Key Points
MADD is a GDP/GTP exchange factor for secretory Rab GTPases and drives their recruitment to WPBs.
MADD facilitates the regulated VWF secretion pathway.
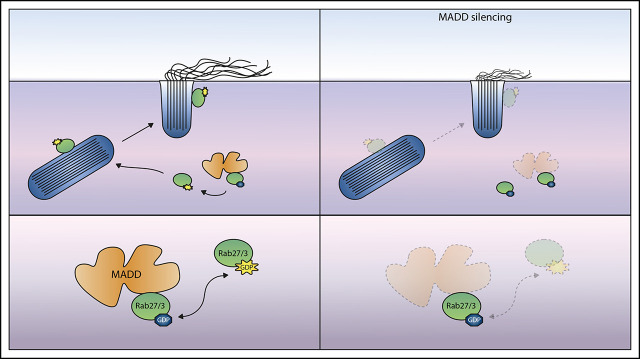
Visual Abstract
Abstract
von Willebrand factor (VWF) is an essential hemostatic protein that is synthesized and secreted by endothelial cells and stored in Weibel-Palade bodies (WPBs). The secretory Rab GTPases Rab27A, Rab3B, and Rab3D have been linked with WPB trafficking and secretion. How these Rabs are activated and recruited to WPBs remains elusive. In this study, we identified MAP kinase-activating death domain (MADD) as the guanine nucleotide exchange factor for Rab27A and both Rab3 isoforms in primary human endothelial cells. Rab activity assays revealed a reduction in Rab27A, Rab3B, and Rab3D activation upon MADD silencing. Rab activation, but not binding, was dependent on the differentially expressed in normal and neoplastic cells (DENN) domain of MADD, indicating the potential existence of 2 Rab interaction modules. Furthermore, immunofluorescent analysis showed that Rab27A, Rab3B, and Rab3D recruitment to WPBs was dramatically decreased upon MADD knockdown, revealing that MADD drives Rab membrane targeting. Artificial mistargeting of MADD using a TOMM70 tag abolished Rab27A localization to WPB membranes in a DENN domain–dependent manner, indicating that normal MADD localization in the cytosol is crucial. Activation of Rab3B and Rab3D was reduced upon Rab27A silencing, suggesting that activation of these Rabs is enhanced through previous activation of Rab27A by MADD. MADD silencing did not affect WPB morphology, but it did reduce VWF intracellular content. Furthermore, MADD-depleted cells exhibited decreased histamine-evoked VWF release, similar to Rab27A-depleted cells. In conclusion, MADD acts as a master regulator of VWF secretion by coordinating the activation and membrane targeting of secretory Rabs to WPBs.
Introduction
von Willebrand factor (VWF) is a multimeric glycoprotein that plays a crucial role in hemostasis.1 Endothelial cells (ECs) synthesize VWF, which is processed by proteolytic cleavage of the VWF propeptide (VWFpp), glycosylation, and multimerization. Mature VWF multimers that emerge from the trans-Golgi network are condensed into tubules and stored in specialized organelles called Weibel-Palade bodies (WPBs).2 In steady-state, VWF from WPBs is continuously secreted into the vascular lumen through the basal pathway to maintain VWF plasma levels.3,4 Upon vascular injury, rapid stimulus-induced WPB exocytosis occurs via the regulated pathway to enable formation of VWF strings under shear stress,5 which become a substrate for adhesion and subsequent activation and aggregation of platelets.6
The family of Rab GTPases, consisting of ∼70 members in humans, plays a variety of roles in vesicle trafficking between intracellular compartments.7 The secretory Rabs, Rab27A and Rab3 isoforms, have been previously implicated in WPB trafficking and secretion.5,8 Rab27A, which is recruited during WPB maturation,9 is able to both promote and inhibit VWF release in a secretagogue- and Rab effector–dependent manner.10-12 Rab3B is present on WPBs independent of their maturation status, but its significance is unclear because Rab3B silencing has no effect on histamine-induced VWF secretion.11,12 Rab3D also localizes to WPBs, and its overexpression results in reduced histamine-induced WPB exocytosis.13 In contrast, Rab3D knockdown seemed to have either an inhibitory effect or no effect on VWF secretion.11,12
Rabs operate as molecular switches cycling between GDP (guanosine diphosphate)-bound (inactive) and GTP-bound (active) states. Inactive Rabs are chaperoned by Rab-GDP dissociation inhibitor (Rab-GDI), which solubilizes hydrophobic regions to retain Rabs in the cytosol.14,15 In their active state, Rabs are targeted to specific organelle membranes, where they subsequently recruit effector proteins.14,16
In ECs, several secretory Rab effectors are known, including Slp4-a, MyRIP, and Munc13 proteins. Each of these effectors is crucial for WPB trafficking and/or exocytosis by mediating interactions with the cytoskeleton (MyRIP),10,11,17,18 as plasma membrane tethering factors (Munc13-2 and Munc13-4),12,19-21 and through association with the SNARE complex that facilitates membrane fusion (Slp4-a).11,22,23 Rab27A is able to bind all of these Rab effectors, whereas Rab3 isoforms interact only with Slp4-a and Munc13-2. Despite the extensive research on the function of Rabs and their associated Rab effectors in different stages of WPB release, the mechanism that controls the initial activation and recruitment of Rabs to WPBs is currently unclear.
Cycling of Rabs between GDP- and GTP-bound states is regulated by Rab-specific guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). The differentially expressed in normal and neoplastic cells (DENN) domain protein family comprises 18 members that each exhibit GEF activity toward specific Rabs.24-26 MAP-kinase activating death domain (MADD), alternatively referred to as DENN or Rab3 guanine exchange protein (Rab3GEP), has been previously identified as a GEF for Rab3 isoforms in neuronal and (neuro)endocrine cells and for Rab27A in melanocytes and parotid acinar cells.27-32 Besides the N-terminal DENN domain, MADD contains a C-terminal death domain (DD) that functions in MAP kinase signaling.33 The physiological significance of MADD is underlined by a recent study from the Undiagnosed Disease Network, which reported that biallelic mutations in MADD are causative for an extremely rare multisystem disorder, delineated by neurologic, endocrine, and exocrine dysfunction, which in some patients also manifests as hematologic abnormalities.34 This positions MADD as a crucial regulator within the neuroendocrine system that may also be involved in Rab27A-controlled release of lysosome-related organelles involved in hemostasis and immunity.6
In this study, we investigated the role of MADD in the regulation of WPB Rabs and VWF secretion. We found that MADD functions as a GEF for Rab27A and Rab3 isoforms in ECs. Furthermore, MADD is required for the recruitment of Rab27A and Rab3D to WPBs. Finally, we show that, as a master regulator of WPB Rabs, MADD controls VWF secretion.
Methods
Antibodies and DNA constructs
Antibodies used for immunoblotting and immunofluorescent stainings are listed in supplemental Table 1. Glutathione S-transferase (GST)-Slac2-b-SHD35 and GST-RIM2-RBD31 expression vectors for Rab activity assays were kind gifts from M. Fukuda, PhD, and R. Regazzi, PhD, respectively. EGFP-tagged human MADD constructs (full length, ΔDENN, ΔDD, TOMM70-EGFP, and TOMM70-EGFP-MADD) have been described previously.27 Lentiviral TOMM70-tagged fusions were made in the LVX-mEGFP-LIC vector22 by swapping mEGFP for TOMM70-EGFP, TOMM70-EGFP-MADD or TOMM70-EGFP-MADD- ΔDENN. The pLKO.1-short hairpin RNA (shRNA) constructs from the TRC MISSION library (supplemental Table 2) were supplied by R. Beijersbergen, MD, and W. Van IJcken, MD. shRNAs targeting MADD and Rabs were cloned into a pLKO.1-puro-CMV-mEGFP-U6-shRNA vector.36 All constructs were verified by DNA sequence analysis.
Cell culture, transfection, and transduction
Pooled, cryopreserved human umbilical cord endothelial cells (HUVECs; Promocell) were cultured on gelatin-coated surfaces in endothelial cell growth medium (EGM-2; Promocell) supplemented with endothelial growth factor mix (Promocell), 18% fetal calf serum (Bodinco), 100 U/mL penicillin, and 100 µg/mL streptomycin (Gibco). Gelatin from porcine skin (Sigma) was dissolved at a final concentration of 1% in phosphate-buffered saline (Fresenius Kabi). HUVECs were kept at 37°C in 5% CO2 and were used in experiments up to passages 5 to 6. Human embryonic kidney 293T (HEK293T) cells were cultured in Dulbecco’s modified eagle medium (DMEM) containing D-glucose and L-glutamine supplemented with 10% fetal calf serum and penicillin-streptomycin. Lentivirus was produced in HEK293T cells using the third-generation lentiviral packaging plasmids pMD2.G, pRSV-REV, and pMDLg/pRRE (Addgene) using TransIT-LT-1 (Mirus Bio), as described previously.37 HUVECs were transduced with 2 batches of fresh virus and selected for 3 days using 0.5 µg/mL puromycin. All other constructs were transfected in HEK293T cells using TransIT-LT-1 or in HUVECs using the Neon transfection system (Invitrogen) delivering 1 pulse of 1300 V and a pulse width of 30 ms. A pool of small interfering RNA (siRNA) oligo duplexes targeting human MADD (ON-TARGET plus SMARTpool, #L004429, Dharmacon) and nontargeting siRNA control (ON-TARGET plus nontargeting pool control, #D001810) were transfected in HUVECs by Nucleofection (Lonza), as described previously.11 Oligo sequences are provided in supplemental Table 3. Additional methods can be found in the supplemental Material.
Results
MADD activates secretory Rabs in a DENN-dependent manner
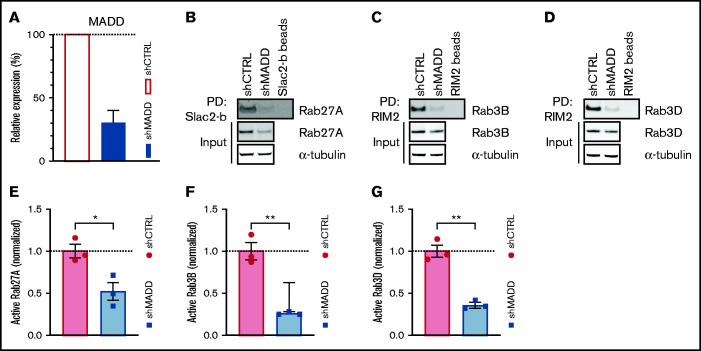
Because MADD has been shown to exhibit GEF activity toward Rab3 isoforms and Rab27A in different cell types,27-32,38 we hypothesized that MADD functions as a secretory RabGEF in ECs. To investigate this, we silenced MADD in HUVECs by using stable expression of shRNAs targeting MADD (shMADD) or a non-targeting control (shCTRL) (Figure 1A; supplemental Figure 1A). We then performed pulldown assays in shCTRL- and shMADD-transduced HUVECs to capture the active GTP-bound fractions of endogenous Rab27A, Rab3B, and Rab3D. GST-tagged Rab effector domains Slp-homolog lacking C2 domains b-Slp homology domain (GST-Slac2-b-SHD) and Rab3-interacting molecule 2-Rab binding domain (GST-RIM2-RBD), which specifically bind to GTP-Rab27A and GTP-Rab3 isoforms, respectively, were used as bait. Upon MADD knockdown, both the amount and the proportion of active GTP-bound Rab27A, Rab3B, and Rab3D were substantially reduced, indicating that MADD is indeed required for their activation in ECs (Figure 1B-G; supplemental Figure 2A-C). Interestingly, Rab27A and Rab3D total protein levels were also significantly decreased upon MADD knockdown (supplemental Figure 2D-F), which corresponds to previous observations that MADD knockout melanocytes have lower Rab27A expression levels.27 This may be attributed to an increased degradation of inactive Rabs,39 decreased messenger RNA (mRNA) expression in case of Rab27A (supplemental Figure 3A-C), or both.
Figure 1.
MADD is a GEF for secretory Rabs. (A) MADD expression was analyzed by quantitative polymerase chain reaction 7 days after transduction of HUVECs with shCTRL or shMADD. Data are from 3 biological replicate experiments. (B) Pulldown (PD) of active, GTP-bound Rab27A using GST-Slac2-b-SHD (Slac2-b)-coupled beads in shCTRL- and shMADD-transduced HUVEC lysates. GTP-bound Rab3B (C) and Rab3D (D) were extracted using GST-RIM2-RBD (RIM2)-coupled beads. PD experiments were performed in biological triplicate, and representative Western blots are shown. (E-G) Bar graphs represent normalized active fractions of Rab27A (E), Rab3B (F), and Rab3D (G) relative to total input levels. Data are shown as mean ± standard error of the mean [SEM] and analyzed using unpaired two-tailed Student t test. Dotted lines indicate activity levels in shCTRL. *P < .05; **P < .01.
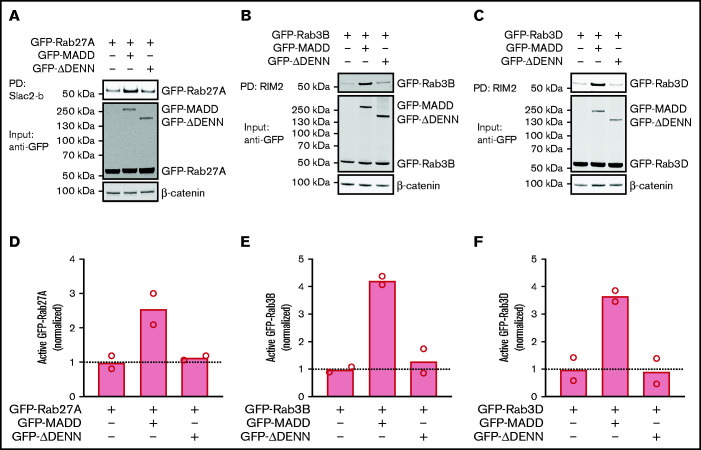
MADD contains DD and DENN domains, which are important for its GEF function.27,31 It is assumed that a Rab binding site is located within the DENN domain, because this is also the case for other DENN domain proteins.24 To determine whether MADD activates all 3 Rabs using its DENN domain, we compared the effect of full-length MADD and a mutant lacking the DENN domain (ΔDENN) on Rab activity in HEK293T cells. Full-length MADD markedly increased the active green fluorescent protein (GFP)-Rab27A, GFP-Rab3B, and GFP-Rab3D fractions, whereas the ΔDENN mutant did not (Figure 2A-F). However, Rab activation was not completely abolished by ΔDENN, but a reduction to the basal activation level was observed, indicating that the truncation mutant is not dominant negative. Notably, we found that full-length MADD as well as ΔDENN co-precipitated with GTP-bound Rabs (supplemental Figure 4A-C), implying that the DENN domain is not crucial for the Rab-GEF interaction. Thus, MADD has the capacity to activate Rab27A, Rab3B, and Rab3D, and its exchange activity depends on the DENN moiety.
Figure 2.
MADD activates secretory Rabs in a DENN domain–dependent manner. HEK293T cells were transfected with GFP-Rab27A (A), GFP-Rab3B (B), or GFP-Rab3D (C) alone or co-transfected with GFP-MADD-full-length or GFP-MADD-ΔDENN. GTP-bound Rabs were extracted from lysates using Slac2-b or RIM2 PD as indicated. GFP-tagged proteins were visualized using an anti-GFP antibody. Experiments were performed in technical duplicates, and representative Western blots are shown. (D-F) Bar graphs represent mean normalized active fractions of Rab27A (D), Rab3B (E), and Rab3D (F) relative to input levels. Dotted lines indicate activity levels in cells transfected with GFP-Rabs without GFP-MADD variants.
MADD drives recruitment of secretory Rabs to WPBs
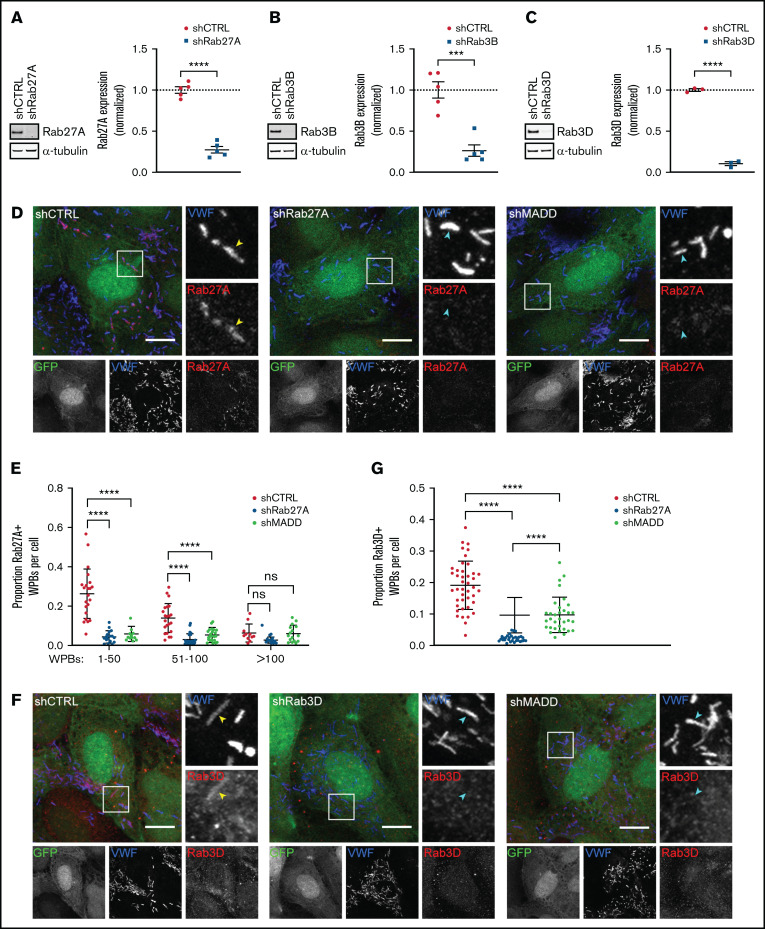
To determine whether MADD is involved in targeting Rab27A and Rab3B and Rab3D to WPBs, we evaluated their localization after MADD depletion in HUVECs. For comparison, we also silenced expression of these Rabs directly with specific shRNAs targeting Rab27A, Rab3B, or Rab3D (Figure 3A-C; supplemental Figure 1B-D). As expected, WPB-localized Rab27A was significantly reduced in shRab27A-transduced ECs when compared with shCTRL (Figure 3D-E). Strikingly, in MADD-depleted cells, Rab27A staining no longer colocalized with VWF, indicating that MADD is involved in Rab27A targeting to WPBs. It has also previously been documented that Rab27A immunoreactivity on WPBs seemed less intense in cells with a high number of WPBs, which is explained by a limited pool of Rab27A that distributes over an increasing number of WPBs, thereby diluting the Rab27A signal.11 Indeed, quantification revealed that higher WPB numbers clearly decreased the proportion of Rab27A+ WPBs in shCTRL cells, a pattern that was not observed after Rab27A or MADD depletion (Figure 3E).
Figure 3.
Rab27A, Rab3B, and Rab3D recruitment to WPBs is decreased upon MADD silencing. (A-C) HUVECs were transduced with pLKO-shRNAs targeting Rab27A (A), Rab3B (B), and Rab3D (C) or nontargeting control (shCTRL); representative western blots and quantifications of 3 to 5 biological replicates are shown confirming knockdown after 7 days. Data are shown as mean ± SEM and were analyzed by using an unpaired Student t test. Dotted lines indicate activity levels in shCTRL. ***P < .001; ****P < .0001. (D,F) HUVECs were transduced with a pLKO-GFP-shRNA co-expression construct containing shCTRL, shRab27A, shRab3D, or shMADD (all shown in green) and immunostained for VWF (blue) and Rab27A or Rab3D (red) as indicated. Individual channels are shown in grayscale below. Boxed areas are magnified on the right. Yellow arrowheads indicate Rab+ and cyan arrowheads indicate Rab– WPBs. Scale bars represent 10 µm. (E,G). The Rab+ proportion of WPBs per cell was quantified. (E) The proportion of Rab27A+ WPBs was divided over 3 bins based on the amount of WPBs in shCTRL (n = 56) and was compared with shRab27A (n = 55) and shMADD (n = 54). Data are shown as mean ± standard deviation (SD) and were analyzed by using two-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. ****P < .0001. (G) The proportion of Rab3D+ WPBs in shCTRL (n = 42) is compared with shRab3D (n = 31) and shMADD (n = 33) without binning. Data are shown as mean ± SD and were analyzed by using one-way ANOVA with Tukey’s multiple comparisons test. ****P < .0001. ns, not significant.
Because MADD also exhibits GEF activity on Rab3B and Rab3D, we tested whether their targeting to WPBs was also dependent on MADD. Rab3D staining was visible on WPBs in shCTRL cells, but not in shRab3D cells, and was significantly decreased upon MADD knockdown (Figure 3F-G). Silencing MADD using siRNAs (siMADD) and comparing with a nontargeting siRNA control (siCTRL) also showed a clear depletion of Rab27A, Rab3B, and Rab27A-effector MyRIP from WPBs (supplemental Figure 5A-C), complementary to our observations in shRNA-transduced ECs. Moreover, phase partitioning showed that upon MADD knockdown, Rab27A, Rab3B, and Rab3D levels were decreased in the detergent phase (ie, membrane-bound), although not significantly in the case of Rab27A (supplemental Figure 6A-B), suggesting that Rab membrane association was reduced. Taken together, we conclude that impaired targeting of Rabs to WPBs upon MADD silencing consists of a reduction of (active) Rab levels (Figure 1; supplemental Figure 2) and decreased Rab recruitment, pointing toward a role for MADD in directing secretory Rabs to WPBs.
MADD has been observed in the cytosol of different cell types, whereas its target Rab27A localized to secretory granules.27,28 Because there is a lack of an antibody that detects endogenous MADD, we determined the intracellular localization in HUVECs by ectopic expression of EGFP-MADD or EGFP-MADD-ΔDENN. Both constructs exhibited primarily cytosolic distribution and were not enriched on WPBs, suggesting that another mechanism is responsible for recruiting secretory Rabs to WPBs (supplemental Figure 7A-B). Although we cannot rule out that during WPB Rab activation, the presence of MADD at the WPB membrane is so transient that it precludes detection, our results suggest that MADD does not activate Rabs exclusively in proximity to WPBs.
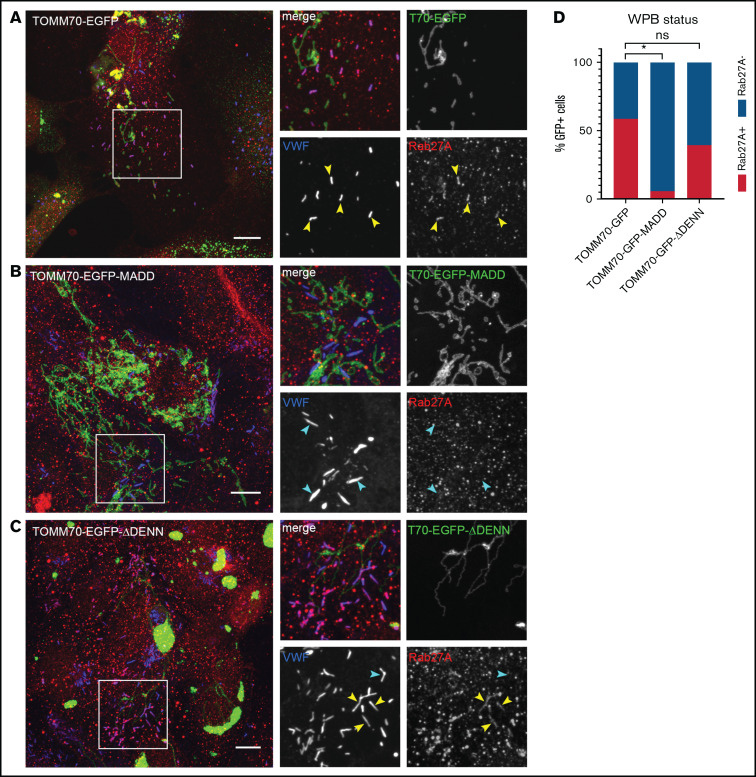
To further substantiate its role in membrane targeting of Rabs, we used EGFP-MADD fused with a TOMM70 tag, which directs MADD to mitochondria by virtue of a mitochondrial targeting motif,27 and we tested the contribution of MADD localization to correct targeting of one of its substrates, Rab27A. The TOMM70-EGFP control localized primarily to (aggregated) mitochondria (Figure 4A), as was seen previously,27 whereas endogenous Rab27A was localized to WPBs as shown by scoring cells exhibiting Rab27A co-staining with VWF (Figure 4A,D). However, in cells expressing TOMM70-EGFP-MADD, WPBs were no longer positive for Rab27A (Figure 4B,D). Intriguingly, TOMM70-EGFP-MADD-ΔDENN did not affect Rab27A localization, suggesting that endogenous MADD function remains unrestricted by this truncation mutant and that the DENN moiety is required for the dislocation of Rab27A (Figure 4C-D). These data reveal that aberrant targeting of MADD to mitochondria prevents DENN domain–dependent recruitment of Rab27A to WPBs, indicating that correct localization of MADD is essential.
Figure 4.
Targeting MADD to mitochondria displaces Rab27A from WPBs. HUVECs were transduced with TOMM70-EGFP (A) or TOMM70-EGFP-MADD (B) or TOMM70-EGFP-MADD-ΔDENN (C) (all shown in green) and immunostained for VWF (blue), Rab27A (red), and VE cadherin (not shown). Boxed areas are magnified on the right. Yellow arrowheads indicate Rab27A+and cyan arrowheads indicate Rab27A– WPBs. Scale bars represent 10 µm. (D) Quantification of the percentage of transduced (GFP+) HUVECs containing Rab27A+ WPBs in TOMM70-EGFP (175 cells analyzed), TOMM70-EGFP-MADD (96 cells analyzed), and TOMM70-EGFP-MADD-ΔDENN (38 cells analyzed). Data are shown as mean and were analyzed by using one-way ANOVA with Dunnet’s multiple comparisons test. *P < .05.
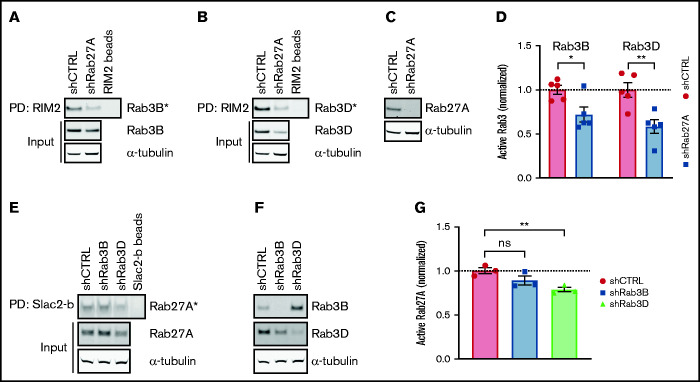
In ECs, Rab27A is present on mature WPBs, but Rab3B had already been detected at an earlier stage.11 This raises the question of whether sequential or cooperative activation of Rab3 and Rab27A underpin their recruitment during WPB maturation. To explore the possibility that secretory Rabs facilitate each other’s activation, we determined Rab3B and Rab3D activation after Rab27A silencing (Figure 5A-C). Interestingly, levels of GTP-Rab3B and GTP-Rab3D were both significantly decreased upon Rab27A knockdown, implying that Rab27A augments activation of Rab3 isoforms (Figure 5D; supplemental Figure 8A-B). In the reverse setup, Rab27A activity remained unchanged upon Rab3B silencing, whereas Rab3D depletion resulted in slightly lower GTP-Rab27A levels, suggesting that Rab3D might also enhance Rab27A GDP/GTP exchange (Figure 5E-G; supplemental Figure 8C-D). Analysis of Rab localization in immunofluorescent images showed no difference in Rab3D presence on WPBs upon Rab27A knockdown; in addition, Rab27A localization remained unchanged in Rab3B- and Rab3D-depleted cells, suggesting that normal recruitment of active Rabs still occurs (supplemental Figure 9A-D). Taken together, the levels of active Rab3B and Rab3D seem to be dependent on the presence of (active) Rab27A, which suggests that activation of Rab27A precedes or promotes Rab3B and Rab3D activation.
Figure 5.
Rab27A augments Rab3B and Rab3D activation. GTP-bound Rab3B (A) and Rab3D (B) fractions (Rab3B* and Rab3D*) were extracted by RIM2 PD in shCTRL- and shRab27A-transduced HUVEC lysates. (C) Western blot analysis of Rab27A expression in shCTRL and shRab27A. (D) Normalized Rab3B and Rab3D activity levels are plotted relative to total input levels. Dotted line indicates activity levels in shCTRL. (E) GTP-bound Rab27A fractions (Rab27A*) were extracted by Slac2-b PD in shCTRL-, shRab3B-, and shRab3D-transduced HUVEC lysates. (F) Western blot analysis of Rab3B and Rab3D expression in shCTRL, shRab3B, and shRab3D. (G) Normalized Rab27A activity levels are plotted relative to total input levels. Representative western blots are shown for 3 Slac2-b PD and 5 RIM2 PD biological replicates. Dotted line indicates activity levels in shCTRL. Statistical analysis used an unpaired Student t test comparing each condition to shCTRL. Data are shown as mean ± SEM. *P < .05; **P < .01.
MADD is crucial for histamine-evoked WPB exocytosis
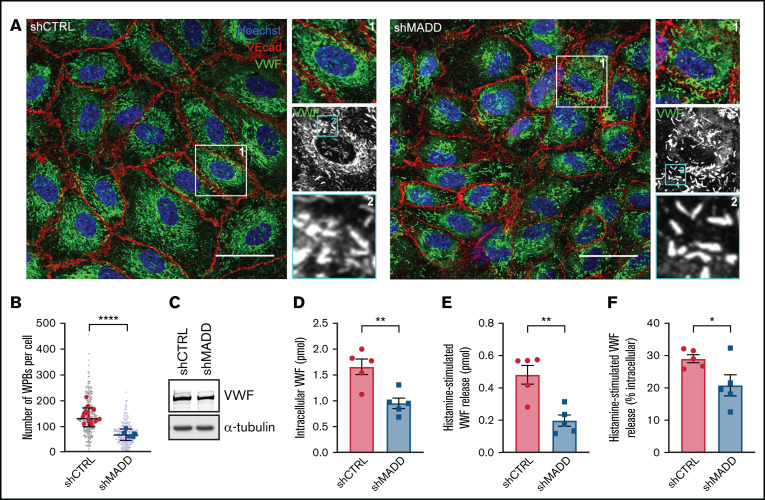
Rab3 B, Rab3D, and Rab27A have been shown to coordinate secretory organelle maturation, transport, and exocytosis through interactions with a variety of effectors.40 Because MADD is necessary for their activation and recruitment to WPBs (Figures 1-4), we investigated the role of MADD in the endothelial secretory pathway. MADD or individual Rab depletion did not have an effect on WPB morphology because the classical cigar-shaped organelles were observed, suggesting that individually they are dispensable for normal WPB formation (Figure 6A; supplemental Figure 10). Nonetheless, we noted a decrease in the number of WPBs per cell upon MADD knockdown, pointing toward a defect in VWF storage (Figure 6B), although differences in total VWF levels between shCTRL and shMADD were not as clear on western blot (Figure 6C).
Figure 6.
MADD promotes VWF secretion in endothelial cells. (A) HUVECs were transduced with shCTRL or shMADD and immunostained for VWF (green), vascular endothelial cadherin (VEcad) (red) and nuclei (blue). Boxed areas are magnified on the right (1) and below (2). Scale bars represent 10 µm. (B) The number of WPBs was quantified per cell (small dots) and averaged per image (large dots) in 15 shCTRL and 6 shMADD images. (C) Western blot analysis for VWF expression in shCTRL- and shMADD-transduced HUVECs. Data from enzyme-linked immunosorbent assay (ELISA) show (D) intracellular VWF content in unstimulated cells, (E) 30-minute histamine-stimulated VWF secretion in absolute amounts (expressed in pmol), and (F) as a percentage of intracellular content of resting or nonstimulated cells (data are from 5 biological replicates). (D-F) Data are shown as mean ± SEM and were analyzed by using an unpaired Student t test. *P < .05; **P < .01; ****P < .0001.
To determine whether MADD is involved in WPB maturation, we examined the maturation status of WPB by electron microscopy. In both shCTRL and shMADD ECs, classical cigar-shaped organelles could be distinguished throughout the cytoplasm, including immature WPBs in close proximity to the Golgi apparatus and WPBs in different stages of maturation as judged by the condensation of VWF bundles (supplemental Figure 11A-B), with no difference in their proportions between control and MADD-depleted cells (supplemental Figure 11C). Collectively, these results indicate that MADD does not play an indispensable role in biogenesis and maturation of WPBs.
Because secretory Rabs and their effectors facilitate WPB exocytosis,11,12 we performed secretion assays to test whether MADD, as an upstream regulator of Rab function, is involved in VWF secretion. MADD silencing significantly reduced intracellular VWF content in unstimulated cells, as well as histamine-stimulated VWF secretion, both in absolute levels and as a percentage of the (unstimulated) intracellular content (Figure 6D-F). These data indicate that in addition to facilitating regulated WPB exocytosis, MADD could play an additional role in VWF storage or turnover of WPBs. Similar to previous reports that used siRNA-based knockdown,11,12 Rab27A knockdown using shRNAs resulted in a significant reduction of the absolute histamine-evoked VWF secretion, and a reduction, although not significant, in the secreted VWF as a percentage of intracellular content (supplemental Figure 12A-D). Rab3B and Rab3D had no significant effect on absolute levels of intracellular VWF or on histamine-stimulated VWF secretion; however, the percentage of VWF secreted by shRab3B cells was significantly increased, opposite to MADD knockdown. The latter result may be explained by a secondary effect of long-term Rab3B knockdown, which seems to upregulate Rab27A expression (supplemental Figure 12D), perhaps to compensate for the depletion of Rab3B. In summary, on the basis of our data and previous reports,11,12 we speculate that the effect of MADD on regulated WPB exocytosis is mainly attributed to its crucial role in Rab27A activation and recruitment.
Discussion
Thus far, it is unknown how secretory Rabs in ECs are activated and targeted to WPBs. In this study, we identified MADD as the GEF for 3 WPB-localized Rabs: Rab27A, Rab3B, and Rab3D. We have shown that MADD is responsible for the activation and targeting of these Rabs to WPBs, thereby promoting VWF secretion. Our findings are in line with reports showing that MADD exhibits GDP and GTP exchange activity on Rab27A27,28,32 and on all 4 Rab3 isoforms in other cell types.30,31 MADD controls Rab27A activation and recruitment to melanosomes in melanocytes and amylase-containing granules in parotid acinar cells.27,28,32 Through regulation of Rab3, MADD mediates neurotransmitter and hormone release from (neuro)endocrine cells.41-43 MADD knockout mice exhibited impaired synaptic vesicle transport and reduced neurotransmitter and insulin release.42,44,45 Overall, MADD functions in the transport and/or exocytosis of (secretory) organelles through regulation of secretory Rab function, which is consistent with our data in ECs.
Although GEFs typically localize to intracellular compartment(s) where Rabs are active,26,46 we observed that EGFP-MADD was not enriched on WPBs but instead showed localization throughout the cytosol. Microscopy data from melanocytes27 and subcellular fractionation experiments in parotid acinar cells also showed the vast majority of MADD in the cytosolic fraction.28 Although the presence of MADD on WPBs does not seem to be required for correct localization of secretory Rabs, artificial sequestering of MADD to the mitochondrial membrane had a severe impact on Rab27A localization, hinting that mobility of MADD in the cytosol may be crucial. Thus, despite the predominantly cytosolic localization of EGFP-MADD, the possibility remains that transient association of MADD with WPBs is needed for delivery of the secretory Rabs to these organelles.
Apart from MADD, Rab-GAPs may also control the localization of these secretory Rabs on WPBs.14,47 A RabGAP screen performed in ECs found that TBC1D10A inhibits VWF secretion via inactivating and presumably removing Rab35 from WPBs, indicating that RabGAPs can also influence Rab localization.48 Recently, the RabGAP TBC1D22A was detected by proximity-labeling in a proteomics study investigating the Rab27A-Rab3B interactomes in ECs, suggesting that this RabGAP could be a candidate for inactivating these Rabs, thereby destabilizing association with the WPB membrane.19
A number of reports have identified MADD as a specific GEF for Rab3 and Rab27A by screening the GDP release or GTP loading of different purified Rab proteins25,30,32 or via isolating the activated Rab fraction from cell lysates using effector pulldown assays,27,28,31,32 which is similar to our approach. However, many of these studies focused solely on its role in activation of either Rab330,31,43,45 or Rab27A,27,28,32,49 with the exception of 1 study in Caenorhabditis elegans neurons,50 showing that MADD homolog AEX-3 activates RAB-3 and RAB-27, which in turn both regulate synaptic transmission. Because multiple secretory Rabs are concurrently expressed in ECs, our system offers the opportunity to study the intricate balance between them in mammalian cells, with Rab isoforms adding an additional layer of complexity.
Previous evidence for a sequential Rab27A-Rab3A activation cascade was found in sperm cells, in which Rab27A and effector Rabphilin3-a, both present on dense core granules, recruit the GEF for Rab3A (GRAB) that in turn recruits and activates Rab3A.38,51 We showed that in ECs, Rab27A, Rab3B, and Rab3D activity levels are interrelated judging from attenuation of Rab3B and Rab3D activation when Rab27A is silenced, which suggests that a positive feedback mechanism is normally present. Furthermore, in vitro GDP dissociation assays showed that MADD by itself cannot fully activate Rab3D and is completely unable to activate Rab3B,30 whereas Rab activation assays in the cellular context presented here and also by others31 revealed that activation of Rab3B and Rab3D was unequivocally dependent on MADD. This points to the requirement for additional cellular factors for allosteric regulation of MADD activity, for instance, by activated Rab27A. However, further studies are needed to elucidate the complete mechanism.
We have shown in ECs that MADD promotes histamine-stimulated VWF secretion to an extent similar to that of Rab27A in absolute levels, suggesting that MADD acts as an upstream regulator of WPB exocytosis. Other Rabs have also been shown to influence WPB exocytosis, including Rab15, Rab35, and Rab46,12,48,52 which could be mobilized by other GEFs such as DENND1 for Rab3525 functioning alongside MADD in regulation of Rabs on WPBs. There is no consensus in the literature regarding the role of Rab3 isoforms in stimulated VWF release.11-13 And our data did not give a conclusive answer to the question of whether Rab3B and Rab3D independently play a role in stimulated VWF secretion because long-term Rab knockdown seems to influence expression of other (redundant) Rabs, which in turn may (partially) compensate for the depletion. The question remains of whether Rab3B and Rab3D are redundant in ECs, like in neurons,53 because they are highly similar with >80% amino acid sequence identity and they recruit the same effector Slp4-a.11
Low levels of circulating VWF, such as in von Willebrand disease (VWD; VWF <30 IU/dL) or in case of low VWF (30-50 IU/dL), give rise to an increased risk of bleeding.54,55 VWF deficiency can be caused by mutations in VWF that affect its biosynthesis and/or clearance. Yet, in ∼25% of VWD type 1 patients and in ∼60% of low VWF patients, no causative mutation in VWF can be detected, and the pathogenic mechanisms that underpin their reduced VWF levels often remain unresolved. In the plasma of the normal population, VWF levels are distributed over a wide range and have been shown to be largely genetically determined.56,57 The heritability of VWF plasma levels consists predominantly of quantitative trait loci outside the VWF gene58; thus, it is to be expected that some will overlap with non-VWF loci responsible for unexplained quantitative VWF deficiencies in VWD1 and in patients with low VWF. Genome-wide association studies of modifiers of VWF levels have identified links with genetic variants in SNARE proteins,59-61 indicating that alterations in secretory processes are responsible for some of the variability in levels of VWF in plasma. However, a significant portion of the heritable component of VWF levels is still unaccounted for,58 and we speculate that additional (pathogenic) mechanisms exist that impair the capacity of endothelial cells to secrete VWF. It is possible that genetic variants in (regulators of) MADD exist that may affect its efficacy in recruiting Rab GTPases and their downstream effectors (including SNAREs) to the exocytic machinery of WPBs, thereby reducing basal and/or vascular injury–induced release of VWF by endothelial cells.
In a recent report, Schneeberger et al34 described a cohort of pediatric patients with biallelic mutations in MADD who suffered from a severe pleiotropic disorder with a spectrum of developmental, neurologic, and hematologic anomalies and poor life expectancy. Cellular studies with patient fibroblasts indicate that a subgroup of these patients show a phenotype consistent with disturbed vesicular trafficking. Some of these patients have mutations in the DENN domain, which we and others have shown to be essential for Rab27A activation and recruitment to WPBs. Interestingly, several patients with biallelic MADD mutations present with clinical symptoms and laboratory phenotypes that are consistent with an increased risk of bleeding, such as frequent nosebleeds and prolonged Ivy bleeding time and abnormal platelet degranulation (unpublished observations, K. Freson, PhD). In platelets, formation and degranulation of alpha and dense granules involves Rab27A and Rab27B,62-64 which are also substrates for MADD exchange activity.25 Whether degranulation of WPBs is also affected in these patients is currently unknown, but in future studies, we will focus on establishing whether an endothelial (secretory) component also exists in the pathology of this multisystem disorder.
In conclusion, the discovery of MADD as a WPB-Rab GEF adds a new component to the VWF-WPB secretory machinery in secretory organelle exocytosis. MADD depletion results in decreased levels of activated secretory Rabs on the WPB membrane, ultimately leading to reduced VWF secretion. Therefore, we propose a model in which MADD coordinates activation and recruitment of Rab27A, Rab3B, and Rab3D to WPBs as an upstream master regulator in the endothelial VWF secretory pathway.
Supplementary Material
Acknowledgments
The authors thank R. Beijersbergen, PhD and B. Morris (The Netherlands Cancer Institute, Amsterdam, The Netherlands), W. van IJcken, PhD (Erasmus MC, Rotterdam, The Netherlands), M. Fukuda, PhD (Tohoku University, Miyagi, Japan), and R. Regazzi, PhD (University of Lausanne, Lausanne, Switzerland) for plasmids and gratefully acknowledge K. Freson, PhD (Katholieke Universiteit Leuven, Leuven, Belgium) for sharing unpublished clinical data on patients with MADD mutations.
This work was supported by grants from the Landsteiner Stichting voor Bloedtransfusie Research (LSBR-1707) and the Dutch Thrombosis Foundation (TSN 2017-01).
Authorship
Contribution: M.K., P.E.B., H.J., I.M.D.C., and I.L.C. performed research and analyzed data; A.N.H. and T.C. contributed vital reagents and expertise; and M.K., J.V., C.M., and R.B. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruben Bierings, Department of Hematology, Erasmus University Medical Center, Rotterdam, Postbus 2040, Rotterdam 3000CB, The Netherlands; e-mail: r.bierings@erasmusmc.nl.
References
- 1.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67(1):395-424. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA. von Willebrand factor, Jedi knight of the bloodstream. Blood. 2014;124(9):1412-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giblin JP, Hewlett LJ, Hannah MJ. Basal secretion of von Willebrand factor from human endothelial cells. Blood. 2008;112(4):957-964. [DOI] [PubMed] [Google Scholar]
- 4.Lopes da Silva M, Cutler DF. von Willebrand factor multimerization and the polarity of secretory pathways in endothelial cells. Blood. 2016;128(2):277-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schillemans M, Karampini E, Kat M, Bierings R. Exocytosis of Weibel-Palade bodies: how to unpack a vascular emergency kit. J Thromb Haemost. 2019;17(1):6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karampini E, Bierings R, Voorberg J. Orchestration of primary hemostasis by platelet and endothelial lysosome-related organelles. Arterioscler Thromb Vasc Biol. 2020;40(6):1441-1453. [DOI] [PubMed] [Google Scholar]
- 7.Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci. 2015;128(17):3171-3176. [DOI] [PubMed] [Google Scholar]
- 8.Nightingale T, Cutler D. The secretion of von Willebrand factor from endothelial cells; an increasingly complicated story. J Thromb Haemost. 2013;11(suppl 1):192-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannah MJ, Hume AN, Arribas M, et al. Weibel-Palade bodies recruit Rab27 by a content-driven, maturation-dependent mechanism that is independent of cell type. J Cell Sci. 2003;116(pt 19):3939-3948. [DOI] [PubMed] [Google Scholar]
- 10.Nightingale TD, Pattni K, Hume AN, Seabra MC, Cutler DF. Rab27a and MyRIP regulate the amount and multimeric state of VWF released from endothelial cells. Blood. 2009;113(20):5010-5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bierings R, Hellen N, Kiskin N, et al. The interplay between the Rab27A effectors Slp4-a and MyRIP controls hormone-evoked Weibel-Palade body exocytosis. Blood. 2012;120(13):2757-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zografou S, Basagiannis D, Papafotika A, et al. A complete Rab screening reveals novel insights in Weibel-Palade body exocytosis. J Cell Sci. 2012;125(pt 20):4780-4790. [DOI] [PubMed] [Google Scholar]
- 13.Knop M, Aareskjold E, Bode G, Gerke V. Rab3D and annexin A2 play a role in regulated secretion of vWF, but not tPA, from endothelial cells. EMBO J. 2004;23(15):2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller MP, Goody RS. Molecular control of Rab activity by GEFs, GAPs and GDI. Small GTPases. 2018;9(1-2):5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeffer SR, Dirac-Svejstrup AB, Soldati T. Rab GDP dissociation inhibitor: putting rab GTPases in the right place. J Biol Chem. 1995;270(29):17057-17059. [DOI] [PubMed] [Google Scholar]
- 16.Oesterlin LK, Pylypenko O, Goud B.. Effectors of rab GTPases: rab binding specificity and their role in coordination of rab function and localization. In: Wittinghofer A, ed. Ras Superfamily Small G Proteins: Biology and Mechanisms 2. Cham, Switzerland: Springer; 2014:39-66. [Google Scholar]
- 17.Conte IL, Hellen N, Bierings R, et al. Interaction between MyRIP and the actin cytoskeleton regulates Weibel-Palade body trafficking and exocytosis. J Cell Sci. 2016;129(3):592-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojo Pulido I, Nightingale TD, Darchen F, Seabra MC, Cutler DF, Gerke V. Myosin Va acts in concert with Rab27a and MyRIP to regulate acute von-Willebrand factor release from endothelial cells. Traffic. 2011;12(10):1371-1382. [DOI] [PubMed] [Google Scholar]
- 19.Holthenrich A, Drexler HCA, Chehab T, Naß J, Gerke V. Proximity proteomics of endothelial Weibel-Palade bodies identifies novel regulator of von Willebrand factor secretion. Blood. 2019;134(12):979-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chehab T, Santos NC, Holthenrich A, et al. A novel Munc13-4/S100A10/annexin A2 complex promotes Weibel-Palade body exocytosis in endothelial cells. Mol Biol Cell. 2017;28(12):1688-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Criado Santos N, Chehab T, Holthenrich A, Gerke V. Analysis of Ca2+-dependent Weibel-Palade body tethering by live cell TIRF Microscopy: Involvement of a Munc13-4/S100A10/Annexin A2 complex. Methods Mol Biol. 2019;1929:437-445. [DOI] [PubMed] [Google Scholar]
- 22.van Breevoort D, Snijders AP, Hellen N, et al. STXBP1 promotes Weibel-Palade body exocytosis through its interaction with the Rab27A effector Slp4-a. Blood. 2014;123(20):3185-3194. [DOI] [PubMed] [Google Scholar]
- 23.Schillemans M, Karampini E, van den Eshof BL, et al. Weibel-Palade body localized syntaxin-3 modulates von Willebrand factor secretion from endothelial cells. Arterioscler Thromb Vasc Biol. 2018;38(7):1549-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marat AL, Dokainish H, McPherson PS. DENN domain proteins: regulators of Rab GTPases. J Biol Chem. 2011;286(16):13791-13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimura S, Gerondopoulos A, Linford A, Rigden DJ, Barr FA. Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J Cell Biol. 2010;191(2):367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishida M, Oguchi ME, Fukuda M. Multiple types of guanine nucleotide exchange factors (GEFs) for Rab small GTPases. Cell Struct Funct. 2016;41(2):61-79. [DOI] [PubMed] [Google Scholar]
- 27.Sanzà P, Evans RD, Briggs DA, et al. Nucleotide exchange factor Rab3GEP requires DENN and non-DENN elements for activation and targeting of Rab27a. J Cell Sci. 2019;132(9):jcs212035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai A, Ishida M, Fukuda M, Nashida T, Shimomura H. MADD/DENN/Rab3GEP functions as a guanine nucleotide exchange factor for Rab27 during granule exocytosis of rat parotid acinar cells. Arch Biochem Biophys. 2013;536(1):31-37. [DOI] [PubMed] [Google Scholar]
- 29.Niwa S, Tanaka Y, Hirokawa N. KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat Cell Biol. 2008;10(11):1269-1279. [DOI] [PubMed] [Google Scholar]
- 30.Wada M, Nakanishi H, Satoh A, et al. Isolation and characterization of a GDP/GTP exchange protein specific for the Rab3 subfamily small G proteins. J Biol Chem. 1997;272(7):3875-3878. [DOI] [PubMed] [Google Scholar]
- 31.Coppola T, Perret-Menoud V, Gattesco S, et al. The death domain of Rab3 guanine nucleotide exchange protein in GDP/GTP exchange activity in living cells. Biochem J. 2002;362(pt 2):273-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figueiredo AC, Wasmeier C, Tarafder AK, Ramalho JS, Baron RA, Seabra MC. Rab3GEP is the non-redundant guanine nucleotide exchange factor for Rab27a in melanocytes. J Biol Chem. 2008;283(34):23209-23216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schievella AR, Chen JH, Graham JR, Lin L-L. MADD, a novel death domain protein that interacts with the type 1 tumor necrosis factor receptor and activates mitogen-activated protein kinase. J Biol Chem. 1997;272(18):12069-12075. [DOI] [PubMed] [Google Scholar]
- 34.Schneeberger PE, Kortüm F, Korenke GC, et al. ; Undiagnosed Diseases Network . Biallelic MADD variants cause a phenotypic spectrum ranging from developmental delay to a multisystem disorder. Brain. 2020;143(8):2437-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda TS, Fukuda M, Ariga H, Mikoshiba K. The Slp homology domain of synaptotagmin-like proteins 1-4 and Slac2 functions as a novel Rab27A binding domain. J Biol Chem. 2002;277(11):9212-9218. [DOI] [PubMed] [Google Scholar]
- 36.Lenzi C, Stevens J, Osborn D, Hannah MJ, Bierings R, Carter T. Synaptotagmin 5 regulates Ca2+-dependent Weibel-Palade body exocytosis in human endothelial cells. J Cell Sci. 2019;132(5):jcs221952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karampini E, Schillemans M, Hofman M, et al. Defective AP-3-dependent VAMP8 trafficking impairs Weibel-Palade body exocytosis in Hermansky-Pudlak Syndrome type 2 blood outgrowth endothelial cells. Haematologica. 2019;104(10):2091-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bustos MA, Lucchesi O, Ruete MC, Mayorga LS, Tomes CN. Rab27 and Rab3 sequentially regulate human sperm dense-core granule exocytosis. Proc Natl Acad Sci USA. 2012;109(30):E2057-E2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi T, Minami S, Tsuchiya Y, et al. Cytoplasmic control of Rab family small GTPases through BAG6. EMBO Rep. 2019;20(4):e46794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65(18):2801-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyoshi J, Takai Y. Dual role of DENN/MADD (Rab3GEP) in neurotransmission and neuroprotection. Trends Mol Med. 2004;10(10):476-480. [DOI] [PubMed] [Google Scholar]
- 42.Li LC, Wang Y, Carr R, et al. IG20/MADD plays a critical role in glucose-induced insulin secretion. Diabetes. 2014;63(5):1612-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oishi H, Sasaki T, Nagano F, et al. Localization of the Rab3 small G protein regulators in nerve terminals and their involvement in Ca2+-dependent exocytosis. J Biol Chem. 1998;273(51):34580-34585. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi K, Tanaka M, Mizoguchi A, et al. A GDP/GTP exchange protein for the Rab3 small G protein family up-regulates a postdocking step of synaptic exocytosis in central synapses. Proc Natl Acad Sci USA. 2002;99(22):14536-14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka M, Miyoshi J, Ishizaki H, et al. Role of Rab3 GDP/GTP exchange protein in synaptic vesicle trafficking at the mouse neuromuscular junction. Mol Biol Cell. 2001;12(5):1421-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25(4):414-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seabra MC, Wasmeier C. Controlling the location and activation of Rab GTPases. Curr Opin Cell Biol. 2004;16(4):451-457. [DOI] [PubMed] [Google Scholar]
- 48.Biesemann A, Gorontzi A, Barr F, Gerke V. Rab35 protein regulates evoked exocytosis of endothelial Weibel-Palade bodies. J Biol Chem. 2017;292(28):11631-11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarafder AK, Wasmeier C, Figueiredo AC, et al. Rab27a targeting to melanosomes requires nucleotide exchange but not effector binding. Traffic. 2011;12(8):1056-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahoney TR, Liu Q, Itoh T, et al. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol Biol Cell. 2006;17(6):2617-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quevedo MF, Bustos MA, Masone D, Roggero CM, Bustos DM, Tomes CN. Grab recruitment by Rab27A-Rabphilin3a triggers Rab3A activation in human sperm exocytosis. Biochim Biophys Acta Mol Cell Res. 2019;1866(4):612-622. [DOI] [PubMed] [Google Scholar]
- 52.Miteva KT, Pedicini L, Wilson LA, et al. Rab46 integrates Ca2+ and histamine signaling to regulate selective cargo release from Weibel-Palade bodies. J Cell Biol. 2019;218(7):2232-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlüter OM, Schmitz F, Jahn R, Rosenmund C, Südhof TC. A complete genetic analysis of neuronal Rab3 function. J Neurosci. 2004;24(29):6629-6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leebeek FWG, Eikenboom JCJ. Von Willebrand’s disease. N Engl J Med. 2016;375(21):2067-2080. [DOI] [PubMed] [Google Scholar]
- 55.Lavin M, Aguila S, Schneppenheim S, et al. Novel insights into the clinical phenotype and pathophysiology underlying low VWF levels. Blood. 2017;130(21):2344-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadler JE. von Willebrand factor: two sides of a coin. J Thromb Haemost. 2005;3(8):1702-1709. [DOI] [PubMed] [Google Scholar]
- 57.Desch KC, Ozel AB, Siemieniak D, et al. Linkage analysis identifies a locus for plasma von Willebrand factor undetected by genome-wide association. Proc Natl Acad Sci USA. 2013;110(2):588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swystun LL, Lillicrap D. Genetic regulation of plasma von Willebrand factor levels in health and disease. J Thromb Haemost. 2018;16(12): 2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith NL, Chen M-H, Dehghan A, et al. ; Wellcome Trust Case Control Consortium . Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: The CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation. 2010;121(12):1382-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Loon J, Dehghan A, Weihong T, et al. Genome-wide association studies identify genetic loci for low von Willebrand factor levels. Eur J Hum Genet. 2016;24(7):1035-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sabater-Lleal M, Huffman JE, de Vries PS, et al. ; INVENT Consortium; MEGASTROKE Consortium of the International Stroke Genetics Consortium (ISGC) . Genome-wide association transethnic meta-analyses identifies novel associations regulating coagulation factor VIII and von Willebrand factor plasma levels. Circulation. 2019;139(5):620-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barral DC, Ramalho JS, Anders R, et al. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J Clin Invest. 2002;110(2):247-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tiwari S, Italiano JE Jr, Barral DC, et al. A role for Rab27b in NF-E2-dependent pathways of platelet formation. Blood. 2003;102(12):3970-3979. [DOI] [PubMed] [Google Scholar]
- 64.Tolmachova T, Abrink M, Futter CE, Authi KS, Seabra MC. Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci USA. 2007;104(14):5872-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.