Key Points
Umbralisib is a unique PI3Kδ/casein kinase-1ε inhibitor with a tolerable safety profile in relapsed/refractory lymphoid malignancies.
Low rates of immune-mediated toxicities were observed with umbralisib.
Visual Abstract
Abstract
Phosphoinositide 3-kinase-δ (PI3Kδ) inhibitors are active in lymphoid malignancies, although associated toxicities can limit their use. Umbralisib is a dual inhibitor of PI3Kδ and casein kinase-1ε (CK1ε). This study analyzed integrated comprehensive toxicity data from 4 open-label, phase 1 and 2 studies that included 371 adult patients (median age, 67 years) with relapsed/refractory non-Hodgkin lymphoma (follicular lymphoma [n = 147]; marginal zone lymphoma [n = 82]; diffuse large B-cell lymphoma/mantle cell lymphoma [n = 74]; chronic lymphocytic leukemia [n = 43]; and other tumor types [n = 25]) who were treated with the recommended phase 2 dose of umbralisib 800 mg or higher once daily. At data cutoff, median duration of umbralisib treatment was 5.9 months (range, 0.1-75.1 months), and 107 patients (28.8%) received umbralisib for ≥12 months. Any-grade treatment-emergent adverse events (AEs) occurred in 366 (98.7%) of 371 patients, with the most frequent being diarrhea (52.3%), nausea (41.5%), and fatigue (31.8%). Grade 3 or higher treatment-emergent AEs occurred in 189 (50.9%) of 371 patients and included neutropenia (11.3%), diarrhea (7.3%), and increased aminotransferase levels (5.7%). Treatment-emergent serious AEs occurred in 95 (25.6%) of 371 patients. AEs of special interest were limited and included pneumonia (29 of 371 [7.8%]), noninfectious colitis (9 of 371 [2.4%]), and pneumonitis (4 of 371 [1.1%]). AEs led to discontinuation of umbralisib in 51 patients (13.7%). Four patients (1.1%) died of AEs, none of which was deemed related to umbralisib. No cumulative toxicities were reported. The favorable long-term tolerability profile and low rates of immune-mediated toxicities support the potential use of umbralisib for the benefit of a broad population of patients with lymphoid malignancies.
Introduction
The phosphatidylinositol 3-kinase (PI3K) family of kinases is at the center of many signaling pathways, including the B-cell receptor pathway.1 Dysregulated PI3K signaling drives abnormal cellular programming that characterizes several B-cell malignancies.2,3 The class I PI3K comprises 4 distinct isoforms (α, β, δ, and γ) that control diverse and important cellular functions,4,5 with the δ and γ isoforms having a more restricted pattern of expression to cells of hematopoietic origin.6,7 PI3Kδ is highly expressed in leukocytes and plays an essential role in normal B-cell development, survival, and function,8 whereas PI3Kγ functions as a molecular switch between immune stimulation and suppression, and, when inhibited, increases inflammation.6,9 In patients with B-cell malignancies, PI3Kδ signaling is often constitutively active, making inhibition of this isoform an attractive target for non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL) treatment.10
The first 3 PI3K inhibitors, idelalisib, duvelisib, and copanlisib, have all shown efficacy across indolent NHL subtypes, with overall response rates ranging from 43.7% to 57%.11-13 Idelalisib and duvelisib are also active in CLL, with overall response rates ranging from 56% to 85.5%.14-17 Use of these PI3K inhibitors is limited by high discontinuation rates due to a host of toxicities, particularly immune-mediated adverse events (AEs).11-13 Idelalisib and duvelisib carry black box warnings for infections (21%-48%), diarrhea or colitis (14%-20%), and pneumonitis (4%-5%). Idelalisib also carries a black box warning for hepatotoxicity (16%-18%) and intestinal perforation (0.5%), whereas serious cutaneous reactions have been reported in ∼5% of patients treated with duvelisib.18-20 Copanlisib has been associated with grade 3/4 hyperglycemia (41%), grade 3 hypertension (26%), grade 3/4 neutropenia (24%), serious and/or fatal infections (19%), noninfectious pneumonitis (5%), and grade 3/4 cutaneous reactions (2.8%/0.6%).21 These findings have been underscored in the pivotal clinical trials of idelalisib, duvelisib, and copanlisib, in which investigators reported grade 3 or higher treatment-emergent AEs (TEAEs) in 54% to 88% of patients, high TEAE-related discontinuation rates (up to 52%), and treatment-related deaths (3.9%-8.8%) despite short median follow-up times in most studies (6-32 months).11-13,17 Although the mechanistic basis for the toxicities observed with these PI3K inhibitors is not fully understood, simultaneous inhibition of PI3Kδ and PI3Kγ may contribute to hematologic and immune-mediated toxicities.22 Variations in the PI3K-isoform inhibition profile of each agent may explain, in part, the differences observed in incidence and severity of toxicities.11-13,22,23 It has been suggested that some toxicities may be isoform specific24 and not a class effect.23
Umbralisib is a novel, oral, and selective dual inhibitor of PI3Kδ and casein kinase-1ε (CK1ε). Compared with other approved PI3K inhibitors, umbralisib has a unique chemical structure. Preclinical analysis has shown potent PI3Kδ isoform inhibition at clinically achievable concentrations.25 Umbralisib exhibits >1500-fold greater selectivity (Kd) for PI3Kδ over the α and β isoforms and ≈225 times greater selectivity over the γ isoform. Furthermore, umbralisib uniquely inhibits CK1ε. This enzyme plays an important role in protein translation of oncogenes such as MYC, BCL2, and CCND1 (cyclin D1).26-28 Umbralisib has a pharmacokinetic profile with a half-life that allows for once-daily dosing, with no known clinically relevant drug–drug interactions.25 As of January 2021, more than 2000 patients with hematologic malignancies have been treated with umbralisib monotherapy or in combination with other agents. As previously reported, umbralisib has shown efficacy across various lymphoma subtypes.25,29 Fowler et al29 recently reported that with a median follow-up of 27.7 months, patients with relapsed or refractory (R/R) marginal zone lymphoma (MZL), follicular lymphoma (FL), or small lymphocytic lymphoma (SLL) achieved an overall response rate of 47.1% after treatment with umbralisib monotherapy. Tumor reduction was observed in 86.4%. Furthermore, Gribben et al30 reported that umbralisib in combination with ublituximab significantly improved progression-free survival compared with standard-of-care chemoimmunotherapy in patients with treatment-naive or R/R CLL.
To better describe the safety profile of umbralisib, we performed an integrated safety analysis of pooled data obtained from the four phase 1 and phase 2 monotherapy clinical trials with the longest follow-up.
Methods
Trial oversight
Trial protocols were approved by the institutional review board or ethics committee at each participating site, and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. All patients provided written informed consent. All trials were sponsored by TG Therapeutics, Inc. Site investigators collected the data for trials in which they were involved. All study investigators vouch for the accuracy and completeness of reported data and confirm the trial’s adherence to the protocol (supplemental Materials). All authors had access to the integrated data and take responsibility for the completeness and accuracy of the analyses.
Study design and participants
Study data were obtained from a pooled analysis of adult patients (≥18 years of age) from 4 open-label, phase 1 and 2 clinical trials. All patients in the safety population were required to have been treated with ≥1 dose of umbralisib monotherapy (≥800 mg daily), with treatment administered until disease progression, unacceptable toxicity, or study withdrawal. The pivotal UNITY-NHL trial (UTX-TGR-205; #NCT02793583) is being conducted in the United States, Australia, Israel, Italy, Poland, Slovakia, South Korea, Spain, and the United Kingdom, whereas the other trials were, or are, being conducted only in the United States. A list of participating study sites and details of the studies are provided in the supplemental Materials and supplemental Table 1. The 4 studies include:
TGR-1202-101 (#NCT01767766) was a phase 1 dose-escalation trial evaluating the safety and efficacy of umbralisib in patients with R/R, histologically confirmed, hematologic malignancies (B-cell NHL, CLL, peripheral T-cell lymphoma, and Hodgkin lymphoma). A 3 + 3 design was used to determine the maximum tolerated dose of oral umbralisib taken once daily during all cycles (1 cycle = 28 days), with doses ranging from 50 mg to 1800 mg daily (only patients treated with ≥800 mg from this study were included in the analysis).
Patients who completed the TGR-1202-101 study wereallowed to continue umbralisib in UTX-TGR-501 (#NCT032- 07256), an ongoing phase 2, long-term, open-label extension trial evaluating the safety and efficacy of umbralisib 800 mg or 1200 mg daily in patients with B-cell NHL or CLL.
TGR-1202-202 (#NCT03364231) is an ongoing phase 2 clinical trial evaluating the efficacy and safety of umbralisib in patients with R/R MZL or Waldenström macroglobulinemia.
UNITY-NHL (UTX-TGR-205) is an ongoing phase 2b multicohort trial evaluating the efficacy and safety of umbralisib in patients with previously treated R/R indolent NHL (follicular lymphoma, SLL, and MZL), mantle cell lymphoma, and diffuse large B-cell lymphoma (DLBCL).
Umbralisib 800 mg was administered orally daily with food in 28-day cycles. Concomitant granulocyte colony–stimulating factor support was allowed, except in the TGR-1202-101 study, in which initiation or escalation of cytokine therapy was disallowed during the first month of study treatment of dose-limiting toxicity-evaluable patients. Patients were required to start prophylaxis for Pneumocystis jirovecii pneumonia and antiviral therapy within 7 days before cycle 1/day 1, with the exception of the TGR-1202-101 and UTX-TGR-501 studies, in which prophylaxis was at investigator discretion.
Key eligibility criteria were similar for all 4 trials. Patients were required to have a diagnosis of R/R NHL or CLL and to have received ≥1 or ≥2 prior treatment regimens. In these trials, refractory was defined as progression during therapy or within 6 months of completing immediate prior therapy. All patients were required to have an Eastern Cooperative Oncology Group status score of ≤2. Key exclusion criteria included major surgery, chemotherapy, or immunotherapy within the past 21 days; evidence of hepatitis B or C virus or known HIV infection; prior autologous hematologic stem cell transplant within 3 months (TGR-1202-101 and UTX-TGR-501) or 6 months (TGR-1202-202 and UTX-TGR-205) of study entry; or prior allogeneic hematologic stem cell transplant within 12 months (TGR-1202-101 and UTX-TGR-501) or any allogeneic hematologic stem cell transplant (TGR-1202-202 and UTX-TGR-205). Full inclusion and exclusion criteria are presented in the supplemental Materials.
Safety assessments
Safety was assessed based on duration of exposure (months) to drug, number of treatment cycles, and dose modifications. AEs were graded according to Common Terminology Criteria for Adverse Events, version 4.03. Each AE was mapped to a system organ class and preferred term using the Medical Dictionary for Regulatory Activities, version 22.1.31 This terminology is the international medical terminology developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Laboratory parameters in each study included, at a minimum, the following: hemoglobin; hematocrit; platelet, white blood cell, absolute neutrophil, and lymphocyte counts; and serum chemistry parameters (sodium, magnesium, potassium, calcium, aspartate aminotransferase [AST], alanine aminotransferase [ALT], serum creatinine, total bilirubin, and alkaline phosphatase). A serious AE was defined as any untoward medical occurrence that resulted in death, was immediately life-threatening, required at least a 24-hour inpatient hospitalization or prolongation of existing hospitalization, and/or resulted in persistent or significant disability/incapacity.
Statistical analysis
All analyses were based on the umbralisib safety population (all patients who received ≥1 dose ≥800 mg of single-agent umbralisib). The last nonmissing measurement prior to the first study drug administration was considered the baseline. Data were summarized by using descriptive statistics, and results are reported both overall and according to disease type, as appropriate. Due to the heterogeneity of assessment schedules across studies, no pooled analyses were performed for vital signs, physical examinations, or electrocardiograms. All analyses were conducted by using SAS version 9.4.
Results
As of September 1, 2019, data from 371 patients were available for analysis (follicular lymphoma, n = 147; MZL, n = 82; DLBCL/mantle cell lymphoma, n = 74; CLL/SLL, n = 43; other tumor types, n = 25) (Table 1). The median age was 67 years (range, 22-95 years); 56.3% were male, 82.7% were White, and 96.0% had an Eastern Cooperative Oncology Group performance status score of 0 or 1. Fifty-four patients (14.6%) had received prior Bruton tyrosine kinase (BTK) inhibitor therapy, and 44 patients (11.9%) had received prior lenalidomide (alone or in combination). Overall, the median number of prior systemic therapies was 2 (range, 1-14), and the median time to progression from most recent therapy was 2.1 months (range, 0.3-447.4 months). In addition, 145 (39.1%) of 371 patients were refractory to their most recent line of prior treatment.
Table 1.
Summary of demographic, baseline, and pretreatment disease characteristics (umbralisib safety population)
| Parameter/statistic | FL (n = 147) | MZL (n = 82) | DLBCL/MCL (n = 74) | CLL/SLL (n = 43) | Other (n = 25) | Total (N = 371) |
|---|---|---|---|---|---|---|
| Age, median (range), y | 65 (29-87) | 68 (34-88) | 72 (41-95) | 64 (43-86) | 62 (22-85) | 67 (22-95) |
| Sex, n (%) | ||||||
| Female | 56 (38.1) | 43 (52.4) | 36 (48.6) | 16 (37.2) | 11 (44.0) | 162 (43.7) |
| Male | 91 (61.9) | 39 (47.6) | 38 (51.4) | 27 (62.8) | 14 (56.0) | 209 (56.3) |
| Race, n (%) | ||||||
| White | 120 (81.6) | 67 (81.7) | 68 (91.9) | 35 (81.4) | 17 (68.0) | 307 (82.7) |
| Non-White | 13 (8.8) | 10 (12.2) | 4 (5.4) | 7 (16.3) | 6 (24.0) | 40 (10.8) |
| Unknown | 1 (0.7) | 0 | 0 | 0 | 2 (8.0) | 3 (0.8) |
| Not reported | 13 (8.8) | 5 (6.1) | 2 (2.7) | 1 (2.3) | 0 | 21 (5.7) |
| ECOG performance status score, n (%) | ||||||
| 0 | 80 (54.4) | 42 (51.2) | 29 (39.2) | 22 (51.2) | 4 (16.0) | 177 (47.7) |
| 1 | 63 (42.9) | 38 (46.3) | 37 (50.0) | 21 (48.8) | 20 (80.0) | 179 (48.2) |
| 2 | 4 (2.7) | 2 (2.4) | 8 (10.8) | 0 | 1 (4.0) | 15 (4.0) |
| Time since initial diagnosis (mo) | ||||||
| Median (min, max) | 69.6 (4.1, 374.9) | 70.7 (4.9, 340.7) | 43.4 (3.6, 446.5) | 63.8 (6.0, 184.3) | 65.2 (7.3, 193.3) | 63.8 (3.6, 446.5) |
| Stage of disease at screening or most recent, n (%)* | ||||||
| I | 14 (9.5) | 5 (6.1) | 2 (2.7) | 1 (2.3) | 0 | 22 (5.9) |
| II | 18 (12.2) | 7 (8.5) | 4 (5.4) | 1 (2.3) | 0 | 30 (8.1) |
| III | 43 (29.3) | 13 (15.9) | 18 (24.3) | 6 (14.0) | 0 | 80 (21.6) |
| IV | 55 (37.4) | 47 (57.3) | 27 (36.5) | 13 (30.2) | 3 (12.0) | 145 (39.1) |
| Unknown | 1 (0.7) | 5 (6.1) | 2 (2.7) | 1 (2.3) | 7 (28.0) | 16 (4.3) |
| No. of prior systemic therapies | ||||||
| Median (min, max) | 3 (1, 10) | 2 (1, 7) | 3 (1, 10) | 2 (1, 8) | 4 (1, 14) | 2 (1, 14) |
| No. of prior lines of therapy, n (%) | ||||||
| <2 | 21 (14.3) | 38 (46.3) | 14 (18.9) | 11 (25.6) | 3 (12.0) | 87 (23.5) |
| ≥2 | 126 (85.7) | 44 (53.7) | 60 (81.1) | 32 (74.4) | 22 (88.0) | 284 (76.5) |
| Select prior systemic therapy, n (%) | ||||||
| Anti-CD20 antibody | 147 (100.0) | 82 (100.0) | 74 (100.0) | 43 (100.0) | 13 (52.0) | 359 (96.8) |
| Anti-CD20 monotherapy only | 3 (2.0) | 18 (22.0) | 0 | 3 (7.0) | 2 (8.0) | 26 (7.0) |
| Anti-CD20–based chemoimmunotherapy | 144 (98.0) | 61 (74.4) | 74 (100.0) | 40 (93.0) | 6 (24.0) | 325 (87.6) |
| Lenalidomide (monotherapy or in combination) | 20 (13.6) | 5 (6.1) | 11 (14.9) | 2 (4.7) | 6 (24.0) | 44 (11.9) |
| Lenalidomide + anti-CD20 antibody | 16 (10.9) | 4 (4.9) | 8 (10.8) | 1 (2.3) | 0 | 29 (7.8) |
| BTK inhibitor | 12 (8.2) | 5 (6.1) | 22 (29.7) | 6 (14.0) | 9 (36.0) | 54 (14.6) |
| Prior treatment relapsed/refractory status, n (%) | ||||||
| Relapsed | 92 (62.6) | 54 (65.9) | 34 (45.9) | 23 (53.5) | 4 (16.0) | 207 (55.8) |
| Refractory† | 55 (37.4) | 20 (24.4) | 40 (54.1) | 19 (44.2) | 11 (44.0) | 145 (39.1) |
| Prior CD20 treatment relapsed/refractory status, n (%) | ||||||
| Relapsed | 80 (54.4) | 52 (63.4) | 29 (39.2) | 11 (25.6) | 0 | 172 (46.4) |
| Refractory† | 41 (27.9) | 15 (18.3) | 16 (21.6) | 7 (16.3) | 0 | 79 (21.3) |
| Not applicable | 10 (6.8) | 2 (2.4) | 8 (10.8) | 4 (9.3) | 0 | 24 (6.5) |
| Time since most recent progression, median (mo) | ||||||
| Median (min, max) | 2.0 (0.4, 235.8) | 1.8 (0.3, 176.1) | 1.7 (0.3, 447.4) | 10.9 (0.6, 182.4) | 15.8 (0.3, 194.3) | 2.1 (0.3, 447.4) |
ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; max, maximum; MCL, mantle cell lymphoma; min, minimum.
Patients with NHL were staged by using the Ann Arbor staging system; patients with CLL were staged by using the Rai staging system.
Defined as progression during or within 6 months of completing immediate prior therapy.
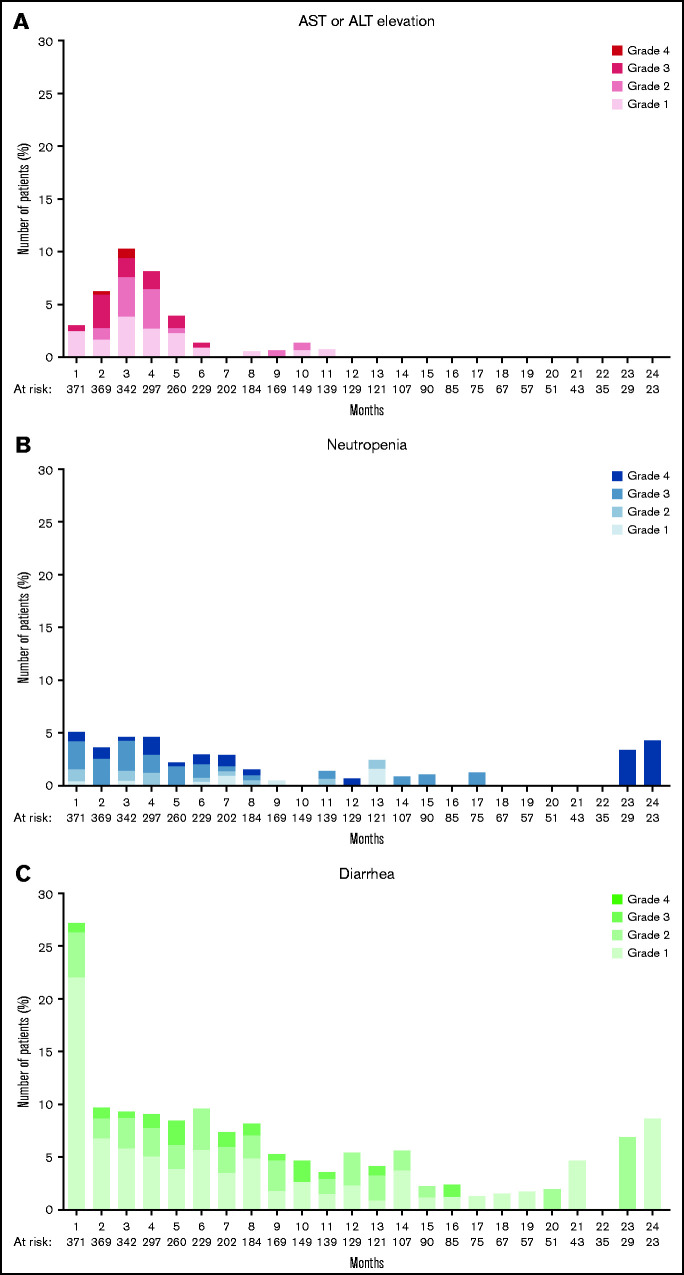
Patients received umbralisib for a median duration of 5.9 months (range, 0.1-75.1 months), with 92 (24.8%) of 371 patients receiving treatment of 12 to <24 months, and 15 (4.0%) of 371 patients receiving treatment of ≥24 months (Table 2). The incidence of TEAEs overall and according to NHL subtype are summarized in Table 3. The most common TEAEs (of any grade) were diarrhea (52.3%), nausea (41.5%), and fatigue (31.8%). The incidence of TEAEs was similar among patients with ≤3 months, 3 to 6 months, 6 to 12 months, or 1 to 2 years of exposure (supplemental Table 2). The most common key TEAEs for patients treated with umbralisib for >1 year (n = 107) were diarrhea (60.7%), neutropenia (17.8%), AST increase (16.8%), and ALT increase (15.0%). Treatment-emergent AEs with a median onset time ≤1 month included constipation (median onset, 1.0 month; median duration, 1.1 months) and diarrhea (median onset, 1.0 month; median duration, 0.6 month); with median onset ≤2 months, TEAEs included infectious colitis (median onset, 1.4 months; median duration, 0.4 month) and transaminase elevation (median onset, 1.9 months; median duration, 1.1 months). Adverse reactions with median onset time of ≥3 months included noninfectious colitis (median onset, 6.0 months; median duration, 0.7 month) and pneumonia (median onset, 3.7 months; median duration, 0.5 month) (Table 4). Toxicities of interest over time are shown in Figure 1. The prevalence of increased AST/ALT peaked by month 3 of umbralisib administration and occurred only rarely after month 6. Although the prevalence of diarrhea was high during the first month of administration of umbralisib (101 of 371 [≈27%]), the vast majority of events were grade 1 (82 of 101). Thirty patients (8.1%) received steroids for the management of AEs that were potentially immune-mediated, including diarrhea or colitis (4%), hepatic dysfunction (2%), rash (2%), and pneumonitis (0.5%).
Table 2.
Extent of exposure to umbralisib
| Extent of exposure | FL (n = 147) | MZL (n = 82) | DLBCL/MCL (n = 74) | CLL/SLL (n = 43) | Other (n = 25) | Total (N = 371) |
|---|---|---|---|---|---|---|
| Duration (mo) | ||||||
| Mean ± SD | 9.8 ± 9.8 | 10.3 ± 7.0 | 4.7 ± 8.6 | 14.7 ± 13.1 | 3.9 ± 3.6 | 9.1 ± 9.7 |
| Median (min, max) | 7.3 (0.1, 75.1) | 9.4 (0.2, 24.6) | 2.3 (0.1, 65.5) | 12.5 (0.7, 55.2) | 2.3 (0.2, 14.0) | 5.9 (0.1, 75.1) |
| Duration, n (%) | ||||||
| <3 mo | 30 (20.4) | 16 (19.5) | 45 (60.8) | 6 (14.0) | 14 (56.0) | 111 (29.9) |
| 3 to <6 mo | 32 (21.8) | 16 (19.5) | 15 (20.3) | 6 (14.0) | 7 (28.0) | 76 (20.5) |
| 6 to <12 mo | 44 (29.9) | 14 (17.1) | 7 (9.5) | 9 (20.9) | 3 (12.0) | 77 (20.8) |
| 12 to <18 mo | 23 (15.6) | 19 (23.2) | 4 (5.4) | 9 (20.9) | 1 (4.0) | 56 (15.1) |
| 18 to <24 mo | 13 (8.8) | 16 (19.5) | 1 (1.4) | 6 (14.0) | 0 | 36 (9.7) |
| ≥24 mo | 5 (3.4) | 1 (1.2) | 2 (2.7) | 7 (16.3) | 0 | 15 (4.0) |
Duration of exposure (mo) = (date of last dose – date of first dose + 1)/30.4375.
MCL, mantle cell lymphoma; SD, standard deviation.
Table 3.
Summary of common TEAEs (≥10% total)
| System organ class/preferred term | FL (n = 147) | MZL (n = 82) | DLBCL/MCL (n = 74) | CLL/SLL (n = 43) | Other (n = 25) | Total (N = 371) |
|---|---|---|---|---|---|---|
| Any TEAE | 144 (98.0) | 82 (100.0) | 72 (97.3) | 43 (100.0) | 25 (100.0) | 366 (98.7) |
| Gastrointestinal disorders | ||||||
| Diarrhea | 83 (56.5) | 50 (61.0) | 27 (36.5) | 24 (55.8) | 10 (40.0) | 194 (52.3) |
| Nausea | 62 (42.2) | 28 (34.1) | 33 (44.6) | 23 (53.5) | 8 (32.0) | 154 (41.5) |
| Vomiting | 35 (23.8) | 16 (19.5) | 19 (25.7) | 15 (34.9) | 1 (4.0) | 86 (23.2) |
| Constipation | 14 (9.5) | 7 (8.5) | 10 (13.5) | 6 (14.0) | 2 (8.0) | 39 (10.5) |
| Abdominal pain | 16 (10.9) | 8 (9.8) | 7 (9.5) | 6 (14.0) | 1 (4.0) | 38 (10.2) |
| General disorders and administration site conditions | ||||||
| Fatigue | 47 (32.0) | 26 (31.7) | 22 (29.7) | 16 (37.2) | 7 (28.0) | 118 (31.8) |
| Pyrexia | 14 (9.5) | 10 (12.2) | 10 (13.5) | 8 (18.6) | 5 (20.0) | 47 (12.7) |
| Edema peripheral | 14 (9.5) | 13 (15.9) | 7 (9.5) | 3 (7.0) | 2 (8.0) | 39 (10.5) |
| Nervous system disorders | ||||||
| Dizziness | 27 (18.4) | 13 (15.9) | 9 (12.2) | 12 (27.9) | 4 (16.0) | 65 (17.5) |
| Headache | 21 (14.3) | 19 (23.2) | 8 (10.8) | 8 (18.6) | 4 (16.0) | 60 (16.2) |
| Infections and infestations | ||||||
| Upper respiratory tract infection | 21 (14.3) | 11 (13.4) | 5 (6.8) | 8 (18.6) | 1 (4.0) | 46 (12.4) |
| Metabolism and nutrition disorders | ||||||
| Decreased appetite | 25 (17.0) | 18 (22.0) | 9 (12.2) | 7 (16.3) | 6 (24.0) | 65 (17.5) |
| Hypokalemia | 18 (12.2) | 7 (8.5) | 8 (10.8) | 2 (4.7) | 4 (16.0) | 39 (10.5) |
| Respiratory, thoracic, and mediastinal disorders | ||||||
| Cough | 25 (17.0) | 18 (22.0) | 7 (9.5) | 13 (30.2) | 9 (36.0) | 72 (19.4) |
| Investigations | ||||||
| ALT increased | 23 (15.6) | 20 (24.4) | 5 (6.8) | 5 (11.6) | 3 (12.0) | 56 (15.1) |
| AST increased | 18 (12.2) | 23 (28.0) | 6 (8.1) | 5 (11.6) | 3 (12.0) | 55 (14.8) |
| Blood creatinine increased | 17 (11.6) | 10 (12.2) | 7 (9.5) | 3 (7.0) | 2 (8.0) | 39 (10.5) |
| Blood and lymphatic system disorders | ||||||
| Neutropenia | 19 (12.9) | 11 (13.4) | 8 (10.8) | 12 (27.9) | 3 (12.0) | 53 (14.3) |
| Anemia | 13 (8.8) | 7 (8.5) | 10 (13.5) | 7 (16.3) | 4 (16.0) | 41 (11.1) |
| Psychiatric disorders | ||||||
| Insomnia | 21 (14.3) | 11 (13.4) | 5 (6.8) | 8 (18.6) | 2 (8.0) | 47 (12.7) |
Data are expressed as n (%). TEAE sorting is done by decreasing frequency of system organ class and preferred term based on the total column. MCL, mantle cell lymphoma.
Table 4.
Onset and duration of AEs
| AE | Any grade (n) | Time to onset (mo) | Duration (mo) |
|---|---|---|---|
| Blood and lymphatic system disorders | |||
| Anemia* | 40 | 1.9 (0.0, 32.5) | 0.9 (0.0, 12.8) |
| Neutropenia (including febrile neutropenia)† | 53 | 1.9 (0.0, 16.6) | 0.5 (0.0, 11.7) |
| Gastrointestinal disorders | |||
| Colitis (noninfectious)‡ | 9 | 6.0 (2.8, 28.4) | 0.7 (0.2, 3.0) |
| Constipation | 39 | 1.0 (0.0, 37.7) | 1.1 (0.0, 54.2) |
| Diarrhea | 194 | 1.0 (0.0, 22.8) | 0.6 (0.0, 53.7) |
| Hepatobiliary disorders | |||
| Transaminase elevation§ | 63 | 1.9 (0.0, 9.5) | 1.1 (0.0, 17.1) |
| Infections and infestations | |||
| Colitis (infectious)‖‖ | 3 | 1.4 (0.4, 1.6) | 0.4 (0.0, 0.9) |
| Pneumonia¶ | 29 | 3.7 (0.8, 25.2) | 0.5 (0.1, 18.0) |
Data are expressed as median (minimum, maximum) unless otherwise indicated. Each occurrence of the same AE was counted as an independent AE. In cases in which 2 or more AEs overlapped or continued from one to another, they were combined as 1 AE in duration calculation. If the end date was missing (eg, ongoing AE), it was imputed as the earliest of (safety data cutoff date, last treatment date + 30 days, end-of-study date, death date).
Grouped term for reactions with multiple preferred terms:
Anemia, hemoglobin decreased.
Neutropenia, febrile neutropenia.
Colitis, colitis microscopic.
Alanine aminotransferase increased, aspartate aminotransferase increased, transaminases increased, hepatic enzyme increased.
Clostridium difficile colitis and enterocolitis viral.
Lower respiratory tract infection, pneumonia, pneumonia Haemophilus, pneumonia Legionella.
Figure 1.
Prevalence of select adverse events of clinical interest over time. Percentage of patients at risk for ALT or AST elevation (A), neutropenia (B), and diarrhea (C) according to month and grade (1-4).
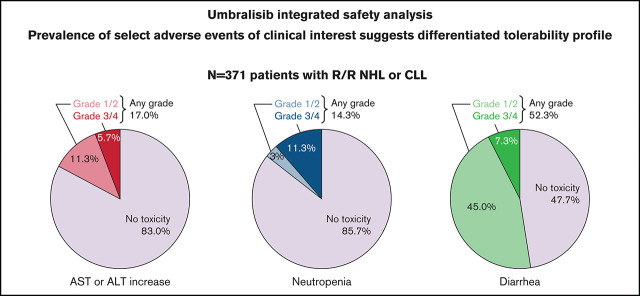
Grade 3 or higher TEAEs occurred in 189 (50.9%) of 371 patients (Table 5). The most common grade 3 or higher TEAEs included neutropenia (11.3%), diarrhea (7.3%), and ALT or AST increase (5.7%). Adverse events of special interest (of any grade) included pneumonia (7.8%), noninfectious colitis (2.4%), and pneumonitis (1.1%). No opportunistic infections (eg, Pneumocystis jirovecii pneumonia or cytomegalovirus reactivation) were reported.
Table 5.
Overview of TEAEs
| TEAE | FL (n = 147) | MZL (n = 82) | DLBCL/MCL (n = 74) | CLL/SLL (n = 43) | Other (n = 25) | Total (N = 371) |
|---|---|---|---|---|---|---|
| Any TEAE | 144 (98.0) | 82 (100.0) | 72 (97.3) | 43 (100.0) | 25 (100.0) | 366 (98.7) |
| Related to umbralisib | 129 (87.8) | 79 (96.3) | 50 (67.6) | 38 (88.4) | 18 (72.0) | 314 (84.6) |
| Grade 3 or higher TEAE | 71 (48.3) | 47 (57.3) | 38 (51.4) | 26 (60.5) | 7 (28.0) | 189 (50.9) |
| Related to umbralisib | 43 (29.3) | 37 (45.1) | 18 (24.3) | 21 (48.8) | 5 (20.0) | 124 (33.4) |
| TEAE outcome of death* | 0 | 0 | 1 (1.4) | 2 (4.7)† | 0 | 3 (0.8)† |
| Serious TEAE | 34 (23.1) | 27 (32.9) | 17 (23.0) | 13 (30.2) | 4 (16.0) | 95 (25.6) |
| Related to umbralisib | 12 (8.2) | 21 (25.6) | 5 (6.8) | 6 (14.0) | 0 | 44 (11.9) |
| Grade 3 or higher serious TEAE | 29 (19.7) | 24 (29.3) | 17 (23.0) | 10 (23.3) | 2 (8.0) | 82 (22.1) |
| Related to umbralisib | 12 (8.2) | 19 (23.2) | 5 (6.8) | 5 (11.6) | 0 | 41 (11.1) |
| TEAE leading to umbralisib withdrawal | 18 (12.2) | 18 (22.0) | 9 (12.2) | 7 (16.3) | 5 (20.0) | 57 (15.4) |
| Related to umbralisib | 16 (10.9) | 15 (18.3) | 6 (8.1) | 6 (14.0) | 3 (12.0) | 46 (12.4) |
| TEAE leading to dose reduction | 18 (12.2) | 9 (11.0) | 4 (5.4) | 4 (9.3) | 4 (16.0) | 39 (10.5) |
| Related to umbralisib | 16 (10.9) | 7 (8.5) | 3 (4.1) | 4 (9.3) | 4 (16.0) | 34 (9.2) |
| TEAE leading to umbralisib interruption | 67 (45.6) | 47 (57.3) | 25 (33.8) | 23 (53.5) | 6 (24.0) | 168 (45.3) |
| Related to umbralisib | 52 (35.4) | 42 (51.2) | 15 (20.3) | 17 (39.5) | 5 (20.0) | 131 (35.3) |
Data are expressed as n (%). MCL, mantle cell lymphoma.
No deaths were related to umbralisib.
One additional death (pneumonia Legionella, in Study 1202-101) was recorded in the end-of-treatment summary case report form. AE outcome was not recorded as fatal in the AE case report form and is not included in this table.
Any-grade TEAEs deemed by the investigators to be related to umbralisib occurred in 84.6% of patients (Table 5). The most common TEAEs of any severity related to umbralisib were diarrhea (165 of 371 [44.5%]), nausea (121 of 371 [32.6%]), and fatigue (78 of 371 [21.0%]) (supplemental Table 3). Grade 3 or higher TEAEs deemed related to umbralisib occurred in 124 (33.4%) of 371 patients, the most common of which were neutropenia (33 of 371 [8.9%]), diarrhea (25 of 371 [6.7%]), ALT increase (20 of 371 [5.4%]), and AST increase (20 of 371 [5.4%]).
Serious TEAEs were reported in 95 (25.6%) of 371 patients and included pneumonia (11 of 371 [3.0%]) and diarrhea (9 of 371 [2.4%]). Serious TEAEs deemed related to umbralisib occurred in 44 (11.9%) of 371 patients and included pneumonia (7 of 371 [1.9%]), diarrhea (7 of 371 [1.9%]), and sepsis (4 of 371 [1.1%]) (supplemental Table 4).
Any-grade TEAEs were reported in 320 (98.5%) of 325 patients who had received prior anti-CD20–based chemoimmunotherapy, 44 (100.0%) of 44 patients who had received prior lenalidomide, and 53 (98.1%) of 54 patients who had received a prior BTK inhibitor. Grade 3 or higher TEAEs occurred in 168 (51.7%) of 325 patients who had received prior anti-CD20–based chemoimmunotherapy, 20 (45.5%) of 44 patients who had received prior lenalidomide, and 21 (38.9%) of 54 patients who had received a prior BTK inhibitor. Serious TEAEs were reported in 84 (25.8%) of 325 patients who had received prior anti-CD20–based chemoimmunotherapy, 8 (18.2%) of 44 patients who had received prior lenalidomide, and 9 (16.7%) of 54 patients who had received a prior BTK inhibitor (supplemental Table 5).
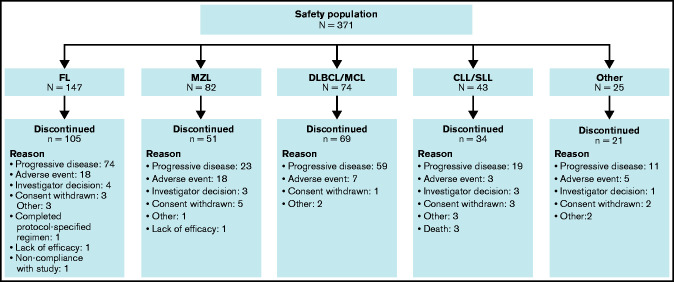
AEs that led to dose interruptions, reductions, and discontinuation occurred in 162 of 371 (43.7%), 49 of 371 (13.2%), and 55 of 371 (14.8%) patients, respectively. Overall, umbralisib treatment was discontinued by 280 patients (75.5%) (Figure 2). The most common reason for discontinuation was progressive disease (186 of 371 [50.1%]), followed by AEs (51 of 371 [13.7%]). The most common TEAEs leading to umbralisib discontinuation were diarrhea (14 of 371 [3.8%]) and ALT or AST increase (10 of 371 [2.7%]); all were considered related to umbralisib except for 1 event of diarrhea that was attributed by the investigator to infection (supplemental Table 6). Death due to AEs was reported in 4 (1.1%) of 371 patients; none was deemed related to umbralisib. Specific causes of death included pleural effusion, septic shock, myocardial infarction, and Legionella pneumophila pneumonia. An additional 22 (5.9%) of 371 patients died due to progressive disease, and 3 (0.8%) of 371 patients had other or unknown causes of death.
Figure 2.
Patient disposition. MCL, mantle cell lymphoma.
The most common laboratory abnormalities in patients on umbralisib treatment (reported by ≥20% of patients overall and ≥5% of patients with grade 3 or higher AEs) were leukopenia (143 of 369 [38.8%]; grade 3, 19 of 369 [5.1%]); neutropenia (135 of 368 [36.7%]; grade 3, 33 of 368 [9.0%]); anemia (decreased hemoglobin) (112 of 369 [30.4%]; grade 3, 20 of 369 [5.4%]); increased AST (111 of 369 [30.1%]; grade 3, 22 of 369 [6.0%]), increased ALT (107 of 369 [29.0%]; grade 3, 21 of 369 [5.7%]); and lymphopenia (89 of 369 [24.1%]; grade 3, 37 of 369 [10.0%]). End-of-study grade 3 and 4 abnormalities were reported for anemia (4.0% and none), lymphopenia (13.7% and 3.1%), neutropenia (3.6% and 3.6%), leukopenia (5.3% and 0.4%), ALT (2.7% and none), and AST (1.8% and none), respectively. ALT or AST ≥3× upper limit of normal (ULN) was reported in 46 of 371 patients (12.4%), and concurrent ALT or AST ≥3× ULN and total bilirubin >2× ULN were reported in 2 patients (1 with MZL and 1 with DLBCL) (supplemental Table 7). No clinically meaningful differences were observed in hematologic or serum chemistry parameters from baseline to study end or in analyses according to subgroup: sex, age, race, geographic region, or prior lines of therapy (data not shown). Additional information is provided in supplemental Tables 8 and 9.
Discussion
This integrated safety analysis of 371 patients with relapsed or refractory lymphoid malignancies found that single-agent umbralisib is associated with a favorable tolerability profile, including low rates of serious immune-mediated toxicities. The most common TEAEs attributed to umbralisib (diarrhea, nausea, and fatigue) were reported at similar rates, regardless of duration of exposure. With a median duration of exposure of 5.9 months and ∼29% of patients on treatment for ≥12 months, our data suggest a favorable long-term safety profile, with no cumulative toxicity over time. Importantly, despite the relatively favorable safety profile we observed with umbralisib, it also seems to provide efficacy similar to that of other PI3K inhibitors.11-13,25,29
About one-third of patients treated with umbralisib experienced a grade 3 or higher TEAE attributed to umbralisib at some point in their course of treatment. The most common of these were neutropenia (8.9%), diarrhea (6.7%), and increased aminotransferase levels (5.4%). These rates are substantially lower than those reported for other PI3K inhibitors in similar patient populations. For example, in pivotal studies, 8% to 27% of patients treated with idelalisib experienced grade 3 or higher neutropenia, diarrhea, or aminotransferase elevations,11 and the overall incidence of grade 3 or higher TEAEs in patients treated with idelalisib, duvelisib, or copanlisib in pivotal studies was 54%, 88%, and 53%, respectively.11-13
In the current analysis, 13.7% of patients discontinued treatment due to AEs, which seems to be favorable in light of the 20.0%, 25%, and 52.0% discontinuation rates due to AEs in clinical trials of idelalisib, copanlisib, and duvelisib, respectively.11,12,17 Furthermore, in a large retrospective analysis, 94% (58 of 62) of patients treated with idelalisib discontinued therapy at a median of 6 months, suggesting that discontinuation rates with these drugs may be even higher in the real-world setting.32 Toxicities from these PI3K inhibitors have also been associated with death in 3.9% to 8.8% of patients.11,13 In our analysis, deaths due to TEAEs were limited to 4 patients (1.1%), with none deemed by the investigator to be related to umbralisib.
The favorable safety profile of umbralisib is particularly relevant for patients with indolent NHL, many of whom have a chronic history that often requires ongoing treatment for prolonged periods.33 For these patients, an important goal of treatment is to achieve durable disease control and reduce future relapse risk.34 As with other promising new therapies in this space, such as bispecific antibodies and next-generation BTK inhibitors,35-37 minimizing toxicity risk, which would lead to a low rate of treatment discontinuations, will allow safe use in the long term. We observed no difference in the rate of AEs based on prior therapy. Although not a comparative study, our analysis suggests that umbralisib may have a more favorable safety profile than other PI3K inhibitors, especially as it relates to hepatic toxicity, colitis, and immune-mediated AEs. In addition, fatal and/or serious pneumonitis is known to occur in ≈4% and ≈5% of patients receiving idelalisib or duvelisib, respectively.18,19 Of the 4 cases (1.1%) of pneumonitis reported with umbralisib, all had confounding factors: 1 case was attributed to progression of disease; 1 patient had a chronic history of bronchitis, asthma, and previous pneumonia; 1 patient had pleural effusion noted 6 months before the first dose of umbralisib, with cultures suggesting infection; and 1 patient had pneumonia 1 week before the diagnosis of pneumonitis. Serious AEs of diarrhea or colitis occurred in 14% to 20% of patients receiving idelalisib and 18% of patients receiving duvelisib.18,19 Although colitis was observed infrequently in patients treated with umbralisib (noninfectious, 2.4%; infectious, <1%), a causal association between umbralisib and colitis cannot be excluded.
Our pooled safety analysis is consistent with other ongoing studies of umbralisib. For example, a phase 2 multicenter trial reported a favorable safety profile in patients with CLL (N = 51) intolerant to previous BTK inhibitor or PI3K inhibitor therapy.38 In that study, umbralisib was well tolerated, with only 6 (12%) patients discontinuing due to an AE. No fatal AEs occurred, and only 1 patient discontinued due to a recurrent AE, which the patient also had experienced with a prior kinase inhibitor. At a median follow-up of 23 months, 32% of patients remained on study. At the cutoff date, 58% of patients had been on umbralisib for a longer duration than their prior kinase inhibitor.38
As noted, immune-mediated toxicities occur frequently in patients treated with idelalisib, duvelisib, or copanlisib.12,39,40 It has been suggested that these toxicities may be linked to a decrease in the percentage of regulatory T cells relative to effector T cells.20,39 One hypothesis regarding the potentially lower rates of immune-mediated toxicities observed with umbralisib is that idelalisib and duvelisib simultaneously inhibit both PI3Kδ and PI3Kγ isoforms, whereas umbralisib selectively inhibits PI3Kδ, with little to no PI3Kγ inhibition.25 PI3Kγ functions as a molecular switch between immune stimulation and suppression and, when inhibited, increases inflammation.9 In preclinical models, the loss of PI3Kδ alone was less likely to result in severe autoimmunity.41,42 However, combined loss of PI3Kδ and PI3Kγ in T cells resulted in severe autoimmunity and inflammation,43 suggesting that the lack of PI3Kγ inhibition by umbralisib may be advantageous. An additional hypothesis relates to the selective dual inhibition of both PI3Kδ and CK1ε by umbralisib. These unique features may contribute to the lower incidence of immune-mediated toxicities, as the inhibition of CK1ε, which is known to regulate β-catenin and thus WNT signaling in part, may have an influence on regulatory T-cell numbers and function.27,28,44 Future studies will elucidate the relative contribution of each of these proposed mechanisms of action.
One important implication of our data showing the favorable safety profile of umbralisib is that it suggests that umbralisib is an agent which may combine well with other novel agents approved and in development for B-cell malignancies. Indeed, umbralisib is currently being explored in combination with 16 other commonly used therapies,25,45-49 and the safety profile of umbralisib combination regimens is generally consistent with the known safety profile of each individual drug, with some patients receiving daily umbralisib for up to 5 years.45,50 Additional studies evaluating umbralisib in combination with other therapies in patients with R/R lymphoid malignancies are ongoing (ClinicalTrials.gov identifiers #NCT03801525, #NCT04016805, and #NCT03671590). Moreover, the feasibility of using umbralisib in combination with ublituximab (an anti-CD20 antibody) in treatment-naive patients with CLL was recently reported, which is particularly notable because prior PI3K inhibitors showed prohibitive toxicities in the frontline setting in CLL.30
A limitation of this integrated analysis is that these data were generated from 4 open-label, nonrandomized, phase 1 and 2 trials with unique study designs and eligibility criteria. However, a benefit of this strategy is that pooled, long-term data increase the potential for identifying less-common events. Although a significant number of patients have had >6 months of follow-up, the extended time to onset for immune-mediated adverse events with other PI3K inhibitors may preclude full assessment of these adverse events in the current analysis. Another significant limitation is that our study does not directly compare umbralisib vs other PI3K inhibitors. Such prospective, randomized clinical trial data would be needed to definitively confirm differences among these drugs, although it is uncertain whether such trials will ever be run. As such, despite its limitations, our study represents a robust data set to help inform the choice of PI3K inhibitor used in clinical practice.
In summary, this integrated safety analysis shows that continuous treatment with umbralisib is generally well tolerated in patients with B-cell malignancies, with low discontinuation rates and without significant treatment-limiting toxicities.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients, their families, and caregivers who participated in this study; the investigators and coordinators at the clinical sites; those who contributed to the design, implementation, and data analyses; and Luminology Scientific Communications (Marina P. Gehring, Medical Writer; Suryanshi Nayyar, Medical Fellow) for editorial assistance in the preparation of the manuscript (funded by TG Therapeutics, Inc). M.S.D. is a Scholar in Clinical Research from the Leukemia & Lymphoma Society. O.A.O. is an American Cancer Society Research Professor. D.M.B. is a National Comprehensive Cancer Network Guidelines panel member.
This study was supported by research funding from TG Therapeutics, Inc.
Authorship
Contribution: M.S.D., O.A.O., M.R.P., R.A., R.P., J.E., H.P.M., P.S., and M.S.W. designed the protocol; O.A.O., W.J., P.L.Z., M.R.P., N.G., B.D.C., E.D., J.A.R., J.N.A., T.S.F., P.F.C., E.L.-M., J.M.B., R.A., R.P., L.A.L., C.Y.C., G.F., J.E., J.C.C., J.M.P., P.S., and I.W.F. acquired data; M.S.D., O.A.O., M.R.P., J.A.R., R.A., R.P., G.F., J.M.P., H.P.M., and P.S. supervised the study; M.S.D., O.A.O., M.R.P., N.G., R.A., R.P., Y.H., H.P.M., P.S., and M.S.W. performed statistical analyses and/or provided input on the analysis and data interpretation; and all authors had access to the study data, critically reviewed or edited the manuscript, and approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: M.S.D. has received grants from AbbVie, Ascentage Pharma, AstraZeneca, Genentech, MEI Pharma, Novartis, Pharmacyclics, Surface Oncology, TG Therapeutics, and Verastem; and personal fees from AbbVie, Adaptive Biotechnologies, Ascentage Pharma, AstraZeneca, BeiGene, Celgene, Eli Lilly, Genentech, Gilead Sciences, Janssen, MEI Pharma, Merck, Novartis, Pharmacyclics, Research to Practice, Syros Pharmaceuticals, TG Therapeutics, Verastem, and Zentalis. O.A.O. is an employee of and has an equity interest in TG Therapeutics. W.J. has received grant support from TG Therapeutics. F.S. has received personal fees from Astex Pharmaceuticals and ADC Therapeutics. T.S.F. has received research grants from TG Therapeutics, Millennium, Novartis, Kyowa, Portola, and Curis; and personal fees from BeiGene, Genentech, Adaptive Biotechnologies, AbbVie, Verastem, Kite, MorphoSys, AstraZeneca, Pharmacyclics, Sanofi, Seattle Genetics, Celgene, and Bristol Myers Squibb. P.L.Z. has received personal fees from Verastem, Celltrion, Gilead, Janssen-Cilag, Bristol Myers Squibb, Servier, Sandoz, MSD, Immune Design, Celgene, Portola, Roche, EUSA Pharma, Kyowa Kirin, and Sanofi. M.R.P. has received institutional research funding for conduct of this trial from TG Therapeutics and other support from Janssen, EMD Serono, Pfizer, Pharmacyclics, Bayer, and Genentech. N.G. has received research grants from Bristol Myers Squibb, TG Therapeutics, Pharmacyclics, Genentech, and Gilead; and personal fees from Bristol Myers Squibb, TG Therapeutics, Seattle Genetics, Janssen, Pharmacyclics, AbbVie, Gilead, AstraZeneca, Karyopharm, Genmab, Incyte, and Epizyme. B.D.C. has received institutional grants from TG Therapeutics, AbbVie, AstraZeneca, Roche-Genentech, Seattle Genetics, Bristol Myers Squibb, Trillium, and Epizyme; and personal fees from TG Therapeutics, AbbVie, BeiGene, MorphoSys, Karyopharm, and Symbio. E.D. has received research grants from TG Therapeutics and ADC Therapeutics; and personal fees from AstraZeneca. D.M.B. has received institutional grants from AbbVie, ArQule, Ascentage, AstraZeneca, BeiGene, DTRM, Genentech, Juno/Celgene/BMS, LOXO, MEI Pharma, Novartis, Pharmacyclics, and TG Therapeutics; and personal fees from AbbVie, Genentech, Pharmacyclics, Pfizer, TG Therapeutics, and Verastem; and nonfinancial support from Pfizer and Teva. J.A.R. has received institutional grants from Sarah Cannon Research Institute, Eli Lilly, Tesaro, TG Therapeutics, Genentech, Celgene, Merck, Bristol Myers Squibb, Boston Biomedical Inc, AstraZeneca, NovoCure, Calithera Biosciences, Novartis, Guardant Health, Acerta Pharma, Rhizen Pharmaceuticals, Takeda Pharmaceuticals, Onconova Therapeutics, Sanofi, CTI Biopharma, Eisai, and Janssen. J.N.A. has received grants from TG Therapeutics, Janssen, Celgene, and Genentech; and personal fees from AbbVie, Pharmacyclics, Janssen, AstraZeneca/Acerta, BeiGene, Genentech, Sunesis, and Ascentage Pharma. P.F.C. has received personal fees from TG Therapeutics. E.L.-M. has received other support from Roche, Janssen, Amgen, Takeda, Novartis, AbbVie, Gilead, Sanofi, and Astellas. J.M.B. has received personal fees from Bristol Myers Squibb, Roche, AbbVie, Seattle Genetics, Tempus Labs, Gilead, Bayer, AstraZeneca, Verastem, MorphoSys, Adaptive Biotechnologies, Epizyme, and Kura. L.A.L. has received personal fees from Kite Pharma, TG Therapeutics, Celgene/BMS, BeiGene, PCYC/Janssen, AbbVie, AstraZeneca, Seattle Genetics, ADC Therapeutics, Epizyme, and Karyopharm. C.Y.C. has received grants from Roche, Celgene, and AbbVie; and personal fees from TG Therapeutics, Roche, Janssen, MSD, Gilead, Ascentage Pharma, Acerta, and Loxo Oncology; and nonfinancial support from Roche. G.F. has received personal fees from Celgene, Pharmacyclics, Gilead, and Amgen. J.C.C. has received personal fees from Kite/Gilead, Novartis, MorphoSys, Bayer, Epizyme, AstraZeneca, Genentech, Karyopharm, and Celgene/Juno. J.M.P. has received other support from Gilead, AstraZeneca, BeiGene, and Loxo Oncology. J.P.S. has received personal fees from AstraZeneca, Acerta, AbbVie, Pharmacyclics, Janssen, TG Therapeutics, MEI Pharmaceuticals, Verastem, and Genentech. Y.H., H.P.M., P.S., and M.S.W. are employees of and have equity interest in TG Therapeutics. I.W.F. has received institutional grants from AbbVie, AstraZeneca, BeiGene, Gilead Sciences, Janssen, Juno Therapeutics, Kite Pharma, MorphoSys, Pharmacyclics, Roche, Seattle Genetics, Takeda, TG Therapeutics, Unum Therapeutics, and Verastem, Acerta Pharmaceuticals, Agios, ArQule, Calithera Biosciences, Celgene, Constellation Pharmaceuticals, Curis, F. Hoffmann–La Roche Ltd, Forma Therapeutics, Forty Seven, Genentech, IGM Biosciences, Incyte, Infinity Pharmaceuticals, Karyopharm Therapeutics, Loxo, Merck, Novartis, Pfizer, Portola Pharmaceuticals, Rhizen Pharmaceuticals, Teva, Trillium Therapeutics, and Triphase Research & Development Corp; and other institutional support from AbbVie, AstraZeneca, BeiGene, Curio Science, Great Point Partners, Iksuda Therapeutics, Gilead Sciences, Janssen, Juno Therapeutics, Kite Pharma, MorphoSys, Nurix Therapeutics, Pharmacyclics, Roche, Seattle Genetics, Takeda, TG Therapeutics, Unum Therapeutics, Verastem, and Yingli Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Matthew S. Davids, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: matthew_davids@dfci.harvard.edu.
References
- 1.Blachly JS, Baiocchi RA.. Targeting PI3-kinase (PI3K), AKT and mTOR axis in lymphoma. Br J Haematol. 2014;167(1):19-32. [DOI] [PubMed] [Google Scholar]
- 2.Dienstmann R, Rodon J, Serra V, Tabernero J.. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. 2014;13(5):1021-1031. [DOI] [PubMed] [Google Scholar]
- 3.Reif K, Okkenhaug K, Sasaki T, Penninger JM, Vanhaesebroeck B, Cyster JG.. Cutting edge: differential roles for phosphoinositide 3-kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J Immunol. 2004;173(4):2236-2240. [DOI] [PubMed] [Google Scholar]
- 4.Bilanges B, Posor Y, Vanhaesebroeck B.. PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev Mol Cell Biol. 2019;20(9):515-534. [DOI] [PubMed] [Google Scholar]
- 5.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B.. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329-341. [DOI] [PubMed] [Google Scholar]
- 6.Miller MS, Thompson PE, Gabelli SB.. Structural determinants of isoform selectivity in PI3K inhibitors. Biomolecules. 2019;9(3):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh P, Dar MS, Dar MJ.. p110α and p110β isoforms of PI3K signaling: are they two sides of the same coin? FEBS Lett. 2016;590(18):3071-3082. [DOI] [PubMed] [Google Scholar]
- 8.Vanhaesebroeck B, Welham MJ, Kotani K, et al. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci USA. 1997; 94(9):4330-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneda MM, Messer KS, Ralainirina N, et al. PI3Kγ is a molecular switch that controls immune suppression [published correction appears in Nature. 2017;542(7639):124]. Nature. 2016;539(7629):437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali AY, Wu X, Eissa N, et al. Distinct roles for phosphoinositide 3-kinases γ and δ in malignant B cell migration. Leukemia. 2018;32(9):1958-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898-3905. [DOI] [PubMed] [Google Scholar]
- 13.Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma [published correction appears in J Clin Oncol. 2019;37(16):1448]. J Clin Oncol. 2019;37(11):912-922. [DOI] [PubMed] [Google Scholar]
- 14.Sharman JP, Coutre SE, Furman RR, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37(16):1391-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flinn IW, O’Brien S, Kahl B, et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies. Blood. 2018;131(8):877-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davids MS, Fisher DC, Tyekucheva S, et al. A phase 1b/2 study of duvelisib in combination with FCR (DFCR) for frontline therapy for younger CLL patients. Leukemia. 2020;35(4):1064-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davids MS, Kuss BJ, Hillmen P, et al. Efficacy and safety of duvelisib following disease progression on ofatumumab in patients with relapsed/refractory CLL or SLL in the DUO Crossover Extension Study. Clin Cancer Res. 2020;26(9):2096-2103. [DOI] [PubMed] [Google Scholar]
- 18.COPIKTRA® (duvelisib) [package insert]. Las Vegas, NV: Verastem, Inc; 2018. [Google Scholar]
- 19.ZYDELIG® (idelalisib) [package insert]. Foster City, CA: Gilead Sciences, Inc; 2018. [Google Scholar]
- 20.Coutré SE, Barrientos JC, Brown JR, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56(10):2779-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ALIQOPA™ (copanlisib) [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2017. [Google Scholar]
- 22.Buchanan CM, Lee KL, Shepherd PR.. For better or worse: the potential for dose limiting the on-target toxicity of PI 3-kinase inhibitors. Biomolecules. 2019;9(9):402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curigliano G, Shah RR.. Safety and tolerability of phosphatidylinositol-3-kinase (PI3K) inhibitors in oncology. Drug Saf. 2019;42(2):247-262. [DOI] [PubMed] [Google Scholar]
- 24.Nunnery SE, Mayer IA.. Management of toxicity to isoform α-specific PI3K inhibitors. Ann Oncol. 2019;30(suppl 10):x21-x26. [DOI] [PubMed] [Google Scholar]
- 25.Burris HA III, Flinn IW, Patel MR, et al. Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol. 2018;19(4):486-496. [DOI] [PubMed] [Google Scholar]
- 26.Deng C, Lipstein MR, Scotto L, et al. Silencing c-Myc translation as a therapeutic strategy through targeting PI3Kδ and CK1ε in hematological malignancies. Blood. 2017;129(1):88-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maharaj K, Powers JJ, Achille A, et al. The dual PI3Kδ/CK1ε inhibitor umbralisib exhibits unique immunomodulatory effects on CLL T cells. Blood Adv. 2020;4(13):3072-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Loosdregt J, Fleskens V, Tiemessen MM, et al. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity. 2013;39(2):298-310. [DOI] [PubMed] [Google Scholar]
- 29.Fowler NH, Samaniego F, Jurczak W, et al. Umbralisib, a dual PI3Kδ/CK1ε inhibitor in patients with relapsed/refractory indolent lymphoma. J Clin Oncol. 2021;39(15):1609-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gribben JG, Jurczak W, Jacobs R, et al. 543 umbralisib plus ublituximab (U2) is superior to obinutuzumab plus chlorambucil (O+Chl) in patients with treatment naïve (TN) and relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): results from the phase 3 Unity-CLL Study. Presented at the 62nd American Society of Hematology Annual Meeting and Exposition (ASH). 5 December 2020. Virtual meeting.
- 31.Medical Dictionary for Regulatory Activities. https://www.meddra.org. Accessed 1 September 2020.
- 32.Mato AR, Hill BT, Lamanna N, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol. 2017;28(5):1050-1056. [DOI] [PubMed] [Google Scholar]
- 33.Kritharis A, Sharma J, Evens AM.. Current therapeutic strategies and new treatment paradigms for follicular lymphoma. Cancer Treat Res. 2015;165:197-226. [DOI] [PubMed] [Google Scholar]
- 34.Lunning MA, Vose JM.. Management of indolent lymphoma: where are we now and where are we going. Blood Rev. 2012;26(6):279-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rader C. Bispecific antibodies in cancer immunotherapy. Curr Opin Biotechnol. 2020;65:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers KA, Thompson PA, Allan JN, et al. Phase II study of acalabrutinib in ibrutinib-intolerant patients with relapsed/refractory chronic lymphocytic leukemia. Haematologica. 2021;106(9):2364-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mato AR, Shah NN, Jurczak W, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021; 397(10277):892-901. [DOI] [PubMed] [Google Scholar]
- 38.Mato AR, Ghosh N, Schuster SJ, et al. Phase 2 study of the safety and efficacy of umbralisib in patients with CLL who are intolerant to BTK or PI3Kδ inhibitor therapy. Blood. 2021;137(20):2817-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lampson BL, Kasar SN, Matos TR, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128(2):195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali K, Soond DR, Pineiro R, et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer [published correction appears in Nature. 2016;535(7613):580]. Nature. 2014;510(7505):407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stark AK, Davenport ECM, Patton DT, et al. Loss of phosphatidylinositol 3-kinase activity in regulatory T cells leads to neuronal inflammation. J Immunol. 2020;205(1):78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji H, Rintelen F, Waltzinger C, et al. Inactivation of PI3Kgamma and PI3Kdelta distorts T-cell development and causes multiple organ inflammation. Blood. 2007;110(8):2940-2947. [DOI] [PubMed] [Google Scholar]
- 44.Cruciat CM. Casein kinase 1 and Wnt/β-catenin signaling. Curr Opin Cell Biol. 2014;31:46-55. [DOI] [PubMed] [Google Scholar]
- 45.Davids MS, Flinn IW, Mato AR, et al. Long-term integrated safety analysis of umbralisib (TGR-1202), a PI3Kδ/CK1ε inhibitor with a differentiated safety profile, in patients with relapsed/refractory lymphoid malignancies. Poster presented at 23rd Congress of the European Hematology Association (EHA). 15 June 2018. Stockholm, Sweden.
- 46.Lunning M, Vose J, Nastoupil L, et al. Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2019;134(21):1811-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nastoupil LJ, Lunning MA, Vose JM, et al. Tolerability and activity of ublituximab, umbralisib, and ibrutinib in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: a phase 1 dose escalation and expansion trial. Lancet Haematol. 2019;6(2):e100-e109. [DOI] [PubMed] [Google Scholar]
- 48.Davids MS, Kim HT, Nicotra A, et al. ; Blood Cancer Research Partnership of the Leukemia and Lymphoma Society . Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: a multicentre phase 1-1b study. Lancet Haematol. 2019;6(1):e38-e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheah CY, Wickham N, Jurczak W, et al. Clinical activity of TG-1701, as monotherapy and in combination with ublituximab and umbralisib (U2), in patients with B-cell malignancies. Presented at 62nd American Society of Hematology Annual Meeting and Exposition (ASH). 5 December 2020. Virtual meeting.
- 50.Davids MS, Kim HT, Savell A, et al. Long term results of a phase I/Ib study of ibrutinib in combination with umbralisib in patients with relapsed/refractory CLL or MCL. e-Poster presented at 25th European Hematology Association (EHA). 11-21 June 2020. Virtual meeting. Abstract EP689.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.