Abstract
Background:
The majority of countries with the highest rotavirus-associated death rates are in sub-Saharan Africa. In 2009, the World Health Organization (WHO) recommended routine vaccination against rotavirus worldwide, with unique age recommendations to administer the first dose before 15 weeks of age and last dose by 32 weeks of age. These age restrictions were relaxed in January 2013, but they may still lead to lower rotavirus vaccine coverage.
Methods:
Children age-eligible to have received rotavirus vaccine that were enrolled in Ghana, Zimbabwe, Rwanda or Burkina Faso’s active rotavirus surveillance platforms from 2013 to 2017 and had a stool specimen that tested rotavirus-negative were included in the analysis. Proportion vaccinated and timeliness of rotavirus vaccine versus DTPw-HepB-Hib (pentavalent) first dose and last dose were compared at weeks 15 and 32, respectively, using Chi-square analyses. Odds ratios were calculated using logistic regression.
Results:
Among children who received rotavirus vaccine dose 1, 96–99% received this dose by 15 weeks of age and among children who received the last dose, 98–99% received it by 32 weeks of age. In all four countries, there was no significant difference in the proportion of children who received first dose rotavirus versus pentavalent vaccine by week 15, or last dose rotavirus versus concordant pentavalent vaccine by week 32. Delayed administration of first dose pentavalent vaccine was significantly associated with missing first dose of rotavirus vaccine in 3 of the 4 countries studied, although delays in administration were rare (1–4%).
Conclusions:
Rotavirus vaccination was timely among sentinel sites in these four early rotavirus vaccine-introducing countries in Africa. Late presentation for vaccination may have resulted in some children with access to care missing first dose of rotavirus vaccine; however, vaccination delays were infrequent and therefore the potential impact of the age restrictions on overall proportion vaccinated was minimal.
Keywords: Surveillance, Validation, Acute gastroenteritis, Methods
1. Background
Rotavirus is the leading cause of severe childhood gastroenteritis and causes >200,000 deaths annually, mostly in low-income countries [1]. The majority of countries with the highest rotavirus-associated death rates are in sub-Saharan Africa. Two oral live attenuated rotavirus vaccines are currently licensed for global use: RotaTeq (RV5), a 3-dose vaccine administered at 6, 10, and 14 weeks of age, and Rotarix (RV1), a 2-dose vaccine administered at 6 and 10 weeks of age. In 2009, the World Health Organization (WHO) recommended routine vaccination against rotavirus worldwide. Due to concern for intussusception, a rare adverse event associated with an early-generation rotavirus vaccine no longer in use, WHO initially recommended that the first and last doses of RV5 and RV1 be given by 15 and 32 weeks of age, respectively [2]. In January 2013, after reassessing the potential benefits and risks of rotavirus vaccination, WHO recommended removing these age restrictions [3]. However, we anticipate that implementation of this recommendation has been challenging in both already existing and subsequent rotavirus vaccination programs as countries would need to adopt this recommendation, update their national immunization program guidelines, and retrain vaccinators.
We examined the timeliness of rotavirus vaccination and compared it with that of DTPw-HepB-Hib (pentavalent) vaccine, which is concurrently administered without age restrictions, in two RV1-introducing countries (Ghana and Zimbabwe) and two RV5-introducing countries (Rwanda and Burkina Faso) that implemented vaccination between 2012 and 2014. As these countries made the decision to implement rotavirus vaccination prior to or soon after WHO’s 2013 decision to remove age restrictions, we hypothesized that the previous age restrictions might have had some impact on the timeliness of rotavirus vaccination.
2. Methods
2.1. Data collection
Rotavirus and pentavalent vaccine timeliness were assessed using data from ongoing multi-site active rotavirus surveillance programs in Ghana, Rwanda, Zimbabwe, and Burkina Faso. These countries introduced rotavirus vaccine at different times: Ghana (April 2012), Rwanda (May 2012), Burkina Faso (October 2013) and Zimbabwe (May 2014). Surveillance and enrollment periods varied by country (Table 1). Active surveillance for acute gastroenteritis hospitalizations was conducted using the WHO generic protocol and a fecal specimen was obtained to test for rotavirus [4]. Vaccination data for enrolled children were obtained through review of each child’s vaccination card. The active surveillance sentinel sites were described previously [5-8].
Table 1.
Characteristics of children and vaccination programs, by country.
| Variables | Ghana | Rwanda | Zimbabwe | Burkina Faso |
|---|---|---|---|---|
| Total | 464 | 985 | 1592 | 527 |
| Demographic | ||||
| Age in weeks (Median, 25, 75 IQR) | 45 (30, 56) | 45 (34, 61) | 49 (34, 68) | 43 (32, 59) |
| Sex (Female N, %) | 196 (42.2) | 429 (43.5) | 669 (42.0) | 211 (40.0) |
| Vaccination information | ||||
| Vaccine introduction date | April 2012 | May 2012 | May 2014 | October 2013 |
| Vaccine type | Rotarix | RotaTeq | Rotarix | RotaTeq |
| Schedule | 6, 10 weeks | 6, 10, 14 weeks | 6, 10 weeks | 2, 3, 4 months |
| Admission dates | February 2013–April 2015 | February 2013–September 2015 | October 2014–December 2016 | April 2014–January 2017 |
| Median months between RVV introduction and enrollment (IQR) | 22 (18, 27) | 26 (17, 31) | 24 (18, 29) | 27 (20, 33) |
Abbreviations: RVV = Rotavirus vaccine.
2.2. Study population
This analysis includes infants between 14 weeks and 36 months of age enrolled in an active rotavirus surveillance program who (1) tested negative for rotavirus, (2) had a vaccination card available for review, and (3) were born at least 2 months after rotavirus vaccine was introduced into the national vaccination program. Forty two children were excluded from the analysis because of illegible or incorrect vaccine administration dates.
Sites conducted vaccine effectiveness evaluations using the test-negative case-control study design; we exclusively used vaccination data from rotavirus-negative acute gastroenteritis cases for the purpose of this evaluation. Severe diarrhea during childhood due to non-rotavirus etiologies is prevalent in sub-Saharan African countries and is not associated with receipt of rotavirus vaccine, so this was a convenient and representative sample for estimating rotavirus vaccine timeliness [9].
2.3. Analysis
Demographic characteristics of children were described. Proportion of vaccinated infants and timeliness were calculated as cumulative percent frequencies. Among children meeting inclusion criteria, we compared the proportion who received rotavirus vaccine to those same children who received pentavalent vaccine. We then applied historical rotavirus vaccine-specific age at administration cutoffs to compare timeliness of vaccination among children who received rotavirus vaccine as compared to children who received pentavalent vaccine. For the last dose of RV1, the concordant pentavalent dose is the second dose in the series and for the last dose of RV5, it is the third dose in the pentavalent series.
Associations between delayed pentavalent vaccine and missed rotavirus vaccine opportunity were determined using logistic regression. We fit an unadjusted model of the probability of rotavirus vaccine administration (the outcome variable) as a function of whether or not pentavalent vaccine administration was delayed (the exposure variable), and determined statistical significance using Wald test. We screened for potential confounders individually by determining whether each potential confounder was associated with the outcome variable among the unexposed and with the exposure variable. The following potential confounders were screened: age on admission, months between vaccine introduction and admission date, and sex. A missed opportunity for rotavirus vaccine was defined as a pentavalent vaccine dose administration without concordant rotavirus vaccine dose administration. Delayed vaccine administration was defined as vaccine administration at least 1 week after the recommended rotavirus vaccination schedule.
Sensitivity analyses were conducted to determine whether associations remained significant after cohort restrictions were applied. These analyses included restricting the age of the cohort to children at least 32 weeks of age to ensure all children had the opportunity to be fully vaccinated with rotavirus vaccine, and excluding children born in the first 4 months after rotavirus vaccine introduction to allow for the rotavirus vaccine program to stabilize after introduction.
3. Results
A total of 3568 rotavirus-negative children enrolled in the surveillance programs were included in these analyses: 464 children from Ghana, 985 children from Rwanda, 1592 children from Zimbabwe, and 527 children from Burkina Faso (Table 1). The age and sex distributions were similar across all four countries; median age ranged from 45 to 49 weeks old, and percent female ranged from 40% to 44%. The median number of months between the date of rotavirus vaccine introduction and the date of admission was highest for children enrolled in Burkina Faso (27 months) and lowest for children in Ghana (22 months).
The proportion of children who received rotavirus versus pentavalent vaccine by dose and age in weeks was plotted to compare rotavirus to pentavalent dose-specific administration trends over the first year of life, and of rotavirus vaccine-specific age cutoffs for administration (Fig. 1). In all countries, the proportion of children who received rotavirus and pentavalent vaccine increased at similar rates over the first year of life, with a sharp increase occurring during scheduled vaccine administration periods.
Fig. 1.
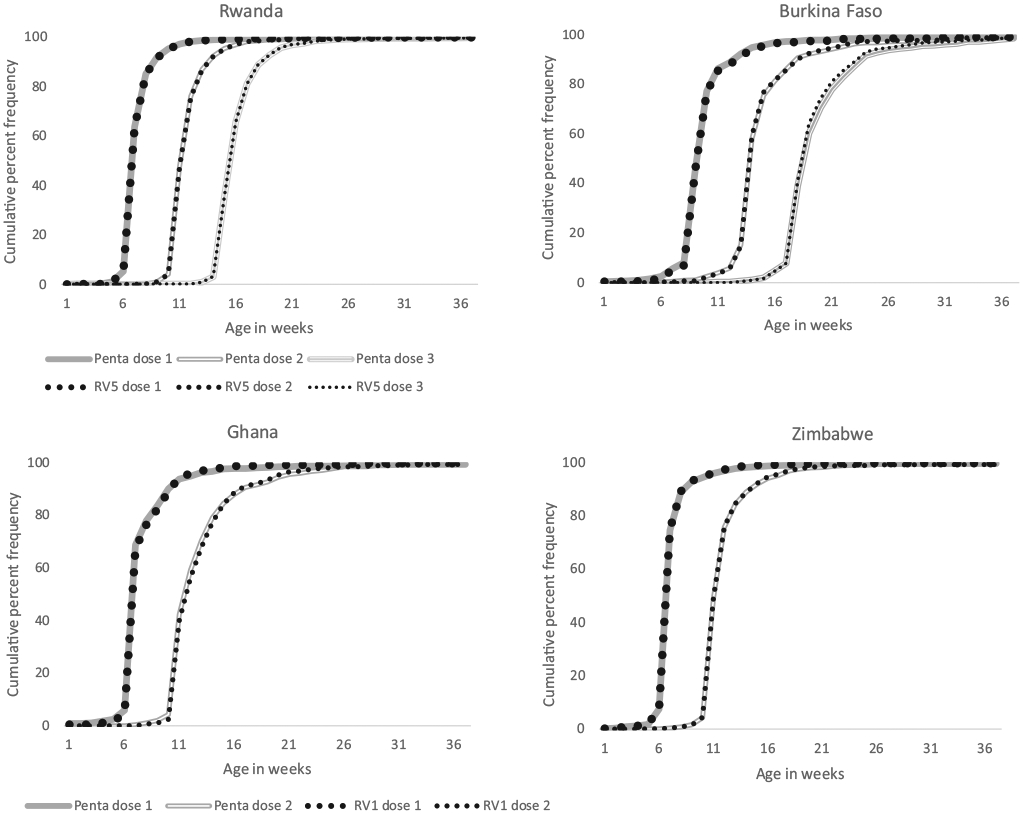
Cumulative proportion of children vaccinated with rotavirus vaccine versus pentavalent vaccine by age in weeks and dose. Rwanda and Burkina Faso used RV5; Ghana and Zimbabwe used RV1.
The proportion of children who received the first dose rotavirus vaccine by one year of age was high in all four countries, ranging from 89% in Burkina Faso to 99% in Rwanda (Table 2). In Burkina Faso, the difference between the proportion of children who received the first dose of rotavirus vaccine and the proportion who received the first dose of pentavalent vaccine was most pronounced (8%). Among children who received the first dose of rotavirus vaccine, the proportion who received it by 15 weeks of age ranged from 96% in Burkina Faso to 99% in Rwanda. In all four countries, the proportion of vaccinated children who had received the first dose of rotavirus vaccine by 15 weeks of age was similar to that of pentavalent vaccine. (Table 3).
Table 2.
Overall proportion of children who received RVV vs. Penta, by dose.
| Country | Received first dose (95% CI) |
Received last/concordant dosea (95% CI) |
||
|---|---|---|---|---|
| RVV | Penta | RVV | Penta | |
| Ghana | 449/464; 96.8% (94.7, 98.0) | 452/464; 97.4% (95.5, 98.5) | 438/464; 94.4% (91.9, 96.2) | 447/464; 96.3% (94.2, 97.7) |
| Rwanda | 973/985; 98.8% (97.9, 99.3) | 981/985; 99.6% (99.0, 99.8) | 952/985; 96.6% (95.3, 97.6) | 964/985; 97.8% (96.8, 98.6) |
| Zimbabwe | 1583/1592; 99.4% (98.9, 99.7) | 1592/1592; 100.% (99.8, 100.0) | 1536/1592; 96.5% (95.5, 97.3) | 1571/1592; 98.7% (98.0, 99.1) |
| Burkina Faso | 467/527; 88.6% (85.6, 91.1) | 509/527; 96.6% (94.7, 97.8) | 401/527; 76.1% (72.3, 79.5) | 448/527; 85.% (81.7, 87.8) |
Abbreviations: RVV = rotavirus vaccine; penta = pentavalent vaccine.
For the RVV last dose, the concordant penta dose was 2nd dose for Rotarix-introducing countries, and 3rd dose for RotaTeq-introducing countries.
Table 3.
Proportion of children vaccinated with RVV who received RVV on-time vs. proportion of children vaccinated with penta who received penta on-time, by dose.
| Country | Received first dose by 15 weeks of age (95% CI) |
Received last/concordanta dose by 32 weeks of age (95% CI) |
||
|---|---|---|---|---|
| RVV | Penta | RVV | Penta | |
| Ghana | 441/449; 98.2% (96.5, 99.1) | 441/452; 97.6% (95.7, 98.6) | 434/438; 99.1% (97.7, 99.6) | 444/447; 99.3% (98.1, 99.8) |
| Rwanda | 963/973; 99.% (98.1, 99.4) | 971/981; 99.% (98.13, 99.45) | 947/952; 99.5% (98.8, 99.8) | 959/964; 99.5% (98.8, 99.8) |
| Zimbabwe | 1565/1583; 98.9% (98.2, 99.3) | 1566/1592; 98.4% (97.62, 98.88) | 1522/1536; 99.1% (98.5, 99.5) | 1561/1571; 99.4% (98.8, 99.7) |
| Burkina Faso | 447/467; 95.7% (93.5, 97.2) | 489/509; 96.1% (94.01, 97.44) | 391/401; 97.5% (95.5, 99.5) | 432/448; 96.4% (94.3, 97.8) |
Abbreviations: RVV = rotavirus vaccine; penta = pentavalent vaccine; 95% CI = 95% Confidence Interval.
For the RVV last dose, the concordant penta dose was 2nd dose for Rotarix-introducing countries, and 3rd dose for RotaTeq-introducing countries.
The proportion of children who received the last dose rotavirus vaccine by one year of age varied by country and ranged from 76% in Burkina Faso to 97% in Rwanda (Table 2). In Burkina Faso, the difference between the proportion of children who received the last dose of rotavirus vaccine and the proportion who received the last dose of pentavalent vaccine was most pronounced (9%). Among children who received the last dose of rotavirus vaccine, the proportion who received it by 32 weeks of age ranged from 97% in Burkina Faso to 100% in Rwanda (Table 3). In all four countries, the proportion of vaccinated children who had received the last dose of rotavirus by 32 weeks of age was similar to that of the concordant pentavalent vaccine dose. This finding remained unchanged when restricting the cohort to children at least 32 weeks of age (see Supplemental Materials, Appendix A, Table 1a and b).
In Ghana, Zimbabwe, and Burkina Faso, children who had received a delayed first dose of pentavalent vaccine (penta) were significantly more likely to miss the first dose of rotavirus vaccine (RVV) as compared to children who had received the first dose of penta on time (Table 4). For example, in Ghana, the odds were 32 times higher that a child receiving penta dose 1 at 15 weeks of age or later (delayed dosing) would miss RVV dose as compared to a child receiving penta dose1 before 15 weeks of age (on-time dosing). However, the confidence intervals around this and other estimate were wide, indicating a high degree of uncertainty around the exact measure of association. No confounders, including age on admission, months between vaccine introduction and admission date, or sex, were identified using the aforementioned screening method (See Supplemental Materials, Appendix A, Table 2a and b for explanatory variable bivariate analyses). This association was most pronounced in Zimbabwe (odds ratio 56.7, 95% confidence interval 14.3–225.7). A sensitivity analysis excluding children born in the four months following vaccine introduction yielded similar findings (see Supplemental Materials, Appendix A, Table 3). Children who received a delayed concordant dose of pentavalent vaccine were also more likely to miss a last dose of rotavirus vaccine as compared to children who had received concordant pentavalent dose on time, and findings were not confounded by age on admission, months between vaccine introduction and admission date, and sex. However, when restricting the cohort to children who had received first dose of rotavirus vaccine and pentavalent vaccine, there was no association between missed last dose of rotavirus vaccine and timeliness of concordant pentavalent dose in any country (Supplemental materials, Appendix A, Table 4).
Table 4.
Association between delay in pentavalent vaccine dose and missed opportunity for rotavirus vaccine dose among infants receiving pentavalent vaccine dose.
| Country | RVV first dose missed |
RVV first dose received |
OR (95% CI) | RVV last dose missed |
RVV last dose received |
OR (95% CI) | |
|---|---|---|---|---|---|---|---|
| Ghana | Delayed penta concordant dose* | 2 | 9 | 32.4 (4.8, 218.0) | 1 | 2 | 21.7 (1.8, 258.8) |
| On-time penta concordant dose* | 3 | 438 | Ref | 10 | 434 | Ref | |
| Rwanda** | Delayed penta concordant dose* | 0 | 10 | – | 0 | 5 | – |
| On-time penta concordant dose* | 8 | 963 | Ref | 12 | 947 | Ref | |
| Zimbabwe | Delayed penta concordant dose* | 4 | 22 | 56.7 (14.3, 225.7) | 2 | 8 | 11.2 (2.3, 54.9) |
| On-time penta concordant dose* | 5 | 1561 | Ref | 34 | 1527 | Ref | |
| Burkina Faso | Delayed penta concordant dose* | 5 | 15 | 3.3 (1.1–9.5) | 6 | 10 | 4.8 (1.7, 13.8) |
| On-time penta concordant dose* | 45 | 444 | Ref | 48 | 384 | Ref |
Abbreviation: RVV = rotavirus vaccine; penta = pentavalent vaccine; OR = Odds Ratio; 95% CI = 95% Confidence Interval.
For the RVV first dose, the concordant penta dose was the penta first dose. For the RVV last dose, the concordant penta dose was 2nd dose for Rotarix-introducing countries, and 3rd dose for RotaTeq-introducing countries.
logistic regression not completed due to quasi separation of datapoints.
4. Discussion
Rotavirus vaccine administration was timely among children enrolled at sentinel sites in these four early rotavirus vaccine-introducing countries in Africa. In all four countries, the proportion of vaccinated children who received the first rotavirus vaccine dose by 15 weeks of age, and the proportion of vaccinated children who received the last rotavirus vaccine dose by 32 weeks of age were over 95%. Delayed administration of pentavalent vaccine at ages beyond the historical age restrictions for rotavirus vaccination were associated with lower rotavirus vaccine use. However, in all four countries, delays in administration of pentavalent vaccine were infrequent and therefore the impact of age restrictions on overall rotavirus vaccination coverage in the population is likely minimal.
Delayed pentavalent vaccine administration was clearly associated with missed rotavirus vaccination opportunity for the first dose alone. When restricting the cohort to children who had received first dose of rotavirus vaccine and first dose of pentavalent vaccine, there was no association between missed last dose of rotavirus vaccine and timeliness of concordant pentavalent vaccine dose in any country. This indicates that rotavirus vaccine lastdose findings were driven primarily by missed rotavirus vaccine first doses. Thus, age restrictions may have a greater impact on the proportion of children receiving the first dose of rotavirus vaccine as compared to last dose.
The association between delayed pentavalent vaccine administration and missed rotavirus vaccine opportunity may have been related to vaccine availability during the surveillance period. However, the time since vaccine introduction was examined to account for issues with vaccine rollout, and findings were consistent after excluding children born in the four months after vaccine introduction period. Continued rotavirus surveillance will be helpful in determining whether these country-specific trends persist many years after rotavirus vaccine introduction.
The proportion of children in our evaluation who received rotavirus vaccine was higher than the most recent Demographic and Health Survey (DHS) countrywide estimates for rotavirus vaccine coverage and pentavalent vaccine coverage, where available, even after restricting the cohort to age-eligible children (Supplemental materials, Appendix A, Table 5) [10-13]. This may relate to the type and number of sentinel sites participating in active surveillance. Active surveillance sentinel sites were chosen in large part due to the broad population they serve, but overall proportion of children vaccinated and timeliness estimates could still be skewed based on the locations of sentinel sites and patient populations. Timing of DHS data collection may also be playing a role; in Zimbabwe, the most recent DHS survey reported rotavirus vaccine coverage during the first year after vaccine introduction (2015). Our analysis also used vaccine cards to assess vaccination status for all cases enrolled in surveillance, which is not susceptible to caregiver recall bias and provides individual-level vaccine administration dates.
This study has a number of limitations. Only children who had access to healthcare were enrolled in the rotavirus surveillance program and the analysis was restricted to children who had a vaccine card. Thus, timeliness could have been overestimated (assuming that children without access to health care or without a vaccine card available are also less likely to be vaccinated on time). The proportion of children vaccinated in our evaluation may not be representative of the general population coverage and should not be used to estimate rotavirus vaccination coverage in the general population; country surveys using a more complete sampling strategy are better suited for this purpose. Furthermore, certain factors typically associated with delayed access to health care, such as residence of child (urban vs rural), could not be assessed.
5. Conclusions
Rotavirus vaccine administration was timely among children enrolled at sentinel sites in these four early rotavirus vaccine-introducing countries in Africa, and age restrictions had minimal impact on rotavirus vaccine use due to timely vaccine administration. In African countries with less timely vaccine administration, disparities in coverage between rotavirus vaccine and pentavalent vaccine may be more prominent if age restrictions continue to be followed to some extent and this should be examined in future evaluations.
Supplementary Material
Acknowledgements
We would like to acknowledge the Expanded Program on Immunisation, and the following individuals for their important contributions to this study: Aaron Curns, Division of Viral Diseases, CDC, USA; Dr. Fred OSEI SARPONG, WHO country office, Ghana; Ghana rotavirus surveillance sites; Regional Rotavirus Reference Laboratory (Ghana); Dr. Marie Rosette Nahimana, WHO country office, Rwanda; Dr. Maxwel RUPFUTSE, WHO country office, Zimbabwe; Dr. Ma Ouattara, WHO country office, Burkina Faso; Burkina rotavirus team surveillance team; Dr. Joseph Nsiari-muzeyi Biey, WHO Inter Country Support Team (IST), West Africa; Dr. Goitom G. WELDEGEBRIEL and Dr. Mutale MUMBA, WHO IST, East and Southern Africa, Kusum Nathoo, Harare Central Hospital and Department of Paediatrics and Child Health, University of Zimbabwe; Ismail Ticklay, Parirenyatwa Group Hospitals and Department of Paediatrics and Child Health, University of Zimbabwe; Nhamo A Gonah, Chitungwiza Central Hospital, Chitungwiza, Arnold Mukaratirwa and Chipo Berejena, National Virology Laboratory, Harare, Zimbabwe.
All authors attest they meet the ICMJE criteria for authorship. Financial support for this evaluation was provided by Gavi, the Vaccine Alliance, through the CDC Foundation and the World Health Organization.
6. Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2019.08.008.
References
- [1].Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 2016;62(Suppl 2):S96–s105. [DOI] [PubMed] [Google Scholar]
- [2].WHO. Rotavirus vaccines: an update. Week Epidemiolog Rec 2009;84:533–40. [PubMed] [Google Scholar]
- [3].WHO. Rotavirus vaccines WHO Position paper–january 2013. Week Epidemiolog Rec 2013;88(5):49–64. [PubMed] [Google Scholar]
- [4].World Health Organization; Generic protocols for (i) hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children and (ii) a community-based survey on utilization of health care services for gastroenteritis in children: field test version; Geneva; Switzerland: WHO; 2002; Available from: http://www.vaccineresources.org/files/Generic_protocol_for_surveillance_WHO.pdf; accessed on 3 July 2018. [Google Scholar]
- [5].Armah G, Pringle K, Enweronu-Laryea CC, et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in ghana. Clin Infect Dis 2016;62(Suppl 2):S200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bonkoungou IJO, Aliabadi N, Leshem E, et al. Impact and effectiveness of pentavalent rotavirus vaccine in children <5years of age in Burkina Faso. Vaccine 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mujuru HA, Yen C, Nathoo KJ, et al. Reduction in diarrhea- and rotavirusrelated healthcare visits among children <5 years of age after national rotavirus vaccine introduction in Zimbabwe. Pediatr Infect Dis J 2017;36(10):995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tate JE, Ngabo F, Donnen P, et al. Effectiveness of pentavalent rotavirus vaccine under conditions of routine use in Rwanda. Clin Infect Dis 2016;62(Suppl 2):S208–12. [DOI] [PubMed] [Google Scholar]
- [9].Schwartz LM, Halloran ME, Rowhani-Rahbar A, Neuzil KM, Victor JC. Rotavirus vaccine effectiveness in low-income settings: An evaluation of the test-negative design. Vaccine 2017;35(1):184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ghana Statistical Service (GSS); Ghana Health Service (GHS), and ICF International. Ghana Demographic and Health Survey 2014; Rockville, Maryland; USA: GSS; GHS, and ICF International; 2015: Available from: https://www.dhsprogram.com/publications/publication-FR307-DHS-Final-Reports.cfm; accessed on 3 July 2018. [Google Scholar]
- [11].National Institute of Statistics of Rwanda; Ministry of Finance and Economic Planning/Rwanda; Ministry of Health/Rwanda, and ICF International; 2015; Rwanda Demographic and Health Survey 2014-15. Kigali; Rwanda: National Institute of Statistics of Rwanda; Ministry of Finance and Economic Planning/Rwanda, Ministry of Health/Rwanda, and ICF International; 2015: Available from: https://www.dhsprogram.com/publications/publication-FR316-DHS-Final-Reports.cfm; accessed on 3 July 2018. [Google Scholar]
- [12].Zimbabwe National Statistics Agency and ICF International; Zimbabwe Demographic and Health Survey 2015: Final Report. Rockville; Maryland; USA: Zimbabwe National Statistics Agency (ZIMSTAT) and ICF International; 2016: Available from: https://www.dhsprogram.com/publications/publication-FR322-DHS-Final-Reports.cfm; accessed on 3 July 2018. [Google Scholar]
- [13].Institut National de la Statistique et de la Démographie; Ministère de l’Économie et des Finances; Enquête Démographique et de Santé et à Indicateurs Multiples (EDSBF-MICS IV) 2010; Ouagadougu, Burkina Faso; Available from: https://www.dhsprogram.com/publications/publication-FR256-DHS-Final-Reports.cfm; accessed on 15 January 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


