Abstract
Chemotherapy-induced thrombocytopenia (CIT) is a common complication of the treatment of non-hematologic malignancies. Many patient-related variables (e.g., age, tumor type, number of prior chemotherapy cycles, amount of bone marrow tumor involvement) determine the extent of CIT. CIT is related to the type and dose of chemotherapy, with regimens containing gemcitabine, platinum, or temozolomide producing it most commonly. Bleeding and the need for platelet transfusions in CIT are rather uncommon except in patients with platelet counts below 25x109/L in whom bleeding rates increase significantly and platelet transfusions are the only treatment. Nonetheless, platelet counts below 70x109/L present a challenge. In patients with such counts, it is important to exclude other causes of thrombocytopenia (medications, infection, thrombotic microangiopathy, post-transfusion purpura, coagulopathy and immune thrombocytopenia). If these are not present, the common approach is to reduce chemotherapy dose intensity or switch to other agents. Unfortunately decreasing relative dose intensity is associated with reduced tumor response and remission rates. Thrombopoietic growth factors (recombinant human thrombopoietin, pegylated human megakaryocyte growth and development factor, romiplostim, eltrombopag, avatrombopag and hetrombopag) improve pretreatment and nadir platelet counts, reduce the need for platelet transfusions, and enable chemotherapy dose intensity to be maintained. National Comprehensive Cancer Network guidelines permit their use but their widespread adoption awaits adequate phase III randomized, placebo-controlled studies demonstrating maintenance of relative dose intensity, reduction of platelet transfusions and bleeding, and possibly improved survival. Their potential appropriate use also depends on consensus by the oncology community as to what constitutes an appropriate pretreatment platelet count as well as identification of patient-related and treatment variables that might predict bleeding.
Introduction
Thrombocytopenia is a common problem in patients with cancer, whether due to the underlying disease, infection, other medications or cancer treatment with chemotherapy or radiation. Thrombocytopenia creates a number of problems in the care of the cancer patient. At platelet counts less than 10x109/L, spontaneous bleeding may be increased. At platelet counts less than 50x109/L, surgical procedures are often complicated by bleeding. At platelet counts under 100x109/L, chemotherapy and radiation therapy may be administered with caution thereby decreasing dose intensity and clinical outcome.1 Therapeutic and prophylactic platelet transfusions create the additional risk of infusion-related complications and might be immuno-suppressive.2 Finally, thrombocytopenia instills in the patient a sense of anxiety and fear of bleeding which exacerbates that due to the cancer diagnosis itself.
The clinician’s response to thrombocytopenia in a cancer patient is varied. Reduction of relative dose intensity (RDI) of chemotherapy or radiation is common; less effective regimens may be chosen; treatment may even be precluded. For some, treatment of another cause of thrombocytopenia (e.g., stopping the offending antiviral agent) may be effective. Platelet transfusion is often the only immediately available treatment. There is increasing interest in using recombinant human thrombopoietin (rhTPO) or thrombopoietin receptor agonists (TPO-RA) such as romiplostim, eltrombopag, avatrombopag, lusutrombopag, and hetrombopag to enhance platelet production and platelet counts.3
Recognizing that chemotherapy creates other hematologic problems (e.g., neutropenia) that may also limit the ability to administer chemotherapy, the focus here will be primarily on situations in which thrombocytopenia is a major limiting variable. This review will discuss the general approach to the patient with a non-hematologic malignancy receiving non-myeloablative chemotherapy, the pathophysiology of chemotherapy-induced thrombocytopenia (CIT), and options for treating CIT including TPO-RA. The use of TPO-RA therapy in myeloablative settings (stem cell transplantation and acute myeloid leukemia induction) has been discussed separately.4 A discussion of thrombocytopenia secondary to therapeutic irradiation or treatment of hematologic malignancies is beyond the scope of this review.
CIT is here defined as a platelet count less than 100x109/L and divided into grades as follows; grade 1: 75x109/L to less than 100x109/L; grade 2: 50x109/L to less than 75x109/L; grade 3: 25x109/L to less than 50x109/L; and grade 4: less than 25x109/L.5,6
The platelet count is an imprecise predictor of bleeding risk in cancer patients
The main reason to check the platelet count in a cancer patient receiving chemotherapy is to attempt to predict the bleeding risk. A biological estimate of the lowest effective platelet count for effective hemostasis comes from the work of Slichter and colleagues,7- 9 who used chromium-51 labeled red blood cells to quantify fecal blood loss in stable thrombocytopenic aplastic patients treated only with anabolic steroids. At platelet counts above 10x109/L, blood loss was normal at less than 5 mL/day. At platelet counts of 5x109/L to 10x109/L, blood loss rose slightly to 9±7 mL/day; however at platelet counts below 5x109/L, the loss was markedly elevated to 50±20 mL/day. Subsequent platelet kinetic studies10 found a fixed minimum requirement for 7.1x109 platelets/L/day to maintain vascular integrity; 18% of the normal daily turnover of 41.2x109 platelets/L/day. This is consistent with in vitro data showing that thrombin generation appears to be maximal as long as the platelet count is above 10x109/L; below that value thrombin generation declines in direct proportion to the platelet count.11,12
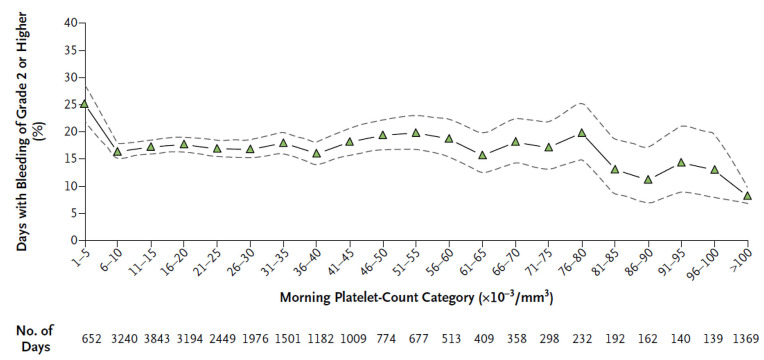
A recent trial assessed the relation of platelet count to bleeding (using a validated bleeding scale6) in thrombocytopenic patients undergoing myeloablative chemotherapy for leukemia or stem cell transplantation (Figure 1).13
It clearly showed bleeding of grade 2 or higher on 25% of days with platelet counts of 5x109/L or less, on 17% of days with platelet counts from 6–80x109/L (P<0.001 for platelet counts ≤5x109/L vs. counts of 6–80x109/L), on 13% of days with platelet counts of 81–100x109/L (P=0.001 for platelet counts of 81–100x109/L vs. counts of 6–80x109/L), and on 8% of days with platelet counts above 100x109/L (P<0.001 for platelet counts >100x109/L vs. counts of 6–80x109/L). In cancer patients undergoing chemotherapy, thrombocytopenic bleeding and bleeding grade have been inadequately studied; in general both increase once the platelet count drops below 75x109/L (odds ratio=3.1; 95% confidence interval [95% CI]: 1.9–5.1).14 Roughly, when the platelet count falls below 50x109/L the probability of bleeding is 0–9.6%; rises to 10.1–17.7% when the count is below 20x109/L; and rises again to 18.4–40.1% when below 10x109/L.15 The incidence of CIT rises with each subsequent cycle of chemotherapy.16
Unfortunately, using just the platelet count to predict bleeding risk for the cancer patient is an over simplification. Platelet function may be altered by other medications, antipyretics, chemotherapy drugs themselves, and renal insufficiency. Platelets in patients with CIT lack the increased size and function of the "young" platelets in immune thrombocytopenia (ITP) which tends to mitigate the bleeding risk seen at comparably low platelet counts in ITP.17 Other patient-related variables markedly affect the hemostatic risk (Table 1). The bleeding risk for each patient needs to be personalized
The importance of maintaining chemotherapy dose intensity
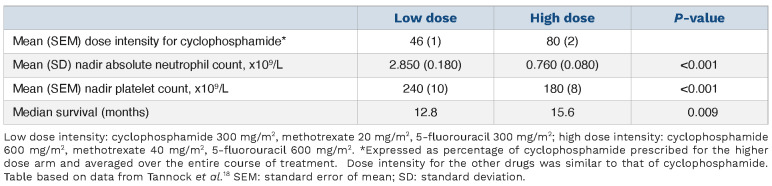
Most chemotherapy regimens have been developed to provide the greatest therapeutic benefit with acceptable toxicity. As demonstrated in Table 2, in patients with metastatic breast cancer, chemotherapy dose intensity is important; when cyclophosphamide/methotrexate/5-fluorouracil dose intensity was decreased by 50%, there was less thrombocytopenia but there was also a significant decrease in median survival.18
Reductions in RDI (due to either reductions in dose or dosing frequency) decrease response rate and survival. In a retrospective study of patients with metastatic colorectal disease being treated with FOLFIRI (folinic acid, fluorouracil, irinotecan) or modified FOLFOX6 (folinic acid, fluorauracil, oxaliplatin), patients receiving a high RDI of irinotecan had a median performance-free survival of 9.9 months compared with 5.6 months for those receiving a low RDI of irinotecan (hazard ratio [HR]=3.18; 95% CI: 1.47–6.88; P<0.01); median overall survival was also reduced from 26.7 months down to 12.9 months for the same two groups (HR=2.72; 95% CI: 1.22–6.04; P=0.01].19
Figure 1.
Relation between bleeding (measured using the World Health Organization bleeding scale) and the platelet count in patients with hypoproliferative thrombocytopenia. The percentage of days on which patients had bleeding of grade 2 or greater is shown, along with the associated 95% confidence intervals (dashed lines), according to the morning platelet count category. (From Slichter et al.13 and reproduced with the permission of the Massachusetts Medical Society.)
Similar effects of reduction in RDI leading to decreased overall survival have been demonstrated in the adjuvant treatment of non-small cell lung cancer,20,21 breast cancer,22 ovarian cancer,22 and non-Hodgkin lymphoma.23 In elderly patients with advanced non-small cell lung cancer, those receiving a RDI of 80% or more had a higher response rate (55.2% vs. 33.3%) and overall survival than those who received a RDI less than 80%.24 In advanced epithelial ovarian cancer25 and metastatic breast cancer26 improved survival was associated with a RDI of 85% or more.
Many factors other than thrombocytopenia determine the feasibility of delivering an adequate RDI for any particular cancer patient: anemia, neutropenia, mucositis, nausea, intravenous access, need for concurrent radiation therapy, and performance status. However, for a significant number of chemotherapy recipients, thrombocytopenia is the major limiting variable, as discussed below.
So, for the cancer patient whose chemotherapy regimen is primarily limited by thrombocytopenia, the potential bleeding risk with chemotherapy becomes a complex calculation in which the platelet count and other hemostatic variables need to be weighed in the context of potential benefit from maintaining chemotherapy RDI. Furthermore, there is no reliable predictor of the degree of nadir thrombocytopenia (and hence risk of bleeding) based on pretreatment platelet count or other patient-related or chemotherapy regimen variables. In general, chemotherapy is administered at pretreatment platelet counts over 100x109/L with many chemotherapy regimens and physicians challenged when platelet counts are below 70x109/L, in particular below 50x109/L. There is no evidence as to what constitutes an "adequate pretreatment platelet count" for any specific chemotherapy for solid tumors; although 70x109/L or higher is widely accepted.
Not all cases of thrombocytopenia in chemotherapy recipients are due to chemotherapy: the clinical approach to thrombocytopenia in the cancer patient
Although chemotherapy and radiation are by far the major causes of thrombocytopenia in the cancer patient, other etiologies should be considered in all patients. In general, the following “checklist” should be considered in cancer patients with platelet counts less than 100x109/L.
• Is the underlying disease the cause of the thrombocytopenia?
Tumor that metastasizes to bone marrow is common in patients with breast and lung cancer as well as in those with primary hematologic malignancies such as lymphoma. Most such patients also demonstrate pancytopenia and these cytopenias generally occur when over 80% of the bone marrow is infiltrated. As discussed below, patients with infiltrated bone marrow respond poorly to TPO-RA.27
• Is there an associated immune thrombocytopenia?
Up to 1% of patients with Hodgkin disease,28,29 2 to 10% of patients with chronic lymphocytic leukemia,30-32 and 0.76% (range, 0–1.8%) of patients with other non-Hodgkin lymphomas29 develop a secondary ITP. These patients respond to steroids, rituximab, splenectomy, and TPO-RA just like patients with primary ITP,33 although treatment of the underlying lymphoma may be effective.29
• Has there been a recent infection?
While infection may produce consumptive coagulopathies (e.g., disseminated intravascular coagulation), some bacteria release neuraminidase that actually reduces platelet survival by removing the sialic acids coating platelets and thereby increasing their clearance by the liver Küpffer cell type C lectin receptor (CLEC4F) or hepatic Ashwell-Morell receptor.34-36 Viral infections (e.g., cytomegalovirus) in compromised patients may inhibit bone marrow production of platelets. Such thrombocytopenias improve with adequate treatment of the infection.
• Has the patient received a new medication? Heparin-induced thrombocytopenia should be considered.37 Antibiotics (e.g., vancomycin,38 linezolid39) and antiviral agents (e.g., ganciclovir40,41) commonly induce thrombocytopenia either by direct bone marrow toxicity or by immune drug-dependent antibody clearance.38,42
• Has there been a recent transfusion? Post-transfusion purpura is a rare complication of transfusion of red blood cells and platelets with the platelet count usually dropping below 10x109/L.43,44 Post-transfusion purpura occurs in the 1% of patients who lack the common platelet antigen PLA-1 (HPA-1a) and is usually seen in women previously sensitized by pregnancy. Upon transfusion of HPA-1a-positive platelets into sensitized HPA-1a-negative patients, antibody destroys the transfused platelets and by an as yet unclear mechanism also destroys the patient’s own HPA-1a-negative platelets. This under-recognized complication of transfusion responds readily to intravenous immunoglobulin.
• Does the patient have a coagulopathy?
In addition to infections, some tumors (e.g., gastric and pancreatic adenocarcinomas) can cause chronic disseminated intravascular coagulation.45,46 Such thrombocytopenic patients usually have elevated D-dimer and prothrombin fragment 1.247 with a low fibrinogen, but often have minimally prolonged prothrombin time and partial thromboplastin time.48 Treatment of this is often difficult. Heparin may improve the coagulopathy, but most patients do not improve without effective treatment of the underlying tumor.
• Is there a chemotherapy- or transplant-related thrombotic microangiopathy?
Mitomycin-C and gemcitabine induce endothelial injury with a resultant thrombotic microangiopathy whose major manifestation is renal failure and thrombocytopenia, best referred to as a chemotherapy-related hemolytic uremic syndrome.49 Patients with such a microangiopathy usually have thrombocytopenia, microangiopathic hemolytic anemia, and organ dysfunction with a normal level of ADAMTS13;50 most improve with supportive care and discontinuation of the chemotherapy. Plasma exchange, rituximab, or steroids are not indicated.51 It is unclear whether complement inhibition with eculizumab or ravulizumab will help.52
• Is the thrombocytopenia temporally related to chemotherapy or radiation therapy?
When was the last chemotherapy or radiation therapy administered? The platelet has a normal lifespan of 8 to 10 days. After many types of chemotherapy, the platelet count generally starts to drop by day 7 and reaches its nadir at day 14 with a gradual return back to baseline by day 28 to 35.53
Depending upon the dose and duration of radiation therapy, the onset of thrombocytopenia is generally at day 7 to day 10 with a longer duration of thrombocytopenia, sometimes lasting for 30 to 60 days.
• What dose and type of chemotherapy was given? As reviewed next, the incidence, severity, and duration of thrombocytopenia vary with the chemotherapy regimen. Most non-myeloablative chemotherapy regimens were developed to minimize thrombocytopenia and the need for platelet transfusions. As such, most standard regimens have relatively low rates of dose-limiting thrombocytopenia; when thrombocytopenia occurs, it is often of short duration (4 to 6 days). Most cases respond well to platelet transfusion.
Table 1.
Variables potentially increasing bleeding risk in cancer patients.
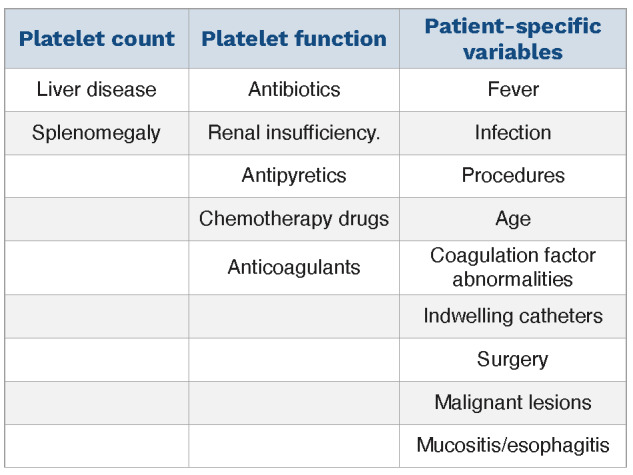
Table 2.
The effect of dose intensity on platelet count and chemotherapy response.
The incidence of thrombocytopenia and the use of platelet transfusions vary with the type of chemotherapy
Although many factors may lead to reduction of chemotherapy RDI, it is difficult to identify how much is attributed to thrombocytopenia. In a review of different chemotherapy regimens in 614 patients with solid tumors, CIT (a platelet count <100x109/L) was seen in 21.8% of all subjects; thrombocytopenia was unaccompanied by other cytopenias in 6.2%.54 Grade 3 thrombocytopenia was seen in 3.6% and grade 4 thrombocytopenia in 3.3%. CIT occurred in 82% of those receiving only carboplatin, and in 58%, 64% and 59% of those receiving combination therapies with carboplatin, gemcitabine or paclitaxel, respectively.
In a retrospective analysis of 43,995 patients (including those with solid tumors or hematologic malignancies) who received 62,071 chemotherapy regimens in the USA between 2000 and 2007,55 CIT occurred in 13,304 (21.4%) regimens. Grade 3 and grade 4 thrombocytopenia occurred in 2,660 (4.3%) and 2,087 (3.4%) regimens, respectively: 7.8% and 3.4% of gemcitabine-based regimens; 6.5% and 4.1% of platinum-based regimens; 3.0% and 2.2% of anthracycline-based regimens; and 1.4% and 0.5% of taxane-based regimens.
In a more recent analysis of 15,521 patients with solid tumors,56 12.8% (95% CI: 12.3–13.4%) had CIT: grade 2 in 6.4%; grade 3 in 4.2% and grade 4 in 1.9%. CIT was more common in patients with solid tumors who received gemcitabine- and platinum-based regimens (14.8% and 13.5%, respectively) than in patients treated with anthracyclineor taxane-based regimens (9.3% and 6.5%, respectively). With regard to tumor type, CIT occurred in 21.4% of melanoma patients, 14.3% of lung cancer patients, 13.5% of colorectal cancer patients, 12.9% of pancreatic cancer patients, and 9.6% of breast cancer patients. Grade 3 (13.3%) and 4 (5.0%) thrombocytopenia occurred most commonly in melanoma patients. The median time to the first decline in platelet count was about 1 to 2 weeks after starting chemotherapy, except for platinum-based regimens which was usually longer than 2 weeks. As expected, other cytopenias also occurred in most patients but 17.7% of CIT patients had only thrombocytopenia. In comparison, CIT was much more common in patients with hematologic malignancies (28% of 2,537 patients) with grade 3 and grade 4 thrombocytopenia in 16.3% and 12.4%, respectively.
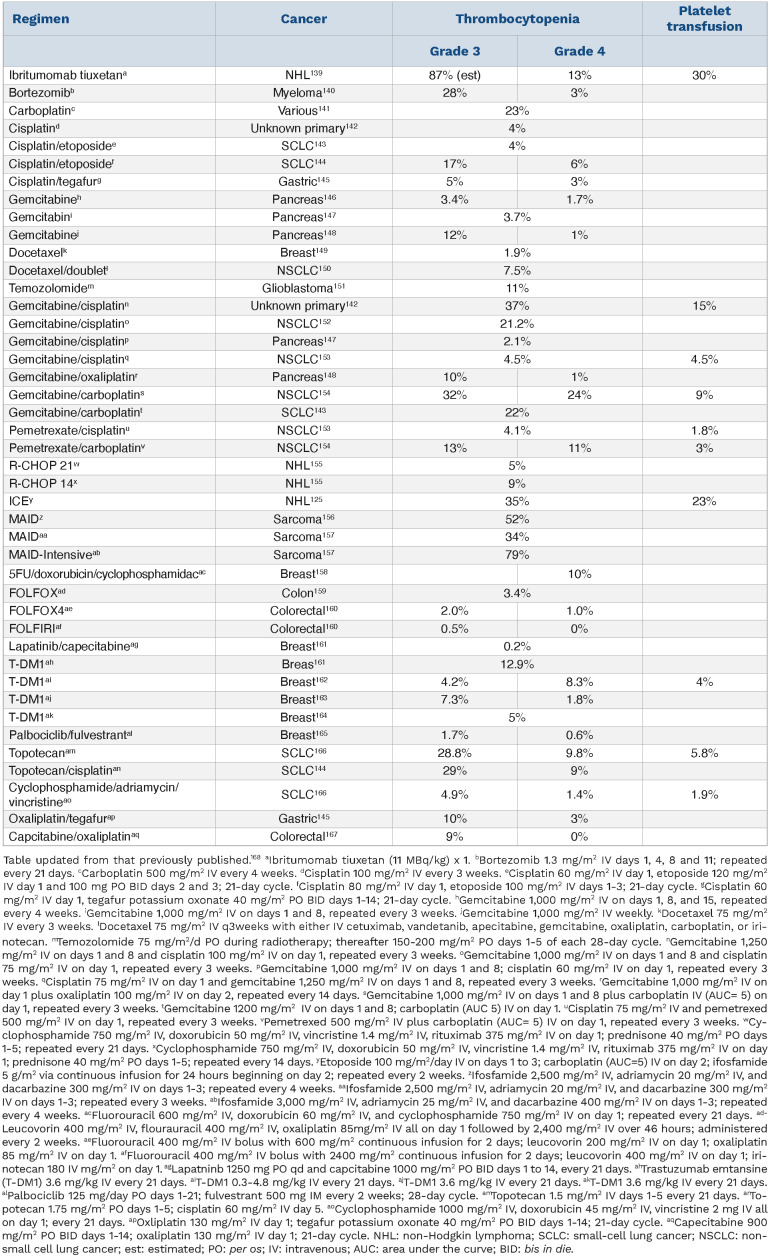
The incidence of thrombocytopenia-related bleeding and platelet transfusions has been very poorly studied in CIT. Bleeding events were not measured for the 62,071 chemotherapy regimens described above, but platelet transfusion data were available for 10,582; transfusions occurred in 2.5% of patients (1.0% for platinum-based regimens, 0.6% for anthracycline-based regimens, 1.8% for gemcitabine-based regimens, and 0.3% for taxane-based regimens). Table 3 provides an overview of the reported frequencies of thrombocytopenia and platelet transfusions with various chemotherapy regimens.
Pathophysiology of chemotherapy-induced thrombocytopenia
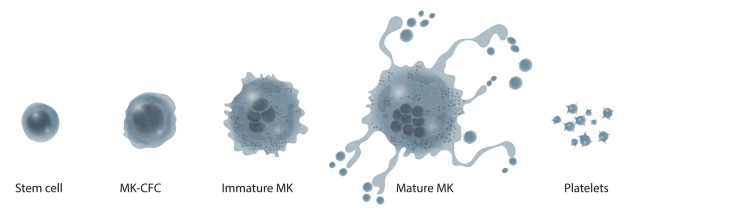
Not all chemotherapy drugs cause thrombocytopenia in the same way. In reviewing the mechanism of CIT, it is helpful to understand how platelets are made (Figure 2). Stem cells differentiate into cells committed to mega-karyocyte differentiation (megakaryocyte colony-forming cells). At some stage, these cells stop their mitotic divisions and enter a process called endomitosis, in which DNA replication occurs but with no subsequent division of the nucleus or the cell. This gives rise to polyploid precursor cells with 2, 4, 8, 16, or 32 times the normal diploid DNA content. These polyploid megakaryocyte precursor cells then stop synthesizing DNA and mature into large, morphologically identifiable megakaryocytes. Mature megakaryocytes then produce platelets by a mechanism that is still poorly defined. In its simplest iteration, portions of the megakaryocyte membrane bud off into the bone marrow sinusoid to produce platelets.57-59 Other models suggest that mature megakaryocytes extrude long cytoplasmic processes through endothelial cells and large strands of platelet material (proplatelets) are released into the circulation, eventually becoming mature platelets, possibly through fragmentation in the lung.60 More recently it has been suggested that some intact megakaryocytes migrate to the lung where they may account for up to 50% of platelet production.61 If not consumed in hemostasis, the mature platelet undergoes programmed cell death (apoptosis) determined by a “platelet clock”.62 This platelet clock depends upon the presence of the anti-apoptotic protein Bcl-x(L), a protein that restrains the pro-apoptotic proteins Bax and Bak.62-65 When Bcl-x(L) declines, the activity of Bax and Bak increases, which triggers platelet apoptosis. The apoptotic platelets are cleared by the reticuloendothelial cell system, probably in the liver; the spleen plays little role in normal platelet homeostasis.66 Different chemotherapy drugs affect the megakaryocyte and platelet production pathway at different steps (Figure 2). Alkylating agents such as busulfan and carboplatin affect pluripotential stem cells.67,68 Cyclophosphamide spares hematopoietic stem cells because of their abundant levels of aldehyde dehydrogenase, but affects later megakaryocyte progenitors.69 The antibody-drug conjugate T-DM1 (trastuzumab [T] coupled to the microtubule toxin emtansine [DM1]) causes grade 3 or higher thrombocytopenia within 1 week in about 13% of patients by inhibiting megakaryocyte growth and differentiation. T-DM1 is internalized into megakaryocytes via the FcγRIIa receptor or by pinocytosis where it releases DM1, which inhibits megakaryocyte polyploidization and growth.70 Bortezomib has no effect on stem cells71 or megakaryocyte maturation but does inhibit NF-κB, a critical regulator of platelet shedding.72 This probably explains the relatively short duration of thrombocytopenia following its administration.72 Not all chemotherapy drugs reduce platelet production; some can actually increase the rate of platelet destruction. Indeed, platelet survival itself may be altered by some chemotherapy agents. The experimental chemotherapy agent ABT-737 reduces the activity of the platelet clock Bcl-xL and rapidly induces platelets to undergo apoptosis.63,73 After a single dose of ABT-737, platelets dropped to 30% of baseline by 2 h, to 5% of baseline by 6 h, started to recover to 10% of baseline by 24 h, and returned to baseline after 72 h.73 This was not due to platelet activation. Rather, caspase-mediated apoptosis was induced with rapid appearance of phosphatidylserine on the platelet surface and clearance of these cells from the circulation by the reticuloendothelial system in the liver. Although not evaluated for most standard chemotherapy drugs, etoposide also increases platelet apoptosis by reducing Bcl-x(L) activity.73
The antibody-drug conjugates gemtuzumab ozogamicin and inotuzumab ozogamicin are both associated with acute thrombocytopenia (platelet counts dropping by 86% in 3–4 days in monkeys) and sinusoidal obliteration syndrome due to acute hepatic sequestration of platelets.74 Finally, chemotherapy may enhance platelet clearance by immune mechanisms. In the treatment of many lymphomas, the administration of single-agent fludarabine has been noted to produce an antiplatelet antibody-mediated ITP in up to 4.5% of patients.75 This ITP is commonly responsive to rituximab.76 Platelet destruction is also increased when chemotherapy drugs produce a drug-dependent antiplatelet antibody-mediated secondary ITP, but this effect is uncommon.
Current approaches to the treatment of chemotherapy-induced thrombocytopenia
The response to significant CIT has not been codified in guidelines and there are few studies to describe the appropriate approach to CIT. Much depends upon the underlying goals of the treatment of the cancer patient; different levels of risk assessment need to be brought into play for those being treated for a cure compared to those being treated for palliation. Overall, it is reasonable when confronted with CIT first to assess the underlying need for chemotherapy and the goals of treatment for that particular patient. A clinical assessment of bleeding risk for patients is also important to undertake, especially if patients are receiving anticoagulant drugs or other therapies that might increase bleeding. What follows is the synthesis of the data and the author's personal experience over the past four decades:
• If possible, treat any other underlying cause of thrombocytopenia: stop antibiotics, treat infections, and control coagulopathy.
• Reduce chemotherapy dose, frequency or alter the chemotherapy regimen, especially if chemotherapy is not standard or not of curative intent.
• Platelet transfusion support can be used if maintenance of dose intensity is vital for response or survival. Platelet transfusions are indicated if the patient is bleeding or to prevent major bleeding if platelet counts are less than 10x109/L (<20x109/L if febrile).13,77 However, in the outpatient setting, this transfusion trigger needs to be reconsidered; transfusing at higher platelet counts on the Friday before a long weekend has its advantages.
• Antifibrinolytic agents such as ε-aminocaproic acid (Amicar®) or tranexamic acid (Lysteda®) have been used in some thrombocytopenic cancer patients to improve hemostasis when platelet transfusions do not work, but are of unproven benefit.78-80 Total daily doses of 2–24 g (mean: 6 g) of ε-aminocaproic acid given in three or four divided doses have been used.79 Tranexamic acid doses of 4–6 g/day given as three or four divided doses have also been studied.80 However, the use of antifibrinolytic agents in cancer patients might exacerbate the underlying increased risk of thrombosis. A recent randomized, prospective, blinded study reported that addition of tranexamic acid (1,000–1,300 mg every 8 h) had no benefit in reducing grade ≥2 bleeding, platelet transfusions, or days without grade ≥2 bleeding in thrombocytopenic (platelets <30x109/L) patients undergoing treatment for hematologic malignancies. Of interest, while there was no increase in non-catheter thromboses, an increased incidence of central line occlusion requiring clearing with tissue plasminogen activator was observed.81
Table 3.
Thrombocytopenia and platelet transfusions in commonly used chemotherapy regimens.
Figure 2.
The production of platelets from bone marrow stem cells. Stem cells differentiate into cells committed to megakaryocyte differentiation (megakaryocyte colony-forming cells, MK-CFC) which are mitotically active. MK-CFC then stop mitosis and start endomitosis producing immature megakaryocytes (MK) with polyploid nuclei. The immature MK then stop their endomitosis and mature into large, morphologically identifiable MK that then migrate to the bone marrow sinusoids and produce platelets.
• Recombinant interleukin 11 (oprelvekin, Neumega®) was shown to reduce the need for platelet transfusions from 96% to 70% of patients who had been transfused with platelets in a prior cycle and who then received additional chemotherapy; but it has significant adverse effects.82 This drug was approved by the Food and Drug Administration for the prevention of thrombocytopenia with chemotherapy but is no longer manufactured for use in North America; it is still available in other parts of the world.
• Despite the lack of Food and Drug Administration approval for these agents, rhTPO and TPO-RA might be considered in patients for whom thrombocytopenia prevents maintenance of dose intensity crucial for remission or survival, and who cannot be supported by platelet transfusions. Recently the National Comprehensive Cancer Network endorsed the use of TPO-RA, notably romi plostim, for treatment of CIT. They recommended initiation of TPO-RA for platelet counts 30– 50x109/L with discontinuation when the platelet count recovers to 50–100x109/L based on an unsupported concern over thrombosis.83
The use of thrombopoietin and thrombopoietin receptor agonists in chemotherapy-induced thrombocytopenia
The pathophysiology of thrombopoietin in chemotherapy-induced thrombocytopenia
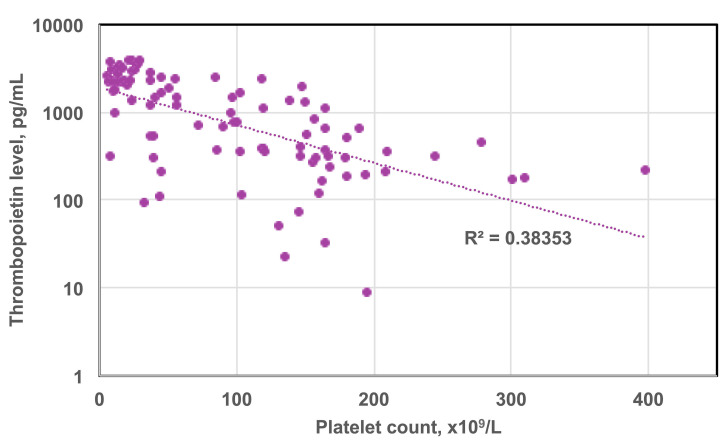
Thrombopoietin (TPO) is the major regulator of platelet production. In animals or humans deficient in TPO or its receptor, the platelet count is 10–15% of normal.84,85 Megakaryocyte, erythroid, and myeloid precursor cells are all reduced in such knock-out animals but their white blood cell and red blood cell counts are normal.86 TPO is produced by the liver usually at a constant rate and its production is decreased by liver disease87 and increased by interleukin-6 in rare conditions such as that associated with ovarian cancer.88,89 TPO has no storage form and is secreted into the circulation and cleared by TPO receptors on platelets. In disorders such as in CIT, TPO levels are inversely related to the rate of platelet production and rise in a log-linear fashion (Figure 3).90-93
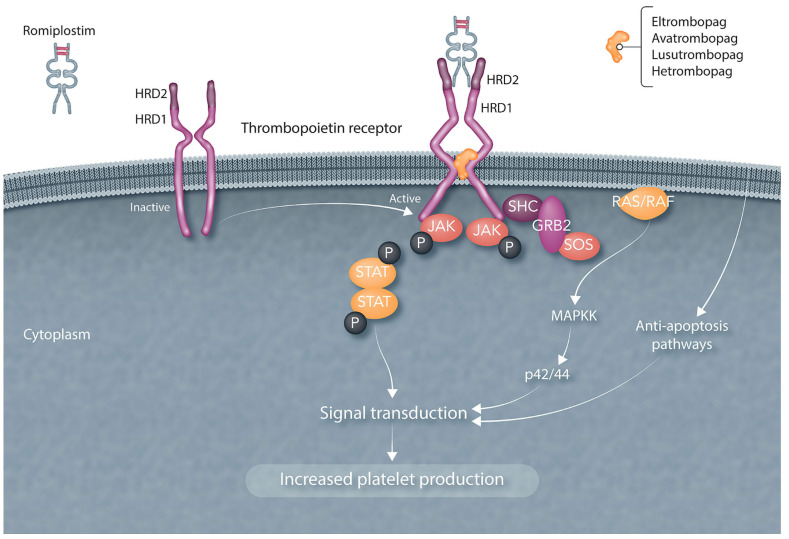
TPO binds to its receptor on many hematopoietic cells (Figure 4) and exerts its effects on most stages of megakaryocyte growth (Figure 2). It is necessary for the viability of hematopoietic stem cells; when the TPO receptor is absent, humans are born with thrombocytopenia and develop pancytopenia over subsequent years.94-96 TPO stimulates mitosis of megakaryocyte colony-forming cells. Its major effect (at exceedingly low concentrations) is to increase megakaryocyte endomitosis and increase megakaryocyte ploidy, greatly expanding the megakaryocyte mass. It then stimulates megakaryocyte maturation. It is unclear whether TPO plays any role in platelet shedding.97 An under-appreciated effect of TPO is that it prevents apoptosis of early and late megakaryocytes,98 an effect that may play a major protective role in patients receiving radiation and chemotherapy.
Before considering the use of TPO agents in patients with cancer, it is important to note that solid tumors appear not to possess functional TPO receptors.99,100 In one study using reverse transcriptase polymerase chain reaction on 39 human cell lines and 20 primary normal and malignant human tissues, TPO receptor (c-mpl) transcripts were found in all megakaryocytic cell lines tested (DAMI, CMK, CMK-2B, CMK-2D, SO), the CD34+ leukemia cell line KMT-2, and the hepatocellular carcinoma cell line Hep3B.100 While fetal liver and brain cells had detectable levels of c-mpl mRNA, none was found in primary tumors. In a more extensive study, microarray detected TPO receptor mRNA in 0/118 breast tumors and at very low levels in 14/29 lung tumors.99 Low but detectable levels of TPO receptor mRNA were found by quantitative polymerase chain reaction in some normal (14–43%) and malignant (3–17%) breast, lung, and ovarian tissues but in none of these tissues was TPO receptor protein detectable by immuno histochemistry. Culture of breast, lung, and ovarian carcinoma cell lines with TPO-RA showed no stimulation of growth. Finally, in none of the human clinical studies described next was there any stimulation of tumor growth by the administration of rhTPO or TPO-RA.
Development of recombinant thrombopoietin and thrombopoietin receptor agonists
The development of clinically relevant TPO molecules has occurred in two phases: the early recombinant TPO molecules and the recent TPO-RA.3,101
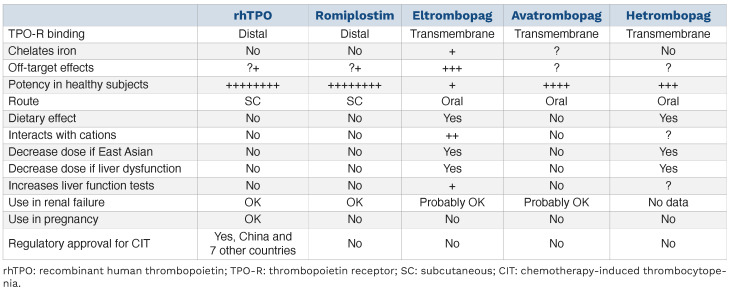
With the discovery of TPO in 1994, two recombinant TPO molecules were developed (Table 4). rhTPO is a fully glycosylated TPO protein made in Chinese hamster ovary cells. The other, pegylated recombinant human mega-karyocyte growth and development factor (PEG-rhMGDF), is a non-glycosylated protein comprising the first 153 amino acids of TPO coupled to polyethylene glycol. Both molecules are potent stimulators of platelet production with half-lives of about 40 h. In healthy volunteers both demonstrated the same time course of platelet response after a single dose: by day 3 megakaryocyte ploidy increased, by day 5 platelet counts started to rise, by days 10–14 a peak platelet count was obtained, and by day 28 platelet counts returned to their baseline.102
Figure 3.
Log-linear correlation of thrombopoietin level with platelet counts. Patients undergoing double umbilical cord blood transplantation had serial platelet counts and thrombopoietin levels determined over their hospital course. As platelet counts declined, thrombopoietin levels rose. Figure created from data in the study by deFilipp et al.91
Between 1995 and 2000, both recombinant TPO underwent extensive clinical development in CIT.103 As discussed more below, both produced an earlier and higher nadir platelet count, shortened the duration of thrombocytopenia, reduced platelet transfusions, and enabled non-myeloablative chemotherapy to be given on schedule.
Development of both was stopped in the West around 2000 because of concerns over neutralizing antibody formation against PEG-rhMGDF.104 In 525 healthy volunteers given up to three monthly doses of PEG-rhMGDF, 13 (2.5%) developed thrombocytopenia due to the formation of antibodies to PEG-rhMGDF that cross-reacted with endogenous TPO, creating TPO deficiency and thrombocytopenia. All subjects recovered, but some required immunosuppressive treatment.105,106 Development of rhTPO (TPIAO®) continued in China where it is now licensed for treatment of CIT (https://www.mims.com/thailand/drug/info/tpiao) and ITP.107 Despite the failure of one of the recombinant TPO molecules, interest turned to developing newer TPO molecules (now called TPO-RA) with novel properties and less risk of antibody formation.1,3 Romiplostim is a “peptibody” created by inserting a 14 amino acid peptide that activates the TPO receptor into an IgG4 heavy chain (Table 4).108 Romiplostim is approved in many countries for the treatment of ITP and in Japan for the treatment of aplastic anemia.
Figure 4.
Recombinant human thrombopoietin and the thrombopoietin receptor agonists bind to and activate the thrombopoietin receptor in different ways. The thrombopoietin (TPO) receptor has been proposed to exist as an inactive preformed dimer (left side) with a proximal and distal hematopoietic receptor domain (HRD1 and HRD2, respectively). Upon binding of romiplostim or recombinant human TPO (not pictured) to the distal HRD2, the conformation of the receptor changes (right side) and a number of signal transduction pathways are activated which increase platelet production. The other TPO receptor agonists bind to the transmembrane region of the receptor and activate many of the same signal transduction pathways. TPO: thrombopoietin; HRD: hematopoietic receptor domain protein; STAT: signal transducer and activator of transcription; JAK: Janus kinase; GRP2: growth receptor bound protein 2; SOS: son of sevenless (a guanine nucleotide exchange factor); RAS: rat sarcoma virus (a small GTP-ase); RAF: rapidly accelerated fibrosarcoma (a serine/threonine kinase); MAPK: mitogen-activated protein kinase.
Table 4.
Thrombopoietic therapies under development for treating chemotherapy-induced thrombocytopenia.
A separate approach identified a number of small molecules (eltrombopag, avatrombopag, lusutrombopag, hetrombopag) that bind and activate the TPO receptor (Table 4).109 All of these small molecule TPO-RA bind the TPO receptor in the transmembrane region, an area different from where TPO or romiplostim binds, and activate the TPO receptor in a different fashion (Figure 4).110-112 All of these small molecules have undergone extensive clinical development and most (eltrombopag, avatrombopag, hetrombopag) are licensed for treating patients with ITP, of whom they increase the platelet count in over 85%.113-119 Eltrombopag is also approved for the treatment of thrombocytopenia in patients with hepatitis C infection requiring anti-viral treatment120 and in patients with aplastic anemia in whom immunosuppressive therapy has failed.121,122 In the latter disease, treatment was also associated with an increase in white blood cells and red blood cells. Avatrombopag and lusutrombopag are both approved for treating thrombocytopenic patients with chronic liver disease about to undergo procedures.109 PEG-rhMGDF, rhTPO, romiplostim, eltrombopag, avatrombopag and hetrombopag have all been studied in patients with CIT. Although a 2017 meta-analysis stated that there was insufficient evidence to support their use in CIT,15 considerable new evidence has emerged since then which challenges that conclusion. Clearly all TPO increase the platelet count in CIT patients but the question is whether that translates into clinical benefit in maintaining RDI, reducing bleeding, and improving response or survival. What follows is a summary of current data.
Treatment of chemotherapy-induced thrombocytopenia with pegylated recombinant human growth and development factor
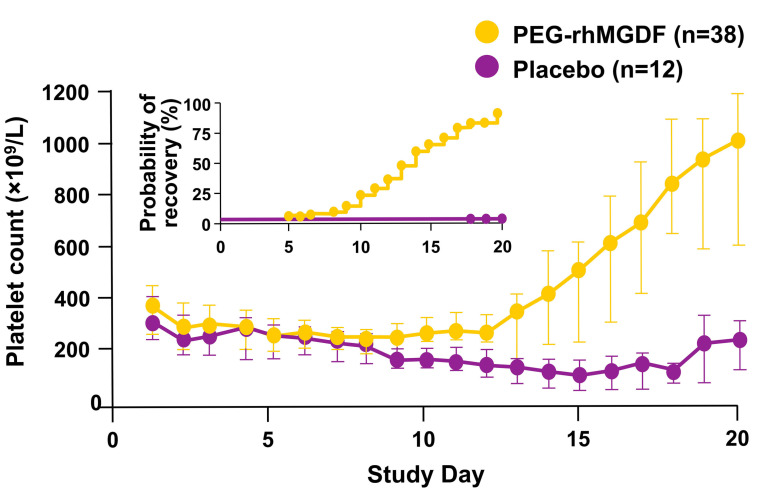
When PEG-rhMGDF was administered for up to 16 days after treatment of lung cancer patients with carboplatin and paclitaxel, the median platelet count nadir was 188x109/L (range, 68–373x109/L) versus the count in the placebo-treated group of 111x109/L (range, 21–307x109/L; P=0.013) (Figure 5). The nadir platelet count occurred earlier in the patients treated with PEG-rhMGDF; the median time to nadir was 7 versus 15 days (P<0.001). The platelet count recovered to baseline in 14 days in the patients given PEGrhMGDF as compared with more than 21 days in those receiving placebo (P<0.001).123 There was no effect on platelet transfusions or bleeding; only one patient in the placebo group required a platelet transfusion. Thromboses were not increased and the patients’ survival was not affected.
In a second major study,124 patients with advanced malignancy were treated with intravenous carboplatin 600 mg/m2 and cyclophosphamide 1,200 mg/m2 in their first cycle. In subsequent cycles they received, in addition, PEG-rhMGDF for 1, 3 or 7 days after chemotherapy. Those receiving the same chemotherapy dose on a subsequent cycle had a significantly higher platelet nadir than in cycle 1, (48x109/L vs. 36x109/L; P=0.003) and the duration of grade 3 or 4 thrombocytopenia was significantly shorter (0 vs. 3 days; P=0.004). However, there was no difference in the time to platelet recovery. Administration of PEGrhMGDF prior to chemotherapy did not show any benefit. A third study has suggested a possible survival benefit from treatment with PEG-rhMGDF.125 In the treatment of patients with relapsed non-Hodgkin lymphoma with ifosfamide, carboplatin, and etoposide (ICE) chemotherapy, maintenance of RDI correlates with improved survival. In a study of 38 non-Hodgkin lymphoma patients randomized to placebo (n=16) or PEG-rhMGDF (n=22), ICE was given on schedule to 42% of those on placebo and 75% of those on PEG-rhMGDF (P=0.008) with overall survival of 21% and 31%, respectively (P=0.06), after a median follow-up of 8.5 years. Patients on placebo were 4.4 times more likely to have a dose delay, which was due to thrombocytopenia in 83%. Grade 4 thrombocytopenia was seen in 35% of placebo recipients versus 15% of patients given PEG-rhMGDF (P=0.02) with platelet nadirs of 20x109/L and 49x109/L (P=0.008), respectively. Platelet transfusions were administered in 23% of placebo cycles and 8% of PEG-rhMGDF cycles (P=0.04).
Figure 5.
Pegylated recombinant human megakaryocyte growth and development factor increases the platelet count in patients undergoing chemotherapy. Lung cancer patients being treated with carboplatin and paclitaxel were also given either placebo (purple circles) or pegylated recombinant human megakaryocyte growth and development factor (yellow circles) in a double-blind, randomized study.123 Platelet counts were measured daily. The inset shows the probability of recovery of the platelet count back to baseline in the two treatment groups. Figure adapted from published data.123 PEG-rhMGDF: pegylated recombinant human megakaryocyte growth and development factor.
As described above, PEG-rhMGDF development stopped in 2000 due to the appearance of antibodies against PEGrhMGDF which cross-reacted with endogenous TPO and caused thrombocytopenia.105
Treatment of chemotherapy-induced thrombocytopenia with recombinant human thrombopoietin
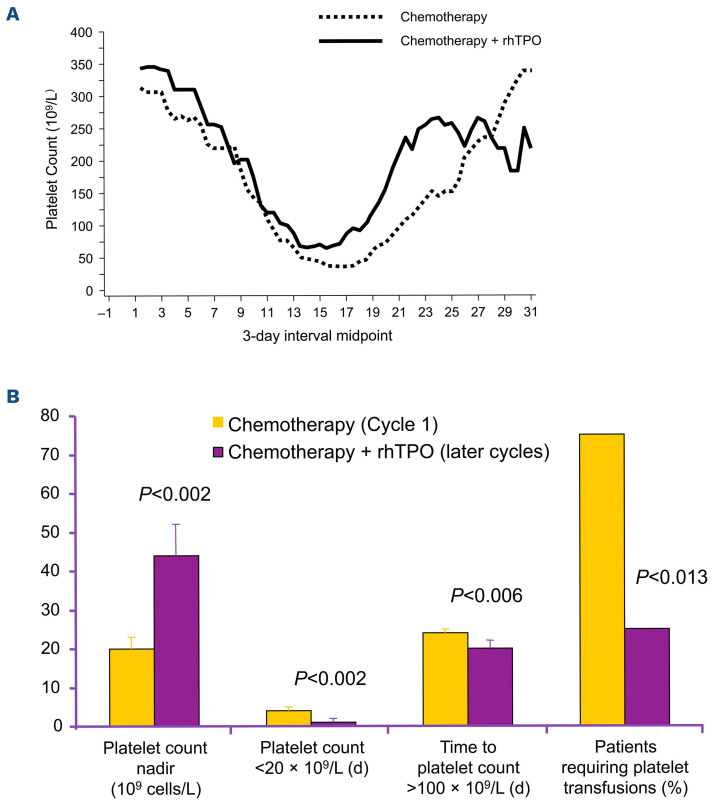
In an early study, rhTPO was administered on days 2, 4, 6, and 8 after a second cycle of carboplatin chemotherapy for patients with a gynecological malignancy (Figure 6). The mean platelet count nadir in the second cycle was higher than that in the first cycle, during which no rhTPO was administered (44x109/L vs. 20x109/L; P=0.002); the number of days with platelet count less than 20x109/L was lower (1 vs. 4 days, P=0.002); the number of days with a platelet count less than 50x109/L was lower (4 vs. 7 days; P=0.006). The need for platelet transfusion in the group receiving rhTPO was reduced from 75% of patients in cycle 1 to 25% of patients in cycle 2 (P=0.013). Recovery to a platelet count 100x109/L or greater was faster (20 days for
Figure 6.
Recombinant human thrombopoietin reduces the need for platelet transfusions in patients undergoing carboplatin chemotherapy for gynecological malignancy. (A) Platelet count time course for patients in cycle 2 (treated with recombinant human thrombopoietin [rhTPO]) compared to that in patients in cycle 1 (treated without rhTPO). Figure provided by Pharmacia, Inc. (Peapack, NJ, USA). (B) Platelet counts and platelet transfusions in cycle 2 (treated with rhTPO) compared with cycle 1 (treated without rhTPO). Figure created from data in the study by Vadhan-Raj et al.126 d: days.
Table 5.
Outcomes of 62 patients with chemotherapy-induced thrombocytopenia in a cross-over study who received placebo in one chemotherapy cycle and recombinant human thrombopoietin in an adjacent cycle. Study data on file at 3sBIO Inc, Shenyang, China.
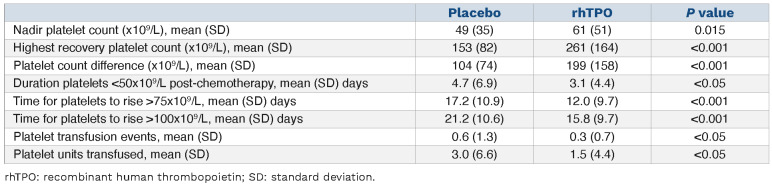
Numerous CIT studies have been conducted in China with rhTPO but with few results published in non-Asian medical journals.127,128 In one study (Study 005) made available to this author in English, 62 cancer patients (42% with lymphoma and the rest with solid tumors) with a platelet count less than 75x109/L on a prior cycle and requiring two more chemotherapy cycles at the same dose were randomly treated in a crossover study of CIT prophylaxis. Twenty-eight patients in group A (n=28) received rhTPO treatment (1 mg/kg rhTPO subcutaneously daily for 14 days starting 6–24 h after chemotherapy) during cycle 1 and received no rhTPO during cycle 2. Group B (n=34) received no rhTPO in the first cycle but received rhTPO treatment during cycle 2. As shown in Table 5 there was a modest improvement in nadir platelet count, with a shorter duration of platelet count below 50x109/L, a much higher recovery platelet count by day 24, and fewer transfusion events. No data were provided regarding maintenance of RDI, bleeding, or whether platelet transfusions were standardized. Similar improvements in platelet counts were obtained in an additional crossover study (Study 006) done in 213 cancer patients.
In a study of 58 lymphoma patients undergoing high-dose cytarabine therapy, two different forms of prophylactic rhTPO were prospectively evaluated. A group that was given rhTPO daily for 10 days after chemotherapy was compared to a group that was given two doses prior to chemotherapy and eight doses after. Those receiving rhTPO before the chemotherapy were less likely to have grade 4 thrombocytopenia (26.9% vs. 48.1%), experienced a shorter duration of grade 4 thrombocytopenia (0.58 days vs. 1.23 days), and required fewer transfusions (13.5% vs. 25%).129 In a small study of rhTPO in 30 patients undergoing adjuvant chemotherapy for gastric or colorectal cancer with regimens containing gemcitabine and capecitabine, patients were randomized to receive either prophylactic treatment with rhTPO 15,000 U/day beginning 4 days before chemotherapy or to receive treatment only when platelet counts after chemotherapy dropped below 75x109/L. Those receiving prophylactic rhTPO experienced better outcomes in terms of a higher mean [standard deviation, SD] nadir platelet count (76x109/L [27x109/L] vs. 53x109/L [17x109/L]; P<0.001], fewer days of dose interruption (1.72 [2.78] vs. 3.72 [3.38]; P=0.002), and a shorter recovery to platelet counts over 100x109/L (4.6 [4.7] vs. 8.9 [2.3] days; P<0.001).130 In a recent meta-analysis131 of 12 CIT studies, mostly in China, when compared with patients treated with placebo or interleukin-11, those treated with rhTPO had modest decreases in the duration of days with platelet counts under 50x109/L and 75x109/L but no difference in days with platelet counts under 100x109/L. Other clinical endpoints were not reported.
Although rhTPO is approved for CIT in China, the studies above document a modest increase in platelet count with treatment but it is hard to assess from published data whether any clinical endpoints such as RDI, remission rate, bleeding or survival were affected.
Treatment of chemotherapy-induced thrombocytopenia with romiplostim
In one retrospective study, cancer patients were selected who had a platelet count less than 100x109/L and who had a more than 4-week delay in their chemotherapy or had dose reductions/modifications in two or more prior cycles of chemotherapy.132 These patients were treated with 2 mg/kg of romiplostim weekly. Platelet counts improved in all and 19/20 had platelet counts of 100x109/L or more. Fifteen patients resumed chemotherapy and all but one of these continued for two or more further cycles without dose modifications. Three of 20 patients developed deep vein thrombosis.
In a phase II, randomized prospective trial (NCT02052882: An Open Label Phase II Study of Romiplostim for Chemotherapy Induced Thrombocytopenia) in solid tumor patients with CIT, patients with platelet counts below 100x109/L for more than 4 weeks despite dose reduction or delay were randomized to receive either romiplostim weekly or observation.133 The primary endpoint was attaining a platelet count of 100x109/L or more by week 3. After enrolling 23 patients, it was found that 14/15 (93%) patients who received romiplostim attained the primary end-point, whereas only 1/8 (12.5%) of those on observation did so (P<0.001). After 2–3 weeks of treatment, those receiving romiplostim had a mean platelet count of 141x109/L versus 57x109/L for those on observation. The randomized portion of the study was discontinued and 37 subsequent patients all received romiplostim. Of the 52 patients who received romiplostim, 44 (85%) met the primary endpoint. All of these 44 patients resumed chemotherapy supported with romiplostim and only three (6.8%) developed subsequent CIT. Twenty-eight patients continued on romiplostim for more than 6 months at a mean dose of 3.3 mg/kg. Six of the 59 patients (10.2%) developed a venous thromboembolism during the first year of romiplostim therapy; none discontinued romiplostim.
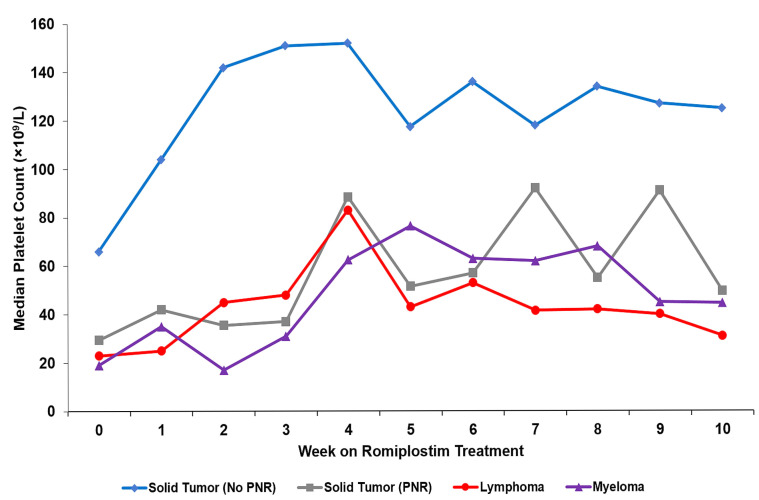
At four Boston cancer centers, supportive care with romiplostim has been utilized for almost 10 years for CIT patients.27,134 Patients eligible for this program had to have a platelet count below 100x109/L for at least 3 weeks after their last chemotherapy treatment or a dose delay of longer than 1 week. Overall, 173 CIT patients (153 with solid tumors, 20 with lymphoma or myeloma) were treated, with 170 (90%) undergoing a median of four (range, 1–36) chemotherapy cycles on romiplostim. The primary outcome was a platelet response defined as a median on-romiplostim platelet count of 75x109/L or more and at least 30x109/L higher than pretreatment baseline. Among all the solid tumor patients 71% had a platelet response, 79% avoided chemotherapy dose reductions/delays and 89% avoided platelet transfusions. The median baseline platelet count of 60x109/L rose to 116x109/L (P=0.001). The median weekly romiplostim dose was 3 mg/kg (interquartile range, 3–5 mg/kg). Solid tumor patients who failed to respond were characterized by extensive bone marrow involvement by tumor, prior pelvic radiotherapy or treatment with temozolomide and their low response rate (<10%) was comparable to that of patients with lymphoma and myeloma (Figure 7). Bleeding rates (7.1/100 patient-years on romiplostim) were less than those of historical controls and there was no apparent increase in thrombosis (11/100 patient-years on romiplostim). Two different dosing algorithms were explored: weekly romiplostim dosing including on days of chemotherapy administration versus a less intense regimen with weekly dosing except on days of chemotherapy administration. Patients on weekly dosing had a significantly higher median platelet count (143x109/L vs. 106x109/L; P<0.001) and a higher rate of achieving a platelet response (81% vs. 63%; P=0.006). Other clinical outcomes including the extent of chemotherapy RDI reduction and bleeding were better in patients receiving weekly treatment.
There are two ongoing trials of romiplostim in CIT with the primary endpoint being the incidence of either a chemotherapy dose delay or reduction (defined as “no thrombocytopenia-induced modification [dose delay, reduction, omission or chemotherapy treatment discontinuation due to platelet counts <100 x 109/L] of any myelosuppressive agent in the second and third cycles of the planned on-study chemotherapy regimen”): NCT03937154: A Phase 3 Randomized Placebo-controlled Double-blind Study of Romiplostim for the Treatment of Chemotherapy-induced Thrombocytopenia in Patients Receiving Chemotherapy for Treatment of Non-small Cell Lung Cancer (NSCLC), Ovarian Cancer, or Breast Cancer; and NCT03362177: A Phase 3 Randomized Placebo-controlled Double-blind Study of Romiplostim for the Treatment of Chemotherapy-Induced Thrombocytopenia in Patients Receiving Oxaliplatin-based Chemotherapy for Treatment of Gastrointestinal, Pancreatic, or Colorectal Cancer.
Figure 7.
Platelet counts of patients with chemotherapy-induced thrombocytopenia treated with romiplostim. Median weekly platelet counts for solid tumor patients (n=122, blue) with no predictors of non-response (no PNR); solid tumor patients (n=31, gray) with predictors of non-response (PNR: bone marrow invasion by tumor, prior pelvic irradiation, or prior temozolomide treatment); aggressive lymphoma patients (n=13, red); and myeloma patients (n=7, purple). Data reproduced with permission from Al-Samkari H et al.27
Treatment of chemotherapy-induced thrombocytopenia with eltrombopag
In a CIT prophylaxis study (NCT00102726), 183 patients received either placebo or eltrombopag 50 mg, 75 mg or 100 mg on days 2 through 11 for at least two 21-day chemotherapy cycles. Eltrombopag was well tolerated. The primary endpoint (the difference in platelet count from day 1 in cycle 2 to the platelet nadir in cycle 2) was not met but postnadir platelet counts were higher for cycles 1 and 2 than in patients in the placebo group.135
In an early but informative blinded, placebo-controlled phase I study to prevent CIT, patients with solid tumors and a platelet count 300x109/L or below receiving up to six cycles of either gemcitabine alone (9 patients) or gemcitabine plus either cisplatin or carboplatin (10 patients) were randomized (3:1) to receive eltrombopag 100 mg or placebo on days -5 to -1 and days 2-6 starting from cycle 2; no study drug was administered for cycle 1.136 For patients receiving gemcitabine alone, the mean (SD) nadir platelet count for cycles 2-6 was 143x109/L (82x109/L) for eltrombopag versus 103x109/L (64x109/L) for placebo; for those receiving gemcitabine plus cisplatin or carboplatin the nadir was 115x109/L (83x109/L) versus 53x109/L (7x109/L) for placebo; 14% of all eltrombopag patients and 50% of placebo patients required dose reductions or delays in cycles 3-6. Three thromboembolic events were reported and felt to be related to other disease characteristics. Platelet counts greater than 400x109/L were reported more frequently in eltrombopag-treated versus control patients (92/19 [4.8 events/patient] vs. 18/7 [2.6 events/patient]) and the decision was made not to increase the dose of eltrombopag above 100 mg/day.
A larger phase II study investigated eltrombopag prophylaxis for the prevention of CIT in patients receiving either gemcitabine alone (42 patients) or gemcitabine with carboplatin or cisplatin (32 patients) over six cycles of chemotherapy. Patients were randomized (1:2) to receive either placebo or eltrombopag 100 mg/day for 5 days before and again daily for 5 days after the chemotherapy.137 The primary endpoint was the mean pretreatment platelet count over six cycles of chemotherapy. The treatment was well tolerated with no increased risk of thrombosis (5/52 [9.6%] on eltrombopag and 2/23 [8.7%] on placebo) but was complicated by a 65% withdrawal rate. The geometric mean platelet count of the 48 eltrombopag–treated patients was 246x109/L compared with 193x109/L for the 23 placebo patients, but the difference did not attain statistical significance (P=0.103). Patients receiving eltrombopag had a slightly lower rate of grade 3/4 thrombocytopenia (27/50 [54%] vs. 16/23 [70%]) and slightly higher nadir platelet counts than patients receiving placebo. There were fewer dose reductions, dose delays and missed doses due to thrombocytopenia in cycles 2-6 for eltrombopag (15/38 [39%]) than for placebo (10/19 [53%]).
A real-world retrospective observational study assessed the response of lymphoma patients whose platelet counts dropped below 30x109/L and who were then treated with eltrombopag (n=51), rhTPO (n=50) or no platelet growth factor support (n=52).138 The baseline platelet counts for all three groups was 24x109/L. After 10 days there was a significantly higher median [SD] platelet count in those on eltrombopag and rhTPO than the untreated patients (131x109/L [71x109/L], 147x109/L [68x109/L], and 76x109/L [40x109/L], respectively; P<0.001); the median (SD) duration of platelet counts <50x109/L was 6.25 (2.61), 5.48 (2.62), and 8.33 (3.98) days, respectively (P=0.036); the mean (SD) days required for recovery to greater than 50x109/L was 6.33 (2.31), 5.44 (2.57), and 8.32 (2.53) days, respectively (P=0.001). Patients receiving eltrombopag or rhTPO were less likely to have grade 2/3 bleeding (5.9% and 4.0%) compared with untreated patients (11.5%); with fewer platelet transfusions (55% and 50%) compared with untreated patients (75%).
One eltrombopag CIT study is currently being conducted: NCT04600960: Eltrombopag for Chemotherapy-induced Thrombocytopenia: A Prospective Single-center One-arm Study.
Treatment of chemotherapy-induced thrombocytopenia with avatrombopag
Avatrombopag has been studied in patients with CIT: NCT03471078: Randomized, Double-blind, Placebo-controlled Study With Open-label Extension to Evaluate the Efficacy and Safety of Avatrombopag for the Treatment of Chemotherapy-induced Thrombocytopenia in Subjects With Active Non-Hematological Cancers. This was a double-blind, placebo-controlled phase III prospective study assessing the safety and efficacy of avatrombopag in patients with CIT (grade ≥2 in a prior cycle) who were receiving chemotherapy for ovarian cancer, small cell lung cancer, non-small cell lung cancer, or bladder cancer. One hundred twenty-two patients were enrolled who had developed grade 3 or 4 thrombocytopenia following treatment with chemotherapy in a prior cycle. Preliminary results of the study showed that avatrombopag failed to meet the composite primary endpoint (avoiding platelet transfusions, chemotherapy dose reductions by ≥15%, and chemotherapy dose delays by ≥4 days). In the intent-to-treat population, 69.5% of patients who received avatrombopag and 72.5% of those who received placebo were responders for the primary endpoint (P=0.72). In the perprotocol population, these rates were 85.0% and 84.4% for avatrombopag and placebo, respectively, (P=0.96).
Other avatrombopag CIT studies are being planned with different entry requirements.
Treatment of chemotherapy-induced thrombocytopenia with hetrombopag
CIT studies are ongoing in China and will soon be started in the West. The current ongoing study is: NCT03976882: A Randomized, Double-blind, Placebo-controlled Multicentre Study With an Open-label Extension to Evaluate the Efficacy and Safety of Hetrombopag for the Treatment of Chemotherapy-induced Thrombocytopenia in Subjects With Malignancy.
Conclusion
CIT is a common complication of non-myeloablative chemotherapy in patients with solid tumors and its incidence varies with the chemotherapy regimen used. Bleeding is generally associated with the degree of thrombocytopenia. Studies that include other variables in addition to the platelet count are needed to predict bleeding in chemotherapy patients; artificial intelligence predictive algorithms may help here.
Other non-chemotherapy-related causes for thrombocytopenia should be assessed and treated in all patients. Platelet transfusions are the main therapy for bleeding in CIT patients. Chemotherapy dose or frequency reductions are the mainstay of CIT treatment but may compromise RDI and therapeutic effect. rhTPO and TPO-RA increase the pretreatment and nadir platelet counts in most patients with
CIT but their beneficial effect on RDI, tumor response, transfusion, bleeding or survival have not yet been adequately demonstrated. It is too early to assess the cost-effectiveness of this form of supportive care. There remains a need for consensus as to what is an adequate pretreatment platelet count in patients being given chemotherapy; such a count will certainly vary with the chemotherapy regimen and patient’s pretreatment variables. This reviewer feels that in most situations, a pretreatment platelet count over 50x109/L is usually adequate.
Acknowledgments
The author is pleased to acknowledge the many helpful discussions and comments on this topic with Dr. Gerald Sof, Dr. Hanny Al-Samkari, Dr. Irene Kuter and the members of the Massachusetts General Hospital Cancer Center.
Funding Statement
Funding: The author has received no financial or other support for the preparation of this article.
References
- 1.Kuter D. General aspects of thrombocytopenia, platelet transfusions, and thrombopoietic growth factors. In: Kitchens CS, Kessler CM, Konkle BA, et al. editors. Consultative Hemostasis and Thrombosis, 4th edition. Philadelphia: Elsevier; 2019:108-126. [Google Scholar]
- 2.Schmied L, Hoglund P, Meinke S. Platelet-mediated protection of cancer cells from immune surveillance - possible implications for cancer immunotherapy. Front Immunol. 2021;12:640578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuter D. Clinical applications of thrombopoietic growth factors. In: Plow T, editor. UpToDate. http://www.uptodate.com (accessed October 26, 2021). [Google Scholar]
- 4.Kuter DJ. What is the role of novel thrombopoietic agents in the management of acute leukemia? Best Pract Res Clin Haematol. 2016;29(4):372-378. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health NCI. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. In: U.S: Department of Health and Human Services, 2017. [Google Scholar]
- 6.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207-214. [DOI] [PubMed] [Google Scholar]
- 7.Slichter SJ. Optimizing platelet transfusions in chronically thrombocytopenic patients. Semin Hematol. 1998;35(3):269-278. [PubMed] [Google Scholar]
- 8.Slichter SJ, LeBlanc R, Jones MK. Quantitative analysis of bleeding risk in cancer patients (PTS) prophylactically transfused at platelet (PLT) counts (CTS) of, 5,000, 10,000, or 20,000. Blood. 1999;94(3):376a.10428550 [Google Scholar]
- 9.Slichter SJ, Harker LA. Thrombocytopenia: mechanisms and management of defects in platelet production. Clin Haematol. 1978;7(3):523-539. [PubMed] [Google Scholar]
- 10.Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood. 1985;66(5):1105-1109. [PubMed] [Google Scholar]
- 11.Chantarangkul V, Clerici M, Bressi C, Giesen PL, Tripodi A. Thrombin generation assessed as endogenous thrombin potential in patients with hyper- or hypo-coagulability. Haematologica. 2003;88(5):547-554. [PubMed] [Google Scholar]
- 12.Chantarangkul V, Clerici M, Bressi C, Tripodi A. Standardization of the endogenous thrombin potential measurement: how to minimize the effect of residual platelets in stored plasma. Br J Haematol. 2004;124(3):355-357. [DOI] [PubMed] [Google Scholar]
- 13.Slichter SJ, Kaufman RM, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362(7):600-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19(4):1137-1146. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Chuai Y, Nie W, Wang A, Dai G. Thrombopoietin receptor agonists for prevention and treatment of chemotherapy-induced thrombocytopenia in patients with solid tumours. Cochrane Database Syst Rev. 2017;11(11):CD012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan BA, Yusoff ZB, Hassali MA, Bin Othman S. Treatment patterns and outcomes in management of solid cancer patients suffering from thrombocytopenia in Penang hospital. Asian Pac J Cancer Prev. 2011;12(11):2841-2845. [PubMed] [Google Scholar]
- 17.Harker LA, Slichter SJ. The bleeding time as a screening test for evaluation of platelet function. N Engl J Med. 1972;287(4):155-1559. [DOI] [PubMed] [Google Scholar]
- 18.Tannock IF, Boyd NF, DeBoer G, et al. A randomized trial of two dose levels of cyclophosphamide, methotrexate, and fluorouracil chemotherapy for patients with metastatic breast cancer. J Clin Oncol. 1988;6(9):1377-1387. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama G, Tanaka C, Uehara K, et al. The impact of dose/time modification in irinotecan- and oxaliplatin-based chemotherapies on outcomes in metastatic colorectal cancer. Cancer Chemother Pharmacol. 2014;73(4):847-855. [DOI] [PubMed] [Google Scholar]
- 20.Ramsden K, Laskin J, Ho C. Adjuvant chemotherapy in resected stage II non-small cell lung cancer: evaluating the impact of dose intensity and time to treatment. Clin Oncol (R Coll Radiol). 2015;27(7):394-400. [DOI] [PubMed] [Google Scholar]
- 21.Crawford J, Denduluri N, Patt D, et al. Relative dose intensity of first-line chemotherapy and overall survival in patients with advanced non-small-cell lung cancer. Support Care Cancer. 2020;28(2):925-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denduluri N, Lyman GH, Wang Y, et al. Chemotherapy dose intensity and overall survival among patients with advanced breast or ovarian cancer. Clin Breast Cancer. 2018;18(5):380-386. [DOI] [PubMed] [Google Scholar]
- 23.Bosly A, Bron D, Van Hoof A, et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol. 2008;87(4):277-283. [DOI] [PubMed] [Google Scholar]
- 24.Luciani A, Bertuzzi C, Ascione G, et al. Dose intensity correlate with survival in elderly patients treated with chemotherapy for advanced non-small cell lung cancer. Lung Cancer. 2009;66(1):94-96. [DOI] [PubMed] [Google Scholar]
- 25.Hanna RK, Poniewierski MS, Laskey RA, et al. Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer. Gynecol Oncol. 2013;129(1):74-80. [DOI] [PubMed] [Google Scholar]
- 26.Loibl S, Skacel T, Nekljudova V, et al. Evaluating the impact of relative total dose intensity (RTDI) on patients' short and longterm outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer- a pooled analysis. BMC Cancer. 2011;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Samkari H, Parnes AD, Goodarzi K, Weitzman JI, Connors JM, Kuter DJ. A multicenter study of romiplostim for chemotherapy-induced thrombocytopenia in solid tumors and hematologic malignancies. Haematologica. 2021;106(4):1148-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimou M, Angelopoulou MK, Pangalis GA, et al. Autoimmune hemolytic anemia and autoimmune thrombocytopenia at diagnosis and during follow-up of Hodgkin lymphoma. Leuk Lymphoma. 2012;53(8):1481-1487. [DOI] [PubMed] [Google Scholar]
- 29.Hauswirth AW, Skrabs C, Schutzinger C, et al. Autoimmune thrombocytopenia in non-Hodgkin's lymphomas. Haematologica. 2008;93(3):447-450. [DOI] [PubMed] [Google Scholar]
- 30.Zent CS, Ding W, Reinalda MS, et al. Autoimmune cytopenia in chronic lymphocytic leukemia/small lymphocytic lymphoma: changes in clinical presentation and prognosis. Leuk Lymphoma. 2009;50(8):1261-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyasa MJ, Parrish RS, Schichman SA, Zent CS. Autoimmune cytopenia does not predict poor prognosis in chronic lymphocytic leukemia/small lymphocytic lymphoma. Am J Hematol. 2003;74(8):1-8. [DOI] [PubMed] [Google Scholar]
- 32.Hodgson K, Ferrer G, Pereira A, Moreno C, Montserrat E. Autoimmune cytopenia in chronic lymphocytic leukaemia: diagnosis and treatment. Br J Haematol. 2011;154(1):14-22. [DOI] [PubMed] [Google Scholar]
- 33.Visco C, Rodeghiero F, Romano A, et al. Eltrombopag for immune thrombocytopenia secondary to chronic lymphoproliferative disorders: a phase 2 multicenter study. Blood. 2019;134(20):1708-1711. [DOI] [PubMed] [Google Scholar]
- 34.Grewal PK, Aziz PV, Uchiyama S, et al. Inducing host protection in pneumococcal sepsis by preactivation of the Ashwell-Morell receptor. Proc Natl Acad Sci U S A. 2013;110(50):20218-20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grewal PK, Uchiyama S, Ditto D, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14(6):648-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuter DJ. Novel therapies for immune thrombocytopenia. Br J Haematol. 2022;196(6):1311-1328. [DOI] [PubMed] [Google Scholar]
- 37.Kuter DJ, Konkle BA, Hamza TH, et al. Clinical outcomes in a cohort of patients with heparin-induced thrombocytopenia. Am J Hematol. 2017;92(8):730-738. [DOI] [PubMed] [Google Scholar]
- 38.Von Drygalski A, Curtis BR, Bougie DW, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007;356(9):904-910. [DOI] [PubMed] [Google Scholar]
- 39.Kuter DJ, Tillotson GS. Hematologic effects of antimicrobials: focus on the oxazolidinone, linezolid. Pharmacotherapy. 2001;21(8):1010-1013. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Smith KP. Safety of alternative antiviral agents for neonatal herpes simplex virus encephalitis and disseminated infection. J Pediatr Pharmacol Ther. 2014;19(2):72-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danziger-Isakov L, Mark Baillie G. Hematologic complications of anti-CMV therapy in solid organ transplant recipients. Clin Transplant. 2009;23(3):295-304. [DOI] [PubMed] [Google Scholar]
- 42.Reese JA, Li X, Hauben M, et al. Identifying drugs that cause acute thrombocytopenia: an analysis using 3 distinct methods. Blood. 2010;116(12):2127-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez CE, Pengetze YM. Post-transfusion purpura. Curr Hematol Rep. 2005;4(2):154-159. [PubMed] [Google Scholar]
- 44.Hawkins J, Aster RH, Curtis BR. Post-transfusion purpura: current perspectives. J Blood Med. 2019;10:405-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levi M. Cancer and DIC. Haemostasis. 2001;31(Suppl 1):47-8. [PubMed] [Google Scholar]
- 46.Saba HI, Morelli GA, Saba RI. Disseminated intravascular coagulation (DIC) in cancer. Cancer Treat Res. 2009;148:137-156. [DOI] [PubMed] [Google Scholar]
- 47.Rosen EA, Vallurupalli M, Choy E, Lennerz JK, Kuter DJ. Management of disseminated intravascular coagulation in a patient with hepatic angiosarcoma: a case report. Medicine (Baltimore). 2018;97(47):e13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuter DJ, Rosenberg RD. Disorders of hemostasis. In: Beck WS, editor. Hematology. Cambridge, Mass: MIT Press. 1991:577-598. [Google Scholar]
- 49.Humphreys BD, Sharman JP, Henderson JM, et al. Gemcitabine-associated thrombotic microangiopathy. Cancer. 2004;100(12):2664-2670. [DOI] [PubMed] [Google Scholar]
- 50.Weitz IC. Thrombotic microangiopathy in cancer. Semin Thromb Hemost. 2019;45(4):348-353. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz J, Winters JL, Padmanabhan A, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher. 2013;28(3):145-284. [DOI] [PubMed] [Google Scholar]
- 52.Jodele S, Fukuda T, Vinks A, et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2014;20(4):518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimazaki C, Inaba T, Uchiyama H, et al. Serum thrombopoietin levels in patients undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1997;19(8):771-775. [DOI] [PubMed] [Google Scholar]
- 54.Ten Berg MJ, van den Bemt PM, Shantakumar S, et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: results from a retrospective hospital-based cohort study. Drug Saf. 2011;34(12):1151-1160. [DOI] [PubMed] [Google Scholar]
- 55.Wu Y, Aravind S, Ranganathan G, Martin A, Nalysnyk L. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large outpatient oncology practice database, 2000-2007. Clin Ther. 2009;31(Pt 2):2416-2432. [DOI] [PubMed] [Google Scholar]
- 56.Shaw JL, Nielson CM, Park JK, Marongiu A, Soff GA. The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies. Eur J Haematol. 2021;106(54):662-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potts KS, Farley A, Dawson CA, et al. Membrane budding is a major mechanism of in vivo platelet biogenesis. J Exp Med. 2020;217(9):e20191206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright JH. A rapid method for the differential staining of blood films and malarial parasites. J Medical Res. 1902;7(1):138-144. [PMC free article] [PubMed] [Google Scholar]
- 59.Wright JH. The origin and nature of blood plates. Boston Med Surg J. 1906;154:643-645. [Google Scholar]
- 60.Machlus KR, Thon JN, Italiano JE. Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation. Br J Haematol. 2014;165(2):227-236. [DOI] [PubMed] [Google Scholar]
- 61.Lefrancais E, Ortiz-Munoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):10510-10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dowling MR, Josefsson EC, Henley KJ, Hodgkin PD, Kile BT. Platelet senescence is regulated by an internal timer, not damage inflicted by hits. Blood. 2010;116(10):1776-1778. [DOI] [PubMed] [Google Scholar]
- 63.Josefsson EC, James C, Henley KJ, et al. Megakaryocytes possess a functional intrinsic apoptosis pathway that must be restrained to survive and produce platelets. J Exp Med. 2011;208(10):2017-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Josefsson EC, White MJ, Dowling MR, Kile BT. Platelet life span and apoptosis. Methods Mol Biol. 2012;788:59-71. [DOI] [PubMed] [Google Scholar]
- 65.White MJ, Schoenwaelder SM, Josefsson EC, et al. Caspase-9 mediates the apoptotic death of megakaryocytes and platelets, but is dispensable for their generation and function. Blood. 2012;119(18):4283-4290. [DOI] [PubMed] [Google Scholar]
- 66.Berger G, Hartwell DW, Wagner DD. P-selectin and platelet clearance. Blood. 1998;92(11):4446-4452. [PubMed] [Google Scholar]
- 67.Fitchen JH, Deregnaucourt J, Cline MJ. An in vitro model of hematopoietic injury in chronic hypoplastic anemia. Cell Tissue Kinet. 1981;14(1):8590. [PubMed] [Google Scholar]
- 68.McManus PM, Weiss L. Busulfan-induced chronic bone marrow failure: changes in cortical bone, marrow stromal cells, and adherent cell colonies. Blood. 1984;64(5):1036-1041. [PubMed] [Google Scholar]
- 69.DeZern AE, Petri M, Drachman DB, et al. High-dose cyclophosphamide without stem cell rescue in 207 patients with aplastic anemia and other autoimmune diseases. Medicine (Baltimore). 2011;90(2):89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uppal H, Doudement E, Mahapatra K, et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin Cancer Res. 2015;21(1):123-133. [DOI] [PubMed] [Google Scholar]
- 71.Fitzgerald M, Fraser C, Webb I, Schenkein D, Esseltine D, Weich N. Normal hematopoietic stem cell function in mice following treatment with bortezomib. Biol Blood Marrow Transplant. 2003;3:193. [Google Scholar]
- 72.Lonial S, Waller EK, Richardson PG, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106(12):3777-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang H, Nimmer PM, Tahir SK, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14(5):943-951. [DOI] [PubMed] [Google Scholar]
- 74.Guffroy M, Falahatpisheh H, Biddle K, et al. Liver microvascular injury and thrombocytopenia of antibody-calicheamicin conjugates in Cynomolgus monkeys-mechanism and monitoring. Clin Cancer Res. 2017;23(7):1760-1770. [DOI] [PubMed] [Google Scholar]
- 75.Leach M, Parsons RM, Reilly JT, Winfield DA. Autoimmune thrombocytopenia: a complication of fludarabine therapy in lymphoproliferative disorders. Clin Lab Haematol. 2000;22:175-178. [DOI] [PubMed] [Google Scholar]
- 76.Hegde UP, Wilson WH, White T, Cheson BD. Rituximab treatment of refractory fludarabine-associated immune thrombocytopenia in chronic lymphocytic leukemia. Blood. 2002;100(3):2260-2262. [PubMed] [Google Scholar]
- 77.Rebulla P, Finazzi G, Marangoni F, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. N Engl J Med. 1997;337(3):1870-1875. [DOI] [PubMed] [Google Scholar]
- 78.Antun AG, Gleason S, Arellano M, et al. Epsilon aminocaproic acid prevents bleeding in severely thrombocytopenic patients with hematological malignancies. Cancer. 2013;119(21):3784-3787. [DOI] [PubMed] [Google Scholar]
- 79.Kalmadi S, Tiu R, Lowe C, Jin T, Kalaycio M. Epsilon aminocaproic acid reduces transfusion requirements in patients with thrombocytopenic hemorrhage. Cancer. 2006;107(1):136-140. [DOI] [PubMed] [Google Scholar]
- 80.Wardrop D, Estcourt LJ, Brunskill SJ, et al. Antifibrinolytics (lysine analogues) for the prevention of bleeding in patients with haematological disorders. Cochrane Database Syst Rev. 2013;(7):CD009733. [DOI] [PubMed] [Google Scholar]
- 81.Gernsheimer TB, Brown SP, Triulzi DJ, et al. Effects of tranexamic acid prophylaxis on bleeding outcomes in hematologic malignancy: the a-TREAT trial. Blood. 2020;136(Suppl 1):1-2.32430499 [Google Scholar]
- 82.Tepler I, Elias L, Smith JW, 2nd, et al. A randomized placebo-controlled trial of recombinant human interleukin-11 in cancer patients with severe thrombocytopenia due to chemotherapy. Blood. 1996;87(9):3607-3614. [PubMed] [Google Scholar]
- 83.Griffiths EA, Alwan LM, Bachiashvili K, et al. Considerations for use of hematopoietic growth factors in patients with cancer related to the COVID-19 pandemic. J Natl Compr Canc Netw. 2020. Sep 1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Sauvage FJ, Carver-Moore K, Luoh SM, et al. Physiological regulation of early and late stages of megakaryocytopoiesis by thrombopoietin. J Exp Med. 1996;183(2):651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Sauvage FJ, Villeval JL, Shivdasani RA. Regulation of megakaryocytopoiesis and platelet production: lessons from animal models. J Lab Clin Med. 1998;131(6):496-501. [DOI] [PubMed] [Google Scholar]
- 86.Carver-Moore K, Broxmeyer HE, Luoh SM, et al. Low levels of erythroid and myeloid progenitors in thrombopoietin- and c-mpl-deficient mice. Blood. 1996;88(3):803-808. [PubMed] [Google Scholar]
- 87.Peck-Radosavljevic M, Wichlas M, Zacherl J, et al. Thrombopoietin induces rapid resolution of thrombocytopenia after orthotopic liver transplantation through increased platelet production. Blood. 2000;95(3):795-801. [PubMed] [Google Scholar]
- 88.Kaser A, Brandacher G, Steurer W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98(9):2720-2275. [DOI] [PubMed] [Google Scholar]
- 89.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang C, Li J, Kuter DJ. The physiological response of thrombopoietin (c-Mpl ligand) to thrombocytopenia in the rat. Br J Haematol. 1999;105(2):478-485. [PubMed] [Google Scholar]
- 91.DeFilipp Z, Makar RS, Brown J, et al. Endogenous thrombopoietin levels are elevated following double cord blood unit transplantation. Bone Marrow Transplant. 2020;55(6):1178-1180. [DOI] [PubMed] [Google Scholar]
- 92.Kuter DJ. The physiology of platelet production. Stem Cells. 1996;14 (Suppl 1):88-101. [DOI] [PubMed] [Google Scholar]
- 93.Emmons RV, Reid DM, Cohen RL, et al. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood. 1996;87(10):4068-4071. [PubMed] [Google Scholar]
- 94.Muraoka K, Ishii E, Tsuji K, et al. Defective response to thrombopoietin and impaired expression of c-mpl mRNA of bone marrow cells in congenital amegakaryocytic thrombocytopenia. Br J Haematol. 1997;96(2):287-292. [DOI] [PubMed] [Google Scholar]
- 95.Ihara K, Ishii E, Eguchi M, et al. Identification of mutations in the c-mpl gene in congenital amegakaryocytic thrombocytopenia. Proc Natl Acad Sci U S A. 1999;96(6):3132-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ballmaier M, Germeshausen M, Schulze H, et al. c-mpl mutations are the cause of congenital amegakaryocytic thrombocytopenia. Blood. 2001;97(1):139-146. [DOI] [PubMed] [Google Scholar]
- 97.Choi ES, Hokom MM, Chen JL, et al. The role of megakaryocyte growth and development factor in terminal stages of thrombopoiesis. Br J Haematol. 1996;95(2):227-233. [DOI] [PubMed] [Google Scholar]
- 98.Zauli G, Vitale M, Falcieri E, et al. In vitro senescence and apoptotic cell death of human megakaryocytes. Blood. 1997;90(6):2234-2243. [PubMed] [Google Scholar]
- 99.Erickson-Miller CL, Pillarisetti K, Kirchner J, et al. Low or undetectable TPO receptor expression in malignant tissue and cell lines derived from breast, lung, and ovarian tumors. BMC Cancer. 2012;12:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Columbyova L, Loda M, Scadden DT. Thrombopoietin receptor expression in human cancer cell lines and primary tissues. Cancer Res. 1995;55(16):3509-3512. [PubMed] [Google Scholar]
- 101.Kuter DJ. Milestones in understanding platelet production: a historical overview. Br J Haematol. 2014;165(2):248-258. [DOI] [PubMed] [Google Scholar]
- 102.Harker LA, Marzec UM, Hunt P, et al. Dose-response effects of pegylated human megakaryocyte growth and development factor on platelet production and function in nonhuman primates. Blood. 1996;88(2):511-521. [PubMed] [Google Scholar]
- 103.Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood. 2002;100(10):3457-3469. [DOI] [PubMed] [Google Scholar]
- 104.Li J, Xia Y, Bertino A, Coburn J, Kuter DJ. Characterization of an anti-thrombopoietin antibody that developed in a cancer patient following the injection of PEG-rHuMGDF. Blood. 1999;94:51a. [Google Scholar]
- 105.Li J, Yang C, Xia Y, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98(12):3241-3248. [DOI] [PubMed] [Google Scholar]
- 106.Basser RL, O'Flaherty E, Green M, et al. Development of pancytopenia with neutralizing antibodies to thrombopoietin after multicycle chemotherapy supported by megakaryocyte growth and development factor. Blood. 2002;99(7):2599-2602. [DOI] [PubMed] [Google Scholar]
- 107.Kong Z, Qin P, Xiao S, et al. A novel recombinant human thrombopoietin therapy for the management of immune thrombocytopenia in pregnancy. Blood. 2017;130(9):1097-1103. [DOI] [PubMed] [Google Scholar]
- 108.Molineux G. The development of romiplostim for patients with immune thrombocytopenia. Ann N Y Acad Sci. 2011;1222:55-63. [DOI] [PubMed] [Google Scholar]
- 109.Virk ZM, Kuter DJ, Al-Samkari H. An evaluation of avatrombopag for the treatment of thrombocytopenia. Expert Opin Pharmacother. 2021;22(3):273-280. [DOI] [PubMed] [Google Scholar]
- 110.Erickson-Miller C, Delorme E, Iskander M, et al. Species specificity and receptor domain interaction of a small molecule TPO receptor agonist. Blood. 2004;104(11):2909. [Google Scholar]
- 111.Erickson-Miller CL, Delorme E, Tian SS, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009;27(2):424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Erickson-Miller CL, DeLorme E, Tian SS, et al. Discovery and characterization of a selective, nonpeptidyl thrombopoietin receptor agonist. Exp Hematol. 2005;33(1):85-93. [DOI] [PubMed] [Google Scholar]
- 113.Kuter DJ. Biology and chemistry of thrombopoietic agents. Semin Hematol. 2010;47(3):243-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98(1):10-23. [DOI] [PubMed] [Google Scholar]
- 115.Kuter DJ. General aspects of thrombocytopenia, platelet transfusions, and thrombopoietic growth factors. In: Kitchens C, Kessler C, Konkle B, editors. Consultative Hemostasis and Thrombosis. 3rd ed. Philadelphia: Elsevier Saunders. 2013:103-116. [Google Scholar]
- 116.Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161(3):411-423. [DOI] [PubMed] [Google Scholar]
- 117.Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363(20):1889-1899. [DOI] [PubMed] [Google Scholar]
- 118.Bussel JB, Buchanan GR, Nugent DJ, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118(1):28-36. [DOI] [PubMed] [Google Scholar]
- 119.Syed YY. Hetrombopag: first approval. Drugs. 2021;81(13):1581-1585. [DOI] [PubMed] [Google Scholar]
- 120.McHutchison JG, Dusheiko G, Shiffman ML, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357(22):2227-2236. [DOI] [PubMed] [Google Scholar]
- 121.Desmond R, Townsley DM, Dumitriu B, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014;123(12):1818-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367(1):11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fanucchi M, Glaspy J, Crawford J, et al. Effects of polyethylene glycol-conjugated recombinant human megakaryocyte growth and development factor on platelet counts after chemotherapy for lung cancer. N Engl J Med. 1997;336(6):404-409. [DOI] [PubMed] [Google Scholar]
- 124.Basser RL, Underhill C, Davis I, et al. Enhancement of platelet recovery after myelosuppressive chemotherapy by recombinant human megakaryocyte growth and development factor in patients with advanced cancer. J Clin Oncol. 2000;18(15):2852-2861. [DOI] [PubMed] [Google Scholar]
- 125.Moskowitz CH, Hamlin PA, Gabrilove J, et al. Maintaining the dose intensity of ICE chemotherapy with a thrombopoietic agent, PEG-rHuMGDF, may confer a survival advantage in relapsed and refractory aggressive non-Hodgkin lymphoma. Ann Oncol. 2007;18(11):1842-1850. [DOI] [PubMed] [Google Scholar]
- 126.Vadhan-Raj S, Verschraegen CF, Bueso-Ramos C, et al. Recombinant human thrombopoietin attenuates carboplatin-induced severe thrombocytopenia and the need for platelet transfusions in patients with gynecologic cancer. Ann Intern Med. 2000;132(5):364-368. [DOI] [PubMed] [Google Scholar]
- 127.Bai CM, Xu GX, Zhao YQ, Han SM, Shan YD. [A multi-center clinical trial of recombinant human thrombopoietin in the treatment of chemotherapy-induced thrombocytopenia in patients with solid tumor]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004;26(4):437-441. [PubMed] [Google Scholar]
- 128.Bai CM, Zou XY, Zhao YQ, Han SM, Shan YD. Thrombopoietin Clinical Trial Cooperation Group. [The clinical study of recombinant human thrombopoietin in the treatment of chemotherapy-induced severe thrombocytopenia]. Zhonghua Yi Xue Za Zhi. 2004;84(5):397-400. [PubMed] [Google Scholar]
- 129.Wang Z, Fang X, Huang H, et al. Recombinant human thrombopoietin (rh-TPO) for the prevention of severe thrombocytopenia induced by high-dose cytarabine: a prospective, randomized, self-controlled study. Leuk Lymphoma. 2018;59(12):2821-2828. [DOI] [PubMed] [Google Scholar]
- 130.Li Q, Jin G, Jiang C, et al. Prophylactic administration of recombinant human thrombopoietin attenuates XELOX or SOX regimen-induced thrombocytopaenia. Arch Med Sci. 2021;17(5):1440-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abudurousuli R, Luo T, Liu R, et al. Research on the recombinant human thrombopoietin in the treatment of thrombocytopenia caused by tumor chemotherapy: a meta-analysis. In J Intell Syst. 2121;3:184-192. [Google Scholar]
- 132.Parameswaran R, Lunning M, Mantha S, et al. Romiplostim for management of chemotherapy-induced thrombocytopenia. Support Care Cancer. 2014;22(5):1217-1222. [DOI] [PubMed] [Google Scholar]
- 133.Soff GA, Miao Y, Bendheim G, et al. Romiplostim treatment of chemotherapy-induced thrombocytopenia. J Clin Oncol. 2019;37(31):2892-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Al-Samkari H, Marshall AL, Goodarzi K, Kuter DJ. The use of romiplostim in treating chemotherapy-induced thrombocytopenia in patients with solid tumors. Haematologica. 2018;103(4):e169-e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kellum A, Jagiello-Gruszfeld A, Bondarenko IN, Patwardhan R, Messam C, Mostafa Kamel Y. A randomized, double-blind, placebo-controlled, dose ranging study to assess the efficacy and safety of eltrombopag in patients receiving carboplatin/paclitaxel for advanced solid tumors. Curr Med Res Opin. 2010;26(10):2339-2346. [DOI] [PubMed] [Google Scholar]
- 136.Winer ES, Safran H, Karaszewska B, et al. Eltrombopag with gemcitabine-based chemotherapy in patients with advanced solid tumors: a randomized phase I study. Cancer Med. 2015;4(1):16-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Winer ES, Safran H, Karaszewska B, et al. Eltrombopag for thrombocytopenia in patients with advanced solid tumors receiving gemcitabine-based chemotherapy: a randomized, placebo-controlled phase 2 study. Int J Hematol. 2017;106(6):765-776. [DOI] [PubMed] [Google Scholar]
- 138.Zhu Q, Yang S, Zeng W, et al. A real-world observation of eltrombopag and recombinant human thrombopoietin (rhTPO) in lymphoma patients with chemotherapy induced thrombocytopenia. Front Oncol. 2021;11:701539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wiseman GA, Gordon LI, Multani PS, et al. Ibritumomab tiuxetan radioimmunotherapy for patients with relapsed or refractory non-Hodgkin lymphoma and mild thrombocytopenia: a phase II multicenter trial. Blood. 2002;99(12):4336-4342. [DOI] [PubMed] [Google Scholar]
- 140.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609-2617. [DOI] [PubMed] [Google Scholar]
- 141.Budd GT, Ganapathi R, Adelstein DJ, et al. Randomized trial of carboplatin plus amifostine versus carboplatin alone in patients with advanced solid tumors. Cancer. 1997;80(6):1134-1140. [PubMed] [Google Scholar]
- 142.Gross-Goupil M, Fourcade A, Blot E, et al. Cisplatin alone or combined with gemcitabine in carcinomas of unknown primary: results of the randomised GEFCAPI 02 trial. Eur J Cancer. 2012;48(5):721-727. [DOI] [PubMed] [Google Scholar]
- 143.Lee SM, James LE, Qian W, et al. Comparison of gemcitabine and carboplatin versus cisplatin and etoposide for patients with poor-prognosis small cell lung cancer. Thorax. 2009;64(1):75-80. [DOI] [PubMed] [Google Scholar]
- 144.Eckardt JR, von Pawel J, Papai Z, et al. Open-label, multicenter, randomized, phase III study comparing oral topotecan/cisplatin versus etoposide/cisplatin as treatment for chemotherapy-naive patients with extensive-disease small-cell lung cancer. J Clin Oncol. 2006;24(13):2044-2051. [DOI] [PubMed] [Google Scholar]
- 145.Lee KW, Chung IJ, Ryu MH, et al. Multicenter phase III trial of S-1 and cisplatin versus S-1 and oxaliplatin combination chemotherapy for first-line treatment of advanced gastric cancer (SOPP trial). Gastric Cancer. 2021;24(1):156-167. [DOI] [PubMed] [Google Scholar]
- 146.Ozaka M, Matsumura Y, Ishii H, et al. Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC-01 study). Cancer Chemother Pharmacol. 2012;69(5):1197-204. [DOI] [PubMed] [Google Scholar]
- 147.Choi JH, Oh SY, Kwon HC, et al. Gemcitabine versus gemcitabine combined with cisplatin treatment locally advanced or metastatic pancreatic cancer: a retrospective analysis. Cancer Res Treat. 2008;40(1):22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27(23):3778-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stemmler HJ, Harbeck N, Groll de Rivera I, et al. Prospective multicenter randomized phase III study of weekly versus standard docetaxel (D2) for first-line treatment of metastatic breast cancer. Oncology. 2010;79(3-4):197-203. [DOI] [PubMed] [Google Scholar]
- 150.Jin Y, Sun Y, Shi X, et al. Meta-analysis to assess the efficacy and toxicity of docetaxel-based doublet compared with docetaxel alone for patients with advanced NSCLC who failed first-line treatment. Clin Ther. 2014;36(12):1980-1990. [DOI] [PubMed] [Google Scholar]
- 151.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 152.Inal A, Kaplan MA, Kucukoner M, Urakci Z, Karakus A, Isikdogan A. Cisplatin-based therapy for the treatment of elderly patients with non-small-cell lung cancer: a retrospective analysis of a single institution. Asian Pac J Cancer Prev. 2012;13(5):1837-1840. [DOI] [PubMed] [Google Scholar]
- 153.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543-3551. [DOI] [PubMed] [Google Scholar]
- 154.Gronberg BH, Bremnes RM, Flotten O, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(19):3217-3224. [DOI] [PubMed] [Google Scholar]
- 155.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide doxorubicin vincristine and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817-1826. [DOI] [PubMed] [Google Scholar]
- 156.Ogura K, Goto T, Imanishi J, et al. Neoadjuvant and adjuvant chemotherapy with modified mesna, adriamycin, ifosfamide, and dacarbazine (MAID) regimen for adult high-grade non-small round cell soft tissue sarcomas. Int J Clin Oncol. 2013;18(1):170-176. [DOI] [PubMed] [Google Scholar]
- 157.Fayette J, Penel N, Chevreau C, et al. Phase III trial of standard versus dose-intensified doxorubicin, ifosfamide and dacarbazine (MAID) in the first-line treatment of metastatic and locally advanced soft tissue sarcoma. Invest New Drugs. 2009;27(5):482-489. [DOI] [PubMed] [Google Scholar]
- 158.Jones SE, Schottstaedt MW, Duncan LA, et al. Randomized double-blind prospective trial to evaluate the effects of sargramostim versus placebo in a moderate-dose fluorouracil, doxorubicin, and cyclophosphamide adjuvant chemotherapy program for stage II and III breast cancer. J Clin Oncol. 1996;14(11):2976-2983. [DOI] [PubMed] [Google Scholar]
- 159.Allegra CJ, Yothers G, O'Connell MJ, et al. Initial safety report of NSABP C-08: a randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27(19):3385-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kilpatrick K, Shaw JL, Jaramillo R, et al. Occurrence and management of thrombocytopenia in metastatic colorectal cancer patients receiving chemotherapy: secondary analysis of data from prospective clinical trials. Clin Colorectal Cancer. 2021;20(2):170-176. [DOI] [PubMed] [Google Scholar]
- 161.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28(16):2698-2704. [DOI] [PubMed] [Google Scholar]
- 163.Krop IE, LoRusso P, Miller KD, et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2012;30(26):3234-3241. [DOI] [PubMed] [Google Scholar]
- 164.Krop IE, Kim SB, Gonzalez-Martin A, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689-699. [DOI] [PubMed] [Google Scholar]
- 165.Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209-219. [DOI] [PubMed] [Google Scholar]
- 166.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17(2):658-667. [DOI] [PubMed] [Google Scholar]
- 167.Chiu J, Tang V, Leung R, et al. Efficacy and tolerability of adjuvant oral capecitabine plus intravenous oxaliplatin (XELOX) in Asian patients with colorectal cancer: 4-year analysis. Asian Pac J Cancer Prev. 2014;14(11):6585-6590. [DOI] [PubMed] [Google Scholar]
- 168.Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology (Williston Park). 2015;29(4):282-294. [PubMed] [Google Scholar]