Key points
Description of 2 cases with 11q aberrations including a cytogenetic double-hit constellation and an HIV+ case.
Evidence that 11q aberrations can occur in high-grade B-cell lymphoma that do not necessarily resemble typical BLL.
Abstract
The recent characterization of a group of non-MYC rearranged aggressive B-cell lymphomas, resembling Burkitt lymphoma (BL), characteristically harboring a telomeric 11q loss or combined 11q proximal gains/loss pattern has led to the introduction of the provisional entity of Burkitt-like lymphoma with 11q aberration (BLL-11q). Prompted by the discovery of a telomeric 11q loss in an HIV+ high-grade B-cell lymphoma patient, we investigated an extended cohort of aggressive B-cell lymphomas, enriched for cases with histopathological features intermediate between DLBCL and BL, including double- and triple-hit lymphomas (n = 47), for 11q loss/combined 11q proximal gains/loss pattern by fluorescence in situ hybridization. We provide first evidence that 11q aberrations can be found in both BLL in the context of an underlying HIV infection as well as in high-grade B-cell lymphomas with MYC, BCL2, and/or BCL6 rearrangements. We therefore propose that the clinicopathological spectrum of malignancies carrying this aberration may be broader than previously assumed.
Introduction
MYC-rearrangements by the t(8;14)(q24;q32) involving the immunoglobulin heavy-chain gene or its variants pose the genetic hallmark in Burkitt lymphoma (BL).1 Recently, a group of aggressive B-cell lymphomas, resembling BL histomorphologically and transcriptomically but lacking MYC-rearrangements, were found to feature a 11q-telomeric loss/11q-proximal gains/loss pattern (Burkitt-like lymphoma with 11q aberration [BLL-11q]). As a result, this provisional entity among aggressive lymphomas has been added to the World Health Organization (WHO) classification.2,3 Although BLL-11q cases have been observed in posttransplant/immunocompromised patients as well as in selected cases with concurrent MYC-rearrangements, there are to date no reports in HIV+ or double-hit (DH) lymphoma patients.3-5
The identification of an 11q aberration in a biopsy from an HIV+ patient prompted us to investigate an extensive cohort of aggressive B-cell lymphomas, focusing on cases with histopathological features intermediate between diffuse large B-cell lymphoma (DLBCL) and BL, including DH and triple-hit (TH) lymphomas, thereby encompassing entities closely related to BLL-11q. We provide first evidence that 11q aberrations can be found in both BLL in the context of an HIV infection and high-grade B-cell lymphomas (HGBLs) with MYC, BCL2, and/or BCL6 rearrangements. These observations indicate a wider spectrum than previously assumed.
Methods
This retrospective study was approved by the ethics committee of the University of Lübeck (reference no. 18-356) and conducted in accordance with the Declaration of Helsinki. Patients had given written informed consent regarding routine diagnostic and academic assessment of their biopsy specimen at the Reference Center for Hematopathology and transfer of their clinical data. We reviewed our institutional databases (Hematopathology Lübeck and Department for Hematology and Oncology UKSH Lübeck) to identify patients with HGBL and data on MYC, BCL2, and/or BCL6 rearrangements. Hereby, we identified 47 patients with HGBL with MYC, BCL2, and/or BCL6 rearrangements (n = 29) or triple wild-type DLBCL/HGBL (histomorphologically exhibiting BL-like or blastoid features that would have been categorized as intermediate between BL and DLBCL in the previous edition of the WHO classification; n = 18) with material available for fluorescence in situ hybridization (FISH) for 11q gains/losses. Clinicopathological features of the cohort are summarized in Table 1. Successful cytogenetic analyses/FISH studies were conducted in 47 of 55 cases as outlined in the supplemental Methods. The Mitelman database has been reviewed in order to identify aberrations on chromosome 11 in aggressive B-cell lymphomas (supplemental Methods; supplemental Table 1). All cases were studied for aberrations in MYC, BCL2, and BCL6 by FISH. In selected cases, whenever fresh biopsy material was available, additional conventional cytogenetic analysis was performed, which recurrently (including case 1) revealed a complex karyotype, as was expected for a cohort of aggressive B-cell lymphomas enriched for DH and TH cases.6 In total, conventional cytogenetics were available in 17 cases (HGBL-DH/TH = 13; triple wild-type DLBCL/HGBL = 4) and revealed a complex karyotype in 8 of 17 cases. One of these cytogenetically complex cases concomitantly harbored an 11q aberration.
Table 1.
Baseline clinicopathological characteristics in patients with aggressive B-cell lymphomas included in the current study
| Characteristics | 11q aberration (n = 4) |
HGBL-DH/TH (n = 28)* |
Triple wild-type DLBCL/HGBL† (n = 15) |
|---|---|---|---|
| Age, y (median + range) | 52.5 (34-72) | 69.5 (35-89) | 64 (18 − 87) |
| Sex (%) | |||
| Female | — | 16 (57.1) | 7 (46.7) |
| Male | 4 (100.0) | 12 (42.9) | 8 (53.3) |
| R-IPI (%) | |||
| 0 | — | 1 (4.5) | 1 (6.7) |
| 1-2 | 1 (25.0) | 9 (40.9) | 8 (53.3) |
| >2 | 3 (75.0) | 12 (54.5) | 6 (40.0) |
| Stage (Ann Arbor) (%) | |||
| I | 1 (25.0) | 5 (22.7) | 1 (6.7) |
| II | 1 (25.0) | 5 (22.7) | 7 (46.7) |
| III | 1 (25.0) | 3 (13.6) | 3 (20.0) |
| IV | 1 (25.0) | 9 (40.9) | 4 (26.7) |
| B-symptoms (%) | |||
| Yes | 2 (50.0) | 9 (40.9) | 6 (40.0) |
| No | 2 (50.0) | 13 (59.1) | 9 (60.0) |
| Immunohistochemistry (%) | |||
| Ki-67 | 93% (90-97) | 85 (40-100) | 85% (70-95) |
| CD10 | 3 (75.0) | 19 (67.9) | 1 (6.7) |
| BCL2 | 1 (25.0) | 18 (64.3) | — |
| GCB | 3 (75.0) | 19 (67.9) | 1 (6.7) |
| Non-GCB | 1 (25.0) | 3 (10.7) | 14 (93.3) |
| Cytogenetics (%) | |||
| MYC | 1 (25.0) | 28 (100.0) | — |
| BCL2 | 2 (50.0) | 18 (64.3) | — |
| BCL6 | — | 14 (50.0) | — |
| 11q aberration | 4 (100.0) | — | — |
| Tetrasomy 11 | — | 1 (3.6) | — |
| Trisomy 11 | — | 1 (3.6) | 4 (26.7) |
| Partial trisomy 11 | — | — | 1 (6.7) |
| Monosomy 11 | — | 1 (3.6) | |
| Extranodal sites (%) | |||
| 0 | 1 (25.0) | 6 (27.3) | 4 (26.7) |
| 1-2 | 3 (75.0) | 16 (72.7) | 11 (73.3) |
| ECOG PS | |||
| 0-1 | 1 (25.0) | 11 (50.0) | 10 (66.7) |
| ≥2 | 3 (75.0) | 11 (50.0) | 5 (33.3) |
| LDH (%) | |||
| Normal | 2 (50.0) | 6 (27.3) | 6 (40.0) |
| Elevated | 2 (50.0) | 16 (72.7) | 9 (60.0) |
| CNS involvement at diagnosis (%) | |||
| Yes | 1 (25.0) | 1 (4.5) | — |
| No | 3 (75.0) | 21 (95.5) | 15 (100.0) |
| Frontline therapy regimen (%) | |||
| CHOP-like | 3 (75.0) | 14 (63.6) | 14 (93.3) |
| R-based | 4 (100.0) | 18 (81.8) | 14 (93.3) |
| Others | — | 3 (13.6) | — |
| Refusal of treatment | — | 1 (4.5) | 1 (6.7) |
| Frontline therapy response rates (%) | |||
| CR | 2 (50.0) | 7 (25.0) | 8 (53.3) |
| PR | 1 (25.0) | 8 (28.6) | 5 (33.3) |
| SD | — | 2 (7.1) | — |
| PD | 1 (25.0) | 5 (17.9) | — |
CHOP, cyclophosphamide/hydroxydaunoribicin/vincristine/prednisolone; CNS, central nervous system; CR, complete remission; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B cell; LDH, lactate dehydrogenase; PPD, progressive disease; PR, partial remission; PS, performance status; R, rituximab; R-IPI, revised International Prognostic Score; SD, stable disease; tnDLBCL (NOS), triple-negative diffuse large B-cell lymphoma (not otherwise specified); VGPR, very good partial remission.
Comprehensive clinical follow-up was available in n = 22 HGBL-DH/TH patients.
Histomorphologically exhibiting BL-like or blastoid features.
Results and discussion
In our cohort (n = 47), we identified 4 new cases harboring 11q aberrations (supplemental Table 2). As expected, we found 2 Epstein-Barr virus (EBV)− cases of BLL-11q among our triple wild-type DLBCL/HGBL group, negative for MYC by immunohistochemistry. These were originally diagnosed prior to the introduction of the current WHO classification as well as the introduction of BLL-11q and displayed the canonical 11q gain/loss constellation with 3 hybridization signals in the 11q23.2∼q23.3 region, 1 signal in the 11q24.3, and a normal centromere signal constellation. Of note and slightly irregular, one of these cases was CD10− and of non-GCB type according to the Hans classifier. In all 4 cases, 11q aberrations were identified in ≥80% of tumor cells by FISH. Cytogenetic findings are depicted in Table 1 and supplemental Tables 3.1 and 3.2. In addition, 4 cases of aggressive B-cell lymphoma presented trisomy 11. Our analysis of the Mitelman database further revealed aberrations on chromosome 11, predominantly trisomies followed by whole-chromosomal deletions, to be a recurrent feature among different B-cell lymphoma entities. These observations require careful interpretation, as many cases were diagnosed prior to current WHO criteria, rendering the specific diagnosis questionable. Moreover, none of the described alterations correspond to the typical 11q gain/loss pattern (requiring array comparative genomic hybridization [aCGH] or target FISH for detection), which would have escaped conventional cytogenetic analysis. Intriguingly, we identified 11q aberrations in 2 additional unusual cases that we present here in detail. Both cases met neither established WHO criteria nor the recently proposed diagnostic approach by Horn et al13 for BLL-11q with strong BCL2 expression by immunohistochemistry (both cases), pleomorphic, plasmacytoid morphology (case 1), and cytogenetic DH constellation with rearrangements affecting MYC and BCL2 (case 2).
Case 1
A 34-year-old patient with pancytopenia, suppression of CD4+ T cells, rapidly progressive lymphadenopathy, suspected hepatic involvement, elevated LDH, and inflammatory markers presented to our department. Cerebrospinal fluid showed infiltration of lymphoid blasts with rounded nuclei, finely clumped chromatin and basophilic as well as distinctly vacuolated cytoplasm (L3 cytomorphology according to FAB classification). An HIV1 infection was newly diagnosed.
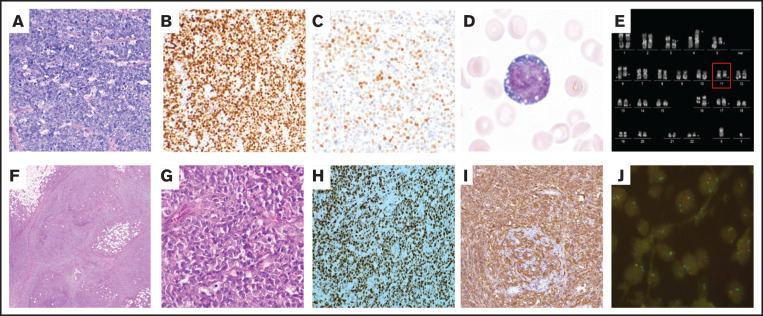
Histopathological LN examination revealed dense, sheetlike infiltrates of lymphoid blasts with a starry-sky pattern and extended necrosis not unlike BL. The infiltrates showed a mature germinal-center B-cell immunophenotype (CD20+, CD79+, CD10+, TdT−) with high proliferation up to 100% and negativity for EBV (Epstein-Barr virus-encoded RNAs [EBER]). Pleomorphic and partially plasmacytoid cytology and strong BCL2 expression were atypical for classic BL. In the LN biopsy, R-banding revealed a complex aberrant karyotype and 4 subclones with, in particular, a deletion in 11q (Figure 1E). Presence of MYC-, IGH-, BCL2-, BCL6-rearrangements or a cryptic MYC-IGH fusion were excluded by FISH, which further confirmed a sole 11q24.3-qter deletion without 11q proximal gain. Although an alternative/cryptic MYC-rearrangement cannot be completely excluded, additional FISH analyses for alternative far telomeric and centromeric MYC-breakpoints and minimal expression of MYC by immunohistochemistry argue in favor of wild-type MYC status (Figure 1C).7 The possibility of false negative immunohistochemistry for MYC, especially in DH lymphomas, is nevertheless acknowledged.8
Figure 1.
Representative images of histopathological and (molecular) cytogenetic findings of case 1 and case 2. Case 1: Histopathological lymph node (LN) examination revealed dense, sheetlike infiltrates of lymphoid blasts presenting with a starry-sky pattern and extended areas of necrosis not unlike BL (A; Giemsa stain, original magnification ×20). The infiltrates showed a mature B-cell immunophenotype (CD20+, CD79+, CD10+, BCL2+, Tdt−) with high proliferative activity (B; Ki-67, 70%-97%; original magnification ×20) and minimal reactivity for MYC by immunohistochemistry, in keeping with MYC wild-type status by FISH and rendering an oversight of a cryptic yet functionally relevant MYC rearrangement relatively unlikely (C; original magnification ×20). Bone marrow smear showed significant infiltration of lymphoid blasts with L3 cytomorphology (D; original magnification ×100). R-banded chromosomes showed a complex aberrant karyotype with 4 subclones (E): 46,XY,t(3;16)(p14;q23)?c,t(4;5)(p16;q21), add(6)(q22),add(11)(q23∼24), add(13)(q14),add(22)(q13)[5]/46,sl,–add(6)(q22),add(6)(q27)[4]/46,sdl1,del(6)(q13q14), –add(13)(q14),t(13;17)(q31;q24)[8]/46,sdl2, del(2)(q11.2q24),t(7;17)(p11;q21)[4]/47,sdl2,+der(5)t(4;5)[2].nuc ish(FOXP1x2)[100],(far-cenMYCx2)[100],(cenMYCx2)[100],(MYCx2) [100],(MYCfar-telx2)[100],(D11Z1, ATM,FDX)x2[100],(D11Z1x2,RP11-414G21x2, ETS1x1)[83/100],(KMT2Ax2)[100],(IGHx2)[100],(P53,MPO)x2[100]. Case 2: Excision biopsy revealed compact infiltrates of an aggressive lymphoma, morphologically and immunophenotypically resembling DLBCL with elevated proliferative activity and segmental starry-sky pattern (F-G; hematoxylin and eosin stain, original magnification ×10; 40×) alongside a high proliferative activity (H; Ki-67, 90%-95%; original magnification ×20) and GCB-like immunophenotype; CD20+ (I; original magnification ×20), CD79+, CD10+, BCL2+, Tdt−. Cytogenetic analysis exhibited rearrangements affecting both MYC and BCL2 genes. FISH image at diagnosis with a custom assay with probes RP11-414G21 (labeled in spectrum green), RP11-629A20 (spectrum orange), and CEP11 for centromere of chromosome 11 (spectrum aqua; Abbott-Vysis, Abbott Park, IL) shows the canonical signal constellation for 11q aberration cases: gain of 11q23.2∼q23.3 evidenced by 3 green signals, loss of 11q24.3 evidenced by 1 red signal, and 2 blue signals of the control probe for the centromere region of chromosome 11 per cell. The 11q aberration pattern was present in 80 of 100 malignant cells, indicating a primary genomic lesion (J; ×63).
Because of CNS involvement, we initiated high-dose methotrexate, cytarabine, thiotepa, and rituximab and highly active antiretroviral therapy while planning for subsequent crossover into the short course SC-EPOCH-RR (etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin, and double dose of rituximab) regimen.9,10 Following 2 courses of methotrexate, cytarabine, thiotepa, and rituximab, the patient developed prolonged neutropenia, pneumonia, delirium, and sepsis, requiring invasive mechanical ventilation and vasopressors. Chemotherapy was therefore suspended. Subsequently, cerebrospinal fluid again revealed meningeosis, and persistent pancytopenia was found to be secondary to near total marrow infiltration. Shortly thereafter, the patient succumbed to septic shock and progressive lymphoma.
Case 2
A 73-year-old male presented with cervical lymphadenopathy, elevated LDH, and impaired performance status (Eastern Cooperative Oncology Group 2) because of comorbidities. Excision biopsy revealed compact infiltrates of an aggressive lymphoma, morphologically and immunophenotypically resembling DLBCL/HGBL (elevated proliferative activity and segmental starry-sky pattern alongside a germinal-center B-cell–like immunophenotype and EBV-negativity [EBER]; CD20+, CD79+, CD10+, BCL2+, Tdt−). FISH revealed rearrangements affecting MYC and BCL2 genes. FISH for the detection of 11q aberration displayed the canonical 11q gain/loss signal constellation as demonstrated in the previous 2 cases (Figure 1J).
Despite advanced age and comorbidities, the patient received cytoreductive treatment with 6 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone), which was well tolerated, leading to a rapid complete response. The patient has been in complete remission for 50 months, which constitutes an unexpectedly encouraging outcome.11,12
BLL-11q reflects a provisional entity of aggressive B-cell lymphoma with a characteristic pattern of 11q imbalance, lack of MYC-rearrangements, Burkitt-like histopathology, and a mutational pattern, distinct from BL.3,4 To date, the putative oncogenic drivers located within the described 11q abnormalities, however, remain speculative.
In addition to 2 new cases of BLL-11q with a typical immunophenotypic profile, expectedly identified within our group of DLBCL/HGBL, we present 2 unusual cases of aggressive lymphoma harboring 11q aberration patterns, which challenge the currently established spectrum of aggressive B-cell lymphomas with an 11q aberration pattern twofold.2,13,14
First, we argue that the clinicopathological presentation among these cases appears more heterogeneous than previously appreciated, now encompassing both HIV-associated cases as well as HGBL-DH/TH. This is, however, basically in keeping with earlier studies questioning the concept of 11q alterations being BLL-11q exclusive, as these aberrations, including gain/loss patterns, are recurrently encountered in MYC+ lymphomas. In their pivotal study, Salaverria et al established the incidence of 11q gain/loss patterns in a group of 514 samples from the MMML project with available aCGH data to be 0.7% in DLBCL (2/267) and 1.6% in IG-MYC molecularly defined Burkitt lymphoma (mBL) (1/63).2,15,16 Moreover, the latter study alongside a recent publication by Gonzalez-Farre et al report on cases with isolated terminal 11q deletion.4 Further studies are needed to refine clinicopathological criteria for this provisional entity. Our findings, however, support the recently proposed diagnostic screening for 11q aberrations in MYC− HGBL with features of BL.13
Second, the isolated 11q24.3-qter deletion rather than the more common gain/loss pattern observed in the otherwise prototypic BLL-11q case 1 (beyond the underlying HIV-infection) further accentuates the need for a definitive minimal cytogenetic definition potentially narrowed down to cases with isolated 11q deletion. These were included in a recent study on the genomic landscape of BLL-11q and exhibited no distinct genomic or clinical features.3 Moreover, the alleged 11q driver genes (ETS1, NFRKB) map to this region. The possibility that this definition may be too permeable as it potentially neglects the oncogenic role of gains affecting 11q is, however, acknowledged, and the recurrent encounter of 11q deletions in aggressive B-cell lymphomas, especially those without Burkitt-like features, underscores the need for caution when including aggressive B-cell lymphomas with an isolated 11q deletion into the BLL-11q category.
In light of an integrative interpretation of the Mitelman database entries regarding 11q aberrations in B-cell neoplasms, revealing predominantly trisomies followed by whole chromosomal deletions, to be a recurrent feature among different B-cell lymphoma entities, previous reports on the incidence of typical gain/loss 11q aberrations in DLBCL and IG-MYC mBL as well as our current observations, we acknowledge the fact that our findings might merely represent uncommon and coincidental varieties and do not necessarily challenge the independence of the BLL-11q entity. However, as case 2, which presented with the typical 11q gain/loss pattern and not only an isolated 11q deletion, should preferably remain within the HGBL-DH/TH category according to the current WHO classification, we still argue that our observations expand on the previously described spectrum of B-cell lymphomas with typical 11q gain/loss patterns and isolated 11q deletions (encompassing ETS1 and NFRKB) in the histopathologically atypical case 1 and warrant further critical evaluation regarding the independence of the BLL-11q entity. Given the oftentimes complex karyotypes of HGBL-DH/TH, a random 11q deletion would appear plausible, whereas the early clonal (≥80% tumor cells showed the aberrations by FISH) and incidental acquisition of an 11q gain/loss pattern appears far less probable.6 Current observations from conventional cytogenetics support the assumption that 11q aberrations are not a coincidental finding in the context of complex aberrant karyotypes in aggressive B-cell lymphomas.
Furthermore, previous efforts strongly emphasized the sizable overlap between the group of HGBL-DH/TH and Burkitt(-like) lymphoma. From our own institutional experience, performing additional FISH testing for BCL2 and BCL6 in 99 cases with Burkitt(-like) morphology and a known MYC rearrangement, we gather a frequency of 15% HGBL-DH/TH, which is in keeping with previous reports by Dave et al and others.17,18 Of further interest, there is scarce evidence from 2 studies analyzing cytogenetics of HIV-related HGBL illustrating that 11q alterations, yet predominantly isolated gains, were detectable by aCGH in a minor subset of patients.19,20
Gene-expression studies suggest that BLL-11q is germinal center–derived and mutationally bears closer resemblance to HGBL/DLBCL than to BL.4
From a clinical perspective, the course of BLL-11q has only been systematically reported in pediatric patients, where it was shown to compare favorably to other aggressive lymphomas.21 In contrast, Sevilla et al reported MYC-negative BLL, prior to the discovery of the 11q gain/loss pattern, to harbor an inferior outcome, compared with MYC+ BL.22 The aggressive course of disease in case 1 allows room for speculation as to why the patient did not benefit from treatment. Immunochemotherapy of lymphomas in HIV+ patients, however, remains challenging, and T-cell depletion prior to the initiation of highly active antiretroviral-therapy and immunochemotherapy adversely affect clinical outcome.23
Here, we present evidence that 11q alterations can occur in HGBL, which do not necessarily resemble typical BLL. We derive the questions, whether the clinicopathological spectrum of malignancies carrying this aberration may be broader than previously assumed and whether BLL-11q indeed constitutes a distinct entity.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Tanja Oelterman and Claudia Becher for skilled technical assistance with sample preparation, immunohistochemical workup, FISH analyses, and photographic documentation.
Authorship
Contribution: N.G. and E.M.M.P. designed the study; A.C.F., H.M., I.O., L.T., and W.K. performed the histopathological workup; E.M.M.P., A.C., and M.S. performed the FISH studies; N.G. and H.M.W. performed the clinical workup; N.G., E.M.M.P., N.v.B., and M.S. performed the data analysis; N.G., I.O., H.M.W., and E.M.M.P. wrote the manuscript; and all authors critically revised final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Niklas Gebauer, Department of Hematology and Oncology, UKSH Campus Lübeck, Ratzeburger Allee 160, 23538 Lübeck, Germany: e-mail: Niklas.Gebauer@uksh.de.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues, vol. 2. Lyon: IARC; 2017 [Google Scholar]
- 2.Salaverria I, Martin-Guerrero I, Wagener R, et al. ; Berlin-Frankfurt-Münster Non-Hodgkin Lymphoma Group . A recurrent 11q aberration pattern characterizes a subset of MYC-negative high-grade B-cell lymphomas resembling Burkitt lymphoma. Blood. 2014;123(8):1187-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagener R, Seufert J, Raimondi F, et al. The mutational landscape of Burkitt-like lymphoma with 11q aberration is distinct from that of Burkitt lymphoma. Blood. 2019;133(9):962-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Farre B, Ramis-Zaldivar JE, Salmeron-Villalobos J, et al. Burkitt-like lymphoma with 11q aberration: a germinal center-derived lymphoma genetically unrelated to Burkitt lymphoma. Haematologica. 2019;104(9):1822-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreiro JF, Morscio J, Dierickx D, et al. Post-transplant molecularly defined Burkitt lymphomas are frequently MYC-negative and characterized by the 11q-gain/loss pattern. Haematologica. 2015;100(7):e275-e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319-2331. [DOI] [PubMed] [Google Scholar]
- 7.Hummel M, Bentink S, Berger H, et al. ; Molecular Mechanisms in Malignant Lymphomas Network Project of the Deutsche Krebshilfe . A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354(23):2419-2430. [DOI] [PubMed] [Google Scholar]
- 8.Collinge B, Ben-Neriah S, Chong L, et al. The impact of MYC and BCL2 structural variants in tumors of DLBCL morphology and mechanisms of false-negative MYC IHC. Blood. 2021;137(16):2196-2208. [DOI] [PubMed] [Google Scholar]
- 9.Ferreri AJM, Cwynarski K, Pulczynski E, et al. ; International Extranodal Lymphoma Study Group (IELSG) . Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017;4(11):e510-e523. [DOI] [PubMed] [Google Scholar]
- 10.Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369(20):1915-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cucco F, Barrans S, Sha C, et al. Distinct genetic changes reveal evolutionary history and heterogeneous molecular grade of DLBCL with MYC/BCL2 double-hit. Leukemia. 2020;34(5):1329-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenwald A, Bens S, Advani R, et al. Prognostic significance of MYC rearrangement and translocation partner in diffuse large B-cell lymphoma: a study by the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol. 2019;37(35):3359-3368. [DOI] [PubMed] [Google Scholar]
- 13.Horn H, Kalmbach S, Wagener R, et al. A diagnostic approach to the identification of Burkitt-like lymphoma with 11q aberration in aggressive B-cell lymphomas. Am J Surg Pathol. 2021;45(3):356-364. [DOI] [PubMed] [Google Scholar]
- 14.Rymkiewicz G, Grygalewicz B, Chechlinska M, et al. A comprehensive flow-cytometry-based immunophenotypic characterization of Burkitt-like lymphoma with 11q aberration. Mod Pathol. 2018;31(5):732-743. [DOI] [PubMed] [Google Scholar]
- 15.Grygalewicz B, Woroniecka R, Rymkiewicz G, et al. The 11q-gain/loss aberration occurs recurrently in MYC-negative Burkitt-like lymphoma with 11q aberration, as well as MYC-positive Burkitt lymphoma and MYC-positive high-grade B-cell lymphoma, NOS. Am J Clin Pathol. 2017;149(1):17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havelange V, Ameye G, Théate I, et al. The peculiar 11q-gain/loss aberration reported in a subset of MYC-negative high-grade B-cell lymphomas can also occur in a MYC-rearranged lymphoma. Cancer Genet. 2016;209(3):117-118. [DOI] [PubMed] [Google Scholar]
- 17.Rieken J, Bernard V, Witte HM, et al. Exhaustion of tumour-infiltrating T-cell receptor repertoire diversity is an age-dependent indicator of immunological fitness independently predictive of clinical outcome in Burkitt lymphoma. Br J Haematol. 2021;193(1):138-149. [DOI] [PubMed] [Google Scholar]
- 18.Dave SS, Fu K, Wright GW, et al. ; Lymphoma/Leukemia Molecular Profiling Project . Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006;354(23):2431-2442. [DOI] [PubMed] [Google Scholar]
- 19.Vaghefi P, Martin A, Prévot S, et al. Genomic imbalances in AIDS-related lymphomas: relation with tumoral Epstein-Barr virus status. AIDS. 2006; 20(18):2285-2291. [DOI] [PubMed] [Google Scholar]
- 20.Capello D, Scandurra M, Poretti G, et al. Genome wide DNA-profiling of HIV-related B-cell lymphomas. Br J Haematol. 2010;148(2):245-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Au-Yeung RKH, Arias Padilla L, Zimmermann M, et al. Experience with provisional WHO-entities large B-cell lymphoma with IRF4-rearrangement and Burkitt-like lymphoma with 11q aberration in paediatric patients of the NHL-BFM group. Br J Haematol. 2020;190(5):753-763. [DOI] [PubMed] [Google Scholar]
- 22.Sevilla DW, Gong JZ, Goodman BK, et al. Clinicopathologic findings in high-grade B-cell lymphomas with typical Burkitt morphologic features but lacking the MYC translocation. Am J Clin Pathol. 2007;128(6):981-991. [DOI] [PubMed] [Google Scholar]
- 23.Clark E, Royse KE, Dong Y, et al. Stable incidence and poor survival for HIV-related Burkitt lymphoma among the US veteran population during the antiretroviral era. J Acquir Immune Defic Syndr. 2020;84(1):18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.