Abstract
Exosomes include plasma-transported vesicles that are secreted by human tissues and reflect metabolic status. The profile of exosomes (particularly microRNA content) is altered in metabolic disease. In type 2 diabetes mellitus, exosomes circulating in plasma induce transcriptional changes related to tumour progression and pro-metastatic phenotypes in target cancer cells, potentially linking obesity to cancer progression and metastasis.
Exosomes were first visualized by electron microscopy nearly 40 years ago as vesicles that are shed by cultured cells that contain enzymatic activities1. For the next 20 years, exosomes were largely ignored by researchers, as critical opinion regarded them as little more than disposal systems for constitutive removal of plasma membrane-encapsulated waste, and their origin and purpose was therefore of limited interest. In 2007, however, a breakthrough paper2 described exosomal mRNAs and microRNAs (miRNAs) in human and mouse cell lines and primary cells, which suggested these potential instructions for gene regulation could be transported to new tissue targets to reprogramme cells. A report of exosomal mitochondrial DNA secreted by glioblastoma cells and astrocytes generated further interest3. Exosomes are now recognized to be extracellular vesicles of 50–200 nm in size that are released by most, or all, somatic cells and undertake more functions than garbage disposal. Importantly, they can contain bioactive molecules that are influenced by the metabolic status of the cell of origin. The ability of exosomes to circulate in blood and lymph suggest that these metabolic signals can travel throughout the body to deliver instructions to target tissues4–6.
Discoveries made over the past 15 years highlight the potential importance of this new mechanism of intercellular communication. Preliminary work published by our group in a pre-print paper, not yet peer-reviewed, suggests that plasma exosomes from adults with type 2 diabetes mellitus (T2DM) reprogrammed a prostate cancer cell line (DU145) to a more aggressive and pro-metastatic phenotype in vitro, unlike plasma exosomes from control individuals without T2DM4. Strikingly, plasma exosomes from different adults with T2DM generated changes in the target DU145 cells, each of them unique, as determined by principal component analysis of RNA-sequencing data. However, non-diabetic control individuals strongly resemble each other. The miRNA profiles of exosomes from plasma obtained from adults with T2DM are also distinct from control individuals without diabetes mellitus. These preliminary findings suggest that miRNA-374 and miRNA-93, which were upregulated in exosomes from adults with T2DM, are critical to upregulate transcription of prominent genes in DU145 cells that are involved in immune exhaustion and mesenchymal phenotypes associated with tumour metastasis4.
Another study from our group reported the first evidence for how exosomes derived from adipocytes of individuals with insulin resistance or T2DM can reprogramme epithelial-like breast cancer cell lines to become more aggressive, mesenchymal-like cells in vitro5. These findings suggest that exosomes not only reflect the metabolic status of a patient, but also carry functional instructions that could differentially drive tumour progression. This hypothesis could explain why patients with cancer and comorbid obesity and/or metabolic disease show more advanced cancer7 and worse outcomes (these include shorter disease-free survival and greater risk of recurrence of obesity-related cancers) than metabolically normal patients with cancer. The adipose microenvironment of patients with breast cancer and obesity with metabolic complications, and associated inflammation, probably engages in cytokine, chemokine, metabolite and exosome crosstalk with malignant cell clones. We suggest that this microenvironment might elicit more transcriptional plasticity and pro-metastatic behaviour than the adipose microenvironment of patients who are metabolically normal.
Increased BMI8 and T2DM6 have been established to be associated with increased circulating concentrations of exosomes and altered payload content. Circulating exosomes from individuals with T2DM can be internalized by monocytes and, because of their different payload, alter signalling pathways related to cell survival, oxidative stress, inflammation and immune function6. This mechanism could link adipose tissue and tumour cells to the immune microenvironment and specific leukocyte infiltrates in obesity-related cancers (FIG. 1). Finally, the incidence of gestational diabetes mellitus (GDM), which is defined as glucose intolerance occurring in the second or third trimester of pregnancy, is rising. Several studies have characterized plasma exosomal microRNAs from pregnant people with GDM and reported upregulated levels of miRNA-144, miRNA-16 and miRNA-103 (REF.9). These miRNAs are associated with solid tumour malignancies and metastatic progression in human studies and in vitro cell-line models. This finding suggests that systemic exosomal crosstalk in GDM might exacerbate risks of obesity-related cancers10.
Fig. 1 |. Circulating exosomes in metabolic disorders could induce tumour progression.
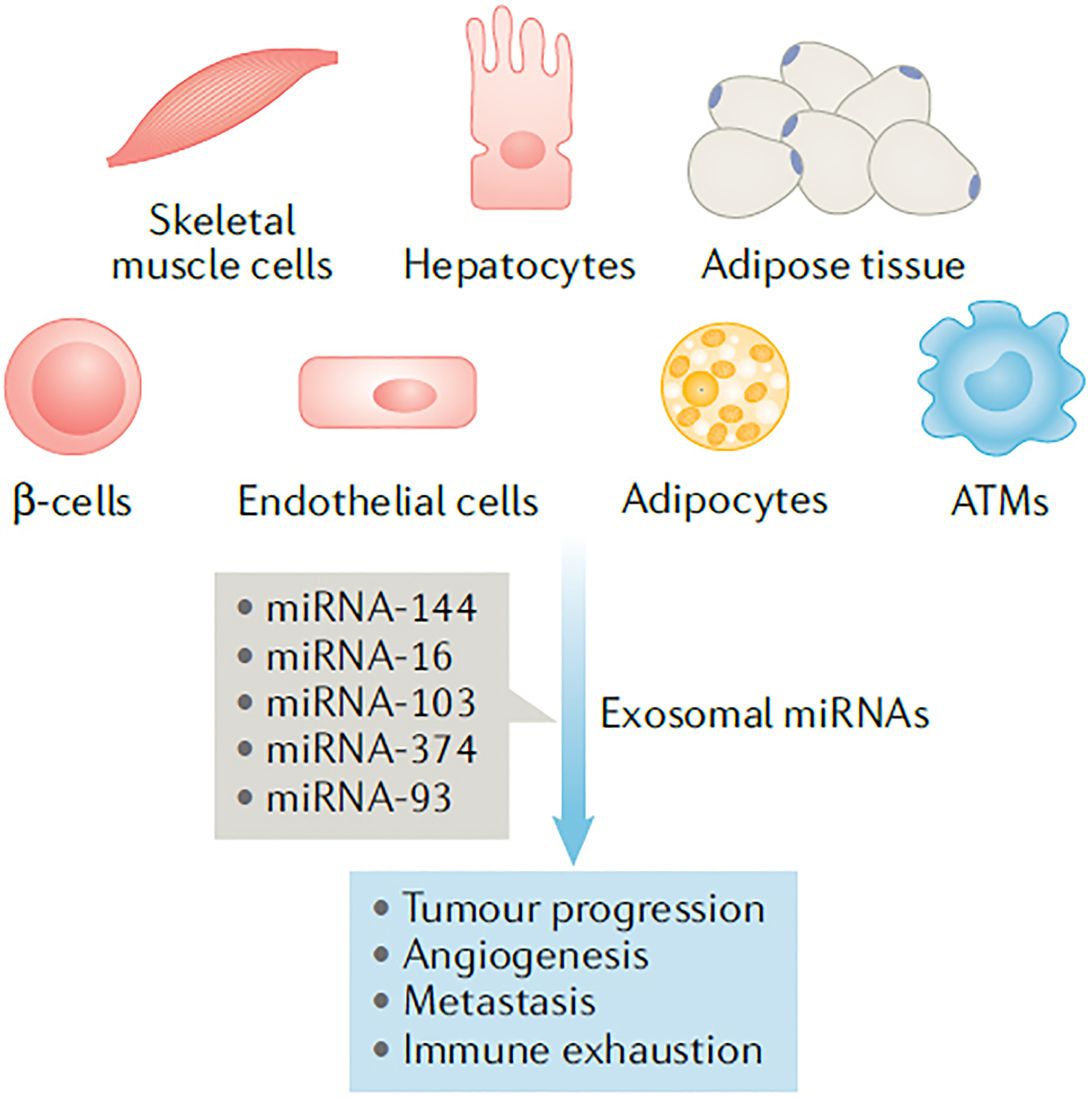
Metabolic disorders, such as type 2 diabetes mellitus, are characterized by a profile of circulating exosomes (secreted from various cells and tissues) with miRNA cargo distinct from individuals who are metabolically normal. Initial studies suggest that this abnormal miRNA cargo associates with tumour progression, angiogenesis and immune exhaustion. ATMs, adipose tissue macrophages; miRNA, microRNA.
The field has reached the stage where it might be practical to design exosomal profiling methods, which can evaluate plasma from patients for exosomal miRNAs and assess their importance for cancer progression. Such noninvasive plasma exosome biomarkers of abnormal metabolism and chronic inflammation in patients with cancer could be formulated into a kit and combined with validated diagnostic modalities, such as imaging and tumour histology, to improve risk assessment and clinical decision-making. This approach might be particularly important for patients with comorbid medical conditions who are not well served by the current standard of care. Patients who do not want to undergo repeated biopsies might be more willing to give blood (or other body fluids that are obtained even less invasively, such as urine and saliva) for exosome profiling.
In conclusion, exosomes are circulating, nanometre-diameter vesicles secreted by all cell types. Individual metabolic status determines the unique miRNA profile of the circulating exosomes. Exosomes released into plasma from the tissues of adults with insulin resistance or T2DM express miRNAs related to tumour progression, angiogenesis, metastasis and immune exhaustion, unlike the tissues of metabolically normal adults. Exosome content is regulated by nutritional inputs, cell stress and metabolic states6. The exosomal miRNA cargo can affect distant cells by reprogramming transcriptional networks. We propose that the use of plasma exosomes as noninvasive biomarkers might assist clinicians to better manage treatment strategies for patients with cancer, and endocrinologists to better manage treatment of patients with metabolic disease. In addition, drug delivery is a promising application of exosomes; for example, new approaches are being developed to engineer and develop exosomes for therapeutic targeting. The field of exosome biology is progressing rapidly; however, the optimal utility of exosomes in precision medicine has not yet been defined.
Footnotes
Competing interests
G.V.D. and N.J. are inventors on US patent 63/171,689 to use exosomes as a cancer diagnostic. P.L. declares no competing interests.
References
- 1.Trams EG, Lauter CJ, Salem N Jr & Heine U, Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 645, 63–70 (1981). [DOI] [PubMed] [Google Scholar]
- 2.Valadi H et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol 9, 654–659 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Guescini M, Genedani S, Stocchi V & Agnati LF Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm 117, 1–4 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Jafari N et al. Novel plasma exosome biomarkers for prostate cancer progression in co-morbid metabolic disease. Preprint at bioRxiv 10.1101/2022.02.01.478722 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jafari N et al. Adipocyte-derived exosomes may promote breast cancer progression in type 2 diabetes. Sci. Signal 14, eabj2807 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman DW et al. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes 67, 2377–2388 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overbeek JA et al. Type 2 diabetes, but not insulin (analog) treatment, is associated with more advanced stages of breast cancer: a national linkage of cancer and pharmacy registries. Diabetes Care 42, 434–442 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Elfeky O, Longo S, Lai A, Rice GE & Salomon C Influence of maternal BMI on the exosomal profile during gestation and their role on maternal systemic inflammation. Placenta 50, 60–69 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Yang X & Wu N MicroRNAs and exosomal microRNAs may be possible targets to investigate in gestational diabetes mellitus. Diabetes Metab. Syndr. Obes 15, 321–330 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrin MC et al. Gestational diabetes as a risk factor for pancreatic cancer: a prospective cohort study. BMC Med. 5, 25 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]


