Abstract
Introduction
Major haemorrhage after injury is the leading cause of preventable death for trauma patients. Recent advancements in trauma care suggest damage control resuscitation (DCR) should start in the prehospital phase following major trauma. In Italy, Helicopter Emergency Medical Services (HEMS) assist the most complex injuries and deliver the most advanced interventions including DCR. The effect size of DCR delivered prehospitally on survival remains however unclear.
Methods and analysis
This is an investigator-initiated, large, national, prospective, observational cohort study aiming to recruit >500 patients in haemorrhagic shock after major trauma. We aim at describing the current practice of hypotensive trauma management as well as propose the creation of a national registry of patients with haemorrhagic shock. Primary objective: the exploration of the effect size of the variation in clinical practice on the mortality of hypotensive trauma patients. The primary outcome measure will be 24 hours, 7-day and 30-day mortality. Secondary outcomes include: association of prehospital factors and survival from injury to hospital admission, hospital length of stay, prehospital and in-hospital complications, hospital outcomes; use of prehospital ultrasound; association of prehospital factors and volume of first 24-hours blood product administration and evaluation of the prevalence of use, appropriateness, haemodynamic, metabolic and effects on mortality of prehospital blood transfusions. Inclusion criteria: age >18 years, traumatic injury attended by a HEMS team including a physician, a systolic blood pressure <90 mm Hg or weak/absent radial pulse and a confirmed or clinically likely diagnosis of major haemorrhage. Prehospital and in-hospital variables will be collected to include key times, clinical findings, examinations and interventions. Patients will be followed-up until day 30 from admission. The Glasgow Outcome Scale Extended will be collected at 30 days from admission.
Ethics and dissemination
The study has been approved by the Ethics committee ‘Comitato Etico di Area Vasta Emilia Centro’. Data will be disseminated to the scientific community by abstracts submitted to international conferences and by original articles submitted to peer-reviewed journals.
Trial registration number
Keywords: trauma management, accident & emergency medicine, intensive & critical care
Strengths and limitations of this study.
The main strength of this study relies on its inclusive approach, aiming at recruiting a large number of patients from any region in the country. This will provide a detailed description of patient characteristics, management and their association with clinical outcomes.
The primary endpoint is to describe the current clinical practice and assess the effectiveness of current practice on patients’ 30 days mortality.
Due to the observational nature of this study, we will not be able to strongly confirm the causal effects between prehospital damage control resuscitation and outcomes. This work will serve as a founding basis to design future targeted randomised control trials.
Introduction
Background and rationale
Major haemorrhage after injury is a global health burden and remains the leading cause of immediate and early preventable death for trauma patients.1 Traumatic bleeding has challenged civilian and military health systems for many years with extremely high mortality rates.2 Over the past decade, trauma resuscitation practice has changed from large-volume fluid replacement targeting perfusion to ‘damage control resuscitation’ that prioritises early correction of coagulation abnormalities.3–7
Damage control resuscitation (DCR) was introduced in 2007 after the earlier discovery of acute traumatic coagulopathy, aiming to protect the ability of the patient’s blood to form a clot during the acute bleeding episode.8 While previous transfusion protocols were focused on correcting coagulation deficiencies after a massive red blood cell (RBC) and crystalloid transfusion volume, in contrast, prevention of coagulopathy is the priority in the new major haemorrhage protocols. The principles of the DCR are early haemorrhage control, permissive hypotension, prevention of dilutional coagulopathy and the identification and rapid treatment of any trauma-induced coagulopathy. Thus, evolutions in trauma care were the introduction of balanced resuscitation with blood products (plasma, RBC, platelets or, if available, even whole blood),9 early administration of tranexamic acid for the treatment of hyperfibrinolysis,10 correction of coagulopathy with targeted administration of clotting factors (based on point-of-care clotting diagnostics as thromboelastometry) and early transfusion strategies that brought blood products into the prehospital phase.11–15 Improved survival from such strategies has been reported in both military and civilian settings.16–19 Projection of blood products into the prehospital phase of trauma care is intuitively attractive as it reduces time-to-transfusion and improves prehospital survival. However, the use of Pre-Hospital Blood Products (PHBP) is both logistically challenging and resource-intensive and is not without risk. Effective delivery may require significant performance improvement in prehospital services. Moreover, a substantial benefit on long-term mortality is not yet clear.20 The recently published Resuscitation with pre-hospital blood products (RePHILL) study investigated whether packed RBCs and lyophilised plasma was superior to normal saline for improving tissue perfusion and reducing mortality in trauma-related haemorrhagic shock.21 The trial was terminated early due to difficult recruitment during the COVID-19 pandemic and failed to find any difference in the composite outcome (episode mortality and/or failure to clear lactates) between the two treatment arms. The study however presents several complexities such as the inclusion of a broad population of shocked but not necessarily anaemic trauma patients and the use of a composite outcome of episode mortality and/or lactate clearance (two instances that might not be additive per se). Therefore, the results of this trial are difficult to translate to the broad spectrum of trauma-related haemorrhages, particularly in exsanguinating trauma patients.
Experts in the field have called for prospective studies to improve the knowledge on prehospital trauma resuscitation and, in particular, the role and effects of prehospital damage control resuscitation strategies as per the early administration of blood products.12 22–25 Recently the Italian National Institute for Health (Istituto Superiore di Sanità) published national trauma guidelines suggesting that prehospital blood transfusion should be considered in bleeding trauma patients.26
In Italy, there are 55 Helicopter Emergency Medical Service (HEMS) bases currently active. HEMS are managed by dispatch centres that are set up on a regional and geographical basis that often correspond to a level 1 trauma centre hospital. HEMS teams usually include one or two pilots, one doctor, one or two nurses and when a Search and Rescue configuration is in operation an alpine rescue technician. Twenty-four of the 55 HEMS bases work around the clock covering night shifts, while 31 are day-limited. Unfortunately, it is not possible to provide overall data on HEMS interventions throughout Italy as there is no national patient registry. Many systems have a particular configuration either for personnel, logistics or clinical procedures, and no national clinical guidelines or indications exist. Nevertheless, it is estimated that the case volume amounts to about 700 missions per year per base and a nocturnal overall activity of around 7%–8%.27 In October 2020 Bologna and Grosseto HEMS became the first Italian civilian prehospital service to routinely offer prehospital red blood cell transfusion. To our knowledge, selected HEMS teams are the only services currently carrying blood products outside the hospital in Italy. To date, we are aware that four HEMS bases in Italy are currently carrying prehospital blood products, through different configurations and availability, RBCs, pre-thawed plasma, fibrinogen concentrate and prothrombin complex concentrate are in use (Bergamo, Bologna, Foggia and Grosseto HEMS bases). Complex interventions such as prehospital blood administration require strong governance and documentation. A significant component of any trauma system is a trauma registry (TR), which is a comprehensive repository of information on the victims of injuries including received treatments. TRs allow the monitoring and benchmarking of patient care with the ultimate aim of improving and reducing the variability of trauma management, for instance across a complex national system.28 With this study we aim at describing the current practice of hypotensive trauma management as well as propose the creation of a national registry of patients with haemorrhagic shock, intending to investigate the relative influence of practice variation on outcomes and ultimately to foster the development of future randomised studies.
Aim and objectives
This is the first attempt to conduct a national multicentre study describing the current prehospital clinical practice, and its impact on in-hospital outcomes. The primary objective of the SPITFIRE study is the exploration of the effect size of the variation in clinical practice of damage control resuscitation on the mortality of hypotensive trauma patients.29 The primary outcome measure will be 24 hours, 7 days and 30 days mortality.
Secondary objectives are:
Association of prehospital factors and survival from injury to hospital admission, intensive care unit length of stay, hospital length of stay, prehospital complications, in-hospital complications and hospital outcomes.
Prevalence, specificity and sensitivity of clinical examination and prehospital ultrasound when available.30 31
Association of prehospital factors and volume of first 24 hours blood product administration.
Evaluation of the prevalence of use, appropriateness, haemodynamic, metabolic and effects on mortality of prehospital blood transfusions.
Evaluation of 30-day functional status.
Finally, the study data set will be used to prospectively test a previously validated optimal treatment bundle designed to minimise 7-day mortality in haemorrhagic shock using machine learning.32
Methods and analysis
Study design
We have designed an investigator-initiated, large, national, prospective, observational cohort study aiming to recruit >500 patients in haemorrhagic shock after major trauma. The actual study start date was 1 May 2021 and the estimated primary completion date is 1 May 2025.
Patient and public involvement
Patients and the public have not been involved in the study design or in the recruitment and conduct of the study.
Setting
Italy. As of December 2021, a total of 55 HEMS bases are operating in the country (50 all year-long and 5 seasonal, to serve tourist/holiday peaks). Study investigators (MT and LC) performed extensive research to obtain the email addresses of local air ambulance coordinators. As soon as contact details were obtained a standardised invitation email was sent to representatives of each service, with regular interval reminders encouraging study participation.
Currently, 22 bases have agreed to participate and are at various stages of study activation, with 15 actively recruiting patients (Bologna, Pavullo, Grosseto, Massa, Torino, Alessandria, Borgosesia, Cuneo, Udine, Trento, Bolzano, Bressanone, Lasa, Pontives and Treviso).
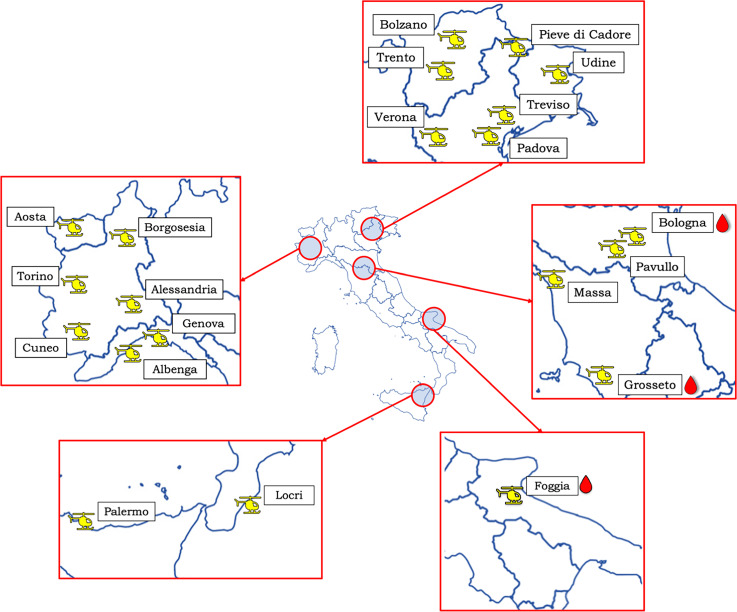
Recruitment for this study started on 1 May 2021 and is expected to last a minimum of 4 years. Additional HEMS bases are encouraged to join the study at any time. On requests from candidate centres, a copy of the study protocol and electronic Case Report Form (eCRF) is sent out for assessment. The geographical distribution of participating centres as of February 2022 is presented in figure 1.
Figure 1.
Current study centres (February 2022). Blood drop represents HEMS bases with blood components availability. The Bolzano icon represents the four Alto Adige provincial bases: Bolzano, Bressanone, Lasa and Pontives. HEMS, Helicopter Emergency Medical Services.
Participants
Inclusion criteria for this study are chosen a priori based on previously published research protocols and trials investigating prehospital major trauma managed by HEMS and prehospital fluid delivery.22 33 34
All patients fulfilling the following inclusion criteria will be recruited:
Age >18 years,
Have sustained a traumatic injury and attended by a prehospital helicopter emergency team which includes a physician,
A systolic blood pressure <90 mm Hg or weak/absent radial pulse during the primary assessment, treatment or transportation to the hospital. In case of upper limb injury any contralateral peripheral pulse is considered acceptable,
A confirmed or clinically likely diagnosis of major haemorrhage.
The decision to only include Italian prehospital helicopter emergency medical services is based on the fact that at the time of writing HEMS teams are the most likely to be exposed to severe trauma patients, deliver the most advanced interventions on scene and are the only (although still uncommon) resources currently carrying blood products prehospitally.
Patients will be excluded if deemed unsalvageable by the HEMS team before starting any resuscitation manoeuvre.
Variables and source of data
Two major sets of variables will be collected: prehospital and hospital data. Prehospital variables will include mission details, times and mechanism of injury. Clinical assessment and interventions performed by the prehospital team (including volume of all fluids, crystalloid and colloid and all types of blood products and concentrates). Hospital variables will include emergency department assessment and interventions, results of diagnostic (imaging and laboratory) and therapeutic procedures (including volumes of fluids and blood products). Patients will be followed up at 24 hours, day 7 and day 30 from admission for organ failures and support. Centres will collect the Glasgow Outcome Scale Extended at 30 days from admission. Death happening at any time will be recorded as well as the primary cause of death. Patient demographics including medical history, gender, age and comorbidities will be extracted from the patient medical records.
Prehospital and hospital variables will be recorded by treating clinicians or study investigators depending on local clinical practice (some centres may share personnel between the two settings, while others will have separate teams for prehospital and in-hospital). All clinical evaluations will be performed as per usual standard of care. Variable selection was based on previously published consensus conferences on prehospital data collection.35–37 Details on collected variables are presented in tables 1 and 2.
Table 1.
Prehospital study variables
| Dispatch and mission | These describe whether the mission includes search and rescue procedures which are known to increase overall prehospital times; type of dispatch (primary, on-scene crew request or secondary transfer); resources and level of resources on scene (BLS type vs ALS with different configurations); evacuation to hospital (air vs road). |
| Times | Key times for the prehospital phase: dispatch centre call-connect time (‘injury time’), HEMS team with patient time. Hospital arrival time (emergency department triage) will be used to determine the overall prehospital time. |
| Mechanism of injury (MOI) | Primary descriptor of the MOI. Road traffic accident (RTA), fall (< or >3 metres), assault (blunt, penetrating knife or gunshot), burns, explosion, crush, electrocution, hanging, animal bite, drowning. If RTA further descriptors are collected: means of transportation, protection (helmet, seat belt, etc), the role of the injured (passenger, pedestrian, etc) and estimated energy (high vs low). Finally, intentionality is recorded (accidental, self-harm, assault, unknown). |
| HEMS clinical examination | Primary clinical assessment including recording of airway, status, lowest SpO2 and arterial systolic/diastolic blood pressure or radial pulse status. Observed or presumed sites of bleeding (long bones, external compressible haemorrhage, penetrating injury, junctional haemorrhage, (sub)amputation, suspected pelvic fracture, haemothorax, hemoperitoneum. Neurological assessment (first recorded Glasgow Coma Scale, HEMS assessed Glasgow Coma Score and sensorimotor deficits). Ultrasound extended Focused Assessment with Sonography for Trauma (eFAST). If eFAST was performed and findings. |
| Prehospital cardiac arrest | Whether the patient was at any time in traumatic cardiac arrest and if a return of spontaneous circulation was obtained. |
| HEMS interventions | Interventions on airways, breathing and circulation including orotracheal intubation or supraglottic device use, use of tourniquets, haemostatic gauzes, pelvic binder, thoracostomies, REBOA or resuscitative thoracotomy and positioning of wide bore vascular access. In case of REBOA a subset of data will be collected (anatomical zone and duration of balloon inflation). Prehospital fluids and blood products. Total volume of prehospital crystalloid, colloid, units of prehospital packed red blood cells, grams of prehospital fibrinogen, volume of prehospital plasma and use of clotting factors (prothrombin complex concentrate). Other drugs. Induction agents, vasopressors, osmotic and hypertonic fluids, electrolytes, tranexamic acid. |
ALS, Advanced Life Support; BLS, Basic Life Support; HEMS, Helicopter Emergency Medical Services; REBOA, Rescuscitative Endovascular Balloon Occlusion of the Aorta; SpO2, Peripheral Oxygen Saturation.
Table 2.
Hospital study variables
| Trauma team activation | Data regarding the presence and activation of a trauma team. Also whether a prehospital activation of a massive transfusion protocol is performed. |
| Emergency department clinical examination | Emergency department clinical assessment including airways, breathing and circulation, estimation of bleeding. Neurological assessment (Glasgow Coma Score, pupils and sensorimotor deficits). Temperature at admission. |
| Biochemical data | First arterial blood gas performed at hospital admission (pH, PaO2, PaCO2, HCO3, lactate, base excess, haemoglobin). ROTEM (if available). INR value at admission. aPTT value at admission. |
| Emergency department interventions | Interventions on airways, breathing and circulation including orotracheal intubation or supraglottic device use, use of tourniquets, haemostatic gauzes, pelvic binder, REBOA or resuscitative thoracotomy and positioning of high flow vascular access. In case of REBOA a subset of data will be collected (anatomical zone and duration of balloon inflation). Emergency department fluids and blood products. Total volume of prehospital crystalloids and colloids, units of prehospital packed red blood cells, grams of prehospital fibrinogen, volume of prehospital plasma. |
| Emergency department diagnostics | Extended Focused Assessment with Sonography for Trauma (if performed and findings). X-Ray (if performed and findings). CT (if performed and findings, date and time of CT). |
| Emergency department outcomes | Haemodynamic status at disposition from the emergency department (systolic blood pressure, heart rate). Lactate and base excess at emergency department disposal. |
| Post-emergency department interventions | The patient pathway following disposition from the emergency department is recorded. Patients might be taken into surgery, angiography, intensive care or die. If taken to surgery or angiography details of the procedure and intraoperative findings are recorded. Eventual intraoperative cardiac arrest and return of spontaneous circulation are recorded. |
| Scores | Injury Severity Score is collected according to international coding standards. Sequential Organ Failure Score at ICU admission. SAPS2 at ICU admission. |
| Intensive care unit (ICU)/high dependency unit (HDU) admission and discharge | Blood gases at ICU/HDU admission. Blood gases 24 hours from ICU/HDU admission. Organ failures within the first 24 hours of hospital admission. Type and duration of organ support (mechanical ventilation, tracheostomy, vasopressors, renal replacement). Total number of packed red blood cells during first 24 hours (including prehospital transfusion if applicable). Total volume (mL) of plasma during first 24 hours (including prehospital transfusion if applicable). Total number of platelets pool during first 24 hours (including prehospital transfusion if applicable). Total grams of fibrinogen concentrate during first 24 hours (including prehospital transfusion if applicable). Total volume (mL) of crystalloids and colloids during first 24 hours (including prehospital transfusion if applicable). Further organ failures and surgical interventions at 7 days from hospital admission. Further organ failures and surgical interventions at 30 days from hospital admission. Glasgow Outcome Scale Extended 30 days from hospital admission. Date, condition and location of hospital discharge. Primary cause of death. |
aPTT, activated Partial Thromboplastin Time; INR, International Normalized Ratio; REBOA, Resuscitative Endovascular Balloon Occlusion of the Aorta; ROTEM, Rotational Thromboelastometry; SAPS2, Simplified Acute Physiology Score 2.
Data collection and management
Anonymised data will be collected via a web-based secure eCRF using the REDCap software (Research Electronic Data Capture, Vanderbilt University, Nashville, Tennessee, USA). The data will be securely stored on the study coordinator’s local health authority research server (AUSL Bologna, REDCap license from IRCCS Bellaria, Bologna, Italy). Data will be regularly checked for consistency and completeness by the study coordinator and steering committee. Reminders will be sent to local investigators to correct potential irregularities. All participating centres will join a data transfer agreement to define the terms for data transfer from the centres to the sponsor. All procedures will comply with the European Union Regulation 2016/279 on data protection.
Study size
The primary endpoint is to describe the current clinical practice and assess the effectiveness of current practice on patients’ 30 days mortality. Major trauma patients experience an overall 20% mortality.2 9 In order to perform a logistic regression analysis using mortality as the dependent variable, 10 events are needed for each covariate inserted in the model as a rule of thumb.
Therefore, a model involving up to 10 covariates will need at least 500 patients.
Plan for analysis
Patients’ characteristics and patterns of lesions will be depicted using descriptive statistics and specific analyses are planned for the primary and secondary objectives. The methodology of analysis is also based on the suggestions from the previous work on multicentre observational prehospital resuscitation on helicopter study by Holcomb et al. 38 39
Primary objective
The variation in clinical practice will be described through descriptive statistics.
The exploration of factors influencing 30 days mortality will be performed as follows: patients will be divided into two groups based on 30 days mortality, and the prehospital and trauma-related variables resulting significantly different between the two groups will be tested as covariates in a univariable logistic regression model. Finally, the multivariable model building will be performed through the least angle regression selection considering as candidate variables all those variables resulting associated with 30 days mortality with a p<0.1 in the univariable analysis. SEs will be adjusted considering single HEMS bases as clusters.
Subsequently, the effect size of single interventions, in particular, the prehospital administration of RBCs, coagulation products and Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) will be studied through propensity score adjusted logistic regression models.
Secondary objectives
Factors associated with survival to hospital admission will be estimated with a multivariable logistic regression model building as described for 30 days mortality.
Prehospital extended Focused Assessment with Sonography for Trauma (eFAST) accuracy will be tested against CT scan and/or operating room findings of hemoperitoneum. The occurrence of a positive eFAST will be tested as an independent factor in a Cox proportional hazards regression model considering time to definitive diagnostics (CT scan) or treatment (operating room access). A propensity score matching will be performed to adjust for the covariates that influence the probability of receiving eFAST in the prehospital setting.
A quantilic regression model will be used to test factors associated with the volume of blood product administration during the first 24 hours after hospital admission.
Discussion
Given that the utility of a TR that includes injured patients across a nationwide trauma system is universally established, we aim to obtain data from a large cohort of trauma patients attended by HEMS. We will provide a detailed description of the patients’ characteristics, bleeding trauma patients’ management strategies, resource use, system organisation and performance and their correlation to clinical outcomes. The differences in the management of bleeding trauma patients nationwide, both prehospital and in-hospital, their treatment and therapeutic strategies together with potential outcome association will also be described.
The results generated from this study will complement other large multicentre studies focusing on bleeding trauma patient practice. In addition, for the first time, we aim to collect large-scale national data on trauma resuscitation from the prehospital HEMS phase to the initial in-hospital management.
Bleeding trauma patients is certainly not a naïve research field, however, the nationwide approach can be considered the main strength and novelty of the study since it allows to explore the clinical practice in geographical regions characterised by very different healthcare organisations.
Concluding, SPITFIRE study protocol provides a timely and unique opportunity to generate high-quality evidence regarding prehospital and in-hospital trauma resuscitation. Evidence that we hope will provide useful information for improving the overall management of the bleeding trauma patient nationally and internationally, moreover, it will provide useful quality improvement data for prehospital helicopter emergency services around the country and lastly, we hope, it will make a significant difference to influencing future research questions and improving patient care.
Ethics and dissemination
Ethics
The study has been approved by the Ethics Committee ‘Comitato Etico di Area Vasta Emilia Centro’ (approval date 22 October 2020). Each participating centre/local Principal Investigator is responsible for obtaining local approval in compliance with the local legislation and rules. The national coordinators will facilitate this process.
Major trauma is unpredictable and often incapacitating and thus immediate prospective informed consent from patients might not be possible. Moreover, in the case patients will retain capacity they will likely need immediate life-saving interventions that cannot be deferred to collect study consent. Consent to participate in the SPITFIRE trial will be sought at the earliest opportunity. Patients or legal representatives will be approached by a local study investigator at a time when they are well and able to receive and process information. All reasonable efforts will be put into place to inform patients or their proxies and to obtain informed consent. In case of patients who die before consent is obtained and with no legal representatives recorded data will be included in the study as per indication of the Italian data protection authority, section on scientific research (section 5.3.2 Provvedimento del Garante n. 146 del 5 Giugno 2019). Patients can be removed from the study, at their own or their legal representative’s request, at any time.
The study will be performed according to the Helsinki Declaration and International Conference on Harmonisation of Good Clinical Practice.
Registration and funding
SPITFIRE is registered with ClinicalTrials.gov. This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The Article Processing Charge for this publication will be offered by the no-profit Fondazione Franca Capurro of Novara.
Dissemination
The study results will be submitted for publication in peer-reviewed international journals and presented at national and international meetings. Study participants will be sent a summary of the final results, including details of their local study population.
Supplementary Material
Footnotes
Twitter: @marco_tarta, @carenzmd, @mazzoli_carlo
Collaborators: SPITFIRE Study Collaborators: Luca Montagnani, Andrea Caglià and Jacopo Pernechele (Aosta HEMS base, Aosta, Italy), Andrea Mina, Simona Cavallo and Roberto Vacca (Torino HEMS base, Torino, Italy), Roberto Gioachin (Borgosesia HEMS base, Borgosesia, Italy), Valeria Bonato (Alessandria HEMS Base, Alessandria, Italy), Claudia Monge (Cuneo HEMS base, Cuneo, Italy), Paolo Frisoni and Luca Nicora (Albenga and Genova HEMS base, Genova, Italy), Giovanna Zilio (Treviso HEMS base, Treviso, Italy), Cristina Barbarino (Pieve di Cadore HEMS base, Pieve di Cadore, Italy), Andrea Paoli, Giacomo Magagnotti and Andrea Spagna (Padova HEMS base, Padova, Italy), Alberto Trincanato and Francesca Verginella (Bolzano, Bressanone, Lasa, Pontives-Laion HEMS bases, Bolzano, Italy), Marta Pescolderung and Stefania Armani (Trento HEMS base, Trento, Italy), Adriano Valerio (Verona HEMS base, Verona, Italy), Giulio Desiderio (Pavullo HEMS base, Pavullo, Italy), Edoardo Picetti (UO Anestesia e Rianimazione, Parma, Italy), Michela Ciminello, Christian Tosato, Yuri Ferrara and Stefano Barbadori (Grosseto HEMS base, Grosseto, Italy), Silvia Pini, Andrea Vignali and Alberto Baratta (Massa HEMS base, Massa, Italy), Davide Durì, Calogero Centonze, Matteo Ciccolini and Alessandra Spasiano (Udine HEMS base, Udine, Italy), Tommaso Marzano, Guido Gambetti (Foggia HEMS base, Foggia, Italy), Domenico Minniti, Marco Tescione (Reggio Calabria/Locri HEMS base, Locri, Italy), Michela Rauseo and Gilda Cinnella (Anesthesia and Intensive Care Unit, Università di Foggia, Foggia, Italy), Tiziana Cena and Rosanna Vaschetto (Anesthesia and Intensive Care Unit, Università del Piemonte Orientale, Novara, Italy), Giacomo Iapichino (Anesthesia and Intensive Care Unit, IRCCS Humanitas Research Hospital, Rozzano, Italy), Tobias Gauss (Anesthésie-Réanimation, CHU Grenoble Alpes, Grenoble, France), Andrea Cortegiani (Anesthesia Intensive Care and Emergency, Università di Palermo, Palermo, Italy), Fabio Genco (Centrale Operativa SUES 118 Pa/Tp, Palermo, Italy), Antonio Iacono (Azienda Ospedali Riuniti Villa Sofia Cervello UOSD Trauma Center, Palermo, Italy), Maria Teresa Strano (Anesthesia and Intensive Care, ARNAS Civico Di Cristina Benfratelli, Palermo, Italy), Annalisa Deiana (Olbia HEMS base, Olbia, Italy), Marco Vidili (Alghero HEMS base, Alghero, Italy), Massimiliano Carta (Cagliari HEMS base, Cagliari, Italy), Alessio Ficarella (Anesthesia and Intensive Care, AO Brotzu, Cagliari, Italy) and Flavia Baccari (Epidemiology and Statistics Unit, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy).
Contributors: MT and LG designed the study (Principal Investigator and co-PI). MT, LC and LG drafted the manuscript; CL, AG, CAM, VC, SC, DL, JBH, GS, CC and GG critically revised the work for important intellectual content; LG and DA are responsible for the statistical and methodological aspects of the study. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Map disclaimer: The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests: JBH is a consultant with Cellphire, Hemostatics and Arsenal, is co-founder, co-CEO and on the Board of Directors of Decisio Health, on the Board of Directors of QinFlow, Zibrio and Oxyband and a co-inventor of the Junctional Emergency Tourniquet Tool. The other authors do not report any competing interest.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
SPITFIRE Study Collaborators:
Luca Montagnani, Andrea Caglià, Jacopo Pernechele, Andrea Mina, Simona Cavallo, Roberto Vacca, Roberto Gioachin, Valeria Bonato, Claudia Monge, Paolo Frisoni, Luca Nicora, Giovanna Zilio, Cristina Barbarino, Andrea Paoli, Giacomo Magagnotti, Andrea Spagna, Alberto Trincanato, Francesca Verginella, Marta Pescolderung, Stefania Armani, Adriano Valerio, Giulio Desiderio, Edoardo Picetti, Michela Ciminello, Christian Tosato, Yuri Ferrara, Stefano Barbadori, Silvia Pini, Andrea Vignali, Alberto Baratta, Davide Durì, Calogero Centonze, Matteo Ciccolini, Alessandra Spasiano, Tommaso Marzano, Guido Gambetti, Domenico Minniti, Michela Rauseo, Gilda Cinnella, Tiziana Cena, Rosanna Vaschetto, Giacomo Iapichino, Tobias Gauss, Andrea Cortegiani, Fabio Genco, Antonio Iacono, Maria Teresa Strano, Annalisa Deiana, Marco Vidili, Massimiliano Carta, Alessio Ficarella, and Flavia Baccari
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Brohi K, Gruen RL, Holcomb JB. Why are bleeding trauma patients still dying? Intensive Care Med 2019;45:709–11. 10.1007/s00134-019-05560-x [DOI] [PubMed] [Google Scholar]
- 2. Stiell IG, Nesbitt LP, Pickett W, et al. The OPALS major trauma study: impact of advanced life-support on survival and morbidity. CMAJ 2008;178:1141–52. 10.1503/cmaj.071154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cole E, Weaver A, Gall L, et al. A decade of damage control resuscitation: new transfusion practice, new survivors, new directions. Ann Surg 2021;273:1215–20. 10.1097/SLA.0000000000003657 [DOI] [PubMed] [Google Scholar]
- 4. Holcomb JB. Major scientific lessons learned in the trauma field over the last two decades. PLoS Med 2017;14:e1002339. 10.1371/journal.pmed.1002339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black JA, Pierce VS, Kerby JD, et al. The evolution of blood transfusion in the trauma patient: whole blood has come full circle. Semin Thromb Hemost 2020;46:215–20. 10.1055/s-0039-3402426 [DOI] [PubMed] [Google Scholar]
- 6. Botteri M, Celi S, Perone G, et al. Effectiveness of massive transfusion protocol activation in pre-hospital setting for major trauma. Injury 2022;53:01064–0. 10.1016/j.injury.2021.12.047 [DOI] [PubMed] [Google Scholar]
- 7. Black JA, Pierce VS, Juneja K, et al. Complications of hemorrhagic shock and massive transfusion-a comparison before and after the damage control resuscitation era. Shock 2021;56:42–51. 10.1097/SHK.0000000000001676 [DOI] [PubMed] [Google Scholar]
- 8. Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma 2007;62:307–10. 10.1097/TA.0b013e3180324124 [DOI] [PubMed] [Google Scholar]
- 9. Sperry JL, Guyette FX, Brown JB, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med 2018;379:315–26. 10.1056/NEJMoa1802345 [DOI] [PubMed] [Google Scholar]
- 10. CRASH-2 trial collaborators, Shakur H, Roberts I, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23–32. 10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 11. Rehn M, Weaver AE, Eshelby S, et al. Pre-hospital transfusion of red blood cells in civilian trauma patients. Transfus Med 2018;28:277–83. 10.1111/tme.12483 [DOI] [PubMed] [Google Scholar]
- 12. Yazer MH, Spinella PC, Bank EA, et al. THOR-AABB working party recommendations for a prehospital blood product transfusion program. Prehosp Emerg Care 2021;271:1–13. 10.1080/10903127.2021.1995089 [DOI] [PubMed] [Google Scholar]
- 13. Ziegler B, Bachler M, Haberfellner H, et al. Efficacy of prehospital administration of fibrinogen concentrate in trauma patients bleeding or presumed to bleed (FIinTIC): a multicentre, double-blind, placebo-controlled, randomised pilot study. Eur J Anaesthesiol 2021;38:348–57. 10.1097/EJA.0000000000001366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spasiano A, Barbarino C, Marangone A, et al. Early thromboelastography in acute traumatic coagulopathy: an observational study focusing on pre-hospital trauma care. Eur J Trauma Emerg Surg 2022;48:431-439. 10.1007/s00068-020-01493-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lockey DJ, Weaver AE, Davies GE. Practical translation of hemorrhage control techniques to the civilian trauma scene. Transfusion 2013;53 Suppl 1:17S–22. 10.1111/trf.12031 [DOI] [PubMed] [Google Scholar]
- 16. Stensballe J, Ostrowski SR, Johansson PI. Haemostatic resuscitation in trauma: the next generation. Curr Opin Crit Care 2016;22:591–7. 10.1097/MCC.0000000000000359 [DOI] [PubMed] [Google Scholar]
- 17. Simmons JW, Powell MF. Acute traumatic coagulopathy: pathophysiology and resuscitation. Br J Anaesth 2016;117:iii31–43. 10.1093/bja/aew328 [DOI] [PubMed] [Google Scholar]
- 18. Voller J, Tobin JM, Cap AP, et al. Joint trauma system clinical practice guideline (JTS CPG): prehospital blood transfusion. 30 October 2020. J Spec Oper Med 2021;21:11–21. [DOI] [PubMed] [Google Scholar]
- 19. Shackelford SA, Del Junco DJ, Powell-Dunford N, et al. Association of prehospital blood product transfusion during medical evacuation of combat casualties in Afghanistan with acute and 30-day survival. JAMA 2017;318:1581–91. 10.1001/jama.2017.15097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rehn M, Weaver A, Brohi K, et al. Effect of prehospital red blood cell transfusion on mortality and time of death in civilian trauma patients. Shock 2019;51:284–8. 10.1097/SHK.0000000000001166 [DOI] [PubMed] [Google Scholar]
- 21. Crombie N, Doughty HA, Bishop JRB, et al. Resuscitation with blood products in patients with trauma-related haemorrhagic shock receiving prehospital care (RePHILL): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Haematol 2022;9:e250–61. 10.1016/S2352-3026(22)00040-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith IM, Crombie N, Bishop JR, et al. RePHILL: protocol for a randomised controlled trial of pre-hospital blood product resuscitation for trauma. Transfus Med 2018;28:346–56. 10.1111/tme.12486 [DOI] [PubMed] [Google Scholar]
- 23. Hashmi ZG, Chehab M, Nathens AB, et al. Whole truths but half the blood: addressing the gap between the evidence and practice of pre-hospital and in-hospital blood product use for trauma resuscitation. Transfusion 2021;61 Suppl 1:S348–53. 10.1111/trf.16515 [DOI] [PubMed] [Google Scholar]
- 24. Holcomb JB, Donathan DP, Cotton BA, et al. Prehospital transfusion of plasma and red blood cells in trauma patients. Prehosp Emerg Care 2015;19:1–9. 10.3109/10903127.2014.923077 [DOI] [PubMed] [Google Scholar]
- 25. Holcomb JB, Swartz MD, DeSantis SM, et al. Multicenter observational prehospital resuscitation on helicopter study. J Trauma Acute Care Surg 2017;83:S83–91. 10.1097/TA.0000000000001484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Linea Guida sulla Gestione Integrata del Trauma Maggiore dalla scena dell’evento alla cura definitiva, 2022. Available: https://snlg.iss.it/?p=2533 [Accessed 01 May 2022].
- 27. Bellini C, Gente M. Helicopter emergency medical service in Italy: a 2021 update. Air Med J 2021;40:419–26. 10.1016/j.amj.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 28. Di Bartolomeo S, Nardi G, Sanson G, et al. The first Italian trauma registry of national relevance: methodology and initial results. Eur J Emerg Med 2006;13:197–203. 10.1097/01.mej.0000217974.54212.a1 [DOI] [PubMed] [Google Scholar]
- 29. Holcomb JB, Moore EE, Sperry JL, et al. Evidence-Based and clinically relevant outcomes for hemorrhage control trauma trials. Ann Surg 2021;273:395–401. 10.1097/SLA.0000000000004563 [DOI] [PubMed] [Google Scholar]
- 30. Press GM, Miller SK, Hassan IA, et al. Prospective evaluation of prehospital trauma ultrasound during aeromedical transport. J Emerg Med 2014;47:638–45. 10.1016/j.jemermed.2014.07.056 [DOI] [PubMed] [Google Scholar]
- 31. Gamberini L, Tartaglione M, Giugni A, et al. The role of prehospital ultrasound in reducing time to definitive care in abdominal trauma patients with moderate to severe liver and spleen injuries. Injury 2022;53:00990–6. 10.1016/j.injury.2021.12.008 [DOI] [PubMed] [Google Scholar]
- 32. Lang E, Neuschwander A, Favé G, et al. Clinical decision support for severe trauma patients: machine learning based definition of a bundle of care for hemorrhagic shock and traumatic brain injury. J Trauma Acute Care Surg 2022;92:135–43. 10.1097/TA.0000000000003401 [DOI] [PubMed] [Google Scholar]
- 33. Naumann DN, Hancox JM, Raitt J, et al. What fluids are given during air ambulance treatment of patients with trauma in the UK, and what might this mean for the future? results from the rescuer observational cohort study. BMJ Open 2018;8:e019627. 10.1136/bmjopen-2017-019627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McManus J, Yershov AL, Ludwig D, et al. Radial pulse character relationships to systolic blood pressure and trauma outcomes. Prehosp Emerg Care 2005;9:423–8. 10.1080/10903120500255891 [DOI] [PubMed] [Google Scholar]
- 35. Krüger AJ, Lockey D, Kurola J, et al. A consensus-based template for documenting and reporting in physician-staffed pre-hospital services. Scand J Trauma Resusc Emerg Med 2011;19:71. 10.1186/1757-7241-19-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tønsager K, Krüger AJ, Ringdal KG, et al. Template for documenting and reporting data in physician-staffed pre-hospital services: a consensus-based update. Scand J Trauma Resusc Emerg Med 2020;28:25. 10.1186/s13049-020-0716-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ringdal KG, Lossius HM, Jones JM, et al. Collecting core data in severely injured patients using a consensus trauma template: an international multicentre study. Crit Care 2011;15:R237. 10.1186/cc10485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeSantis SM, Swartz MD, Greene TJ, et al. Interim monitoring of nonrandomized prospective studies that invoke propensity scoring for decision making. J Trauma Acute Care Surg 2020;88:e46–52. 10.1097/TA.0000000000002474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greene TJ, DeSantis SM, Fox EE, et al. Utilizing propensity score analyses in prehospital blood product transfusion studies: lessons learned and moving toward best practice. Mil Med 2018;183:124–33. 10.1093/milmed/usx137 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.