Abstract
Background
Arthroscopic rotator cuff repair using human dermal matrix allograft augmentation has been widely used. We assessed the effect of acellular human dermal matrix augmentation after arthroscopic repair of large rotator cuff tears through a prospective, single-blinded, randomized controlled trial with a long-term follow-up.
Methods
Sixty patients with large-sized rotator cuff tears were randomly assigned to two groups. Patients in the control group underwent arthroscopic rotator cuff repair. Allograft patch augmentation was additionally performed in the allograft group. All patients were subdivided into a complete coverage (CC) group or an incomplete coverage (IC) group according to footprint coverage after cuff repair. Constant and American Shoulder and Elbow Surgeons (ASES) scores were assessed preoperatively and at final follow-up. Magnetic resonance imaging was also performed at the same time to evaluate the anatomical results.
Results
Forty-three patients were followed up for an average of 5.7 years. Clinical scores (Constant and ASES) increased significantly at the last follow-up in both groups. The increase in ASES score in the allograft group was statistically significantly greater than that in the control group. The degree of Constant score improvement did not differ significantly between the two groups. The retear rate was 9.1% in the allograft group, which was significantly lower than that in the control group (38.1%). In the control group, the CC subgroup had a statistically significantly lower retear rate (16.7%) than did the IC subgroup. There were no retear cases in the CC subgroup of the allograft group.
Conclusions
Long-term follow-up of arthroscopic repair of large rotator cuff tears with allograft patch augmentation showed better clinical and anatomical results. Footprint coverage after rotator cuff repair was an important factor affecting the retear rate. If the footprint was not completely covered after rotator cuff repair, allograft patch augmentation may reduce the retear rate.
Keywords: Rotator cuff tear, Arthroscopy, Allograft, Alloderm, Footprint coverage
Rotator cuff disease is one of the most common causes of shoulder pain and dysfunction. There are various methods used for surgical treatment of large rotator cuff tears. Even with appropriate surgical techniques and methods, complete repair may not be achieved in large rotator cuff tears, and retears have been reported in 40% to 90% of cases.1,2,3) Although the clinical scores for pain relief, range of motion, and strength may improve after surgical repair, clinical outcomes can be poor if a repair failed.4,5)
In recent years, there have been many studies to improve rotator cuff healing. Biological agents such as platelet-rich plasma and bone marrow aspiration have been studied and biological enhancement techniques have also attracted great attention.6) Extracellular matrix (ECM) has also been applied either to substitute for or augment rotator cuff repair, and acellular human dermal allografts have been widely used today. Patch augmentation can be attempted to resurface the uncovered footprint and to reinforce the repaired tendon.7) It is believed that the acellular ECM has a three-dimensional protein structure and can serve as a scaffold and a biological stimulus to recruit host cells to a tendon-like matrix.8,9,10)
With regard to the potential benefit of the application of human dermal matrix (HDM) in patients with rotator cuff tears, some studies have suggested that the use of a dermal matrix could improve the outcomes of rotator cuff repairs.7,11) However, most of the early studies regarding the use of HDM for rotator cuff augmentation have faced shortcomings, such as a small sample size, short follow-up, retrospective design, and even a lack of clear inclusion and exclusion criteria.9,12,13,14)
The purpose of this study was to investigate the effectiveness of acellular HDM augmentation for arthroscopic repair of large rotator cuff tears in a prospective, single-blinded, randomized controlled trial with a long-term follow-up. We hypothesized that patients undergoing surgical repair augmented with an allograft patch would demonstrate improvements in clinical and functional outcomes.
METHODS
We conducted this study in compliance with the principles of the Declaration of Helsinki. The protocol of the study and all procedures were reviewed and approved by the Institutional Review Board of Kangnam Sacred Heart Hospital, Hallym University College of Medicine (IRB No. 2013-08-72). This study complied with clinical research practices. All patients provided informed consent prior to their participation after receiving an explanation of the procedure and aims of the study.
Patient Recruitment and Randomization
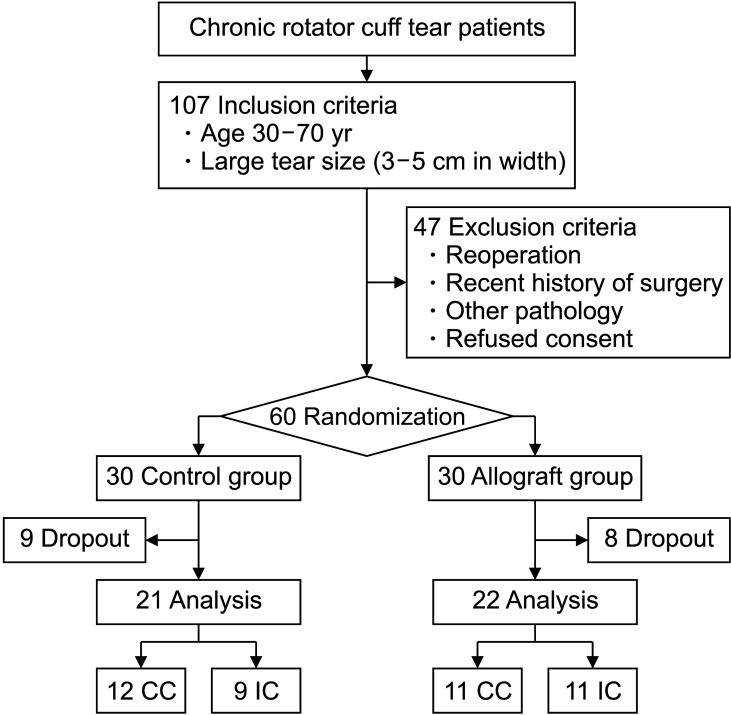
Between October 1, 2013, and February 28, 2015, 107 consecutive patients who failed to respond to conservative management and continued to experience unacceptable pain and weakness in the affected shoulder were enrolled in this study. They were all outpatients of our hospital. The inclusion criteria were patients aged 30–70 years with a large (3–5 cm in width) chronic rotator cuff tear confirmed by preoperative magnetic resonance imaging (MRI). The exclusion criteria were as follows: (1) reoperation due to retear, (2) operation in other body parts within 6 months before and after rotator cuff repair, (3) any other associated shoulder lesion, and (4) other severe medical problems, such as malignancy, respiratory disease, and preexisting coagulopathy. Patients who refused to provide consent were excluded as well (Fig. 1).
Fig. 1. The flowchart of patient enrollment in the study. CC: complete coverage, IC: incomplete coverage.
After exclusion, a total of 60 patients were prospectively and randomly assigned into two groups, each with 30 patients, before rotator cuff surgery. Patients in group 1 (control group) were treated with rotator cuff repair without allograft augmentation, and patients in group 2 (allograft group) underwent surgery with acellular HDM augmentation. A randomization method using blocks of a predetermined size was applied so that the two groups of subjects were balanced. The block size was 4, and each block was randomly composed of two cases in the allograft group and two cases in the control group. Therefore, subjects were randomized into the groups in a 1 : 1 ratio. Subjects were registered in the order in which serial numbers were assigned, and the operation was performed after confirming the assigned random number whenever subjects were registered. All randomization processes were performed by one of the researchers (GWL).
Allograft Material
CGDerm (CGBio, Dae-woong Pharm, Seoul, Korea) is an acellular HDM derived from cadaveric skin. The epidermis and dermal cells have been removed through the acellularization process. The thickness of the allograft product was 1.04–2.29 mm. It was cut to cover the footprint according to the size of the defect during operation. It met the safety criteria of the Ministry of Food and Drug Safety.15,16)
Surgical Technique
All the operations were conducted by the senior surgeon (KCN), with patients placed in the lateral decubitus position under general anesthesia. Interscalene, brachial plexus, and suprascapular nerve blocks were not performed. A biceps long head tenotomy or tenodesis was performed, which was determined according to the patients’ activity level and needs and intraoperative findings. Adequate intra-articular release of rotator cuff was routinely performed. After an examination of the glenohumeral joint, the arthroscope was placed in the subacromial space via the posterolateral portal. Subacromial decompression and acromioplasty were routinely performed when subacromial bursitis was identified. After removal of the bursal tissue, the tear figuration was identified. Release of bursal-side rotator cuff was performed. Footprints were prepared by removing a part of soft tissue and cortical bone.
A margin convergence technique was used as deemed necessary. A rotator cuff repair was performed using two medial anchors and two lateral anchors. Two 4.75 mm BioComposite SwiveLock anchors (Arthrex, Naples, FL, USA) were used as medial anchors. The double pulley technique was used to compress the rotator cuff into the footprint, using two suture strands form each medial anchor.17) The suture-bridge technique was used to repair the rotator cuff using the remaining two suture strands forming each medial anchor and two lateral anchors. Lateral anchors were used with two knotless 4.75 mm BioComposite SwiveLock anchors (Arthrex).18) After the repair procedure, the uncovered footprint defect size was measured using a probe with a scale bar. At this time, the degree of footprint coverage was evaluated.
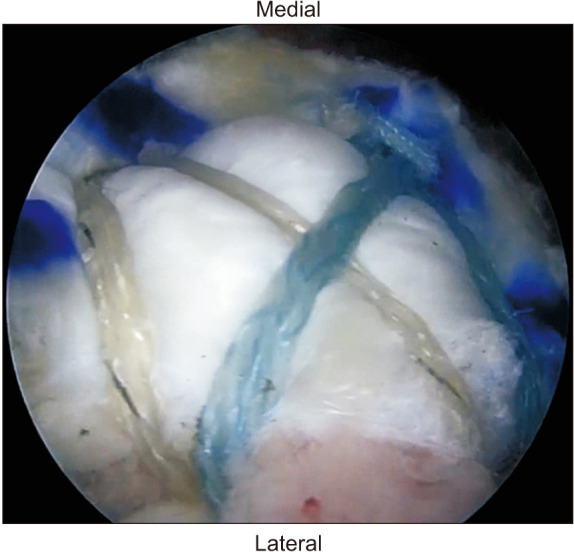
In the allograft group, the HDM patch was augmented, using the remaining sutures without the use of additional sutures. Four strands of each medial anchor were passed through the rotator cuff tendon and the allograft patch. Depending on the shape of the rotator cuff tear, the suture strands of the medial anchors were used to repair in some cases, and in those cases, the sutures were not cut after the knot was formed. The remaining sutures were not cut and passed through the allograft patch. The double-pulley technique and suture-bridge technique were used in the same manner (Fig. 2). Prior to augmentation, the allograft patch was rehydrated in sterile saline or lactated Ringer’s solution at room temperature. After rehydration, the allograft patch had a uniformly soft and pliable consistency within 3 minutes. The graft was cut to cover the medial 1 cm of the bone-tendon interface to the lateral end of footprint.
Fig. 2. Arthroscopic view from the posterolateral portal after repair of the rotator cuff with allograft patch augmentation.
Postoperative Management
An abduction pillow sling (20° of abduction and neutral rotation) was applied for all patients during a period of 6 weeks after the surgery. Patients visited the outpatient clinic regularly. They began performing pendulum exercises 1 week postoperatively and active-assisted range of motion exercises 6 weeks after surgery.
Evaluation
Preoperative demographic data including age, sex, body mass index (BMI), symptom duration (time from symptom onset to surgery), drinking and smoking habits, and history of hypertension and diabetes were assessed. Functional outcomes were evaluated using the Constant score, American Shoulder and Elbow Surgeons (ASES) score. Clinical examinations were performed preoperatively and at least 5 years (mean, 5.7 years) postoperatively. In addition, the anatomical results were simultaneously evaluated using MRI at the last follow-up of each patient.
A musculoskeletal radiologist (IY) with more than 20 years of experience interpreted the MRI findings and determined the results. Fatty infiltration was measured according to the Goutallier classification that was modified by Fuchs et al.19,20) According to the classification of Sugaya et al.,5) types IV and V were classified as retears.
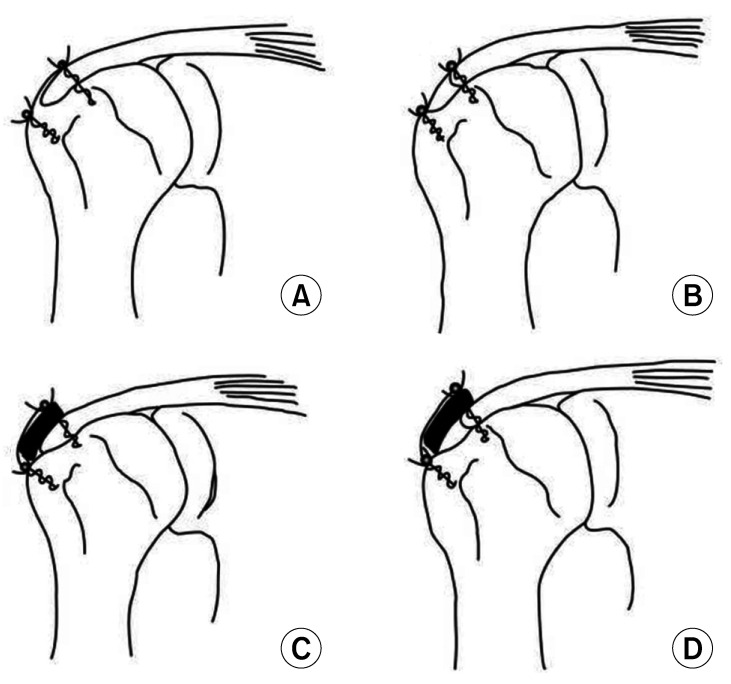
All patients were subdivided into complete coverage (CC) or incomplete coverage (IC) subgroup according to footprint coverage after cuff repair during surgery (Fig. 3). For accuracy, a senior surgeon (KCN) used a 5-mm marked probe to determine the footprint coverage intraoperatively. The decision was agreed by the first assistant (JYK or GWL). CC was defined as > 50% of footprint coverage (repaired to the lateral end of footprint) and IC was defined as less than 50% of the footprint (repaired less than mid-half of footprint).21) In addition to common surgical sequelae, patients were monitored for complications such as wound infection, foreign body reaction, or other complications related to allograft rejection.
Fig. 3. Illustration of the rotator cuff repair with allograft patch augmentation. (A) Complete coverage (CC) in the control group. (B) Incomplete coverage (IC) in the control group. (C) CC in the allograft group. (D) IC in the allograft group.
Statistical Analysis
Based on previous studies that investigated the minimal clinically important difference in patients undergoing rotator cuff surgery, a sample size analysis was conducted using the Constant score (range, 0–100) as the primary outcome variable. Based on a two-sided significance level (α) of 0.05 and a power (β) of 0.90, it was assumed that the anticipated difference in the mean Constant score between the two groups would be 10.4 points and the common standard deviation (SD) would be 10.22) A sample size of at least 21 patients in each group was calculated. We estimated that 30 patients should be recruited, based on an anticipated dropout rate of 30%.
Demographic data were compared between the two groups, using independent Student t-test and Fisher’s exact test. Functional outcomes and fatty infiltration were compared before and after surgery using the dependent Student t-test. Clinical outcomes of CC and IC subgroups within each group were compared using nonparametric Mann-Whitey U-test. The retear rates between two groups and occurrence of retears regarding footprint coverage were evaluated using Fisher’s exact test.
All data are presented as mean ± SD, with a p < 0.05 set as the significance level. The statistical analyses were performed using IBM SPSS ver. 27.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Demographic Data
Patients who were not available for follow-up were further excluded from the study. Total 17 patients were lost to follow-up over the study period. The mean follow-up period was 5.7 ± 1.0 years. Twenty-one patients in the control group and 22 patients in the allograft group were followed up until the end of the study. Among the demographic data, age, sex, BMI, symptom duration, alcohol and smoking use, hypertension, and diabetes mellitus showed no differences between the two groups. Preoperative functional scores, tear size, and fatty infiltration of the rotator cuff showed no differences between the two groups (Table 1).
Table 1. Comparison of Demographic Data between Two Groups.
| Variable | Control group (n = 21) | Allograft group (n = 22) | p-value | |
|---|---|---|---|---|
| Age (yr) | 58.3 ± 7.0 | 60.2 ± 8.4 | 0.427 | |
| Sex (male : female) | 7 : 14 | 7 : 15 | 1.000 | |
| BMI (kg/m2) | 24.6 ± 4.5 | 24.2 ± 2.9 | 0.735 | |
| Symptom duration (wk) | 15.4 ± 10.6 | 16.6 ± 21.9 | 0.827 | |
| Drinking alcohol | 7 (33.3) | 11 (50.0) | 0.358 | |
| Smoking | 3 (14.3) | 1 (4.5) | 0.345 | |
| Hypertension | 7 (33.3) | 6 (27.3) | 0.747 | |
| Diabetes | 2 (9.5) | 3 (13.6) | 1.000 | |
| Preoperative tear size | ||||
| Coronal plane (cm) | 3.30 ± 0.44 | 3.46 ± 0.56 | 0.306 | |
| Sagittal plane (cm) | 3.06 ± 0.50 | 3.03 ± 0.51 | 0.823 | |
| Coronal × sagittal (cm2) | 10.28 ± 3.00 | 10.68 ± 3.50 | 0.685 | |
| Preoperative fatty infiltration* | ||||
| Supraspinatus | 1.81 ± 0.51 | 1.82 ± 0.66 | 0.962 | |
| Infraspinatus | 1.24 ± 0.44 | 1.23 ± 0.43 | 0.935 | |
| Subscapularis | 1.10 ± 0.30 | 1.05 ± 0.21 | 0.533 | |
| GFDI | 1.38 ± 0.28 | 1.36 ± 0.21 | 0.849 | |
| Preoperative functional scores | ||||
| Constant | 58.2 ± 9.0 | 52.8 ± 18.4 | 0.234 | |
| ASES | 54.0 ± 17.1 | 48.7 ± 19.7 | 0.351 | |
| Operation time (min) | 110.9 ± 31.8 | 125.3 ± 24.1 | 0.100 | |
| Operative side, dominant : non-dominant | 15 : 6 | 16 : 6 | 1.000 | |
| Biceps procedure | 16 Tenotomy 5 Tenodesis |
16 Tenotomy 6 Tenodesis |
1.000 | |
| Subacromial decompression | 19 (90.5) | 19 (86.3) | 1.000 | |
Values are presented as mean ± standard deviation or number (%).
BMI: body mass index, GFDI: global fatty degeneration index, ASES: American Shoulder and Elbow Surgeons.
*Fatty infiltration was classified according to Goutallier classification.
Clinical Outcomes
The mean Constant scores improved from 58.2 ± 9.0 preoperatively to 66.8 ± 14.0 at the last follow-up in the control group (p = 0.036) and from 52.8 ± 18.4 to 71.1 ± 7.7 in the allograft group (p = 0.003). There were no significant differences in Constant score increase between the two groups (p = 0.053) (Table 2).
Table 2. Comparison of Functional Outcomes and Fatty Infiltration within Groups and between Two Groups.
| Variable | Control group (n = 21) | Allograft group (n = 22) | p-value* | |||||
|---|---|---|---|---|---|---|---|---|
| Preoperative | Final | p-value | Preoperative | Final | p-value | |||
| Functional score | ||||||||
| Constant | 58.2 ± 9.0 | 66.8 ± 14.0 | 0.036† | 52.8 ± 18.4 | 71.1 ± 7.7 | 0.003† | 0.053 | |
| ASES | 54.0 ± 17.1 | 70.8 ± 20.5 | 0.001† | 48.7 ± 19.7 | 78.9 ± 12.8 | 0.000† | 0.047† | |
| Fatty infiltration‡ | ||||||||
| Supraspinatus | 1.81 ± 0.51 | 1.43 ± 0.98 | 0.088 | 1.82 ± 0.66 | 1.73 ± 1.08 | 0.605 | 0.294 | |
| Infraspinatus | 1.24 ± 0.44 | 1.05 ± 0.38 | 0.104 | 1.23 ± 0.43 | 1.18 ± 0.73 | 0.715 | 0.388 | |
| Subscapularis | 1.10 ± 0.30 | 0.81 ± 1.03 | 0.162 | 1.05 ± 0.21 | 0.68 ± 0.57 | 0.057 | 0.747 | |
| GFDI | 1.38 ± 0.28 | 1.10 ± 0.68 | 0.055 | 1.36 ± 0.21 | 1.19 ± 0.70 | 0.171 | 0.483 | |
Values are presented as mean ± standard deviation.
ASES: American Shoulder and Elbow Surgeons, GFDI: global fatty degeneration index.
*p-values represent the level of significance in the comparison of the changes between the two groups. †Values with statistical significance (p < 0.05). ‡Fatty infiltration was classified according to Goutallier classification.
The mean ASES scores increased from 54.0 ± 17.1 preoperatively to 70.8 ± 20.5 at the last follow-up in the control group (p = 0.001) and from 48.7 ± 19.7 to 78.9 ± 12.8 in the allograft group (p < 0.001). The ASES score increase of the allograft group was significantly greater than that of the control group (p = 0.047) (Table 2). When power analysis was performed, β for the change in ASES score between the two groups was 0.895.
Fatty Infiltration
Supraspinatus fatty infiltration on MRI of the control group was 1.81 ± 0.51 preoperatively and 1.43 ± 0.98 at the final follow-up. It was 1.82 ± 0.66 preoperatively and 1.73 ± 1.01 at the final follow-up in the allograft group. The difference between the two groups was not significant at the final follow-up (p = 0.294) (Table 2). Fatty infiltration of the infraspinatus and subscapularis, and the global fatty degeneration index did not change significantly, and the scores showed no differences between the two groups at the final follow-up (p > 0.05) (Table 2).
Retear Rate
According to the classification by Sugaya et al.,5) types IV and V were considered as retears. The retear rate of the control group was 38.1% and the allograft group had a low retear rate of 9.1% (Fig. 4). There was statistically significant difference between the two groups (p = 0.034) (Table 3). When power analysis was performed, β for retear rates between the two groups was 0.611.
Fig. 4. Postoperative T2 oblique sagittal magnetic resonance images. Intact rotator cuff in the control group (A) and in the allograft group (B). Retear of the rotator cuff in the control group (C) and in the allograft group (D).
Table 3. Retear Rates of Two Groups.
| Variable | Control group (n = 21) | Allograft group (n = 22) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Intact | 13 (61.9) | 20 (90.9) | 4.190 (1.003–17.501) | 0.034* |
| Retear | 8 (38.1) | 2 (9.1) |
Values are presented as number (%) unless otherwise indicated.
OR: odds ratio, CI: confidence interval.
*Statistical significance (p < 0.05).
Footprint Coverage Assessment
In the control group, 12 patients were classified as the CC subgroup and 9 patients as the IC subgroup. In the allograft group, 11 patients were classified as the CC subgroup and 11 patients as the IC subgroup. When clinical outcomes of the CC and IC subgroups were compared within each group, there were no statistically significant differences between the two subgroups (preoperative and last follow-up values and increases in ASES and Constant score).
On final follow-up MRI, in the control group, the CC subgroup showed a lower retear rate (16.7%) than the IC subgroup (66.7%) (p = 0.032) (Table 4). In the allograft group, the retear rate was 0% in the CC subgroup and 18.2% in the IC subgroup (p = 0.476) (Table 4). The CC and IC subgroups of the allograft group had lower retear rates than those of the control group did, but these were not statistically significant (p = 0.214 and 0.065, respectively) (Table 4). There was no specific difference in MRI findings among subgroups according to footprint coverage.
Table 4. Retear Rates Regarding Footprint Coverage after Cuff Repair.
| Group | Control group (n = 21) | Allograft group (n = 22) | ||||||
|---|---|---|---|---|---|---|---|---|
| CC (n = 12) | IC (n = 9) | OR (95% CI) | p-value | CC (n = 11) | IC (n = 11) | OR (95% CI) | p-value | |
| Intact | 10 (83.3) | 3 (33.3) | 2.500 (0.959–6.516) | 0.032*,† | 11 (100) | 9 (81.8) | - | 0.476* |
| Retear | 2 (16.7) | 6 (66.7) | 0 | 2 (18.2) | ||||
Values are presented as number (%) unless otherwise indicated.
CC: complete coverage, IC: incomplete coverage, OR: odds ratio, CI: confidence interval.
*p-values represent the level of significance between the retear rate of the two subgroups within group. †Statistical significance (p < 0.05).
Complications
No nerve damage, wound infections, foreign body reactions, or other complications related to rejection of the allograft were observed in this study. One patient with a retear in the control group underwent reverse total shoulder arthroplasty approximately 1 year after surgery.
DISCUSSION
Depending on the size of the rotator cuff tear, various treatment methods are available, from non-surgical treatment to reverse total shoulder arthroplasty. ECM has received great attention and various materials have been developed and used to reduce the high failure rate of rotator cuff repair and improve tendon healing. It is considered to enhance the repair strength by dispersing shear force at the repair interface.
There have been studies demonstrating benefits of structural augmentation in rotator cuff repair. In a human cadaveric model, Shea et al.23) evaluated enhanced rotator cuff repair with strained reinforcement in the repair, which is similar to the protocol in our study. In that study, gap formation was decreased by 40% in the reinforced specimens compared to the control. The ECM-reinforced group showed a significantly higher ultimate load-to-failure compared with the control group, in which the ECM graft was estimated to afford a load of 35%. Other studies reported mechanical advantages of allograft material and augmentation.24,25) Additionally, allograft material can serve as cell signal molecules, chemoattractants, and cell binding sites for new infiltrating host cells capable of modulating the healing process at the site of implantation.26)
In another prospective, randomized controlled trial (level II study), Barber et al.9) evaluated the effect of HDM patch augmentation as in the current study. They demonstrated better functional outcomes of augmentation when compared with controls. They reported significant differences in the healing rate between the allograft group (85%) and the control group (40%) using gadolinium-enhanced MRI to evaluate rotator cuff healing. Gilot et al.11) reported the better ASES score improvement and lower retear rates after rotator cuff repair with ECM augmentation, compared to non-augmentation. Several studies have previously reported good clinical results with augmented rotator cuff repair, regardless of the operative procedure, approach method, repair technique, rotator cuff tear size, or revision.13,14,27,28,29) However, the quality of the evidence from these studies is limited as it is based on observational and minimally controlled studies. In the current study, the functional score and retear rate of the allograft patch group were improved similar to those in previous studies, which is considered more meaningful in that the patients were followed up for a long time.
For large or massive rotator cuff tears, it may not be possible to cover the entire area of the footprint, resulting in incomplete or partial repair. However, the contact zone and interface pressure between the tendon and bone should been maximized to improve healing or integrity. Two studies have reported that the incomplete footprint coverage showed a relatively low healing quality and a relatively high retear rate,21,30) similar to the results of our study. In the control group, the CC subgroup showed a significantly lower retear rate (16.7%) than did the IC subgroup (66.7%) (p = 0.032). The CC subgroup of the allograft group showed a lower retear rate (0%) than did the IC subgroup, but there was no statistically significant difference (p = 0.476). On the other hand, the CC and IC subgroups of the allograft group showed lower retear rates than those of the subgroups of the control group, but there were no statistically significant differences (p = 0.214 and p = 0.065, respectively). It was assumed that this was because the sample size was not large enough for comparing subgroups. We thought patch augmentation was more important if coverage was incomplete after repair. We also hypothesized that the incompletely repaired footprint was covered by the graft and that it was biochemically bound to the rotator cuff. However, there were no studies evaluating the relationship between footprint coverage and allograft augmentation. We believe that further studies with a larger number of patients are needed to identify the relationship between footprint coverage and the allograft in the future.
Our study has some limitations. First of all, the sample size was small. The sample size of this study was adequate to identify statistical differences in Constant scores (primary outcome), which is comparable to that of the study by Barber et al.9) (total 42 patients), who reported sufficient power to support their retear rate differences between two groups. However, in the current study, β for retear rates between the two groups was 0.611. We think that a large sample size will be required to obtain sufficient power in future studies. Second, there is a possibility of selection bias in the subdivision process according to the footprint coverage area. Third, the amount of DNA remaining in allograft has not yet been studied. Remaining DNA may cause an inflammatory response, which may affect pain or tendon healing, although no serious side effects occurred in this study.
Long-term follow-up of arthroscopic repair of large rotator cuff tears with allograft patch augmentation showed better clinical and anatomical results than did non-augmentation. Footprint coverage after rotator cuff repair is an important factor affecting the retear rate. Although no statistical significance was observed in the current study, if the footprint is not completely covered after rotator cuff repair, allograft patch augmentation may reduce the re-tear rate. Relevant research seems to be necessary.
ACKNOWLEDGEMENTS
The authors would like to thank Prof. Ik Yang (Department of Radiology, Kangnam Sacred Heart Hospital, Hallym University College of Medicine) for his valuable technical assistance and contribution to the evaluation of anatomical results. This research was supported by Hallym University research fund (No. H20200556).
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Kim JR, Cho YS, Ryu KJ, Kim JH. Clinical and radiographic outcomes after arthroscopic repair of massive rotator cuff tears using a suture bridge technique: assessment of repair integrity on magnetic resonance imaging. Am J Sports Med. 2012;40(4):786–793. doi: 10.1177/0363546511434546. [DOI] [PubMed] [Google Scholar]
- 2.Miller BS, Downie BK, Kohen RB, et al. When do rotator cuff repairs fail?: serial ultrasound examination after arthroscopic repair of large and massive rotator cuff tears. Am J Sports Med. 2011;39(10):2064–2070. doi: 10.1177/0363546511413372. [DOI] [PubMed] [Google Scholar]
- 3.Rousseau T, Roussignol X, Bertiaux S, Duparc F, Dujardin F, Courage O. Arthroscopic repair of large and massive rotator cuff tears using the side-to-side suture technique: mid-term clinical and anatomic evaluation. Orthop Traumatol Surg Res. 2012;98(4 Suppl):S1–S8. doi: 10.1016/j.otsr.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393–403. doi: 10.1016/j.arthro.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair: a prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953–960. doi: 10.2106/JBJS.F.00512. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Kim EK, Kim SJ, Song DH. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. J Orthop Surg Res. 2018;13(1):1. doi: 10.1186/s13018-017-0693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalmers PN, Tashjian RZ. Patch augmentation in rotator cuff repair. Curr Rev Musculoskelet Med. 2020;13(5):561–571. doi: 10.1007/s12178-020-09658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19(3):467–476. doi: 10.1016/j.jse.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Barber FA, Burns JP, Deutsch A, Labbe MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8–15. doi: 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Ricchetti ET, Aurora A, Iannotti JP, Derwin KA. Scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2012;21(2):251–265. doi: 10.1016/j.jse.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Gilot GJ, Alvarez-Pinzon AM, Barcksdale L, Westerdahl D, Krill M, Peck E. Outcome of large to massive rotator cuff tears repaired with and without extracellular matrix augmentation: a prospective comparative study. Arthroscopy. 2015;31(8):1459–1465. doi: 10.1016/j.arthro.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Leuzinger J, Sternberg C, Smolen D, Jakob R. Patch augmentation in rotator cuff repair surgery with elder patients. Z Orthop Unfall. 2016;154(5):504–512. doi: 10.1055/s-0042-106475. [DOI] [PubMed] [Google Scholar]
- 13.Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141–147. doi: 10.1177/0363546511422795. [DOI] [PubMed] [Google Scholar]
- 14.Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 Suppl):104–109. doi: 10.1016/j.jse.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Kim SY, Lim SY, Mun GH, Bang SI, Oh KS, Pyon JK. Evaluating the effectiveness of cryopreserved acellular dermal matrix in immediate expander-based breast reconstruction: a comparison study. Arch Plast Surg. 2015;42(3):316–320. doi: 10.5999/aps.2015.42.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Park KR, Kim TG, et al. A comparative study of CG CryoDerm and AlloDerm in direct-to-implant immediate breast reconstruction. Arch Plast Surg. 2013;40(4):374–379. doi: 10.5999/aps.2013.40.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arrigoni P, Brady PC, Burkhart SS. The double-pulley technique for double-row rotator cuff repair. Arthroscopy. 2007;23(6):675. doi: 10.1016/j.arthro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Park MC, Elattrache NS, Ahmad CS, Tibone JE. “Transosseous-equivalent” rotator cuff repair technique. Arthroscopy. 2006;22(12):1360. doi: 10.1016/j.arthro.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures: pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78–83. [PubMed] [Google Scholar]
- 20.Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 21.Koh KH, Lim TK, Park YE, Lee SW, Park WH, Yoo JC. Preoperative factors affecting footprint coverage in rotator cuff repair. Am J Sports Med. 2014;42(4):869–876. doi: 10.1177/0363546513518581. [DOI] [PubMed] [Google Scholar]
- 22.Kukkonen J, Kauko T, Vahlberg T, Joukainen A, Aarimaa V. Investigating minimal clinically important difference for Constant score in patients undergoing rotator cuff surgery. J Shoulder Elbow Surg. 2013;22(12):1650–1655. doi: 10.1016/j.jse.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Shea KP, Obopilwe E, Sperling JW, Iannotti JP. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg. 2012;21(8):1072–1079. doi: 10.1016/j.jse.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20–24. doi: 10.1016/j.arthro.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Aurora A, Gatica JE, van den Bogert AJ, McCarron JA, Derwin KA. An analytical model for rotator cuff repairs. Clin Biomech (Bristol, Avon) 2010;25(8):751–758. doi: 10.1016/j.clinbiomech.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Lorbach O, Baums MH, Kostuj T, et al. Advances in biology and mechanics of rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2015;23(2):530–541. doi: 10.1007/s00167-014-3487-2. [DOI] [PubMed] [Google Scholar]
- 27.Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36–44. doi: 10.4103/0973-6042.96992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petri M, Warth RJ, Horan MP, Greenspoon JA, Millett PJ. Outcomes after open revision repair of massive rotator cuff tears with biologic patch augmentation. Arthroscopy. 2016;32(9):1752–1760. doi: 10.1016/j.arthro.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 29.Steinhaus ME, Makhni EC, Cole BJ, Romeo AA, Verma NN. Outcomes after patch use in rotator cuff repair. Arthroscopy. 2016;32(8):1676–1690. doi: 10.1016/j.arthro.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Lee YS, Jeong JY, Park CD, Kang SG, Yoo JC. Evaluation of the risk factors for a rotator cuff retear after repair surgery. Am J Sports Med. 2017;45(8):1755–1761. doi: 10.1177/0363546517695234. [DOI] [PubMed] [Google Scholar]