Abstract
Background:
Current pharmaceutical treatments for depression are sometimes ineffective and may have unwanted side effects that interfere with patient compliance. This study examined the potential antidepressant-like effects of dietary- and microbial-derived aryl hydrocarbon receptor (AhR) ligands, 3,3′-diindolylmethane (DIM) and 1,4-dihydroxy-2-naphthoic acid (1,4-DHNA).
Methods:
Female C57BL/6 mice were subjected to unpredictable chronic mild stress (UCMS) or were unstressed. For three weeks prior to UCMS mice were fed daily with vehicle or 20 mg/kg DIM, 1,4-DHNA or AhR-inactive isomer 3,7-DHNA; another group was subjected to two weeks UCMS before ligand administration began. Mice were examined for anhedonia-like behavior as measured by the sucrose preference test. Additionally, anxiety levels of the mice were examined before UCMS and ligand administration began and at the end in the open field, light/dark, elevated plus maze, novelty-induced hypophagia, and marble burying tests. At the end of the experiment they were also examined in the Morris water maze (MWM) task.
Results:
Both DIM and 1,4-DHNA, but not 3,7-DHNA, successfully prevented and reversed UCMS-induced anhedonia-like behavior. Furthermore, both DIM and DHNA had little to no effect on anxiety levels and did not induce spatial learning deficits.
Limitations:
Additional studies are required to determine to what degree the antidepressant-like effects of DIM and 1,4-DHNA can be attributed to their activities as AhR ligands.
Conclusions:
Our findings indicate that dietary and microbial-derived AhR ligands may have clinical applications as potential antidepressants. Future studies are necessary to elucidate the role of AhR in depression-like states and the underlying mechanisms of action.
Keywords: Depression; Anxiety; Spatial learning; Aryl hydrocarbon receptor (AhR); 3,3′-Diindolylmethane (DIM); 1,4-Dihydroxy-2-naphthoic acid (1,4-DHNA); 3,7-Dihydroxy-2-naphthoic acid (3,7-DHNA)
1. Introduction
Major depressive disorder (MDD) is a serious mental health disorder that affects over 264 million people worldwide, (World Health Organization, 2018) and it is a leading cause of disability and suicide (Ferrari et al., 2013; Hawton et al., 2013; O’Rourke and Siddiqui, 2019). Approximately one-third of patients achieve remission after trying one of the existing pharmaceutical antidepressants (McIntyre et al., 2014; Kautzky et al., 2019). Unfortunately, the majority of patients have to try several medications or other treatment options, and some patients do not respond to any treatment at all (van Bronswijk et al., 2019). Individuals are typically considered to suffer from treatment resistant depression (TRD) when they fail to respond to two treatment attempts (McIntyre et al., 2014), putting them at much higher risk for disability and mortality (Mrazek et al., 2014; Kautzky et al., 2019). Thus, additional and more effective treatment options or potential supplements are still required.
Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed class of drugs for major depressive disorder (MDD) (Mandrioli et al., 2012). Serotonin (5-hydroxytryptamine, 5-HT) is a product of tryptophan metabolism by the enzymes tryptophan hydroxylase (TPH) and aromatic L-amino acid decarboxylase (AADC), and tryptophan metabolism is thought to be associated with the pathology of mood disorders. Notably, only a small percentage of free tryptophan (that is not designated for protein synthesis) is metabolized into 5-HT (Palego et al., 2016). The majority of free tryptophan is converted by the enzymes indolamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase into kynurenine (KYN). In contrast to 5-HT, KYN can cross the blood–brain barrier (Fukui et al., 1991). KYN synthesized in the periphery, predominantly in the liver, is a major source for the KYN in the brain (Schwarcz et al., 2012). Lower concentrations of KYN in plasma were associated with more severe depressive symptoms (Setoyama et al., 2016; Liu et al., 2018). Importantly, KYN is an endogenous ligand for the aryl hydrocarbon receptor (AhR) (Opitz et al., 2011; Bessede et al., 2014). A variant genotype for AhR, known to be associated with lower expression of AhR and lower KYN concentrations in plasma, was found to be associated with MDD (Liu et al., 2018). However, the role of AhR and tryptophan metabolism in the pathology of MDD is still not fully understood.
The AhR is recognized for its broader physiological role as an environmental sensor, and it responds to AhR-active tryptophan metabolites, dietary components, and microbiota-derived metabolites (Grifka-Walk et al., 2021). The AhR is also thought to play a key role in the interactions between gut microbiota, activation of neural pathways, and mental status, known as the microbiome-gut-brain axis (Lee et al., 2017; Barroso et al., 2021). 3,3′-Diindolylmethane (DIM) is derived from the ingestion and subsequent dimerization of indole-3-carbinol (I3C, also an AhR agonist) from cruciferous vegetables, and it is also a dietary supplement (Vermillion Maier et al., 2021). 1,4-Dihydroxy-2-naphthoic acid (1,4-DHNA) is a bacteria-derived metabolite (Bentley and Meganathan, 1982; Mori et al., 1997; Isawa et al., 2002). Both DIM and 1,4-DHNA exhibit significant AhR agonist activity in multiple cell types (Cheng et al., 2017; Vermillion Maier et al., 2021). DIM stimulated immune function when administered orally in mice, and its precursor I3C stimulated apoptosis in tumorigenic cells while protecting normal cells and inhibiting translocation of p65, a proinflammatory transcription factor, to the nucleus (Aggarwal and Ichikawa, 2005; Xue et al., 2008). Additionally, I3C prevented clonidine-induced despair behaviors in the forced swim test, a measure of despair-like behavior in rodents (El-Naga et al., 2014). 1,4-DHNA is less well-studied than DIM; however, it is known to be protective against colitis (Dolan and Chang, 2017). Given AhR’s established role in inflammation and tryptophan metabolism, two potential etiologies of MDD, and considering the anti- inflammatory potential of DIM and 1,4-DHNA, we hypothesized that the aforementioned ligands would have an effect on depressive-like behaviors.
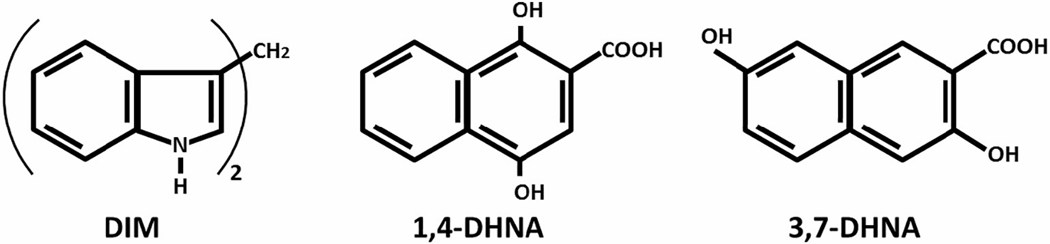
In this study, we examined the effects of DIM and 1,4-DHNA to prevent and reverse the depressive-like effect of unpredictable chronic mild stress (UCMS). UCMS is an established rodent model of depression with high translational potential and relevance to human depression (Willner, 2017). We examined female mice, given that the prevalence of MDD is almost double in females as compared to males (Albert, 2015). Moreover, we compared the effects of 1,4-DHNA to the effects of 3,7- dihydroxy-2-naphthoic acid (3,7-DHNA, Fig. 1), a structurally similar derivative that is predominantly inactive at the AhR (Cheng et al., 2017). Additionally, using a battery of behavioral tests, we examined the effects of DIM and 1,4-DHNA on anxiety-like behaviors as well as on spatial learning acquisition using the Morris water maze (MWM) task.
Fig. 1.
Structures of DIM, 1,4-DHNA and 3,7-DHNA.
2. Methods
2.1. Animals
All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee. Female C57BL/6N mice were purchased from Envigo Laboratories (Houston, Texas, USA) and housed four per cage with food and water ad libitum in a temperature-controlled (21 ± 2 °C, humidity 45%) vivarium with a 12-hour light/12-hour dark cycle (light on at 7:30 AM). Mice arrived in the vivarium at 6–7 weeks old and were acclimated for at least 6 days before beginning the experiment. Unless specifically mentioned, all tests were conducted in rooms containing low illumination (60 W white light) and 40 dB white noise generator.
2.2. Ligands
3,3′-Diindolylmethane (DIM), 1,4-dihydroxy-2-naphthoic acid (1,4- DHNA), and 3,7-dihydroxy-2-naphthoic acid (3,7-DHNA) (Fig. 1) were purchased from Sigma-Aldrich (St. Louis, MO). Ligands were dissolved in Great Value brand corn oil and a 20 mg/kg dose was mixed into melted HEB Select Ingredients Texas peanut butter in VIWIEU brand miniature ice tray molds. The peanut butter pellets were frozen at − 80 °C overnight. Each day mice were placed into individual cages and a peanut butter pellet was placed on the water sipper nozzle for the individual cage. Mice were allowed to stay in the cage for approximately 10–30 min until they finished eating the peanut butter; then they were returned to their home cage. This method was adapted from (Gonzales et al., 2014) and was chosen over the daily gavage injection which is a procedure that when applied daily can be stressful by itself.
2.3. Unpredictable chronic mild stress (UCMS)
UCMS protocol was adapted from Burstein and Doron (2018) and in accordance with other studies (Muscat et al., 1992; Grippo et al., 2006; Delis et al., 2013). This protocol did not include food or water deprivation. Seven stressors were administered weekly over the course of 4–5 weeks: 1) Restrainer — mice were placed in a modified 50 mL falcon tube for 4 h; 2) Wet cage — a mouse cage was filled with ½ in deep lukewarm water and mice were placed in the cage for 4 h; 3) Cage replacement — mice were moved into the home cage of stranger mice for 4 h before being returned to their home cage; 4) Tilted cage — home cages were placed on a 45 degree angle tilt for 4 h; 5) Dampened bedding — lukewarm water was poured into the home cage of the mice until the bedding was thoroughly dampened, and mice stayed in the cage for 4 h before being transferred to a clean cage; 6) Empty cage — mice were placed into a cage with no bedding for 4 h; and 7) Light/dark disruption — one dark cycle of the 12/12 dark/light cycle was spent in light, resulting in 36 consecutive hours of light exposure. The order of appearance and daily schedule of each stressor was altered weekly (see Table 1S in Supplementary Materials).
2.4. Sucrose preference
Mice were monitored once weekly for their preference to consume sucrose. Once weekly, for 2 consecutive days, animals were individually housed for 4 h and had access to one bottle of water and one bottle of 3% sucrose. The bottles were placed side by side, were freely available, and their positions were switched daily in order to account for a side preference. Sucrose preference was calculated as [milliliters sucrose solution consumed] / [milliliters sucrose solution + milliliters water consumed]. For each mouse, data was averaged across the 2 consecutive days.
2.5. Open field test (OFT)
This test was conducted in an automated optical beam activity monitor [40 × 40 × 30.5 (height) cm]. Subjects were placed in the center of the box to begin a 10-min test session. The computerized integration of the data was used to score time spent in the center and in the periphery.
2.6. Light/dark (L/D) test
Based on Bourin and Hascoet (2003), this test is assessed in the same activity boxes as the OFT, split into an 18 × 18 cm dark chamber and a light zone. A mouse was placed in the middle of the light chamber and recorded for 10 min. The computerized integration of the data was used to score time spent in the light and dark zones.
2.7. Elevated plus maze (EPM)
Similar to our previous studies (Hofford et al., 2009), the EPM apparatus consists of four arms (87 mm wide, 155 mm long) elevated 63.8 cm above the ground, with two arms enclosed on two sides by 16.3 cm high opaque walls. Mice were placed in the center of the maze facing toward an enclosed arm and recorded for 10 min by an overhead camera. Behaviors were scored from the video for the duration of time spent in the open arms (defined as all four legs having crossed the entrance line to one of the open arms), and the total number of crosses into the center compartment.
2.8. Novelty-induced hypophagia (NIH)
Based on Hunsberger et al. (2007), mice were introduced in their home cage to diluted condensed milk for 30 min daily for 3 consecutive days. Carnation sweetened condensed milk, diluted 1:3 in water, was provided in plastic serological pipettes (10 mL) with attached sippers and rubber stoppers that are mounted to the wire cage lid. On day 4, mice were tested individually in their home cages with low illumination level (50 lx). Each mouse was removed from the cage while the pipette is installed on the cage lid. Testing began immediately upon returning to the home cage. On day 5, mice were tested in a novel cage free of bedding and in bright illumination level (1200 lx). On both testing days, mice were recorded for the latency to the first sip of milk.
2.9. Marble bury (MB) test
Based on Deacon (2006), each mouse was placed in a large cage (40 × 24 × 20 cm) filled with bedding 5 cm deep from the cage floor and 20 blue marbles (positioned in 4 × 5 grid) for 30 min. Marbles were counted as buried if 2/3 or more was covered by bedding. Number of buried marbles was recorded.
2.10. Morris water maze (MWM)
Based on Vorhees and Williams (2006). A 36′′ diameter pool was used as the maze with a 5 in.2 clear Plexiglas platform placed at the eastern equator 7 in. from the edge of the pool. The room featured distinct visual cues on the northern, eastern, and western walls, and the experimenter stood in a marked position in the southern side of the room. The time to reach the platform was recorded on six consecutive days. On days 1–5, mice were subjected to 4 daily trial sessions; the start position was rotated across days and sessions, and the time to reach the platform over the 4 daily sessions was averaged. On day 6, the challenge day, a considerably different previously unused start position was chosen and the time to reach the platform was recorded.
2.11. Experimental design
2.11.1. Experiment 1 (Fig. 2A)
Fig. 2.
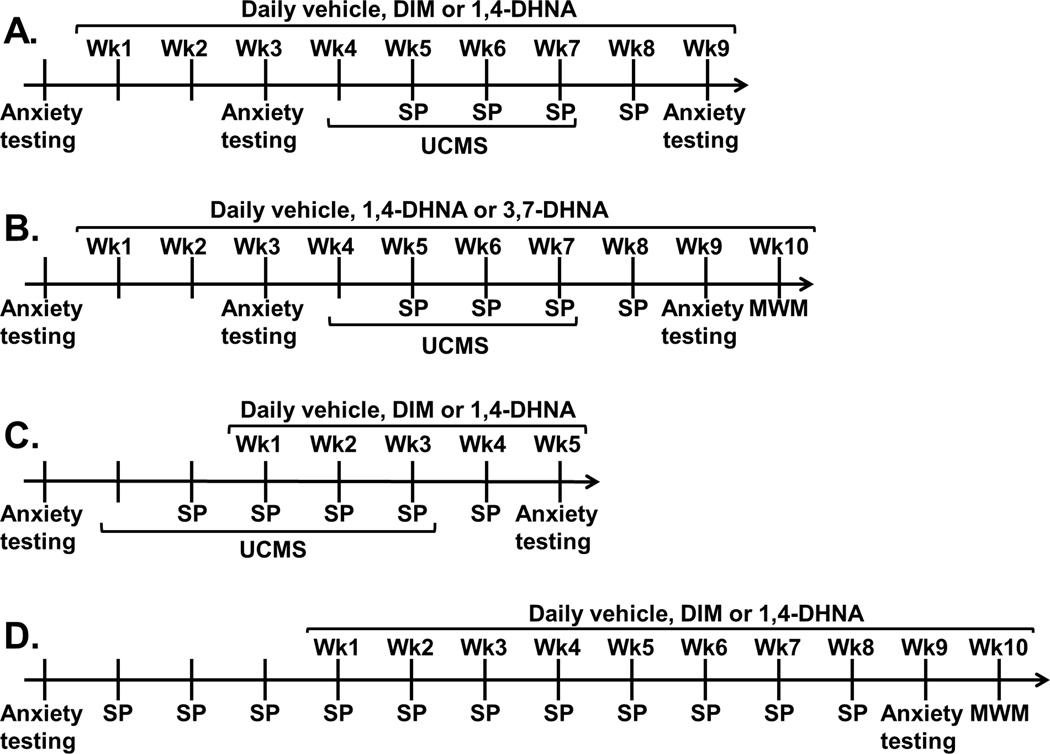
Experimental design. Wk = week; anxiety testing = testing in the open field test (OFT), light/dark (L/D) test, marble burying (MB), novelty-induced hypophagia (NIH) and elevated plus maze (EPM) paradigms; UCMS = unpredictable chronic mild stress; SP = sucrose preference test; MWM = Morris water maze.
Mice (n = 16 per experimental group) were examined for their behaviors in the OFT, L/D test, MB, NIH and EPM paradigms. Then mice were administered daily with vehicle, DIM, or 1,4-DHNA for 9 weeks. On week 3 of ligand administration, mice were re-tested for their behaviors in the OFT, L/D test, MB, NIH and EPM paradigms. During weeks 4–7 of ligand administration, mice either remained unstressed or were subjected to UCMS protocol. Sucrose preference tests were conducted once weekly at the end of weeks 5–8 of ligand administration. During week 9 of ligand administration, mice were re-tested for their behaviors in the OFT, L/D test, MB, NIH and EPM paradigms.
2.11.2. Experiment 2 (Fig. 2B)
Mice (n = 8 per experimental group) were examined for their behaviors in the OFT, L/D test, MB, NIH and EPM paradigms. Then mice were administered daily with vehicle, 1,4-DHNA, or 3,7-DHNA for 10 weeks. On week 3 of ligand administration, mice were re-tested for their behaviors in the OFT, L/D test, MB, NIH and EPM paradigms. During weeks 4–7 of ligand administration, mice remained unstressed or were subjected to the UCMS protocol. Sucrose preference tests were conducted once weekly, at the end of weeks 5–8 of ligand administration. During week 9 of ligand administration, mice were re-tested for their behaviors in the OFT, L/D test, MB, NIH and EPM paradigms. During week 10, mice were tested in the MWM task.
2.11.3. Experiment 3 (Fig. 2C)
Mice (n = 16 per experimental group) were examined for their behaviors in the OFT, L/D test, MB, NIH and EPM paradigms. Then mice remained unstressed or were subjected to UCMS protocol for 5 weeks. Sucrose preference tests were conducted once weekly, starting at the end of the second week of UCMS exposure until one week after the completion of UCMS protocol. Mice were administered with vehicle, DIM, or 1,4-DHNA for 5 weeks, starting on the second week of UCMS exposure. During week 5 of ligand administration, mice were re-tested for their behaviors in the OFT, L/D test, MB, NIH and EPM paradigms.
2.11.4. Experiment 4 (Fig. 2D)
Mice (n = 8–12 per experimental group) were examined for their behaviors in the OFT, L/D test, MB, NIH and EPM paradigms. Mice remained unstressed for the entire duration of the experiment. Sucrose preference tests were conducted once weekly, at the end of each week. After three weeks of baseline sucrose preference tests, ligand administration began and continued daily for 10 weeks. During week 9 of ligand administration, mice were re-tested for their behaviors in the OFT, L/D test, MB, NIH and EPM paradigms. During week 10, mice were tested in the MWM task.
2.12. Data analysis
For each behavioral test, data was analyzed using MANOVA (IBM SPSE Statistics 25) for the between-group factors of experimental group (vehicle, DIM, 1,4-DHNA, 3,7-DHNA) and within-group factors of week or day as applicable. Please note that the graphs for the anxiety testing present the differences between week 3 and baseline (ΔWk 3) and week 9 and baseline (ΔWk 9). Nonetheless, for the ANOVA analyses (in Supplementary Material) the raw data was used. Post hoc contrasts between each treatment group were conducted using the Bonferroni procedure. Differences with p-values of less than 0.05 were deemed statistically significant. Results are presented as mean ± SEM.
3. Results
3.1. Antidepressant-like effects
3.1.1. Prevention
In order to examine the effectiveness of DIM, 1,4-DHNA, and 3,7-DHNA on the prevention of UCMS-induced depressive-like behaviors, mice received vehicle or one of the ligands and were subsequently subjected to UCMS. As expected, mice subjected to UCMS exhibited a significant reduction in sucrose preference as compared to unstressed mice (Fig. 3A, B, and C). Mice that received 20 mg/kg DIM or 1,4-DHNA for 3 weeks before being subjected to UCMS did not exhibit a reduction in sucrose preference (Fig. 3A). In fact, their sucrose preference was indistinguishable from unstressed mice. However, mice that received AhR-inactive isomer 3,7-DHNA for 3 weeks before being subjected to UCMS were not protected from the effects of UCMS and exhibited a similar reduction in sucrose preference to mice subjected to UCMS that received only vehicle (Fig. 3B).
Fig. 3.
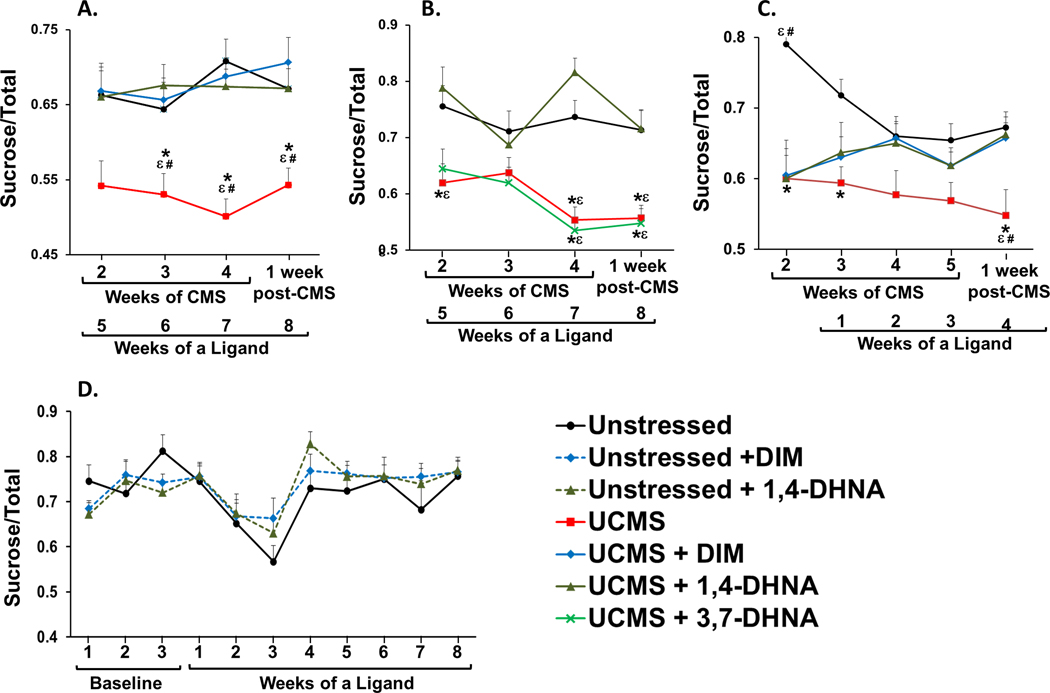
DIM and 1,4-DHNA, but not 3,7-DHNA, prevented and reversed UCMS-induced decrease in sucrose preference. Mice were unstressed or subjected to UCMS for 4 weeks. (A, B) Sucrose preference was monitored weekly starting at the end of the second week of UCMS, and continuing until 1 week post UCMS. Mice received daily vehicle, DIM, 1,4-DHNA, or 3,7-DHNA for 3 weeks prior to the beginning of UCMS, or (C) or starting and at the third week of UCMS, and then continued for the entire duration of the experiment. (D) Sucrose preference was monitored weekly. Baseline sucrose preference was recorded for 3 weeks, followed by 8 weeks of receiving vehicle, DIM or 1,4-DHNA. (A) Two-way repeated ANOVA revealed a main effect of experimental group (F(3,60) = 14.035, p < 0.0001), but no significant interaction between experimental group and week (F(9,180) = 0.636, p > 0.05). (B) Two-way repeated ANOVA revealed a main effect of experimental group (F (3,28) = 22.192, p < 0.0001), a main effect of week (F(3,84) = 2.996, p < 0.05), but no significant interaction between experimental group and week (F(9,84) = 1.762, p > 0.05). (C) Two-way repeated ANOVA revealed a main effect of experimental group (F(3,60) = 9.962, p < 0.0001), and a significant interaction between experimental group and week (F(12,240) = 1.868, p < 0.05). (D) Two-way repeated ANOVA revealed that there was no main effect of experimental group (F(2,29) = 0.228, p > 0.05), and no significant interaction between experimental group and week (F(2,29) = 1.224, p > 0.05). (*) Bonferroni post hoc contrast indicates a significant difference from unstressed mice (p < 0.05); (#) Bonferroni post hoc contrast indicates a significant difference from stressed mice receiving DIM (p < 0.05); (ε) Bonferroni post hoc contrast indicates a significant difference from stressed mice receiving 1,4-DHNA (p < 0.05). Results are presented as mean + SEM.
3.1.2. Reversal
In order to examine the effectiveness of DIM and 1,4-DHNA to reverse UCMS-induced depressive-like behaviors, mice were first subjected to UCMS and then received vehicle or one of the ligands. As expected, before receiving the ligands, all mice demonstrated a reduction in sucrose preference (Fig. 3C). However, subsequently after 4 weeks of receiving DIM or 1,4-DHNA and continuing UCMS their sucrose preference was significantly different from mice subjected to UCMS and vehicle (Fig. 3C). In fact, after 4 weeks of receiving DIM or 1,4-DHNA their sucrose preference was indistinguishable from unstressed mice.
3.1.3. Unstressed mice
Additionally, we tested the effects of the ligands on sucrose preference in unstressed mice. Eight weeks of receiving DIM and 1,4-DHNA did not have a significant effect on the sucrose preference of unstressed mice (Fig. 3D).
3.2. Anxiety tests
3.2.1. Null effects of ligands
DIM and 1,4-DHNA did not significantly affect OFT, MB, NIH behaviors, as compared to vehicle, in unstressed (Fig. 4A–E) or stressed mice (Fig. 5A–E). Some trends were observed, but they did not reach statistical significance. Additionally, in stressed mice, DIM had no significant effect on the L/D test (Fig. 5B) or on anxiety behaviors in the EPM (i.e., no significant differences in time spent in the open arms or number of entries into the open arms, Fig. 5F). Exposure to UCMS itself caused a decrease in total activity in the EPM at 9 weeks as compared to their baseline (i.e., in the number of entries into the close arms and total number of crosses across the middle, Fig. 5F). However, no significant differences in EPM behaviors were observed between vehicle- or ligand- treated mice subjected to UCMS.
Fig. 4.
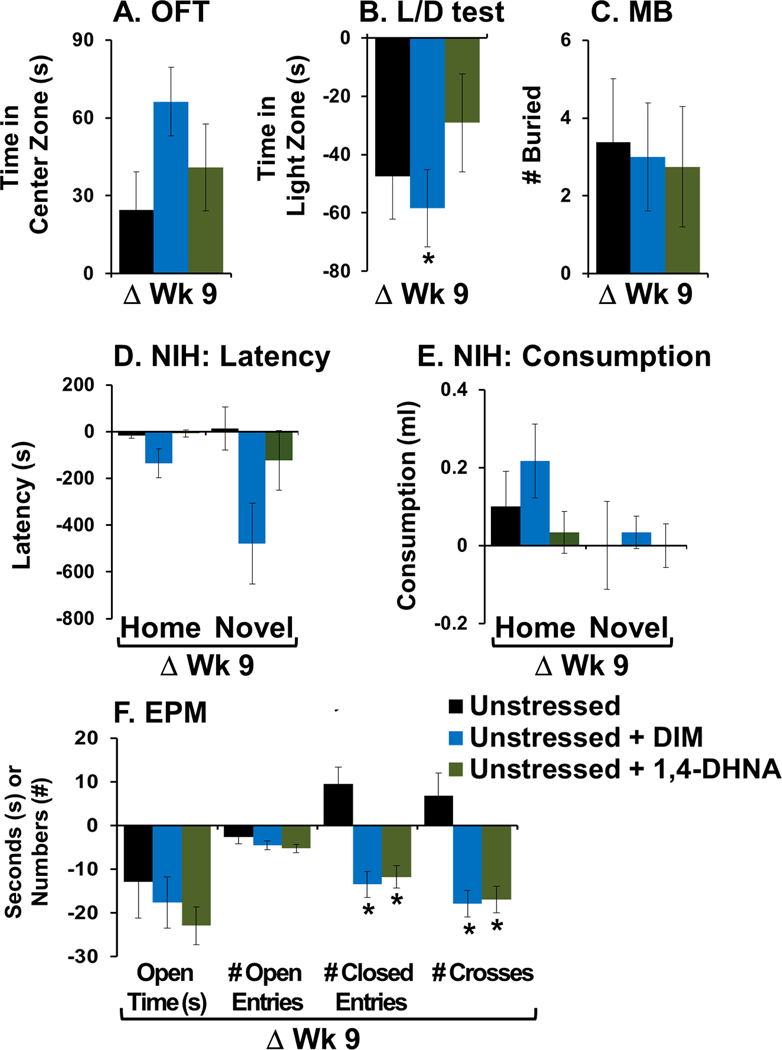
DIM and 1,4-DHNA had little to no effect on anxiety behaviors in unstressed mice. Baseline behaviors were examined. Animals were re-tested after 9 weeks of receiving vehicle or ligand. Data is presented as the difference between the re-test at week 9 and baseline (ΔWk 9). (A) Time spent in the center zone of OFT; (B) time spent in the light zone of L/D apparatus; (C) number of buried marbles; (D) NIH latency to take the first lick; (E) NIH total consumption of milk; and (F) time spent in open arms of the EPM in seconds, number of entries to the open and closed arms, and total crosses in the middle. The ANOVA analyses for each test can be found in Supplementary Material. (*) Bonferroni post hoc contrast indicates a significant difference from unstressed mice (p < 0.05). Results are presented as mean ± SEM.
Fig. 5.
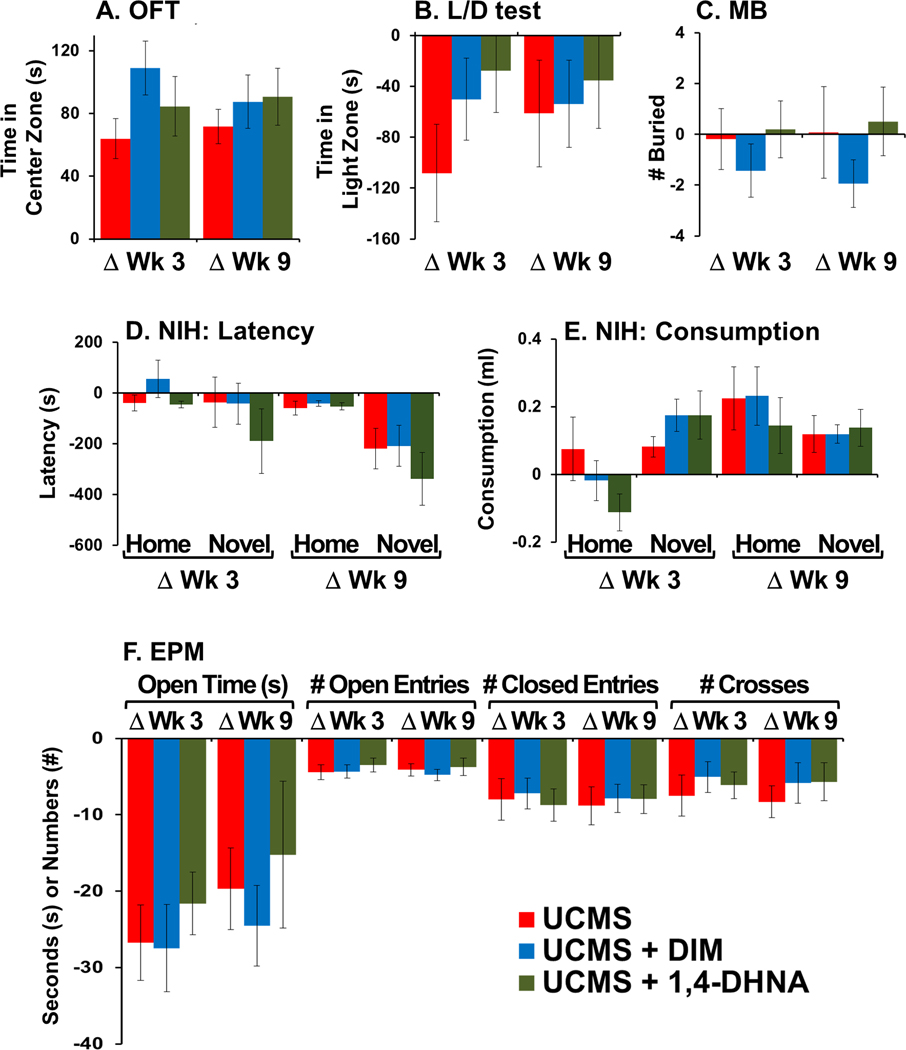
No effect of DIM or 1,4-DHNA on anxiety behaviors in stressed mice. Baseline behaviors were examined. Animals were re-tested after 3 weeks of receiving vehicle or ligand, just before UCMS began. Animals were re-tested again post-UCMS, after 9 weeks of receiving ligands. Data is presented as the difference between the re-test at week 3 and baseline (ΔWk 3) and the re-test at week 9 and baseline (ΔWk 9). (A) Time spent in the center zone of OFT; (B) time spent in the light zone of L/D apparatus; (C) number of buried marbles; (D) NIH latency to take the first lick; (E) NIH total consumption of milk; and (F) time spent in open arms of the EPM in seconds, number of entries to the open and closed arms, and total crosses in the middle. The ANOVA analyses for each test can be found in Supplementary Material.
3.2.2. Ligand effects in unstressed mice
In the L/D test, eight weeks of treatment with DIM had a significant (but small magnitude) effect, on reducing time spent in the light zone as compared to vehicle in unstressed mice (Fig. 4B). Similarly to stressed mice, no significant effect on anxiety was observed in the EPM test (Fig. 4F). However, significant differences in total activity levels were found between unstressed mice treated with vehicle vs. ligand (Fig. 4F). Specifically, at 9 weeks as compared to baseline, unstressed mice treated with vehicle exhibited an increase in total activity, while unstressed mice treated with a ligand exhibited a decrease in total activity.
3.3. Spatial learning acquisition
Toxic AhR ligands, such as TCDD, have effects on cognitive function. Thus, following 9 weeks of receiving vehicle or one of the ligands, with or without being subjected to UCMS, the effects of the AhR ligands on spatial learning in the MWM task was determined. Neither DIM nor 1,4-DHNA had a significant effect on spatial learning in unstressed animals (Fig. 6A) or animals subjected to UCMS (Fig. 6B). As expected, significant reduction in escape latencies were observed in all experimental groups over the six days of trials; however, there were not significant differences between the experimental groups in their escape latencies or the rate to reduce these escape latencies over days (Fig. 6A and B).
Fig. 6.
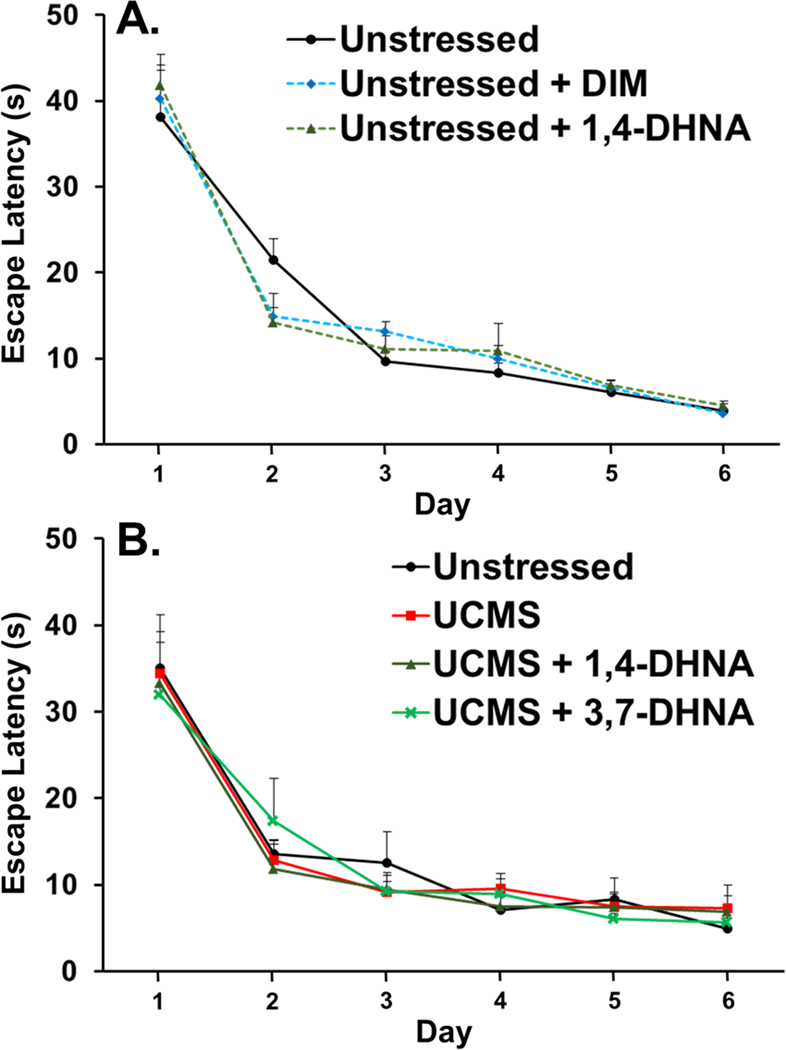
No effects of DIM or 1,4-DHNA on MWM performance. (A) Unstressed mice were examined for their escape latencies in the MWM following 9 weeks of receiving vehicle, DIM or 1,4-DHNA. Two-way repeated ANOVA revealed that there was a main effect of day (F(1,29) = 300.622, p < 0.0001), but no main effect of experimental group (F(2,29) = 0.28, p > 0.05), and no significant interaction between experimental group and day (F(2,29) = 0.056, p > 0.05). (B) Mice subjected to UCMS received daily vehicle, 1,4-DHNA, or 3,7-DHNA beginning 3 weeks prior to UCMS and continued for the entire duration of the experiment. Two-way repeated ANOVA revealed that there was a main effect of day (F(5,140) = 68.151, p < 0.0001), but no main effect of experimental group (F(3,28) = 0.84, p > 0.05), and no significant interaction between experimental group and day (F(15,140) = 0.413, p > 0.05). Results are presented as mean + SEM.
4. Discussion
The AhR was initially discovered as a receptor that bound toxic environmental contaminants, such as TCDD, and structurally related aromatics (Poland et al., 1974). Subsequent studies show that AhR also binds structurally diverse phytochemicals, endogenous biochemicals, microbial metabolites, and pharmaceuticals. Many of these AhR ligands exhibit AhR-dependent health benefits and do not exhibit TCDD-like toxicities, and are selective AhR modulators (SAhRMs) (Safe et al., 2018; Safe et al., 2020; Stockinger et al., 2021). In this study we have used two SAhRMs, namely the phytochemical metabolite DIM and the microbial metabolite 1,4-DHNA. DIM has been previously used in clinical trials for cancer therapy and is commercially available as a nutraceutical, and probiotic preparations that produce 1,4-DHNA are also being marketed (Amare, 2020).
Both DIM and 1,4-DHNA prevented and reversed the UCMS-induced decrease in sucrose preference, which serves as a rodent model to measure anhedonia (Strekalova et al., 2006). Anhedonia is the decreased ability to experience pleasure (Ribot, 1903), and is a core feature of MDD (American Psychiatric Association, 2013). These findings are in line with a previous study showing that I3C, another AhR ligand that is converted to DIM in the gut, prevents clonidine-induced despair behaviors in the forced swim test (El-Naga et al., 2014).
Moreover, this study suggests that the antidepressant-like effects of DIM and 1,4-DHNA might be mediated through the AhR. Both DIM and 1,4-DHNA exhibit significant agonist activity at the AhR (Cheng et al., 2017; Vermillion Maier et al., 2021). 3,7-DHNA is structurally similar to 1,4-DHNA but with hardly any activity at the AhR (Fig. 1, (Cheng et al., 2017). In contrast to 1,4-DHNA, 3,7-DHNA did not prevent the reduction in sucrose preference caused by exposure to UCMS. Thus, for the DHNA isomers, antidepressant activity was observed only for the AhR agonist 1,4-DHNA. However, more studies are required to determine to what degree the antidepressant-like effects can be attributed to their effect at the AhR.
The AhR is highly expressed at barrier sites, i.e. in locations where materials from the environment can be introduced to the organism, such as the skin, lung, and gut (Esser et al., 2013; Guerrina et al., 2018; Barroso et al., 2021). As an environmental sensor, AhR is sensitive to dietary components and tryptophan metabolites and is thought to play a key role in host-gut microbiota interactions (Lee et al., 2017; Dong and Perdew, 2020). Recent preclinical and clinical studies suggested an association between gut microflora and MDD (Eitan et al., 2021), and significant alterations in the relative abundance of gut microbiota were observed in MDD patients (Zheng et al., 2016; Liu et al., 2020a; Sanada et al., 2020). Transplanting fecal microbiota from healthy individuals decreased depression and anxiety symptoms in both preclinical and clinical studies, while depression-like behaviors were observed in germ- free mice transplanted with fecal microbiota from MDD patients (Zheng et al., 2016; Chinna Meyyappan et al., 2020; Liu et al., 2020b). 1,4-DHNA is a microbiota metabolite produced by both Propionibacterium freudenreichii and Lactobacillus casei, and it stimulates bifodobacterial growth (Mori et al., 1997; Isawa et al., 2002). Notably, previous studies have identified lower counts of both Bifidobacterium and Lactobacillus in MDD patients (Aizawa et al., 2016). Moreover, L. casei improved UCMS- induced depressive-like symptoms in rats (Kang et al., 2015; Gu et al., 2020). Similarly, administration of a probiotic supplementation containing Lactobacillus acidophilus, L. casei, and Bifidobacterium bifidum significantly decreased symptoms of depression in MDD patients, suggesting a possible role for 1,4-DHNA and the AhR in treating MDD patients (Akkasheh et al., 2016); however, it is important to note that in placebo-controlled clinical trials probiotic supplementation did not significantly improve symptoms of severe MDD (Romijn et al., 2017).
Mood disorders are also associated with peripheral inflammation and neuroinflammation, and AhR is involved in numerous immune functions (Bauer and Teixeira, 2021; Leite Dantas et al., 2021). Notably, deletion of microglial AhR results in the upregulation of genes associated with microglial activation and central nervous system (CNS) inflammatory response, and systemic administration of commensal-microbe-derived AhR ligands suppresses microglial activation of proinflammatory pathways (Rothhammer et al., 2018). Furthermore, dietary ligands of AhR were demonstrated to have a vital role in modulating immune responses (De Juan and Segura, 2021; Gargaro et al., 2021). Specifically, both DIM and 1,4-DHNA were shown to be anti-inflammatory, to ameliorate colitis, and suppress macrophage-derived proinflammatory cytokines (Cho et al., 2008; Kim et al., 2009; Okada et al., 2013), and DIM suppresses the differentiation of regulatory T (Treg) cells (Yang et al., 2020). These effects were suggested to be mediated by their activity at the AhR (Fukumoto et al., 2014; Yang et al., 2020). Decreased T helper 17 (Th17) cells and increased Treg cells were suggested to contribute to the depressive-like effects of UCMS (Hong et al., 2013). Subsequently, Th17/Treg imbalances were suggested to be involved in the pathophysiology of major depressive disorder (Cui et al., 2021). Thus, more studies are required to determine if the antidepressant-like effect of DIM and 1,4-DHNA is due to their effects on inflammation through inhibition of differentiation of Treg cells and cytokine production.
Additionally, AhR is expressed in multiple brain areas, including in high abundance in the adult hippocampus (Kimura and Tohyama, 2017). Reduced hippocampal neurogenesis is associated with the pathophysiology of major depression, while antidepressants were demonstrated to increase neurogenesis (Bourin, 2021). Indole, a gut microbiota-derived compound produced from tryptophan (Konopelski and Ufnal, 2018), was reported to increase hippocampal neurogenesis in an AhR-dependent manner (Wei et al., 2021); therefore, it is possible that the antidepressant-like effects of the SAhRMs were mediated via effects on hippocampal neuroplasticity. However, the toxic AhR ligand TCDD caused robust spatial learning deficits in the MWM task (Brouillette and Quirion, 2008), and effects of TCDD on hippocampal- dependent spatial tasks were sex-dependent, observed only in female but not male mice, and mediated through alteration of estrogen pathways. I3C, the parent compound of DIM, had no effect on MWM behaviors of male rats (Willeman et al., 2019); however, that study did not examine females. Therefore, in this study we examined the effects of DIM and 1,4-DHNA on spatial learning in the MWM task in females. We demonstrated that unlike TCDD, neither DIM or 1,4-DHNA caused spatial learning deficits in females; these AhR ligand-dependent differences between toxic and non-toxic AhR ligands are frequently observed and are due to their activities as SAhRMs (Safe et al., 2020). More studies are required to fully evaluate the effects of DIM and 1,4-DHNA on cognition and learning.
In this study, both DIM or 1,4-DHNA were provided orally, inside peanut butter pellets, suggesting that AhR-active dietary supplements and microbiota metabolites could play a role in preventing and treating depression. Importantly, after oral administration of DIM, concentrations in plasma and tissue, including the brain, rise rapidly and peak at 0.5 to 1 h before a polyexponential decline (Anderton et al., 2004). Thus, given the prevalence of AhR in many tissues, further studies are required to determine the site of action of DIM and 1,4-DHNA. This importance of determining the site of action is also underscored by the existence of an absorption-enhanced formulation (BioResponse-DIM; Indolplex) of DIM with significantly higher bioavailability in both rodents and humans (Anderton et al., 2004; Reed et al., 2008), which could potentially be more efficacious clinically if the site of action is not within the gut.
Lastly, there is a significant comorbidity between mood and anxiety disorders (Kaufman and Charney, 2000; Hirschfeld, 2001; Nemeroff, 2002). Thus, in this study we also examined whether DIM and 1,4-DHNA alter anxiety sensitivities. We used a battery of established rodent tests, including OFT, L/D test, MB, NIH and EPM paradigms. Some trends were observed; however, only a few significant effects were observed. DIM had a small effect of reducing time spent in the light zone in the L/D test in unstressed, but not stressed, mice. Additionally, in the EPM test DIM and 1,4-DHNA significantly reduced general activity of unstressed mice. Mice subjected to UCMS exhibited reduced activity in the EPM test; however, this reduction did not significantly differ between vehicle and ligand treated mice. Thus, the effects of DIM and 1,4-DHNA on depressive-like behaviors is not likely due to their effect on anxiety levels.
5. Conclusions
Our findings suggest that dietary supplements and microbial metabolites, such as DIM and 1,4-DHNA, respectively, can assist in preventing and treating mood disorders. Given the high prevalence of mood disorders in females, this study was conducted using female mice. Future studies need to determine whether these protective effects of DIM and 1,4-DHNA can be also generalized to males. Additionally, further studies should employ other measures of antidepressant-like properties, such as the forced swim test, which is frequently used to assess despair-like behavior in rodents (Porsolt et al., 1978). Both DIM and 1,4-DHNA had only minor effects on anxiety in unstressed mice, and did not cause deficits in spatial learning in stressed or unstressed mice. More research is required to evaluate the full spectrum of behavioral effects and the underlying molecular mechanisms including to what degree the effects observed in this study can be attributed to the AhR, the site of activity (e.g., gut, immune system, brain), and the cellular signaling mediating these effects.
Supplementary Material
Acknowledgements
This work was supported by a Seed Grant from Texas A&M University (to SE); and supported in part by NIH grant R35 CA197707 (to RSC) and funds from the Allen Endowed Chair in Nutrition & Chronic Disease Prevention (to RSC).
Role of funding source
This work was supported by internal seed grant from Texas A&M University (to SE); and supported in part by NIH grant R35 CA197707 (to RSC) and funds from the Allen Endowed Chair in Nutrition & Chronic Disease Prevention (to RSC). The funding source had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
CRediT authorship contribution statement
Caitlin A. Madison: Investigation, Formal analysis, Writing – original draft. Jacob Kuempel: Investigation, Writing – original draft. Georgia Lee Albrecht: Investigation. Lauren Hillbrick: Investigation. Arul Jayaraman: Conceptualization, Writing – original draft. Stephen Safe: Conceptualization, Writing – original draft. Robert S. Chapkin: Conceptualization, Writing – original draft. Shoshana Eitan: Conceptualization, Supervision, Formal analysis, Writing – original draft.
Declaration of competing interest
The authors have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jad.2022.04.106.
References
- Aggarwal BB, Ichikawa H, 2005. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 4, 1201–1215. [DOI] [PubMed] [Google Scholar]
- Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, Ota M, Koga N, Hattori K, Kunugi H, 2016. Possible association of bifidobacterium and lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord 202, 254–257. [DOI] [PubMed] [Google Scholar]
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, Memarzadeh MR, Asemi Z, Esmaillzadeh A, 2016. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 32, 315–320. [DOI] [PubMed] [Google Scholar]
- Albert PR, 2015. Why is depression more prevalent in women? J. Psychiatry Neurosci 40, 219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amare DE, 2020. Anti-cancer and other biological effects of a dietary compound 3,3′-diindolylmethane supplementation: a systematic review of human clinical trials. Nutr. Diet. Suppl 12, 123–137. [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association. [Google Scholar]
- Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML, Mager DE, 2004. Physiological modeling of formulated and crystalline 3,3’-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab. Dispos 32, 632–638. [DOI] [PubMed] [Google Scholar]
- Barroso A, Mahler JV, Fonseca-Castro PH, Quintana FJ, 2021. The aryl hydrocarbon receptor and the gut-brain axis. Cell. Mol. Immunol 18, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer ME, Teixeira AL, 2021. Neuroinflammation in mood disorders: role of regulatory immune cells. Neuroimmunomodulation 1–9. [DOI] [PubMed]
- Bentley R, Meganathan R, 1982. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol. Rev 46, 241–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, Iannitti R, Tissi L, Volpi C, Belladonna ML, Orabona C, Bianchi R, Lanz TV, Platten M, Della Fazia MA, Piobbico D, Zelante T, Funakoshi H, Nakamura T, Gilot D, Denison MS, Guillemin GJ, DuHadaway JB, Prendergast GC, Metz R, Geffard M, Boon L, Pirro M, Iorio A, Veyret B, Romani L, Grohmann U, Fallarino F, Puccetti P, 2014. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511, 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M, 2021. Neurogenesis and neuroplasticity in major depression: its therapeutic implication. Adv. Exp. Med. Biol 1305, 157–173. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M, 2003. The mouse light/dark box test. Eur. J. Pharmacol 463, 55–65. [DOI] [PubMed] [Google Scholar]
- Brouillette J, Quirion R, 2008. The common environmental pollutant dioxin-induced memory deficits by altering estrogen pathways and a major route of retinol transport involving transthyretin. Neurotoxicology 29, 318–327. [DOI] [PubMed] [Google Scholar]
- Burstein O, Doron R, 2018. The unpredictable chronic mild stress protocol for inducing anhedonia in mice. J. Vis. Exp 140, 58184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Jin U-H, Davidson LA, Chapkin RS, Jayaraman A, Tamamis P, Orr A, Allred C, Denison MS, Soshilov A, Weaver E, Safe S, 2017. Editor’s highlight: microbial-derived 1,4-Dihydroxy-2-naphthoic acid and related compounds as aryl hydrocarbon receptor Agonists/Antagonists: structure-activity relationships and receptor modeling. Toxicol. Sci 155, 458–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinna Meyyappan A, Forth E, Wallace CJK, Milev R, 2020. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatry 20, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Seon MR, Lee YM, Kim J, Kim JK, Kim SG, Park JH, 2008. 3,3’-diindolylmethane suppresses the inflammatory response to lipopolysaccharide in murine macrophages. J. Nutr 138, 17–23. [DOI] [PubMed] [Google Scholar]
- Cui M, Dai W, Kong J, Chen H, 2021. Th17 cells in depression: are they crucial for the antidepressant effect of ketamine? Front. Pharmacol 12, 649144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Juan A, Segura E, 2021. Modulation of immune responses by nutritional ligands of aryl hydrocarbon receptor. Front. Immunol 12, 645168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM, 2006. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat. Protoc 1, 122–124. [DOI] [PubMed] [Google Scholar]
- Delis F, Thanos PK, Rombola C, Rosko L, Grandy D, Wang GJ, Volkow ND, 2013. Chronic mild stress increases alcohol intake in mice with low dopamine D2 receptor levels. Behav. Neurosci 127, 95–105. [DOI] [PubMed] [Google Scholar]
- Dolan KT, Chang EB, 2017. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol. Nutr. Food Res 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Perdew GH, 2020. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes 12, 1859812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan S, Madison CA, Kuempel J, 2021. The self-serving benefits of being a good host: a role for our micro-inhabitants in shaping opioids’ function. Neurosci. Biobehav. Rev 127, 284–295. [DOI] [PubMed] [Google Scholar]
- El-Naga RN, Ahmed HI, Abd Al Haleem EN, 2014. Effects of indole-3-carbinol on clonidine-induced neurotoxicity in rats: impact on oxidative stress, inflammation, apoptosis and monoamine levels. Neurotoxicology 44, 48–57. [DOI] [PubMed] [Google Scholar]
- Esser C, Bargen I, Weighardt H, Haarmann-Stemmann T, Krutmann J, 2013. Functions of the aryl hydrocarbon receptor in the skin. Semin. Immunopathol 35, 677–691. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA, 2013. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 10, e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR, 1991. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem 56, 2007–2017. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Toshimitsu T, Matsuoka S, Maruyama A, Oh-Oka K, Takamura T, Nakamura Y, Ishimaru K, Fujii-Kuriyama Y, Ikegami S, Itou H, Nakao A, 2014. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol. Cell Biol 92, 460–465. [DOI] [PubMed] [Google Scholar]
- Gargaro M, Manni G, Scalisi G, Puccetti P, Fallarino F, 2021. Tryptophan metabolites at the crossroad of immune-cell interaction via the aryl hydrocarbon receptor: implications for tumor immunotherapy. Int. J. Mol. Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales C, Zaleska MM, Riddell DR, Atchison KP, Robshaw A, Zhou H, Sukoff Rizzo SJ, 2014. Alternative method of oral administration by peanut butter pellet formulation results in target engagement of BACE1 and attenuation of gavage-induced stress responses in mice. Pharmacol. Biochem. Behav 126, 28–35. [DOI] [PubMed] [Google Scholar]
- Grifka-Walk HM, Jenkins BR, Kominsky DJ, 2021. Amino acid trp: the far out impacts of host and commensal tryptophan metabolism. Front. Immunol 12, 653208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Weiss RM, Johnson AK, 2006. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol. Psychiatry 59, 309–316. [DOI] [PubMed] [Google Scholar]
- Gu F, Wu Y, Liu Y, Dou M, Jiang Y, Liang H, 2020. Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the BDNF-TrkB signal pathway and the intestinal microbiota. Food Funct. 11, 6148–6157. [DOI] [PubMed] [Google Scholar]
- Guerrina N, Traboulsi H, Eidelman DH, Baglole CJ, 2018. The aryl hydrocarbon receptor and the maintenance of lung health. Int. J. Mol. Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawton K, Casanas ICC, Haw C, Saunders K, 2013. Risk factors for suicide in individuals with depression: a systematic review. J. Affect. Disord 147, 17–28. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RMA, 2001. The comorbidity of major depression and anxiety disorders: recognition and Management in Primary Care. Prim. Care Companion J. Clin. Psychiatry 3, 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Hodgson SR, Roberts KW, Bryant CD, Evans CJ, Eitan S, 2009. Extracellular signal-regulated kinase activation in the amygdala mediates elevated plus maze behavior during opioid withdrawal. Behav. Pharmacol 20, 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Zheng J, Ding ZY, Chen JH, Yu L, Niu Y, Hua YQ, Wang LL, 2013. Imbalance between Th17 and treg cells may play an important role in the development of chronic unpredictable mild stress-induced depression in mice. Neuroimmunomodulation 20, 39–50. [DOI] [PubMed] [Google Scholar]
- Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS, 2007. Antidepressant actions of the exercise-regulated gene VGF. Nat. Med 13, 1476. [DOI] [PubMed] [Google Scholar]
- Isawa K, Hojo K, Yoda N, Kamiyama T, Makino S, Saito M, Sugano H, Mizoguchi C, Kurama S, Shibasaki M, Endo N, Sato Y, 2002. Isolation and identification of a new bifidogenic growth stimulator produced by propionibacterium freudenreichii ET-3. Biosci. Biotechnol. Biochem 66, 679–681. [DOI] [PubMed] [Google Scholar]
- Kang J-E, Kim T-J, Moon G-S, 2015. A novel lactobacillus casei LP1 producing 1,4-Dihydroxy-2-naphthoic acid, a bifidogenic growth stimulator. Prev. Nutr. Food Sci 20, 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Charney D, 2000. Comorbidity of mood and anxiety disorders. Depress. Anxiety 12 (Suppl. 1), 69–76. [DOI] [PubMed] [Google Scholar]
- Kautzky A, Dold M, Bartova L, Spies M, Kranz GS, Souery D, Montgomery S, Mendlewicz J, Zohar J, Fabbri C, Serretti A, Lanzenberger R, Dikeos D, Rujescu D, Kasper S, 2019. Clinical factors predicting treatment resistant depression: affirmative results from the european multicenter study. Acta Psychiatr. Scand 139, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Kwon HS, Kim DH, Shin EK, Kang YH, Park JH, Shin HK, Kim JK, 2009. 3,3’-diindolylmethane attenuates colonic inflammation and tumorigenesis in mice. Inflamm. Bowel Dis 15, 1164–1173. [DOI] [PubMed] [Google Scholar]
- Kimura E, Tohyama C, 2017. Embryonic and postnatal expression of aryl hydrocarbon receptor mRNA in mouse brain. Front. Neuroanat 11, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopelski P, Ufnal M, 2018. Indoles - gut bacteria metabolites of tryptophan with pharmacotherapeutic potential. Curr. Drug Metab 19, 883–890. [DOI] [PubMed] [Google Scholar]
- Lee HU, McPherson ZE, Tan B, Korecka A, Pettersson S, 2017. Host-microbiome interactions: the aryl hydrocarbon receptor and the central nervous system. J. Mol. Med. (Berl.) 95, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite Dantas R, Freff J, Ambrée O, Beins EC, Forstner AJ, Dannlowski U, Baune BT, Scheu S, Alferink J, 2021. Dendritic cells: neglected modulators of peripheral immune responses and neuroinflammation in mood disorders? Cells 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Ray B, Neavin DR, Zhang J, Athreya AP, Biernacka JM, Bobo WV, Hall-Flavin DK, Skime MK, Zhu H, Jenkins GD, Batzler A, Kalari KR, Boakye-Agyeman F, Matson WR, Bhasin SS, Mushiroda T, Nakamura Y, Kubo M, Iyer RK, Wang L, Frye MA, Kaddurah-Daouk R, Weinshilboum RM, 2018. Beta-defensin 1, aryl hydrocarbon receptor and plasma kynurenine in major depressive disorder: metabolomics-informed genomics. Transl. Psychiatry 8, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, Rowan-Nash AD, Sheehan AE, Walsh RFL, Sanzari CM, Korry BJ, Belenky P, 2020a. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav. Immun 31531–31534. S0889–1591. [DOI] [PMC free article] [PubMed]
- Liu S, Guo R, Liu F, Yuan Q, Yu Y, Ren F, 2020b. Gut microbiota regulates depression-like behavior in rats through the neuroendocrine-immune-mitochondrial pathway. Neuropsychiatr. Dis. Treat 16, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrioli R, Mercolini L, Saracino MA, Raggi MA, 2012. Selective serotonin reuptake inhibitors (SSRIs): therapeutic drug monitoring and pharmacological interactions. Curr. Med. Chem 19, 1846–1863. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, Barakat M, Miguelez M, 2014. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J. Affect. Disord 156, 1–7. [DOI] [PubMed] [Google Scholar]
- Mori H, Sato Y, Taketomo N, Kamiyama T, Yoshiyama Y, Meguro S, Sato H, Kaneko T, 1997. Isolation and structural identification of bifidogenic growth stimulator produced by propionibacterium freudenreichii. J. Dairy Sci 80, 1959–1964. [DOI] [PubMed] [Google Scholar]
- Mrazek DA, Hornberger JC, Altar CA, Degtiar I, 2014. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr. Serv 65, 977–987. [DOI] [PubMed] [Google Scholar]
- Muscat R, Papp M, Willner P, 1992. Antidepressant-like effects of dopamine agonists in an animal model of depression. Biol. Psychiatry 31, 937–946. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, 2002. Comorbidity of mood and anxiety disorders: the rule, not the exception? Am. J. Psychiatry 159, 3–4. [DOI] [PubMed] [Google Scholar]
- O’Rourke MC, Siddiqui W, 2019. Suicide Screening and Prevention. StatPearls Publishing LLC, Treasure Island (FL). [PubMed] [Google Scholar]
- Okada Y, Tsuzuki Y, Narimatsu K, Sato H, Ueda T, Hozumi H, Sato S, Hokari R, Kurihara C, Komoto S, Watanabe C, Tomita K, Kawaguchi A, Nagao S, Miura S, 2013. 1,4-Dihydroxy-2-naphthoic acid from propionibacterium freudenreichii reduces inflammation in interleukin-10-deficient mice with colitis by suppressing macrophage-derived proinflammatory cytokines. J. Leukoc. Biol 94, 473–480. [DOI] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M, 2011. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203. [DOI] [PubMed] [Google Scholar]
- Palego L, Betti L, Rossi A, Giannaccini G, 2016. Tryptophan biochemistry: structural, nutritional, metabolic, and medical aspects in humans. J. Amino Acids 2016, 8952520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland AP, Glover E, Robinson JR, Nebert DW, 1974. Genetic expression of aryl hydrocarbon hydroxylase activity. Induction of monooxygenase activities and cytochrome P1–450 formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice genetically “nonresponsive” to other aromatic hydrocarbons. J. Biol. Chem 249, 5599–5606. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M, 1978. “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur. J. Pharmacol 51, 291–294. [DOI] [PubMed] [Google Scholar]
- Reed GA, Sunega JM, Sullivan DK, Gray JC, Mayo MS, Crowell JA, Hurwitz A, 2008. Single-dose pharmacokinetics and tolerability of absorption- enhanced 3,3’-diindolylmethane in healthy subjects. Cancer Epidemiol. Biomark. Prev 17, 2619–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot T, 1903. The psychology of the emotions. Walter Scott, Limited. [Google Scholar]
- Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C, 2017. A double-blind, randomized, placebo-controlled trial of lactobacillus helveticus and bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 51, 810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao C-C, Ardura-Fabregat A, de Lima KA, Gutiérrez-Vázquez C, Hewson P, Staszewski O,Blain M, Healy L, Neziraj T, Borio M, Wheeler M, Dragin LL, Laplaud DA, Antel J, Alvarez JI, Prinz M, Quintana FJ, 2018. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Han H, Goldsby J, Mohankumar K, Chapkin RS, 2018. Aryl hydrocarbon receptor (AhR) ligands as selective AhR modulators: genomic studies. Curr. Opin. Toxicol 11–12, 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Jin UH, Park H, Chapkin RS, Jayaraman A, 2020. Aryl hydrocarbon receptor (AHR) ligands as selective AHR modulators (SAhRMs). Int. J. Mol. Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada K, Nakajima S, Kurokawa S, Barceló-Soler A, Ikuse D, Hirata A, Yoshizawa A, Tomizawa Y, Salas-Valero M, Noda Y, Mimura M, Iwanami A, Kishimoto T, 2020. Gut microbiota and major depressive disorder: a systematic review and meta-analysis. J. Affect. Disord 266, 1–13. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q, 2012. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci 13, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoyama D, Kato TA, Hashimoto R, Kunugi H, Hattori K, Hayakawa K, Sato-Kasai M, Shimokawa N, Kaneko S, Yoshida S, Goto YI, Yasuda Y, Yamamori H, Ohgidani M, Sagata N, Miura D, Kang D, Kanba S, 2016. Plasma metabolites predict severity of depression and suicidal ideation in psychiatric patients-a multicenter pilot analysis. PLoS One 11, e0165267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Shah K, Wincent E, 2021. AHR in the intestinal microenvironment: safeguarding barrier function. Nat. Rev. Gastroenterol. Hepatol 18, 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D, 2006. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav. Pharmacol 17, 271–287. [DOI] [PubMed] [Google Scholar]
- van Bronswijk S, Moopen N, Beijers L, Ruhe HG, Peeters F, 2019. Effectiveness of psychotherapy for treatment-resistant depression: a meta-analysis and meta-regression. Psychol. Med 49, 366–379. [DOI] [PubMed] [Google Scholar]
- Vermillion Maier ML, Siddens LK, Uesugi SL, Choi J, Leonard SW, Pennington JM, Tilton SC, Smith JN, Ho E, Chow HHS, Nguyen BD, Kolluri SK, Williams DE, 2021. 3,3’-diindolylmethane (BioResponse DIM(®)) exhibits significant metabolism following Oral dosing in humans. Drug Metab. Dispos 49, 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT, 2006. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc 1, 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei GZ, Martin KA, Xing PY, Agrawal R, Whiley L, Wood TK, Hejndorf S, Ng YZ, Low JZY, Rossant J, Nechanitzky R, Holmes E, Nicholson JK, Tan EK, Matthews PM, Pettersson S, 2021. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U. S. A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeman MN, Chawla MK, Zempare MA, Biwer LA, Hoang LT, Uprety AR, Fitzhugh MC, De Both M, Coleman PD, Trouard TP, Alexander GE, Mitchell KD, Barnes CA, Hale TM, Huentelman M, 2019. Gradual hypertension induction in middle-aged Cyp1a1-Ren2 transgenic rats produces significant impairments in spatial learning. Physiol. Rep 7 e14010-e14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, 2017. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol. Stress 6, 78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2018. Depression. https://www.who.int/news-room/fact-sheets/detail/depression. Accessed 7 May 2019.
- Xue L, Pestka JJ, Li M, Firestone GL, Bjeldanes LF, 2008. 3,3’-diindolylmethane stimulates murine immune function in vitro and in vivo. J. Nutr. Biochem 19, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Tan L, Chen Y, Liu A, Hong M, Peng Z, 2020. DIM mitigates the development of experimental autoimmune encephalomyelitis by maintaining the stability and suppressive function of regulatory T cells. Cell. Immunol 358, 104238. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P, 2016. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.