SUMMARY
Ferroptosis is a unique type of non-apoptotic cell death resulting from the unrestrained occurrence of peroxidized phospholipids, which are subject to iron-mediated production of lethal oxygen radicals. This cell death modality has been detected across many organisms, including in mammals, where it can be used as a defense mechanism against pathogens or even harnessed by T cells to sensitize tumor cells toward effective killing. Conversely, ferroptosis is considered as one of the main cell death mechanisms promoting degenerative diseases. Emerging evidence suggests that ferroptosis represents a vulnerability in certain cancers. Here, we critically review recent advances linking ferroptosis vulnerabilities of dedifferentiating and persister cancer cells to the dependency of these cells on iron, a potential Achilles’ heel for small molecule intervention. We provide a perspective on the mechanisms reliant on iron that contribute to the persister cancer cell state and how this dependency may be exploited for therapeutic benefits.
eTOC Blurb
Rodriguez et al. discuss how iron allows cells to acquire a drug-tolerant persister cancer state and how this addiction confers a high vulnerability to ferroptosis. This feature therefore opens up unprecedented opportunities to identify new predictors of ferroptosis sensitivity and provides the basis for the development of next-generation therapeutics.
INTRODUCTION
Iron is one of the most abundant metals on Earth and an essential element of life. Unlike noble metals such as gold and platinum whose stability towards corrosion is highly desirable in the jewelry industry, it is the ability of iron to readily adopt various oxidation states through the loss of electrons and to undergo or to mediate chemical reactions that makes life possible. As a result of its particular reactivity, iron has become indispensable in academic research and the chemical industry, in particular for the selective transformation of a wide range of organic substrates (Chen and White, 2007, 2010; Schreiber, 1980). In biology, iron is central to the function of iron-sulfur cluster-containing proteins including those involved in proteins of the electron transport chain (ETC) in mitochondria. These clusters allow electrons to shuttle across the inner mitochondrial membrane (IMM) to reduce oxygen, thereby establishing a proton gradient over the IMM, which is necessary for the production of energy stored as adenosine triphosphate (ATP). Iron is also central to the function of many other classes of proteins involved in DNA replication, DNA repair, telomere maintenance and ribosome assembly (Xu et al., 2013). Most importantly, upon binding to a porphyrin core to form heme, iron can reversibly interact with diatomic gases enabling red blood cells to supply molecular oxygen to organs and is central to the redox activity of enzymes with distinct activities including catalases, peroxidases and nitric oxide synthases (Muckenthaler et al., 2017).
While metals in biology are commonly referred to as co-factors required for the catalytic activity of enzymes, iron is in many cases the catalyst itself. It promotes redox reactions, while the proteins and heme cores stabilize iron and its oxidized intermediates to enhance the catalytic turn-over of reactions, providing substrate specificity, chemo-, regio- and stereo-selectivity of substrate oxidation. For example, lipoxygenases (LOX) are a family of iron-dependent enzymes that catalyze the dioxygenation of polyunsaturated fatty acids (PUFAs), leading to the production of signaling molecules such as leukotrienes and hepoxilins via intermediate lipid peroxides. Iron-containing cyclooxygenases are responsible for the synthesis of another class of oxidized lipids, i.e., prostanoids, that include prostaglandins, prostacyclins and thromboxanes. Hypoxia-inducible factor (HIF)-proline dioxygenase uses iron catalyst, 2-ketoglutarate as an electron donor and molecular oxygen as a substrate to promote hydroxylation and degradation of the transcription factors HIF. Through a comparable catalytic cycle, iron- and 2-ketoglutarate-dependent demethylases of histones and nucleic acids regulate epigenetic landscapes to orchestrate the expression of specific genes. Related to this, free iron can promote Fenton chemistry in a non-enzymatic manner, whereby the metal can directly reduce molecular oxygen, hydrogen peroxide and lipid hydroperoxides to yield oxygen-containing radicals in cells (Schreiber, 1980). Thus, because of its versatile reactivity, cellular iron homeostasis is tightly regulated to avoid the uncontrolled production of otherwise deleterious highly reactive species (Muckenthaler et al., 2017; Pantopoulos et al., 2012).
Here, we briefly describe the main molecules involved in the regulation of cellular iron homeostasis illustrating how cells exploit and deal with its multifaceted chemical reactivity. Next, we discuss the importance of iron for the regulation of key cellular processes and how the presence of iron may become a threat. In particular, we focus on cancer cell populations refractory to conventional therapy, whose physiology thoroughly rely on iron, conferring a vulnerability of these cells to ferroptosis. Finally, we provide an account on small molecules susceptible to take advantage of the reactivity of iron to eradicate these cells, which not only present valuable tools for understanding ferroptosis mechanisms in cell biology studies, but also provide the basis for the design of next-generation therapeutics for the clinical management of cancer.
Regulation of cellular iron homeostasis
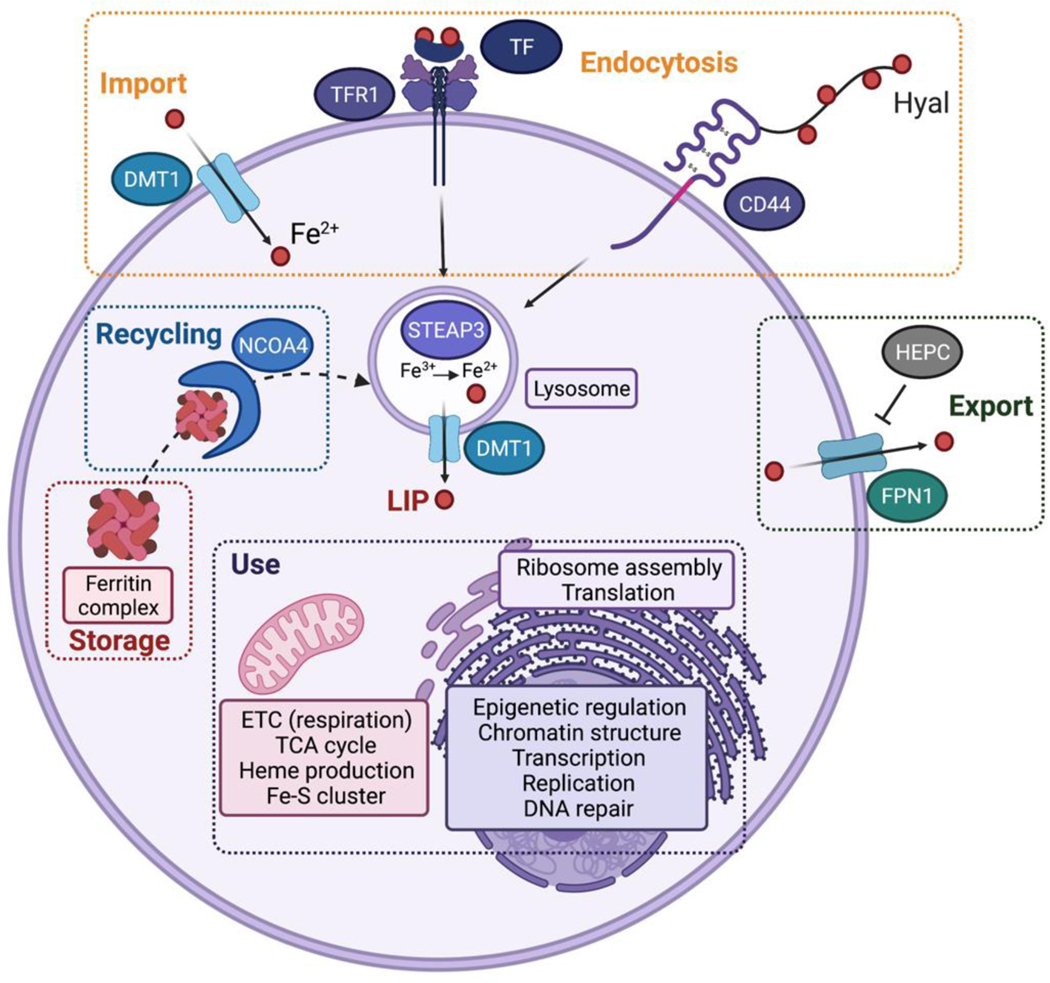
Various cellular mechanisms of iron uptake have been reported. For example, enterocytes use the divalent metal transporter 1 (DMT1) to shuttle iron across the plasma membrane, while most cells take up iron by means of endocytosis (Fig. 1). Ferric iron-loaded transferrin (TF) interacts with transferrin receptor 1 (TFR1) at the plasma membrane and this complex is internalized through the formation of endocytic vesicles, whose acidification upon maturation into functional lysosomes unloads iron from TF. Ferric iron is subsequently reduced by metalloreductases including STEAP3 (Six-transmembrane epithelial antigen of prostate 3), and translocates from the lumen of vesicles to the cytosol via DMT1 as ferrous iron. This reactive labile iron pool (LIP) can traffic to distant organelles where iron is required for the activity of specific proteins. Notably, in iron biology the LIP remains a poorly understood cellular feature likewise, mechanisms by which iron is distributed in cells remain elusive. For instance, iron-dependent histone demethylases operate in the nucleus, but it is not clear whether these proteins assemble with iron in the cytosol or in the nucleus. Iron can be stored as a poorly reactive iron oxide form within a multimeric ferritin complex to limit undesired chemical reactions, and the LIP may be replenished through autophagic degradation of ferritin involving the cargo protein nuclear receptor coactivator 4 (NCOA4) via a mechanism known as ferritinophagy (Gao et al., 2016; Hou et al., 2016). Alternatively, the membrane protein ferroportin-1 (FPN1), which is under control of the liver hormone hepcidin (HEPC), can export iron outside of cells thereby limiting accumulation and unnecessary storage of excess cellular iron. Importantly, iron uptake, storage and export are tightly regulated post-transcriptionally by complex mechanisms occurring at the mRNA level. These involve iron responsive elements (IRE) localized in the untranslated regions (UTRs) of RNAs coding for proteins controlling the balance of cellular iron (Hentze et al., 2010).
Figure 1. Cellular iron homeostasis.
In mammalian cells, iron can be imported through divalent metal transporter 1 (DMT1, alias SLC11A2) or internalized by means of endocytosis involving the canonical transferrin receptor 1 (TFR1)/transferrin (TF) or the alternative CD44/Hyaluronate (Hyal) pathways. Upon maturation of endocytic vesicles, ferric iron is released from TF, reduced by six-transmembrane epithelial antigen of prostate 3 (STEAP3) and ferrous iron translocates to the cytosol. Iron can be stored as ferritin complexes, which can be recycled by means of ferritinophagy involving nuclear receptor coactivator 4 (NCOA4.) Alternatively, iron can be exported outside of cells through ferroportin-1 (FPN1, alias SLC40A1), whose level is regulated by hepcidin (HEPC). Iron traffics towards distinct compartments where it is required to mediate key cellular processes.
The activity of iron-dependent enzymes to produce useful oxidized metabolites and the existence of a LIP are, however, inevitably associated with the accumulation of undesired by-product of oxidation that can be toxic or even lethal to cells. Thus, cells have evolved sophisticated mechanisms of ‘detoxification’, that consist of, as an example, importing and building molecules with high reducing potential such as cysteine (Cys) and glutathione (GSH), respectively, that can be used to scavenge peroxides and peroxidized lipids. In particular, the cystine-glutamate antiporter (also termed system xc-) enables cellular uptake of cystine, which upon intracellular reduction to cysteine is the main building block for GSH biosynthesis. GSH itself is the requisite co-substrate of glutathione peroxidase 4 (GPX4) consumed to directly reduce peroxidized phospholipids (Ingold et al., 2018; Seiler et al., 2008; Yang et al., 2014). Recently, solute carrier family 25 member 39 (SLC25A39) has been shown to mediate GSH transport into the mitochondrial matrix and to regulate assembly of iron-sulfur clusters in this organelle, indicating a key regulatory role of mitochondrial GSH in iron-sulfur cluster biogenesis rather than modulating redox processes in this organelle (Wang et al., 2021). However, whether this may have an effect on sensitivity of cells towards ferroptosis remains elusive.
The prevalence of iron in the biology of the cell raises questions as to whether and how defective cellular iron homeostasis, or alternatively the pharmacological manipulation of iron chemistry and the handling of related reaction products lead to cell death. For example, TF and TfR1 have been implicated in the regulation of ferroptosis presumably by modulating the cellular iron content (Gao et al., 2015). Knocking out NCOA4 interferes with ferritin degradation and iron remobilization, which in turn can have a protective effect against ferroptosis (Gao et al., 2016; Gryzik et al., 2021; Hou et al., 2016). Furthermore, inhibiting DMT1 leads to the production of reactive oxygen species, lipid peroxidation and cell death (Turcu et al., 2020). Thus, tight control of iron uptake, storage and distribution is key to prevent ferroptosis.
Iron: the missing link in the dogma of ferroptosis
Unrestrained iron-dependent lipid peroxidation and Fenton-like chemistry results in the rupture of lipid membranes including the plasma membrane – a hallmark of ferroptosis (Conrad and Pratt, 2019). Nonetheless, questions as to whether all cellular membranes are equally prone to lipid peroxidation, the subcellular site of lipid peroxidation, and the first event inciting (iron-dependent) lipid peroxidation have remained largely obscure (Aldrovandi et al., 2021). First attempts to decipher lipid peroxidation signatures of cells succumbing to ferroptosis revealed that a subset of glycerophospholipids such as phosphatidylethanolamines (PE) functionalized with certain PUFAs, including arachidonic acid and adrenic acid, may represent prime targets of peroxidation (Doll et al., 2017; Kagan et al., 2017) (Fig. 2). This supports the hypothesis that mitochondria, an organelle known to contain high levels of PE, where iron chemistry is prevalent, might be one of the subcellular sites where lipid peroxidation sets off. This is reinforced by early findings indicating that the guardian of ferroptosis, GPX4, is abundant in the mitochondrial intermembrane space (IMS) (Liang et al., 2009). With the discovery of ferroptosis suppressor protein-1 (FSP1, alias “apoptosis inducing factor mitochondria associated 2”, AIFM2) as the second mainstay in ferroptosis control, this simplistic scenario has been challenged as FSP1 preferentially interacts with several other cellular structures such as the plasma membrane, perinuclear structures, and the Golgi apparatus (Bersuker et al., 2019; Doll et al., 2019). Unlike GPX4 that solely acts on (phospho)lipid hydroperoxides, FSP1 along with extramitochondrial ubiquinone (also referred to as coenzyme Q10, CoQ10) and/or α-tocopherol, halts the process of lipid peroxidation at the level of lipid radicals using electrons from NAD(P)H. Besides PUFA-containing phospholipids, polyunsaturated ether phospholipids (ePLs) might be another tinder for uncontrolled lipid peroxidation (Zou et al., 2020a). Although first considered to counteract lipid peroxidation by acting as a sink of radicals, these peroxisome-born phospholipids may also contribute to the ferroptotic process. This is a plausible hypothesis in light of the prevalence of iron-dependent enzymes in this organelle (Schrader and Fahimi, 2006). Hence, all these findings put forward the idea that depending on the cell’s route of energy production (e.g., glycolytic versus oxidative), the site of generation of the prime radicals inciting lipid peroxidation, particularly upon cellular stress (e.g., ischemia/reperfusion), and the amount of PUFAs esterified in membranous compartments ultimately determines the cell’s predisposition for lipid peroxidation. For instance, an acyl-CoA synthetase long chain family member 4 (ACSL4)- and lysophosphatidylcholine acyltransferase 3 (LPCAT3)-dependent enrichment of membranes with PUFAs positively correlates with a pro-ferroptotic state (Dixon et al., 2015; Doll et al., 2017; Kagan et al., 2017), whereas an ACSL3-mediated enrichment of membranes with chemically less reactive monounsaturated fatty acids (MUFAs) has the opposite effect by promoting an anti-ferroptotic cell state (Magtanong et al., 2019; Ubellacker et al., 2020). Importantly, ferroptosis can be operationally defined as cell death that can be prevented by both iron chelation and a spin trap. This may be confounded by the idea that putative ferroptosis inhibitors may not always localize at subcellular sites where lipid peroxidation is first initiated and propagates.
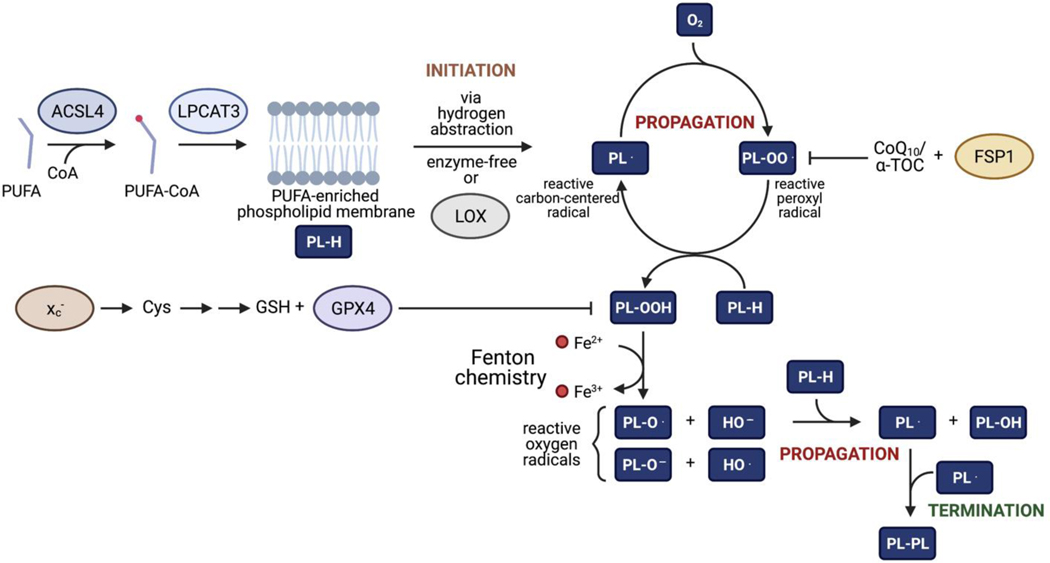
Figure 2. Mechanisms leading to ferroptosis.
Free-radical chain reaction leading to the production of lipid peroxides and ferroptosis. At membranes, phospholipids (PL-H) can be subjected to radical-mediated hydrogen abstraction. Following this initiation step, PL radicals (PL·) can react with molecular oxygen to form the highly reactive PL peroxyl radical (PL-OO·), itself reactive towards PL-H enabling this reaction to propagate through repeated cycles. This further produces PL· and PL hydroperoxides (PL-OOH). Through Fenton chemistry, PL-OOH can form a hydroxyl (HO·) and PL alkoxyl radicals (PL-O·) that can also propagate the chain reaction leading to additional production of PL·. The chain reaction can terminate in a variety of ways including hemolytic cleavage of adjacent carbon–carbon bonds to generate carbon radicals and aldehydes and dimerization of two carbon radicals including PL·. Mechanisms that protect cells from ferroptosis include system xc- that mediates uptake of cystine required for glutathione (GSH) production, which is itself a substrate of glutathione peroxidase 4 (GPX4) to scavenge PL-OOH. Ferroptosis suppressor protein-1 (FSP1) can trap PL-OO· via coenzyme Q10 (CoQ10) and/or α-tocopherol (α -TOC) in a GSH-independent manner.
Even though the number of conjugated dienes in phospholipid acyl chains impacts on the ability of membranes to be oxidized, it remains a matter of intense debate how lipid peroxidation is in fact initiated and what the contribution of iron is in this context. Early findings pointed towards a distinct iron-dependent LOX, i.e., 12/15-lipoxygenase, as the main driver of lipid peroxidation by generating peroxides directly at membranes in form of esterified hydroperoxyeicosatetraenoic acids (HPETEs) (Seiler et al., 2008); however, subsequent studies with transgenic mice challenged this hypothesis (Brutsch et al., 2015; Friedmann Angeli et al., 2014; Matsushita et al., 2015). Similarly, overexpression of certain human LOX isoforms in cells only decreased the threshold for lipid peroxidation to occur, while non-enzymatic lipid autoxidation, which may be initiated by iron in an enzyme-free manner, apparently is a true driver of ferroptosis (Shah et al., 2018). Cytochrome P450 oxidoreductase (POR) and NADH-cytochrome b5 reductase (CYB5R1) have recently been identified to impact on lipid peroxidation and ferroptosis sensitization (Yan et al., 2021; Zou et al., 2020b), although their respective contributions appear to facilitate the cycling of redox cofactors during the propagation stage and do not appear to be direct. Both types of enzymes generate hydrogen peroxide, which upon reaction with ferrous iron yields highly toxic hydroxyl radicals via the so-called Fenton reaction. The hydroxyl radical in turn may abstract a hydrogen atom from membrane PUFAs, thus inciting a lipid peroxidation chain reaction. Therefore, it seems that a single lipid oxidizing enzyme that would be responsible for the first phospholipid PUFAs peroxidation has not been identified yet.
Conceivably, non-enzymatic mechanisms that kick off lipid peroxidation involved in ferroptosis are at play. For instance, physico-chemical stress is sufficient to abstract a labile hydrogen atom from membrane PUFAs leaving a carbon-centered radical behind that would then readily react with molecular oxygen to generate the highly reactive peroxyl lipid radical igniting lipid autoxidation (Fig. 2). Hydroxyl radical generated from hydrogen peroxide and unchaperoned ferrous iron (from the LIP) via the Fenton reaction is still regarded as the prime event in triggering lipid peroxidation. Yet, what remains a matter of controversy is that the highly reactive hydroxyl radical and/or ferrous iron might not come in sufficiently close proximity with membrane PUFAs given the highly lipophilic environment of lipid bilayers, which would argue against a major role of, at least, hydroxyl radical in priming lipid peroxidation. However, experimental data arguing against a direct role of iron are lacking.
Unlike hydroxyl radical, hydroperoxyl radical, the protonated form of superoxide, which can readily form in the IMS of mitochondria due to the higher abundance of superoxide and the acidic nature of this suborganellar compartment, may trigger lipid peroxidation. In the context of tissue ischemia/reperfusion injury (IRI)-related pathologies, such as organ transplantation, cardiac infarction and stroke, this might be of particular relevance. It is known that succinate accumulation during ischemia and rapid re-oxidation of succinate by succinate dehydrogenase during the reperfusion phase can cause the extensive generation of superoxide by reverse electron transport at mitochondrial complex I (Chouchani et al., 2014; Chouchani et al., 2016). In addition, cysteine starvation-induced ferroptosis was recently linked to a massive accumulation of superoxide at mitochondrial complex III (Homma et al., 2021), with a rather marginal implication of complex I under these pro-ferroptotic conditions. Protonation of superoxide and hydroperoxyl radical-mediated lipid peroxidation might thus be a highly relevant mechanism that could cause ferroptosis in IRI, which warrants further investigation. As with the implication of iron in ferroptosis, which is still largely based on cell-based studies, the use of iron chelating drugs and the known chemical reactivity of iron, substantial work remains to be done in order to characterize both the chemical and biological roles played by iron in the ferroptotic process.
Iron addiction of cancer stem cells
The paradigm of cancer stem cells (CSCs), also termed cancer stem-like cells and tumor-initiating cells (TIC), defines the existence of a long-lived population of cancer cells prone to self-renewal that fuel tumor growth. CSCs represent a fraction of the cellular content of tumors, and they have been associated with cancer recurrence after chemo- or radiation therapy, tumor dormancy and metastasis (Batlle and Clevers, 2017). Cancer cell plasticity defines the capacity of cells to reversibly undergo dynamic changes between phenotypically distinct states, which has been linked to the acquisition of stemness properties with tumor-seeding capacity and resistance to therapy (Boumahdi and de Sauvage, 2020). This includes epithelial-mesenchymal plasticity (EMP) where epithelial carcinoma cells can dedifferentiate to adopt a mesenchymal or mixed epithelial–mesenchymal (EM) phenotype (Lambert and Weinberg, 2021; Nieto et al., 2016; Williams et al., 2019; Yang et al., 2020). Intriguingly, this therapy-resistant state has been reported in other tissue-types that do not have an epithelial origin including melanoma, glioblastoma, prostate cancer and sarcoma (Viswanathan et al., 2017). We suggest that this pan-cancer form of resistance converges on a high PUFA phospholipid content, yet distinct cell types use distinct transcriptional programs to converge on the ferroptosis-vulnerable state. It is for this reason that the pan-cancer resistant state transcends EMT and escaped detection by transcriptomic analyses. Realization of its existence instead required a perturbational approach – studying common features of cells stressed with many distinct mechanisms-of-action (MoA) perturbagens.
The implication of iron in cancer progression has been known for over half a century (Torti and Torti, 2013, 2020), but it is only recently that the dependency of CSCs and cancer cells in the mesenchymal state to iron has been documented. Yet, functional roles of iron in the maintenance of a dedifferentiated therapy-resistant state remains poorly understood. In glioblastoma, CSCs have been shown to be characterized by enhanced iron trafficking involving the TFR1/TF iron-endocytosis pathway (Schonberg et al., 2015). Interestingly, it was shown that knocking down ferritin reduced levels of the transcription factor forkhead box M1 (FoxM1) and signal transducer and activator of transcription 3 (STAT3) signaling, which correlated with alterations of cell cycle progression. It was postulated that the STAT3-FoxM1 signaling axis depends on iron and/or requires direct ferritin binding. Conceivably, excess of free iron, whose handling is impaired upon ferritin knockdown conditions, promotes the formation of free radicals involved in FoxM1 transcriptional activity.
In a study investigating TIC in vitro, it was shown that levels of FPN were reduced in high-grade ovarian tumors, while that of TFR1 were increased compared to low grade serous ovarian cancer (Basuli et al., 2017). Pharmacological alteration of iron levels impaired cell proliferation and metastatic dissemination and this involved expression of matrix metalloproteases and interleukin-6 (IL-6). Importantly, it was shown that the iron chelator deferoxamine (DFO) impacted on cell viability in a model of ovarian TIC. This was consistent with a higher iron load in these cells advocating for a dependency of these cells to iron, which was confirmed by the effect of knocking down the iron-homeostasis regulatory protein iron-regulatory protein 2 (IRP2).
In another study, it was shown that HMLER CD44high cells, a model of tumorigenic breast cancer cells recapitulating features of CSC, were more vulnerable than their epithelial counterpart to salinomycin (Gupta et al., 2009), a natural product that has been shown to sequester iron in the lysosomal compartment (Mai et al., 2017). This work revealed that total cellular iron as well as the LIP was higher in CD44high cells compared to their epithelial counterparts, consistent with the dependency of the mesenchymal state of cancer cells to iron (Muller et al., 2020). In agreement with this, supplementing HMLER epithelial cells with ferric iron promoted epithelial-mesenchymal transition (EMT), whereas knocking down ferritin heavy chain (FTH1) blocked oncostatin M (OSM)-induced EMT. In a subsequent study, it was shown that triple negative breast cancer cells in the mesenchymal state preferentially mediate iron endocytosis by an alternative TFR1-independent pathway involving hyaluronates bound to the recycling membrane protein CD44 (Muller et al., 2020), which had previously been defined as a CSC marker (Stuelten et al., 2010). In the work by Muller et al., it was shown that levels of the mitochondrial iron-sulfur cluster-containing protein aconitase 2 (Aco2) increase during EMT along with other enzymes of the Krebs cycle, indicating a key metabolic role of iron for the acquisition of the mesenchymal cell state. Levels of 2-ketoglutarate, a metabolite that can be produced by the Krebs cycle, increased accordingly, thus providing a rationale for the higher cellular iron load as observed for that cell state (Muller et al., 2020). Additionally, iron- and 2-ketoglutarate-dependent demethylases were found to be up-regulated upon induction of EMT in cancer cells of several lineages and this correlated with an increase of CD44 and ferritin. The cellular content of iron increased along with these demethylases, thereby promoting the depletion of repressive histone marks, including H3K27me3 and H3K9me2, leading to increased expression of mesenchymal and EMT genes. Since histone methylation is a competing chemical process, it was argued that to transiently shift the cellular equilibrium towards a mixed EM or mesenchymal state, cells increase the concentration of reagents that promote demethylation (e.g., iron, 2-ketoglutarate, demethylases), very much akin to physical chemistry processes subjected to Le Chatelier’s principle, defining a fundamental chemical biology paradigm.
In an important study, a chromatin-mediated reversible drug-tolerant state implicating the H3K4me3 lysine demethylase 5B (KDM5A) was reported in non-small-cell lung carcinoma (NSCLC) (Sharma et al., 2010). In another study, it was shown in pancreatic ductal adenocarcinoma (PDAC) that large-scale epigenetic reprogramming of H3K9me2 takes place in distant metastasis, supporting the prevalent role of iron and iron-dependent demethylases in the regulation of cancer cell plasticity and cancer cell dissemination (McDonald et al., 2017; Muller et al., 2020). It is noteworthy that PDAC is a notoriously difficult-to-treat disease characterized by high EMT potential driven by the transcription factor zinc finger E-box binding homeobox 1 (ZEB1), like in TNBC (Krebs et al., 2017). In melanoma cells, single cell transcriptomic analysis revealed high transcriptional variability that can predict drug resistance (Shaffer et al., 2017). Drug treatment was accompanied by epigenetic reprogramming that controlled the loss of differentiating factors such as SRY-box transcription factor 10 (SOX10) and activation of new signaling pathways, which may involve the slow-cycling iron-dependent demethylase KDM5B. This work revealed the multistage nature of acquisition of drug resistance and supported the existence of rare populations of cells susceptible to exploit such reprogramming. Interestingly, like for triple negative breast cancer (TNBC), NSCLC, melanoma and PDAC are characterized by cells expressing high levels of CD44 in the CSC niche and drug-tolerant populations (McDonald et al., 2017; Shaffer et al., 2017; Sharma et al., 2010), pointing towards heightened iron trafficking as a driver of cell plasticity and drug resistance (Muller et al., 2020). Aberrant demethylation of histones is a hallmark of cancer recurrence and correlates with poor survival. Regulation of the epigenetic landscape directly implicates iron-dependent demethylases (Greer and Shi, 2012). This is also supported by data showing that pharmacological inhibition of iron-dependent demethylases targeting the permissive mark H3K4me3 (e.g., KDM5) reduced survival of drug-tolerant cancer cells in various tissue-types (Vinogradova et al., 2016). This body of work illuminates a direct role of iron in the regulation of cancer cell plasticity pointing towards a vulnerability of these cells to iron chemistry.
Vulnerability of cancer cells to ferroptosis
Persister cancer cells and drug-tolerant cancer cells define populations of cells refractory to treatment in the academic and clinical settings. These cells can sustain tumor progression during treatment and inexorably lead to cancer relapse post-therapy; for example, when the optimization of their fitness proceeds over time (Marine et al., 2020; Shen et al., 2020). We suggest that this intermediate phase common to many cancer patients, where there is evidence of tumor debulking yet survival of the patient requires ongoing treatment, is the basis of the clinical term “minimal residual disease”. While these distinct terminologies encompass somewhat overlapping features, persister cancer cells per se may carry or acquire genetic alterations providing them with a clonal advantage over other cancer cells to escape the selective pressure imposed by cancer therapy. In contrast, it is increasingly recognized that cancer cells can dedifferentiate, exploiting metabolic and epigenetic plasticity to reversibly acquire resistance to therapy independently of genetic alterations. These biological features contribute to the heterogenous nature of tumors making it challenging to identify effective therapeutic strategies (Fig. 3). In particular, small molecules able to target the CSC niche and populations of cells that exhibit inherent tolerance to therapy or acquire drug tolerance are scarce. The vulnerability of cancer cell populations to ferroptosis reflecting the longstanding implication of iron in tumor progression has recently been reviewed (Torti and Torti, 2020). Such vulnerability is consistent with the dependency of CSCs to iron and the prevalent role of this metal in the regulation of epigenetic plasticity underlying EMT with the acquisition of a therapy-resistant state (Fig. 3).
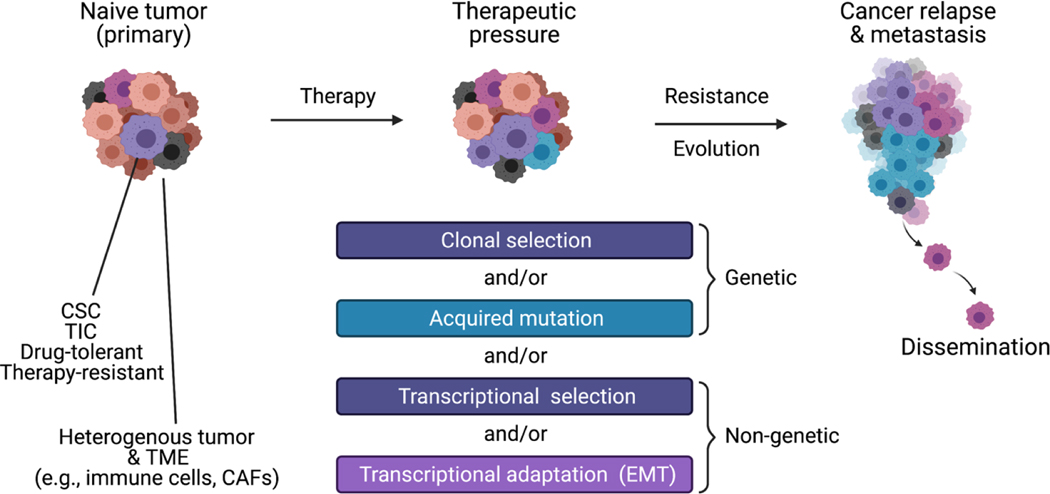
Figure 3. Paradigm of persister cancer cells.
Tumors are heterogenous by nature and composed of cancerous and non-cancerous cells. Notably, the latter includes cancer associated fibroblasts (CAFs) and immune cells constituting the tumor microenvironment (TEM). Therapy promotes evolution of the tumor composition towards the selection of cells that are refractory to treatment. This can lead to metastasis and cancer relapse.
In line with this, it has been shown that autophagic degradation of ferritin resulting in the release of labile iron primes PDAC for ferroptosis (Kremer et al., 2021), and that cysteine depletion in mice models of PDAC leads to ferroptosis (Badgley et al., 2020). Remarkably, the dependency of a drug-tolerant persister state of cancer cells to a lipid peroxidase pathway (i.e., GPX4) has been documented (Hangauer et al., 2017; Viswanathan et al., 2017). This work revealed that cells in an EM or mesenchymal-like state are characterized by the biosynthesis and esterification of PUFAs at membranes, which are exquisite substrates of peroxidation by iron-dependent LOX or non-enzymatic (iron-dependent) processes (Fig. 2). This finding is consistent with the higher iron load and iron-dependency of the mesenchymal state of cells (Muller et al., 2020). Whether PUFAs are structurally or functionally required in the mesenchymal state of cells remains unclear. This increased dependency on PUFA-enriched membranes and a dramatic shift in metabolic activity mirrors what was reported for astrocytic cells forced to undergo direct neuronal reprogramming, which was intriguingly associated with a highly increased sensitivity towards ferroptosis (Gascon et al., 2016).
Regardless, peroxidized lipids are chemically unstable substrates that can readily be transformed into reactive oxygen radicals in the presence of iron and that leads to the formation of lipid peroxidation breakdown products such as truncated phospholipids and even toxic aldehydes. Their occurrence ultimately promotes ferroptotic cell death if not counteracted by the phospholipid hydroperoxidase GPX4 and/or the FSP1 system. Notably, this vulnerability was shown across cancer cells of distinct origins in a drug-tolerant state, including ZEB1-driven cells prone to epithelial-mesenchymal plasticity, TGF-β-induced therapy-resistant melanoma cells, as well as sarcomas which are inherently fixed in a mesenchymal state. Consistently, pharmacological inhibition of GPX4 promotes ferroptosis in a wide range of cancer types in vitro and in vivo, paving the way towards the development of small molecules effective against cancer cells capable of adopting a therapy-resistant state.
Therapeutic opportunities
Targeting pathways that impact on the pool of peroxidized lipid
Uncontrolled lipid peroxidation of cellular membranes is the main driver of ferroptosis. As such, interfering with related protective systems is considered as one of the most promising approaches to eradicate tumor cells, and in particular of those in a therapy-resistant state (as outlined in (Friedmann Angeli et al., 2019). Since the cyst(e)ine/glutathione/GPX4 axis still constitutes the “guardian” of ferroptosis, compounds that inhibit the activity of any of these molecules might be regarded as the most powerful tools. Erastin was the first described ferroptosis-inducing agent (Dixon et al., 2012; Dolma et al., 2003) (Table 1 and Fig. 4). This small molecule irreversibly and specifically inhibits system xc- (Sato et al., 2018), similar to the FDA-approved drug sulfasalazine (though with much less potency) and millimolar concentrations of extracellular L-glutamate among others (Conrad and Sato, 2012; Gout et al., 2001). Inhibition of system xc- ultimately causes cysteine starvation, GSH depletion and GPX4 inactivation. While erastin works extremely well in cells that depend on cysteine taken up by system xc- in the form of cystine, it is metabolically unstable and thus not suitable for in vivo use. Therefore, improved analogues of erastin were developed in the meantime including piperazine-erastin and imidazole ketone erastin (IKE) (Larraufie et al., 2015; Zhang et al., 2019). Nonetheless, not all cells require system xc- for survival and proliferation and a careful stratification is mandatory to determine whether a given cancer cell actually depends on this amino acid antiporter. Moreover, genetic loss of the substrate-specific subunit of system xc-, Slc7a11, is well-tolerated in mice given that the majority of cyst(e)ine is present in its reduced form cysteine in the extracellular space in vivo, which can be easily taken up by neutral amino acid transporters thereby bypassing the need for SLC7A11 (Sato et al., 2005). Evidently, this is in stark contrast to cell and organoid cultures where almost all cysteine is present in its oxidized, dimeric form, necessitating the expression of system xc-. Thus, system xc- presents one of the most promising molecular targets in triggering ferroptosis in the cancer context, as repeatedly demonstrated in cells and mice with reduced or abrogated expression of SLC7A11 (Arensman et al., 2019; Badgley et al., 2020; Lim et al., 2019; Sato et al., 2020). Not only is tumor growth hampered by the lack of SLC7A11, but also the metastasizing potential of B16F10 melanoma cells was dramatically impaired in Slc7a11 knockout cells (Badgley et al., 2020; Sato et al., 2020). Yet, it seems to be at odds that SLC7A11 deficiency can be bypassed in vivo by neutral amino acid transporters taking up cysteine from the extracellular space and thereby feeding tumor cells with the building block of GSH. Whether impaired glutamate secretion or other metabolic constraints accounts for the impaired tumor growth observed under SLC7A11-depleted conditions thus warrants further experimentation. An alternative way to deprive cells and mice from cyst(e)ine is the use of cyst(e)inase, an engineered and pharmacologically optimized human enzyme that degrades extracellular cyst(e)ine and thereby suppresses tumor growth in various tumor entities (Cramer et al., 2017).
Table 1.
Main inducers of iron-dependent cell death
| Small molecules | Cell death | Targets/MoA | Preclinical models | References |
|---|---|---|---|---|
| Erastin | Ferroptosis | Irreversibly inhibits system xc- | BJ, TIP5 transformed cell lines | (Dixon et al., 2012; Dolma et al., 2003) |
| Imidazole ketone erastin (IKE) | BJ transformed cell lines, DLBCL xenografts model | (Larraufie et al., 2015; Zhang et al., 2019) | ||
| Sulfasalazine | Lymphoma cell lines | (Gout et al., 2001) | ||
| L-buthionine sulfoximine (BSO) | Sensitizes to ferroptosis | Inhibits γ-glutamylcysteine synthetase (γ-GCS) and GSH production | Not defined | (Griffith and Meister, 1979) |
| Piperlongumine (PL) | Ferroptosis | Acts partly by modulating GSH levels | Various in vitro and in vivo models including pancreatic cancer | (Tripathi and Biswal, 2020; Yamaguchi et al., 2018) |
| Phenethylisocyanate (PEITC) | Sensitizes to ferroptosis | Various in vitro and in vivo models including ovarian and pancreatic cancer | (Kasukabe et al., 2016; Trachootham et al., 2006) | |
| ML162 | Ferroptosis | Inhibits GPX4 | BJ transformed cell line | (Weiwer et al., 2012) |
| RSL3 | BJ transformed cell line | (Yang et al., 2014; Yang and Stockwell, 2008) | ||
| Masked nitrile-oxides (e.g., ML210) and propiolamides | Various cancer cell lines including melanoma, renal, fibrosarcoma, colon, pancreatic cancers | (Eaton et al., 2020a; Eaton et al., 2020b) | ||
| iFSP1 | Synergizes with RSL3-and dimethyl fumarate-induced ferroptosis | Inhibits FSP1 | Various cancer cell lines including DLBCL cell lines | (Doll et al., 2019; Schmitt et al., 2021) |
| Brequinar | Ferroptosis in GPX4low cells | Inhibits DHODH and synergizes with sulfasalazine in GPX4high cells | Various in vitro and in vivo models including fibrosarcoma and lung squamous cell carcinoma | (Mao et al., 2021) |
| Fin56 | Ferroptosis | Promotes GPX4 degradation and activates squalene synthase | BJ transformed cell lines | (Shimada et al., 2016) |
| FINO2 | Promotes iron oxidation and indirect inhibition of GPX4 | BJ transformed cell line, fibrosarcoma | (Abrams et al., 2016; Gaschler et al., 2018) | |
| Ferroptocide | Inhibits thioredoxin | Various cancer cell lines and primary cancer cells | (Llabani et al., 2019) | |
| Methotrexate | Synergizes with GPX4 inhibition to induce ferroptosis | Inhibits dihydrofolate reductase | Jurkat cells | (Soula et al., 2020) |
| Salinomycin, ironomycin | Lysosomal cell death | Sequester lysosomal iron | HMLER transformed cell line, breast cancer xenografts | (Mai et al., 2017; Muller et al., 2020) |
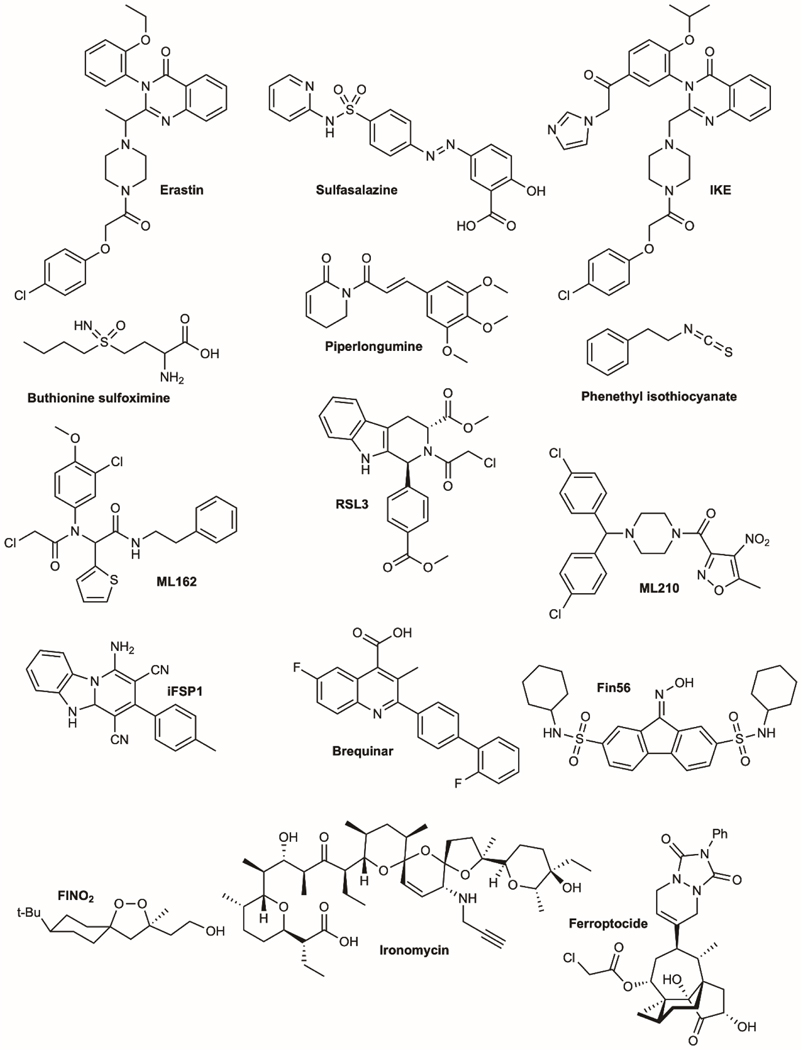
Figure 4.
Molecular structures of cell death inducers
Further downstream in the cascade, cellular depletion of GSH might be another powerful means to sensitize cells towards ferroptosis by indirectly inhibiting GPX4. L-buthionine sulfoximine (BSO), an old clinically approved drug that specifically inhibits γ-glutamylcysteine synthetase (γ-GCS), the enzyme catalyzing the first and rate-limiting step in GSH biosynthesis, remains a valid tool to modulate GSH levels in cells and tissues and might be at least considered as a ferroptosis-sensitizing compound (Griffith and Meister, 1979). In addition, piperlongumine, a biologically active alkaloid/amide phytochemical that may also act by depleting intracellular GSH, or perhaps other electrophilic compounds, might be further explored in the context of ferroptosis induction (Tripathi and Biswal, 2020; Yamaguchi et al., 2018). The naturally occurring isothiocyanate, ß-phenethyl isothiocyanate (PEITC), an ingredient of cruciferous vegetables and known to be an anticancer drug, should be investigated more carefully in the context of ferroptosis due to its GSH modulating activity (Kasukabe et al., 2016; Trachootham et al., 2006). Since all these upstream events ultimately converge on the phospholipid peroxidase GPX4, inhibitors targeting this important enzyme also represents a promising strategy, although challenging in light of the poorly druggable nature of this protein. In fact, a series of GPX4 inhibitors, such as (1S,3R)-RSL3 (RSL3), ML162 and ML210 and various derivatives thereof, have been identified that very efficiently trigger ferroptosis, at least in a cellular context (Moosmayer et al., 2021; Weiwer et al., 2012; Yang et al., 2014; Yang and Stockwell, 2008). Due to their highly electrophilic nature, the mechanism of action of these compounds relies on the covalent alkylation of the active site selenocysteine of GPX4, although a recent report indicated that RSL3 not only targets GPX4, but almost all other selenocysteine containing proteins, of which 24 are present in humans in addition to GPX4 (Chen et al., 2018). Another caveat of these compounds is their inherently poor selectivity and/or unfavorable pharmacokinetic properties strongly hindering their use in vivo. This, however, might have been overcome with the development of masked electrophiles which undergo chemical transformations in cells to yield reactive propiolamide- and nitrile-oxide warheads (Eaton et al., 2020a; Eaton et al., 2020b). This buffered chemical reactivity enables a more selective targeting of GPX4, despite these compounds still being covalent GPX4 inhibitors. Yet, targeting GPX4 by non-covalent inhibitors has not been reported, which is most likely based on its overall protein structure that is known to lack a classical binding pocket. Reversible GPX4 inhibitors, in turn, might be preferred as GPX4 is an essential protein for many tissues in mice including kidney, liver and certain regions in the brain (Conrad et al., 2021).
Unlike GPX4, knocking out the FSP1-coding gene (i.e., Aifm2) does not cause any apparent phenotype in mice akin to animals lacking Slc7a11 (B6.129-Aifm2tm1Marc/https://www.infrafrontier.eu/search; (Mei et al., 2006; Tonnus et al., 2021)), which argues in favor of a likely broad therapeutic window for inhibitors targeting either FSP1 or system xc-. However, due to the dispensable role for FSP1 in cell proliferation and survival across a large number of tumor cell lines (Doll et al., 2019), it can be expected that targeting FSP1 alone might not be sufficient to efficiently kill tumor cells. In fact, the first described FSP1 inhibitor iFSP1 does not cause tumor cell death but sensitizes tumor cells towards RSL3-induced ferroptosis in culture, as well as when xenotransplanted in fish when combined with dimethyl fumarate (Schmitt et al., 2021). This is consistent with the first discovery that the NAD(P)H/CoQ10/FSP1 is a highly efficient backup system when GPX4 function is perturbed or disrupted (Bersuker et al., 2019; Doll et al., 2019). In contrast, inactivation of dihydroorotate dehydrogenase (DHODH) leads to mitochondrial lipid peroxidation and ferroptosis in GPX4low cancer cells, while inhibiting DHODH with brequinar synergizes with sulfasalazine to induce ferroptosis and to reduce tumor growth supporting the protective role of DHODH against ferroptosis (Mao et al., 2021).
Beyond these, an impressive arsenal of ferroptosis inducers has been described over the past decade directed towards distinct targets in the ferroptotic pathway. For instance, Fin56 has been reported to exhibit a dual activity causing both GPX4 deprivation and activation of squalene synthase (Shimada et al., 2016), while the endoperoxide-containing 1,2-dioxolane FINO2 causes ferroptosis by a mechanism likely involving iron oxidation and GPX4 inhibition (Abrams et al., 2016; Gaschler et al., 2018). Chemical modification of the natural product pleuromutilin, an antibiotic originally isolated from the fungus Clitopilus passeckerianus, yielded ferroptocide as an efficient ferroptosis inducer presumably by inhibiting the small redox-active protein thioredoxin (Caneque and Rodriguez, 2019; Llabani et al., 2019). This is interesting as thioredoxin is an essential component of the second mainstay in mammalian redox control, i.e., the thioredoxin-dependent system (besides the GSH-dependent system). In addition, the tetrahydrobiopterin (BH4) biosynthesis pathway including GTP cyclohydrolase I (GTPCH) was recently identified as another backup system for compromised GPX4 activity (Kraft et al., 2020). It was shown that BH4 acts as a radical trapping antioxidant (similar to ferrostatin-1 and liproxstatin-1 (Zilka et al., 2017)), involving regeneration of oxidized BH4 (i.e., dihydrobiopterin, BH2) by dihydrofolate reductase (DHFR) (Soula et al., 2020). Accordingly, methotrexate, an FDA-approved drug and inhibitor of DHFR, was shown to synergize with GPX4 inhibitors to kill cancer cells (Soula et al., 2020). Finally, overloading cancer cells with iron-packed nanoparticles used either alone or in conjunction with lipid hydroperoxides and/or heat might be another strategy to sensitize to ferroptosis or even kill cancer cells efficiently. This strategy, however, requires functionalization of these toxic cargoes to reach their actual targets to eradicate the desired population of cells with sufficient therapeutic windows (Kim et al., 2016; Xie et al., 2021; Xu et al., 2020).
Exploiting the labile iron pool
The dependency of CSCs on iron and the finding that iron is required for the regulation of EMT, together with the vulnerability of the mesenchymal drug-tolerant persister state to ferroptosis, suggest that iron chemistry may directly be exploited for therapeutic benefits. While strategies designed to circumvent accumulation of peroxidized lipids represent effective approaches to induce ferroptosis, it was shown that sequestering iron in the lysosomal compartment can kill cells undergoing EMT in vitro and eradicates a TIC population in patient-derived xenografts of breast cancer (Mai et al., 2017). Since most cells internalize iron by endocytic processes and that the CSC marker CD44, which correlates with cancer recurrence and poor outcome, mediates iron endocytosis in the mesenchymal state of cancer cells, lysosomes represent the hub where iron can be strategically intercepted prior to reaching the relevant cellular compartments. Because the iron load is high in these cells overexpressing CD44, the effect of sequestering iron in lysosomes is expected to be more pronounced compared to drug-sensitive epithelial counterparts (Table 1 and Fig. 4).
Two major phenotypic consequences may arise from sequestering iron in this compartment. The first is a depletion of iron in various subcellular compartments, which inevitably impacts on multiple processes in particular ribosomal function, mitochondrial metabolism and in the nucleus where epigenetic transition involved in the regulation of EMT require iron-dependent demethylases among other enzymes. The second is that iron accumulation in lysosomes can lead to the production of reactive oxygen radicals overtime through Fenton chemistry, leading to lipid membrane peroxidation and lysosome membrane permeabilization (LMP), a trigger of lysosomal cell death. Importantly, it is the lysosomal biochemical content that governs mechanisms of cell death modalities, which is expected to be cell-type dependent.
In TNBC cells, it was shown that ironomycin, a potent synthetic derivative of salinomycin, accumulates in lysosomes, thereby promoting the production of reactive oxygen species (ROS) and inducing lysosomal leakage and cell death reminiscent of ferroptosis (Mai et al., 2017; Muller et al., 2020). Specifically, it was shown that the iron chelator DFO, the cysteine donor and reducing agent N-acetyl cysteine (NAC), as well as the ferroptosis inhibitor ferrostatin-1 partly protected CD44high HMLER tumorigenic breast cells and EGF-induced mesenchymal TNBC cells from ironomycin. Additionally, liproxstatin-1 blocked lipid peroxidation induced by ironomycin in TNBC. The absence of complete protection against cell death may stem from the fact that lysosomal dysfunction can trigger other cell death modalities that are kinetically favored, should a specific vulnerability be pharmacologically diverted (Galluzzi et al., 2014). While this mechanism is consistent to some extent with ferroptotic cell death (e.g., viability rescue permitted by DFO and ferrostatin-1), it may not fully comply with all the features of canonical ferroptosis vulnerability. The chemical reaction induced by iron in ironomycin-treated cells takes place in a distinct cellular compartment (e.g., the lysosome) compared to ferroptosis that may occur as a result of system xc- or GPX4 inhibition, and the types of toxic oxygen radicals produced may also be of distinct nature. Thus, it can be seen as a chemical induction of toxic species manipulating cellular iron (active mechanism) rather than a blockade of the production of agents that would normally interfere with the accumulation of such species (passive mechanism), thereby conferring a particular vulnerability. Regardless, the work by Mai et al. and Muller et al. highlight lysosomal iron as a vulnerability point of cancer cells in the mesenchymal state (Mai et al., 2017; Muller et al., 2020). Pharmacological reprogramming of iron sub-cellular localization, thereby diverting metabolism and epigenetic plasticity, and re-directing the chemistry of iron to instead promote Fenton chemistry, may thus represent a promising therapeutic angle that warrants further investigation. Interestingly, it was shown that supplementing cells undergoing lysosomal dysfunction with iron rescues cell proliferation and that treating cancer cells with an iron chelator alters cancer cell proliferation (Weber et al., 2020). Furthermore, persister cancer cells can maintain proliferative capacity by up-regulating antioxidant gene programs (Oren et al., 2021). These studies hint towards iron being a valuable target to interfere with the proliferation of cancer cells in the persister state.
CONCLUSION
Contrary to belief, iron is not a trace element. Unlike many other metals, it is abundant in cells and tissues and plays distinct roles essential for the biology of the cell. The fact that iron is implicated in cancer progression has been established beyond reasonable doubt. Recent literature converges towards the idea that iron is involved in metabolic and epigenetic processes enabling specific populations of cells to escape treatments including chemo- and radio-therapy. At the same time, these cells inevitably acquire a high vulnerability to chemical processes promoted by iron including lipid peroxidation, which, if not carefully controlled can lead to ferroptotic cell death. A key role of iron in the regulation of EMT has recently been uncovered (Muller et al., 2020), implicating the CSC marker CD44 along with hyaluronates, which are abundantly found in some of the most aggressive solid tumors, in iron uptake and regulation of epigenetic plasticity. This raises the question as to whether CD44 or the hyaluronate content of tumors may be used as predictors of ferroptosis vulnerability. In support of this, CD44 has been shown to stabilize system xc- regulating the redox status of cancer cells and promoting tumor growth (Ishimoto et al., 2011). This raises the question of a coordinated regulation of cystine uptake concomitant with iron endocytosis. Furthermore, ePLs, which are dynamically regulated during cell-state transitions and are potential substrates of peroxidation have recently been implicated in ferroptosis (Aldrovandi and Conrad, 2020; Zou et al., 2020a). Whether these lipids play a structural role in membranes of mesenchymal cells or are involved in a particular signaling axis of drug-tolerant persister cancer cells remains to be fully characterized.
Nature is driven by chemically favored processes. Reactive oxygen radicals are likely to be formed when iron chemistry is at work, independently of whether these radicals are useful or toxic for cells. While biology is constantly subjected to evolution, universal principles of physical chemistry are not, at least not on the same time scale. It is our opinion that life has evolved mechanisms to circumvent undesired chemistry. For instance, cells have implemented proteins such as system xc-, GPX4 and FSP1 to deal with potentially deleterious molecules produced as a result of a high iron-load, where iron is required for cells to adopt distinct identities, one that may protect them from therapy. In this context, small molecules offer the means to manipulate the chemistry at work to dissect iron biology and provide the basis for the development of innovative and powerful next-generation therapeutics (Schreiber et al., 2015).
ACKNOWLEDGMENTS
R.R. is funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 647973), the Fondation Charles Defforey-Institut de France, Ligue Contre le Cancer (Equipe Labellisée) and Région Ile-de-France. S.L.S. is partly supported by the National Cancer Institute’s Cancer Target Discovery and Development (CTD2) Network (grant number U01CA217848). M.C. is supported by the Deutsche Forschungsgemeinschaft (DFG) CO 291/7–1, CO 297/9–1 and CO 297/10–1, the German Federal Ministry of Education and Research (BMBF) VIP+ program NEUROPROTEKT (03VP04260), the Ministry of Science and Higher Education of the Russian Federation (075–15-2019–1933) and the Else Kröner-Fresenius-Stiftung (2020_EKTP19). M.C. is further funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. GA 884754). We apologize to those whose work could not be discussed here due to space restrictions.
DECLARATION OF INTERESTS
R.R. is a founder of Adrestia Therapeutics and SideROS. S.L.S. is a shareholder and serves on the Board of Directors of Jnana Therapeutics and Kojin Therapeutics; is a shareholder of Forma Therapeutics and Decibel Therapeutics; is a shareholder and advises Kisbee Therapeutics, Belharra Therapeutics, Vividian Therapeutics, Exo Therapeutics, and Eikonizo Therapeutics; serves on the Scientific Advisory Boards of Eisai Co., Ltd., Ono Pharma Foundation, Biogen, Inc., F-Prime Capital Partners and the Board of Advisers of the Genomics Institute of the Novartis Research Foundation; and is a Novartis Faculty Scholar. M.C. is a founder and shareholder of ROSCUE Therapeutics GmbH and is a member of the Molecular Cell Advisory Board.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abrams RP, Carroll WL, and Woerpel KA (2016). Five-Membered Ring Peroxide Selectively Initiates Ferroptosis in Cancer Cells. ACS Chem Biol 11, 1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrovandi M, and Conrad M. (2020). Ferroptosis: the Good, the Bad and the Ugly. Cell research 30, 1061–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrovandi M, Fedorova M, and Conrad M. (2021). Juggling with lipids, a game of Russian roulette. Trends Endocrinol Metab 32, 463–473. [DOI] [PubMed] [Google Scholar]
- Arensman MD, Yang XS, Leahy DM, Toral-Barza L, Mileski M, Rosfjord EC, Wang F, Deng S, Myers JS, Abraham RT, et al. (2019). Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proc Natl Acad Sci U S A 116, 9533–9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J, Firl CEM, et al. (2020). Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368, 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basuli D, Tesfay L, Deng Z, Paul B, Yamamoto Y, Ning G, Xian W, McKeon F, Lynch M, Crum CP, et al. (2017). Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene 36, 4089–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, and Clevers H. (2017). Cancer stem cells revisited. Nat Med 23, 1124–1134. [DOI] [PubMed] [Google Scholar]
- Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al. (2019). The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumahdi S, and de Sauvage FJ (2020). The great escape: tumour cell plasticity in resistance to targeted therapy. Nat Rev Drug Discov 19, 39–56. [DOI] [PubMed] [Google Scholar]
- Brutsch SH., Wang CC., Li L., Stender H., Neziroglu N., Richter C., Kuhn H., and Borchert A. (2015). Expression of inactive glutathione peroxidase 4 leads to embryonic lethality, and inactivation of the alox15 gene does not rescue such knock-in mice. Antioxid Redox Signal 22, 281–293. [DOI] [PubMed] [Google Scholar]
- Caneque T, and Rodriguez R. (2019). Diverse engineering . Nature chemistry 11, 499–500. [DOI] [PubMed] [Google Scholar]
- Chen MS, and White MC (2007). A predictably selective aliphatic C-H oxidation reaction for complex molecule synthesis. Science 318, 783–787. [DOI] [PubMed] [Google Scholar]
- Chen MS, and White MC (2010). Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science 327, 566–571. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu Y, Lan T, Qin W, Zhu Y, Qin K, Gao J, Wang H, Hou X, Chen N, et al. (2018). Quantitative Profiling of Protein Carbonylations in Ferroptosis by an Aniline-Derived Probe. J Am Chem Soc 140, 4712–4720. [DOI] [PubMed] [Google Scholar]
- Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, et al. (2014). Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C, Krieg T, and Murphy MP (2016). A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab 23, 254–263. [DOI] [PubMed] [Google Scholar]
- Conrad M, Lorenz SM, and Proneth B. (2021). Targeting Ferroptosis: New Hope for As-Yet-Incurable Diseases. Trends Mol Med 27, 113–122. [DOI] [PubMed] [Google Scholar]
- Conrad M, and Pratt DA (2019). The chemical basis of ferroptosis. Nat Chem Biol 15, 1137–1147. [DOI] [PubMed] [Google Scholar]
- Conrad M, and Sato H. (2012). The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (−) : cystine supplier and beyond. Amino Acids 42, 231–246. [DOI] [PubMed] [Google Scholar]
- Cramer SL, Saha A, Liu J, Tadi S, Tiziani S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson S, et al. (2017). Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med 23, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, Superti-Furga G, and Stockwell BR (2015). Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem Biol 10, 1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Grocin AG, Xavier da Silva TN, Panzilius E, Scheel CH, et al. (2019). FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698. [DOI] [PubMed] [Google Scholar]
- Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, et al. (2017). ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolma S, Lessnick SL, Hahn WC, and Stockwell BR (2003). Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296. [DOI] [PubMed] [Google Scholar]
- Eaton JK, Furst L, Cai LL, Viswanathan VS, and Schreiber SL (2020a). Structure-activity relationships of GPX4 inhibitor warheads. Bioorg Med Chem Lett 30, 127538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JK, Furst L, Ruberto RA, Moosmayer D, Hilpmann A, Ryan MJ, Zimmermann K, Cai LL, Niehues M, Badock V, et al. (2020b). Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat Chem Biol 16, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann Angeli JP, Krysko DV, and Conrad M. (2019). Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer 19, 405–414. [DOI] [PubMed] [Google Scholar]
- Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, et al. (2014). Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16, 1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Bravo-San Pedro JM, and Kroemer G. (2014). Organelle-specific initiation of cell death. Nat Cell Biol 16, 728–736. [DOI] [PubMed] [Google Scholar]
- Gao M, Monian P, Pan Q, Zhang W, Xiang J, and Jiang X. (2016). Ferroptosis is an autophagic cell death process. Cell research 26, 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Monian P, Quadri N, Ramasamy R, and Jiang X. (2015). Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell 59, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS, Bos PH, Reznik E, et al. (2018). FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol 14, 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon S., Murenu E., Masserdotti G., Ortega F., Russo GL., Petrik D., Deshpande A., Heinrich C., Karow M., Robertson SP., et al. (2016). Identification and Successful Negotiation of a Metabolic Checkpoint in Direct Neuronal Reprogramming. Cell stem cell 18, 396–409. [DOI] [PubMed] [Google Scholar]
- Gout PW, Buckley AR, Simms CR, and Bruchovsky N. (2001). Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia 15, 1633–1640. [DOI] [PubMed] [Google Scholar]
- Greer EL, and Shi Y. (2012). Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 13, 343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW, and Meister A. (1979). Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254, 7558–7560. [PubMed] [Google Scholar]
- Gryzik M, Asperti M, Denardo A, Arosio P, and Poli M. (2021). NCOA4-mediated ferritinophagy promotes ferroptosis induced by erastin, but not by RSL3 in HeLa cells. Biochim Biophys Acta Mol Cell Res 1868, 118913. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, and Lander ES (2009). Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138, 645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, et al. (2017). Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Galy B, and Camaschella C. (2010). Two to tango: regulation of Mammalian iron metabolism. Cell 142, 24–38. [DOI] [PubMed] [Google Scholar]
- Homma T, Kobayashi S, Sato H, and Fujii J. (2021). Superoxide produced by mitochondrial complex III plays a pivotal role in the execution of ferroptosis induced by cysteine starvation. Arch Biochem Biophys 700, 108775. [DOI] [PubMed] [Google Scholar]
- Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, and Tang D. (2016). Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12, 1425–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, et al. (2018). Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 172, 409–422 e421. [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al. (2011). CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell 19, 387–400. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al. (2017). Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasukabe T, Honma Y, Okabe-Kado J, Higuchi Y, Kato N, and Kumakura S. (2016). Combined treatment with cotylenin A and phenethyl isothiocyanate induces strong antitumor activity mainly through the induction of ferroptotic cell death in human pancreatic cancer cells. Oncol Rep 36, 968–976. [DOI] [PubMed] [Google Scholar]
- Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, Conrad M, Turker MZ, Gao M, Jiang X, et al. (2016). Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol 11, 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Muller C, Zandkarimi F, Merl-Pham J, Bao X, Anastasov N, Kossl J, et al. (2020). GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci 6, 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs AM., Mitschke J., Lasierra Losada M., Schmalhofer O., Boerries M., Busch H., Boettcher M., Mougiakakos D., Reichardt W., Bronsert P., et al. (2017). The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol 19, 518–529. [DOI] [PubMed] [Google Scholar]
- Kremer DM, Nelson BS, Lin L, Yarosz EL, Halbrook CJ, Kerk SA, Sajjakulnukit P, Myers A, Thurston G, Hou SW, et al. (2021). GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nature communications 12, 4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AW, and Weinberg RA (2021). Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer 21, 325–338. [DOI] [PubMed] [Google Scholar]
- Larraufie MH, Yang WS, Jiang E, Thomas AG, Slusher BS, and Stockwell BR (2015). Incorporation of metabolically stable ketones into a small molecule probe to increase potency and water solubility. Bioorg Med Chem Lett 25, 4787–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Yoo SE, Na R, Walter CA, Richardson A, and Ran Q. (2009). Short form glutathione peroxidase 4 is the essential isoform required for survival and somatic mitochondrial functions. J Biol Chem 284, 30836–30844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JKM, Delaidelli A, Minaker SW, Zhang HF, Colovic M, Yang H, Negri GL, von Karstedt S, Lockwood WW, Schaffer P, et al. (2019). Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc Natl Acad Sci U S A 116, 9433–9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llabani E, Hicklin RW, Lee HY, Motika SE, Crawford LA, Weerapana E, and Hergenrother PJ (2019). Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis. Nature chemistry 11, 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, Tarangelo A, Ward CC, Cho K, Patti GJ, Nomura DK, et al. (2019). Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem Biol 26, 420–432 e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai TT, Hamai A, Hienzsch A, Caneque T, Muller S, Wicinski J, Cabaud O, Leroy C, David A, Acevedo V, et al. (2017). Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nature chemistry 9, 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, Koppula P, Wu S, Zhuang L, Fang B, et al. (2021). DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marine JC, Dawson SJ, and Dawson MA (2020). Non-genetic mechanisms of therapeutic resistance in cancer. Nat Rev Cancer 20, 743–756. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, and Kopf M. (2015). T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med 212, 555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald OG, Li X, Saunders T, Tryggvadottir R, Mentch SJ, Warmoes MO, Word AE, Carrer A, Salz TH, Natsume S, et al. (2017). Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet 49, 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei J, Webb S, Zhang B, and Shu HB (2006). The p53-inducible apoptotic protein AMID is not required for normal development and tumor suppression. Oncogene 25, 849–856. [DOI] [PubMed] [Google Scholar]
- Moosmayer D, Hilpmann A, Hoffmann J, Schnirch L, Zimmermann K, Badock V, Furst L, Eaton JK, Viswanathan VS, Schreiber SL, et al. (2021). Crystal structures of the selenoprotein glutathione peroxidase 4 in its apo form and in complex with the covalently bound inhibitor ML162. Acta Crystallogr D Struct Biol 77, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckenthaler MU, Rivella S, Hentze MW, and Galy B. (2017). A Red Carpet for Iron Metabolism. Cell 168, 344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Sindikubwabo F, Caneque T, Lafon A, Versini A, Lombard B, Loew D, Wu TD, Ginestier C, Charafe-Jauffret E, et al. (2020). CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nature chemistry 12, 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Huang RY, Jackson RA, and Thiery JP (2016). Emt: 2016. Cell 166, 21–45. [DOI] [PubMed] [Google Scholar]
- Oren Y, Tsabar M, Cuoco MS, Amir-Zilberstein L, Cabanos HF, Hutter JC, Hu B, Thakore PI, Tabaka M, Fulco CP, et al. (2021). Cycling cancer persister cells arise from lineages with distinct programs. Nature 596, 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantopoulos K, Porwal SK, Tartakoff A, and Devireddy L. (2012). Mechanisms of mammalian iron homeostasis. Biochemistry 51, 5705–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Shiiya A, Kimata M, Maebara K, Tamba M, Sakakura Y, Makino N, Sugiyama F, Yagami K, Moriguchi T, et al. (2005). Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem 280, 37423–37429. [DOI] [PubMed] [Google Scholar]
- Sato M., Kusumi R., Hamashima S., Kobayashi S., Sasaki S., Komiyama Y., Izumikawa T., Conrad M., Bannai S., and Sato H. (2018). The ferroptosis inducer erastin irreversibly inhibits system xc- and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci Rep 8, 968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Onuma K, Domon M, Hasegawa S, Suzuki A, Kusumi R, Hino R, Kakihara N, Kanda Y, Osaki M, et al. (2020). Loss of the cystine/glutamate antiporter in melanoma abrogates tumor metastasis and markedly increases survival rates of mice. Int J Cancer 147, 3224–3235. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Xu W, Bucher P, Grimm M, Konantz M, Horn H, Zapukhlyak M, Berning P, Brandle M, Jarboui MA, et al. (2021). Dimethyl fumarate induces ferroptosis and impairs NF-kappaB/STAT3 signaling in DLBCL. Blood 138, 871–884. [DOI] [PubMed] [Google Scholar]
- Schonberg DL, Miller TE, Wu Q, Flavahan WA, Das NK, Hale JS, Hubert CG, Mack SC, Jarrar AM, Karl RT, et al. (2015). Preferential Iron Trafficking Characterizes Glioblastoma Stem-like Cells. Cancer Cell 28, 441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M, and Fahimi HD (2006). Peroxisomes and oxidative stress. Biochim Biophys Acta 1763, 1755–1766. [DOI] [PubMed] [Google Scholar]
- Schreiber SL (1980). Fragmentation reactions of .alpha.-alkoxy hydroperoxides and application to the synthesis of the macrolide (.+−.)-recifeiolide. Journal of the American Chemical Society 102, 6163–6165. [Google Scholar]
- Schreiber SL, Kotz JD, Li M, Aube J, Austin CP, Reed JC, Rosen H, White EL, Sklar LA, Lindsley CW, et al. (2015). Advancing Biological Understanding and Therapeutics Discovery with Small-Molecule Probes. Cell 161, 1252–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W, et al. (2008). Glutathione Peroxidase 4 Senses and Translates Oxidative Stress into 12/15-Lipoxygenase Dependent- and AIF-Mediated Cell Death. Cell Metab 8, 237–248. [DOI] [PubMed] [Google Scholar]
- Shaffer SM, Dunagin MC, Torborg SR, Torre EA, Emert B, Krepler C, Beqiri M, Sproesser K, Brafford PA, Xiao M, et al. (2017). Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 546, 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R, Shchepinov MS, and Pratt DA (2018). Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent Sci 4, 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, et al. (2010). A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Vagner S, and Robert C. (2020). Persistent Cancer Cells: The Deadly Survivors. Cell 183, 860–874. [DOI] [PubMed] [Google Scholar]
- Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ, and Stockwell BR (2016). Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol 12, 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F, Molina H, Garcia-Bermudez J, Pratt DA, and Birsoy K. (2020). Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol 16, 1351–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuelten CH, Mertins SD, Busch JI, Gowens M, Scudiero DA, Burkett MW, Hite KM, Alley M, Hollingshead M, Shoemaker RH, et al. (2010). Complex display of putative tumor stem cell markers in the NCI60 tumor cell line panel. Stem Cells 28, 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnus W, Meyer C, Steinebach C, Belavgeni A, von Massenhausen A, Gonzalez NZ, Maremonti F, Gembardt F, Himmerkus N, Latk M, et al. (2021). Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nature communications 12, 4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti SV, and Torti FM (2013). Iron and cancer: more ore to be mined. Nat Rev Cancer 13, 342–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti SV, and Torti FM (2020). Iron: The cancer connection. Mol Aspects Med 75, 100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D., Zhou Y., Zhang H., Demizu Y., Chen Z., Pelicano H., Chiao PJ., Achanta G., Arlinghaus RB., Liu J., et al. (2006). Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 10, 241–252. [DOI] [PubMed] [Google Scholar]
- Tripathi SK, and Biswal BK (2020). Piperlongumine, a potent anticancer phytotherapeutic: Perspectives on contemporary status and future possibilities as an anticancer agent. Pharmacol Res 156, 104772. [DOI] [PubMed] [Google Scholar]
- Turcu AL, Versini A, Khene N, Gaillet C, Caneque T, Muller S, and Rodriguez R. (2020). DMT1 Inhibitors Kill Cancer Stem Cells by Blocking Lysosomal Iron Translocation. Chemistry 26, 7369–7373. [DOI] [PubMed] [Google Scholar]
- Ubellacker JM, Tasdogan A, Ramesh V, Shen B, Mitchell EC, Martin-Sandoval MS, Gu Z, McCormick ML, Durham AB, Spitz DR, et al. (2020). Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova M, Gehling VS, Gustafson A, Arora S, Tindell CA, Wilson C, Williamson KE, Guler GD, Gangurde P, Manieri W, et al. (2016). An inhibitor of KDM5 demethylases reduces survival of drug-tolerant cancer cells. Nat Chem Biol 12, 531–538. [DOI] [PubMed] [Google Scholar]
- Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, et al. (2017). Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yen FS, Zhu XG, Timson RC, Weber R, Xing C, Liu Y, Allwein B, Luo H, Yeh HW, et al. (2021). SLC25A39 is necessary for mitochondrial glutathione import in mammalian cells. Nature 599, 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RA, Yen FS, Nicholson SPV, Alwaseem H, Bayraktar EC, Alam M, Timson RC, La K, Abu-Remaileh M, Molina H, et al. (2020). Maintaining Iron Homeostasis Is the Key Role of Lysosomal Acidity for Cell Proliferation. Mol Cell 77, 645–655 e647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiwer M, Bittker JA, Lewis TA, Shimada K, Yang WS, MacPherson L, Dandapani S, Palmer M, Stockwell BR, Schreiber SL, et al. (2012). Development of small-molecule probes that selectively kill cells induced to express mutant RAS. Bioorg Med Chem Lett 22, 1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ED, Gao D, Redfern A, and Thompson EW (2019). Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat Rev Cancer 19, 716–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Sun W, Zhang C, Dong B, Yang J, Hou M, Xiong L, Cai B, Liu X, and Xue W. (2021). Metabolic Control by Heat Stress Determining Cell Fate to Ferroptosis for Effective Cancer Therapy. ACS Nano 15, 7179–7194. [DOI] [PubMed] [Google Scholar]
- Xu W, Barrientos T, and Andrews NC (2013). Iron and copper in mitochondrial diseases. Cell Metab 17, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Qin Z, Ma J, Cao W, and Zhang P. (2020). Recent progress in nanotechnology based ferroptotic therapies for clinical applications. Eur J Pharmacol 880, 173198. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Kasukabe T., and Kumakura S. (2018). Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int J Oncol 52, 1011–1022. [DOI] [PubMed] [Google Scholar]
- Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J, Zhang Z, and Wang X. (2021). Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol Cell 81, 355–369 e310. [DOI] [PubMed] [Google Scholar]
- Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, et al. (2020). Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 21, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, et al. (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, and Stockwell BR (2008). Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15, 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, Brown LM, Uchida K, O’Connor OA, and Stockwell BR (2019). Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cell Chem Biol 26, 623–633 e629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, and Pratt DA (2017). On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent Sci 3, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, Paradkar S, Boehnke N, Deik AA, Reinhardt F, et al. (2020a). Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 585, 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W, Sandoval-Gomez G, Clish CB, Doench JG, and Schreiber SL (2020b). Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol 16, 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]