Abstract
Non-surgical techniques with hyaluronic acid fillers to rejuvenate face are very popular treatment due to ease of administration and achievement of faster improvement. Although they are considered to be safe procedures, few rare adverse effects have been reported in literatures. The number of these cosmetic procedures being performed is increasing every year. Simultaneously, the rate of filler complications has also increased.
This case series presents three clinical cases of delayed onset nodules after hyaluronic acid fillers after 1–5 months post-procedure. Juvederm Volift was used in all the mentioned patients.
Granulomatous reactions can occur with any of the hyaluronic acid fillers. These are generally localized immunological reactions. An awareness about these rare side effects helps us in the assessment and ensurance of accurate diagnosis and treatment.
Keywords: Delayed onset nodules, filler complications, granulomatous reactions due to hyaluronic acid fillers, Juvederm Volift
INTRODUCTION
Hyaluronic acid fillers are used to fill volume deficits and efface wrinkles with nearly no recovery time and high rates of patient satisfaction, thus leading to increase in demand.[1] Many different hyaluronic acid-injectable fillers are available on the market and differ in terms of hyaluronic acid concentration, particle size, cross-linking density, requisite needle size, duration, stiffness, hydration, presence of lidocaine, type of cross-linking technology, and cost. Hyaluronic acid is a natural component of many soft tissues and is identical across species minimizing immunogenicity, which has been linked to wound healing and skin regeneration. The biomechanical and biochemical effects of hyaluronic acid (HA) on the local microenvironment of the injected site are the key to its success as a soft tissue filler.[2]
In spite of hyaluronic molecule being an inert molecule, delayed onset immune mediated nodules have become a rare but challenging complication.
Hypersensitivity reactions can be classified as acute or delayed, depending on the time of onset.[3] Type I hypersensitivity reactions occur within minutes or hours after injections due to an immunoglobulin E-mediated immune response and manifest as angioedema or anaphylactic reactions occurring after initial or repeated exposure.[4,5,6,7]
Delayed hypersensitivity reactions are characterized by induration, erythema, and edema and are mediated by T-lymphocytes rather than antibodies. They typically occur 48–72 h after injection but may be seen as late as several weeks after injection and may persist for many months.[8,9] Late-onset reactions occur at least 3 months after the uneventful injection of a dermal filler.
CASE 1
A 61-year-old patient visited our OPD with concerns of loss of volume in cheeks and sunken eyes. She was treated with 1 mL of Juvederm Voluma and 1 mL of Juvederm Volift (8-point lift) following MD codes. Pre-procedure pictures and consent were taken before starting the procedure. Topical EMLA cream was applied before treatment. History of any infections or recent dental procedure was taken. All sterile precautions were taken before executing the procedure. Bolus injections were performed at all points (1–2 U) except in the submalar area (CK4) where retrograde fanning was done in the deep dermal plane. The post-procedure period was uneventful.
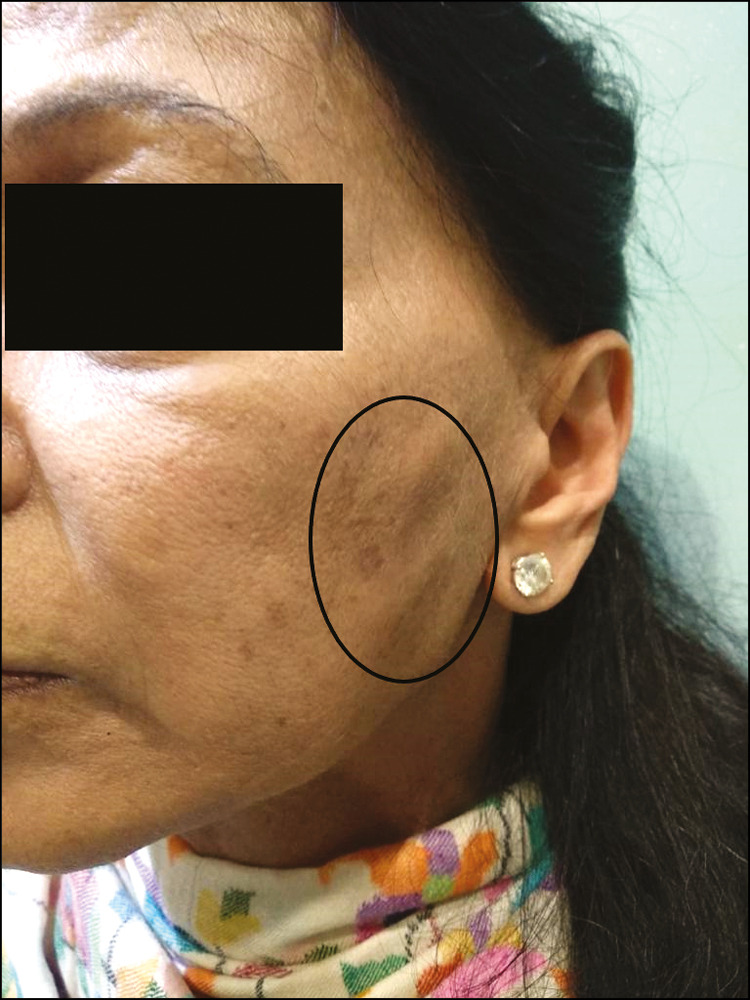
She presented with nodules in the submalar area on both cheeks after 5 months. The patient had earlier visited an ENT specialist when the nodules were tender. Fine needle aspiration cytology had been performed which was inconclusive. The nodules were only restricted to the submalar area where Juvederm Volite was used.
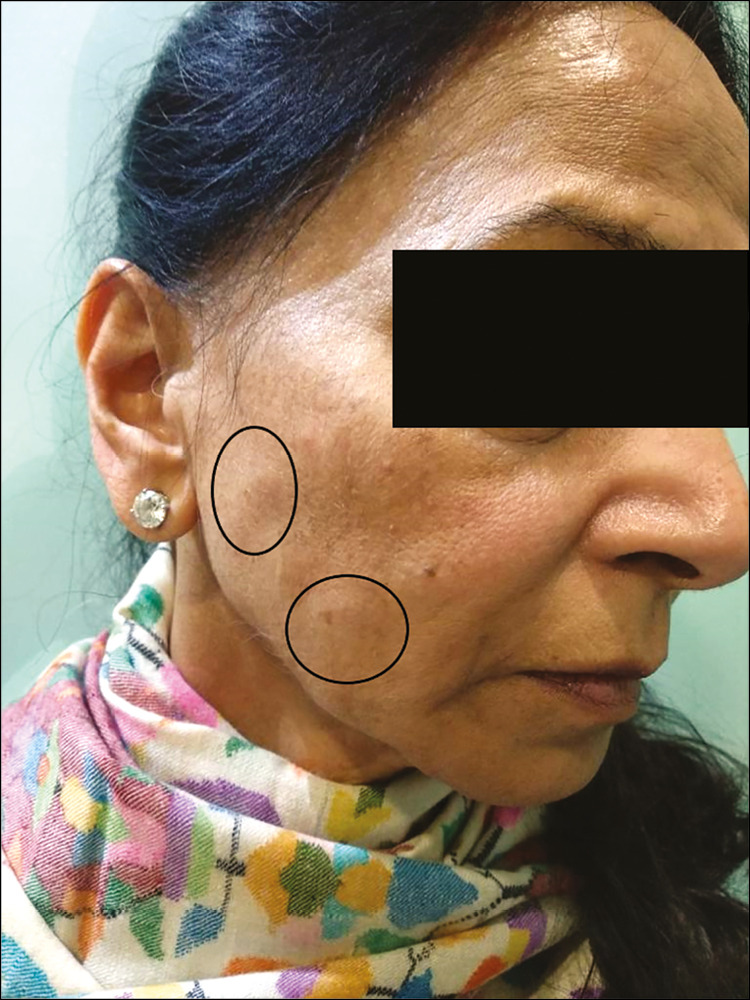
The patient was prescribed ciprofloxacin 500 mg bid for 3 weeks and oral prednisolone 20 mg for 2 weeks. She was lost to follow-up.
CASE 2
The second patient is a 44-year-old patient whose past cosmetic history includes multiple injectable procedures for various indications and products from Juvederm (Allergan) and Restylane were used. Juvederm Volite was used to correct her tear trough. Pre-procedure pictures and consent were taken before starting the procedure. Topical EMLA cream was not applied before treatment. History of any infections or recent dental procedure was taken. All sterile precautions were taken before executing the procedure. The injections were performed according to MD codes for tear trough. Bolus injections (2–3 U) were given using a needle. The post-procedure period was uneventful.
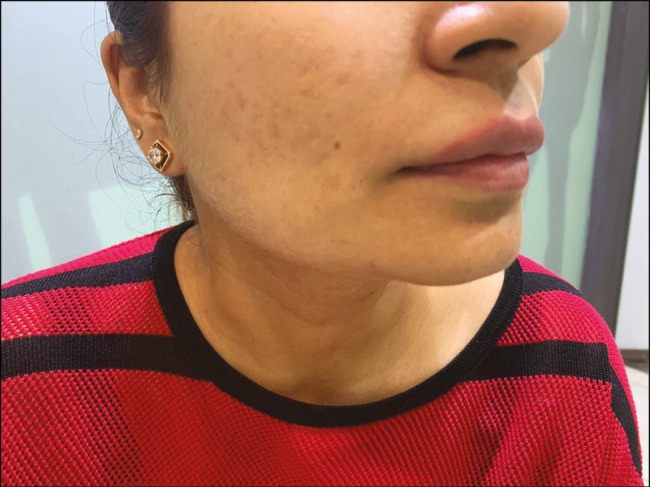
The patient presented with non-tender nodules at Tt1 and Tt2 points after 3 weeks of procedure. Slight massaging and mild radiofrequency (three sessions) were used to resolve them but there was no improvement. As mentioned earlier, the patient takes hyaluronic acid fillers regularly and has never experienced any nodule formation. On taking detailed history, the patient had complained of flu-like symptoms a week before the appearance of nodules. A short course of ciprofloxacin 500 mg and methyl prednisolone was given for a week with mild improvement. Hyaluronidase was used to resolve the nodules which were not responsive to treatment.
CASE 3
A 36-year-old female patient presented with thin lips and was recommended Juvederm Volift for lip rejuvenation. She had taken Juvederm Voluma XC for cheek augmentation 6 months earlier. Satisfied with the results, she wanted to consider lip rejuvenation. The patient had complained of a few lumps in the earlier session for cheek augmentation with Juvederm Voluma XC which settled on their own.
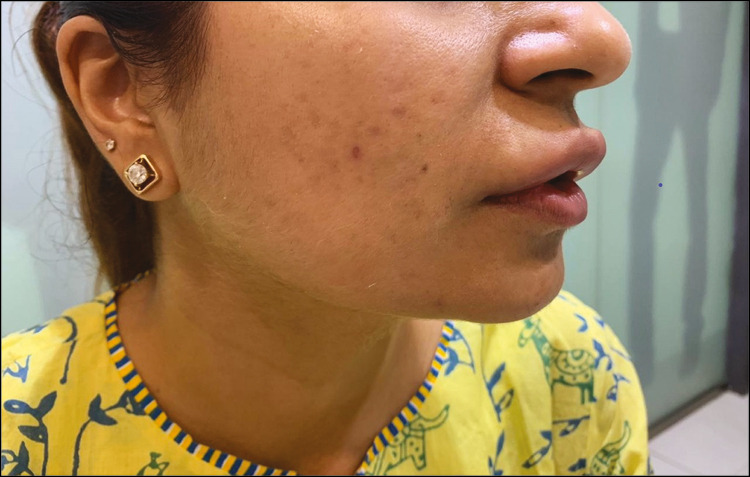
Keeping this under consideration, history of any infections or dental work was taken and she was prescribed azithromycin 500 mg for 3 days and valacyclovir 1000 mg stat dose as prophylactic. Pre-procedure pictures and consent were taken before starting the procedure. Topical EMLA cream was applied before treatment. All sterile precautions were taken before executing the procedure. Injections were performed according to the MD codes. All points except Lp5, 7, and 8 were done. She complained of mild swelling and discomfort for 3 days after procedure which resolved subsequently.
The patient presented with edema and tender nodules (Lp1 on upper lip) after a month of the procedure. There was no history of any infection according to the patient. She was prescribed ciprofloxacin 500 mg bid for 3 weeks and oral prednisolone 20 mg for 3 weeks. There was a mild relief with medication but the edema kept recurring when the dose of steroid was tapered. With little-to-no improvement, we decided to hyalase the material. There was a relief in the edema in the following days. We continued the antibiotics for another week and stopped the steroids.
DISCUSSION
Late-onset inflammatory reactions are rare complications, and their cause may be infectious or immune-mediated. Hyaluronic acid fillers have been considered inert immunologically as their structure is similar to the naturally occurring HA disaccharide polymers in the extracellular matrix and also they lack a protein moiety.[10]
In all the cases, the presentation seems to be immunological in nature. Delayed type IV hypersensitivity mediated by T lymphocytes and CD4+ cells is the most likely explanation for late-onset nodule formation.[5,11] Late-onset reactions can occur from weeks to many months after administration of HA fillers. It can present with inflammatory nodules and edema. They can occur in both first time and previously injected patients. Certain influencing factors are previous infections and trauma, injection technique, filler volume, repeated treatments, intramuscular implantation, and various properties of fillers.[5,6,12]
The Juvederm family of fillers is composed of homogeneous monophasic gel produced through either the Hylacross (HYC) or Vycross (VYC) technology. In HYC, high molecular weight HA is used in the cross-linking process in contrast to VYC technology in which both high and low molecular weight HA is used for crosslinking.[10,13,14]
High molecular weight HA is primarily anti-inflammatory, whereas low molecular weight HA is proinflammatory and can activate the innate immune system.[10] According to Beleznay et al.,[15] systemic inflammatory response to an unknown antigen can release proinflammatory low molecular weight HA fragments during an accelerated breakdown of HA gels.
Another theory is the formation of biofilm, which are heterogenous structures that comprise bacteria embedded within a strong extracellular matrix of secreted polysaccharides including hyaluronic acid.[16] They function as a self-maintaining organism with a resistant homeostatic environment.[17] The histologic appearance of hyaluronic acid reactions displays a bluish material surrounded by numerous macrophages and giant cells rarely mixed with neutrophils and bacteria.[18,19,20]
Delayed reactions are difficult to diagnose and treat due to the time lapsed. Steroids help in reducing the inflammatory signs. Antibiotic course can be included if the patient complains of any systemic infection. Hyaluronidase should be injected in lumps which do not resolve.
CONCLUSION
The risk of delayed hypersensitivity to HA fillers was considered to be very low. With the introduction of differentially linked HA fillers, the rate of these reactions seems to be on a rise.[10]
As the incidence may vary depending on the manufacturing technology, the patient should not be injected with the same molecule in the next session.
Identification and prompt treatment can resolve the inflammatory symptoms with medication.[21] These reactions can be self-limiting but oral steroids are needed for rapid improvement and to reduce the risk of recurrence. In case of lump formation, hyaluronidase is needed for resolution.[22,23,24]
Thus, informing the patients about the rare complications and to follow up in case of the occurrence of the same before treatment can help us avoid disappointed patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rohrich RJ, Ghavami A, Crosby MA. The role of hyaluronic acid fillers (restylane) in facial cosmetic surgery: Review and technical considerations. Plast Reconstr Surg. 2007;120:41S–54S. doi: 10.1097/01.prs.0000248794.63898.0f. [DOI] [PubMed] [Google Scholar]
- 2.Greene JJ, Sidle DM. The hyaluronic acid fillers: Current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423–32. doi: 10.1016/j.fsc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Rzany B, DeLorenzi C. Understanding, avoiding, and managing severe filler complications. Plast Reconstr Surg. 2015;136:196S–203S. doi: 10.1097/PRS.0000000000001760. [DOI] [PubMed] [Google Scholar]
- 4.Ozturk CN, Li Y, Tung R, Parker L, Piliang MP, Zins JE. Complications following injection of soft-tissue fillers. Aesthet Surg J. 2013;33:862–77. doi: 10.1177/1090820X13493638. [DOI] [PubMed] [Google Scholar]
- 5.Funt D, Pavicic T. Dermal fillers in aesthetics: An overview of adverse events and treatment approaches. Plast Surg Nurs. 2015;35:13–32. doi: 10.1097/PSN.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 6.Alijotas-Reig J, Fernández-Figueras MT, Puig L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45:97–108. doi: 10.1007/s12016-012-8348-5. [DOI] [PubMed] [Google Scholar]
- 7.Dayan SH. Complications from toxins and fillers in the dermatology clinic: Recognition, prevention, and treatment. Facial Plast Surg Clin North Am. 2013;21:663–73. doi: 10.1016/j.fsc.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Funt D, Pavicic T. Dermal fillers in aesthetics: An overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316. doi: 10.2147/CCID.S50546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arron ST, Neuhaus IM. Persistent delayed-type hypersensitivity reaction to injectable non-animal-stabilized hyaluronic acid. J Cosmet Dermatol. 2007;6:167–71. doi: 10.1111/j.1473-2165.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 10.Sadeghpour M, Quatrano NA, Bonati LM, Arndt KA, Dover JS, Kaminer MS. Delayed-onset nodules to differentially crosslinked hyaluronic acids comparative incidence and risk assessment. Dermatol Surg. 2019;45:1085–94. doi: 10.1097/DSS.0000000000001814. [DOI] [PubMed] [Google Scholar]
- 11.DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33:561–75. doi: 10.1177/1090820X13484492. [DOI] [PubMed] [Google Scholar]
- 12.Rongioletti F. Complications granulomateuses des techniques de comblement. Ann Dermatol Venerol. 2008;135:59–65. doi: 10.1016/S0151-9638(08)70213-8. [DOI] [PubMed] [Google Scholar]
- 13.Baeva LF, Lyle DB, Rios M, Langone JJ, Lightfoote MM. Different molecular weight hyaluronic acid effects on human macrophage interleukin 1β production. J Biomed Mater Res A. 2014;102:305–14. doi: 10.1002/jbm.a.34704. [DOI] [PubMed] [Google Scholar]
- 14.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: An information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Beleznay K, Carruthers JD, Carruthers A, Mummert ME, Humphrey S. Delayed-onset nodules secondary to a smooth cohesive 20 mg/ml hyaluronic acid filler: Cause and management. Dermatol Surg. 2015;41:929–39. doi: 10.1097/DSS.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 16.Rohrich RJ, Monheit G, Nguyen AT, Brown SA, Fagien S. Soft-tissue filler complications: The important role of biofilms. Plast Reconstr Surg. 2010;125:1250–6. doi: 10.1097/PRS.0b013e3181cb4620. [DOI] [PubMed] [Google Scholar]
- 17.Rohrich RJ, Nguyen AT, Kenkel JM. Lexicon for soft tissue implants. Dermatol Surg. 2009;35(Suppl 2):1605–11. doi: 10.1111/j.1524-4725.2009.01337.x. [DOI] [PubMed] [Google Scholar]
- 18.Duranti F, Salti G, Bovani B, Calandra M, Rosati ML. Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatol Surg. 1998;24:1317–25. doi: 10.1111/j.1524-4725.1998.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Cossío S, Castaño-Oreja MT. Biocompatibility of two novel dermal fillers: Histological evaluation of implants of a hyaluronic acid filler and a polyacrylamide filler. Plast Reconstr Surg. 2006;117:1789–96. doi: 10.1097/01.prs.0000214656.07273.b0. [DOI] [PubMed] [Google Scholar]
- 20.Ghislanzoni M, Bianchi F, Barbareschi M, Alessi E. Cutaneous granulomatous reaction to injectable hyaluronic acid gel. Br J Dermatol. 2006;154:755–8. doi: 10.1111/j.1365-2133.2005.07074.x. [DOI] [PubMed] [Google Scholar]
- 21.Bhojani-Lynch T. Late-onset inflammatory response to hyaluronic acid dermal fillers. Plast Reconstr Surg Global Open. 2017;5:e1532. doi: 10.1097/GOX.0000000000001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Signorini M, Liew S, Sundaram H, De Boulle KL, Goodman GJ, Monheit G, et al. Global Aesthetics Consensus Group. Global Aesthetics Consensus: Avoidance and management of complications from hyaluronic acid fillers—Evidence- and opinion-based review and consensus recommendations. Plast Reconstr Surg. 2016;137:961e–71e. doi: 10.1097/PRS.0000000000002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alijotas-Reig J, Fernández-Figueras MT, Puig L. Pseudocystic encapsulation: A late noninflammatory complication of hyaluronic acid filler injections. Dermatol Surg. 2013;39:1726–8. doi: 10.1111/dsu.12316. [DOI] [PubMed] [Google Scholar]
- 24.Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893–7. doi: 10.1097/00042728-200508000-00001. [DOI] [PubMed] [Google Scholar]


