Abstract
Renal impairment (RI) is common in patients with multiple myeloma (MM) and new therapies that can improve renal function are needed. The phase III IKEMA study (clinicaltrials gov. Identifier: NCT03275285) investigated isatuximab (Isa) with carfilzomib and dexamethasone (Kd) versus Kd in relapsed MM. This subgroup analysis examined results from patients with RI, defined as estimated glomerular filtration rate <60 mL/min/1.73 m². Addition of Isa prolonged progression-free survival (PFS) in patients with RI (hazard ratio: 0.27; 95% confidence interval [CI]: 0.11–0.66; median PFS not reached for Isa-Kd versus 13.4 months for Kd [20.8-month follow-up]). Complete renal responses occurred more frequently with Isa-Kd (52.0%) versus Kd (30.8%) and were durable in 32.0% versus 7.7% of patients, respectively. Treatment exposure was longer with Isa-Kd, with median number of started cycles and median duration of exposure of 20 versus 9 cycles and 81.0 versus 35.7 weeks for Isa-Kd versus Kd, respectively. Among patients with RI, the incidence of patients with grade ≥3 treatment-emergent adverse events was similar between the two arms (79.1% in Isa-Kd vs. 77.8% in Kd). In summary, the addition of Isa to Kd improved clinical outcomes with a manageable safety profile in patients with RI, consistent with the benefit observed in the overall IKEMA study population.
Introduction
Multiple myeloma (MM) is characterized by abnormal proliferation of plasma cells and production of M-protein, a monoclonal immunoglobulin (Ig). Renal impairment (RI) affects up to 50% of MM patients, depending on how RI is defined. MM-related RI is multifactorial, but mainly caused by precipitation of Ig-free light chains in the distal tubules, leading to tubule obstruction and cast nephropathy.1 RI is a major cause of morbidity and an adverse predictor of survival in MM patients.2,3 As renal function recovery is associated with improved clinical outcomes, it is one of the main therapeutic goals in MM patients with RI. Urgent therapy is required to achieve reversal of severe RI, since renal failure established for >2 weeks would substantially compromise the possibility of recovery.4-7
Newly introduced anti-myeloma therapies such as proteasome inhibitors (i.e., bortezomib, carfilzomib)3,8-12 and immunomodulatory drugs (i.e., lenalidomide, pomalidomide)13-20 aid in the recovery of renal function.21,22 Carfilzomib is a next-generation proteasome inhibitor approved as monotherapy or in combination with dexamethasone (Kd), lenalidomide/ dexamethasone, or daratumumab/dexamethasone for relapsed/refractory MM (RRMM).8,23 The phase III ENDEAVOR study demonstrated superiority of Kd versus bortezomib/ dexamethasone (Vd) in RRMM patients with 1–3 prior treatment lines.8 Median progression-free survival (PFS) was 18.7 months with Kd versus 9.4 months with Vd (hazard ratio [HR]: 0.53; 95% confidence interval [CI]: 0.44–0.65; P<0.0001]. Median overall survival (OS) was 47.6 months with Kd versus 40.0 months with Vd (HR: 0.791; 95% CI: 0.65–0.96; one-sided P=0.010). A post-hoc exploratory subgroup analysis of ENDEAVOR reported complete renal response in 15.3% of Kd-treated patients, with longer median PFS and OS in patients achieving complete renal responses.9 The ENDEAVOR study results showed activity in patients with renal function impairment, supporting Kd as a therapeutic option for MM patients with RI.7- 9 However, Kd treatment in patients with RI may present challenges, as carfilzomib has been associated with renal toxicity and hypertension, and may require repeated administration of intravenous fluids compared with oral or subcutaneous alternatives.7-9,23
Based on the phase III ICARIA-MM study, isatuximab (Isa), an anti-CD38 monoclonal antibody, is approved in a number of countries in combination with pomalidomide/ dexamethasone for the treatment of RRMM patients who have received ≥2 prior therapies, including lenalidomide and a proteasome inhibitor.24-27 Based on the phase III IKEMA study, Isa to date is also approved in combination with Kd in the United States for patients with relapsed MM who have received 1–3 prior treatment lines and in the European Union for MM patients who have received ≥1 prior therapy.24,25,28
A pre-specified IKEMA interim analysis showed that PFS was prolonged by the addition of Isa (median PFS, not reached for Isa-Kd versus 19.2 months with Kd; stratified HR: 0.53; 99% CI: 0.32–0.89; one-sided log-rank test P=0.0007), crossing the pre-specified efficacy boundary (P=0.005).28 This pre-specified subgroup analysis of IKEMA examined efficacy, renal response, and safety in patients with RI, at the time of the interim analysis.
Methods
Study design
IKEMA (clinicaltrials gov. Identifier: NCT03275285) was a prospective, multinational, randomized, open-label, parallel-group, phase III study conducted at 69 study centers in 16 countries.29 Institutional ethics committees or independent review boards approved the study protocol for each center. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent.
Patients
Details of the study methodology have been reported previously.28,29 Briefly, eligible patients had relapsed MM with 1–3 prior lines of therapy. Patients were excluded if they had primary refractory MM or serum free-light chain measurable disease only, had received prior carfilzomib treatment, were refractory to anti-CD38 antibody therapy, or presented with left ventricular ejection fraction <40%. Patients with a baseline estimated glomerular filtration rate (eGFR) as low as 15 mL/min/1.73m² were eligible for enrolment.30 Patients with prior pulmonary comorbidities, including chronic obstructive pulmonary disease, could
be enrolled.29
Randomization
Patients were randomly assigned in a 3:2 ratio to receive Isa-Kd or Kd. Randomization was stratified by number of prior treatment lines (1 vs. >1) and Revised International Staging System (R-ISS) stage I or II versus stage III versus not classified, at study entry.28
Treatment
Patients in the Isa-Kd arm received Isa intravenously at 10 mg/kg on days 1, 8, 15, and 22 in the first 28-day cycle; and days 1 and 15 in subsequent cycles. In both arms, carfilzomib was administered intravenously at 20 mg/m2 on days 1 and 2; 56 mg/m2 on days 8, 9, 15, and 16 of cycle 1; and then 56 mg/m2 on days 1, 2, 8, 9, 15, and 16 of subsequent cycles.28 Dexamethasone 20 mg was administered intravenously or orally on days 1, 2, 8, 9, 15, 16, 22, and 23. Treatment continued until unacceptable adverse event (AE), disease progression, or other discontinuation criteria.
Study endpoints and measured outcomes
The primary efficacy endpoint was PFS, as per blinded independent response committee (IRC). The IRC reviewed disease assessments for response and progression (central radiological evaluation, M-protein quantification from central laboratory, and local bone marrow aspiration for plasma cell infiltration when needed). Key secondary efficacy endpoints included overall response rate (ORR) according to the International Myeloma Working Group (IMWG) response criteria,31 very good partial response (VGPR) or better rate, measurable residual disease (MRD) negativity rate, complete response (CR) rate, and OS.32-34 MRD was assessed by central laboratory using next-generation sequencing (NGS) Adaptive clonoSEQ Assay (Adaptive Biotechnologies, Seattle, WA) with a minimum sensitivity of 1/105 nucleated cells in patients reaching ≥VGPR.
Efficacy assessments were performed on day 1 of every cycle and at end of treatment. Safety assessments included recording of AE (graded per NCI-CTCAE v4.03), laboratory parameters (including complete blood, neutrophil, and platelet counts; and hemoglobin values, graded per NCI-CTCAE version 4.03), vital signs, electrocardiograms, and Eastern Cooperative Oncology Group performance status (ECOG PS). Safety was regularly reviewed by an Independent Data Monitoring Committee.
Renal response
Both renal function impairment and renal response were analyzed. The eGFR was assessed using the modification of diet in renal disease (MDRD) equation on days 1, 2, 8, 9, 15, 16, and 22 of cycle 1; days 1, 8, and 15 of cycle 2; days 1 and 15 of each subsequent treatment cycle, and as clinically indicated. eGFR results were classified as RI (<60 mL/min/1.73 m²) or no RI (≥60 mL/min/1.73 m²). Based on IMWG criteria, complete renal response was defined as an increase in eGFR from <50 mL/min/1.73 m² at baseline to ≥60 mL/min/1.73 m² (no RI) in ≥1 post-baseline assessment.6,7 Responses were considered durable when lasting ≥60 days.6 A minor renal response was defined as an improvement in eGFR from ≥15 to <30 mL/min/1.73 m² at baseline to ≥30 mL/min/1.73 m² in ≥1 assessment during treatment.7
Statistical analysis
Sample size calculation was based on the primary efficacy endpoint; 159 events were needed to detect a 41% lower risk of disease progression (HR: 0.59) using a log-rank test (one-sided significance level of 0.025, 90% power). An interim PFS analysis was pre-planned when 65% of the 159 PFS events (103 events) were observed to detect overwhelming efficacy.
All efficacy analyses were conducted in the intent-to-treat population and summarized by randomized treatment. Extent of study treatment and treatment-emergent AE (TEAE) analyses were conducted in the safety population. Median PFS, probabilities of being progression-free, and corresponding CI were estimated using the Kaplan-Meier method. HR estimates were determined using the Cox proportional-hazard model by subgroup. Comparisons between patients with and without RI were observational only, with no formal statistical analysis performed. SAS 9.4 (SAS, Cary, NC) was used for all analyses.
Results
Patients
A total of 302 patients were randomized to Isa-Kd (n=179) or Kd (n=123). Baseline eGFR values could be calculated for 165 patients in the Isa-Kd arm and 111 in the Kd arm. Baseline eGFR was not evaluable for 14 patients in Isa-Kd and 12 in Kd, due to local legal restrictions on collecting racial group information. Among evaluable patients, 43 (26.1%) in the Isa-Kd arm and 18 (16.2%) in the Kd arm had RI (eGFR <60 mL/min/1.73 m2). Of these, 39 (23.6%) patients in Isa-Kd and 15 (13.5%) in Kd had moderate RI (eGFR ≥30 to <60 mL/min/1.73 m²); 2.4% of patients in IsaKd and 2.7% in Kd had severe RI (eGFR ≥15 to <30 mL/min/1.73 m²).
Among patients with RI at baseline, characteristics were generally well balanced between study arms (Table 1), except for more patients aged ≥75 years in the Isa-Kd than the Kd arm (14.0% vs. 5.6%, respectively) and more patients refractory to lenalidomide (25.6% Isa-Kd vs. 50.0% Kd) or to immunomodulatory drugs and proteasome inhibitors (18.6% Isa-Kd vs. 44.4% Kd) in the control arm. Patients with RI in both the Isa-Kd and Kd arms tended to be older, had more ISS stage III disease, and received more prior therapy lines than patients without RI (Table 1). Patient flow was described previously.28
Efficacy
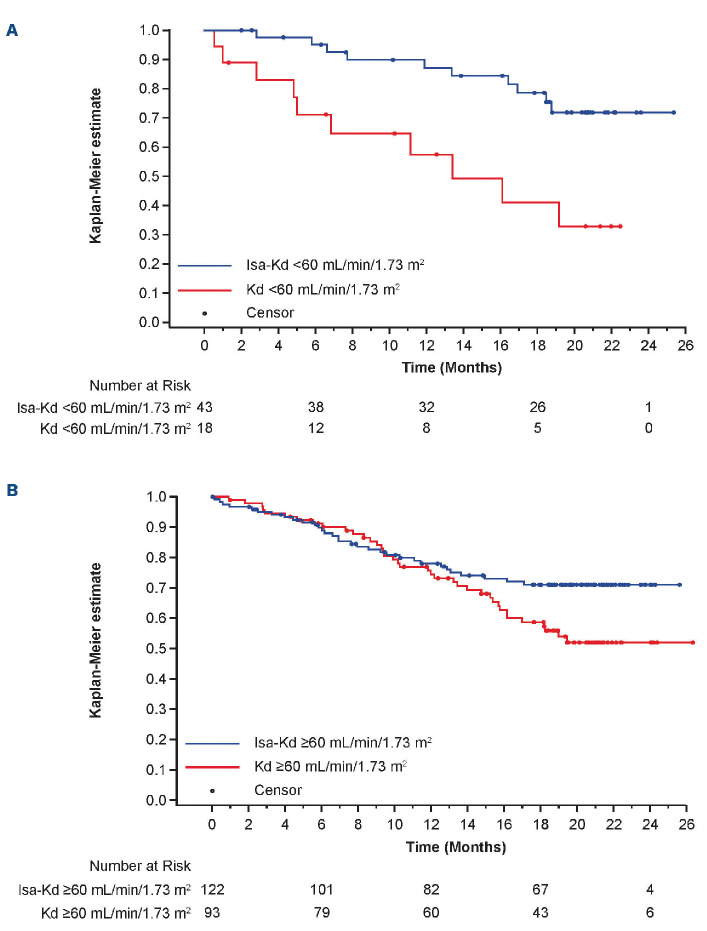
At a median overall follow-up of 20.7 months, the PFS benefit of Isa-Kd versus Kd in patients with and without RI, according to the assessment by the IRC, was consistent with that seen for the overall IKEMA study population (Figure 1). The addition of Isa prolonged PFS in patients with RI (HR: 0.27; 95% CI: 0.11–0.66; median PFS not reached for Isa-Kd vs. 13.4 months for Kd) and in patients without RI (HR: 0.63; 95% CI: 0.39–1.00; medians not reached). Probability to be free of a PFS event at 18 months was 79% with Isa-Kd versus 41% with Kd in patients with RI and 71% with Isa-Kd versus 59% with Kd in those without RI.
Multivariate analysis of PFS for patients with RI was performed to adjust the imbalance at baseline between IsaKd and Kd, including ISS stage, gain(1q21), refractory to PI or IMiD therapy, sex, and regulatory region as covariates. Adjusted HR was equal to 0.21 (95% CI: 0.07–0.68), suggesting that the imbalance did not influence the treatment effect in favor of Isa-Kd for PFS.
Consistent treatment effect was also observed in patients with the most severe RI at baseline (eGFR <45 mL/min/1.73 m²), as an exploratory analysis, in favor of patients treated in Isa-Kd (HR: 0.16; 95% CI: 0.04–0.67; median PFS, not reached for Isa-Kd [n=19] versus 11.14 months for Kd [n=10]) and in patients with eGFR ≥45 mL/min/1.73 m² (HR: 0.60; 95% CI: 0.39–0.93; medians not reached, n=146 versus n=101).
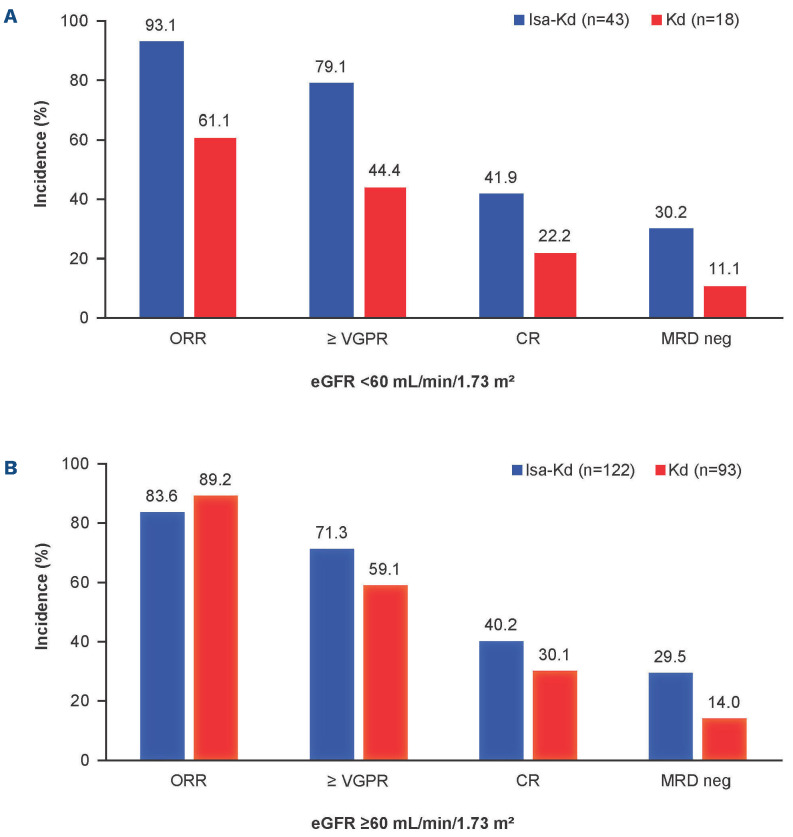
In the intent-to-treat population, the ORR was higher with Isa-Kd versus Kd for patients with RI (93.1% vs. 61.1%, respectively; Figure 2). Although the ORR was 83.6% with IsaKd versus 89.2% with Kd for patients without RI, the ≥VGPR rate for patients with RI was 79.1% with Isa-Kd versus 44.4% with Kd, and for patients without RI, it was 71.3% versus 59.1%, respectively. The MRD negativity rate, assessed by NGS at 10-5 sensitivity level in bone marrow aspirates from patients who achieved ≥VGPR, was 30.2% with Isa-Kd versus 11.1% with Kd for patients with RI and 29.5% with IsaKd versus 14.0% with Kd for patients without RI. In addition, the CR rate for patients with RI was 41.9% with Isa-Kd versus 22.2% with Kd, and for patients without RI, it was 40.2% versus 30.1%, respectively (Figure 2).
Table 1.
Baseline characteristics in patients with and without renal impairment in the isatuximab (Isa) carfilzomib (K) dexamethasone (d) (Isa-Kd) and Kd arms – intent-to-treat population
Although OS data were not mature at the interim analysis, 17% and 20% of patients died in the Isa-Kd and Kd arms, respectively (among patients with RI: 12% in Isa-Kd versus 39% in Kd and among patients without RI: 18% in Isa-Kd versus 15% in Kd).
Renal response
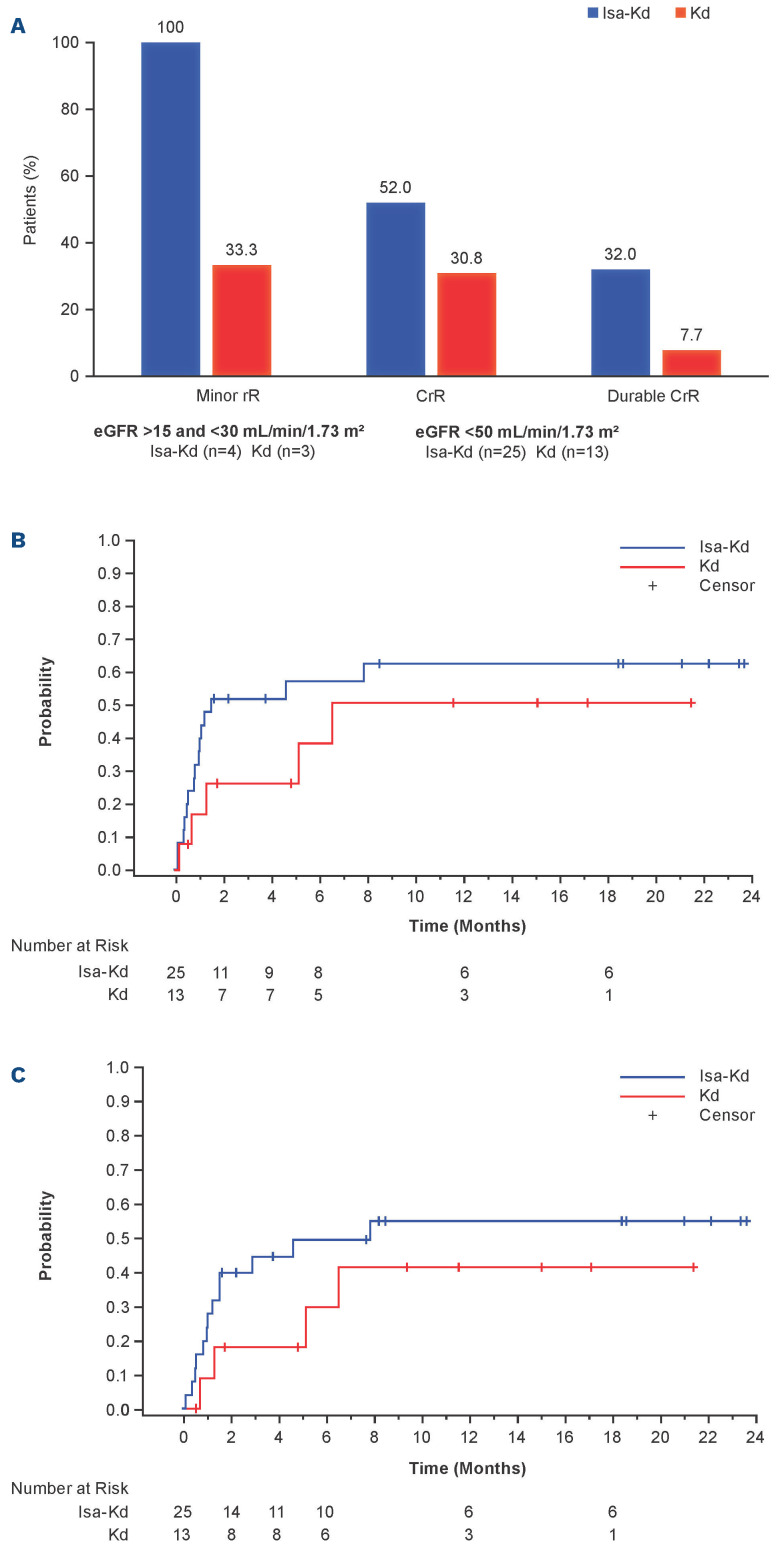
Among the 25 and 13 patients in the Isa-Kd and Kd arms, respectively, with eGFR <50 mL/min/1.73 m² at baseline, more patients in the Isa-Kd than the Kd arm had a complete renal response (52.0% vs. 30.8%; Figure 3A). Durable complete renal response occurred in eight of 25 (32.0%) Isa-Kd versus one of 13 (7.7%) Kd patients. In patients with severe RI at baseline (eGFR ≥15 to <30 mL/min/1.73 m²), all patients in the Isa-Kd arm achieved minor renal response compared with only one patient in the Kd arm (4/4 [100%] versus 1/3 [33.3%], respectively) (Figure 3A). Moreover, the time to first renal response and time to complete renal response were shorter in patients with baseline eGFR <50 mL/min/1.73 m² treated with Isa-Kd. Median time (95% CI) to first renal response was 1.51 (0.82–not calculable [NC]) months in the Isa-Kd arm versus 6.51 (0.69–NC) months in the Kd arm (Figure 3B). Median time (95% CI) to complete renal response was 7.82 (1.22–NC) months in the Isa-Kd arm versus NC (1.28–NC) months in the Kd arm (Figure 3C).
A similar incidence of patients experienced, at least once, end-stage RI (eGFR <15 mL/min/1.73 m²) during treatment with Isa-Kd versus Kd (1.8% vs. 2.7%, respectively). In the safety population, the number of patients with ≥1 TEAE mapped in the acute renal failure Standardized MedDRA Queries (SMQ) narrow terms was nine of 177 (5.1%, of which 1.1% were grade ≥3) in the Isa-Kd arm versus ten of 122 (8.2%, 2.5% grade ≥3). Acute kidney injury was observed in five of 177 (2.8%, 1.1% grade ≥3) Isa-Kd patients versus seven of 122 (5.7%, 1.6% grade ≥3) Kd patients.
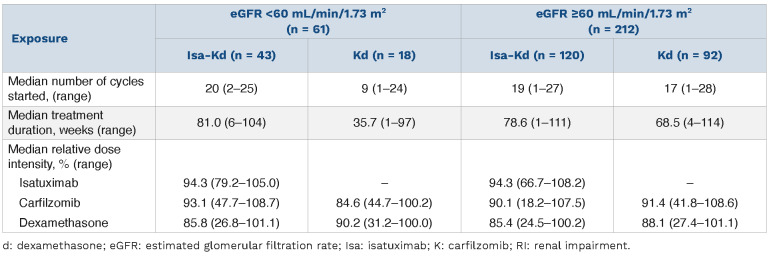
Treatment exposure
Longer treatment duration was observed with Isa-Kd versus Kd in patients with and without RI (Table 2). The median (range) number of cycles for Isa-Kd was 20 (2–25) for patients with RI and 19 (1–27) cycles for those without RI, and for Kd it was 9 (1–24) cycles for patients with RI and 17 (1–28) cycles for those without RI. Median duration of exposure for patients with and without RI was 81.0 (6– 104) and 78.6 (1–111) weeks with Isa-Kd versus 35.7 (1–97) and 68.5 (4–114) weeks with Kd. More patients were still on treatment at the cut-off date in the Isa-Kd arm (55.8% with RI and 54.1% without RI) versus the Kd arm (16.7% with RI and 36.6% without RI). The reasons for definitive treatment discontinuation in patients with RI were progressive disease (27.9% in the Isa-Kd vs. 33.3% in the Kd arm) and AE (7.0% in the Isa-Kd vs. 27.8% in the Kd arm). In patients without RI, 26.2% in the Isa-Kd versus 37.6% in the Kd arm discontinued treatment due to progressive disease and 9.8% in the Isa-Kd versus 9.7% in the Kd arm due to AE.
Figure 1.
Progression-free survival with isatuximab (Isa) carfilzomib (K) dexamethasone (d) (Isa-Kd) compared with Kd. (A) Patients with renal impairment (RI) (eGFR <60 mL/min/1.73 m²) or (B) without RI (eGFR ≥60 mL/min/1.73 m²), (ITT population). Progression-free survival (PFS) as per blinded independent response committee. d: dexamethasone; eGFR: estimated glomerular filtration rate; Isa: isatuximab; ITT: intent to treat; K: carfilzomib; PFS: progression-free survival.
The median relative dose intensity of Isa was similar in patients with or without RI; thus, RI did not impact Isa administration. The relative dose intensity of carfilzomib in patients with RI was lower in the Kd arm (84.6%) than in the Isa-Kd arm (93.1%), but similar in patients without RI (90.1% in the Isa-Kd vs. 91.4% in the Kd arm), indicating that more carfilzomib doses were delayed, reduced, or omitted in patients with RI who received Kd (Table 2).
Safety
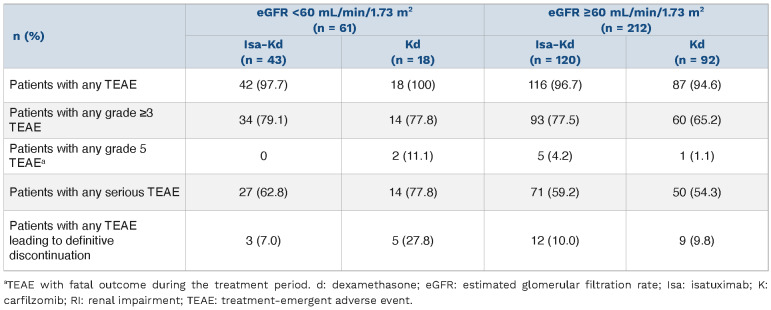
TEAE were experienced in 97.7% of Isa-Kd versus 100% of Kd patients with RI, whereas 93.7% versus 94.6% of patients without RI experienced TEAE in the Isa-Kd versus Kd arms, respectively (Table 3). In patients with RI, grade ≥3 TEAE were reported in 79.1% of Isa-Kd versus 77.8% of Kd patients and serious TEAE in 62.8% of Isa-Kd versus 77.8% of Kd patients. In patients with RI, treatment with Isa-Kd did not increase the incidence of TEAE with fatal outcome during treatment (Isa-Kd, 0% vs. Kd, 11.1% [2/18]) nor of TEAE leading to treatment discontinuation (Isa-Kd, 7.0% vs. Kd, 27.8%, Table 3).
Figure 2.
Response rates with isatuximab (Isa) carfilzomib (K) dexamethasone (d) (Isa-Kd) compared with Kd. (A) Patients with renal impairment (RI) (eGFR <60 mL/min/1.73 m²) or (B) without RI (eGFR ≥60 mL/min/1.73 m²), (ITT population). CR: complete response; d: dexamethasone; eGFR: estimated glomerular filtration rate; Isa: isatuximab; ITT: intent to treat; K: carfilzomib; MRD neg: minimal residual disease negativity; ORR: overall response rate; PR: partial response; VGPR: very good partial response. MRD was assessed by next-generation sequencing with a sensitivity level 10-5.
Figure 3.
Renal response in the isatuximab (Isa) carfilzomib (K) dexamethasone (d) (Isa-Kd) compared with Kd arms. (A) Complete and durable (≥60 days) renal responses in patients with eGFR <50 mL/min/1.73 m² at baseline and minor renal responses in patients with eGFR ≥15 and <30 mL/min/1.73 m² at baseline, (ITT population). (B) Time to first renal response and (C) time to first complete renal response in patients with eGFR <50 mL/min/1.73 m² at baseline. CrR: complete renal response; d: dexamethasone; eGFR: estimated glomerular filtration rate; Isa: isatuximab; ITT: intent to treat; K: carfilzomib; rR: renal response.
Table 2.
Overall extent of exposure in patients with and without renal impairment in the isatuximab (Isa) carfilzomib (K) dexamethasone (d) (Isa-Kd) and Kd arms – safety population
Table 3.
Safety summary in patients with and without renal impairment in the isatuximab (Isa) carfilzomib (K) dexamethasone (d) (Isa-Kd) and Kd arms – safety population
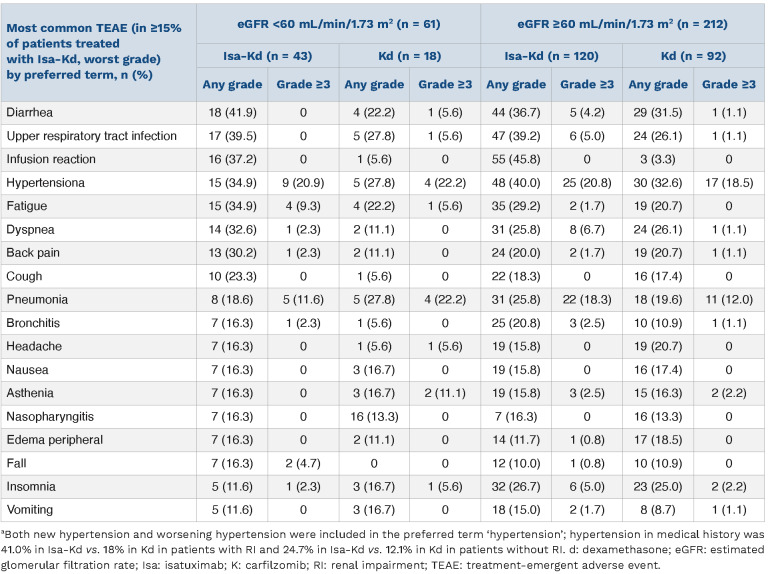
TEAE occurring in ≥15% of patients treated with Isa-Kd are shown in Table 4, by renal function group and treatment arm. In patients with RI, the most common TEAE with Isa-Kd versus Kd were diarrhea (41.9% vs. 22.2%), upper respiratory tract infection (39.5% vs. 27.8%), hypertension (34.9% vs. 27.8%), and fatigue (34.9% vs. 22.2%). The most common TEAE with Isa-Kd versus Kd in patients without RI were hypertension (40.0% vs. 32.6%), upper respiratory tract infections (39.2% vs. 26.1%), and diarrhea (36.7% vs. 31.5%). Infusion reactions were observed in 37.2% of Isa-Kd versus 5.6% of Kd patients with RI and 45.8% of Isa-Kd versus 3.3% of Kd patients without RI, but no grade ≥3 infusion reactions were reported. Hypertension was the most common grade ≥3 TEAE independently of renal function: 20.9% with Isa-Kd versus 22.2% with Kd in patients with RI and 20.8% with Isa-Kd versus 18.5% with Kd in patients without RI (Table 4).
Carfilzomib has been reported to cause cardiac complications.35 Cardiac failure (by standardized MedDRA query) was observed in 11.6% (2.3% grade ≥3) of Isa-Kd patients with RI versus 5.6% (5.6% grade ≥3) of Kd patients with RI. In patients without RI, cardiac failure was observed in 5.8% (4.2% grade ≥3) of Isa-Kd patients versus 6.5% (3.3% grade ≥3) of Kd patients.
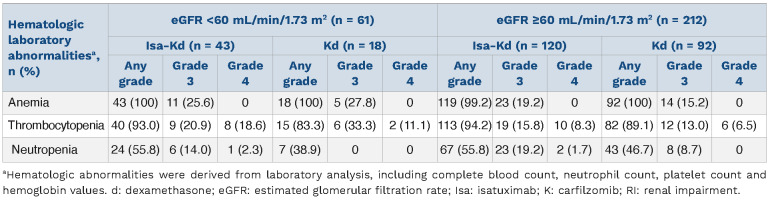
The most common hematologic abnormalities based on laboratory results in treated patients with RI were anemia (all patients in both arms) and thrombocytopenia (93.0% with Isa-Kd versus 83.3% with Kd, Table 5). In patients without RI, incidence of anemia was 99.2% with Isa-Kd versus 100% with Kd, whereas incidence of thrombocytopenia was 94.2% with Isa-Kd versus 89.1% with Kd.
Importantly, the incidence of grade 3–4 anemia and thrombocytopenia were comparable in all subgroups, while the incidence of grade 3 neutropenia was higher in the Isa-Kd arm in patients with or without RI (Table 5).
Table 4.
Treatment-emergent adverse events occurring in ≥15% of patients treated with isatuximab (Isa) carfilzomib (K) dexamethasone (d) (Isa-Kd), according to the renal impairment status – safety population.
Table 5.
Hematologic abnormalities determined by laboratory analysis in patients with and without renal impairment in the isatuximab (Isa) carfilzomib (K) dexamethasone (d) (Isa-Kd) and Kd arms – safety population.
Discussion
In MM patients, RI is considered a poor prognostic factor, being associated with earlier mortality and worse OS.5,22,36 As such, there is a critical need for anti-MM therapies that also improve renal function. This prespecified subgroup analysis of the phase III IKEMA study demonstrated that Isa-Kd is efficacious and has a manageable safety profile in patients with RI.
The risk of disease progression or death in patients with RI was 73% lower in the Isa-Kd arm, indicated by the very low HR (HR: 0.27; 95% CI: 0.11–0.66). This result is consistent with the PFS benefit of Isa-Kd observed in the overall IKEMA study population (HR: 0.53; 99% CI: 0.32–0.89)28 and in patients without RI (HR: 0.63; 95% CI: 0.39–1.00). In IKEMA, median PFS observed among patients with RI (eGFR <60 mL/min/1.73 m²) receiving Kd (13.4 months) was similar to results of the Kd arm in the ENDEAVOR subgroup analysis, with a median PFS of 14.9 months in patients with creatinine clearance ≥15 to <50 mL/min (severe/moderate RI).9 Consistent with the PFS results, duration of study treatment exposure was similar in Isa-Kd patients with or without RI (81 and 78.6 months, respectively), whereas it was shorter in Kd patients with RI versus those without RI (35.7 vs. 68.5 months, respectively).
The ORR was greater with Isa-Kd than Kd in patients with RI (93.1% vs. 61.1%), whereas patients without RI showed similar ORR in the two study arms (83.6% vs. 89.2%), consistent with the overall IKEMA study population (86.6% Isa-Kd vs. 82.9% Kd).28 Of note, depth of response was superior with Isa-Kd versus Kd independently of RI status, with respect to CR rate (41.9% Isa-Kd vs. 22.2% Kd in patients with RI; 40.2% Isa-Kd versus 30.1% Kd in patients without RI), ≥VGPR rate (79.1% Isa-Kd vs. 44.4% Kd in patients with RI; 71.3% Isa-Kd vs. 59.1% Kd in patients without RI), and MRD negativity rate (30.2% Isa-Kd vs. 11.1% Kd in patients with RI; 29.5% Isa-Kd vs. 14.0% Kd in patients without RI).
Remarkably, compared with Kd, Isa-Kd increased the proportion of patients with RI who achieved both complete (52.0% Isa-Kd vs. 30.8% Kd) and durable (≥60 days; 32.0% Isa-Kd vs. 7.7% Kd) renal responses, and decreased time to first (1.5 months Isa-Kd vs. 6.5 months Kd) and to complete (7.8 months Isa-Kd vs. NC Kd) renal response, suggesting that Isa-Kd allows the achievement of sustainable reversal of RI. Similarly, compared with Kd, fewer patients in the Isa-Kd arm experienced worsening of renal function or progression to end-stage RI.
The addition of Isa to Kd was associated with a manageable safety profile in MM patients with and without RI. Among patients with RI, there was a similar incidence of patients with grade ≥3 TEAE between the two arms (79.1% Isa-Kd vs. 77.8% Kd), whereas this incidence was higher in patients without RI (77.5% Isa-Kd vs. 65.2% Kd). Furthermore, in patients with RI there was a similar incidence of patients with serious TEAE or TEAE leading to death during study treatment or treatment discontinuation. The higher treatment exposure observed with Isa-Kd versus Kd might have contributed to the higher incidence of grade ≥3 TEAE in patients without RI. Cardiac failure in the overall population was similar between study arms (7.3% all grades and 4.0% grade ≥3 in Isa-Kd versus 6.6% all grades and 4.1% grade ≥3 in Kd), but incidence of any-grade cardiac failure was higher in patients with RI in Isa-Kd (11.6% vs. 5.6%). This can be related to a longer treatment exposure in Isa-Kd (median number of cycles was 20 in Isa-Kd vs. 9 in Kd) and higher carfilzomib relative dose intensity in Isa-Kd (93.1% vs. 84.6%). This difference in incidence disappeared for grade ≥3 events.
The most common TEAE in patients with RI treated with Isa-Kd versus Kd were diarrhea, upper respiratory tract infection, hypertension, and fatigue with similar frequency observed in the overall IKEMA population.28 There was no increased incidence of infusion reactions in the RI (37.2% Isa-Kd vs. 5.6% Kd) compared with non-RI (45.8% Isa-Kd vs. 3.3% Kd) subgroups.
There are few reports in the literature analyzing the efficacy and toxicity of anti-CD38 monoclonal antibodies in patients with RI. The results of this IKEMA subgroup analysis reinforce the findings of the ICARIA-MM RI subgroup analysis, which showed that addition of Isa to Pd also improved clinical outcomes in patients with RI.27 Median PFS was 9.5 months with Isa-Pd versus 3.7 months with Pd (HR: 0.50; 95% CI: 0.30–0.85) for patients with RI. Isa-Pd also showed greater depth of response in patients with RI, with a 56% ORR with Isa-Pd versus 25% with Pd. Complete renal response rates were achieved in 23 of 32 (71.9%) patients treated with Isa-Pd and eight of 21 (38.1%) treated with Pd; these were durable in ten of 32 (31.3%) and four of 21 (19.0%) of patients treated with Isa-Pd versus Pd, respectively.27 Data about efficacy and safety of daratumumab, a different CD38 monoclonal antibody, in patients with RI are limited. A few, isolated case reports with single dialysis-dependent patients have been published.37-40 Results from a retrospective, multicenter, open-label study designed to evaluate safety and efficacy of daratumumab in RRMM patients with end-stage RI requiring hemodialysis (n=15) reported a median PFS of 8.7 months, OS of 12.2 months, and ORR of 40%.41 The most common grade 3–4 hematologic AE included thrombocytopenia (n=5), anemia (n=4), and neutropenia (n=4). Infusion reactions (n=6) were the most frequent non-hematologic AE.41 Results of an interim analysis of the phase II DARE study, a multicenter, single-arm, open-label study in RRMM patients with severe RI (eGFR <30 mL/min/1.73 m2) or in need of hemodialysis were reported recently.42 Eligible patients had received ≥2 prior treatment lines (including bortezomib- and lenalidomide-based regimens) and presented with ECOG PS score ≤2. At the cut-off date, 35 patients treated with daratumumab and dexamethasone showed a 12-month PFS probability of 50%, an ORR of 45.7%, and a renal response rate of 17.1%. The most common grade 3–4 AE were anemia (17.1%) and hyperglycemia (8.6%). A total of 48.6% of patients had ≥1 grade 3–4 AE and 25.7% of patients experienced ≥1 serious AE.42 Limitations of this IKEMA subgroup analysis include: (i) less than 3% of patients in each arm had severe RI, so the results presented here are mainly applicable to patients with moderate RI, and (ii) RI status at baseline was not a stratification factor, likely resulting in small differences in subgroup size between treatment arms.
In summary, addition of Isa to Kd improved PFS and depth of response in patients with relapsed MM and RI, with a manageable safety profile, consistent with the benefit observed in the overall IKEMA study population. More patients treated with Isa-Kd showed reversal of RI and durable renal responses compared with those treated with Kd. Based on these findings, Isa-Kd represents a valuable addition to the therapies used to treat patients with MM-related renal dysfunction.
Acknowledgments
The IKEMA study was sponsored by Sanofi. We thank the participating patients and their caregivers, and the study centers and investigators for their contributions to the study. Medical writing support was provided by C. Semighini Grubor, PhD, and S. Mariani, MD, PhD, of Elevate Medical Afairs, contracted by Sanofi Genzyme, for publication support services.
References
- 1.Clark AD, Shetty A, Soutar R. Renal failure and multiple myeloma: pathogenesis and treatment of renal failure and management of underlying myeloma. Blood Rev. 1999;13(2):79-90. [DOI] [PubMed] [Google Scholar]
- 2.Eleutherakis-Papaiakovou V, Bamias A, Gika D, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma. 2007;48(2):337-341. [DOI] [PubMed] [Google Scholar]
- 3.San-Miguel JF, Richardson PG, Sonneveld P, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia. 2008;22(4):842-849. [DOI] [PubMed] [Google Scholar]
- 4.Yadav P, Cook M, Cockwell P. Current trends of renal impairment in multiple myeloma. Kidney Dis (Basel). 2016;1(4):241-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fotiou D, Dimopoulos MA, Kastritis E. Managing renal complications in multiple myeloma. Expert Rev Hematol. 2016;9(9):839-850. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Terpos E, Chanan-Khan A, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28(33):4976-4984. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos MA, Sonneveld P, Leung N, et al. International Myeloma Working Group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol. 2016;34(13):1544-1557. [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulos M, Siegel D, White DJ, et al. Carfilzomib vs bortezomib in patients with multiple myeloma and renal failure: a subgroup analysis of ENDEAVOR. Blood. 2019;133(2):147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimopoulos MA, Richardson PG, Schlag R, et al. VMP (bortezomib, melphalan, and prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27(36):6086-6093. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Reversibility of renal impairment in patients with multiple myeloma treated with bortezomib-based regimens: identification of predictive factors. Clin Lymphoma Myeloma. 2009;9(4):302-306. [DOI] [PubMed] [Google Scholar]
- 12.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Bortezomib-based triplets are associated with a high probability of dialysis independence and rapid renal recovery in newly diagnosed myeloma patients with severe renal failure or those requiring dialysis. Am J Hematol. 2016;91(5):499-502. [DOI] [PubMed] [Google Scholar]
- 13.Weisel KC, Dimopoulos MA, Moreau P, et al. Analysis of renal impairment in MM-003, a phase III study of pomalidomide + low-dose dexamethasone versus high-dose dexamethasone in refractory or relapsed and refractory multiple myeloma. Haematologica. 2016;101(7):872-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavriatopoulou M, Terpos E, Dimopoulos MA. IMiDs for myeloma induced renal impairment. Oncotarget. 2018;9(84):35476-35477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimopoulos M, Weisel K, van de Donk N, et al. Pomalidomide plus low-dose dexamethasone in patients with relapsed/refractory multiple myeloma and renal impairment: results from a Phase II trial. J Clin Oncol. 2018;36(20):2035-2043. [DOI] [PubMed] [Google Scholar]
- 16.Siegel DS, Weisel KC, Dimopoulos MA, et al. Pomalidomide plus low-dose dexamethasone in patients with relapsed/refractory multiple myeloma and moderate renal impairment: a pooled analysis of three clinical trials. Leuk Lymphoma. 2016;57(12):2833-2838. [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulos M, Alegre A, Stadtmauer EA, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer. 2010;116(16):3807-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimopoulos MA, Cheung MC, Roussel M, et al. Impact of renal impairment on outcomes with lenalidomide and dexamethasone treatment in the FIRST trial, a randomized, open-label phase 3 trial in transplant-ineligible patients with multiple myeloma. Haematologica. 2016;101(3):363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimopoulos MA, Christoulas D, Roussou M, et al. Lenalidomide and dexamethasone for the treatment of refractory/relapsed multiple myeloma: dosing of lenalidomide according to renal function and effect on renal impairment. Eur J Haematol. 2010;85(1):1-5. [DOI] [PubMed] [Google Scholar]
- 20.Dimopoulos MA, Terpos E, Goldschmidt H, Alegre A, Mark T, Niesvizky R. Treatment with lenalidomide and dexamethasone in patients with multiple myeloma and renal impairment. Cancer Treat Rev. 2012;38(8):1012-1019. [DOI] [PubMed] [Google Scholar]
- 21.Dimopoulos MA, Roussou M, Gkotzamanidou M, et al. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia. 2013;27(2):423-429. [DOI] [PubMed] [Google Scholar]
- 22.Park S, Han B, Kim K, et al. Renal insufficiency in newly-diagnosed multiple myeloma: analysis according to International Myeloma Working Group consensus statement. Anticancer Res. 2014;34(8):4299-4306. [PubMed] [Google Scholar]
- 23.Kyprolis® (carfilzomib) for injection for intravenous use [prescribing information] Amgen Thousand Oaks, CA: (March 2021). [Google Scholar]
- 24.Sarclisa® (isatuximab-irfc) injection for intravenous use [prescribing information] Sanofi Bridgewater, NJ: (March 2021). [Google Scholar]
- 25.European Medicines Agency. Sarclisa, INN-Ixatuximab. Summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/sarclisa-epar-product-information_en.pdf. Accessed May 13, 2021. [Google Scholar]
- 26.Attal M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I; ICARIA-MM study group. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096-2107. [DOI] [PubMed] [Google Scholar]
- 27.Dimopoulos MA, Leleu X, Moreau P, et al. Isatuximab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma patients with renal impairment: ICARIA-MM subgroup analysis. Leukemia. 2021;35(2):562-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreau P, Dimopoulos M-A, Mikhael J, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. 2021;397(10292):2361-2371. [DOI] [PubMed] [Google Scholar]
- 29.Moreau P, Dimopoulos MA, Yong K, et al. Isatuximab plus carfilzomib/dexamethasone versus carfilzomib/dexamethasone in patients with relapsed/refractory multiple myeloma: IKEMA Phase III study design. Future Oncol. 2020;16(2):4347-4358. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470. [DOI] [PubMed] [Google Scholar]
- 31.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-346. [DOI] [PubMed] [Google Scholar]
- 33.Lahuerta JJ, Paiva B, Vidriales MB, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35(25):2900-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paiva B, Puig N, Cedena MT, et al. Measurable residual disease by next-generation flow cytometry in multiple myeloma. J Clin Oncol. 2020;38(8):784-792. [DOI] [PubMed] [Google Scholar]
- 35.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1(7):449-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United kingdom Medical Research Council trials between 1980 and 2002--Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23(36):9219-9226. [DOI] [PubMed] [Google Scholar]
- 37.Rocchi S, Tacchetti P, Pantani L, et al. Safety and efficacy of daratumumab in dialysis-dependent renal failure secondary to multiple myeloma. Haematologica. 2018;103(6):e277-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smyth E, Glavey S, Melotti D, et al. Dialysis independence following single-agent daratumumab in refractory myeloma with renal failure. Ir J Med Sci. 2019;188(3):1079-1080. [DOI] [PubMed] [Google Scholar]
- 39.Moore DC, Arnall JR, Janes A, Pineda-Roman M. Dialysis independence following combination daratumumab, thalidomide, bortezomib, cyclophosphamide, and dexamethasone in multiple myeloma with severe renal failure. Clin Lymphoma Myeloma Leuk. 2020;20(7):e395-398. [DOI] [PubMed] [Google Scholar]
- 40.Jeyaraman P, Bhasin A, Dayal N, Pathak S, Naithani R. Daratumumab in dialysis-dependent multiple myeloma. Blood Res. 2020;55(1):65-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cejalvo MJ, Legarda M, Abella E, et al. Single-agent daratumumab in patients with relapsed and refractory multiple myeloma requiring dialysis: results of a Spanish retrospective, multicentre study. Br J Haematol. 2020;190(5):e289-292. [DOI] [PubMed] [Google Scholar]
- 42.Kastritis E, Terpos E, Symeonidis A, et al. Daratumumab with dexamethasone in patients with relapsed/refractory multiple myeloma and severe renal impairment: results on efficacy and safety of the Phase 2 Dare study. Blood. 2020;136(Suppl 1):S48-49. [Google Scholar]