Abstract
Macrophage infiltration has been identified as an independent poor prognostic factor for several cancers. Macrophages also orchestrate various tumor-promoting processes. This observation sparked an interest to therapeutically target these plastic innate immune cells. To date, blockade of colony-stimulating factor 1 (CSF1) or its receptor represents one of the selective approaches to manipulate tumor-associated macrophages. In this review, I discuss the efficacy and safety of various CSF1 receptor tyrosine kinase inhibitors, anti–CSF1 receptor monoclonal antibodies, and anti-CSF1 monoclonal antibodies in clinical development for patients with cancer and highlight potential combination partners, mainly anti–program cell death protein 1 (PD-1) and program cell death protein ligand 1 (PD-L1) antibodies.
Keywords: tumor-associated macrophages, colony-stimulating factor 1 receptor, tenosynovial giant cell tumor
INTRODUCTION
Two major pathways of macrophage activation have been described associated with the activation of distinct T-lymphocyte immune responses. Classical activation is associated with the actions of interferon-γ and directed toward killing of intracellular pathogens.[1] Alternative activation, involving responses to interleukin (IL) 4, IL-5, or IL-13, has been associated with helminths and allergy.[2] These states of macrophage activation have also been called M1 and M2, respectively, linked to the activation of Th1 and Th2 cells.[3] Tumor-associated macrophage (TAM) phenotype is anti-inflammatory, immune-regulatory, and therefore tumor promoting (alternatively activated or M2 macrophages) as opposed to proinflammatory and tumoricidal (classically activated or M1 macrophages).[4] TAMs are a poor prognostic factor in a number of cancers.[5,6] However, macrophage differentiation is a continuum rather than a dichotomy.[7] Among many targets in macrophages (e.g., calreticulin,[8] programmed cell death protein-1 [PD-1][9]), the most extensively studied TAM-directed therapies are targeting CD47[10,11] (and other “do not eat me” signals such as CD24[12]) and colony-stimulating factor 1 receptor (CSF1R).[13]
CSF1R, also known as macrophage colony-stimulating factor receptor (M-CSFR or CD115), which belongs to the type III protein tyrosine kinase receptor family, is mainly restricted to cells of the mononuclear phagocyte lineage and macrophages in particular. There are two ligands of CSF1R, macrophage colony-stimulating factor (M-CSF or CSF1)[14] and IL-34.[15] CSF1R signaling regulates the differentiation of myeloid cells toward an M2 phenotype of macrophage, which within the tumor microenvironment promotes survival, proliferation,[16] and metastatic potential of tumor cells,[17] along with suppressing antitumor immunity.[18] In animal models, CSF1R inhibition strongly reduces F4/80+ tumor-associated macrophages accompanied by an increase of the CD8+/CD4+ T-cell ratio.[19]
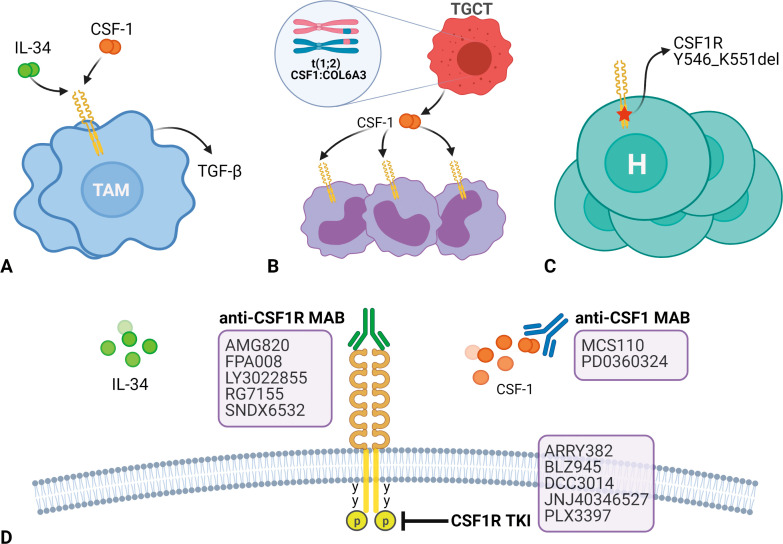
In addition to TAMs, the CSF1-CSF1R pathway plays an important role in other diseases. Tenosynovial giant cell tumor (TGCT, previously known as pigmented villonodular synovitis) is a benign neoplasm that develops in the synovial lining of joints, tendon sheaths, and bursae. TGCTs harbor a reciprocal somatic chromosomal translocation, t(1;2)(p13;q37), resulting in fusion of the type VI collagen α-3 promoter upstream of the coding sequence of the MCSF gene. As a result, the synovial cells overexpress CSF1, which leads to the recruitment of CSF1R-expressing macrophages that constitute the “tumor” in TGCT (Fig. 1B).[20]
Figure 1.
Pathogenic roles of colony-stimulating factor 1 receptor (CSF1R). A. Tumor-associated macrophages (TAMs); B. Tenosynovial giant cell tumors (TGCTs) with translocation t(1;2) CSF1:COL6A3 with recruitment of CSF1R-expressing cells including macrophages, giant cells, and osteoclasts. C. Histiocytosis (H) with CSF1R Y546_K551 del. D. CSF1R blockade strategies. MAB: monoclonal antibody; TKI: tyrosine kinase inhibitor. Figure created with biorender.com.
Histiocytic neoplasms (including Langerhans cell histiocytosis, Erdheim-Chester disease, juvenile xanthogranuloma [JXG], Rosai-Dorfman disease, and histiocytic sarcoma) are a spectrum of clonal proliferations of a special type of immature dendritic cell called the Langerhans cell. Most histiocytic neoplasms have gene mutations or fusions, with CSF1R mutations being more common in JXG (Fig. 1C).[21]
A variety of tyrosine kinase inhibitors (TKIs) and monoclonal antibodies (MABs) directed at CSF1R or CSF1 are in clinical development both as monotherapy or in combination with immunotherapy (e.g., anti–PD-1/L1 antibodies) (because preclinical studies showed that cancer cell lines consistently produced CSF1 after exposure to CD8+ T cells or CD8+ T-cell–derived cytokines, coenrichment of CD8+ T cells with CSF1 in human cancer was associated with nonresponsiveness to PD-1 checkpoint blockade, and combination of anti–PD-1 and anti-CSF1R antibodies induced the regression of BRAFV600E-driven, transplant mouse melanomas dependent on the effective elimination of TAMs)[22] or chemotherapy (because preclinical studies revealed that targeting TAMs by CSF1R blockade in the transgenic mouse model for breast cancer stimulated intratumoral type I interferon signaling, which enhanced the anticancer efficacy of platinum-based chemotherapies).[23] In this review, I evaluate the state of the art of CSF1R inhibitors, focusing on the limitations and potential side effects.
METHODS
A systematic review of the English literature from 2010 was undertaken in December 2020 using the terms colony stimulating factor 1, colony stimulating factor 1 receptor, tyrosine kinase inhibitors, monoclonal antibodies, phase I trials, phase II trials, phase III trials, and clinical trials. Ovid, Medline (PubMed.gov), and Embase were evaluated. The abstracts of American Association of Cancer Research (AACR) Annual Meeting, American Society of Clinical Oncology Annual Meeting, European Society of Medical Oncology Congress, and AACR-National Cancer Institute-European Organization for Research and Treatment of Cancer Molecular Targets and Cancer Therapeutics Symposium from 2010 were also evaluated. The reference lists of the included manuscripts were also reviewed to ensure all the relevant trials were enrolled.
RESULTS
CSF1R Tyrosine Kinase Inhibitors (Tables 1 and 2)
Table 1.
Summary of biochemical IC50 values of CSF1R tyrosine kinase inhibitor in clinical development
|
Code
|
IC50
|
|||||
|
CSF1R, nM
|
KIT, nM
|
FLT3β, nM
|
PDGFRβ, nM
|
LCK
|
ABL
|
|
| ARRY382[24] | 9 | NA | NA | NA | NA | NA |
| BLZ945[25] | 1 | 3200 | 9100 | 4800 | > 10 μM | > 10 μM |
| DCC3014[28] | 3 | 1600 | NA | NA | 2800 nM | 2900 nM |
| JNJ40346527 (edicotinib)[29] | 3.2 | 20 | 190 | NA | NA | NA |
| PLX3397 (pexidartinib)[30] | 13 | 27 | 160 | NA | 860 nM | NA |
ABL: Abelson; CSF1R: colony-stimulating factor 1 receptor; FLT3: fms-like tyrosine kinase 3; IC50: half maximal inhibitory concentration; KIT: stem cell factor receptor; LCK: lymphocyte-specific protein tyrosine kinase; NA: not available; PDGFRβ: platelet-derived growth factor receptor β.
Table 2.
CSF1R tyrosine kinase inhibitors in clinical development
|
Code
|
Generic
|
Phase I Trial
|
Phase Ib Trial
|
|||
|
DLT
|
MTD, mg/d
|
RP2D, mg/d
|
Response
|
ICI Partner
|
||
| ARRY382[24] | CK, pyrexia, AST | 400 | 400 | None | Pembrolizumab | |
| BLZ945[26] | Amylase, lipase, AST, ALP, sudden death | 1200 | 1200 | 1 PR in GBM | Spartalizumab (1 PR in HNSCC) | |
| DCC3014[28] | Lipase, hypocalcemia | 1 PR in TGCT | Avelumab | |||
| JNJ40346527[29] | Edicotinib | None | None | None | 1 CR in HL | |
| PLX3397*[30,31] | Pexidartinib | None | None | 1000 | 12 PRs in TGCT[12] | Durvalumab Pembrolizumab |
ALP: alkaline phosphatase; AST: aspartate aminotransferase; CK: creatinine kinase; CR: complete response; CSF1R: colony-stimulating factor 1 receptor; DLT: dose-limiting toxicity; FLT3: fms-like tyrosine kinase 3; GBM: glioblastoma multiforme; HL: Hodgkin lymphoma; HNSCC: head and neck squamous cell carcinoma; ICI: immune checkpoint inhibitor; MTD: maximum tolerated dose; PR: partial response; RP2D: recommended phase II dose; TGCT: tenosynovial giant cell tumor.
PLX3397 (pexidartinib) is a CSF1, KIT, FLT3 inhibitor.
ARRY382
ARRY382 is an investigational, selective, oral inhibitor of CSF1R tyrosine kinase (half maximal inhibitory concentration [IC50], concentration required for obtaining 50% of a maximum effect) = 9 nM). A phase I trial was conducted in refractory solid malignancies. The sites of primary cancer were colorectal (n = 8), breast (n = 2), pancreatic (n = 2), prostate (n = 2), non–small cell lung cancer (NSCLC; n = 2), and others (n = 10). The median number of prior treatments was five (range, two–16). Dose-limiting toxicities (DLTs) were creatinine kinase (CK) increase, pyrexia, and aspartate aminotransferase (AST) increase. The maximum tolerated dose (MTD) was 400 mg once a day with biologic activity observed at doses of 200 mg or greater once a day. There was a dose-proportional, predictable pharmacokinetics with good target coverage. The half-life (t1/2) of ARRY382 was ∼18 hours. No objective responses were observed.[24] A phase Ib trial of ARRY382 plus pembrolizumab (anti–PD-1 antibody) is ongoing.
BLZ945
BLZ945 is an investigational, selective, oral, brain-penetrant inhibitor of CSF1R tyrosine kinase. In animal models, including intracranial glioblastoma multiforme (GBM), CSF1 blockade by BLZ945 may reduce TAM recruitment to the tumor microenvironment, inhibit tumor growth, and overcome resistance to PD-1 inhibitors.[25] BLZ945 was used as a single agent and in combination with spartalizumab (anti–PD-1 antibody), in patients with cancers that are linked to the upregulation of TAMs, including glioblastoma (GBM) and pancreatic cancer. A phase I trial of BLZ945 or BLAZ945 + spartalizumab (anti–PD-1 antibody) was presented. Patients received different schedules of BLZ945 including weekly (300–1600 mg/d) or 4 days on/10 days off (300–1200 mg/d); or BLZ945 weekly (150–1400 mg/d) or 4 days on/10 days off (300–1200 mg/d) + spartalizumab (400 mg every 4 weeks). A total of 146 patients receiving BLZ945 (n = 77) or the combination (n = 69) were reported. The sites of primary cancer were GBM (n = 24), pancreatic cancer (n = 19), and colorectal cancer (n = 10) and others (breast cancer, mesothelioma, soft tissue sarcoma). The median number of prior treatments was not reported. DLTs occurred in 7/77 patients in the BLZ945 arm (increases in amylase, lipase, AST, alkaline phosphatase [ALP]; sudden death) and 7/69 patients in the combination arm (increases in amylase, AST, alanine aminotransferase [ALT]; dizziness, hyperuricemia). Treatment-related adverse events (AEs) (≥ 20%) were AST increase (35%), nausea (29%), and vomiting (23%) in the BLZ945 arm; and AST increase (38%), ALT increase (25%), vomiting (23%), and nausea (20%) in the combination arm. Grade 3 or greater treatment-related AEs were reported in 19/77 (25%) patients in the BLZ945 arm and 23/69 (33%) patients in the combination arm. BLZ945 exposure increases were less than dose proportional after 600/700 mg. The t1/2 of BLZ945 was 15–24 hours without interaction with spartalizumab. Per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, there was a partial response (PR) in one patient with head and neck cancer in the combination arm. In evaluable patients with relapsed/refractory GBM (n = 18; 7 in BLZ945 arm, 11 in combination arm), two PRs were reported per Response Assessment in Neuro-Oncology (RANO) criteria (one in BLZ945 arm, one in combination arm). Recommended phase II dose (RP2D) was 1200 mg/d (4 days on/10 days off) for single-agent BLZ945. The MTD was 700 mg/d (4 days on/10 days off) for BLZ945 + spartalizumab.[26,27]
DCC3014
DCC3014 is an investigational, selective, oral inhibitor of CSF1R tyrosine kinase. DCC3014 was designed to selectively bind to the CSF1R switch pocket. DCC3014 has greater than 100-fold selectivity for CSF1R over the closely related kinases FLT3, KIT, PDGFRα, PDGFRβ, and VEGFR2. In preclinical animal studies, DCC3014 reduced M2 macrophages. In a phase I trial of DCC3014, DCC3014 was generally well tolerated in patients with malignant solid tumors and diffuse-type TGCT. The sites of primary cancer were colorectal (n = 8), pancreatic (n = 5), ovarian (n = 4), TGCT (n = 3), prostate (n = 3), and others (n = 16). The median number of prior treatments was not reported. Loading doses used in the cohort 2 and subsequent cohorts were based on pharmacokinetic (PK) profiles observed in the cohort 1. There were 2 DLTs in the cohort 1 (10 mg once daily), grade 4 lipase increase, and grade 3 hypocalcemia. Any grade of asymptomatic serum enzyme elevation and grade 3 hypocalcemia were excluded from DLTs for evaluation of subsequent cohorts. Grade 1 or 2 AST elevations were seen in 92% of patients. Grade 1 ALT elevations were observed in 29% of patients. The t1/2 of DCC3014 was not reported. Exposure to DCC3014 was associated with an increase in plasma CSF1 and IL-34 in plasma. A rapid, sustained reduction of CD16+ monocytes was observed in peripheral blood. One patient had a confirmed PR that sustained for 9 months with improved mobility and reduced pain. Dose escalation part is ongoing to determine the RP2D.[28] These data have recently been updated at Connective Tissue Oncology Society 2021 Virtual Annual Meeting with 25 patients with TGCT. In addition, a phase Ib trial of DCC3014 plus avelumab (anti–PD-L1 [programmed cell death-ligand 1] antibody) is ongoing.
JNJ40346527 (edicotinib)
JNJ40346527 is an investigational, selective, oral inhibitor of CSF1R tyrosine kinase. The clinical development of JNJ40346527 mainly focused on noncancer indications. A phase I/II trial of JNJ40346527 in relapsed or refractory classical Hodgkin lymphoma was conducted. The median number of prior treatments was six (range, three–14). Patients (n = 21) were assigned to sequential cohorts (including 150, 300, 450, and 600 mg once a day) of JNJ40346527. No DLTs were observed and MTD was not established. Most common drug-related AEs were nausea, headache, and pyrexia. The t1/2 of JNJ40346527 was not reported. JNJ40346527 exposure increased in near dose proportionality from 150 to 450 mg once a day, but plateaued at 600 mg once a day. Target engagement (> 80% inhibition of CSF1R phosphorylation, 4 hours after dosing) was confirmed. Preliminary antitumor activity was limited with a complete response (CR) in 1 patient lasting for almost 1 year and ongoing.[29]
PLX3397 (pexidartinib)
PLX3397 is a multi-targeted receptor TKI of CSF1R, KIT, and FLT3 with IC50 of 13 nM, 27 nM, and 160 nM, respectively. Strictly speaking, PLX3397 is a conformation-specific inhibitor, not a TKI, of CSF1R. PLX3397 binds the autoinhibited state of CSF1R and makes direct contact with the juxtamembrane region. The first-in-human phase I trial of PLX3397 was conducted. In the dose escalation, the dose of PLX3397 was escalated in patients with solid malignancies (n = 41). The sites of primary cancer were colorectal (n = 10), soft tissue sarcoma (n = 4), breast (n = 3), ovarian (n = 3), prostate (n = 3), unknown primary (n = 3), pancreatic (n = 2), non–small cell lung (n = 2), osteosarcoma (n = 2), endometrial (n = 2), and others (n = 9). The median number of prior treatments was not reported. In the cohort expansion, PLX3397 at the RP2D was evaluated in patients with TGCT (n = 23). The RP2D of PLX3397 was 1000 mg per day. In the cohort-expansion part, 12 patients with TGCTs had a PR. The median duration of response exceeded 8 months. The most common AEs included fatigue, change in hair color, nausea, dysgeusia, and periorbital edema. AEs rarely led to discontinuation of treatment.[30] A phase I trial of PLX3397 in Asian patients with advanced solid malignancies was conducted. The sites of primary cancer were urothelial (n = 2), salivary gland (n = 2), trophoblastic tumor, gallbladder, liver cancer, soft tissue sarcoma, renal cell carcinoma, chordoma, and TGCT (n = 1 each). Nine of 11 patients had received at least one prior systemic therapy. Patients received PLX3397: cohort 1, 600 mg/d (n = 3); cohort 2, 1000 mg/d for 2 weeks and then 800 mg/d (n = 8). The most common grade 3 or greater AEs were increased AST, ALT, γ-glutamyl transferase, and anemia. RP2D was 1000 mg/d for 2 weeks and then 800 mg/d. Preliminary antitumor activity was observed in one patient with TGCT (PR).[31] A randomized phase III trial of PLX3397 in patients with symptomatic, advanced TGCT for whom surgery was not recommended was conducted. PLX3397 was given as 1000 mg per day for the first 2 weeks, followed by 800 mg per day for 22 weeks. The primary endpoint was centrally reviewed overall response rate by RECIST 1.1 at week 25. Patients were randomly assigned to receive PLX3397 (n = 61) or placebo (n = 59). The overall response rate was higher for PLX3397 than placebo at week 25 (39% [n = 24/61] vs 0% [n = 0/59], p < 0.0001). Serious AEs occurred in eight of 61 (13%) patients in the PLX3397 group and one of 59 (2%) patients in the placebo group. Hair color changes (67%), fatigue (54%), AST increase (39%), nausea (38%), ALT increase (28%), and dysgeusia (25%) were the most frequent treatment-related AEs. Three patients given PLX3397 had AST/ALT elevations of at least 3 times the upper limit of normal with total bilirubin and ALP elevations of at least 2 times the upper limit of normal indicative of mixed or cholestatic hepatotoxicity. PLX3397 is the first systemic therapy to show a robust tumor response in TGCT with improved patient symptoms and functional outcomes.[32]
A phase Ib trial of PLX3397 + durvalumab (anti–PD-L1 antibody) in patients with pancreatic or colorectal cancer was conducted. A total of 19 patients were enrolled in the dose escalation with four dose levels (400, 600, 800, and 1000 mg/d) of PLX3397 plus durvalumab 1400 mg intravenously every 4 weeks. Two DLTs (1 AST/ALT elevation and 1 AST/ALT elevation plus bilirubin increase) occurred in eight patients who received PLX3397 1000 mg/d. The RP2D is PLX3397 800 mg/d. There was no objective response. The cohort expansion is ongoing. Results of the phase Ib trial of PLX3397 with paclitaxel in advanced solid malignancies had been reported.[33]
In addition to PLX3397, PLX5622 is an investigational, selective, oral, brain-penetrant inhibitor of CSF1R tyrosine kinase (IC50 = 16 nM). The clinical development of PLX5622 mainly focused on noncancer indications. PLX73086 (AC708) is a potent compound that inhibits CSF1R and targets TAMs as it prevents CSF1-mediated phosphorylation of CSF1R. It shows significant selectivity for CSF1R as compared with other protein kinases. A phase I trial of PLX73086 was initiated in 2016 but terminated in 2018.
Anti-CSF1R (Receptor) Monoclonal Antibodies (Table 3)
Table 3.
Anti-CSF1/CSF1R monoclonal antibodies in clinical development
|
Code
|
Generic
|
Phase I Trial
|
Phase Ib Trial
|
|||
|
DLT
|
MTD
|
RP2D
|
Responder
|
ICI Partner
|
||
| CSF1R | ||||||
| AMG820[34,35] | Deafness | None | 1100 mg | None | Pembrolizumab (2 PRs in CRC, 1 PR in NSCLC) | |
| FPA008[36,37] | Cabiralizumab | None | None | 4 mg/kg | 4 PRs in TGCT | Nivolumab (4 PRs in PDAC) |
| LY3022855 (IMC-CS4)[38,39] | LVEF decrease, rhabdomyolysis + AKI, pancreatitis | None | 100 mg | None | Durvalumab | |
| RG7155[42] | Emactuzumab | None | None | 1000 mg | 2 CRs + 22 PRs in TGCT | Avelumab |
| SNDX6532[45,46] | Axatilimab | None | None | 6 mg/kg | None | Durvalumab |
| CSF1 | ||||||
| MCS110[47] | Lacnotuzumab | None | None | None | Spartalizumab (1 PR in PDAC) | |
| PD0360324 | Avelumab | |||||
AKI: acute kidney injury; CSF1: colony-stimulating factor 1; CSF1R: colony-stimulating factor 1 receptor; CR: complete response; CRC: colorectal cancer; DLT: dose-limiting toxicity; ICI: immune checkpoint inhibitor; LVEF: left ventricular ejection fraction; MTD: maximum tolerated dose; PDAC: pancreatic ductal adenocarcinoma; PR: partial response; RP2D: recommended phase II dose; NSCLC: non–small cell lung cancer; TGCT: tenosynovial giant cell tumor
AMG820
AMG820 is an investigational, fully human CSF1R MAB that inhibits binding of the ligands CSF1 and IL-34 and subsequent ligand-mediated receptor activation. A first-in-human phase I trial of AMG820 was conducted. Patients with advanced solid tumor (n = 25) received intravenous AMG820 0.5 mg/kg weekly or 1.5 to 20 mg/kg every 2 weeks. The most common sites of primary cancer were colorectal cancer (n = 11) and NSCLC (n = 3). The MTD was not reached. One DLT was observed at 20 mg/kg (irreversible grade 3 deafness). The most common treatment-related AEs were periorbital edema (44%), increased AST (28%), fatigue (24%), nausea (16%), increased ALP (12%), and blurred vision (12%). Twenty-eight percent of patients had grade 3 or greater treatment-related AEs. AMG820 showed linear pharmacokinetics. Increased concentration of serum CSF1 and reduced numbers of skin macrophages were observed. Elevated CSF1 levels were maintained over a 2-week dosing cycle with apparent maximal levels being reached at AMG820 doses of 6 mg/kg or greater. At the highest administered dose (10 or 20 mg/kg every 2 weeks), AMG820 trough concentrations were at or above the target serum concentration of anti-mouse CSF1R antibody found to be efficacious in a mouse NCI-H1650 tumor xenograft model to reduce tumor volume and deplete TAMs. There were no objective responses.[34] A phase Ib trial of AMG820 plus pembrolizumab in colorectal cancer, pancreatic cancer, or NSCLC was reported. Patients received AMG820 1100 or 1400 mg plus pembrolizumab 200 mg every 3 weeks. Overall, 116 patients received AMG820 plus pembrolizumab (AMG820 1100 mg, n = 98; AMG820 1400 mg, n = 18). Seven patients had DLTs (AMG820 1100 mg, n = 6; AMG820 1400 mg, n = 1). The RP2D is AMG820 1100 mg plus pembrolizumab 200 mg every 3 weeks. The most common AEs were increased AST (60%), fatigue (48%), periorbital edema (48%), and maculopapular rash (37%). The best response was immune-related PR in 3 patients. Among five phase II cohorts (proficient mismatch repair-proficient colorectal cancer [n = 41], pancreatic cancer [n = 31], anti–PD-1 antibody-naïve with PD-L1 < 50% NSCLC [n = 4], anti–PD-1 antibody-failed with PD-L1 < 50% NSCLC [n = 19], and anti–PD-1 antibody-failed with PD-L1 ≥ 50% NSCLC [n = 6]), three patients had immune-related PR, including two patients with colorectal cancer and one patient with NSCLC that had progressed following prior treatment with nivolumab and with low PD-L1 expression (< 50%). Serum CSF1 and IL-34 increased post treatment. There was reduction in nonclassical (CD14 + CD16high) monocytes and increase of PD-L1–expressing, CD4 or CD8-positive cells in on-treatment tumor biopsies.[35]
FPA008 (cabiralizumab)
FPA008 is a humanized immunoglobulin G4 (IgG4) MAB that blocks ligand binding, receptor signaling, and therefore depletes TAMs. A phase I trial of FPA008 in TGCT was conducted. The dose of FPA008 was escalated from 1, 2, to 4 mg/kg (n = 9). There was no DLT. The RP2D is 4 mg/kg, based on maximum reduction of nonclassical monocytes at doses of 2 mg/kg or greater. Among the initial 11 patients with a TGCT at the 4-mg/kg dose, there were four confirmed PRs.[36] A phase I trial of FPA008 or FPA008 + nivolumab (anti–PD-1 antibody) in advanced solid malignancies was conducted. The dose of FPA008 monotherapy was escalated from 2, 4, to 6 mg/kg (n = 24) and the RP2D is 4 mg/kg. FPA008 4 mg/kg every 2 weeks was the minimal dose required to consistently deplete circulating nonclassical monocytes throughout the dosing interval. The dose of FPA008 in combination with nivolumab (3 mg/kg) was escalated from 1, 2, 4, to 6 mg/kg (n = 10) and the RP2D is FPA008 4 mg/kg + nivolumab 3 mg/kg. The most common treatment-related AEs were elevations in CK and AST/ALT (without elevation in bilirubin). Again, FPA008 4 mg/kg every 2 weeks was the minimal dose required to consistently deplete circulating nonclassical monocytes. Among 33 patients who received FPA008 4 mg/kg + nivolumab 3 mg/kg, there were four PRs (all microsatellite stable, low tumor mutation burden [< 10 mutations/megabase] pancreatic cancer).[37] Patients with locally advanced or metastatic pancreatic cancer whose condition progressed after a prior line of chemotherapy were enrolled in the randomized phase II trial: FPA008 + investigator choice of chemotherapy in arm A, FPA008 with nivolumab in arm B, FPA008 + nivolumab + gemcitabine + nab-paclitaxel in arm C, or FPA008 + nivolumab + oxaliplatin/5-fluorouracil/leucovorin (FOLFOX) in arm D. On February 25, 2020, Five Prime Therapeutics announced that the primary endpoint, which is progression-free survival (PFS), was not met.
LY3022855 (IMC-CS4)
LY3022855 is a human IgG1 MAB targeting CSF1R. LY3022855 prevents ligands CSF1 and IL-34 from binding to CSF1R and consequently inhibits CSF1R activation. LY3022885 was administered in a 6-week cycle. Two escalation regimens (part A: weight-based dosing; part B: flat dosing) were investigated in a 3 + 3 design. A total of 35 (part A, n = 29; part B, n = 6) patients with advanced solid malignancies were treated. Common treatment-emergent AEs were fatigue (54%), hypoalbuminemia (40%), nausea (37%), AST increase (37%), anemia (34%), anorexia (34%), CK elevation (29%), and constipation (23%). Most common grade 3/4 treatment-emergent AEs were anemia (11%), fatigue (11%), ascites (9%), and lymphocyte count decrease (9%). There were three DLTs: grade 3 left ventricular systolic dysfunction (n = 1), grade 4 rhabdomyolysis and grade 4 acute kidney injury (n = 1), and grade 3 pancreatitis (n = 1). There was no objective response. Pharmacodynamic analyses revealed dose-dependent increase in serum CSF1 levels and suppression of circulating nonclassical monocytes. The RP2D of LY3022855 monotherapy is 100 mg once weekly.[38] Patients with advanced refractory metastatic breast cancer (n = 22) and metastatic castration-resistant prostate cancer (n = 12) received LY3022885 in 6-week cycles in cohorts: 1.25 mg/kg every 2 weeks; 1.0 mg/kg on weeks 1, 2, 4, and 5; 100 mg once weekly; and 100 mg every 2 weeks. Common treatment-related AEs were fatigue (38%), decreased appetite (27%), nausea (27%), increased lipase (24%), and increased CK (21%). On day 8, circulating CSF1 levels increased and nonclassical monocytes decreased. No response was observed.[39]
RG7155 (emactuzumab)
RG7155 (emactuzumab) is a MAB that inhibits CSF1R activation. High-affinity binding of dimeric CSF1 to CSF1R requires receptor dimerization, which is inhibited by RG7155's blocking the receptor dimerization interface. Consequently, the antibody inhibits binding of both CSF1 and IL-34 to CSF1R in a competitive manner.[19,40] The first-in-human phase I trial of diffuse-type TGCT was conducted. RG7155 at 900, 1350, or 2000 mg every 2 weeks was administered in the dose escalation part (n = 12). No DLTs were noted. Based on pharmacokinetic, pharmacodynamic, and safety information, a dose of 1000 mg every 2 weeks was chosen as the RP2D.[41] Common AEs were facial edema (64%), asthenia (56%), and pruritus (56%). Grade 3 AEs were periorbital edema (n = 1), lupus erythematosus (n = 1), dermatitis (n = 1), mucositis (n = 1), and fatigue (n = 1). Twenty-four of 28 (86%) TGCT patients achieved an objective response (CR, n = 2; PR, n = 22).[42] Results of the phase Ib trial of RG7155 or RG7155 in combination with paclitaxel in advanced solid malignancies had been reported. RG7155 at 100–3000 mg every 2 weeks was administered (n = 29) and 200–2000 mg every 2 weeks plus paclitaxel at 80 mg/m2 weekly (n = 34) in the dose escalation part. No DLTs were noted for monotherapy. Asthenia, anemia, and decreased appetite occurred most often. Two patients treated with emactuzumab and paclitaxel experienced DLTs (one patient at 1350 mg [grade 4 hypokalemia, grade 3 gastrointestinal inflammation, and grade 3 hemorrhagic enterocolitis; 1 patient at 2000 mg [grade 5 gastrointestinal perforation]). Asthenia, periorbital edema, and anemia occurred most often. No MTD was reached in either study arm. No patients receiving emactuzumab monotherapy showed an objective response. Four patients (ER-positive, HER2-negative breast cancer, n = 3; ovarian cancer, n = 1) receiving emactuzumab in combination with paclitaxel showed a PR. Skin macrophages rather than peripheral blood nonclassical monocytes or circulating CSF1 were identified as an optimal pharmacodynamic marker to select the RP2D, which is emactuzumab at 1000 mg every 2 weeks.[43] Results of the phase Ib trial of RG7155 in combination with RG7876 (selicrelumab, CD40 agonist) in triple-negative breast cancer, ovarian cancer, pancreatic cancer, gastric cancer, colorectal cancer, melanoma, and mesothelioma had been reported. Cluster of differentiation 40 (CD40) is a costimulatory molecule of the tumor necrosis factor receptor superfamily. CD40 is expressed on antigen-presenting cells (e.g., dendritic cells, macrophages, B lymphocytes). The main mode of action of anti-CD40 agonistic MABs such as RG7876 may be the induction of increased tumor-specific antigen presentation via activation of antigen-presenting cells, resulting in the production of cytotoxic T cells directed against the tumor. Three DLTs (all infusion-related reactions) were observed at 8, 12, and 16 mg of selicrelumab together with 1000 mg of emactuzumab. The MTD was not reached. The most common AEs were infusion-related reactions (76%), fatigue (54%), facial edema (38%), AST increase (35%), and CK increase (35%). Pharmacodynamic analyses demonstrated an increase of Ki67+CD8+ T cells and a decrease of nonclassical monocytes in peripheral blood. There was no objective response.[44] The optimal dosing interval of RG7155 is not clear. Most patients had been treated with once-every-2-week dosing. A small group of breast cancer patients (n = 5) had been treated with once-every-3-week dosing but PK/pharmacodynamic data were not presented.[43]
SNDX6532 (axatilimab)
SNDX6352 (axatilimab) is a high-affinity, dual-ligand (CSF1 and IL-34) blocking IgG4 MAB targeting CSF1R. In SNDX6532 monotherapy arm (1, 2, 3, 6 mg/kg every 2 weeks, and 6 mg/kg every 4 weeks), the AE profile was dose-dependent transient elevation in AST, ALT, amylase, lipase, and CK. There was no objective response. Dose-proportional increase in plasma concentration with drug accumulation was observed. Circulating nonclassical monocytes were ablated at all doses above 1 mg/kg. Plasma CSF1 and IL-34 concentrations increased with treatment at all doses above 1 mg/kg. The RP2D for monotherapy in advanced solid malignancies is 6 mg/kg every 4 weeks.[45] In the SNDX6532 (1, 2, and 3 mg/kg every 2 weeks) plus durvalumab arm, the incidence of immune-related AEs was not increased. There was no objective response. The RP2D for durvalumab combination in advanced solid malignancies is 3 mg/kg every 2 weeks.[46] The optimal dosing interval of SNDX6532 is not clear. Only every-2-week dosing had been tested and every-4-week dosing is ongoing.
Anti-CSF1 (Ligand) Monoclonal Antibodies (Table 2)
MCS110 (lacnotuzumab)
MCS110 is a humanized IgG1 MAB against CSF1. A phase Ib trial assessed MCS110 with spartalizumab in patients with advanced melanoma, endometrial cancer, pancreatic cancer, or triple-negative breast cancer. During dose escalation, patients received MCS110 at 1 or 3 mg/kg with 100-mg spartalizumab, or MCS110 at 3, 5, 7.5, or 10 mg/kg with 300-mg spartalizumab, every 3 weeks. Fifty patients were enrolled at six combination dose levels. The most common treatment-related AEs were periorbital edema (30%), increased AST (24%), and increased CK (24%). Preliminary antitumor activity was observed in pancreatic cancer (PR, n = 1).[47] The optimal dosing interval is not clear.
PD0360324
PD0360324 is an antibody-dependent cellular cytotoxicity–enhanced, fully human IgG2 MAB that binds CSF1 with high affinity (dissociation constant [KD] = 281 pM) and selectivity to prevent the binding of CSF1 to its receptor. The clinical development of PD036324 mainly focused on noncancer indications. A phase Ib trial of PD036324 plus avelumab (anti–PD-L1 antibody) was planned. The optimal dosing interval of PD036324 is not clear.
DISCUSSION
There had been many CSF1R TKIs, anti-CSF1R MABs, and anti-CSF1 MABs in clinical development as anticancer agents. Although there are two natural ligands (CSF1 and IL-34) for CSF1R, it does not seem to be different among CSF1R TKIs, anti-CSF1R MABs, and anti-CSF1 MABs, based on clinical trial results. For example, two (one head and neck cancer, n = 1 per RECIST; GBM, n = 1 per RANO criteria) of 69 patients with advanced solid malignancies responded to spartalizumab + BLZ945 (a CSF1R TKI) in the phase I trial.[26,27] One (pancreatic cancer) of 50 patients with advanced solid malignancies responded to spartalizumab + MCS110 (an anti-CSF1 MAB).[47] This might be explained by the preclinical study demonstrating that IL-34 compared with CSF1 displays equivalent macrophage differentiation ability but a different polarization potential, with a striking increase in IL-10 secretion in M1 macrophages and CCL17 secretion in M2 macrophages derived from IL-34–stimulated monocytes, compared to CSF1-stimulated monocytes.[15]
The class side effects of these agents are AST increase, ALT increase, CK increase (almost always without organ damage), and periorbital edema. Whether the different dosing intervals/schedule of CSF1R inhibitors is better tolerated by patients while being equally/more active against cancers is not known yet.
Most trials used decreased peripheral blood nonclassical monocytes (CD14 + CD16high) and increased peripheral blood ligands (CSF1 and/or IL-34) as pharmacodynamic target engagement. Nonclassical monocytes have been reported as the actual progenitor of M2-like macrophages as they seem to be involved in tissue repair as well as displaying inflammatory characteristics such as IL-13 and tumor necrosis factor-α production upon activation. Only one trial (RG7155, 100–3000 mg every 2 weeks) used not only peripheral blood markers but also TAMs in pretreatment and on-treatment tumor biopsies as pharmacodynamic biomarkers. In this trial, the pharmacodynamic plateaus for peripheral blood markers (e.g., CSF1) were observed at lower doses (≥ 400 mg) than those for surrogate tumor tissue (e.g., CSF1R+ and CD68+/CD163+ macrophages in tumor tissue) (≥ 900 mg). Since there was no MTD, the optimal biologic dose of RG7155 was 1000 mg every 2 weeks as determined from optimal target saturation, resulting in maximal depletion of TAMs in tissue without altering the safety and tolerability profile. The data of this trial implied that relying exclusively on pharmacodynamic effects in peripheral blood is not sufficient to determine the optimal biologic doses for agents that do not reach an MTD.[43] Whether tumor TAMs' changes or peripheral blood nonclassical monocytes, CSF1, and IL-34 changes are the best pharmacodynamic biomarker to determine the optimal biologic doses translating into better antitumor efficacy needs more studies.
Although all CSF1R pathway inhibitors, regardless of TKIs, MABs against receptor, and MABs against ligand, demonstrated activity as a single agent in diffuse-type TGCT (a proliferative tumor without metastatic potential and a representative CSF1-CSF1R pathway–dependent disease[20]),[27,29,31,35,42] there were only two objective responses in advanced solid “malignancies” (GBM in BLZ945, n = 1[26]; Hodgkin lymphoma in JNJ40346527, n = 1[29]). One explanation is that CSF1R inhibition strongly reduced F4/80+ TAMs, accompanied by an increase of the CD8+/CD4+ T-cell ratio in animal models.[19] However, the CD8+/CD4+ T-cell ratio after TAM depletion did not increase in pretreatment and on-treatment tumor biopsies of patients with advanced solid malignancies.[43]
As for the combination with anti–PD-1/PD-L1 antibodies, there were PRs repeatedly observed across trials in microsatellite-stable pancreatic cancer (e.g., FPA004 + nivolumab, n = 4[37]; MCS110 + spartalizumab, n = 1[47]), considering anti–PD-1/PD-L1 antibody alone not active in pancreatic cancer. The PFS was not prolonged in the randomized phase II trial of FPA004 + nivolumab in pancreatic cancer, dampening the enthusiasm. Although the combinations of CSF1R inhibitors and anti–PD-1/PD-L1 antibodies are the most popular ways of the clinical development, we have to put CSF1R inhibition/TAM modulation in the context of resistance mechanisms of anti–PD-1/PD-L1 antibodies. The induction of an effective anti–PD-1/PD-L1 antibody response requires successful (1) antigen presentation and T-cell activation, (2) T-cell trafficking and tumor infiltration, and (3) T-cell killing activity within the tumor microenvironment. Various immune escape mechanisms present at each of these stages can result in primary (patients who do not respond at all) or acquired (patients who initially respond to anti–PD-1/PD-L1 antibodies, and later have relapse) resistance to anti–PD-1/PD-L1 antibodies.[48] Loss-of-function mutations of Janus kinase 1 or Janus kinase 2 mutations and truncating mutations of β-2-microglobulin, resulting in a lack of response to interferon-γ and loss of surface expression of major histocompatibility complex class I, are well-known examples of acquired resistance mechanisms.[49] Whether CSF1R-expressing TAMs play an important role in primary or acquired resistance of anti–PD-1/PD-L1 antibodies is still uncertain. Future clinical trials of the combination of anti–PD-1/PD-L1 antibodies with CSF1R inhibitors should focus on predictive biomarker-selective patient population. The pretreatment tumor biopsy, rather than archival tumor tissue, will play an important role in determination of predictive biomarkers. This is especially critical in the setting of acquired resistance.
To make matters more complicated, mechanisms of the resistance to CSF1R pathway inhibitors had been elucidated. One preclinical study demonstrated that cancer-associated fibroblasts (CAFs) are the major source of chemokines that recruit granulocytes to tumors. CSF1 produced by tumor cells caused downregulation of granulocyte-specific chemokine expression in CAF, which limited migration of these cells to tumors. Treatment with CSF1R inhibitors disrupted this cross talk and triggered a profound increase in granulocyte recruitment to tumors. This implies that combination of a CSF1R inhibitor with a CXCR2 antagonist is worthy of clinical testing.[50] Another preclinical study showed that recurred GBM reestablished sensitivity to CSF1R inhibition upon transplant to another mice, indicating that resistance was tumor microenvironment driven. Phosphatidylinositol 3-kinase (PI3K) pathway activity was elevated in recurrent GBM, driven by macrophage-derived insulin-like growth factor-1 (IGF-1) and tumor cell IGF-1 receptor (IGF1R). Combining IGF1R or PI3K blockade with CSF1R inhibition in recurrent GBMs significantly prolonged overall survival in mice.[51]
CONCLUSION
There have been at least five CSF1R TKIs, five anti-CSF1R MABs, and two anti-CSF1 MABs in clinical development to target a variety of diseases including (in the decreasing order of evidence) TGCT with translocation t(1;2) CSF1:COL6A3, histiocytosis with CSF1R Y546_K551 del, and TAMs. Pexidartinib had been approved for TGCT. How to optimally modulate TAMs with CSF1R inhibitors and combine CSF1R inhibitors with other immunotherapies (e.g., anti–PD-1/PD-L1 antibodies) needs further investigation.
Acknowledgments
The author thanks Hsin-Ling Peng, Department of Oncology, National Taiwan University Hospital, for assistance creating Figure 1.
Funding Statement
Source of Support: This review was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 106-2314-B-002-225, MOST 108-2314-B-002-077, MOST 109-2321-B-002-037).
Footnotes
Conflict of Interest: Dr Lin serves as an advisor to Blueprint Medicines, Boehringer-Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, and Novartis; receives honorarium from Daiichi Sankyo, Eli Lilly, Novartis, and Roche; and receives travel support from BeiGene and Eli Lilly.
References
- 1.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med . 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med . 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol . 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 4.Biswas SK, Gangi L, Paul S, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood . 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 5.Leek RD, Lewis CE, Whitehouse R, et al. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res . 1996;56:4625–4629. [PubMed] [Google Scholar]
- 6.Väyrynen JP, Haruki K, Lau MC, et al. The prognostic role of macrophage polarization in the colorectal cancer microenvironment. Cancer Immunol Res . 2021;9:8–19. doi: 10.1158/2326-6066.CIR-20-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol . 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 8.Feng M, Chen JY, Weissman-Tsukamoto R, et al. Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. Proc Natl Acad Sci U S A . 2015;112:2145–2150. doi: 10.1073/pnas.1424907112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature . 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A . 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med . 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature . 2019;572:392–396. doi: 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov . 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 14.Guleria I, Pollard JW. Aberrant macrophage and neutrophil population dynamics and impaired Th1 response to Listeria monocytogenes in colony-stimulating factor 1-deficient mice. Infect Immun . 2001;69:1795–1807. doi: 10.1128/IAI.69.3.1795-1807.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boulakirba S, Pfeifer A, Mhaidly R, et al. IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential. Sci Rep . 2018;8:256. doi: 10.1038/s41598-017-18433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aharinejad S, Paulus P, Sioud M, et al. Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res . 2004;64:5378–5384. doi: 10.1158/0008-5472.CAN-04-0961. [DOI] [PubMed] [Google Scholar]
- 17.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med . 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulus P, Stanley ER, Schäfer R, et al. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res . 2006;66:4349–4356. doi: 10.1158/0008-5472.CAN-05-3523. [DOI] [PubMed] [Google Scholar]
- 19.Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell . 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Staals EL, Ferrari S, Donati DM, Palmerini E. Diffuse-type tenosynovial giant cell tumour: current treatment concepts and future perspectives. Eur J Cancer . 2016;63:34–40. doi: 10.1016/j.ejca.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Durham BH, Lopez Rodrigo E, Picarsic J, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med . 2019;25:1839–1842. doi: 10.1038/s41591-019-0653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neubert NJ, Schmittnaegel M, Bordry N, et al. T cell-induced CSF1 promotes melanoma resistance to PD1 blockade. Sci Transl Med . 2018;10 doi: 10.1126/scitranslmed.aan3311. [DOI] [Google Scholar]
- 23.Salvagno C, Ciampricotti M, Tuit S, et al. Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat Cell Biol . 2019;21:511–521. doi: 10.1038/s41556-019-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendell JC, Tolcher AW, Jones SF, et al. Abstract A252: a phase 1 study of ARRY-382, an oral inhibitor of colony-stimulating factor-1 receptor (CSF1R), in patients with advanced or metastatic cancers. Mol Cancer Ther . 2013;12:A252. [Google Scholar]
- 25.Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med . 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin C-C, Gil-Martin M, Bauer TM, et al. Abstract CT171: phase I study of BLZ945 alone and with spartalizumab (PDR001) in patients (pts) with advanced solid tumors. Cancer Res . 2020. 80:CT171.
- 27.Wu J, Lin C-C, Gil-Martin M, et al. Abstract CT172: pharmacodynamic and gene expression profiling of patients treated with BLZ945 + spartalizumab demonstrates on-target peripheral and tumor immune microenvironment modulation. Cancer Res . 2020. 80:CT172.
- 28.Taylor MH, Leong S, Su Y, et al. Abstract C087: phase 1 study of DCC-3014, an oral inhibitor of CSF1R, to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics in patients with advanced solid tumors, including diffuse-type tenosynovial giant cell tumor. Mol Cancer Ther . 2019;18:C087. [Google Scholar]
- 29.von Tresckow B, Morschhauser F, Ribrag V, et al. An open-label, multicenter, phase I/II study of JNJ-40346527, a CSF-1R inhibitor, in patients with relapsed or refractory Hodgkin lymphoma. Clin Cancer Res . 2015;21:1843–1850. doi: 10.1158/1078-0432.CCR-14-1845. [DOI] [PubMed] [Google Scholar]
- 30.Tap WD, Wainberg ZA, Anthony SP, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med . 2015;373:428–437. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Chen TW, Hsu CH, et al. A phase I study of pexidartinib, a colony-stimulating factor 1 receptor inhibitor, in Asian patients with advanced solid tumors. Invest New Drugs . 2020;38:99–110. doi: 10.1007/s10637-019-00745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tap WD, Gelderblom H, Palmerini E, et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial. Lancet . 2019;394:478–487. doi: 10.1016/S0140-6736(19)30764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wesolowski R, Sharma N, Reebel L, et al. Phase Ib study of the combination of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors. Ther Adv Med Oncol . 2019;11:1758835919854238. doi: 10.1177/1758835919854238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadopoulos KP, Gluck L, Martin LP, et al. First-in-human study of AMG 820, a monoclonal anti-colony-stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clin Cancer Res . 2017;23:5703–5710. doi: 10.1158/1078-0432.CCR-16-3261. [DOI] [PubMed] [Google Scholar]
- 35.Razak AR, Cleary JM, Moreno V, et al. Safety and efficacy of AMG 820, an anti-colony-stimulating factor 1 receptor antibody, in combination with pembrolizumab in adults with advanced solid tumors. J Immunother Cancer . 2020;8 doi: 10.1136/jitc-2020-001006. [DOI] [Google Scholar]
- 36.Sankhala KK, Blay J-Y, Ganjoo KN, et al. A phase I/II dose escalation and expansion study of cabiralizumab (cabira; FPA-008), an anti-CSF1R antibody, in tenosynovial giant cell tumor (TGCT, diffuse pigmented villonodular synovitis D-PVNS) J Clin Oncol . 2017;35:11078. [Google Scholar]
- 37.Carleton M, Powers J, Phillips P, et al. Pharmacodynamics (PD) and genomic profiling of pts treated with cabiralizumab (cabira) + nivolumab (NIVO) provide evidence of on-target tumor immune modulations and support future clinical applications. J Clin Oncol . 2018;36:3020. [Google Scholar]
- 38.Dowlati A, Rugo HS, Harvey RD, et al. A phase I study of LY3022855, a colony-stimulating factor-1 receptor (CSF-1R) inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol . 2017;35:2523. [Google Scholar]
- 39.Autio KA, Klebanoff CA, Schaer D, et al. Immunomodulatory activity of a colony-stimulating factor-1 receptor inhibitor in patients with advanced refractory breast or prostate cancer: a phase I study. Clin Cancer Res . 2020;26:5609–5620. doi: 10.1158/1078-0432.CCR-20-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pradel LP, Ooi CH, Romagnoli S, et al. Macrophage susceptibility to emactuzumab (RG7155) treatment. Mol Cancer Ther . 2016;15:3077–3086. doi: 10.1158/1535-7163.MCT-16-0157. [DOI] [PubMed] [Google Scholar]
- 41.Smart K, Bröske AM, Rüttinger D, et al. PK/PD mediated dose optimization of emactuzumab, a CSF1R inhibitor, in patients with advanced solid tumors and diffuse-type tenosynovial giant cell tumor. Clin Pharmacol Ther . 2020;108:616–624. doi: 10.1002/cpt.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cassier PA, Italiano A, Gomez-Roca CA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol . 2015;16:949–956. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 43.Gomez-Roca CA, Italiano A, Le Tourneau C, et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann Oncol . 2019;30:1381–1392. doi: 10.1093/annonc/mdz163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machiels JP, Gomez-Roca C, Michot JM, et al. Phase Ib study of anti-CSF-1R antibody emactuzumab in combination with CD40 agonist selicrelumab in advanced solid tumor patients. J Immunother Cancer . 2020;8 doi: 10.1136/jitc-2020-001153. [DOI] [Google Scholar]
- 45.Azad N, Rasco D, Sharma S, et al. Abstract CT149: SNDX-6352-0502—a phase 1, open-label, dose escalation trial to investigate the safety, tolerability, pharmacokinetics and pharmacodynamic activity of SNDX-6352 monotherapy in patients with unresectable, recurrent, locally-advanced, or metastatic solid tumors. Cancer Res . 2020;80 : CT149. [Google Scholar]
- 46.Tolcher AW, Rasco D, Sharma S, et al. Abstract CT242: SNDX-6352-0502: a phase 1, open-label, dose escalation trial to investigate the safety, tolerability, pharmacokinetics and pharmacodynamic activity of SNDX-6352 in combination with durvalumab in patients with unresectable, recurrent, locally-advanced, or metastatic solid tumors. Cancer Res . 2020. 80:CT242.
- 47.Calvo A, Joensuu H, Sebastian M, et al. Phase Ib/II study of lacnotuzumab (MCS110) combined with spartalizumab (PDR001) in patients (pts) with advanced tumors. J Clin Oncol . 2018;36:3014. [Google Scholar]
- 48.Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol . 2017;18:e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 49.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med . 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar V, Donthireddy L, Marvel D, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell . 2017;32:654–668.e655. doi: 10.1016/j.ccell.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quail DF, Bowman RL, Akkari L, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science . 2016;352 aad3018. [Google Scholar]