31.2) | 361 (30.7) | 2097 (24.4) |
| Location of immunization clinic accessed | |||||
| Within an MSA | 13,616 (43.3) | 1238 (34.1) | 7602 (42.2) | 659 (55.9) | 4117 (47.9) |
| Not within an MSA | 17,806 (56.7) | 2392 (65.9) | 10,420 (57.8) | 519 (44.1) | 4475 (52.1) |
| Vaccination coverage at 24 months | |||||
| Combined 7-vaccine series completion | 19,580 (62.3) | 2053 (56.6)* | 11,592 (64.3)* | 701 (59.5) | 5234 (60.9) |
| Vaccines in combined 7-vaccine series: | |||||
| Hepatitis B, 3 doses | 28,370 (90.3) | 3330 (91.7)* | 16,346 (90.7)* | 1032 (87.6) | 7662 (89.2) |
| Diphtheria-tetanus-acellular pertussis, 4 doses | 23,783 (75.7) | 2399 (66.1)* | 14,102 (78.3)* | 837 (71.1) | 6445 (75.0) |
| Haemophilus influenzae type b, 3 or 4 dosesb | 24,057 (76.6) | 2791 (76.9) | 14,013 (77.8) | 875 (74.3) | 6378 (74.2) |
| Pneumococcal conjugate, 4 doses | 24,199 (77.0) | 2469 (68.0)* | 14,333 (79.5)* | 871 (73.9) | 6526 (76.0) |
| Poliovirus, 3 doses | 28,229 (89.8) | 3194 (88.0)* | 16,351 (90.7)* | 1023 (86.8) | 7661 (89.2) |
| Measles-mumps-rubella, 1 dose | 28,616 (91.1) | 3263 (89.9)* | 16,522 (91.7)* | 1056 (89.6) | 7775 (90.5) |
| Varicella, 1 dose | 27,951 (89.0) | 3215 (88.6)* | 16,164 (89.7)* | 1031 (87.5) | 7541 (87.8) |
* Statistically significant (p < 0.05) difference between AI/AN children and NHW children. P-values were calculated using chi-square tests.
AI/AN, American Indian/Alaska Native; MSA, metropolitan statistical area.
Children of any ethnicity and identified as AI/AN were included in the study as AI/AN. Other known race or ethnicity included children of Asian, Black, Native Hawaiian and other Pacific Islander race and Hispanic ethnicity.
Depending on brand.
4.1. Vaccination coverage and series completion
Among AI/AN children, 56.6% completed the combined 7-vaccine series by age 24 months, compared to 64.3% among NHW children. Completion of all recommended doses for each vaccine series was significantly lower among AI/AN children compared to NHW children (p < 0.05) with the exception of Hib vaccine series (p = 0.25). Among AI/AN children, 66.1% completed the four-dose DTaP series and 68.0% completed the four-dose PCV series by age 24 months, compared to 78.3% and 79.5%, respectively, of NHW children. Among children with missing race/ethnicity, vaccination coverage was more similar to NHW children than AI/AN children or children of other known race/ethnicity (Table 2).
4.2. Timeliness of vaccine receipt
By age 24 months, only 23.1% of AI/AN children had received all doses on-time for the combined 7-vaccine series; 33.4% received all doses, but some or all were late; and 43.4% were missing some or all doses. Among NHW children, 40.4% received all doses on-time; 23.9% received all doses, but some or all were late; 35.7% were missing some or all doses by age 24 months. Across each of the seven vaccine series, a greater proportion of NHW children received all recommended doses on-time as compared with AI/AN children (Table 3).
Table 3.
Timeliness of vaccination by 24 months among American Indian/Alaska Native and non-Hispanic White Montana children born 2015–2017, by race/ethnicity (n = 21,652).
|
American Indian/Alaska Native n = 3,630 |
Non-Hispanic White n = 18,022 |
||||||
|---|---|---|---|---|---|---|---|
| Vaccine series |
All doses, all on time n (column %) |
All doses, but not all on time n (column %) |
Missing some or all doses n (column %) |
All doses, all on time n (column %) |
All doses, but not all on time n (column %) |
Missing some or all doses n (column %) |
Chi-squarep-value |
| Combined 7-vaccine series | 839 (23.1) | 1214 (33.4) | 1577 (43.4) | 7284 (40.4) | 4308 (23.9) | 6430 (35.7) | < 0.001 |
| Vaccines in combined 7-vaccine series: | |||||||
| Hepatitis B, 3 doses | 2881 (79.4) | 449 (12.4) | 300 (8.3) | 15,436 (85.7) | 910 (5.1) | 1676 (9.3) | < 0.001 |
| Diphtheria-tetanus-acellular pertussis, 4 doses | 1077 (29.7) | 1322 (36.4) | 1231 (33.9) | 10,052 (55.8) | 4050 (22.5) | 3920 (21.8) | < 0.001 |
| Haemophilus influenzae type b, 3 or 4 dosesa | 1536 (42.3) | 1255 (34.6) | 839 (23.1) | 11,243 (62.4) | 2770 (15.4) | 4009 (22.3) | < 0.001 |
| Pneumococcal conjugate, 4 doses | 1252 (34.5) | 1217 (33.5) | 1161 (32.0) | 11,045 (61.3) | 3288 (18.2) | 3689 (20.5) | < 0.001 |
| Poliovirus, 3 doses | 1898 (52.3) | 1296 (35.7) | 436 (12.0) | 13,849 (76.8) | 2502 (13.9) | 1671 (9.3) | < 0.001 |
| Measles-mumps-rubella, 1 dose | 2521 (69.5) | 742 (20.4) | 367 (10.1) | 14,252 (79.1) | 2270 (12.6) | 1500 (8.3) | < 0.001 |
| Varicella, 1 dose | 2473 (68.1) | 742 (20.4) | 415 (11.4) | 13,772 (76.4) | 2392 (13.3) | 1858 (10.3) | < 0.001 |
a Depending on brand.
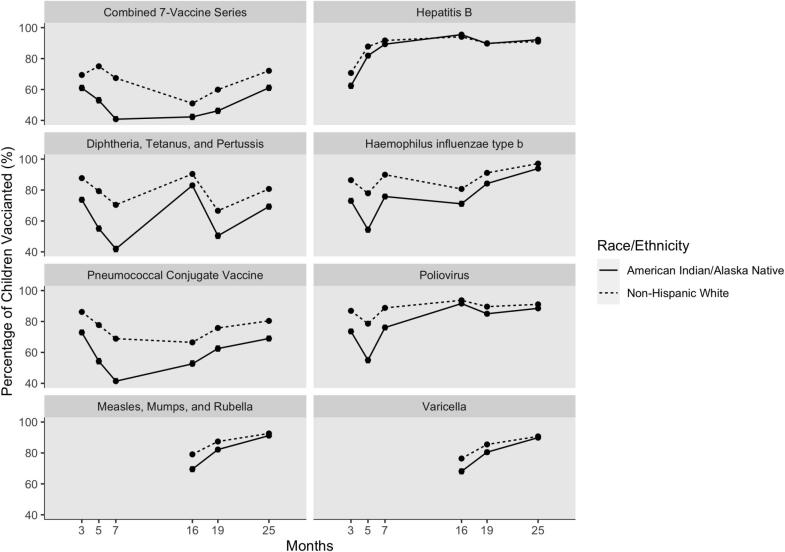
Disparities in immunization coverage existed at each milestone age (Fig. 1), with the greatest disparity demonstrated by DTaP coverage at age seven months (41.9% of AI/AN children up-to-date compared to 70.4% of NHW children). However, across vaccine series, the coverage gap narrowed markedly between the seven month and 16 month milestone ages. For MMR and varicella, which are recommended at age 12 months, the coverage gap also narrowed between ages 16 months and 25 months.
Fig. 1.
Up-to-date vaccination coverage at key milestone ages for American Indian/Alaska Native (n = 3,630) and Non-Hispanic White (n = 18,022) Montana children born 2015–2017.
4.3. Undervaccination patterns
While 23.1% of AI/AN children and 40.4% of NHW children had zero days undervaccinated, the majority of both AI/AN and NHW children were undervaccinated at some point in the first two years of life. Undervaccination patterns indicative of parental vaccine hesitancy were similar across race/ethnicity (AI/AN: 18.4%, NHW: 17.5%). However, 55.3% of AI/AN children had undervaccination patterns indicative of structural barriers to accessing immunization services. In comparison, only 40.7% of NHW children in Montana had undervaccination patterns suggestive of structural barriers. About 30.0% of AI/AN children completed all vaccines doses but were late in receiving some of all doses; on average, these children were one month behind schedule (average days undervaccinated = 35.0 days, SD = 39.2 days). In comparison, NHW children who experienced delays and still completed all doses (21.9%) had a lower average number of days delayed (mean days undervaccinated = 23.8 days, SD = 31.2 days). While a lower overall proportion of the study cohort, delayed starts to vaccination were more common among AI/AN children compared to NHW (AI/AN: 3.3%, NHW: 1.5%) (Table 4).
Table 4.
Proportion of American Indian/Alaska Native and non-Hispanic White Montana children demonstrating specific undervaccination patterns.
|
American Indian/Alaska Native n = 3,630 |
Non-Hispanic White n = 18,022 |
|||
|---|---|---|---|---|
| Pattern | n (column %) |
Average days undervaccinated Mean ± SD |
n (column %) |
Average days undervaccinated Mean ± SD |
| ACIP schedule adherence | ||||
| No days undervaccinated | 839 (23.1) | 0.0 ± 0.0 | 7284 (40.4) | 0.0 ± 0.0 |
| Undervaccination patterns | ||||
| Patterns indicating parental vaccine hesitancy | 666 (18.4) | 297.1 ± 200.1 | 3144 (17.5) | 294.2 ± 213.0 |
| Restrictive shot-limitinga | 54 (1.5) | 211.0 ± 168.3 | 617 (3.4) | 229.3 ± 183.4 |
| Episodic shot-limitingb | 197 (5.4) | 201.5 ± 146.6 | 778 (4.3) | 270.2 ± 189.5 |
| Selective vaccinationc | 415 (11.4) | 353.7 ± 204.9 | 1749 (9.7) | 298.4 ± 190.0 |
| Patterns indicating structural barriers | 2007 (55.3) | 114.0 ± 134.6 | 7327 (40.7) | 66.7 ± 90.2 |
| Initiated all series, but missing doses | 919 (25.3) | 207.5 ± 147.0 | 3381 (18.8) | 116.9 ± 108.8 |
| Completed all doses, but some or all late | 1088 (30.0) | 35.0 ± 39.2 | 3946 (21.9) | 23.8 ± 31.2 |
| Other patterns | ||||
| Delayed startd | 118 (3.3) | 280.2 ± 194.4 | 267 (1.5) | 298.4 ± 190.0 |
ACIP, Advisory Committee on Immunization Practices; AI/AN, American Indian/Alaska Native; SD, standard deviation.
a Restrictive shot-limiting: at least 6 visits with less than or equal to 3 vaccines at each visit.
b Episodic shot-limiting: at least 1 immunization visit with less than or equal to 2 vaccines before age 15 months and no more than 2 immunization visits with greater than or equal to 3 vaccines.
c Selective vaccination: did not start at least one recommended vaccine series.
d Delayed start to vaccination could be indicative of either parental choice or structural barriers accessing immunization services.
Among children with patterns suggestive of parental hesitancy barriers, a slightly greater proportion (14.4%) of AI/AN children still completed the combined 7-vaccine series by age 24 months, while only 9.4% of NHW children with patterns suggestive of parental hesitancy completed all doses of all series. The differences in combined 7-vaccine series completion among children who experienced delayed starts to vaccination were negligible (AI/AN: 25.4%, NHW: 25.1%) (Table 5).
Table 5.
Combined 7-vaccine series completion among American Indian/Alaska Native and non-Hispanic White Montana children, by undervaccination pattern.
|
American Indian/Alaska Native n = 3,630 |
Non-Hispanic White n = 18,022 |
|||
|---|---|---|---|---|
| Pattern | Number of children with pattern | Number that completed the combined 7-vaccine series by age 24 months (row %) | Number of children with pattern | Number that completed the combined 7-vaccine series by age 24 months (row %) |
| ACIP schedule adherence | ||||
| No days undervaccinated | 839 | 839 (100.0) | 7284 | 7284 (100.0) |
| Undervaccination patterns | ||||
| Patterns indicating parental vaccine hesitancy | ||||
| Restrictive shot-limitinga | 54 | 21 (38.9) | 617 | 141 (22.9) |
| Episodic shot-limitingb | 197 | 75 (38.1) | 778 | 154 (19.8) |
| Selective vaccinationc | 415 | 0 (0.0)e | 1749 | 0 (0.0)e |
| Patterns indicating structural barriers | ||||
| Initiated all series, but missing doses | 919 | 0 (0.0)e | 3381 | 0 (0.0)e |
| Completed all doses, but some or all late | 1088 | 1088 (100.0) | 3946 | 3946 (100.0) |
| Other patterns | ||||
| Delayed startd | 118 | 30 (25.4) | 267 | 67 (25.1) |
ACIP, Advisory Committee on Immunization Practices.
Restrictive shot-limiting: at least 6 visits with less than or equal to 3 vaccines at each visit.
Episodic shot-limiting: at least 1 immunization visit with less than or equal to 2 vaccines before age 15 months and no more than 2 immunization visits with greater than or equal to 3 vaccines.
Selective vaccination: did not start at least one recommended vaccine series.
Delayed start to vaccination could be indicative of either parental choice or structural barriers accessing immunization services.
By definition, children who do not initiate a vaccine series or have missing doses will not complete the combined 7-vaccine series.
5. Discussion
In our analysis of Montana’s IIS data, we found disparities in early childhood vaccination coverage, vaccination timeliness, and adherence to the ACIP-recommended schedule in AI/AN children compared to NHW children. While 56.6% of AI/AN children completed the combined 7-vaccine series by age 24 months, only 23.1% of AI/AN children received all vaccine doses by the ages recommended by the ACIP. In comparison, 64.3% of NHW children completed the combined 7-vaccine series by age 24 months, and 40.4% of NHW children received all recommended vaccines doses on-time. Relative to NHW children, more AI/AN children experienced delays to vaccination, and vaccine delays have been shown to be associated with increased VPD risk as well as failure to complete the combined 7-vaccine series (Curran et al., 2016, Glanz et al., 2013a, Newcomer et al., 2021, Phadke et al., 2016, Rane et al., 2021). Lack of vaccine timeliness may be explained by our finding that a greater proportion of AI/AN children demonstrated undervaccination patterns suggestive of structural barriers to accessing immunization services. Our identification of racial/ethnic immunization disparities points to the need for targeted strategies to increase timely vaccination among AI/AN children in Montana.
The milestone analysis completed in this study highlighted vulnerabilities among both AI/AN and NHW communities in Montana; however, AI/AN children fell behind on recommended vaccine doses with greater frequency. Between AI/AN and NHW children, the greatest disparities were in timely vaccination for the DTaP and PCV four-dose series. At age 19 months, only 50.4% of AI/AN and 66.6% of NHW children were up-to-date with four doses of DTaP. Yet at age 16 months, when three doses were considered up-to-date, coverage levels were much higher among both AI/AN children (83.0%) and NHW children (90.4%). Missing doses of the multi-dose DTaP series is associated with increased risk of pertussis (Glanz et al., 2013a, Robison, 2013, Zhao et al., 2017). Results from our milestone analysis highlight opportunities for targeted interventions in Montana, such as initiatives to promote the importance of starting multi-dose immunization series at age two months to increase the likelihood of on-time series completion among AI/AN populations.
Our examination of centralized IIS data allowed for the characterization of vaccination coverage, vaccination timeliness, and undervaccination patterns for racial and ethnic subpopulations. This study’s findings that coverage gaps exist in Montana were consistent with an IIS analysis from neighboring North Dakota, also a large, primarily rural state with AI/AN as the largest racial minority group (Woinarowicz and Howell, 2020). In our study, examination of IIS vaccination records longitudinally allowed us to identify when vaccine delays occurred and barriers to timely vaccination, such as use of alternative vaccination schedules (Daley et al., 2021, Freeman et al., 2022, Glanz et al., 2013b, Hargreaves et al., 2020, Nadeau et al., 2015, Newcomer et al., 2021, Robison et al., 2012). This approach has advantages over standard cross-sectional measures of vaccine coverage that measure the number of doses a child has received by age 24 months. The U.S. National Vaccine Advisory Committee has identified the measurement of timeliness of vaccine administration—an indicator of immunization services quality—as a preventive health priority, and has recommended using state IIS data to monitor vaccine timeliness and coverage with greater geographic precision (NVAC, 2015). While structural barriers to preventive health care services in rural states like Montana are broad, and can include medical provider shortages and transportation challenges, findings from IIS-based analyses can be used to efficiently allocate resources toward improving access to immunization (Groom et al., 2015). Realizing the full potential of IIS data could facilitate more timely and complete vaccination of all U.S. children against VPDs.
5.1. Strengths and limitations
While immunization providers’ reporting rates to ImMTrax are high, there is no state statutory requirement for participation. Therefore, misclassification of vaccination status could be possible due to missing vaccination records. Another related issue is that ImMTrax does not routinely track children with zero vaccine records. While Montana-specific estimates are not available, NIS-Child surveys of children born 2015–2017 estimated that 1.1–1.5% of all U.S. children received zero vaccines by age 24 months (Hill et al., 2021). Additionally, this study was limited to children with ≥ 1 vaccination on or after the 1st birthday. This inclusion criterion may have led to a slight overestimation in vaccination coverage, particularly for vaccine doses given after the 1st birthday. However, requiring ≥ 1 vaccination on or after the 1st birthday likely had a net positive effect on data quality, since it ensured that children vaccinated early in life but with no further vaccine records in ImMTrax (which could occur if a child moves out of state) were not included in analyses. Despite these limitations of the IIS data source, a previous comparison found that vaccination coverage rates measured in ImMTrax were similar to rates measured through NIS-Child, which is considered the gold standard for assessing vaccine coverage at national and state levels in the U.S. (Newcomer et al., 2021).
Missing race/ethnicity data was an additional limitation of our data source. Misclassification of missing race and ethnicity data is a ubiquitous challenge across public health data sources. Federal, state, local, and tribal data systems often do not interface; therefore, ImMTrax serves to facilitate coordination of immunization data. Ultimately, immunization providers may not be comfortable asking about race/ethnicity, or patients may not volunteer their race/ethnicity due to fear of discrimination and medical mistreatment (Shavers et al., 2012). In this study, missing race/ethnicity data were more common in non-I/T/U facilities. Initiatives to improve race/ethnicity data collection and data systems interoperability are needed.
6. Conclusions
The U.S. government has identified eliminating health disparities and promoting health equity across national, state, tribal, and community levels as foundational principles guiding the Healthy People 2030 goals (HHS, 2021). Moreover, the federal trust responsibility to AI/AN peoples confers access to medical and social services to all federally recognized tribes under treaty obligations (Warne and Frizzell, 2014). Assuring immunization equity is not only a moral obligation, but a legal responsibility.
Vaccination coverage and timeliness are key indicators used to evaluate quality of health services delivery (NVAC, 2015). While vaccination coverage and timeliness remain lower among AI/AN children in Montana, understanding disparities in immunization coverage can help to decrease VPD risk. These findings outline actionable steps toward increasing early childhood vaccination coverage rates among children in Montana and better supporting the health and cultural strengths of AI/AN communities.
CRediT authorship contribution statement
Sarah Y. Michels: Conceptualization, Formal analysis, Writing – original draft. Rain E. Freeman: Conceptualization, Formal analysis, Writing – review & editing. Elizabeth Williams: Conceptualization, Writing – review & editing, Supervision. Alexandria N. Albers: Conceptualization, Writing – review & editing. Bekki K. Wehner: Conceptualization, Resources, Writing – review & editing. Annie Rechlin: Conceptualization, Resources, Writing – review & editing. Sophia R. Newcomer: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by a Center for Biomedical Research Excellence award (1P20GM130418) from the National Institute of General Medical Sciences of the National Institutes of Health. The study sponsor was not involved in the study design; data collection, analysis, and interpretation of results; writing of the report; or the decision to submit the report for publication. No potential conflicts of interest were reported by the study authors.
References
- Castor M.L., Smyser M.S., Taualii M.M., Park A.N., Lawson S.A., Forquera R.A. A nationwide population-based study identifying health disparities between American Indians/Alaska Natives and the general populations living in select urban counties. Am. J. Public Health. 2006;96:1478–1484. doi: 10.2105/AJPH.2004.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Impact of vaccines universally recommended for children–United States, 1990–1998. Centers for Disease Control and Prevention. MMWR Morb. Mortal. Wkly Rep. 1999;48:243–248. [PubMed] [Google Scholar]
- CDC Vaccination coverage levels among Alaska Native children aged 19–35 months–National Immunization Survey, United States, 2000–2001. Centers for Disease Control and Prevention. MMWR Morb. Mortal. Wkly Rep. 2003;52:710–713. [PubMed] [Google Scholar]
- CDC Ten great public health achievements–United States, 2001–2010. Centers for Disease Control and Prevention. MMWR Morb. Mortal. Wkly. Rep. 2011;60:619–623. [PubMed] [Google Scholar]
- CDC Progress in immunization information systems - United States, 2012. Centers for Disease Control and Prevention. MMWR Morb. Mortal. Wkly. Rep. 2013;62:1005–1008. [PMC free article] [PubMed] [Google Scholar]
- CDC . NORC at the University of Chicago; 2020. National Immunization Survey-Child: a user’s guide for the 2019 public-use data file. [Google Scholar]
- Curran D., Terlinden A., Poirrier J.E., Masseria C., Krishnarajah G. Vaccine Timeliness: A Cost Analysis of the Potential Implications of Delayed Pertussis Vaccination in the US. Pediatr. Infect. Dis. J. 2016;35:542–547. doi: 10.1097/INF.0000000000001071. [DOI] [PubMed] [Google Scholar]
- Daley M.F., Reifler L.M., Shoup J.A., Narwaney K.J., Kharbanda E.O., Groom H.C., Jackson M.L., Jacobsen S.J., McLean H.Q., et al. Temporal Trends in Undervaccination: A Population-Based Cohort Study. Am. J. Prev. Med. 2021;61:64–72. doi: 10.1016/j.amepre.2021.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favin M., Steinglass R., Fields R., Banerjee K., Sawhney M. Why children are not vaccinated: a review of the grey literature. International Health. 2012;4:229–238. doi: 10.1016/j.inhe.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Freeman R.E., Thaker J., Daley M.F., Glanz J.M., Newcomer S.R. Vaccine timeliness and prevalence of undervaccination patterns in children ages 0–19 months, U.S., National Immunization Survey-Child 2017. Vaccine. 2022;40:765–773. doi: 10.1016/j.vaccine.2021.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz J.M., Narwaney K.J., Newcomer S.R., Daley M.F., Hambidge S.J., Rowhani-Rahbar A., Lee G.M., Nelson J.C., Naleway A.L., et al. Association between undervaccination with diphtheria, tetanus toxoids, and acellular pertussis (DTaP) vaccine and risk of pertussis infection in children 3 to 36 months of age. JAMA Pediatr. 2013;167:1060–1064. doi: 10.1001/jamapediatrics.2013.2353. [DOI] [PubMed] [Google Scholar]
- Glanz J.M., Newcomer S.R., Jackson M.L., Omer S.B., Bednarczyk R.A., Shoup J.A., DeStefano F., Daley M.F. White Paper on studying the safety of the childhood immunization schedule in the Vaccine Safety Datalink. Vaccine. 2016;34(Suppl 1):A1–a29. doi: 10.1016/j.vaccine.2015.10.082. [DOI] [PubMed] [Google Scholar]
- Glanz J.M., Newcomer S.R., Narwaney K.J., Hambidge S.J., Daley M.F., Wagner N.M., McClure D.L., Xu S., Rowhani-Rahbar A., et al. A Population-Based Cohort Study of Undervaccination in 8 Managed Care Organizations Across the United States. JAMA Pediat. 2013;167:274–281. doi: 10.1001/jamapediatrics.2013.502. [DOI] [PubMed] [Google Scholar]
- Groom A.V., Santibanez T.A., Bryan R.T. Vaccination Coverage Among American Indian and Alaska Native Children, 2006–2010. Pediatrics. 2012;130:e1592–e1599. doi: 10.1542/peds.2012-1001. [DOI] [PubMed] [Google Scholar]
- Groom A.V., Washington M.L., Smith P.J., Bryan R.T. Underimmunization of American Indian and Alaska Native children. Pediatrics. 2008;121:938–944. doi: 10.1542/peds.2007-1794. [DOI] [PubMed] [Google Scholar]
- Groom H., Hopkins D.P., Pabst L.J., Murphy Morgan J., Patel M., Calonge N., Coyle R., Dombkowski K., Groom A.V., et al. Immunization information systems to increase vaccination rates: a community guide systematic review. J. Public Health Manag. Pract. 2015;21:227–248. doi: 10.1097/PHH.0000000000000069. [DOI] [PubMed] [Google Scholar]
- Hargreaves A.L., Nowak G., Frew P.M., Hinman A.R., Orenstein W.A., Mendel J., Aikin A., Nadeau J.A., McNutt L.-A., et al. Adherence to Timely Vaccinations in the United States. Pediatrics. 2020;145 doi: 10.1542/peds.2019-0783. [DOI] [PubMed] [Google Scholar]
- Hatcher S.M., Agnew-Brune C., Anderson M., Zambrano L.D., Rose C.E., Jim M.A., Baugher A., Liu G.S., Patel S.V., et al. COVID-19 Among American Indian and Alaska Native Persons - 23 States, January 31-July 3, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:1166–1169. doi: 10.15585/mmwr.mm6934e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Census, 2021. Montana population estimates. United States Census Bureau, URL https://www.census.gov/quickfacts/MT (accessed 8.15.2021).
- HHS, 2020. Defining Rural Population. U.S. Department of Health and Human Services. URL https://www.hhs.gov/guidance/document/defining-rural-population (accessed 8.15.2021).
- HHS . U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion; 2021. Healthy People 2030 Framework. URL https://health.gov/healthypeople/about/healthy-people-2030-framework#:~:text=Healthy%20People%202030’s%20overarching%20goals,and%20well%2Dbeing%20of%20all (accessed 8.15.2021) [Google Scholar]
- Hill H.A., Yankey D., Elam-Evans L.D., Singleton J.A., Pingali S.C., Santibanez T.A. Vaccination Coverage by Age 24 Months Among Children Born in 2016 and 2017 - National Immunization Survey-Child, United States, 2017–2019. MMWR Morb. Mortal. Wkly Rep. 2020;69:1505–1511. doi: 10.15585/mmwr.mm6942a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H.A., Yankey D., Elam-Evans L.D., Singleton J.A., Sterrett N. Vaccination Coverage by Age 24 Months Among Children Born in 2017 and 2018 - National Immunization Survey-Child, United States, 2018–2020. MMWR Morb. Mortal. Wkly Rep. 2021;70:1435–1440. doi: 10.15585/mmwr.mm7041a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman R., Curns A., Cheek J., Singleton R., Anderson L., Pinner R. Infectious Disease Hospitalizations Among American Indian and Alaska Native Infants. Pediatrics. 2003;111:E176–E182. doi: 10.1542/peds.111.2.e176. [DOI] [PubMed] [Google Scholar]
- Holman R.C., Folkema A.M., Singleton R.J., Redd J.T., Christensen K.Y., Steiner C.A., Schonberger L.B., Hennessy T.W., Cheek J.E. Disparities in infectious disease hospitalizations for American Indian/ Alaska Native people. Public Health Rep. 2011;126:508–521. doi: 10.1177/003335491112600407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough-Telford C., Kimberlin D.W., Aban I., Hitchcock W.P., Almquist J., Kratz R., O'Connor K.G. Vaccine Delays, Refusals, and Patient Dismissals: A Survey of Pediatricians. Pediatrics. 2016;138 doi: 10.1542/peds.2016-2127. [DOI] [PubMed] [Google Scholar]
- Jones D.S. The persistence of American Indian health disparities. Am. J. Public Health. 2006;96:2122–2134. doi: 10.2105/AJPH.2004.054262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman E.T., Barker L.E., Shaw K.M., McCauley M.M., Buehler J.W., Pickering L.K. Timeliness of Childhood Vaccinations in the United StatesDays Undervaccinated and Number of Vaccines Delayed. JAMA. 2005;293:1204–1211. doi: 10.1001/jama.293.10.1204. [DOI] [PubMed] [Google Scholar]
- Murphy T.V., Syed S.B., Holman R.C., Haberling D.L., Singleton R.J., Steiner C.A., Paisano E.L., Cheek J.E. Pertussis-Associated Hospitalizations in American Indian and Alaska Native Infants. J. Pediatr. 2008;152:839–843. doi: 10.1016/j.jpeds.2007.11.046. [DOI] [PubMed] [Google Scholar]
- Murthy N.R.L., Pabst L., Fiebelkorn A.P., Ng T. Progress in Childhood Vaccination Data in Immunization Information Systems—United States, 2013–2016. MMWR Morb. Mortal. Wkly Rep. 2017;66:1178–1181. doi: 10.15585/mmwr.mm6643a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau J.A., Bednarczyk R.A., Masawi M.R., et al. Vaccinating my way--use of alternative vaccination schedules in New York State. J. Pediatr. 2015;166(1):151–156. doi: 10.1016/j.jpeds.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Newcomer S.R., Freeman R.E., Wehner B.K., Anderson S.L., Daley M.F. Timeliness of Early Childhood Vaccinations and Undervaccination Patterns in Montana. Am. J. Prev. Med. 2021;61:e21–e29. doi: 10.1016/j.amepre.2021.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NVAC Assessing the State of Vaccine Confidence in the United States: Recommendations from the National Vaccine Advisory Committee - Approved by the National Vaccine Advisory Committee on June 9, 2015 [corrected] Public Health Rep. 2015;130:573–595. doi: 10.1177/003335491513000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke V.K., Bednarczyk R.A., Salmon D.A., Omer S.B. Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States: A Review of Measles and Pertussis. JAMA. 2016;315:1149–1158. doi: 10.1001/jama.2016.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey J.J., Watkins M., Ryman T.K., Sandhu P., Bo A., Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: Findings from a systematic review of the published literature, 1999–2009. Vaccine. 2011;29:8215–8221. doi: 10.1016/j.vaccine.2011.08.096. [DOI] [PubMed] [Google Scholar]
- Rane M.S., Rohani P., Halloran M.E. Association of Diphtheria-Tetanus-Acellular Pertussis Vaccine Timeliness and Number of Doses With Age-Specific Pertussis Risk in Infants and Young Children. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.19118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison S.G. Incomplete Early Childhood Immunization Series and Missing Fourth DTaP Immunizations; Missed Opportunities or Missed Visits? ISRN Prev Med. 2013;2013 doi: 10.5402/2013/351540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison S.G., Groom H., Young C. Frequency of Alternative Immunization Schedule Use in a Metropolitan Area. Pediatrics. 2012;130:32–38. doi: 10.1542/peds.2011-3154. [DOI] [PubMed] [Google Scholar]
- Shavers V.L., Fagan P., Jones D., Klein W.M., Boyington J., Moten C., Rorie E. The state of research on racial/ethnic discrimination in the receipt of health care. Am. J. Public Health. 2012;102:953–966. doi: 10.2105/AJPH.2012.300773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton R., Hammitt L., Hennessy T., Bulkow L., DeByle C., Parkinson A., Cottle T.E., Peters H., Butler J.C. The Alaska Haemophilus influenzae type b experience: lessons in controlling a vaccine-preventable disease. Pediatrics. 2006;118:e421–e429. doi: 10.1542/peds.2006-0287. [DOI] [PubMed] [Google Scholar]
- Singleton R., Holve S., Groom A., McMahon B.J., Santosham M., Brenneman G., O’Brien K.L. Impact of Immunizations on the Disease Burden of American Indian and Alaska Native Children. Arch. Pediatr. Adolesc. Med. 2009;163:446–453. doi: 10.1001/archpediatrics.2009.44. [DOI] [PubMed] [Google Scholar]
- Smith P.J., Battaglia M.P., Huggins V.J., Hoaglin D.C., Rodén A., Khare M., Ezzati-Rice T.M., Wright R.A. Overview of the sampling design and statistical methods used in the National Immunization Survey. Am. J. Prev. Med. 2001;20:17–24. doi: 10.1016/s0749-3797(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Smith P.J., Humiston S.G., Parnell T., Vannice K.S., Salmon D.A. The association between intentional delay of vaccine administration and timely childhood vaccination coverage. Public Health Rep. 2010;125:534–541. doi: 10.1177/003335491012500408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strine T.W., Mokdad A.H., Barker L.E., Groom A.V., Singleton R., Wilkins C.S., Chu S.Y. Vaccination coverage of American Indian/Alaska native children aged 19 to 35 months: findings from the National Immunization Survey, 1998–2000. Am. J. Public Health. 2003;93:2046–2049. doi: 10.2105/ajph.93.12.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne D., Frizzell L.B. American Indian health policy: historical trends and contemporary issues. Am. J. Public Health. 2014;104(Suppl 3):S263–S267. doi: 10.2105/AJPH.2013.301682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger J.D., Zulz T., Bruden D., Singleton R., Bruce M.G., Bulkow L., Parks D., Rudolph K., Hurlburt D., et al. Invasive Pneumococcal Disease in Alaskan Children: Impact of the Seven-Valent Pneumococcal Conjugate Vaccine and the Role of Water Supply. Pediatr. Infect. Dis. J. 2010;29:251–256. doi: 10.1097/INF.0b013e3181bdbed5. [DOI] [PubMed] [Google Scholar]
- Williamson L.L., Harwell T.S., Koch T.M., Anderson S.L., Scott M.K., Murphy J.S., Holzman G.S., Tesfai H.F. COVID-19 Incidence and Mortality Among American Indian/Alaska Native and White Persons - Montana, March 13-November 30, 2020. MMWR Morb. Mortal. Wkly Rep. 2021;70:510–513. doi: 10.15585/mmwr.mm7014a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woinarowicz M., Howell M. Comparing vaccination coverage of American Indian children with White children in North Dakota. Public Health. 2020;186:78–82. doi: 10.1016/j.puhe.2020.06.050. [DOI] [PubMed] [Google Scholar]
- Wolter K.K., Smith P.J., Khare M., Welch B., Copeland K.R., Pineau V.J., Davis N. Statistical Methodology of the National Immunization Survey, 2005–2014. Vital Health Stat. 2017;1:1–107. [PubMed] [Google Scholar]
- Zhao Z., Smith P.J., Hill H.A. Missed opportunities for simultaneous administration of the fourth dose of DTaP among children in the United States. Vaccine. 2017;35:3191–3195. doi: 10.1016/j.vaccine.2017.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]