Abstract
Introduction
To evaluate the impact of Sars-Cov-2 infection on mortality and immune checkpoint inhibitor (ICI) toxicity in patients with cancer receiving ICIs compared to those not receiving ICIs.
Methods
We conducted a retrospective matched cohort study of 25 patients receiving ICIs within 1 year of coronavirus disease 2019 (COVID-19) diagnosis between March 20, 2020, and June 3, 2020, at the Dana-Farber Cancer Institute/Mass General Brigham. Cases were matched 1:1 with controls based on age, sex, and anticancer therapy within the prior 6 months.
Results
Seven of 25 (28%) patients receiving ICIs died from COVID-19 as compared with nine of 25 (36%) controls. Through multivariable analysis adjusting for age, sex, and anticancer therapy, ICI use was not associated with increased risk for COVID-19 death (OR [odds ratio] 0.36, 95% CI 0.07–1.87). Determinants of mortality included age (OR 1.14, 95% CI 1.03–1.27) and chronic obstructive pulmonary disease (OR 12.26, 95% CI 1.76–85.14). Statin use was protective against mortality (OR 0.08, 95% CI 0.01–0.63). Two patients experienced persistent immune-related adverse events (irAEs) (hypophysitis); one had new-onset irAE (hypothyroidism) during their COVID-19 course. Patients with ICIs had significantly higher platelet (p = 0.017) and D-dimer (p = 0.037) levels. Elevated troponin levels (p = 0.01) were associated with COVID-19 death in patients using ICI.
Conclusion
There is insufficient evidence to conclude COVID-19–related outcomes are associated with ICIs, and we did not observe an increased risk of COVID-19–related death associated with ICIs. The potential protective effect of statin therapy and role of laboratory biomarkers warrant further investigation.
Keywords: COVID-19, immune checkpoint inhibitors, programmed death 1, programmed death ligand 1, cytotoxic T-lymphocyte–associated protein 4, immune-related adverse events, cancer
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has presented unique challenges for clinicians caring for patients with cancer.[1,2] Although early studies suggest that patients with cancer may be more vulnerable to COVID-19,[1,3] recent studies have refuted these claims and have shown no increased mortality rates when compared to patients without cancer.[4–6] The impact of immune checkpoint inhibitors (ICIs) on COVID-19–related clinical outcomes remains unclear.[7]
ICIs augment the T-cell response and prevent cancer immune evasion, generating durable responses in difficult-to-treat tumor types.[8] Immune-related adverse events (irAEs) are hypothesized to be driven in part by cytokine dysregulation,[9–11] which may parallel the cytokine storm implicated in COVID-19 cases of acute respiratory distress syndrome (ARDS).[12–16] In contrast, ICIs may also improve viral clearance17 or restore immunocompetency in exhausted T cells during chronic viral infection.[18–20] Notably, T-cell exhaustion and lymphopenia have been proposed as contributors to COVID-19′s immunopathology.[12,21]
Limited studies on the impact of COVID-19 in patients with cancer receiving ICIs have yielded mixed findings.[1,6,22–27] No studies have investigated the concurrent impact of ICI therapy and COVID-19 infection on irAEs. In this retrospective matched cohort study, our primary analysis evaluated the impact of ICIs on COVID-19–related death when compared with patients not receiving ICIs. We also evaluated for new or persistent irAEs in the setting of COVID-19. Finally, we investigated baseline characteristics, concomitant medications, and laboratory results as possible predictors for COVID-19 mortality in patients receiving ICIs.
METHODS
Study Design, Setting, and Participants
The Partners Healthcare Institutional Review Board approved this project (No. 2020P000851). The study was performed in accordance with the Declaration of Helsinki. After institutional review board approval, we performed a retrospective matched cohort study of adult patients receiving ICIs (programmed cell death [PD-1], its ligand [PD-L1], and/or cytotoxic T-lymphocyte–associated protein 4 [CTLA-4] inhibitors) prior to a confirmed COVID-19 diagnosis in the Dana-Farber Cancer Institute/Mass General Brigham network between March 20, 2020 and June 3, 2020. We queried inpatient and outpatient electronic medical records, billing codes, and pharmacy records for patients with cancer receiving ICIs including nivolumab, durvalumab, atezolizumab, cemiplimab, pembrolizumab, tremelimumab, and/or ipilimumab within 1 year of COVID-19 diagnosis confirmed by serology and/or polymerase chain reaction. We chose a 1-year time point to account for the immune-modulating effects of ICIs, which may have an effect on delayed ICI-associated toxicities even after the decline of receptor occupancy.[28] Patients with any malignancy type and those receiving ICI therapy alone and/or in combination with other anticancer agents, were included.
Data Collection
We abstracted electronic medical records for demographics, oncologic history, ICI, other anti–cancer agent therapy within 6 months prior to COVID-19 diagnosis, comorbidities, and concomitant medications. A medical oncologist graded irAEs occurring before and during COVID-19 course on the basis of Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.[29] COVID-19 characteristics were abstracted including presenting symptoms, intensive care unit (ICU) admission, COVID-19 complications, and treatments. Relevant radiologic findings and presenting, peak, and nadir laboratory results were collected. Clinical status at last follow-up was defined as fully recovered, recovered with complications (defined as a new oxygen requirement even after COVID-19 recovery), or death.
Data and Statistical Analysis
The primary outcome was death due to COVID-19. Summaries of patient demographics, comorbidities, cancer and COVID-related disease characteristics, concomitant medications, oncologic history, and irAEs are primarily descriptive. The control group was manually and randomly selected on the basis of three specific criteria: age, sex, and anticancer therapy within the prior 6 months to COVID-19 diagnosis. Comparisons between cases and matched controls for characteristics measured on a continuous scale were conducted with Wilcoxon rank sum tests. Fisher exact test was used to compare categorical variables. Parallel analyses assessed differences according to COVID-19–related death. The Wilcoxon rank sum test compared laboratory results.
The relationships between COVID-related death, patient disease, and prior treatment characteristics were explored by using multivariable logistic regression models. Only comorbidities or concomitant medications at the time of COVID-19 diagnosis with at least a 20% incidence (10 patients) were considered and included hypertension, hyperlipidemia, chronic obstructive pulmonary disease (COPD), obesity, autoimmune disease, smoking status, steroid use, statin use, and vitamin D supplementation. Cancer type, disease stage, prior radiotherapy, and vaccine of any type within 6 months were also considered. Cancer type was classified into four categories: gastrointestinal (GI), skin, thoracic, and other.
All models included the three matching criteria and cancer treatment cohort (ICI versus no ICI). Each of the predictors listed above was modeled individually, with those having statistical significance of p ≤ 0.2 carried forward as a potential predictor in the final multivariable model (hypertension, hyperlipidemia, COPD, smoking status, and statin use). Statistical assessment for the models was based on Wald chi-square tests, with significance defined as p < 0.05. Odds ratio (OR) estimates are presented with 95% Wald confidence intervals. Analyses used SAS 9.4 (SAS Institute Inc).
RESULTS
Clinical Characteristics of Patients
Between March 20, 2020 and June 3, 2020, we queried electronic records to find patients receiving immunotherapy who received inpatient or outpatient care. We reviewed the records of 611 patients with cancer with prior ICI use who were evaluated as inpatients or outpatients at our institutions, although this number is not comprehensive of all patients receiving ICIs at our institutions; 359 of these patients were tested for SARS-CoV-2. The final study population included 25 patients who tested positive for SARS-CoV-2 and received ICI within 1 year of their COVID-19 diagnosis. Sixteen of 25 (64%) patients received other forms of anticancer therapy in addition to ICI, including chemotherapy and targeted therapy, within 6 months of their COVID-19 diagnosis. The median age was 72 years (range 45–83 y) and 11 (44%) patients were female (Tables 1 and 2).
Table 1.
Demographic, clinical, and treatment characteristics of patients with cancer treated with or without ICIs before testing positive for COVID-19
|
|
ICI Use,
N
= 25,
n
(%) |
No ICI Use,
N
= 25,
n
(%) |
p-
Value
|
| Age, years | 0.82 | ||
| Median (range) | 72 (45–83) | 68 (36–87) | |
| Sex | |||
| Female | 11 (44) | 11 (44) | |
| Male | 14 (56) | 14 (56) | |
| Ethnicity | 0.44 | ||
| White | 20 (80) | 17 (68) | |
| Black/African American | 2 (8) | 4 (16) | |
| Hispanic/Latino | 2 (8) | 4 (16) | |
| Other | 1 (4) | 0 | |
| Tobacco use history | 0.57 | ||
| Never smoked tobacco | 9 (36) | 12 (48) | |
| Former tobacco smoker | 12 (48) | 11 (44) | |
| Current tobacco smoker | 4 (16) | 2 (8) | |
| Type of malignancy | 0.0002 | ||
| Thoracica | 10 (40) | 1 (4) | |
| Melanoma and nonmelanoma skinb | 5 (12) | 0 | |
| Gastrointestinalc | 4 (16) | 7 (28) | |
| Otherd | 6 (24) | 17 (68) | |
| Comorbid conditions | |||
| Hypertension | 18 (72) | 11 (44) | |
| Hyperlipidemia | 14 (56) | 6 (24) | |
| Autoimmune disease | 8 (32) | 3 (12) | |
| Cardiac diseasee | 8 (32) | 6 (24) | |
| COPD | 7 (28) | 6 (24) | |
| Obesity (BMI ≥ 30) | 6 (24) | 4 (16) | |
| Diabetes | 2 (8) | 10 (40) | |
| Charlson Comorbidity Index | 0.001 | ||
| Median (range) | 9 (2–18) | 7 (2–11) | |
| Received vaccination in the last year | 0.26 | ||
| No | 16 (64) | 11 (44) | |
| Yes | 9 (36) | 14 (56) | |
| History of autoimmune disease | 0.17 | ||
| No | 17 (68) | 22 (88) | |
| Yes | 8 (32) | 3 (12) | |
| ICI received in last year | |||
| Atezolizumab | 3 (12) | ||
| Cemiplimab | 1 (4) | ||
| Durvalumab | 1 (4) | ||
| Ipilimumab | 1 (4) | ||
| Nivolumab | 3 (12) | ||
| Pembrolizumab | 16 (64) | ||
| Number of prior lines of oncologic therapy | |||
| 1 | 7 (28) | 14 (56) | |
| 2 | 11 (44) | 5 (20) | |
| 3 | 2 (8) | 4 (16) | |
| 4 | 1 (4) | 1 (4) | |
| 5 | 3 (12) | 1 (4) | |
| 6 | 1 (4) | 0 | |
| Time between last dose of ICI therapy and COVID-19 diagnosis | |||
| Actively on therapy | 7 (28) | ||
| < 3 months | 10 (40) | ||
| 3–6 months | 3 (12) | ||
| > 6 months–1 year | 5 (20) | ||
| Cancer staging at initiation of ICI (cases) or anticancer therapy (controls) | < 0.0001 | ||
| I | 0 | 4 (16) | |
| II | 0 | 0 | |
| III | 2 (8) | 0 | |
| IVA-C | 22 (88) | 9 (36) | |
| Not applicable (hematologic) | 1 (4) | 8 (32) | |
| Unknown | 0 | 4 (16) | |
| Cancer status at initiation of ICI (cases) or anticancer therapy (controls) | 0.002 | ||
| Local disease | 1 (4) | 4 (16) | |
| Lymph node metastases | 1 (4) | 0 | |
| Distant metastases | 21 (84) | 9 (36) | |
| No evidence of disease (adjuvant) | 1 (4) | 4 (16) | |
| Not applicable (hematologic) | 1 (4) | 8 (32) | |
| Disease status at last imaging prior to COVID-19 diagnosis | |||
| Progression of disease | 14 (56) | 8 (32) | |
| Stable disease | 4 (16) | 5 (20) | |
| Partial response | 7 (28) | 3 (12) | |
| Complete response | 0 | 9 (36) | |
| Other anticancer therapy in past 6 months (cases) | |||
| No | 9 (36) | ||
| Yes | 16 (64) | ||
| Concurrent with ICI | 12 | ||
| After ICI discontinuation | 4 | ||
| Anticancer therapy in past 6 months (controls) | |||
| No | 9 (36) | ||
| Yes | 16 (64) | ||
| History of radiation therapy prior to ICI | |||
| Yes | 14 (56) | ||
| History of chemotherapy prior to ICI | |||
| Yes | 14 (56) | ||
| Time between initiation of anticancer therapy and COVID-19 diagnosis | 0.52 | ||
| Median, days (range) | 125 (13– 718) | 67 (2–1684) | |
| Time between last dose of anticancer therapy | |||
| Median, days (range) | 29 (0–328) | 12 (0–118) | 0.03 |
| Patient still currently receiving ICI (or anticancer therapy) at time of COVID-19 diagnosis | 0.99 | ||
| Yes | 7 (28) | 6 (24) | |
| No | 18 (72) | 19 (74) | |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; COVID-19: coronavirus disease 2019; ICI: immune checkpoint inhibitor
For cases, thoracic cancers include five squamous cell, four adenocarcinoma, and one non–small-cell lung cancer, not otherwise specified. For controls, one case of small cell lung cancer
For cases, skin cancers include three melanoma and two nonmelanoma skin cancers (basal cell carcinoma and squamous cell carcinoma).
For cases, gastrointestinal cancers include one esophageal, one liver, one colon, and one gastric cancer. For controls, one liver, four colon, one gastric, and one pancreatic cancer
For cases, other cancer types include four oropharyngeal, one breast, and one acute myeloid leukemia (AML). For controls, one breast, one AML, one chronic myeloid leukemia, one acute lymphoblastic leukemia, one myelofibrosis, three B-cell lymphoma, one T-cell lymphoma, one multiple myeloma, two prostate, one ovarian, one thyroid, one bladder, one renal, and one brain cancer
Including coronary artery disease, congestive heart failure, arrhythmia, atrial fibrillation, and cardiac disease not otherwise specified
Table 2.
Patient demographics, oncologic history, comorbidities, and outcomes of 25 patients with COVID-19 and ICI therapy
|
Pt No.
*
|
Age (y)/Sex/ Ethnicity
|
ICI-Treated Malignancy
|
Stage
|
Comorbidities
|
ICI
|
Final COVID-19 Status
**
|
| 1 | 55/M/White | Melanoma | IV | HTN, hemophilia, hepatitis C, syphilis | Nivolumab | Fully recovered |
| 2 | 73/F/White | Lung, squamous cell | IVB | HTN, HL, COPD, pulmonary embolism, CHF, CKD, obesity | Pembrolizumab | Died |
| 3 | 47/F/Black | Breast | IV | Asthma, allergic rhinitis | Nivolumab | Fully recovered |
| 4 | 81/M/White | Esophageal | IVB | HTN, HL, IBD, peripheral neuropathy, iron deficiency anemia | Pembrolizumab | Died |
| 5 | 45/M/Hispanic or Latino | Liver | IV | DM | Atezolizumab | Recovered with complications |
| 6 | 72/F/White | Lung, squamous cell | IV | HTN, HL, COPD, malignant lymphoma, hiatal hernia, low back pain, depression, previous MI, CKD | Pembrolizumab | Died |
| 7 | 51/F/White | Oropharyngeal | IVA | Celiac disease, anemia, MALT lymphoma | Pembrolizumab | Fully recovered |
| 8 | 77/F/White | Lung, adenocarcinoma | IV | HTN, COPD, CAD, CHF, CVA, tuberculosis, CKD, obesity | Pembrolizumab | Died |
| 9 | 74/M/Black | Oropharyngeal, squamous cell | IV | HTN, dementia, HIV, CKD | Pembrolizumab | Died |
| 10 | 66/F/White | Lung, adenocarcinoma | III | HTN, cirrhosis (porphyria and hepatitis C), obesity, osteopenia, prior DVT, depression | Durvalumab | Fully recovered |
| 11 | 80/M/White | Acute myelogenous leukemia | MDS, benign prostatic hyperplasia | Ipilimumab | Died | |
| 12 | 71/M/White | Nonmelanoma skin cancer | IV | Asthma, prior DVT, homelessness, pancreatitis, temporal artery hemorrhage, otitis externa | Pembrolizumab | Fully recovered |
| 13 | 62/F/White | Lung, other | IV | Atrial fibrillation, mitral valve prolapse, iron deficiency anemia | Pembrolizumab | Fully recovered |
| 14 | 61/M/White | Oropharyngeal squamous cell | IV | HTN, HL | Pembrolizumab | Recovered with complications |
| 15 | 47/M/White | Oropharyngeal | III | HTN, oral mucositis (radiation induced), GERD, depression, obstructive sleep apnea | Pembrolizumab | Recovered with complications |
| 16 | 76/M/Hispanic or Latino | Lung, squamous cell | IV | HTN, HL, DM, asthma, COPD, PE, atrial fibrillation, pericardial effusion, prior GI bleed | Nivolumab | Fully recovered |
| 17 | 74/F/White | Lung, squamous cell | IV | HTN, HL, COPD, PE, PVD, GERD, anxiety | Atezolizumab | Recovered with complications |
| 18 | 61/M/White | Melanoma | IV | HL, CVA, eczema, psoriasis, Lyme disease, neurosyphilis, neuromyopathy | Pembrolizumab | Fully recovered |
| 19 | 80/F/White | Lung, adenocarcinoma | IVB | HTN, HL, COPD, atrial fibrillation, obesity, pacemaker, autonomic dysfunction (orthostatic), cytomegalovirus infection, depression, history of breast cancer, history of endometrial cancer, osteoarthritis | Atezolizumab | Recovered with complications |
| 20 | 75/F/White | Colon | IV | HTN, HL, IBD, GERD, arthritis | Pembrolizumab | Fully recovered |
| 21 | 66/M/White | Lung, adenocarcinoma | IV | HTN, HL, COPD, CAD, CVA, alcoholism | Pembrolizumab | Recovered with complications |
| 22 | 83/M/White | Nonmelanoma skin cancer | IV | HTN, HL, atrial fibrillation, prostate cancer, osteoarthritis, lumbar disc disease, GERD, anemia | Cemiplimab | Fully recovered |
| 23 | 54/M/White | Melanoma | IV | HTN, HL, obesity, cognitive impairment (congenital), hearing loss, ankylosing spondylitis, schizophrenia, sarcoma, psoriasis | Pembrolizumab | Fully recovered |
| 24 | 75/M/White | Lung, squamous cell | IV | HTN, HL, cardiomyopathy | Pembrolizumab | Died |
| 25 | 61/F/Other | Oropharyngeal | IVC | HTN, HL, prior PE | Pembrolizumab | Fully recovered |
CAD: coronary artery disease; CHF: congestive heart failure; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; COVID-19: coronavirus disease 2019; CVA: cerebrovascular accident; DM: diabetes mellitus; DVT: deep vein thrombosis; GERD: gastric esophageal reflux disease; GI: gastrointestinal; HIV: human immunodeficiency virus; HL: hyperlipidemia; HTN: hypertension; IBD: inflammatory bowel disease; ICI: immune checkpoint inhibitor; IV, stage 4 malignancy; MALT: mucosa-associated lymphoid tissue; MDS: myelodysplastic syndrome; MI: myocardial infarction; PE: pulmonary embolism; Pt: patient; PVD: peripheral vascular disease
Age at COVID-19 diagnosis
Recovered with complications is defined as patient having a new oxygen requirement even after COVID-19 recovery
The most common malignancy was lung (10/25, 40%), followed by oropharyngeal cancer (4/25, 16%), melanoma (3/25, 12%) and nonmelanoma skin cancer (2/25, 8%). Most patients had a solid tumor malignancy (24/25, 96%) and one patient had a hematologic malignancy (4%). The most common comorbid conditions were hypertension (18/25, 72%) and hyperlipidemia (14/25, 56%), with a median Charlson Comorbidity Index of 9 (range 2–18). The most common concomitant medications at the time of COVID-19 diagnosis were vitamin D (13/25, 52%), proton pump inhibitors (11/25, 44%), corticosteroids (8/25, 32%), and statins (8/25, 32%) (Supplementary Table 1).
The 25 controls without prior ICI use were well matched on the basis of age (68 years, range 36–87 y), sex (44% female), and receipt of anticancer therapy in the past 6 months (64%). Matching from the limited population of patients with cancer diagnosed with COVID-19 resulted in some baseline differences. Type of malignancy (p = 0.0002) and cancer staging (p < 0.0001) significantly differed, and we were unable to match exact anticancer therapy regimens (Supplementary Table 1). The controls had lower Charlson Comorbidity Index of 7 (range 2–11, p = 0.001) and received more recent anticancer therapy prior to COVID-19 diagnosis than did patients receiving ICI (12 vs 29 days, p = 0.03).
Impact of ICI on COVID-19–Related Death
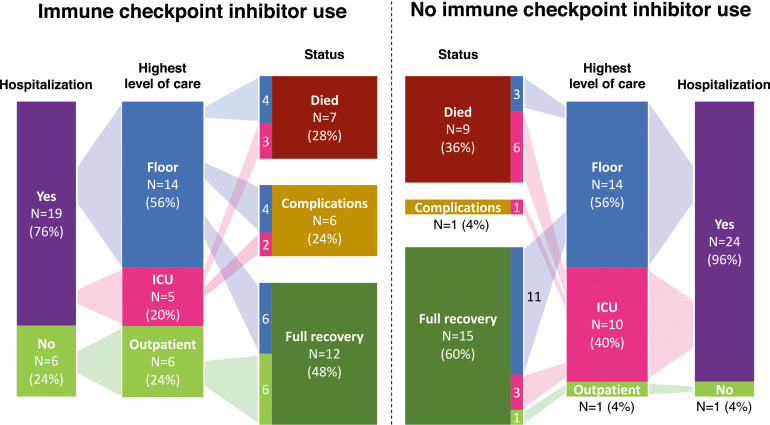
Among 25 patients receiving ICI therapy within 1 year of their COVID-19 diagnosis, the median time between last dose of ICI and COVID-19 diagnosis was 29 days (range 0–328 d) (Table 1). Among 25 control patients not receiving ICIs, the median time between last dose of anticancer therapy (non-ICI) and COVID-19 diagnosis was 12 days (range 0–118 d). Most patients receiving ICIs were treated in the inpatient setting (19/25, 76%) with six (24%) patients treated as outpatients (Fig. 1). Seven (28%) patients died owing to COVID-19, compared with nine (36%) patients in the control group.
Figure 1.
COVID-19 outcomes among 25 patients receiving ICI within 1 year of COVID-19 diagnosis and 25 case control patients. Flow graphic demonstrating the hospitalization status, highest level of care, and final COVID-19 outcomes among the two patient cohorts (25 patients with prior ICI use and 25 controls). Complications are defined as patients who continue to require supplemental oxygen after discharge. COVID-19: coronavirus disease 2019; ICI: immune checkpoint inhibitor.
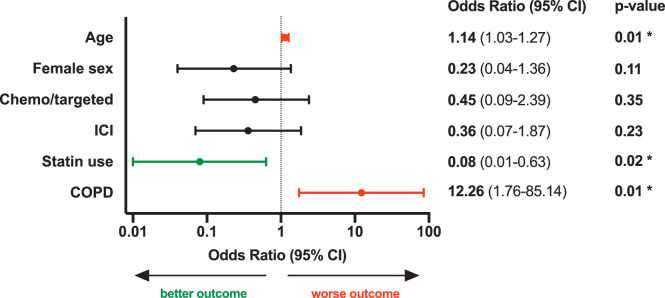
Significant predictors of COVID-19–related death included age (OR [per 1-year increment]: 1.14, 95% CI: 1.03–1.27), COPD (OR [yes versus no]: 12.26, 95% CI: 1.76–85.14), and active statin use (OR [yes versus no]: 0.08, 95% CI: 0.01–0.63) (Fig. 2). After controlling for significant predictors in our matched cohort analysis, history of ICI therapy within 1 year of COVID-19 diagnosis did not significantly impact COVID-19–related death (OR [yes versus no]: 0.36, 95% CI: 0.07–1.87, p = 0.23) (Fig. 2). When history of ICIs and/or anticancer therapy within 6 months prior to COVID-19 diagnosis was removed from the pooled analysis, age (OR: 1.14, 95% CI: 1.04–1.25), COPD (OR: 13.26, 95% CI: 1.81–97.45), and active statin use (OR: 0.09, 95% CI: 0.01–0.72) remained significant predictors for COVID-19–related death.
Figure 2.
Predictors of COVID-19 mortality in multivariable analysis. Odds ratios for the impact of baseline patient characteristics on COVID-19 mortality, including age, sex, use of non-ICI anticancer therapy (chemo/targeted) in the past 6 months, ICI therapy in the past year, active statin use, and COPD. Odds ratios were calculated by multivariable logistic regression. Error bars represent 95% CIs. The x-axis is on a log10 scale. The odds ratio for age is per increasing year. *p-value < .05. COPD: chronic obstructive pulmonary disease; COVID-19: coronavirus disease 2019; ICI: immune checkpoint inhibitor.
COVID-19–Related Clinical Characteristics and Outcomes
Most patients receiving ICIs presented with respiratory (72%) and GI (60%) symptoms (Table 3). Five (20%) patients were admitted to the ICU. Thirteen patients (52%) required supplemental oxygen, with two (8%) requiring intubation. More controls required ICU care (10/25, 40%, p = 0.02) and intubation (9/25, 36%, p = 0.04). The most common respiratory complications in patients receiving ICIs were pleural effusion (36%) and pneumonia (24%).
Table 3.
COVID-19-related outcomes of patients with cancer receiving ICIs or other anticancer therapy before testing positive for COVID-19
|
|
ICI Use,
N
= 25,
n
(%) |
No ICI Use,
N
= 25,
n
(%) |
p-
Value |
| Admission status for COVID-19 | 0.10 | ||
| Managed inpatient | 19 (76) | 24 (96) | |
| Managed outpatient | 6 (24) | 1 (4) | |
| ICU admission | 0.02 | ||
| Yes | 5 (20) | 10 (40) | |
| No | 14 (56) | 14 (56) | |
| Not hospitalized | 6 (24) | 1 (4) | |
| COVID-19 test type | 0.99 | ||
| Polymerase chain reaction | 24 (96) | 25 (100) | |
| Serology | 1 (4) | 0 (0) | |
| Presenting symptoms | |||
| Respiratorya | 18 (72) | 20 (80) | |
| Gastrointestinalb | 15 (60) | 9 (36) | |
| Fatigue/malaise | 13 (52) | 9 (36) | |
| Fever | 11 (44) | 20 (80) | |
| Chills | 5 (20) | 3 (12) | |
| Neurologicalc | 3 (12) | 3 (12) | |
| Anosmia | 2 (8) | 2 (8) | |
| Ageusia | 2 (8) | 0 (0) | |
| Asymptomatic | 1 (4) | 0 (0) | |
| Final clinical status | 0.15 | ||
| Fully recovered | 12 (48) | 15 (60) | |
| Recovered with complications | 6 (24) | 1 (4) | |
| Death | 7 (28) | 9 (36) | |
| Cause of death related to COVID-19 | |||
| Respiratory failure | 4 (57.1) | 5 (55.6) | |
| Multi-organ failure | 3 (42.9) | 3 (33.3) | |
| Sepsis | 0 | 1 (11.1) | |
| Baseline or chronic oxygen requirement | 0.02 | ||
| Yes | 6 (24) | 0 | |
| No | 19 (76) | 25 (100) | |
| Radiologic testing for COVID-19 | |||
| CXR | 17 (68) | 25 (100) | |
| CT | 12 (48) | 10 (40) | |
| Supplemental oxygen interventions for COVID-19 | 0.04 | ||
| Nasal cannula with standard O2 | 13 (52) | 18 (72) | |
| High-flow nasal cannula | 1 (4) | 0 | |
| Non-rebreather | 5 (20) | 1 (4) | |
| CPAP | 1 (4) | 0 | |
| Intubation | 2 (8) | 9 (36) | |
| BIPAP | 0 | 1 (4) | |
| Multi-organ complications related to COVID-19 | |||
| Multi-organ failure | 3 (12) | 2 (8) | |
| Sepsis | 5 (20) | 6 (24) | |
| None | 14 (60) | 18 (72) | |
| Pulmonary complications related to COVID-19 | |||
| Respiratory failure | 2 (8) | 9 (36) | |
| Pleural effusion | 9 (36) | 1 (4) | |
| Pneumonia | 6 (24) | 9 (36) | |
| None | 6 (24) | 10 (40) | |
| ARDS | 3 (12) | 10 (40) | |
| Pneumonitis | 2 (8) | 0 | |
| Pulmonary embolism | 2 (8) | 0 | |
| Unknown | 1 (4) | 0 | |
| Cardiac complications related to COVID-19 | |||
| Hypotension | 6 (24) | 4 (16) | |
| Cardiac ischemia | 2 (8) | 1 (4) | |
| Atrial fibrillation | 4 (16) | 5 (20) | |
| CHF exacerbation | 1 (4) | 0 | |
| Sinus bradycardia | 1 (4) | 0 | |
| Right ventricular conduction delay | 1 (4) | 1 (4) | |
| Cardiomyopathy | 0 | 1 (4) | |
| None | 13 (52) | 16 (64) | |
| Treatments for COVID-19 and related complications | 0.04 | ||
| Supportive care | 15 (60) | 8 (32) | |
| Antibiotics | 13 (52) | 21 (84) | |
| Chloroquine/ hydroxychloroquine | 4 (16) | 7 (28) | |
| Remdesivir | 3 (12) | 10 (40) | |
| Vasopressors | 2 (8) | 0 | |
| Tocilizumab | 2 (8) | 0 | |
| Glucocorticoids | 2 (8) | 0 | |
| Mechanical ventilation | 2 (8) | 9 (36) | |
| Anticoagulants | 2 (8) | 0 | |
| Beta blockers/calcium channel blockers | 1 (4) | 0 | |
| Platelets | 1 (4) | 0 | |
| Antivirals | 1 (4) | 2 (8) | |
ARDS: acute respiratory distress syndrome; BIPAP: Bilevel airway positive pressure; CHF: congestive heart failure; COVID-19: coronavirus; CPAP: continuous positive airway pressure; CT: computed tomography scan; CXR: chest radiograph; ICI: immune checkpoint inhibitor; ICU: intensive care unit
For cases, respiratory symptoms include shortness of breath (12, 48%), dry cough (7, 28%), sore throat (3, 12%), cough with sputum (3, 12%), and nasal congestion (1, 4%). For cases, respiratory symptoms include dry cough (14, 56%), shortness of breath (8, 32%), cough with sputum (2, 8%), sore throat (1, 4%), hemoptysis (1, 4%), and nasal congestion (1, 4%)
For cases, gastrointestinal symptoms include nausea (6, 24%), vomiting (5, 20%), diarrhea (4, 16%), and abdominal pain (3, 12%). For controls, gastrointestinal symptoms include diarrhea (8, 32%), nausea and vomiting (4, 16%), and abdominal pain (2, 8%)
For cases, neurological symptoms include altered mental status (1, 4%) and headache (2, 8%). For controls, neurological symptoms include headache (2, 8%) and altered mental status (1, 4%)
Immune-Related Adverse Events
Eight (32%) patients had a history of confirmed irAEs prior to their COVID-19 diagnosis. The most common prior irAEs were dermatitis (3/8, 37.5%), hepatitis (3/8, 37.5%), and hypophysitis (2/8, 25%). We followed up patients for a median of 57 days (range 21–83 d) after COVID-19 diagnosis. Three (12%) patients had documented grade-3 irAEs during their COVID-19 course, with one irAE (hypothyroidism) being new in onset and two being persistent irAEs (hypophysitis) (Table 4, Supplementary Fig. 1).
Table 4.
Immune-related adverse events occurring in patients before and during COVID-19 course
|
Patient No.
|
Tumor Type
|
Last ICI Regimen
|
Prior to COVID-19 Diagnosis
|
During COVID-19 Diagnosis
|
||
|
irAE No. 1 (Grade)
|
irAE No. 2 (Grade)
|
irAE No. 3 (Grade)
|
irAE No. 1 (Grade)
|
|||
| 2 | Melanoma | Nivolumab | Colitis (3) | Hepatitis (3) | Pancreatitis (2) | |
| 11 | Lung | Durvalumab | Dermatitis (2) | |||
| 27 | Oropharyngeal | Pembrolizumab | Hepatitis (1) | Dermatitis (1) | ||
| 19 | Melanoma | Pembrolizumab | Pancreatitis (3) | Hepatitis (1) | Hypophysitis (3) | Hypophysitis (3)a |
| 23 | Skin cancer | Cemiplimab | Dermatitis (2) | |||
| 9 | Lung | Pembrolizumab | Pneumonitis (3) | |||
| 3 | Lung | Pembrolizumab | Hypophysitis (3) | Hypophysitis (3)a | ||
| 5 | Esophageal | Pembrolizumab | Hypothyroidism (3)b | |||
COVID-19: coronavirus disease 2019; ICI: immune checkpoint inhibitor; irAE: immune-related adverse event
Persistent irAE continuing during COVID-19 course
New irAE developing during COVID-19 course
Laboratory Findings
We investigated differences between laboratory values for cases and controls (Supplementary Fig. 2; Supplementary Table 2). Most hematologic and inflammatory biomarkers did not differ between the two cohorts. Although both ICI and control cohorts had similar percentages of patients on cytotoxic chemotherapy within 6 months prior to COVID-19 diagnosis—60% and 64%, respectively—patients with ICI use had significantly higher presenting platelet count (median 226 vs 173 K/mcL, p = 0.02) and D-dimer level (median 1850 vs 1123 ng/mL, p = 0.04) compared to controls.
We also compared laboratory results between survivors and deaths within each cohort (Supplementary Fig. 3). Elevated presenting, peak, and nadir troponin levels were related to COVID-19 mortality in patients with ICI use (p = 0.04, 0.01, 0.03) but not in controls.
DISCUSSION
A challenge facing oncologists is the uncertainty regarding the impact of ICIs on COVID-19 outcomes in patients with cancer. In this retrospective matched cohort study of 25 patients treated at major academic centers, exposure to ICIs within a year of COVID-19 diagnosis was not associated with increased COVID-19–related mortality. Notably, patients receiving ICIs did not fare worse despite having a higher proportion of underlying thoracic malignancies, smoking history, obesity, metastatic disease, and higher Charlson Comorbidity Index, although they had a lesser proportion of hematologic malignancy and diabetes mellitus, both of which were associated with worse COVID-19 outcomes.[30]
Interestingly, the 28% mortality rate among patients with ICI use in our cohort mirrors the mortality rate for patients with cancer in two cancer cohort studies from Montefiore[27] (New York, NY) and the United Kingdom[26] and is higher than that from studies from the Cancer Consortium (13%)[5] and Mount Sinai (New York, NY)(11%).[4]
Previous studies in patients with cancer have conflicting results, with recent larger studies supporting a favorable impact of ICIs on COVID-19 clinical outcomes, congruous with our findings. Prior ICI use in 31 patients included in a study[6] of 423 patients with cancer in New York predicted hospitalization (hazard ratio [HR] 3.06) and severe respiratory illness (HR 3.03), defined as high-flow oxygen supplementation or mechanical ventilation, when compared with patients with no ICI use In contrast, another case series from France22 and a global registry for thoracic cancers with over 70 patients[23,24] did not find a significant impact of ICI on COVID-19 outcomes. In a UK study of 44 patients, recent ICI therapy within 4 weeks of COVID-19 diagnosis trended toward decreased risk of COVID-19 death (OR 0.59).[26] Similarly, in a study with 41 patients with lung cancer, PD-1 blockade was not significantly associated with increased risk of COVID-19 severity or mortality.[25] In one prospective study among 461 patients, recent anticancer treatment including immunotherapy within 4 weeks of COVID-19 diagnosis was not a significant contributor to COVID-19 mortality.[26] The conflicting results of these studies could be attributed in part to differences in sample size, patient characteristics, and cancer type.
ARDS is a severe COVID-19 complication that is driven by inflammatory cytokines.[14] The interleukin-6 (IL-6) cytokine is elevated in both patients with COVID-19 and patients who develop irAEs, which may suggest a similar mechanism of immune activation. Accordingly, patients with COVID-19 receiving ICIs may be at increased risk for more profound cytokine storm and immune hyperactivation. Notably, our data did not find that this was evident on laboratory evaluation, was significant enough to induce irAEs, or led to higher death rate. ICIs may induce favorable outcomes by improving viral clearance[17] and restoring immunocompetency in exhausted T cells.[18–20] However, this study is retrospective and therefore is not positioned to answer this question; further study of immune correlates is required.
We also evaluated ICI-induced irAEs in the setting of COVID-19. Two patients (8%) developed COVID-19 pneumonitis; despite radiologic overlap,[31] neither were initially suspected of having ICI-induced pneumonitis. Notably, we only observed one new-onset irAE. There were two cases of persistent hypophysitis, which is expected, as this generally requires chronic hormone replacement. Further study is warranted to evaluate whether a longer follow-up period would alter rates of delayed effects of COVID-19 on irAEs.
Age and COPD were independent predictors of COVID-19–related death in our entire cohort. Advanced age[6,27,32,33] and COPD[25,34–36] are known significant predictors of COVID-19 hospitalization and mortality in patients with cancer. Increased mortality rates may be due to higher risk for respiratory failure[35] or increased airway expression of angiotensin-converting enzyme II (ACE2) in patients with COPD, which predisposes to viral entry.[37] Thus, patients with cancer who have COPD, regardless of anticancer therapy, may be at greater risk of COVID-19 mortality.
Active statin use had a significant protective effect, aligning with known evidence. Statin intake has been associated with the absence of COVID-19 symptoms (OR 2.91).[38] Retrospective data from China show a significant decrease in mortality (HR 0.58) in patients taking statins.[39] Statins may have an anti-inflammatory effect and dampen immune response in the setting of severe COVID-19 infection.[40–42] Statin therapy may also stimulate angiopoietin-1 and maintain myeloid differentiation factor 88 levels, the adaptor protein of toll-like receptors, leading to mitigation of NF-κB activation and decreased risk of ARDS.[38,42] In contrast, there is a potential mechanistic negative effect of statins on COVID-19 infection from increasing ACE2 expression.[43] Given the ubiquitous use of statins, larger studies are warranted to explore this relationship.
We did not observe differences in most biomarkers between ICI and non-ICI patients with cancer. Notably, ICI patients had significantly higher platelet counts and D-dimer levels at presentation than non-ICI patients, although a similar proportion of patients in both cohorts had D-dimer levels above the reference range (86.7% vs 83.3%).
The activation of coagulation pathways from the cytokine response to COVID-19 infection[44,45] could be augmented with checkpoint blockade owing to the inflammatory contribution of reinvigorated T cells.[46] Although D-dimer and platelet counts were significantly elevated among ICI patients, the clinical relevance is uncertain because all deaths were related to respiratory failure and not due to hypercoagulable states.[47] Among ICI patients, two (8%) experienced nonfatal pulmonary embolisms compared with zero clotting events among control patients.
Elevated troponin levels predicted COVID-19 deaths in patients receiving ICIs, while this differentiation was not observed in non-ICI patients. The underlying mechanism is still unclear; however, this discrepancy may be due to potential inflammatory differences mediated by ICI treatment, including the increased presenting levels of D-dimer in ICI patients.[48,49]
Limitations
This study is limited by its small sample size and retrospective nature. Our methodology was not all-encompassing in comprehensively searching for all patients receiving ICIs with coexisting COVID-19 diagnosis among our institutions. We may have missed COVID-19–infected patients who tested or received care elsewhere or who did not test owing to asymptomatic or mild symptoms in the time frame. Another possibility for bias is the greater proportion of ICI patients managed as outpatients compared with controls. However, we repeated the multivariable logistic analysis of only inpatients (N = 38) and did not find a significant difference in outcomes or ORs (Supplementary Fig. 4). Furthermore, our patients varied in regard to recency of last ICI dose; most were actively receiving therapy (28%) or had their last ICI dose within 3 months of COVID-19 diagnosis (40%) (Table 1). Receptor occupancy of ICIs decays gradually over the 6 months following ICI discontinuation,[50] and we were unable to control for differences in risk among patients according to recency of their last ICI dose. Moreover, we did not account for Eastern Cooperative Oncology Group (ECOG) status, which could affect survival outcomes, as this information was not documented or available. Lastly, we were limited to 1:1 matching for cases to controls, based on three criteria, given our small total control population of cancer patients with COVID-19 infection. Accordingly, we were unable to control for cancer malignancy stage or cancer types in our primary analysis.
CONCLUSION
This study provides insight on the COVID-19 outcomes and irAEs of patients with cancer receiving ICIs. Substantially, our matched cohort analysis found that ICI treatment is not associated with greater risk of COVID-19 mortality. Consistent with previous reports, age and COPD were significant risk factors for death within our cohort, and statins provided a protective benefit. We observed low rates of new or persistent irAEs within our small sample. Finally, patients receiving ICIs only exhibited differences in elevated platelets and D-dimer at COVID-19 diagnosis. Although larger prospective studies are necessary to evaluate long-term safety of ICIs during COVID-19 infection, our findings support the continuation of ICIs during the COVID-19 pandemic for patients with cancer because we did not observe an increased risk of COVID-19–related death associated with ICI therapy.
Supplementary Material
Acknowledgment
We acknowledge the DFCI Oncology Data Retrieval System (OncDRS) for the aggregation, management, and delivery of the clinical and operational research data used in this project. The content is solely the responsibility of the authors.
Supplemental Material
Supplemental data are available online with the article.
Funding Statement
Source of Support: None.
Footnotes
Conflict of Interest: Isaac A. Klein is a shareholder and member of the Scientific Advisory Board of Dewpoint Therapeutics, a shareholder in Infinite MD, and consultant to Day-to-Day Health. Shilpa Grover is an editor in gastroenterology at UpToDate, Wolters Kluwer Inc. Nicole R. LeBoeuf is a consultant and has received honoraria from Bayer, Seattle Genetics, and Sanofi. Osama Rahma has received research support from Merck; serves as a speaker for activities supported by educational grants from BMS and Merck; consultant for Merck, Celgene, Five Prime, GSK, Bayer, Roche/Genentech, Puretech, Imvax, and Sobi; and has a pending patent titled “Methods of Using Pembrolizumab and Trebananib.” The remaining authors had nothing to disclose.
References
- 1.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov . 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhalabi O, Subbiah V. Managing cancer care during the COVID-19 pandemic and beyond. Trends Cancer . 2020;6:533–535. doi: 10.1016/j.trecan.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Report of the WHOChina Joint Mission on Coronavirus Disease 2019 (COVID19) WHO; February 16–24, 2020. [Google Scholar]
- 4.Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19: an experience in New York City. Ann Oncol . 2020;31:1088–1089. doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet . 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nature Med . 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adashek JJ, Hajjar J, Chemaly RF, Kurzrock R. Are cancer patients at higher risk of death with COVID-19? J Immunother Precis Oncol . 2020;3:49–51. doi: 10.4103/2666-2345.280883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open . 2019;2:e192535. doi: 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med . 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin . 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 11.Khan S, Khan SA, Luo X, et al. Immune dysregulation in cancer patients developing immune-related adverse events. Br J Cancer . 2019;120:63–68. doi: 10.1038/s41416-018-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science . 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 13.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm' in COVID-19. J Infect . 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnaldez FI, O'Day SJ, Drake CG, et al. The Society for Immunotherapy of Cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response. J Immunother Cancer . 2020 May;8:e000930. doi: 10.1136/jitc-2020-000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonomi L, Ghilardi L, Arnoldi E, et al. A rapid fatal evolution of Coronavirus Disease-19 (COVID-19) in an advanced lung cancer patient with a long time response to nivolumab. J Thorac Oncol . 2020;15:E83–E85. doi: 10.1016/j.jtho.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovly CM, Boyd KL, Gonzalez-Ericsson PI, et al. Rapidly fatal pneumonitis from immunotherapy and concurrent SARS-CoV-2 infection in a patient with newly diagnosed lung cancer. medRxiv . Published online May 1, 2020. 2020.04.29.20085738. [DOI]
- 17.Cortese I, Muranski P, Enose-Akahata Y, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med . 2019;380:1597–1605. doi: 10.1056/NEJMoa1815039. [DOI] [PubMed] [Google Scholar]
- 18.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature . 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 19.Schönrich G, Raftery MJ. The PD-1/PD-L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol . 2019;9:207. doi: 10.3389/fcimb.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol . 2018;18:91–104. doi: 10.1038/nri.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther . 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barlesi FF, S. Bayle A, Gachot B, et al. Outcome of cancer patients infected with COVID19 including toxicity of cancer treatments. Presented at: AACR Virtual Meeting I; USA: 2020. [Google Scholar]
- 23.2020 AACR Annual Virtual Meeting I. USA: 2020. G MC. TERAVOLT (Thoracic cancERs international coVid 19 cOLlaboraTion): first results of a global collaboration to address the impact of COVID-19 in patients with thoracic malignancies. Presented at. [Google Scholar]
- 24.Horn L, Whisenant JG, Torri V TERAVOLT Consortium Investigators. 2020 AACR Annual Virtual Meeting I. USA: 2020. Thoracic Cancers International COVID-19 Collaboration (TERAVOLT): impact of type of cancer therapy and COVID therapy on survival. Presented at. [Google Scholar]
- 25.Luo J, Rizvi H, Egger JV, et al. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov . 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee LYW, Cazier JB, Starkey T, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet . 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov . 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med . 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Common terminology criteria for adverse events (ctcae) v5.0. Published on Nov, 27, 2017. Accessed May 21, 2020. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf . [PubMed]
- 30.He W, Chen L, Yuan G, et al. COVID-19 in persons with haematological cancers. Leukemia . 2020;34:1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol . 2017;35:709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo J, Rizvi H, Preeshagul IR, et al. COVID-19 in patients with lung cancer. Ann Oncol . 2020;31:1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol . 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet . 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One . 2020;15:e0233147. doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir Med . 2020;167:105941. doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J . 2020;55:2000688. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Spiegeleer A, Bronselaer A, Teo JT, et al. The effects of ARBs, ACEIs and statins on clinical outcomes of COVID-19 infection among nursing home residents. J Am Med Dir Assoc . 2020;21:909–914. doi: 10.1016/j.jamda.2020.06.018. E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X-J, Qin J-J, Cheng X, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab . 2020;32:176–187. doi: 10.1016/j.cmet.2020.06.015. E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiner Ž, Hatamipour M, Banach M, et al. Statins and the COVID-19 main protease. Arch Med Sci . 2020;16:490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fedson DS, Opal SM, Rordam OM. Hiding in plain sight: an approach to treating patients with severe COVID-19 infection. mBio . 2020;11:e00398–20. doi: 10.1128/mBio.00398-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dashti-Khavidaki S, Khalili H. Considerations for statin therapy in patients with COVID-19. Pharmacotherapy . 2020;40:484–486. doi: 10.1002/phar.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol . 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med . 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood . 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunimasa K, Nishino K, Kimura M, et al. Pembrolizumab-induced acute thrombosis: a case report. Medicine . 2018;97:e10772–e10772. doi: 10.1097/MD.0000000000010772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young A, Chapman O, Connor C, et al. Thrombosis and cancer. Nat Rev Clin Oncol . 2012;9:437–449. doi: 10.1038/nrclinonc.2012.106. [DOI] [PubMed] [Google Scholar]
- 48.Spallarossa P, Tini G, Sarocchi M, et al. Identification and management of immune checkpoint inhibitor–related myocarditis: use troponin wisely. J Clin Oncol . 2019;37:2201–2205. doi: 10.1200/JCO.18.02464. [DOI] [PubMed] [Google Scholar]
- 49.Mir H, Alhussein M, Alrashidi S, et al. Cardiac complications associated with checkpoint inhibition: a systematic review of the literature in an important emerging area. Can J Cardiol . 2018;34:1059–1068. doi: 10.1016/j.cjca.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol . 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.