Abstract
Age-related changes in proprioception are known to affect postural stability, yet the extent to which such changes affect the finger joints is poorly understood despite the importance of finger proprioception in the control of skilled hand movement. We quantified age-related changes in finger proprioception in 37 healthy young, middle-aged, and older adults using two robot-based tasks wherein participants’ index and middle fingers were moved by an exoskeletal robot. The first task assessed finger position sense by asking participants to indicate when their index and middle fingers were directly overlapped during a passive crisscross movement; the second task assessed finger movement detection by asking participants to indicate the onset of passive finger movement. When these tasks were completed without vision, finger position sense errors were 48% larger in older adults compared to young participants (p < 0.05); proprioceptive reaction time was 78% longer in older adults compared to young adults (p < 0.01). When visual feedback was provided in addition to proprioception, these age-related differences were no longer apparent. No difference between dominant and non-dominant hand performance was found for either proprioception task. These findings demonstrate that finger proprioception is impaired in older adults and visual feedback can be used to compensate for this deficit. The findings also support the feasibility and utility of the FINGER robot as a sensitive tool for detecting age-related decline in proprioception.
Keywords: Proprioception, Aging, Joint position sense, Finger function, Robotic evaluation
Introduction
Proprioception, the sense of how our bodies are positioned, is a critical component of voluntary movement control and is important for generating smooth, coordinated movements and for maintaining upright posture and balance (Gandevia et al. 2002). Muscle spindles (Proske et al. 2000; Proske 2006), cutaneous receptors (Collins et al. 2005), and joint mechanoreceptors (Edin 1990; Edin 2001) provide proprioceptive feedback to the central nervous system that is essential for determining the position of distal body segments (Cordo et al. 2011). Not surprisingly, functionally deafferented individuals suffer profound disturbances in arm and hand function (Rothwell et al. 1982; Sainburg et al. 1993), postural control (Messier et al. 2003), and locomotion (Lajoie et al. 1996).
A number of investigations have provided evidence that proprioception is affected by healthy aging and have focused on the ability of older individuals to detect passive motion or reproduce experimentally pre-determined joint positions in the lower limb (Skinner et al. 1984; Pai et al. 1997; Petrella et al. 2014). It has also been well documented that these changes in lower extremity proprioception contribute to the decreases in postural stability often associated with healthy aging (Berg 1989; Goble et al. 2009a). Collectively, these data have been taken as evidence of compromised proprioceptive acuity that is thought to contribute to age-related postural instability (Woollacott et al. 1986), which lends to an increased risk of falls in older adults (Sorock and Labiner 1992; Lord et al. 1999).
Evidence also exists that upper limb static position sense is impaired in older adults, as demonstrated by an object-based spherical hand grasp-matching task (Kalisch et al. 2012) and by limb position reproduction tasks about the elbow (Adamo et al. 2007; Herter et al. 2014) and wrist (Adamo et al. 2009). Additionally, passive movement detection thresholds about the wrist joint are up to twice as high in older compared to young healthy participants (Wright et al. 2012). However, little is known regarding the effect of age on proprioception the finger joints, despite the importance of proprioceptive feedback for coordinated hand and arm control that is of critical use in activities of daily living and in maintaining functional independence (Dukelow et al. 2010; Dukelow et al. 2012). This age-related decline in joint position sense acuity needs further characterization, including direct measurement of finger joint proprioception.
To our knowledge, there are only a few tests designed to assess position sense in finger joints (Ferrell et al. 1992; Wycherley et al. 2005; Kalisch et al. 2012). Clinical assessments of proprioception are commonly based on discriminating the upward or downward position of a passively moved finger (Lincoln et al. 1991; Winward et al. 1999). While traditional evaluations of sensory function often include proprioceptive tasks (Winward et al. 1999) and have proven useful in evaluating the condition of patients with stroke (Carey and Matyas 2011) and other impairments, these assessments are frequently insensitive, unreliable, subjective, and found to lack standardization (Lincoln et al. 1991; Connell and Tyson 2012). In contrast, robotic assessments are quantitative, sensitive, and can detect motor and sensory deficits in patients who receive normal scores on traditional clinical assessment measures (Reinkensmeyer and Boninger 2012; Debert et al. 2012; Simo et al. 2014). For example, KINARM is a device that measures and perturbs shoulder and elbow joint positions and has provided reliable quantitative assessments of deficits in limb position sense for patients with stroke and traumatic brain injury (Scott 1999; Dukelow et al. 2010; Dukelow et al. 2012; Herter et al. 2014). The largest study to date that assessed systematic aging-related declines in position sense with robotics used the robot to move one arm passively to a location in space, then asked the participant to match the location of the arm (Herter et al. 2014). Several age-related declines in shoulder and elbow proprioception were identified, including variability and absolute error. Extending the use of robotics to assessing proprioception in healthy individuals can improve understanding of the effects of healthy aging on human proprioception and dexterity.
In this study we examined finger proprioception in healthy participants through the use of a novel exoskeleton robot called FINGER. FINGER is capable of individually assisting both the index and middle fingers through a natural grasping motion (Taheri et al. 2014). Each finger is individually guided by an 8-bar mechanism that controls the orientation and position of the proximal phalanx and the position of the middle phalanx, thus providing a naturalistic curling motion around the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints (Fig. 1). We designed a novel finger proprioception task, as well as a second task that mimics current neurological practice, and used them to assess young, middle-aged, and older adults. We hypothesized that older participants would generate larger errors in the passive finger position sense test and have delayed proprioceptive reaction times compared to younger participants. Additionally, we hypothesized that adding visual feedback of the hand being tested during these tasks would help participants to compensate for any proprioception errors. As a secondary aim, we also compared the two proprioception tasks across each participant’s dominant and non-dominant finger joints to determine if a relative hand advantage for proprioceptive processing was present.
Fig. 1.
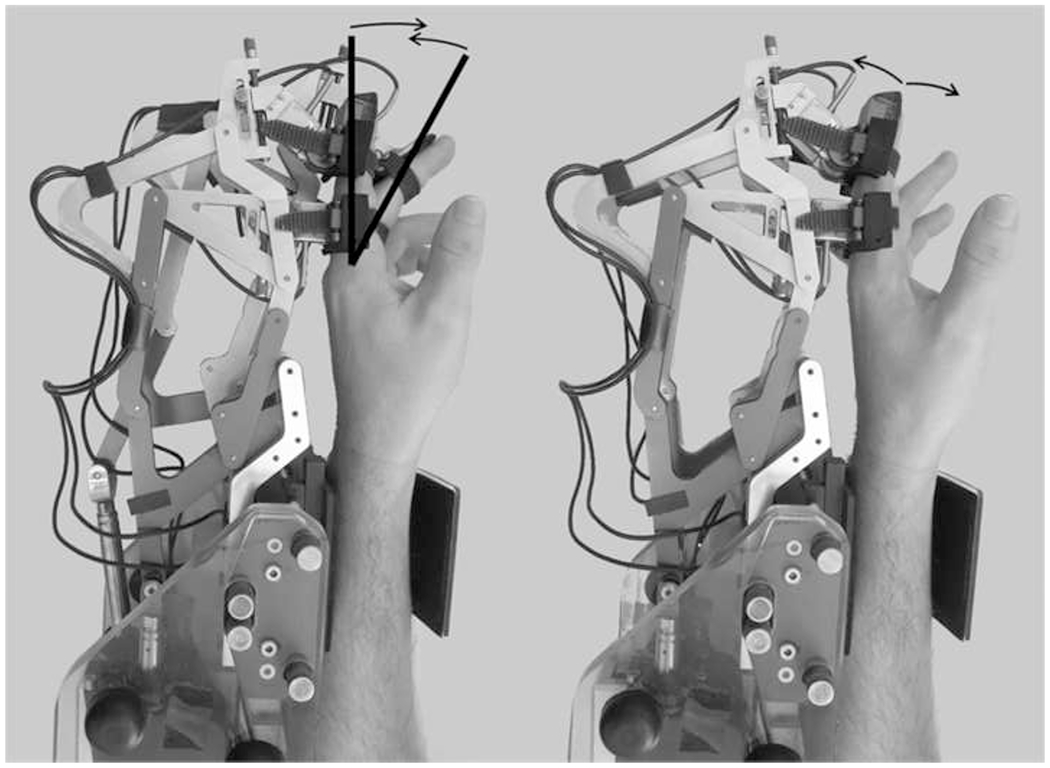
FINGER robot with two 8-bar finger curling mechanisms and two actuators that allow for naturalistic grasping motions by controlling the angle and position of the proximal phalanx and the position of the middle phalanx. The index and middle fingers attach to the robot and are guided through crisscross finger movements during the proprioception tasks; movement stops and reverses directions when fingers are separated at 30% of range of motion (defined by bold lines)
Methods
Participants
Healthy participants, aged 22-87 years were recruited. Exclusion criteria included any history of hand injury (such as wrist, hand, or finger fractures or the presence of surgical hardware) or pathology (such as diabetes, stroke, or arthritis). Handedness was determined using the Edinburgh Handedness Inventory (Oldfield 1971). The local ethics committee approved this study and written informed consent was obtained from each participant prior to participating, following procedures established by the University of California Irvine Institutional Review Board.
Experimental design with FINGER
The experiment took place across a single session and involved use of the exoskeleton rehabilitation robot, FINGER (Fig. 1, Taheri et al. 2014). FINGER is capable of individually moving both the index and middle fingers through a natural grasping motion. Each finger can be individually guided by an 8-bar mechanism that controls the orientation and position of the proximal phalanx and the position of the middle phalanx. Each 8-bar mechanism has a single degree-of-freedom and is actuated by a high bandwidth and low-friction linear electric actuator. In designing FINGER, a regression analysis was used to determine the angular relationship between the middle and proximal phalanges for 7 healthy motion capture participants, using a second-order polynomial equation (Taheri et al. 2014, Fig. 2). As the relationship between the proximal interphalangeal (PIP) and metacarpophalangeal (MCP) joints has been quantified and movement of these joints is highly correlated, for simplicity we reference error with regard to the MCP joint. However, position sense assessments reported here are thought to derive from both the PIP and MCP.
Fig. 2.

Example index and middle finger movement paths during proprioception tasks generated by the FINGER robot. FINGER moved participants’ index and middle fingers in opposing directions to create crisscross motions. One crossover event occurred during each crisscross movement wherein the index and middle fingers were directly overlapping. The position in space where the crossover event occurred varied for each crisscross movement; to create this effect, the fingers alternated between symmetric and asymmetric movements. Each crisscross movement occurred over 5 seconds, followed by a pseudo-random 0-3 second pause. During the pause, index and middle fingers were separated at 30% of the ROM by FINGER. Varying finger velocity profiles and pseudo-random pause times created non-periodic crisscross movements that participants could not predict using timing strategies
In this experiment, we used the FINGER robot to actively move the participant’s passive index and middle fingers through a crisscross motion (Fig. 2). All movements followed minimum jerk trajectories calculated to take the desired finger from its starting point to its target point over the course of 5 seconds, with fingers moving at a MCP angular velocity of 0.24 radians per second. For all crisscrossing movements, FINGER moved participants’ index and middle fingers in opposing directions and always came to pause with the fingers separated by 30% of the natural range of motion (ROM) for these two fingers. Thus, at only one point in time during each crisscross movement were the participants’ index and middle fingers directly aligned. The rate of change of the separation distance between the fingers was identical for all crisscrossing movements (Fig. 2). However, the position in space where the fingers were directly aligned varied for each crisscross movement. In order to achieve this, FINGER alternated between symmetric and asymmetric finger movement paths (Fig. 2). During symmetric movements, the index and middle fingers made mirrored movements through 30% ROM; during asymmetric movement paths, one finger moved through a larger range than the other to create different finger velocity profiles. The magnitude of asymmetry varied between 10-70% ROM before fingers came to rest separated at 30% ROM, with the various asymmetric movement paths presented in a pseudo-randomized order. A pause time with duration pseudo-randomized to be between 0-3 seconds followed each crisscrossing movement in order to generate crisscross finger motions that were non-periodic and therefore unpredictable to participants through use of timing strategies.
Two proprioception tasks were performed using the same robot-controlled finger motions generated by FINGER: a finger overlap task and a movement onset task. For each task, a total of 12 crossover movements occurred over approximately 2 minutes. Participants first performed the finger overlap task, then each was assessed to confirm that they understood the overlap task, and then they performed the movement detection task on either their dominant or non-dominant hand. All experimental procedures were then repeated with their other hand. The order of hand testing was counter-balanced across participants. The timing sequence of each finger movement and each rest period was identical within and across participants. All participants wore noise-canceling headphones throughout testing to neutralize any sound emitted from FINGER.
Passive finger position sense: Overlap task
Passive finger position sense was measured with a finger overlap task. During the overlap task, all participants were properly fitted into FINGER and asked to relax their hand. Test trials were repeated if any evidence of active movement was observed. Participants were instructed to press the spacebar on a keyboard with their free hand when they perceived their index and middle fingers from their test hand were directly overlapped on top of one another. Participants completed the overlap task under two different feedback conditions: first with visual access to their hand and then with vision occluded. Error was defined as the amount of finger separation, measured in degrees about the MCP, that existed when the participant indicated they felt their index and middle fingers were directly overlapped. Error included angles with both negative and positive degrees, as responses occurred both before and after fingers were directly overlapped (0°). However, unless otherwise stated all analyses report group errors as averages of absolute errors.
Following completion of the overlap task, participants completed an assessment to evaluate their comprehension of the overlap task. Participants who were able to describe the desired finger position when they tapped the spacebar using specific keywords (such as “overlapped”, “aligned”, and “in parallel”) were deemed cognitively aware and compliant of the overlap task instructions. This assessment also had participants indicate if they could feel their fingers 1) start moving, 2) stop moving, or 3) cross over during the overlap task. These three questions referenced the index and middle fingers separately and were answered yes/no. The entire assessment was completed for both the dominant and non-dominant hands, directly after concluding the overlap task with vision occluded for each hand.
Passive movement detection: Movement onset task
Passive movement detection was measured with a movement onset task. During passive movement detection testing, which was a form of reaction time test, participants were instructed to press the spacebar on a keyboard with their free hand when they first perceived any passive movement in their fingers. Participants completed the movement onset task under two different feedback conditions: first with visual access to their hand and then with vision occluded. Performance was quantified as the amount of time delay, in milliseconds (ms), between the onset of robot initiated finger movement and the moment the participant pressed the spacebar to indicate perceived motion.
Statistical analysis
Statistical analyses were conducted using JMP 11 software, were 2-tailed, and used α=0.05. Normally distributed data and data that could be transformed to a normal distribution were analyzed using parametric statistics, otherwise non-parametric statistics were used.
Participant performance on the overlap task and on the movement onset task was analyzed separately. Initial analyses examined the effect of age on finger proprioceptive ability. An omnibus mixed effect model, with participants as a random effect and age group category as a fixed effect, was performed to assess the main effect of age on each of the proprioception tasks. The main effects of visual feedback condition and hand dominance were also evaluated to elucidate any differences between the three age groups according to hand dominance and according to the presence or absence of visual feedback. Post hoc analyses were performed using Fisher’s Least Significant Difference test. Within group analyses were performed using a mixed effect model, with participants as a random effect and visual feedback condition and hand dominance as fixed effects. Post hoc analyses were again performed using Fisher’s Least Significant Difference test.
Results
We recruited 37 healthy adult volunteers aged 20 years and above. The measurements were acquired from three groups of adults: 12 young participants (average age: 24.5 ± 1.6 years (mean ± SD), range: 22-28, 5 males), 12 middle-aged participants (average age: 44.5 ± 9.4 years, range: 30-60, 3 males), and 13 older participants (average age: 73.3 ± 6.8 years, range: 67-87, 8 males). Of the 37 participants, 35 were right-handed. Performance on the overlap task and the movement onset task was collected under four different conditions: dominant hand with vision, dominant hand without vision, non-dominant hand with vision, non-dominant hand without vision. Not all participants completed the four different testing conditions as the full protocol was incorporated in stages; the number of participants for each test are given within Figs. 3 and 4.
Fig. 3.
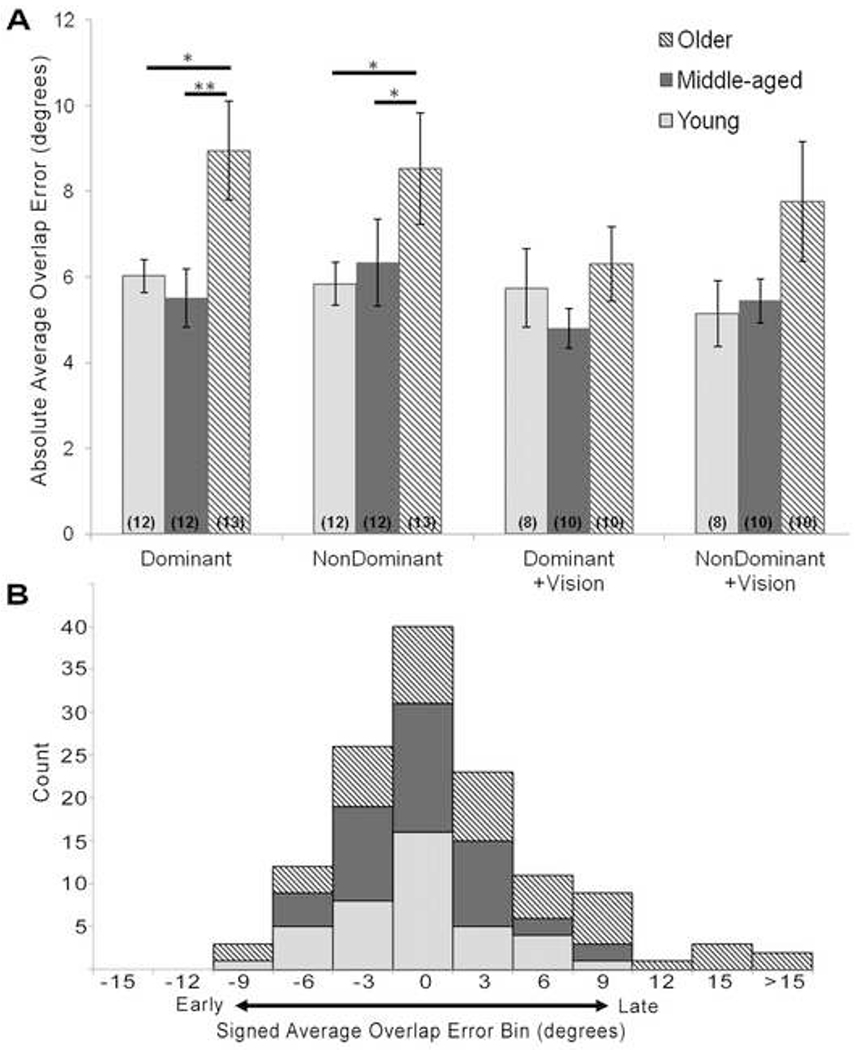
(A) Average absolute error, in degrees about the MCP, made on the overlap task for the dominant and non-dominant hands, with and without vision. The older age group performed significantly worse than the young and middle-aged groups for both the dominant and non-dominant hands without vision. However, no difference existed between the age groups when participants were permitted visual feedback of their hand. Numbers in parentheses indicate the number of participants tested for each condition. Error bars are standard error. * = p<0.05. ** = p<0.01. (B) Histogram distribution of signed average errors made on the overlap task
Fig. 4.
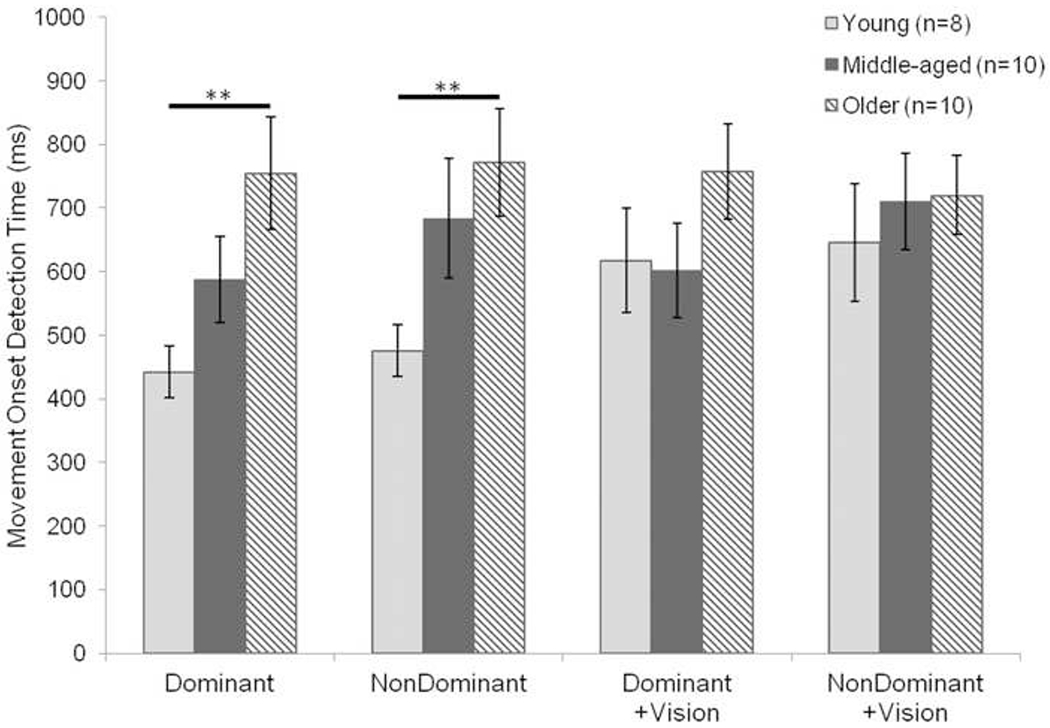
Mean time to detect finger movement onset during the movement onset task for the dominant and non-dominant hands, with and without vision. The older age group performed significantly worse than the young age group for both the dominant and non-dominant hands without vision. However, no difference existed among the groups when participants were permitted visual feedback while completing the task. Error bars are standard error. ** = p<0.01
Overlap Task Results
Results from the assessment immediately following the overlap task revealed that all participants understood the instructions for the overlap task and attempted to press the spacebar when their index and middle fingers were directly overlapped. Likewise, 95% of participants were able to feel their index and middle fingers start moving, stop moving, and crossover during the task. Only one participant in the middle-aged group was unable to feel their index finger start moving; one participant in the older age group reported they could not feel their fingers cross over. Although these participants reported lack of somatosensation, each did demonstrate comprehension of the overlap task, and so data from all participants are included in the following statistical analyses.
For participant average absolute errors, a difference in performance on the overlap task was found to exist as a main effect of age (F(2,31)=5.74, p=0.007, Fig. 3A); the main effect for visual feedback condition was trending (F(1,99)=2.83, p=0.09); and the main effect for hand dominance was not significant (F(1,88)=0.10, p=0.75). Given our hypothesis that vision would help participants compensate for any deficits in proprioception, we proceeded with one-way ANOVAs to evaluate any main effect of age for the four test conditions: dominant hand, dominant hand+vision, non-dominant hand, non-dominant hand+vision (Fig. 3). Difference in overlap error according to age was detected for both hands when participants completed the task with vision occluded (dominant hand: F(2,34)=4.84, p=0.01; non-dominant hand: F(2,33)=3.53, p=0.04). Post-hoc tests for the dominant hand revealed the older age group made significantly larger errors than the young (t(34)=2.05, p=0.04) and middle-aged groups (t(34)=3.04, p=0.004). For example, the older participants made on average 48% larger finger position sense errors compared to young participants. Similarly, post-hoc tests for the non-dominant hand indicated the older group made larger errors than the young (t(33)=2.04, p=0.04) and middle-aged groups (t(33)=2.46, p=0.02). Conversely, a difference in overlap error according to age was not detected when participants completed the task with visual feedback (dominant hand+vision: F(2,25)=0.78, p=0.47; non-dominant hand+vision: F(2,25)=1.73, p=0.19).
The overlap task mixed-effects model did not indicate a significant interaction for categorical age groups and visual feedback condition (F(2,99)=0.52, p=0.6).
Overlap error can also be evaluated by computing signed averages, wherein negative and positive errors reveal if participants responded before or after their fingers were directly overlapped, respectively (Fig. 3B). Signed average overlap errors were calculated for each age group (young: −3.2 ± 2.9° (mean ± SD); middle-aged: −0.01 ± 3.9°; older: 2.6 ± 6.3°). Across all age groups and task conditions, 49% of participants made negative signed average overlap errors, indicating that participants anticipated the moment their fingers would cross.
Movement Onset Task Results
For participant average movement onset detection times, a difference in performance on the movement onset task was found to exist as a main effect of age (F(2,25)=4.16, p=0.03, Fig. 4) and a main effect of visual feedback condition (F(1,82)=6.01, p=0.01); the main effect for hand dominance was not significant (F(1,82)=2.83, p=0.10). Using the same age group divisions from the overlap task analyses, subsequent one-way ANOVAs were performed to detect any differences among age groups for the four testing conditions (Fig. 4). A main effect of age was found for both hands when participants performed the movement onset task with vision occluded (dominant hand: F(2,25)=4.74, p=0.02; non-dominant hand: F(2,25)=4.29, p=0.02). Post-hoc tests for the dominant hand revealed the older age group had significantly longer reaction times than the young age group (t(25)=3.08, p=0.005). For example, proprioceptive reaction time was 78% longer in older adults compared to young adults. Likewise, post-hoc tests for the non-dominant hand indicated the older group had significantly longer reaction times than the young age group (t(25)=2.90, p=0.007). Conversely, a difference in movement onset detection time according to age was not detected when participants had visual input during the task (dominant hand+vision: F(2,25)=1.84, p=0.18; non-dominant hand+vision: F(2,25)=0.59, p=0.56).
Moreover, the movement onset task mixed-effects model indicated a significant interaction for categorical age groups and visual feedback condition (F(2,81)=4.31, p=0.02). Within-group analyses indicated a significant main effect for vision for the young age group (F(1,22)=13.03, p=0.002), but not for the middle-aged or older age groups. A paired t-test revealed detection times for the young age group with visual feedback were longer than those without visual feedback (t(22)=3.6, p=0.002).
Discussion
The primary aim of the present study was to evaluate age-related changes in finger joint position and movement sense by means of a novel robotic proprioception assessment. We hypothesized that passive finger movement tasks with FINGER would detect diminished proprioceptive ability in older adults compared to younger adults. Our results from 37 individuals aged 22-80 years revealed significant age-related declines in PIP and MCP joint proprioception. In the case of the overlap task, wherein index and middle fingers were passively moved in opposing directions and participants indicated when their fingers were directly overlapped, older participants demonstrated diminished finger position sense and made 48% larger errors than young healthy participants (Fig. 3). Moreover, the movement onset task also showed that older participants were 78% slower in detecting the onset of passive finger movements than young participants (Fig. 4). These proprioceptive deficits were masked when older participants were permitted vision of their hand. Additional assessments indicated hand dominance did not affect finger proprioception in either task. These results describe a decline of finger proprioception and finger proprioception reaction time with normal aging, a finding of concern to our aging population given that finger joint proprioceptive ability strongly relates to precise control of hand movements performed during activities of daily living.
The results presented here support the view that age-related proprioception decline is a generalized phenomena that older adults experience throughout multiple effector systems of the body. These data are the first to provide strong evidence that finger joint position sense and movement onset detection are significantly impaired in healthy older adults, a finding that supports observations of generalized declines in proprioceptive ability with aging. Previous research indicates lower limb proprioception, specifically position sense of toes, ankles, and knees (Sorock and Labiner 1992), decreases with normal aging. Declines in active and passive joint movement of the elbow and wrist have been detected in the elderly at comparable rates reported for the lower limb (Adamo et al. 2007; Adamo et al. 2009; Wright et al. 2012; Herter et al. 2014). For example, in an arm position-matching task, errors in hand-based position sense parameters increased 36% across adulthood (Herter et al. 2014). The present findings report a similar decline in proprioception of the finger joints. It is important to note, however, that task design likely has a significant influence in evaluating joint position sense, and thus direct comparisons across protocols are difficult. What is clear is that age-related proprioceptive impairment is pervasive across limbs, including the fingers.
Age-related changes affecting finger proprioception
Numerous peripheral and central level neurophysiological factors might account for the observed age-related changes in proprioception (Goble and Brown 2009). A general loss of sensitivity affecting stretch-sensitive mechanoreceptors (Shaffer and Harrison 2007), age-related alterations in cutaneous receptors, decreased density of Meissner and Pacinian corpuscles per unit of skin area (Vega et al. 2012), and a decline in joint mechanoreceptors (Morisawa 1998) likely contribute to the impaired dynamic position sense detected in the older participants.
In addition to these probable contributions from the peripheral nervous system, it is likely that some component of age-related decline in proprioceptive function is related to changes in the central nervous system (Goble et al. 2009a). This could theoretically be due to some combination of elementary sensory signaling or cognitive decline. Regarding the latter, joint position errors made by the elderly can be modulated by the amount of proprioceptive processing required (Goble et al. 2005; Adamo et al. 2007; Goble and Brown 2007), suggesting task type and experimental design can indeed impact the severity of proprioceptive impairments detected. In the current study, the overlap and movement onset tasks do not rely on proprioceptive memory (as in ipsilateral remembered matching) or interhemispheric transfer (as in mirroring tasks). Thus, we suggest that the proprioception assessments presented here are independent of cognitive attentional resources available to older individuals and likely reflect the neurophysiological underpinnings of finger proprioception for different age groups.
Advantages of proprioception testing with FINGER
Joint position sense is usually assessed in patients with stroke (Carey et al. 1996; Dukelow et al. 2010) or as a test for sensory deficits due to aging or disease (Peixoto et al. 2011; Ochoa and Gomiak 2014). Robotic devices can quantitatively assess sensorimotor dysfunction with heightened sensitivity in these populations (Reinkensmeyer and Boninger 2012). For example, robotic assessments have revealed patients frequently have deficits in motor and sensory functions despite receiving normal scores on traditional clinical measures (Debert et al. 2012). Additionally, seemingly ‘standardized’ somatosensation assessments have in fact been found to be subjective and have poor inter-rate reliability (Lincoln et al. 1991; Connell and Tyson 2012), deeming traditional proprioceptive measurements both unreliable and insensitive. As this study sought to detect minor differences in proprioceptive ability that may exist between healthy individuals as a function of age, we designed proprioception tasks using FINGER. Using this finger-curling robot allowed us to design two passive proprioception tasks.
Various tasks designed to measure the ability to sense joint movement have been developed. An early study in motion sense found that compared to young adults, older participants were less capable of sensing motion of the metacarpophalangeal and metatarsophalangeal joints in the absence of vision (Kokmen et al. 1978). Movement detection thresholds have also been studied in relation to normal aging for lower limb joints, such as the ankle (Xu et al. 2004; Westlake and Culham 2007) and knee (Barrack et al. 1983; Skinner et al. 1984). More recently, robotics have been employed to enhance acuity in studies addressing kinesthesia in the elbow and wrist joints (Scott 1999; Dukelow et al. 2010; Wright et al. 2012; Semrau et al. 2013; Herter et al. 2014; Simo et al. 2014). The present study is the first to our knowledge to extend conventional measurements of passive movement detection to the finger joints using robotics.
The overlap task was designed to address the intrinsic dual functionality of proprioception, which refers to both position sense and movement detection. The ability to monitor position during motion has been termed “dynamic position” sense. Traditional position sense studies have had participants mirror a static position with their free limb (Adamo et al. 2007). While employing robotics in this setting has introduced objective scoring (Dukelow et al. 2010; Wright et al. 2012; Semrau et al. 2013; Herter et al. 2014), testing paradigms that incorporate sense of position and sense of movement are challenging to design. The overlap task presented here introduces a quantitative assessment for dynamic finger position sense (Figs. 1, 2). Because sense of position and sense of movement are both important in proprioception and strongly contribute to fine motor control during voluntary movement execution (Gandevia et al. 1992; Proske and Gandevia 2009), we suggest the overlap task, which tests both senses, provides enhanced insight to the decay of proprioceptive ability as a result of normal aging. Indeed, this task detected decreased proprioception in the older age group compared to both the young and middle-aged groups and therefore proved to be a more sensitive probe than the movement onset task, which characterizes sense of movement alone and did not consistently detect a difference between the older and middle-aged groups.
Role of feedback condition on proprioception
In a number of highly skilled motor activities, responses to kinesthetic stimulus rather than a visual one would seem beneficial since the kinesthetic route is faster to process (Chernikoff and Taylor 1952; Botwinick and Brinley 1962; Keele and Posner 1968; Klein and Posner 1974). Despite this, kinesthetic cues are rarely the only means through which one perceives movement. On both the overlap task and movement onset task, our older age group demonstrated impaired proprioception compared to the young group without vision, but performed comparably when vision was included. Previous studies have confirmed the importance of vision in the control of posture under challenging conditions (Manchester et al. 1989; Lord et al. 1991; Lord and Menz 2000) and suggest that the visual system is relied upon to compensate for diminished proprioception in the lower limbs and upper limbs (Sainburg et al. 1993). It is likely that visual input plays a similar compensatory role in the finger joints, which allows older participants to complete proprioception tasks indistinguishably from younger participants.
It is well-known that reaction times generally increase with aging and this effect is seen in response to stimuli across all sensory modalities (Fozard et al. 1994; Ratcliff et al. 2001; Dykiert et al. 2012). Thus, it may seem possible that such aging-related increases in reaction time would map onto increases observed in overlap error, making it possible that the observed deficit was due to prolonged reaction time rather than impaired proprioception per se. It is important to note that by design, the overlap task is inherently independent of reaction time because it is anticipatory by nature. Longer preparatory intervals are known to reduce reaction time (Dykiert et al. 2012); given that participants could anticipate the timing that their slowly moving fingers would cross, this preparatory interval was extended during the overlap task. Thus, 49% of participants made signed average overlap errors that were negative (Fig. 3B). The prevalence of negative errors on the overlap task reveals that participants anticipated the crossover event and sometimes reacted too early. The overlap task is therefore anticipatory in nature and delays in reaction time due to aging are unlikely to confound the results presented here.
One should also consider the role of reaction time in the movement onset task. It is logical that reaction time does play a role in the movement onset task, as it is in essence a test of proprioceptive reaction time. However, the results from the movement onset task are likely indicative of more than a simple delay of reaction time with age. For example, if these results revealed a delay in reaction time alone, there should be a difference between older and young age groups regardless of visual feedback condition. This is not the case, as all age groups performed similarly on the movement onset task when they were permitted visual access to their hand (Fig. 4). One may be tempted to attribute the similar performances amongst the groups when visual feedback was permitted to the young participants’ seemingly slowed reaction times. Indeed, visual feedback significantly increased movement onset detection time for the young age group, but this is not an atypical observation. Visual responses are known to have slower processing times compared to those of kinesthesia (Chernikoff and Taylor 1952; Botwinick and Brinley 1962; Keele and Posner 1968; Klein and Posner 1974). Moreover, visual input tends to dominate input from somatosensory modalities (Posner et al. 1976). For the young participants, a tendency to depend on visual feedback of the hand rather than proprioception when performing this task resulted in slower response times than when they completed the task without vision, thereby requiring them to utilize their highly attuned proprioceptive abilities. It is likely that the older age group also relied on visual feedback to complete this task when given the opportunity. Yet, the older age group did not demonstrate a significant main effect for visual feedback condition. This suggests that proprioceptive abilities for elderly individuals were diminished and therefore rendered the slower processing time of visual input undetectable. By any means, given the potential confounds of reaction time on the movement onset task, the overlap task is perhaps a more ideal way to parse out the effects of delayed reaction time from the effects of age-related decline in finger proprioception.
Lack of asymmetry due to hand dominance
Studies regarding static position sense about the elbow have indicated asymmetries in younger individuals with the non-dominant arm exhibiting an enhanced ability to utilize limb position feedback (Goble et al. 2006). Interpreted as a specialization of the non-dominant hemisphere system for position-related proprioception processing (Goble et al. 2009a; Goble et al. 2009b), this finding has been seen in older adults for conditions requiring interhemispheric transfer of proprioceptive information (i.e. static limb matching tasks) (Adamo et al. 2007; Adamo et al. 2009; Herter et al. 2014). Although a lifetime of dominant hand use may suggest enhanced dynamic movement onset detection for dominant limbs, dynamic movement reproduction does not differ between the two arms (Goble and Brown 2009; Cusmano et al. 2014) nor does passive movement onset detection in wrist joints differ according to hand dominance for elderly participants (Wright et al. 2012). Likewise, in the present study the main effect of hand dominance failed to reach statistical significance for the overlap and movement onset tasks. This suggests that decay in proprioceptive ability with natural aging is generalized to both upper limbs. Moreover, it highlights the influence of task design in detecting kinesthetic asymmetries.
Conclusions
The results of this study extend our current understanding of the extent of age-related proprioception declines and confirm that such declines are general phenomena affecting the most distal part of the upper extremity. Additionally, they introduce two novel robotic techniques for quantitatively assessing dynamic position sense in the finger joints, one being free of possible reaction time confounds. These results may also have clinical value. The functional consequences of impaired finger joint proprioceptive ability strongly relate to precise control of finger movements performed during activities of daily living (Ghez et al. 1995; Gordon et al. 1995). This is particularly relevant for our aging society where physiological declines in finger proprioception are naturally occurring, and may also be useful for understanding diseases in which sensory function is affected such as stroke.
Acknowledgments
Dr. Cramer served as a consultant for GlaxoSmithKline, MicroTransponder, Dart Neuroscience, RAND Corporation, and Roche, and is a co-founder of personalRN. Dr. Reinkensmeyer is a co-founder of Flint Rehabilitation Devices, and has received payment for consulting and holds equity in Hocoma; both companies are manufacturers of rehabilitation technology. The terms of these interests have been reviewed by the U.C. Irvine Conflict of Interest committee. The work described was supported by R01HD062744 from the National Center for Medical Rehabilitation Research, and the Institute for Clinical and Translational Science University of California, Irvine, with funds provided by the National Center of Research Resources, 5 M011 RR-00827-29, US Public Health Service.
Contributor Information
Morgan L Ingemanson, Department of Anatomy & Neurobiology, University of California, Irvine, CA 92697, USA.
Justin B Rowe, Department of Biomedical Engineering, University of California, Irvine, CA 92697, USA.
Vicky Chan, Department of Mechanical and Aerospace Engineering, University of California, Irvine, CA 92697, USA.
Eric T Wolbrecht, Mechanical Engineering Department, University of Idaho, Moscow, ID 83844, USA.
Steven C Cramer, Department of Anatomy & Neurobiology, University of California, Irvine, CA 92697, USA; Department of Neurology, University of California, Irvine, CA 92697, USA.
David J Reinkensmeyer, Department of Mechanical and Aerospace Engineering, University of California, Irvine, CA 92697, USA; Department of Anatomy & Neurobiology, University of California, Irvine, CA 92697, USA; Department of Biomedical Engineering, University of California, Irvine, CA 92697, USA.
References
- Adamo DE, Alexander NB, Brown SH (2009) The influence of age and physical activity on upper limb proprioceptive ability. J Aging Phys Act 17:272–93. [DOI] [PubMed] [Google Scholar]
- Adamo DE, Martin BJ, Brown SH (2007) Age-related differences in upper limb proprioceptive acuity. Percept Mot Skills 104:1297–309. doi: 10.2466/pms.104.4.1297-1309 [DOI] [PubMed] [Google Scholar]
- Barrack RL, Skinner HB, Cook SD, Haddad RJ (1983) Effect of Articular Disease and Total Knee Arthroplasty on Knee Joint-Position Sense. J Neurophysiol 50:684–687. [DOI] [PubMed] [Google Scholar]
- Berg K (1989) Balance and its measure in the elderly: A review. Physiother Canada 41:240–46. [Google Scholar]
- Botwinick J, Brinley JF (1962) An analysis of set in relation to reaction time. J Exp Psychol 63:568–574. doi: 10.1037/h0048305 [DOI] [PubMed] [Google Scholar]
- Carey LM, Matyas TA (2011) Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting. J Rehabil Med 43:257–263. doi: 10.2340/16501977-0662 [DOI] [PubMed] [Google Scholar]
- Carey LM, Oke LE, Matyas TA (1996) Impaired limb position sense after stroke: A quantitative test for clinical use. Arch Phys Med Rehabil 77:1271–8. [DOI] [PubMed] [Google Scholar]
- Chernikoff R, Taylor FV (1952) Reaction Time To Kinesthetic Stimulation Resulting from Sudden Arm Displacement. J Experimenal Psychol 43:1–8. [DOI] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC (2005) Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol 94:1699–706. doi: 10.1152/jn.00191.2005 [DOI] [PubMed] [Google Scholar]
- Connell LA, Tyson SF (2012) Measures of sensation in neurological conditions: a systematic review. Clin Rehabil 26:68–80. doi: 10.1177/0269215511412982 [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Horn J-L, Künster D, et al. (2011) Contributions of skin and muscle afferent input to movement sense in the human hand. J Neurophysiol 105:1879–88. doi: 10.1152/jn.00201.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusmano I, Sterpi I, Mazzone A, et al. (2014) Evaluation of upper limb sense of position in healthy individuals and patients after stroke. J Healthc Eng 5:145–62. [DOI] [PubMed] [Google Scholar]
- Debert CT, Herter TM, Scott SH, Dukelow SP (2012) Robotic assessment of sensorimotor deficits after traumatic brain injury. J Neurol Phys Ther 36:58–67. doi: 10.1097/NPT.0b013e318254bd4f [DOI] [PubMed] [Google Scholar]
- Dukelow SP, Herter TM, Bagg SD, Scott SH (2012) The independence of deficits in position sense and visually guided reaching following stroke. J Neuroeng Rehabil 9:72. doi: 10.1186/1743-0003-9-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukelow SP, Herter TM, Moore KD, et al. (2010) Quantitative assessment of limb position sense following stroke. Neurorehabil Neural Repair 24:178–87. doi: 10.1177/1545968309345267 [DOI] [PubMed] [Google Scholar]
- Dykiert D, Der G, Starr JM, Deary IJ (2012) Age Differences in Intra-Individual Variability in Simple and Choice Reaction Time: Systematic Review and Meta-Analysis. PLoS One. doi: 10.1371/journal.pone.0045759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB (1990) Finger joint movement sensitivity of non-cutaneous mechanoreceptor afferents in the human radial nerve. Exp Brain Res 82:417–422. [DOI] [PubMed] [Google Scholar]
- Edin BB (2001) Cutaneous afferents provide information about knee joint movements in humans. J Physiol 531:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell WR, Crighton A, Sturrock RD (1992) Position sense at the proximal interphalangeal joint is distorted in patients with rheumatoid arthritis of finger joints. Exp Physiol 77:675–80. [DOI] [PubMed] [Google Scholar]
- Fozard JL, Vercruyssen M, Reynolds SL, et al. (1994) Age differences and changes in reaction time: The Baltimore longitudinal study of aging. J Gerontol 49:179–189. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI, Burke D (1992) Kinaesthetic signals and muscle contraction. Trends Neurosci 15:62–65. doi: 10.1016/0166-2236(92)90028-7 [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Refshauge KM, Collins DF (2002) Proprioception: peripheral inputs and perceptual interactions. Adv Exp Med Biol 508:61–8. [DOI] [PubMed] [Google Scholar]
- Ghez C, Gordon J, Ghilardi MF (1995) Impairments of Reaching Movements in Patients Without Proprioception. II. Effects of Visual Information on Accuracy. J Neurophysiol 73:361–372. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH (2009) Dynamic proprioceptive target matching behavior in the upper limb: effects of speed, task difficulty and arm/hemisphere asymmetries. Behav Brain Res 200:7–14. doi: 10.1016/j.bbr.2008.11.034 [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH (2007) Task-dependent asymmetries in the utilization of proprioceptive feedback for goal-directed movement. Exp Brain Res 180:693–704. doi: 10.1007/s00221-007-0890-7 [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Wenderoth N, et al. (2009a) Proprioceptive sensibility in the elderly: degeneration, functional consequences and plastic-adaptive processes. Neurosci Biobehav Rev 33:271–8. doi: 10.1016/j.neubiorev.2008.08.012 [DOI] [PubMed] [Google Scholar]
- Goble DJ, Lewis CA, Brown SH (2006) Upper limb asymmetries in the utilization of proprioceptive feedback. Exp Brain Res 168:307–11. doi: 10.1007/s00221-005-0280-y [DOI] [PubMed] [Google Scholar]
- Goble DJ, Lewis CA, Hurvitz EA, Brown SH (2005) Development of upper limb proprioceptive accuracy in children and adolescents. Hum Mov Sci 24:155–70. doi: 10.1016/j.humov.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Goble DJ, Noble BC, Brown SH (2009b) Proprioceptive target matching asymmetries in left-handed individuals. Exp brain Res 197:403–8. doi: 10.1007/s00221-009-1922-2 [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Ghez C (1995) Impairments of Reaching Movements in Patients Without Proprioception. I. Spatial Errors. J Neurophysiol 73:347–360. [DOI] [PubMed] [Google Scholar]
- Herter TM, Scott SH, Dukelow SP (2014) Systematic changes in position sense accompany normal aging across adulthood. J Neuroeng Rehabil 11:43. doi: 10.1186/1743-0003-11-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch T, Kattenstroth J-C, Kowalewski R, et al. (2012) Age-related changes in the joint position sense of the human hand. Clin Interv Aging 7:499–507. doi: 10.2147/CIA.S37573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele SW, Posner MI (1968) Processing of visual feedback in rapid movements. J. Exp. Psychol 77:155–158. [DOI] [PubMed] [Google Scholar]
- Klein RM, Posner MI (1974) Attention to visual and kinesthetic components of skills. Brain Res 71:401–411. doi: 10.1016/0006-8993(74)90984-6 [DOI] [PubMed] [Google Scholar]
- Kokmen E, Bossemeyer RJ, Williams W (1978) Quantitative evaluation of joint motion sensatio in aging population. J Gerontol 33:62–7. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Teasdale N, Cole JD, et al. (1996) Gait of a deafferented subject without large myelinated sensory fibers below the neck. Neurology 47:109–15. [DOI] [PubMed] [Google Scholar]
- Lincoln NB, Crow J, Jackson J, et al. (1991) The unreliability of sensory assessments. Clin Rehabil 5:273–282. doi: 10.1177/026921559100500403 [DOI] [Google Scholar]
- Lord SR, Clark RD, Webster IW (1991) Postural stability and associated physiological factors in a population of aged persons. J Gerontol 46:M69–76. [DOI] [PubMed] [Google Scholar]
- Lord SR, Menz HB (2000) Visual contributions to postural stability in older adults. Gerontology 46:306–310. doi: 10.1159/000022182 [DOI] [PubMed] [Google Scholar]
- Lord SR, Rogers MW, Howland A, Fitzpatrick R (1999) Lateral Stability, Sensorimotor Function and Falls in Older People. J Am Geriatr Soc 47:1077–81. [DOI] [PubMed] [Google Scholar]
- Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O (1989) Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol 44:M118–27. [DOI] [PubMed] [Google Scholar]
- Messier J, Adamovich S, Berkinblit M, et al. (2003) Influence of movement speed on accuracy and coordination of reaching movements to memorized targets in three-dimensional space in a deafferented subject. Exp Brain Res 150:399–416. doi: 10.1007/s00221-003-1413-9 [DOI] [PubMed] [Google Scholar]
- Morisawa Y (1998) Morphological study of mechanoreceptors on the coracoacromial ligament. J Orthop Sci 3:102–110. [DOI] [PubMed] [Google Scholar]
- Ochoa N, Gorniak SL (2014) Changes in sensory function and force production in adults with type II diabetes. Muscle Nerve 50:984–90. doi: 10.1002/mus.24261 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Pai YC, Rymer WZ, Chang RW, Sharma L (1997) Effect of age and osteoarthritis on knee proprioception. Arthritis Rheum 40:2260–5. doi: [DOI] [PubMed] [Google Scholar]
- Peixoto JG, Dias JMD, Dias RC, et al. (2011) Relationships between measures of muscular performance, proprioceptive acuity, and aging in elderly women with knee osteoarthritis. Arch Gerontol Geriatr 53:e253–7. doi: 10.1016/j.archger.2011.05.008 [DOI] [PubMed] [Google Scholar]
- Petrella RJ, Lattanzio PJ, Nelson MG (2014) Effect of age and activity on knee joint proprioception. Am J Phys Med Rehabil 76:235–41. [DOI] [PubMed] [Google Scholar]
- Posner MI, Nissen MJ, Klein RM (1976) Visual dominance: an information-processing account of its origins and significance. Psychol Rev 83:157–171. doi: 10.1037/0033-295X.83.2.157 [DOI] [PubMed] [Google Scholar]
- Proske U (2006) Kinesthesia: the role of muscle receptors. Muscle Nerve 34:545–58. doi: 10.1002/mus.20627 [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC (2009) The kinaesthetic senses. J Physiol 587:4139–4146. doi: 10.1113/jphysiol.2009.175372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Wise AK, Gregory JE (2000) The role of muscle receptors in the detection of movements. Prog Neurobiol 60:85–96. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Thapar a, McKoon G (2001) The effects of aging on reaction time in a signal detection task. Psychol. Aging 16:323–341. [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Boninger ML (2012) Technologies and combination therapies for enhancing movement training for people with a disability. J Neuroeng Rehabil 9:17. doi: 10.1186/1743-0003-9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Day BL, et al. (1982) Manual Motor Performance in a Deafferented Man. Brain 105:515–542. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Poizner H, Ghez C (1993) Loss of Proprioception Produces Deficits in Interjoint Coordination. J Neurophysiol 70:2136–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH (1999) Apparatus for measuring and perturbing shoulder and elbow joint positions and torques during reaching. J Neurosci Methods 89:119–127. doi: 10.1016/S0165-0270(99)00053-9 [DOI] [PubMed] [Google Scholar]
- Semrau JA, Herter TM, Scott SH, Dukelow SP (2013) Robotic identification of kinesthetic deficits after stroke. Stroke 44:3414–21. doi: 10.1161/STROKEAHA.113.002058 [DOI] [PubMed] [Google Scholar]
- Shaffer SW, Harrison AL (2007) Perspective Aging of the Somatosensory System: A Translational Perspective. Phys Ther 87:193–207. doi: 10.2522/ptj.20060083 [DOI] [PubMed] [Google Scholar]
- Simo L, Botzer L, Ghez C, Scheldt R a (2014) A robotic test of proprioception within the hemiparetic arm post-stroke. J Neuroeng Rehabil 11:77. doi: 10.1186/1743-0003-11-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HB, Barrack RL, Cook SD (1984) Age-related Decline in Proprioception. Clin Orthop Relat Res 184:208–11. [PubMed] [Google Scholar]
- Sorock GS, Labiner DM (1992) Peripheral neuromuscular dysfunction and falls in an elderly cohort. Am J Epidemiol 136:584–91. [DOI] [PubMed] [Google Scholar]
- Taheri H, Rowe JB, Gardner D, et al. (2014) Design and preliminary evaluation of the FINGER rehabilitation robot: controlling challenge and quantifying finger individuation during musical computer game play. J Neuroeng Rehabil 11:10. doi: 10.1186/1743-0003-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega JA, López-muñiz A, Calavia MG, et al. (2012) Clinical Implication of Meissner’s Corpuscles. CNS Neurol Disord - Drug Targets 11:1–13. [DOI] [PubMed] [Google Scholar]
- Westlake KP, Culham EG (2007) Research Report Sensory-Specific Balance Training in Older Adults: Effect on Proprioceptive Reintegration and Cognitive Demands. Phys Ther 87:1274–83. doi: 10.2522/ptj.20060263 [DOI] [PubMed] [Google Scholar]
- Winward CE, Halligan PW, Wade DT (1999) Current practice and clinical relevance of somatosensory assessment after stroke. Clin Rehabil 13:48–55. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Shumway-Cook A, Nashner LM (1986) Aging and Posture Control: Changes in Sensory Organization and Muscular Coordination. Int J Aging Hum Dev 23:97–114. [DOI] [PubMed] [Google Scholar]
- Wright ML, Adamo DE, Brown SH (2012) Age-related declines in the detection of passive wrist movement. Neurosci Lett 500:108–112. doi: 10.1016/j.neulet.2011.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wycherley AS, Helliwell PS, Bird HA (2005) A novel device for the measurement of proprioception in the hand. Rheumatology 44:638–41. doi: 10.1093/rheumatology/keh568 [DOI] [PubMed] [Google Scholar]
- Xu D, Hong Y, Li J, Chan K (2004) Effect of tai chi exercise on proprioception of ankle and knee joints in old people. Br J Sport Med 38:50–54. doi: 10.1136/bjsm.2002.003335 [DOI] [PMC free article] [PubMed] [Google Scholar]


