Key Points
Cytogenetic lesions in pediatric AML differ by race-ethnicity including higher rates of specific poor prognosis lesions among Black children.
Racial-ethnic minorities experience worse outcomes in pediatric AML regardless of genetic disease features.
Visual Abstract
Abstract
Black and Hispanic children with acute myeloid leukemia (AML) have worse outcomes compared with White children. AML is a heterogeneous disease with numerous genetic subtypes in which these disparities have not been specifically investigated. In this study, we used the Therapeutically Applicable Research to Generate Effective Treatments (TARGET) database to examine the association of race-ethnicity with leukemia cytogenetics, clinical features, and survival outcomes within major cytogenetic subgroups of pediatric AML. Compared with White non-Hispanic patients, t(8;21) AML was more prevalent among Black (odds ratio [OR], 2.22; 95% confidence interval [CI], 1.28-3.74) and Hispanic patients (OR, 1.74; 95% CI, 1.05-2.83). The poor prognosis KMT2A rearrangement t(6;11)(q27;q23) was more prevalent among Black patients (OR, 6.12; 95% CI, 1.81-21.59). Among those with KMT2Ar AML, Black race was associated with inferior event-free survival (EFS) (hazard ratio [HR], 2.31; 95% CI, 1.41-3.79) and overall survival (OS) (HR, 2.54; 1.43-4.51). Hispanic patients with KMT2Ar AML also had inferior EFS (HR, 2.20; 95% CI, 1.27-3.80) and OS (HR, 2.07; 95% CI, 1.09-3.93). Similarly, among patients with t(8;21) or inv(16) AML (ie, core-binding factor [CBF] AML), Black patients had inferior outcomes (EFS HR, 1.93; 95% CI, 1.14-3.28 and OS HR, 3.24; 95% CI, 1.60-6.57). This disparity was not detected among patients receiving gemtuzumab ozogamicin (GO). In conclusion, racial-ethnic disparities in survival outcomes among young people with AML are prominent and vary across cytogenetic subclasses. Future studies should explore the socioeconomic and biologic determinants of these disparities.
Introduction
Black and Hispanic children with acute myeloid leukemia (AML) have inferior outcomes compared with White children.1-4 The root cause of these disparities is likely multifactorial. Proposed contributing factors include fewer socioeconomic resources, higher prevalence of medical comorbidities, higher risk of adverse drug events, higher disease acuity at presentation, lower enrollment in clinical trials, and less access to matched hematopoietic stem cell donors among Black and Hispanic children vs White children.1,2,5-8
Although racial-ethnic disparities are well-described in pediatric AML, the malignancy’s rarity limits in-depth investigations into this phenomenon. Pediatric AML is a heterogeneous disease characterized by numerous genetic and phenotypic subtypes, and its prognosis varies widely based on these differences.4,9,10 Studies of the relative prevalence of clinically significant AML cytogenetics among racial-ethnic backgrounds, as well as those exploring racial-ethnic outcome disparities within these cytogenetic subgroups, are limited.3,11 Whether the antileukemic effect of specific chemotherapeutic agents is modified by race-ethnicity within AML subtypes is also unknown.
The Therapeutically Applicable Research to Generate Effective Treatments (TARGET) database is a public resource with data from over 900 patients diagnosed with AML between 1996 and 2013 that was originally developed to characterize the molecular genetics of pediatric malignancies.12 The objective of the current study was to leverage the TARGET AML database to identify differences in cytogenetic features, disease phenotype, and survival among pediatric patients of diverse racial-ethnic backgrounds. We further investigated disease phenotype and survival within the most common cytogenetic groups of pediatric AML: KMT2A-rearranged (KMT2Ar) AML and core-binding factor (CBF) AML. We hypothesized that Hispanic and Black patients have inferior survival compared with White non-Hispanic patients regardless of AML cytogenetics.
In the AAML0531 trial, the addition of gemtuzumab ozogamicin (GO) to standard therapy improved event-free survival (EFS) and reduced relapse rates overall, but the impact of GO varied among risk groups.9 As data regarding GO use were available in the TARGET database, we also investigated whether GO was associated with a differential outcome within specific racial-ethnic groups.
Methods
Study design and data collection
We conducted a retrospective cohort study using the TARGET AML database. Data were downloaded directly from the National Cancer Institute.13 The database contains prospective data from patients with de novo AML enrolled in three pediatric AML clinical trials: (1) Children’s Cancer Group (CCG) 2961 (1996-2006)10; (2) Children’s Oncology Group (COG) AAML03p1 (2003-2013)14; and (3) COG AAML0531 (2006-2013).9 All analyses in this study were based on patients’ original presentation with de novo AML (rather than at the time of potential relapse).
Data extracted included age, sex, self-reported race and ethnicity, primary cytogenetic lesion (eg, KMT2Ar), presence or absence of specific recurrent mutations (eg, FLT3, NPM1, CEBPA), clinical features at presentation (eg, initial white blood cell [WBC] count), clinical response (eg, induction failure), treatment protocol, use of GO, and survival time. Patients coded as KMT2Ar(+) were identified as having KMT2A, MLL, or a known KMT2A fusion partner listed among cytogenetic data available in the dataset. Similarly, patients with CBF were identified as having t(8;21) or inv(16) listed among cytogenetic data.
Owing to its original purpose to describe AML genetics, the TARGET dataset is divided into discovery and validation subsets. The discovery set was enriched for, though not exclusive to, patients with relapse; the validation set was a random sample of patients with or without relapse from AAML0531.13 For this study, the two subsets were merged, and numeric identifiers were used to remove duplicate entries of patients (ie, patients captured in both the discovery and validation samples; n = 93).
Inclusion/exclusion criteria
The analysis was limited to patients aged less than 21 years at diagnosis. To avoid analyses with limited power, we restricted the study to White non-Hispanic, Hispanic, and Black non-Hispanic patients. Patients identifying as both Black and Hispanic were included within the Hispanic group. Other racial-ethnic groups each accounted for less than 5% of the entire cohort. Patients with unknown race-ethnicity were also excluded.
Statistical analysis
Associations between race-ethnicity and categorical variables such as primary cytogenetic lesion were determined with univariable logistic regression. Continuous variables were compared by race-ethnicity using the Kruskal-Wallis and Mann-Whitney U tests.
EFS was calculated as the time in days from study enrollment until the earliest of induction failure, relapse, or death. Overall survival (OS) was calculated as the time from study enrollment until death. EFS and OS estimates are reported at 3 years from enrollment. Patients were right-censored at the earliest of 3 years from diagnosis, study withdrawal, or loss to follow-up. Survival curves were calculated using the Kaplan-Meier method, and the log-rank test was used to evaluate for differences in survival among subgroups. Univariable Cox proportional-hazards modeling was used to quantify differential survival among racial-ethnic subgroups. White non-Hispanic patients were designated as the reference group.
Finally, we conducted a post hoc causal mediation analysis based on the findings of the primary survival analysis in order to better understand the relationship between Black race, risk cytogenetics, and survival. In causal mediation analysis, the total effect of a primary exposure (eg, Black race) is broken down into a natural indirect effect mediated by an identified variable (eg, high-risk cytogenetics) and a natural direct effect which is the sum of all other causal pathways between the primary exposure and outcome.15,16
RNA sequencing analysis
To identify potential determinants of GO response among patient subgroups, we leveraged RNA sequencing data available within the TARGET dataset to compare myeloblast CD33 expression among patients. Normalized RNA sequencing data were generated as previously described.12 TARGET identifier codes from clinical data were matched to those within available mRNAseq open access files. Reads per kilobase of transcript per million (RPKM) values representing CD33 mRNA expression (ENSG00000105383) were compared among patient subgroups using the Mann-Whitney U test for pairwise comparison and the Kruskal-Wallis test for comparisons among groups.
Analyses were performed in R version 4.0.2 with specific use of the Survival package.17,18 Statistical significance was set at P < .05. All data analyses were conducted between July and November 2020. The study followed guidelines as outlined by Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria.19 The study was exempted from institutional review board approval due to its exclusive use of deidentified, publicly available data.
The data used for this analysis are available at https://portal.gdc.cancer.gov/projects/TARGET-AML.
Results
Patient characteristics
At the time of data abstraction, the TARGET AML dataset included clinical data from 957 patients. We identified 843 White non-Hispanic, Hispanic, and Black patients. Eight patients aged 21 years or older at diagnosis were excluded. Twenty-one patients with missing race or ethnicity data were also excluded. In total, 552 White non-Hispanic, 154 Hispanic, and 108 Black non-Hispanic patients were included in the study, representing 85% of the entire TARGET AML clinical database (Table 1). From the original discovery and validation datasets comprising the TARGET database, 289 (35.6%) patients were in the discovery set, 431 (53.0%) in the validation set, and 94 (11.5%) were in both. The distribution of race and ethnicity among children in the discovery vs validation set was similar (Pearson’s χ2 P = .71).
Table 1.
Characteristics of the study population
| White non-Hispanic (N=552) | Black (N=108) |
Hispanic (N=154) | P | |
|---|---|---|---|---|
| Male sex | 301 (54.5) | 53 (49.1) | 83 (53.9) | .58 |
| Age at diagnosis | 10.1 (3.7, 14.9) | 11.3 (3.7, 13.4) | 8.9 (2.6, 14.2) | .23 |
| Primary cytogenetic lesion | ||||
| KMT2Ar | 124 (22.5) | 25 (23.1) | 21 (13.6) | .05 |
| t(8;21) | 60 (10.9) | 23 (21.3) | 27 (17.5) | <.01 |
| inv(16) | 75 (14.3) | 11 (10.2) | 22 (15.4) | .46 |
| −7/-7q | 5 (0.9) | 2 (1.9) | 1 (0.6) | .59 |
| −5/-5q | 22 (4.0) | 9 (8.3) | 8 (5.2) | .15 |
| Normal | 139 (25.2) | 17 (15.7) | 28 (18.2) | .04 |
| Other | 133 (24.1) | 35 (32.4) | 47 (30.5) | .088 |
| Unknown | 27 (4.9) | 0 | 11 (7.1) | .024 |
| Prominent mutations | ||||
| FLT3 | 34 (6.2) | 7 (6.6) | 10 (6.5) | .98 |
| NPM1 | 41 (7.4) | 4 (3.7) | 12 (7.8) | .54 |
| CEBPA | 30 (5.4) | 7 (6.5) | 6 (3.9) | .67 |
| cKIT (exon 8) | 22 (4.0) | 7 (6.5) | 4 (2.6) | .11 |
| cKIT (exon 17) | 16 (2.9) | 0 | 6 (3.9) | .08 |
| Treatment protocol | .01 | |||
| AAML0531 | 465 (84.2) | 85 (78.7) | 138 (89.6) | |
| AAML03P1 | 52 (9.4) | 13 (12.0) | 16 (10.4) | |
| CCG-2961 | 35 (6.3) | 10 (9.3) | 0 | |
| WBC at diagnosis (median, IQR) |
31.1 (10.9, 86.7) | 34.2 (15.9, 96.9) | 33.6 (11.8, 90.8) | .34 |
| CNS (+) at diagnosis | 35 (6.3) | 12 (11.1) | 8 (5.2) | .14 |
| MRD at end of course 1 | .07 | |||
| Positive | 133 (24.1) | 33 (30.6) | 32 (20.8) | |
| Negative | 295 (53.4) | 48 (44.4) | 96 (62.3) | |
| Unknown | 124 (22.5) | 27 (25.0) | 26 (16.9) | |
| MRD at end of course 2 | <.01 | |||
| Positive | 66 (12.0) | 15 (13.9) | 21 (13.6) | |
| Negative | 304 (55.1) | 44 (40.7) | 99 (64.3) | |
| Unknown | 182 (33.0) | 49 (45.4) | 34 (22.1) | |
| HSCT received in CR1 | 88 (15.9) | 10 (9.3) | 23 (14.9) | .20 |
Categorical data are n (%); continuous data are median (interquartile range).
CNS, central nervous system; CR1, first complete remission; HSCT, hematopoietic stem cell transplant; MRD, minimal residual disease.
The median age at diagnosis was 10.1 years (interquartile range, 3.3-14.7). The median time under observation was 4.5 years (interquartile range, 1.4-6.0). Cytogenetic data were missing for 38 patients (4.7%); otherwise, data were complete.
AML cytogenetics and clinical features by race-ethnicity
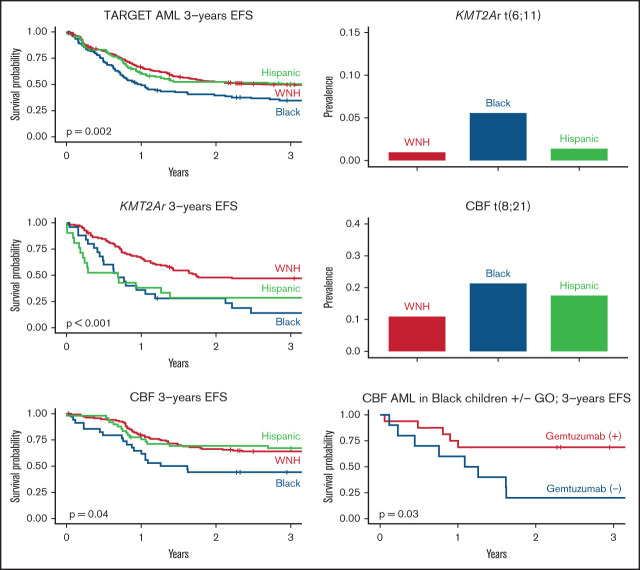
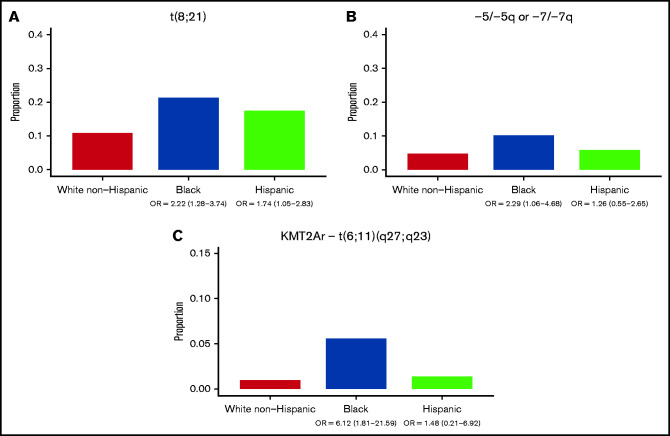
The prevalence of AML cytogenetic subtypes differed significantly by race-ethnicity. Relative to White non-Hispanic patients, both Black ([OR], 2.22; 95% CI, 1.28-3.74) and Hispanic patients (OR, 1.74; 95% CI, 1.05-2.83) were more likely to have t(8;21) AML (Figure 1A). Associations between inv(16) AML and race-ethnicity were not detected. 5q deletion/monosomy 5 or 7q deletion/monosomy 7 was more common among Black patients (Figure 1B; OR, 2.29; 95% CI, 1.06-4.68).
Figure 1.
Primary cytogenetic lesions by race-ethnicity. Data represent 525 White non-Hispanic, 143 Hispanic, and 108 Black patients with cytogenetic data available. (A) Proportion of patients with t(8;21) cytogenetics by race-ethnicity. (B) Proportion of patients with monosomy 5, 5q deletion, monosomy 7, or 7q deletion by race/ethnicity. (C) Proportion of patients with t(6;11)(q27;q23) by race-ethnicity.
KMT2Ar AML was less common among Hispanic patients relative to White non-Hispanic patients (OR, 0.54; 95% CI, 0.32-0.88). While KMT2Ar AML was not broadly associated with Black race, the specific poor prognosis KMT2Ar t(6;11)(q27;q23) was more common among Black patients (Figure 1C; OR, 6.12; 95% CI, 1.81-21.59). No racial-ethnic associations were detected among patients with KMT2A fusions t(9;11), t(10;11), or t(11;19).
Across the cohort, presenting phenotypic features of AML did not differ by race-ethnicity, including: age, WBC count, myeloblast percentage, central nervous system (CNS) disease, and presence of chloromas. When KMT2Ar was interrogated specifically, Black patients were older at diagnosis, presenting at a median (interquartile range) of 11.7 years (2.3-15.1) compared with 3.2 years (1.0-11.8) among other patients (P = .01). There were no differences in presenting features by race-ethnicity in CBF AML (supplemental Tables 1 and 2).
Outcomes by race-ethnicity; overall cohort
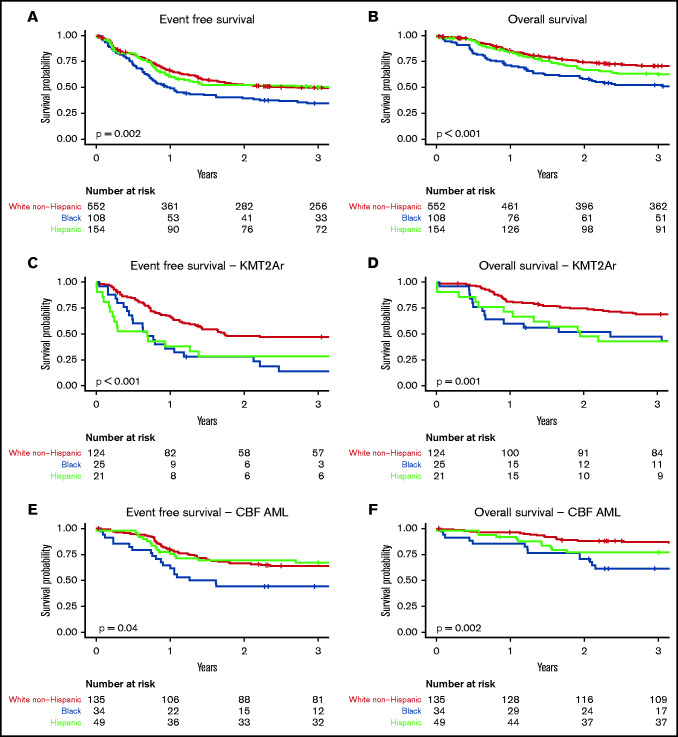
Survival differed by race-ethnicity across the entire cohort as well as within cytogenetic subgroups (Table 2). Black patients were at highest risk of events (hazard ratio [HR], 1.57; 95% CI, 1.21-2.04) with an EFS of 35% (95% CI, 27-45) compared with 50% (95% CI, 43-59) among Hispanic patients and 50% (95% CI, 46-54) among White non-Hispanic patients (Figure 2A-B). Similarly, risk of death was highest among Black patients (HR, 1.97; 95% CI, 1.47-2.65) with OS of 52% (95% CI, 43-63) compared with 63% (95% CI, 55-71) among Hispanic patients and 71% (95% CI, 67-75) among White non-Hispanic patients.
Table 2.
Cox proportional-hazards model of the association between race-ethnicity and 3-year survival among cytogenetic subgroups
| Race-ethnicity | Event-free survival HR (95% CI) |
Overall survival HR (95% CI) |
|---|---|---|
| All patients | ||
| White non-Hispanic | ref | ref |
| Black | 1.57 (1.21-2.04) | 1.97 (1.47-2.65) |
| Hispanic | 1.04 (0.81-1.34) | 1.22 (0.91-1.63) |
| KMT2Ar | ||
| White non-Hispanic | ref | ref |
| Black | 2.31 (1.41-3.79) | 2.54 (1.43-4.51) |
| Hispanic | 2.20 (1.27-3.80) | 2.07 (1.09-3.93) |
| t(8;21) or inv(16) | ||
| White non-Hispanic | ref | ref |
| Black | 1.93 (1.14-3.28) | 3.24 (1.60-6.57) |
| Hispanic | 0.99 (0.57-1.71) | 2.03 (1.00 – 4.10) |
Statistically significant results are bolded.
Figure 2.
Survival in patients with AML by race-ethnicity within the TARGET cohort. (A) EFS across entire TARGET cohort by race-ethnicity. (B) OS across entire TARGET cohort by race-ethnicity. (C) EFS among patients with KMT2Ar AML by race-ethnicity. (D) OS among patients with KMT2Ar AML by race-ethnicity. (E) EFS among patients with CBF AML by race-ethnicity. (F) OS among patients with CBF AML by race-ethnicity.
Neither induction failure nor relapse as the first event was associated with race-ethnicity. Death as the first event (rather than induction failure or relapse) was more likely among Black patients (OR, 2.83; 95% CI, 1.46-5.29). Early death, defined as death within 50 days of diagnosis, was also more common among Black patients (OR, 4.55; 95% CI, 1.44-13.96).
Proportionally fewer Black patients received stem cell transplants after first complete remission (10/108, 9.3%) compared with Hispanic (23/154, 14.9%) and White non-Hispanic (88/552, 15.9%) patients. The relative odds of Black patients receiving a transplant compared with White non-Hispanic patients approached but did not reach statistical significance (OR, 0.54; 95% CI, 0.25-1.03).
Outcomes by race-ethnicity; KMT2Ar AML
Across the entire cohort, KMT2Ar was associated with inferior EFS (HR, 1.30; 95% CI, 1.04-1.62), but not OS. Survival differences by race-ethnicity were prominent within this cytogenetic subset (Figure 2C-D). Black patients had the lowest EFS at 14% (95% CI, 5-39) compared with 29% in Hispanic patients (95% CI, 15-56) and 47% in White non-Hispanic patients (95% CI, 39-57). Both Black race (HR, 2.31; 95% CI, 1.41-3.79) and Hispanic ethnicity (HR, 2.20; 95% CI, 1.27-3.80) were associated with inferior EFS in patients with KMT2Ar AML.
OS among Black patients with KMT2Ar AML was 47% (95% CI, 31-72) compared with 43% in Hispanic patients (95% CI, 26-70) and 69% in White non-Hispanic patients (95% CI, 61-78). Both Black race (HR, 2.54; 95% CI, 1.43-4.51) and Hispanic ethnicity (HR, 2.07; 95% CI, 1.09-3.93) were associated with inferior OS.
In the KMT2Ar subgroup, induction failure was more common among Hispanic vs other patients (OR, 9.52; 95% CI, 2.58-36.88). Death was more likely to be the first event in Black patients (OR, 10.08; 95% CI, 2.30-52.36). Neither early death nor relapse were associated with race-ethnicity.
Outcomes by race-ethnicity; CBF AML
Patients with CBF AML had superior EFS (HR, 0.54; 95% CI, 0.42-0.68) and OS (HR, 0.41; 95% CI, 0.30-0.56) compared with non-CBF AML. Similar to KMT2Ar AML, survival differed by race-ethnicity. Black patients had the lowest EFS at 44% (95% CI, 30-64) and OS at 61% (95% CI, 47-80) (Figure 2E-F). Survival of Hispanic patients was intermediate with EFS of 67% (95% CI, 55-82) and OS of 77% (95% CI, 66-90). White non-Hispanic patients had an EFS of 64% (95% CI, 56-73) and OS of 87% (95% CI, 82-93). Black race was associated with inferior EFS (HR, 1.93; 95% CI, 1.14-3.28) and OS (HR, 3.24; 95% CI, 1.60-6.57). Neither EFS nor OS was significantly associated with Hispanic ethnicity, but lower OS among Hispanic patients was notably close (HR, 2.03; 95% CI, 1.00-4.10).
We also analyzed outcomes within the two cytogenetic subgroups of CBF AML. For those with inv(16), Black patients had the lowest EFS at 36% (95% CI, 17-79), Hispanic patients had the highest EFS at 73% (95% CI, 56-94), and White non-Hispanic patients had an EFS of 52% (95% CI, 41-64). Similarly, Black patients had an OS of 73% (95% CI, 51-100) compared with 86% (95% CI, 73-100) in Hispanics and 85% (95% CI, 77-94) in White non-Hispanic patients. Differences in survival within this subgroup were not statistically significant.
For patients with t(8;21) AML, EFS was lowest among Black patients at 48% (95% CI, 31-73) followed by Hispanic patients at 63% (95% CI, 47-84) and White non-Hispanic patients at 80% (95% CI, 70-91). Black race (HR, 3.15 95% CI, 1.44-6.92) was associated with inferior EFS. Similarly, OS was lowest among Black patients at 56% (95% CI, 39-81), followed by Hispanic patients at 70% (95% CI, 54-90) and White non-Hispanic patients at 90% (95% CI 82-98). Both Black race (HR, 4.43; 95% CI, 1.69-11.66) and Hispanic ethnicity (HR, 2.82; 95% CI, 1.02-7.80) were associated with inferior OS.
Within the CBF AML cohort, Black patients were more likely to have death as their first event (OR, 4.48; 95% CI, 1.18-17.11). Early death was also more common among Black patients (OR, 12.87; 95% CI, 1.59-265.05). There were no associations between race-ethnicity in relapse or induction failure.
Association of GO use with AML outcomes by race and ethnicity
Similar to the results from COG AAML0531, EFS was higher with GO; although within this cohort, the association between GO and survival did not reach statistical significance (HR, 0.84; 95% CI, 0.69-1.03). GO had no significant effect on OS (HR, 0.91; 95% CI, 0.71-1.16).
Given differences in survival by race-ethnicity, we investigated whether GO differentially affected survival within specific racial-ethnic groups in patients enrolled on AAML03P1 and AAML0531 (Table 3). Black patients had the greatest improvement in EFS (HR, 0.62; 95% CI, 0.38-1.03) and OS (HR, 0.57; 95% CI, 0.33-0.99) in the GO arm, although statistical significance was marginal in the case of EFS. There was little difference due to GO observed in Hispanic and White non-Hispanic patients.
Table 3.
Cox proportional hazards model of association between gemtuzumab and survival among racial-ethnic and cytogenetic subgroups
| Cytogenetic subgroup Race-ethnicity |
Event free survival HR (95% CI) |
Overall survival HR (95% CI) |
|---|---|---|
| All cytogenetic groups | ||
| All patients | 0.84 (0.69-1.03) | 0.91 (0.71-1.16) |
| Black | 0.62 (0.38-1.03) | 0.57 (0.33-0.99) |
| Hispanic | 0.99 (0.63-1.55) | 0.94 (0.56-1.57) |
| WNH | 0.85 (0.66-1.10) | 1.01 (0.74-1.39) |
| KMT2Ar AML | ||
| All patients | 0.64 (0.43-0.95) | 0.78 (0.48-1.27) |
| Black | 0.67 (0.24-1.86) | 0.36 (0.12-1.06) |
| Hispanic | 0.42 (0.15-1.18) | 0.84 (0.25-2.81) |
| WNH | 0.60 (0.36-0.98) | 0.79 (0.42-1.46) |
| Core binding factor AML | ||
| All patients | 0.79 (0.50-1.24) | 1.00 (0.53-1.87) |
| Black | 0.30 (0.10-0.92) | 0.44 (0.12-1.64) |
| Hispanic | 1.66 (0.63-4.35) | 1.19 (0.40-3.54) |
| WNH | 0.75 (0.42-1.35) | 1.22 (0.47-3.22) |
Statistically significant values are bolded.
WNH, White non-Hispanic
Similar to the cohort as a whole, patients with KMT2Ar AML had increased EFS (HR, 0.64; 95% CI, 0.43-0.95) but not OS (HR, 0.78; 95% CI, 0.48-1.27) when receiving GO. When broken down by race, GO showed a significant effect on EFS among White non-Hispanic patients only (HR, 0.60; 95% CI, 0.36-0.98). GO had no significant effect on OS among any race-ethnic group with KMT2Ar AML; however, Black patients notably had the greatest effect estimate of GO on OS (HR, 0.36; 95% CI, 0.12-1.06) albeit failing to reach statistical significance.
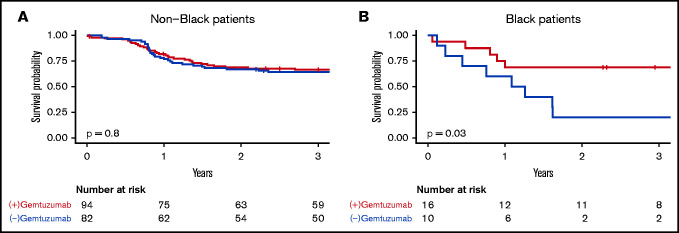
In CBF AML, no significant effects on EFS or OS were observed among all patients together but GO again showed the greatest effect on EFS among Black patients (HR, 0.30; 95% CI, 0.10-0.92) with no other racial-ethnic group showing significant effects (Figure 3). When survival outcomes by race-ethnicity were reexamined among only patients with CBF AML who received GO (n = 110), differences in EFS and OS by race-ethnicity seen previously were absent. EFS was 69% (95% CI, 49-96) among Black patients, 60% (95% CI, 44-83) among Hispanic patients, and 69% (95% CI, 59-81) among White non-Hispanic patients. OS was 75% (95% CI, 57-100) among Black patients, 76% (95% CI 61-95) among Hispanic patients, and 88% (95% CI, 81-96) among White non-Hispanic patients.
Figure 3.
EFS by receipt of gemtuzumab in Black vs non-Black patients with CBF AML. (A) Non-Black patients. (B) Black patients.
Given the known association between CD33 expression and response to GO, we examined mRNA sequencing data of patients from the TARGET database to determine if myeloblast CD33 mRNA expression at diagnosis differed by race-ethnicity.20 Data were available for 160 (29%) White non-Hispanic, 44 (29%) Hispanic, and 28 (26%) Black patients. CD33 expression did not differ significantly by race-ethnicity in the cohort as a whole (P = .67); however, compared with other patients, White non-Hispanic patients had higher CD33 expression within the CBF subgroup (RPKM = 22.1 vs 9.1, P < .01).
Sensitivity and mediation analysis
We observed that a higher proportion of Black patients were treated on the CCG-2961 protocol (Table 1), the oldest of the three protocols with the highest treatment-related mortality.10 In this dataset, treatment on CCG-2961 was associated with inferior EFS (HR, 1.69; 95% CI, 1.19-2.39) and OS (HR, 1.66; 95% CI, 1.09-2.53). Therefore, for each of the survival analyses described above, we conducted post hoc sensitivity analyses with and without patients treated on CCG-2961 in order to determine if poor prognosis among Black children was due to a higher likelihood to be treated on CCG-2961. Measured association of race-ethnicity with survival was clinically identical with and without the CCG-2961 patients, supporting the robustness of the original analysis (supplemental Table 3). Similar to the original analysis, Black race remained associated with lower EFS (HR, 1.61; 95% CI, 1.22-2.12) and OS (HR, 2.10, 95% CI, 1.54-2.87).
We performed a similar analysis with and without patients experiencing early death within 50 days of diagnosis given the association between Black race and early mortality. We found effect sizes to be similar but attenuated, with wider confidence intervals given the smaller sample size of the subanalysis (supplemental Table 4). Similar to the original analysis, Black race was associated with lower EFS (HR, 1.49; 95% CI, 1.14-1.95) and lower OS (HR, 1.86; 95% CI, 1.36-2.54) among those children surviving beyond 50 days from diagnosis (supplemental Table 4).
Given Black race was associated with the poor prognostic KMT2A translocation t(6;11)(q27;q23), and that t(6;11)(q27;q23) was associated with poor EFS (HR, 2.48; 95% CI, 1.4-4.4) and OS (HR, 2.89; 95% CI, 1.54-5.44), we performed a causal mediation analysis to determine the proportion of poor OS due to Black race mediated by t(6;11)(q27;q23) (supplemental Figure 1). The measured effect of Black race mediated by t(6;11)(q27;q23) was HRindirect = 1.04 (95% CI, 1.00-1.19), and the measured effect of Black race through all other pathways was HRdirect = 1.93 (95% CI, 1.87-2.10). In the analysis, the proportion of Black race’s effect on OS mediated by the effect of t(6;11)(q27;q23) was 6%.
Discussion
Inferior survival of pediatric AML among racial-ethnic minorities has been described for several decades.4,21,22 Socioeconomic status, early mortality, and accessibility to stem cell transplant are likely component causes of these disparities.2,4,6,21 To date, associations between race-ethnicity and genetic features of AML have not been investigated as they relate to outcome, potentially owing to the relatively recent understanding of the complex role of genetic features in pediatric AML biology and response to therapy.12
Using the TARGET database, we found that the genetic landscape of AML varies by race-ethnicity. Black patients were twice as likely to have high-risk genetic features −5/5q- or −7/7q- compared with non-Black patients. Black patients were also over five times as likely to have the KMT2A rearrangement t(6;11)(q27;q23), a lesion carrying an EFS and OS of less than 20%.23 These findings suggest that, while variables such as availability of transplant donors likely contribute to outcome disparities in pediatric AML, underlying biology predisposing patients to more severe disease phenotype may also contribute.
Outcome disparities by race-ethnicity were evident even among cytogenetic subgroups typically associated with favorable prognosis. Black patients were more likely to have the favorably prognostic t(8;21) AML, yet the 3-year OS among Black patients with t(8;21) was considerably lower than that of White non-Hispanic patients: 61% compared with 87%. Among patients with CBF AML generally, the 61% OS among Black patients was equivalent to the OS of patients without this favorable cytogenetic subtype within the broader cohort. Notably, survival in Black patients with CBF AML was more similar to outcomes in intermediate- or high-risk AML as a whole than to favorable-risk AML.9 These findings are similar to recent studies of adult AML where Black patients with favorable-risk NPM1-mutated FLT3-ITDlo/no disease had significantly inferior survival to White patients, although differences in outcomes specifically of CBF AML were not identified.24
Racial-ethnic disparities within groups with poorly prognostic lesions revealed dismal outcomes among Black and Hispanic patients, suggesting a potentially additive negative effect of race-ethnicity on survival among these subsets.23 EFS among Hispanic patients with KMT2Ar AML was 29% and only 14% among Black patients compared with 47% among White non-Hispanic patients.
Black patients were also more likely to have the KMT2A rearrangement t(6;11)(q27;q23), a cytogenetic lesion associated with older age at diagnosis and poor prognosis.23 Differential phenotypic features among Black and Hispanic patients such as older age at presentation, a finding in this study among Black patients with KMT2Ar AML, may therefore be driven in part by variation in the prevalence of specific AML cytogenetic lesions by race-ethnicity. Nonetheless, our data suggest that t(6;11)(q27;q23) AML appears to play only a partial role in the poor prognosis of Black patients relative to others, mediating <10% of the observed effect on survival.
In contrast to previous studies demonstrating higher WBC at presentation among Black patients, there was no difference in WBC at diagnosis in this cohort as a whole or within specific cytogenetic groups.25 As the TARGET database includes only patients enrolled in clinical trials, it is possible that higher acuity at presentation with an associated higher WBC is a deterrent from clinical trial enrollment. Therefore, Black patients with higher WBC at presentation were possibly underrepresented in this analysis.
Apart from survival alone, patterns of events contributing to poor survival differed by race-ethnicity. While both Black and Hispanic patients had unfavorable outcomes, their disease courses differed. For example, Hispanic patients with KMT2Ar AML were more likely to experience induction failure, whereas Black patients across the cohort were more likely to have early death within 50 days of treatment initiation relative to other patients. These data support previous studies by Dr. Winestone and colleagues showing higher AML mortality among Black patients soon after diagnosis.5,6 This study also shows that the association between Black race and higher mortality extended beyond the initial phase of treatment.
A relative lack of stem cell transplant donors may contribute to poor outcomes in minority patients.4,6 Black patients in this study were less likely to receive a hematopoietic stem cell transplant vs White non-Hispanic patients (OR, 0.54; 95% CI, 0.25-1.03), although the confidence interval was wide. In the case of CBF AML, transplant is no longer standard of care; thus, the persistence of disparities in CBF AML, particularly those of EFS, is not likely dependent on access to stem cell transplant.9,10 Ultimately, the TARGET AML dataset is insufficient to assess whether the lack of available transplant donors contributed to fewer transplants or to outcome disparities. Data identifying patients for whom transplant was indicated, such as the presence of minimal residual disease, was often unavailable.9,10
One of the most striking findings in this study is the preferential improvement with GO therapy among Black patients with CBF AML (Figure 3). The COG AAML0531 trial reported modest improvements in EFS with decreased relapse risk among patients who received GO, with the greatest effects in low- and intermediate-risk disease.9 While the effect size of GO on EFS within the current study was small, Black patients with CBF AML in this cohort had substantial benefit from the addition of GO to therapy compared with White non-Hispanic or Hispanic patients. These findings suggest biologic differences may exist that influence GO responsiveness within certain AML subtypes or that other factors contributing to the care of patients receiving an investigational agent may be at play.
While hypothesis-generating, these data should be interpreted cautiously. AAML0531 and AAML03P1 were not designed or powered to identify the modification of GO’s effect on survival by race-ethnicity, particularly within individual cytogenetic subtypes. Therefore, only large effect sizes within common cytogenetic subclasses such as CBF AML could be detected within this cohort. Due to small samples sizes, the finding of no association between GO and race-ethnicity within other subgroups is inconclusive.
Several studies have investigated biologic determinants that alter the effectiveness of GO.20,26,27 Patients with higher CD33 expression, the most important identified predictor of GO efficacy, significantly benefit from GO therapy regardless of risk group, whereas those with low CD33 expression do not benefit from the addition of GO to therapy.20 Two single-nucleotide polymorphisms in genes ABCB1 and CD33 also impact response to GO. The CD33 SNP rs12459419 increases expression of a CD33 isoform with decreased binding of GO at the cell surface, and the ABCB1 SNP rs1045642 decreases the efflux of calicheamicin, leading to accumulation of the active drug within the cell and increased cell death.26,27 Functional variation in these genes and/or differing CD33 expression profiles among different racial-ethnic groups may contribute to differences in GO-related outcomes seen in this study. Further studies directed at pharmacogenomics as a component cause of differences in outcome by race-ethnicity among patients with AML may be beneficial.
Strengths and limitations
This study is among the largest studies of racial-ethnic disparities in pediatric AML, and its conclusions are strengthened by high-quality, prospectively collected data from three large randomized clinical trials.
Conclusions drawn from the TARGET AML dataset are limited by important selection biases. The dataset is comprised of a discovery dataset combined with a validation dataset.12 While the validation dataset was a random selection of patients from AAML0531, the discovery set was disproportionately enriched for patients experiencing a relapse. Therefore, this study is biased toward patients with inferior outcomes. While race-ethnicity was distributed similarly between the discovery and validation datasets, the effect sizes and confidence intervals reported here may differ in a general population of patients with AML. This study only included patients enrolled in clinical trials, a population known to underrepresent minority populations.8
This study was also limited by small numbers of patients within certain cytogenetic and racial-ethnic subgroups such that extensive multivariable analyses could not be conducted, and confidence intervals of some effect sizes were very wide. A necessary follow-up to this study will be to replicate these findings in a larger, more generalizable pediatric AML cohort for the reasons mentioned above, as well as because many newly defined genetic risk factors are rare in the overall AML population.
Finally, we recognize that self-reported race-ethnicity is an imprecise tool for categorizing patients by genetic and non-genetic risk factors. Race-ethnicity is better understood as a framework to represent socioeconomic, cultural, environmental, and genetic-ancestral similarities among broad populations. There is considerable genetic admixture among patients self-identified as White non-Hispanic, Hispanic, and Black; therefore, this study is subject to these limitations.28 Future studies should leverage genetic as well as self-reported ancestry as tools to reveal determinants of AML outcomes. Nonetheless, reliance on broad, self-reported racial-ethnic groups used in this and other studies has revealed a multitude of important disparities across pediatric oncology and beyond.29
Conclusion
The genetic landscape, phenotype, and survival outcomes of pediatric AML vary by race-ethnicity. Racial-ethnic minorities are more likely to have unfavorable cytogenetic features than White non-Hispanic peers in pediatric AML and experience inferior outcomes regardless of genetic disease features. In addition, racial-ethnic differences may also modify the benefit of GO therapy, with the greatest benefit in this analysis observed in Black patients with CBF AML. Although Black and Hispanic patients are more likely to have certain unfavorable genetic features, Black children with AML experience inferior survival even in the presence of favorable cytogenetic features. As such, further investigation into the root cause(s) of the unacceptable racial-ethnic disparities in childhood AML outcomes must be identified and addressed.
Potential causes of these disparities begin at the level of the patient and the biologic features of their leukemia, expand outward to the health care systems in which they are treated, and ultimately reach into the society in which they live. We should continue to study the epidemiology of high-risk AML cytogenetics, epigenetics, and pharmacogenomics of AML therapy as potential causes of racial-ethnic disparities in childhood AML.
However, we must also systematically test the hypothesis that structural inequities disproportionately affecting racial-ethnic minorities contribute to these outcome disparities. All children treated for AML in high-income countries receive essentially identical anti-neoplastic treatment regimens with putatively standardized approaches to supportive care. Therefore, every aspect of clinical care, from that received before and leading up to diagnosis, during inpatient and outpatient therapy, and through to surveillance in long-term survivorships, should be rigorously studied from the perspective of the racial-ethnic outcome disparities described in this and similar studies.30-32
Supplementary Material
Acknowledgments
The results published here are in whole derived from data generated by the Therapeutically Applicable Research to Generate Effective Treatments (https://ocg.cancer.gov/programs/target) initiative phs000218. We would like to thank C. Gillespie, S.M. Blaney, and S.L. Berg for their critical review of this manuscript. This project was funded in part by the NIH CA183252-07/10 GHCHD supplement.
This project was funded in part by the NIH CA183252-07/10 GHCHD supplement.
Authorship
Contributions: R.E.R. was responsible for study concept; R.E.R., S.E.C., and C.L.M. were responsible for study design; S.E.C. and C.L.M. performed data extraction; C.L.M. conducted the statistical analysis and created figures; S.E.C., C.L.M., and R.G. wrote the manuscript; M.E.S., J.L., and R.E.R. supervised the study; and S.E.C., C.L.M., R.G., J.L., M.E.S., and R.E.R. edited the manuscript and agreed on the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rachel E. Rau, Baylor College of Medicine, 1 Baylor Plaza, Houston, TX 77030, e-mail: rachel.rau@bcm.edu.
References
- 1.Winestone LE, Aplenc R.. Disparities in survival and health outcomes in childhood leukemia. Curr Hematol Malig Rep. 2019;14(3):179-186. [DOI] [PubMed] [Google Scholar]
- 2.Winestone LE, Getz KD, Miller TP, et al. The role of acuity of illness at presentation in early mortality in black children with acute myeloid leukemia. Am J Hematol. 2016;92(2):141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gramatges MM, Deshpande A, Lupo PJ, et al. Ethnic disparities relative to disease features and outcomes in children with acute myeloid leukemia. Pediatr Blood Cancer. 2017;64(9):e26487. [DOI] [PubMed] [Google Scholar]
- 4.Aplenc R, Alonzo TA, Gerbing RB, et al. ; Children’s Oncology Group . Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2006;108(1):74-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Newton JG, Getz KD, et al. Comparable on-therapy mortality and supportive care requirements in Black and White patients following initial induction for pediatric acute myeloid leukemia. Pediatr Blood Cancer. 2018;66(4):e27583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winestone LE, Getz KD, Miller TP, et al. Complications preceding early deaths in Black and White children with acute myeloid leukemia. Pediatr Blood Cancer. 2017;64(12):e26712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher BT, Zaoutis TE, Leckerman KH, Localio R, Aplenc R.. Risk factors for renal failure in pediatric patients with acute myeloid leukemia: a retrospective cohort study. Pediatr Blood Cancer. 2010;55(4):655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winestone LE, Getz KD, Rao P, et al. Disparities in pediatric acute myeloid leukemia (AML) clinical trial enrollment. Leuk Lymphoma. 2019;60(9):2190-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32(27):3021-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children’s oncology group. Blood. 2008;111(3):1044-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubnitz JE, Lensing S, Razzouk BI, Pounds S, Pui CH, Ribeiro RC.. Effect of race on outcome of white and black children with acute myeloid leukemia: the St. Jude experience. Pediatr Blood Cancer. 2006;48(1):10-15. [DOI] [PubMed] [Google Scholar]
- 12.Bolouri H, Farrar JE, Triche T Jr, et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med. 2017;24(1):103-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Health NI of. TARGET Data Matrix. 2020.
- 14.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia. Cancer. 2011;118(3):761-769. [DOI] [PubMed] [Google Scholar]
- 15.VanderWeele TJ. Causal mediation analysis with survival data. Epidemiology. 2011;22(4):582-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange T, Hansen KW, Sørensen R, Galatius S.. Applied mediation analyses: a review and tutorial. Epidemiol Health. 2017;39:e2017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therneau T. A Package for Survival Analysis in R. 2020.
- 18.Team RC. R: A language and environment for statistical computing. n.d.
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative .The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4): 344-349. [DOI] [PubMed] [Google Scholar]
- 20.Pollard JA, Loken M, Gerbing RB, et al. CD33 expression and its association with gemtuzumab ozogamicin response: results from the Randomized Phase III Children’s Oncology Group Trial AAML0531. J Clin Oncol. 2016;34(7):747-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pui C-H, Pei D, Pappo AS, et al. Treatment outcomes in black and white children with cancer: results from the SEER database and St Jude Children’s Research Hospital, 1992 through 2007. J Clin Oncol. 2012;30(16):2005-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linabery AM, Ross JA.. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975-1999. Cancer. 2008;113(9):2575-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balgobind BV, Raimondi SC, Harbott J, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114(12):2489-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatnagar B, Kohlschmidt J, Mrózek K, et al. Poor survival and differential impact of genetic features of black patients with acute myeloid leukemia. Cancer Discov. 2020;11(3):626-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winestone LE, Getz KD, Li Y, et al. Increased disease burden among black children compared to white children with newly diagnosed acute myeloid leukemia. Blood. 2018;132(suppl 1):369.29895666 [Google Scholar]
- 26.Rafiee R, Chauhan L, Alonzo TA, et al. ABCB1 SNP predicts outcome in patients with acute myeloid leukemia treated with Gemtuzumab ozogamicin: a report from Children’s Oncology Group AAML0531 Trial. Blood Cancer J. 2019;9(6):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamba JK, Chauhan L, Shin M, et al. CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo acute myeloid leukemia: report from Randomized Phase III Children’s Oncology Group Trial AAML0531. J Clin Oncol. 2017;35(23):2674-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galanter JM, Fernandez-Lopez JC, Gignoux CR, et al. ; LACE Consortium . Development of a panel of genome-wide ancestry informative markers to study admixture throughout the Americas. PLoS Genet. 2012;8(3):e1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheurer ME, Lupo PJ, Schüz J, et al. An overview of disparities in childhood cancer: report on the Inaugural Symposium on Childhood Cancer Health Disparities, Houston, Texas, 2016. Pediatr Hematol Oncol. 2018;35(2):95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogedegbe G. Responsibility of edical journals in addressing racism in health care. JAMA Netw Open. 2020;3(8):e2016531. [DOI] [PubMed] [Google Scholar]
- 31.Beck AF, Edwards EM, Horbar JD, Howell EA, McCormick MC, Pursley DM.. The color of health: how racism, segregation, and inequality affect the health and well-being of preterm infants and their families. Pediatr Res. 2020;87(2):227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C, Zhang C, Wang Q, Li Z, Lin J, Wang H.. Differences in stage of cancer at diagnosis, treatment, and survival by race and ethnicity among leading cancer types. JAMA Netw Open. 2019;3(4):e202950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.