Abstract
Cocaine and ethanol are two commonly co-abused substances; however, the neuropathology following chronic dual consumption is poorly understood. Neural stem cells (NSCs) are a subpopulation of cells within the adult brain that are integral to brain maintenance and repair making them an appealing target to reverse neurodegeneration associated with abused substances. Yet, knowledge about NSC response to chronic poly-drug administration of ethanol and cocaine is minimal. Here, we developed a novel chronic poly-drug administration paradigm of ethanol and cocaine using a transgenic mouse model to trace endogenous NSC survival and differentiation in three brain regions from both male and female mice. We report significant and distinct patterns of NSC survival and differentiation among brain regions, as well as between sexes. Additionally, poly-drug administration had synergistic effects on NSC survival. Altered cognitive and hedonic behaviors were also observed, however the extent of these behavioral changes was not proportional to the NSC changes. With this mouse model we can effectively examine cognitive and behavioral changes and correlate them with pathological changes in the brain in response to chronic poly-drug administration, which is of great value in understanding the progression of neurodegeneration in polysubstance use disorders and evaluation potential therapeutics on neuroregeneration.
Keywords: Neural stem cells, Cocaine, Ethanol, Poly-drug, Neurogenesis, Tanycyte layer
1. Introduction
Ethanol and cocaine are two commonly co-abused substances. In a 2007 survey, 96% of cocaine users reported ethanol use within the same month (concurrently), and 85% reported using the two drugs simultaneously (Midanik et al., 2007). Co-abuse of ethanol and cocaine has been shown to produce psychotic episodes, increase suicidal and homicidal ideation, and enhance symptoms of psychiatric disorders, such as depression (Salloum et al., 1996; Goldstein et al., 2004; Salloum et al., 2004; Garlow et al., 2007; Velasquez et al., 2007). Despite these strong negative consequences, cocaine and ethanol are preferentially co-abused and people who report co-abuse show poorer prognosis after treatment and higher risk of relapse (Schmitz et al., 1997; Mengis et al., 2002; Lyne et al., 2010). In clinical studies, individuals report ethanol ameliorates negative side effects of “coming down” after using cocaine, and females generally report stronger euphoric feelings compared to males (Magura and Rosenblum, 2000; Graziani et al., 2014). Aside from clinical studies, very little work has been done evaluating the chronic effect of ethanol and cocaine poly-drug administration on the brain. This presents a large gap in knowledge regarding mechanistic and neuropathological changes that occur in the brain following chronic poly-drug administration, which impedes efforts to develop effective treatments or management strategies.
It is well established that ethanol and cocaine individually contribute to neurodegeneration and negatively affect the adult endogenous neural stem cell population (Crews and Nixon, 2003; Kempermann et al., 2015; McGrath et al., 2017). Endogenous neural stem cells (NSCs) in the adult brain have gained attention as a potential therapeutic target due to their critical role in maintaining neurogenesis throughout life and possibly mediating brain repair and functional recovery (Crews, 2008; Mandyam and Koob, 2012). The subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus are the two most commonly studied regions of adult NSCs, and individual administration of either cocaine or ethanol affects NSCs in these regions (Crews and Nixon, 2003; Kempermann et al., 2015; Xu et al., 2016; McGrath et al., 2017). Furthermore, sustained neurogenesis in the hippocampus is believed to contribute to contextual memory formation (Xu et al., 2016). The tanycyte layer (TL) of the third ventricle also contains cells with NSC-like properties (Haan et al., 2013; Robins et al., 2013; McGrath et al., 2017); however, the impact of drugs and ethanol on the cells in this region in relatively unknown. To date, there is a lack of report on the combined use of ethanol and cocaine on any of these NSC populations. Considering the therapeutic implications of adult NSCs in a variety of fields, it is important to evaluate the impact of simultaneous poly-drug use on the NSC populations in order to advance translational efficacy of poly-drug abuse interventions.
To achieve this, we utilized a genetic inducible fate mapping transgenic mouse model to trace the fate of endogenous NSC populations (Lagace et al., 2007; McGrath et al., 2017) in response to chronic ethanol, cocaine, or combination treatment. By developing a novel poly-drug administration paradigm, we investigated the effect of ethanol and cocaine co-use on the fate of NSCs in three regions of interest (SVZ, SGZ, and TL). Responses between male and female mice, in each respective region, were compared to determine if sex altered NSC response to drug treatment. Finally, behavioral tests were conducted to assess memory and homeostatic/hedonic feeding behaviors.
2. Results
2.1. Novel mouse model of chronic cocaine and ethanol co-exposure
Adult Nestin-CreERT2:R26R-YFP bi-transgenic mice were used to trace the fate of endogenous NSCs, following tamoxifen induction, in response to chronic ethanol feeding, in a calorically-balanced liquid diet, and/or cocaine administration, via intraperitoneal injections (Fig. 1A). Animals with similar ages were used in this study, and males and females were randomly assigned to a treatment group. The age of mice was chosen to represent an adult population of approximately 25–30 years of age (Dutta and Sengupta, 2016) (Fig. 1B). We were interested in evaluating the impact of poly-drug use in the adult population, as we were interested in the effect poly-drug abuse has on the fully matured brain, which in humans is approximately 25–30 years of age (Sowell et al., 2003; Arain et al., 2013). Tamoxifen was administered when animals were 2 months of age, in this study, then 1 month lapsed prior to drug treatment to enable maximum recombination of the GFP transgene, as well as to allow washout of the immediate effects of tamoxifen.
Fig. 1.
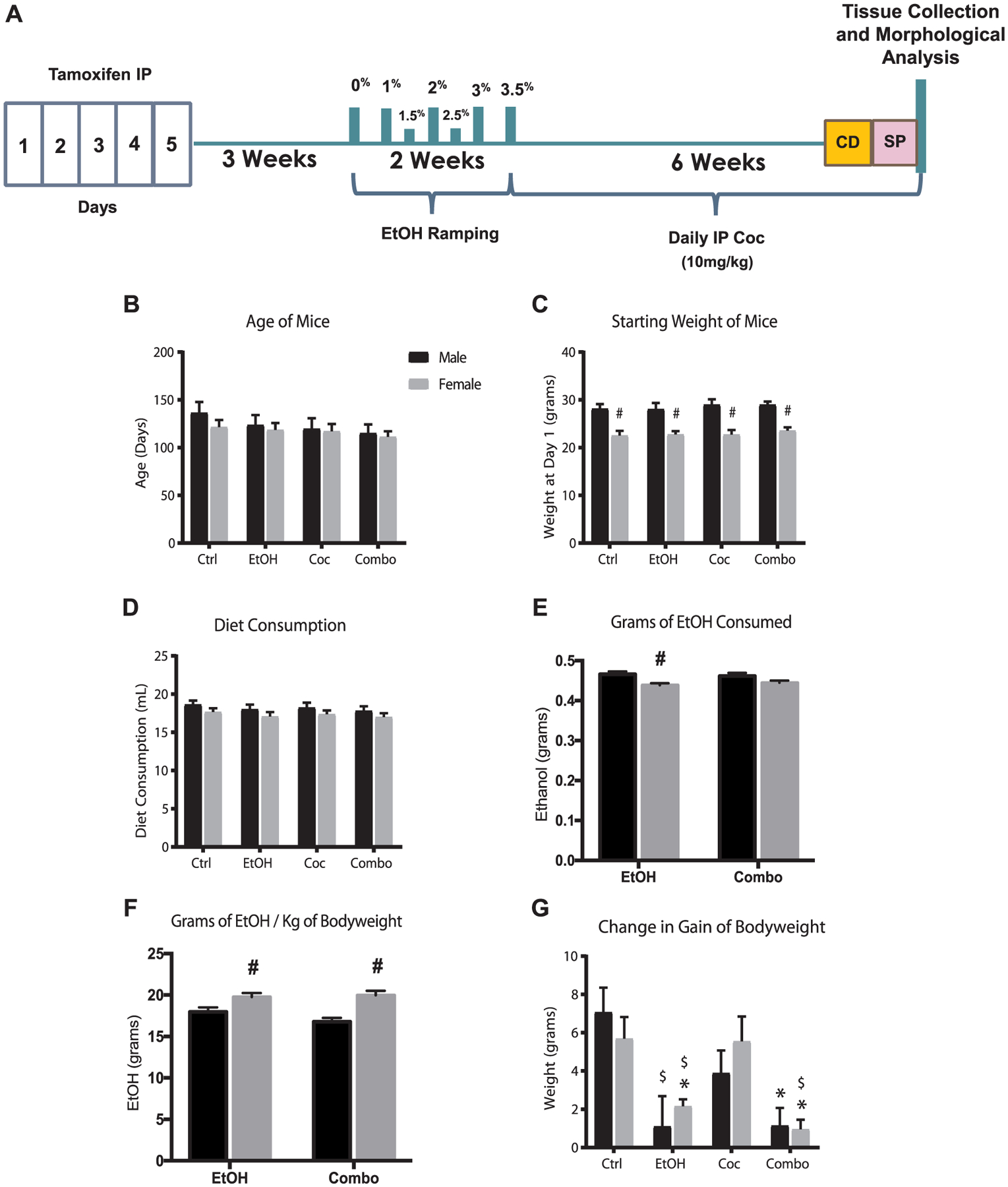
Chronic drug treatment model. (A) Schematic of experimental paradigm. CD, context fear discrimination learning; IP, intraperitoneal; SP, sucrose preference test. (B) Average age of mice at the start of experiment. (C) Average bodyweight at the start of experiment. (D) Average daily diet consumption. (E) Average grams of ethanol consumed daily. (F) Average grams of ethanol consumed per kilogram of mouse bodyweight. (G) Average changes in gain of bodyweight. Values are shown as mean ± SEM, #p < 0.05 compared to other sex in the same group, *p < 0.05 compared to control, $p < 0.05 compared to cocaine; control (Ctrl), ethanol (EtOH), cocaine (Coc), and combination (Combo). Ctrl male n = 7, Ctrl female n = 13, EtOH male n = 10, EtOH female n = 13, Coc male n = 10, Coc female n = 14, Combo male n = 10, Combo female n = 15.
Male mice weighed more than females, however, there were no significant differences among bodyweights of control and treatment groups (Fig. 1C). Male mice tended to have higher average daily diet consumption compared to their female counterparts. In the ethanol group, males consumed more diet than females; therefore, males consumed significantly more grams of ethanol per day (Fig. 1D and E). However, females in both the ethanol and combination treatment groups consumed more grams of ethanol per kilogram of body weight (Fig. 1F). Mice in the control and cocaine groups exhibited similar growth rates in bodyweight; however, in the ethanol and combination groups, both males and females had a significant reduction in gain of bodyweight (Fig. 1G).
The metabolism of cocaine in the presence of ethanol produces a unique metabolite, cocaethylene, which is known to be cardiotoxic (O’Leary, 2002; Cittadini et al., 2015). Thus, liquid chromatography coupled to mass spectrometry (LC-MS) was used to measure cocaine and its metabolites in whole blood at one hour and 24 h following cocaine injection (Supplementary Fig. 1). There were increased levels of cocaine (Coc), benzoylecgonine (BCE), and cocaethylene (CE) one hour after administration; however, after 24 h these metabolites were undetectable. Daily intraperitoneal injections of cocaine were used to maintain active cocaine metabolism and blood levels.
2.2. Effect of drug treatment on SVZ NSCs
The SVZ of the rostral lateral ventricle was chosen because it harbors endogenous NSCs and is an area of active neurogenesis in adult mammalian brains (Lim and Alvarez-Buylla, 2014). Tamoxifen enabled expression of YFP was enhanced by immunohistochemistry using a green fluorescent protein (GFP) antibody. We examined three phases of NSCs, including NSCs (GFP+) and newly differentiated NSCs which were doublecortin (DCX) positive (DCX+GFP+) or glial fibrillary acidic protein (GFAP) positive (GFAP+GFP+). Since NSCs also express GFAP, cautions were made to exclude those GFAP+ cells with DAPI-stained nuclei located in the SVZ, and other NSC regions below. DCX is a marker for neuronal lineage and GFAP is for glial lineage. The third phase we examined was total neuronal or astrocyte populations (DCX+ or GFAP+), including those differentiated cells before tamoxifen induction. Cells that were double-labeled with GFP and a marker of differentiation (DCX or GFAP) represent newly differentiated cells after induction of YFP expression in Nestin+ cells. Cells that differentiated prior to tamoxifen induction and no longer expressed Nestin would not be co-labeled with GFP. We quantified positively stained cells in the SVZ, particularly focusing on the origin of the rostral migratory stream (oRMS).
There were no differences in GFP+ cells between control male and female mice (Fig. 2A and E). All drug treatments resulted in a reduction of GFP+ cells (Fig. 2). Males and females in the ethanol group had fewer GFP+ cells compared to control and cocaine mice (Fig. 2A–C and E). Combination treatment had the greatest impact on GFP+ cells (Fig. 2D and E). Next, we examined the neurogenic capacity of SVZ NSCs after drug treatment using the DCX antibody. Male and female control mice had the similar number of DCX expressing cells (Fig. 2A and F). Interestingly, while ethanol decreased total number of DCX+ cells, cocaine did not reduce the DCX+ population (Fig. 2B, C and F). Combination treatment caused the largest reduction in DCX+ cells (Fig. 2D and F), which were significantly greater than ethanol alone.
Fig. 2.
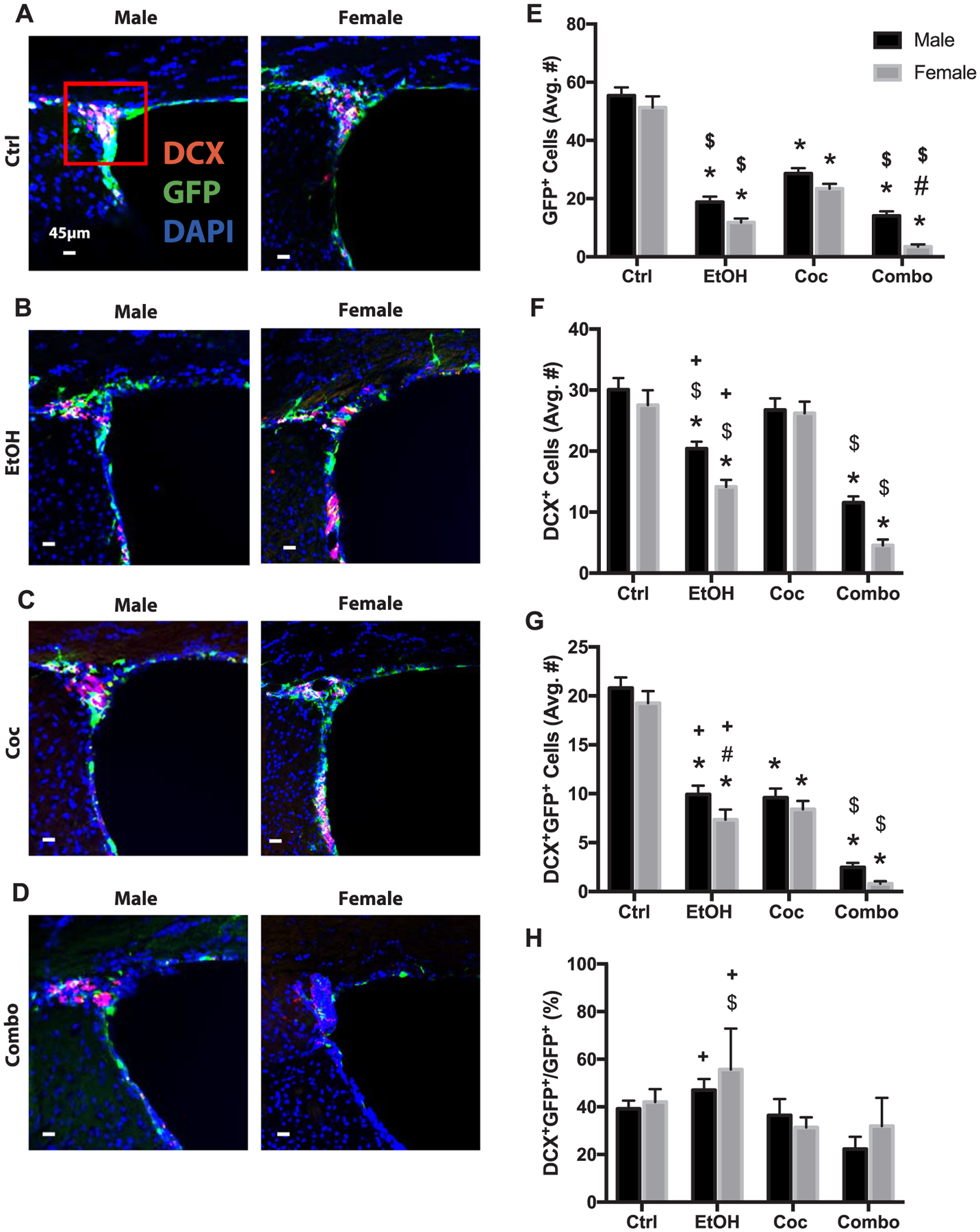
Neural stem cell and neurogenesis in the subventricular zone (SVZ). (A–D) Representative brain images of control (Ctrl), ethanol (EtOH), cocaine (Coc), and combination (Combo) treated mice, respectively, immunostained with stem cell marker (GFP, green), neuronal marker (DCX, red), and merged with nuclear marker DAPI (Bregma 0.5 through 1.08). (E–H) Quantification of GFP+, DCX+, DCX+GFP+, and the percentage of DCX+GFP+ over total GFP+ cells in the SVZ and origin of the rostral migratory stream (area inside red square). Values are shown as mean ± SEM, *p < 0.05 compared to control, #p < 0.05 compared to other sex in the same group, $p < 0.05 compared to cocaine group, +p < 0.05 compared to combination group, n = 3 mice per sex per group. Scale bars, 45 μm.
All drug treatments significantly reduced the newly differentiated neurons (DCX+GFP+) (Fig. 2B–D and G). Combination treatment decreased DCX+GFP+ cells in male and females (Fig. 2G); and both sexes exhibited significantly more severe damage than their ethanol counterparts. In terms of the percentage of GFP+ cells becoming DCX+ (DCX+GFP+/GFP+), none of the treatment groups had a significant change when compared to controls (Fig. 2H). However, both sexes in the ethanol group showed significantly higher values than those in the combination group.
To further verify a reduction in neurogenesis, the olfactory bulb of control and combination mice were stained for GFP and mature neuronal marker, NeuN (Supplementary Fig. 2). There was a significant decrease in both GFP+ and NeuN+ cells in the combination group, which was more pronounced in the females. Additionally, there was a dramatic reduction in NeuN+GFP+ cell populations compared to control.
We further assessed the effect of drug consumption on astroglial differentiation in the oRMS by using GFAP as a glial marker. No significant differences were found in the number of GFAP+, GFAP+GFP+, or the percentage of GFP+ cells becoming GFAP+ (GFAP+GFP+/GFP+) between males and females in control mice (Fig. 3A and E–G). Cocaine had no effect on the total population of GFAP+ cells; however, ethanol and combination treatment significantly reduced this population (Fig. 3A–E). All treatments reduced the newly differentiated GFAP+GFP+ cells (Fig. 3B–D and F). Both males and females in the ethanol and combinations groups had significantly fewer cells than those in the cocaine group. There were no significant changes among the groups with regards to the percentage of GFP+ cells becoming GFAP+ (Fig. 3G). Individual channels of all stains presented in Figs. 2 and 3 can be found in Supplementary Figs. 3 and 4.
Fig. 3.
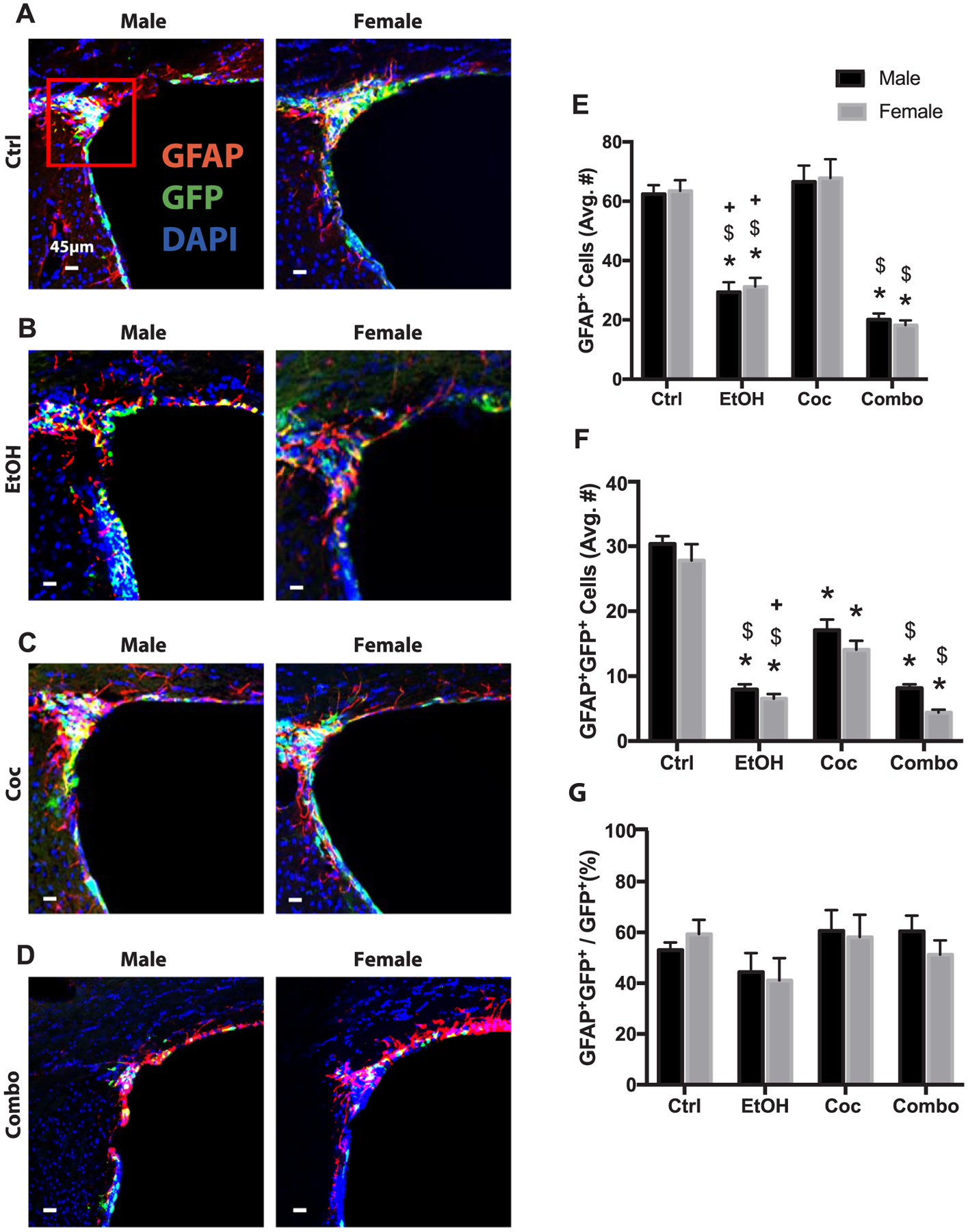
Astrogliogenesis in the SVZ. (A–D) Representative brain images of control (Ctrl), ethanol (EtOH), cocaine (Coc), and combination (Combo) treated mice, respectively, immunostained with stem cell marker (GFP, green), astrocyte marker (GFAP, red), and merged with nuclear maker DAPI (Bregma 0.5 through 1.08). (E–G) Quantification of GFAP+, GFAP+GFP+, and the percentage of GFAP+GFP+ over total GFP+ cells in the SVZ and origin of the rostral migratory stream (area inside red square). Values are shown as mean ± SEM, *p < 0.05 compared to control, #p < 0.05 compared to other sex in the same group, $p < 0.05 compared to cocaine group, +p < 0.05 compared to combination group, n = 3 mice per sex per group. Scale bars, 45 μm.
In summary, these data show the NSCs in the SVZ are sensitive to chronic ethanol and cocaine treatment but were most sensitive to the combination drug treatment. Additionally, female NSCs (GFP+) are more sensitive to combination drug treatment when compared to males. Migratory neurons (DCX+) and astrocytes (GFAP+) present before tamoxifen induction were not affected by cocaine treatment but are sensitive to combination drug treatment. Newly differentiated neurons and astrocytes (DCX+GFP+ and GFAP+GFP+) seem to be the most vulnerable to combination drug treatment.
2.3. Effect of drug treatment on SGZ NSCs
Next, we examined the SGZ in the dentate gyrus of the dorsal hippocampus, given its role in active adult neurogenesis and cognitive function (Kempermann et al., 2015). Control males seemed to have fewer GFP+ cells compared to control females (Fig. 4A and E). Ethanol reduced GFP+ cells in both males and females however, cocaine did not significantly impact the number of GFP+ cells (Fig. 4B, C and E). Interestingly, there was a change in the morphology of many GFP+ cells, showing loss of arborization in both cocaine and combination groups (Fig. 4C). Combination treatment almost completely depleted GFP+ cells in males and females (Fig. 4B, D and E).
Fig. 4.
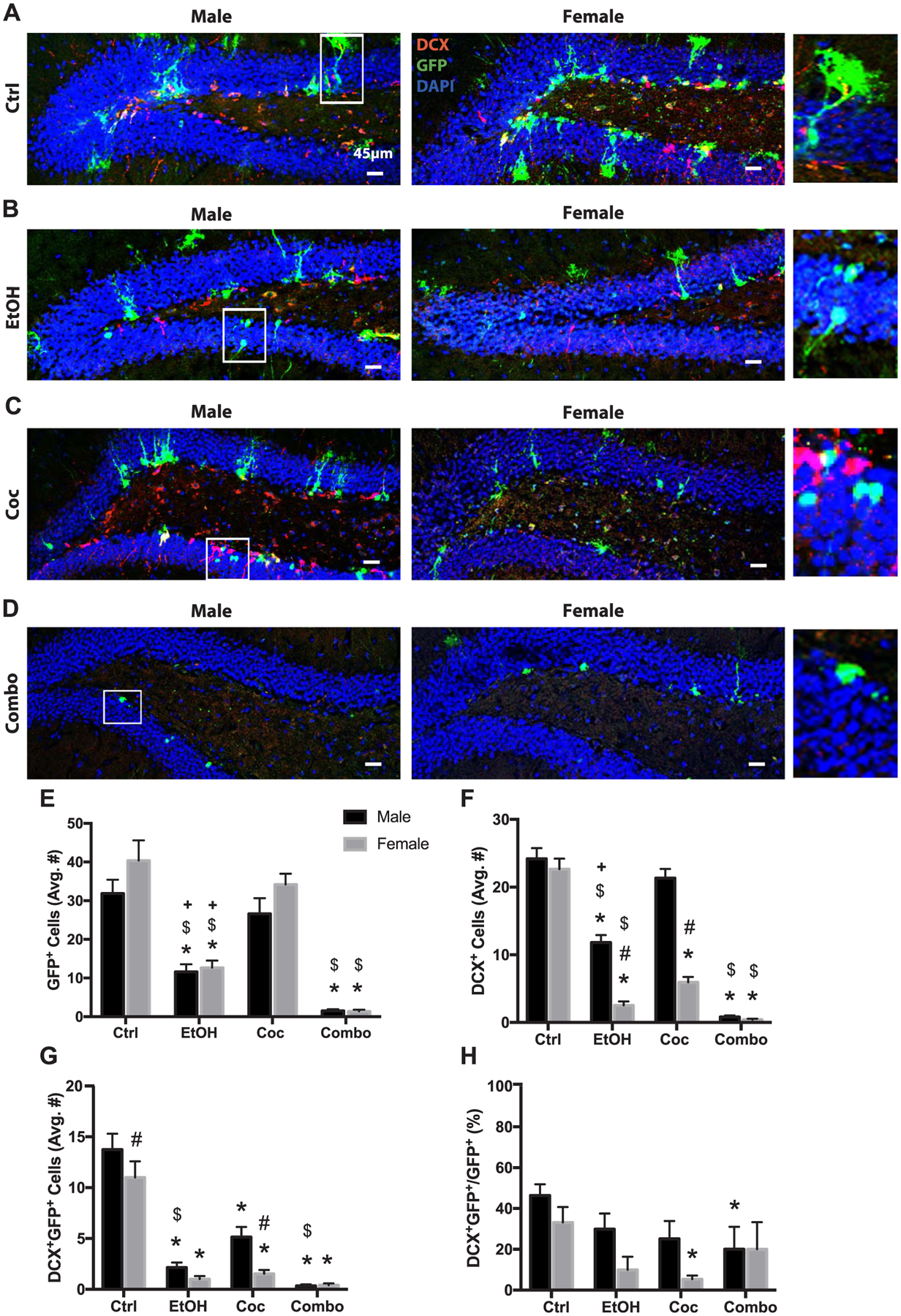
Neural stem cell and neurogenesis in the subgranular zone (SGZ). (A–D) Representative brain images of control (Ctrl), ethanol (EtOH), cocaine (Coc), and combination (Combo) treated mice, respectively, stained with stem cell marker (GFP green), neuronal marker (DCX red), and merged with nuclear maker DAPI (Bregma 0.5 through 1.08). White boxes represent increased magnification of selected cells as seen on the right side of panel. (E–H) Quantification of GFP+, DCX+, DCX+GFP+, and the percentage of DCX+GFP+ over total GFP+ cells, in the SGZ. Values are shown as mean ± SEM, *p < 0.05 compared to control, #p < 0.05 compared to other sex in the same group, $p < 0.05 compared to cocaine group, +p < 0.05 compared to combination group, n = 3 mice per sex per group. Scale bars, 45 μm.
Furthermore, ethanol, cocaine and combination treatment significantly reduced DCX+ cells in female mice (Fig. 4B–D and F). Males in the cocaine group did not show reduction in DCX+ cells, whereas males in the ethanol and combination groups had a significant decrease (Fig. 4B–D and F). Female mice in the ethanol and cocaine groups had significantly fewer DCX + cells compared to their male counterparts (Fig. 4B, C and F). Although control female mice tended to have more GFP+ cells, they had significantly fewer DCX+GFP+ cells compared to control males (Fig. 4G). All drug treatments reduced DCX+GFP+ cell populations in both male and female mice (Fig. 4G). Cocaine significantly reduced the percentage of NSCs differentiating into neurons (DCX+GFP+/GFP+) in female mice, while combination treatment significantly reduced this percentage in males (Fig. 4H). Individual channels of all stains presented in Fig. 4 can be found in Supplementary Fig. 5.
In summary, combination drug treatment almost completely obliterated NSCs, newly differentiated neurons and total DCX+ immature neurons in the SGZ of both males and females. Newly differentiated neurons are most susceptible to drug treatment, and the SGZ neurons of female mice are more sensitive to cocaine compared to males.
2.4. Effect of drug treatment on TL NSCs
We also evaluated the TL of the third ventricle because this region harbors a subpopulation of cells known as tanycytes which possess NSC-like characteristics and is becoming known as a new neurogenic niche (Haan et al., 2013; Robins et al., 2013). In this study, there was little evidence of newly differentiating neurons in the adult TL, therefore our primary focus was on GFP+ and GFAP+ cell populations. Control male and female mice had similar numbers of GFP+ and GFAP+ cells; whereas males had more GFAP+GFP+ (Fig. 5A and E–G). Ethanol and combination treatment reduced GFP+ cells in the TL in males and females (Fig. 5E). Cocaine did not significantly alter female GFP+ cell populations compared to control; however, males had a decrease (Fig. 5C and E). In all three treatment groups there was a noticeable change in morphology of GFP+ cells, with loss of arborization and flattening of cells (Fig. 5B–D). Ethanol and cocaine reduced total GFAP+ cells in males and females (Fig. 5F). Interestingly, combination treatment did not impact the total GFAP+ population in this region (Fig. 5D and E). As shown in Fig. 5G, ethanol reduced the GFAP+GFP+ population in males and females. Cocaine, on the other hand, decreased GFAP+GFP+ cells in males, but increased these cells in females. Combination treatment reduced GFAP+GFP+ cells in both males and females. Despite the reduction of GFAP+GFP+ cells in male cocaine-treated mice (Fig. 5G), they had showed an increase in the percentage of GFP+ cells becoming GFAP+ cells (Fig. 5H). Males in the combination group had a reduction in this percentage compared to both cocaine and control mice (Fig. 5H). Individual channels of all stains presented in Fig. 5 can be found in Supplementary Fig. 6.
Fig. 5.
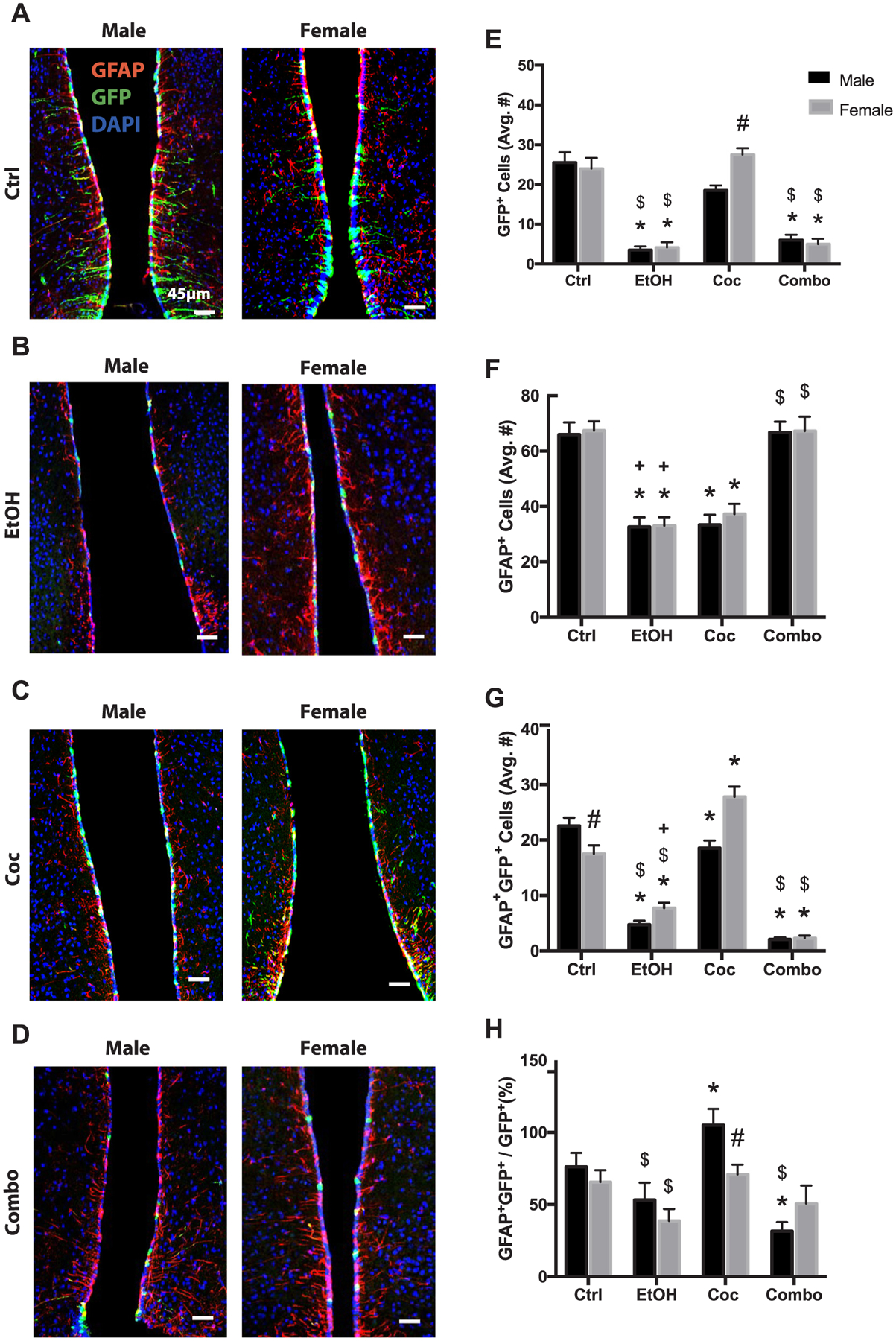
Neural stem cell and astrogliogenesis in the tanycyte layer (TL). (A–D) Representative brain images of control (Ctrl), ethanol (EtOH), cocaine (Coc), and combination (Combo) treated mice, respectively, stained with stem cell marker (GFP green), astrocyte marker (GFAP red), and merged with nuclear maker DAPI (Bregma 0.5 through 1.08). (E–H) Quantification of GFP+, GFAP+, GFAP+GFP+, and the percentage of GFAP+GFP+ over total GFP+ cells, in the SGZ. Values are shown as mean ± SEM, *p < 0.05 compared to control, #p < 0.05 compared to other sex in the same group, $p < 0.05 compared to cocaine group, +p < 0.05 compared to combination group, n = 3 mice per sex per group. Scale bars, 45 μm.
In summary, combination treatment reduced GFP+ and GFAP+GFP+ cell populations but did not affect total GFAP+ cell populations that include both newly differentiated and pre-existing astrocytes. In addition, GFP+ and GFAP+GFP+ cells in the female TL are more resilient than males to cocaine treatment.
2.5. Effect of drug treatment on animal behavior
To determine whether single and poly-drug chronic administration altered behavioral outcomes, we conducted a fear conditioning contextual discrimination test for cognitive function, and a sucrose preference test for hedonic behavior.
Context fear discrimination learning has been related to hippocampal neurogenesis and function (Cortez et al., 2017). Here we found that all four groups of animals gradually increased the time of freezing in the shock context (Fig. 6A and B). Control and ethanol groups reached a mean discrimination ratio above 0.5 by day 3, but it took 4 days for cocaine and combination groups to discriminate (Fig. 6C). Compared to the control, there was a decreasing trend in the mean ratio after drug treatment on day 4, albeit statistically insignificant. A further analysis on the performance of each individual mouse revealed that the control group (n = 20) had only 10% of mice unable to discriminate (i.e., ratio ≤ 0.5), which was increased to 20% in the ethanol group, 40% in cocaine, and 26% in combination (Fig. 6D and E). In addition, females of both cocaine and combination groups tended to perform worse than males.
Fig. 6.
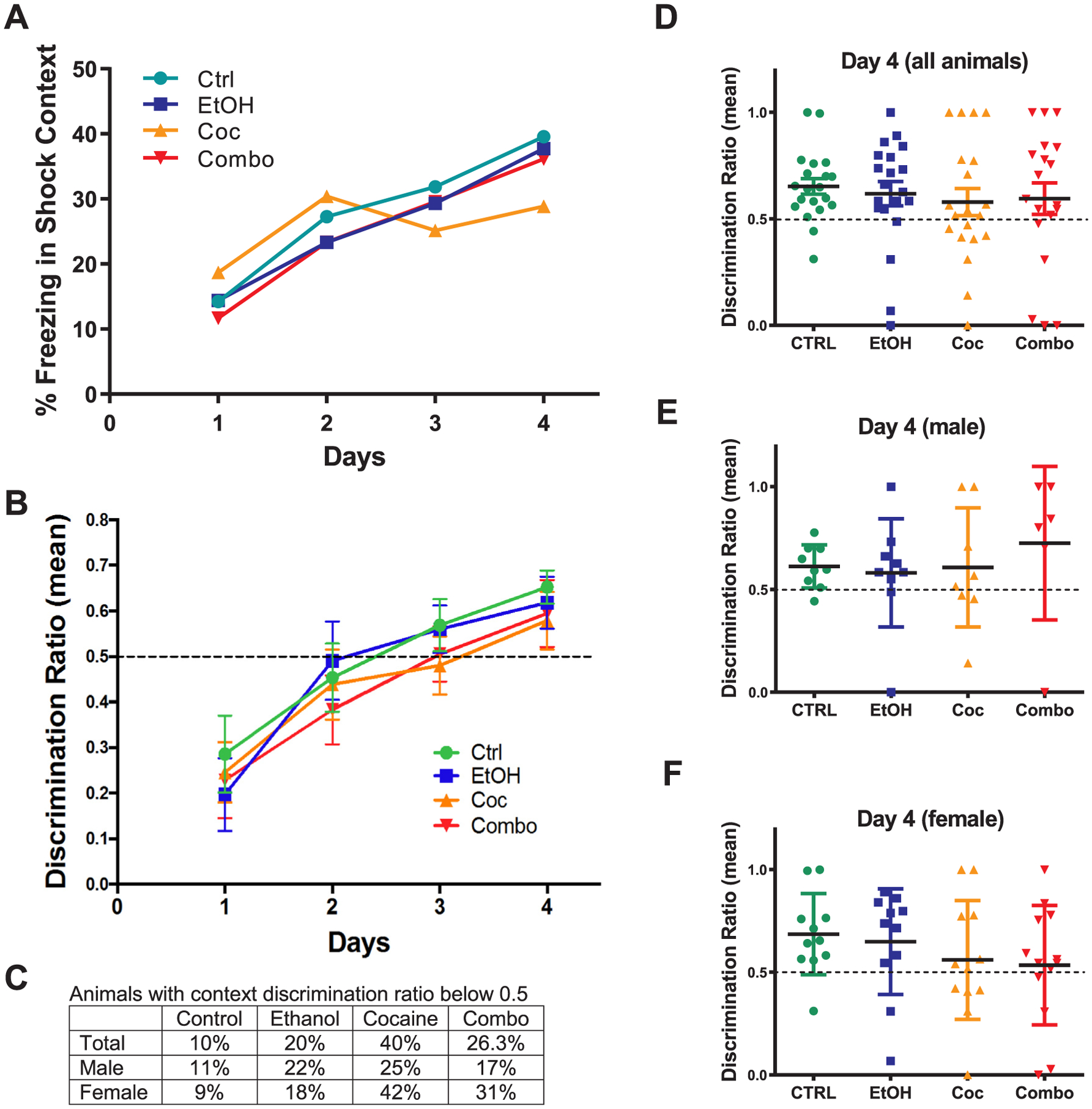
Context fear discrimination learning paradigm. (A, B) Analysis of percent freezing (b) and discrimination ratio (c) for each treatment group from day 1–4. (C) Chart comparing the percentage of male and female in each treatment group with a discrimination ratio below 0.5. (D–F) plots of the discrimination ratio for individual animal in each group on day 4. Control (Ctrl) n = 20, ethanol (EtOH) n = 20, cocaine (Coc) n = 20, and combination (Combo) n = 19. Values are shown as mean ± SEM.
Furthermore, to determine if changes in the tanycyte layer correlated with changes in hedonic behavior, we conducted a modified form of the sucrose preference task (Fig. 7). It has been shown that hypothalamic tanycytes play a role in glucose-sensing via G protein coupled receptors and glucose transporters (Benford et al., 2017; Lazutkaite et al., 2017), suggesting alterations of tanycytes may affect feeding-related behaviors based on communication between these cells and the arcuate nucleus of the hypothalamus (Lee et al., 2012). In our modified sucrose preference paradigm, mice had access to two bottles of their respective diets, which were identical in every way except one bottle had a percentage of sucrose replacing the equivalent amount of maltose-dextrin (Fig. 7A). In this way, mice were tested on their preference for sucrose over maltose-dextrin, since the diet was calorically balanced. As shown in Fig. 7B, control, ethanol and cocaine groups did not show preference for sucrose diet at 0.5% sucrose, but the combination group consumed approximately 62% more sucrose diet. As expected, increasing sucrose to 1% led all groups to exhibit sucrose-preference; however, there are no significant differences among groups (Fig. 7C). This data suggests that combination mice were able to detect a difference in sugar substitution at a lower concentration than other groups. Although preliminary, this behavioral study suggested a potential role of hypothalamic tanycytes and the effect of poly-drug use on hedonic feeding-related behaviors.
Fig. 7.
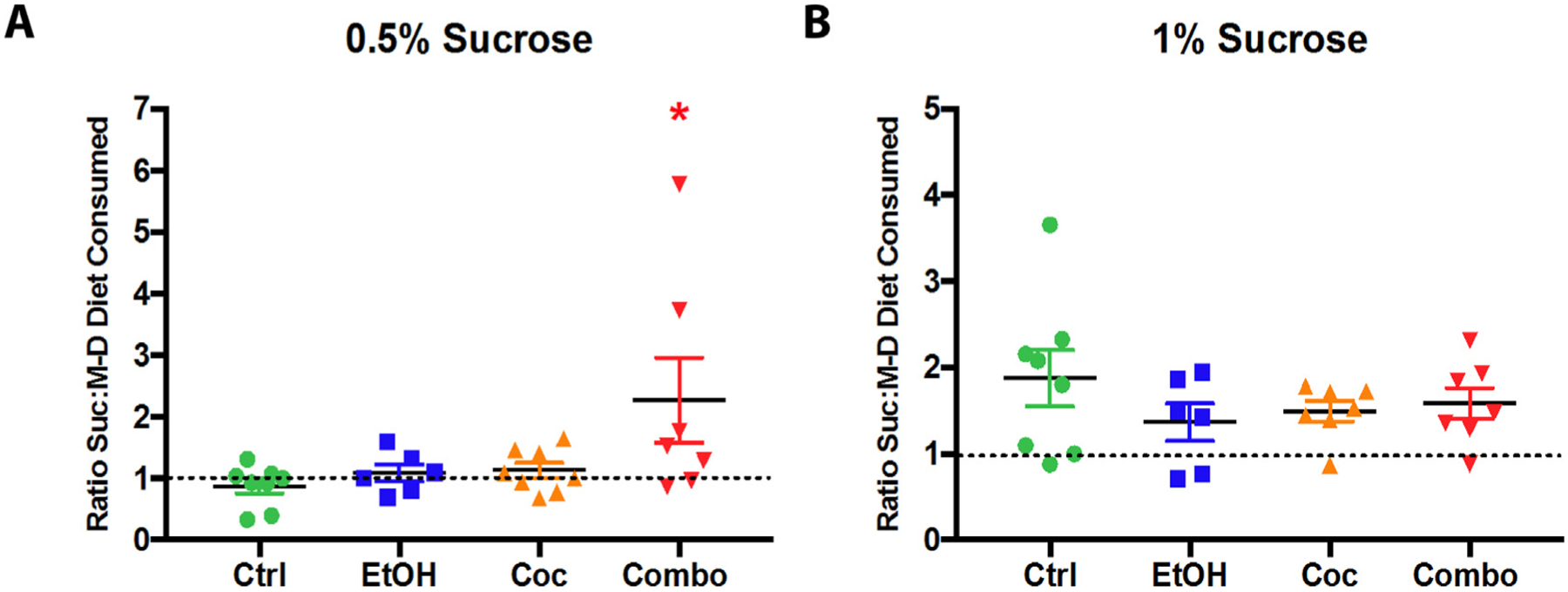
Modified sucrose preference test. (A, B) Analysis of sucrose to maltose-dextrin diet consumed at 0.5% sucrose and 1% sucrose. Control (Ctrl) n = 6, ethanol (EtOH) n = 6, cocaine (Coc) n = 8, and combination (Combo) n = 6. Values are shown as mean ± SEM, *p < 0.05 compared to control.
3. Discussion
This study employs a novel poly-drug administration paradigm using a genetic inducible fate-mapping rodent model to study the effects of chronic ethanol and cocaine co-administration on NSCs and their progeny, and to assess behavioral outcomes following poly-drug administration. To the best of our knowledge this is the first study to report findings from a chronic poly-drug administration of ethanol and cocaine. Additionally, the primary advantage of this model is the ability to trace a distinct population of NSCs over time, in response to treatment, and characterize behavioral changes. Along this line, we found that chronic treatment of poly-drugs caused more severe damage on NSCs than ethanol alone, while cocaine led to only mild or no damage of NSCs. Single and combined drug treatments all significantly reduced neurogenesis and females were more vulnerable. Chronic exposure to cocaine and poly-drugs tend to alter some cognitive function and hedonic behavior, respectively. However, the severity of drug-induced NSC damage did not correlate completely with the changes of these behavior.
Previously, we have characterized the blood ethanol concentration (BEC) following ethanol liquid diet consumption compared to control (McGrath et al., 2017). We found that BEC was highly variable and did not correlate with behavioral changes. Peak BEC occurs 1–2 h following feeding which is a measure of acute intoxication (Grahame and Grose, 2003). In our study, mice were given ad libitum access to the ethanol liquid diet and did most of their feeding in the first few hours of the dark cycle. Therefore, during the day, BEC could vary drastically. However, mice still exhibited behavioral changes, due to chronic consumption of an ethanol diet.
The main goal of this study was to evaluate the effect of co-administration of ethanol and cocaine on NSCs in the adult mouse brain. It has been well established that ethanol interacts with cocaine to form cocaethylene (Bunney et al., 2001; Althobaiti and Sari, 2016). Typically, cocaine is metabolized by carboxylesterase-1 to produce the inactive metabolite benzoylecgonine (BCE). In the presence of ethanol, carboxylesterase metabolizes cocaine, into cocaethylene. It has also been shown that ethanol does not change cocaine pharmacokinetics, suggesting that the enhanced effect on NSCs seen in poly-drug use here could be attributed to a synergistic effect of both drugs taken simultaneous and/or the cocaethylene metabolite (Fowler et al., 1992). Future experiments will be focused on understanding the direct effect of cocaethylene on NSCs.
In the SVZ, female NSCs were more sensitive to the toxic effects of poly-drug treatment, which also caused the most dramatic reduction of newly differentiated neurons. It is important to note that the female mice in this study were free cycling. While we acknowledge that the estrous cycle and sex hormones play a role in NSC behavior (Ponti et al., 2018), free cycling females were included to increase the translational value of our findings. Furthermore, poly-drug treatment was almost equally detrimental to NSCs and newly differentiated neurons in both the SVZ and SGZ; however, neurons that differentiated before tamoxifen induction were more sensitive to poly-drug treatment in the SGZ. One limitation in our study was the lack of a NSC proliferation marker, which would have established if the lack of differentiation was due to truly reduced differentiation versus an overall reduction in the number of progenitor cells. Previous work in our lab with chronic administration of ethanol alone also produced sex- and region dependent effects and resulted in a much more dramatic impairment of NSCs in the SVZ as well as high mortality and adverse health events (McGrath et al., 2017). This discrepancy is most likely due to the higher dose of ethanol (4%) used in our previous study as compared to the 3.5% in this study. The lower dosing of ethanol in this study was critically important in order to observe subtle effects of ethanol alone as well as evaluate the synergistic effect of poly-drug use.
Interestingly, we did not observe a correlation between context discrimination performance and degree of NSC loss and neurogenesis in the SGZ of the hippocampus. It is well established that adult NSCs contribute to spatial learning and memory (Imayoshi et al., 2008; Zhang et al., 2008); however, these studies were conducted with selective ablation or suppression of NSCs growth. In our study the brain was grossly affected by cocaine and ethanol administration which may influence neurocircuitry in the hippocampus and the amygdala (Antoniadis and McDonald, 2000). The latter may not have been suf-ficiently impaired following combined administration and was able to produce a compensatory fear response, explaining the maintained performance in the task. It is of interest in future studies to conduct more refined and challenging behavioral tests to better isolate brain functions associated with neurogenic regions.
A reduction in hypothalamic NSCs was detected in the TL following combination treatment; however, the reduction was not quite as dramatic as that seen in the SGZ or SVZ. Further investigation would be beneficial to determine if there are protective factors in the micro-environment surrounding the TL. Another noteworthy finding was that, despite significant reduction of new astrocytic differentiation, the total number of astrocytes (GFAP+) were unaffected in the TL following combination treatment. The heightened resiliency of astrocyte populations in the TL warrants further investigation into the mechanisms of resilience. To interrogate whether changes in the TL affected hedonic feeding-related behavior we conducted a modified sucrose preference test and found that the combination group exhibited an increased sensitivity to this hedonic stimulation. While it has been demonstrated that hypothalamic tanycytes have a glucose-sensing function, their role in poly-drug use and feeding-related behaviors remains ambiguous (Antoniadis and McDonald, 2000; Benford et al., 2017). Our modified sucrose preference test thus offers a new way for further dissecting and understanding their roles in regulating hedonic preference in the context of drug abuse. Furthermore, a study conducted by Jones and colleagues in 2006 shows that there were sex-dependent differences on ethanol and cocaine induced taste aversions (Jones et al., 2006). The TL presents a unique opportunity into examining how hypothalamic tanycytes communicate signals from cerebrospinal fluid to the hypothalamus.
While this study provides important information on the neuropathology of poly-drug exposure, one limitation was that drug administration was involuntary; therefore, conclusions about drug dependency cannot be drawn. In order to accurately control confounding variables such as dosing and caloric intake, mice were not given a choice between ethanol or control diet, and cocaine was administered via daily intraperitoneal injections. While this treatment paradigm provides a well-controlled dosing scheme, there can be no assessment of drug seeking. It is of interest to evaluate drug-seeking and dependency in this model in future studies
Endogenous adult NSCs are significantly impacted following chronic ethanol and cocaine co-exposure in a regional and sex-dependent manner. Given the adverse effects of neurodegeneration in substance using populations, targeting NSCs regeneration and neurogenic repair may be a promising approach to improve clinical efficacy of treatments and patient quality of life.
4. Experimental procedure
4.1. Animals
Nestin-CreERT2:R26R-YFP bi-transgenic male and female mice were generated by crossing the C57BL/6-Tg(Nes-cre/ERT2)KEisc/J strain with the B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J strain (both obtained from the Jackson Laboratory), and have been described elsewhere (Lagace et al., 2007). Mice were genotyped by PCR using genomic DNA from tail snip to confirm presence of Cre and YFP trans-genes. Upon induction, tamoxifen will allow for constitutive expression of enhanced yellow fluorescent protein (eYFP) in all nestin expressing cells of adult mice. Nestin is an intermediate filament protein expressed in quiescent and proliferative neural stem cells. Tamoxifen was administered intraperitoneally for a total of 5 days at 75 mg/kg. Female mice were free cycling during the duration of this study. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch and conducted in accordance with the Guide for the Care and Use of Laboratory Animals, and maintained on a 12-hour light/dark cycle.
4.2. Experimental design
Mice, 3–4 months old, were randomly assigned using the “randomize” (RAND) function in Microsoft Excel to one of four groups: control (male n = 7, female n = 13), cocaine (male n = 10, female n = 14), ethanol (male n = 10, female n = 13), or combination (male n = 10, female n = 15). Mice in each group were age and sex matched to control for variability. Following randomized group assignment, mice were individually housed and given ad libitum access to water and a Lieber-DeCarli liquid diet containing complete nutrients for rodents (Dyets Inc., Bethlehem, PA, CAT #710260) as previously described50. Calorically-matched feeding was done in which ethanol calories replaced the equivalent calories of maltose-dextrin in the diet for ethanol and combination mice. The amount of ethanol in the liquid diet was slowly increased from 1% to 3.5% over the course of two weeks. Mice in the ethanol and combination groups were maintained on 3.5% ethanol liquid diet for a total of 8 weeks, while mice in the control and cocaine groups were given calorie balanced liquid diet without ethanol. Cocaine treatment was given over a total of 6 weeks (Fig. 1A). The 8-week treatment course was chosen as a chronic paradigm as previously defined by Bertola et al (Bertola et al., 2013). All diet was made fresh daily. Each day, between 9:00–11:00 am, the feeding bottles were removed from the cage, the remaining liquid diet was measured and subtracted from the original diet administered. Blood ethanol concentrations (BEC) following chronic liquid diet consumption were monitored as described before (McGrath et al., 2017). On the first day of 3.5% ethanol in diet, the combination and cocaine mice received daily 10 mg/kg intraperitoneal injections (i.p.) of (−)-Cocaine (National Institute on Drug Abuse), dissolved in 0.9% NaCl, every day for six weeks. Mice in the control and ethanol group received vehicle injection of 20 μL of 0.9% saline. Animals were weighed once a week. All data presented represents ± SEM.
4.3. Analysis of cocaine & metabolites using LC-MS
An Agilent 1260 HPLC system with a binary gradient pump, auto-sampler and column oven (50 °C) was used for the chromatographic separation. An Agilent SB-C18 1.8uM 2.1 × 50 mm column was used. Mobile phase A was water + 0.1% formic acid and mobile phase B acetonitrile + 0.1% formic acid. A flow rate 500 μL/min was used. The gradient with a total run time of 12 min 0–1 min 98% A, 1–7 min a linear gradient to 35% A, 7–7.1 min to 2% A, 7.1–9 min 2% A, 9–9.1 min 98% A, 9.1–12 min 98% A. The injection volume was set to 10 μL.
A SCIEX QTRAP 6500 system with Turbo V source with ESI probe was used. The target compounds were detected in positive polarity. The ion source parameters were optimized for the new LC conditions using the Compound Optimization (FIA) function in Analyst® software and are as follows: Curtain gas (40), CAD set to high, Ionspray voltage 4500, Temperature 50C, GS1 50, GS2 50, DP ranged from 76 to 81 V, CE ranged from 27 to 41 V, CXP ranged from 10 to 16 V. Two characteristic MRM transitions were monitored for each analyte, and 1 MRM transition for each internal standard.
4.4. Behavioral assessment
Context fear discrimination learning was conducted as we described previously (Cortez et al., 2017). All procedures were carried out between 7:00 am and 6:00 pm Each day before testing, mice were allowed to acclimate to the testing room for 1 h. Control (n = 20), ethanol (n = 20), cocaine (n = 20), and combination (n = 19) were exposed to the shock context on training day (Day 0), before being returned to their home cage. Starting Day 1 through the rest of the experiment, mice were placed in both the shock (Context A) and a safe (Context B) context. Animals were randomly selected from each group and the order of context presented was randomized each day to avoid animals adjusting to the schedule. There was no foot shock in Context B. Percent freezing was recorded in each context. Because contextual fear-discrimination is a hippocampal-dependent task, it measures an animal’s ability to distinguish between two environmental contexts. Each context used a standard mouse fear conditioning chamber (MedAssociates). Context A had no modifications to the chamber with light and fan. Context B also had a grid floor, however it also contained cardboard inserts, vanilla extract, and the chamber light and fan were shut off. Mice spend equal amounts of time in both chambers, and were randomly assigned to which context they would experience first. A four-hour interval was left in between contexts for each subject on each day of testing. Chambers were cleaned with 70% ethanol in between each subject. Digital video-based data capture and analysis with Free-zeFrame software (Actimetrics) was used to assess freezing behavior. Discrimination ratios (% freezing in Context A ÷ % freezing in Context A + B) were calculated for each group on each test day.
4.5. Sucrose preference task
In order to assess if changes observed in cell populations in the TL correlated with behavioral changes, a sucrose preference test was employed. Mice were given two bottles, one containing only maltose-dextrin, the other containing the substituted percentage of sucrose. In order to assess if drug treatment affected taste preference for sucrose compared to maltose-dextrin, sucrose was increased in the liquid diets of control (n = 6), ethanol (n = 8), cocaine (n = 6), and combination (n = 8) mice over the course of 4 days. Sucrose was increased incrementally every 2 days from 0.5% to 1%. The equivalent amount of maltose-dextrin was removed from the liquid diet corresponding to the amount of sucrose added in order to maintain the caloric balance of the diets. Diet consumption was measured daily and used to calculate the ratio of sucrose:maltose-dextrin consumption.
4.6. Immunohistochemistry
At the termination of the experiments, mice were anesthetized with ketamine/xylazine (90 mg/kg and 10 mg/kg), and blood samples were collected prior to perfusion with 5 mL PBS and 30 mL of cold 4% paraformaldehyde via an intra-cardial injection. Mouse brains were collected, post-fixed in 4% paraformaldehyde for 1 day at 4 °C, and then infiltrated with 30% sucrose for 5 days at 4 °C before embedding in OCT media. Tissue from three mice per treatment group was serially sectioned, coronally, at a thickness of 30 μm using a Leica cryostat machine. Every fifth section was collected on the same slide with four sections per slide, and five slides per region (each slide covered a distance of 480 μm). Therefore, for every region and each cell marker, four tissue sections were counted spanning a distance of 480 μm. Slides were randomized for staining and double blinded for stereology using anonymous identification numbers. Detection of induced-YPF was enhanced by immunohistochemical staining with chicken anti-GFP antibodies (Aves Labs, Tigard, OR, CAT# GFP-1020). Additionally, sections were incubated with antibodies against GFAP (Thermo Fisher Scientific, Waltham, MA, CAT#PA110019), DCX (San Francisco, CA, Abcam, CAT#ab18723), or NeuN (Millipore, Billercia MA CAT#MAB377). Following primary antibody incubation at 4 °C overnight, sections were incubated with AlexaFluor secondary antibodies (goat-anti-chicken 488, CAT#A11039; goat-anti-rabbit 568, CAT#A11011; and goat-anti-mouse 568 CAT#AB175473).
4.7. Imaging and cell counting
All images were acquired on a Nikon D-ECLIPSE C1 Confocal Microscope, using the Nikon EZ-C1 3.91 software. Stereology was performed as previously described (Coggeshall and Lekan, 1996). Blinded cell counting was performed using either NIS Elements or ImageJ software. Positive identification of cells was based on both immunostaining and morphological characteristics, and only DAPI+ nucleated cell profiles were counted. The SVZ of the lateral ventricle was counted from bregma 0.50 mm through 1.08 mm, the origin of the rostral migratory stream was counted from bregma 1.5 mm through 2 mm, and both the SGZ of the hippocampus and the TL of the third ventricle were counted from bregma −1.58 mm through −2.16 mm. Four sections (both hemispheres) from each region, spanning a total of 480 μm longitudinally, were counted for each antibody. Average total positive cells were performed using triplicate mice for each region and antibody.
4.8. Statistical analysis
The number of mice required for analysis was calculated based on our previous results and power analyses using G*Power 3.1.7. An n = 3 was needed to provide statistical significance at p < 0.05 with a power of 0.80 for the histological data, and an n = 11 for behavioral analysis based on pilot studies. For morphological analyses, 2-way ANOVA (effects of treatment and sex) with a post hoc Tukey test assisted by GraphPad Prism v7 software were applied to analyze the effects of treatment on brain cells both individually and combined. A 2-way ANOVA was conducted to analyze morphological alterations in the olfactory bulb. Each group had three mice being used for each immunostaining, a p value less than 0.05 was considered statistically significant and a p value greater than 0.05 but less than 0.1 was considered a trend. Context discrimination and sucrose preference was analyzed using a 2-way repeated measures ANOVA (effects of treatment and sex) with a post hoc Tukey’s test.
Supplementary Material
HIGHLIGHTS.
Novel mouse model to study chronic co-administration of alcohol and cocaine.
Altered neural stem cell response across neurogenic areas in adult mouse brain.
Sex-dependent responses of neural stem cells to poly-drug co-use.
Hypothalamic neural stem cells affected by alcohol and cocaine co-exposure.
Acknowledgments
Thanks to Auston C. Grant and Javier Allende Labastida for input regarding this project. All in vivo rodent behavior was performed in the UTMB Rodent In Vivo Assessment Core, directed by Dr. Kelly Dineley, housed in the Center for Addiction Research, directed by Dr. Kathryn Cunningham.
Funding statement
This work was supported by the NIDA T32 Fellowship (Grant#: 5T32DA007287-17 and 3T32DA007287-18S1 to ELM), the Center for Addiction Research (KAC), and the John S. Dunn Research Foundation (PW).
Abbreviations:
- NSCs
neural stem cells
- SVZ
subventricular zone
- SGZ
subgranular zone
- TL
tanycyte layer
- Ctrl
control
- EtOH
ethanol
- Coc
cocaine
- Combo
combination
- YFP
yellow fluorescent protein
- GFP
green fluorescent protein
- GFAP
glial fibrillary acidic protein
- DCX
doublecortin
- LC-MS
liquid chromatography coupled to mass spectrometry
- BCE
benzoylecgonine
- CE
cocaethylene
- oRMS
orbital rostral migratory stream
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brainres.2019.146425.
References
- Althobaiti YS, Sari Y, 2016. Alcohol interactions with psychostimulants: an overview of animal and human studies. J. Addict. Res. Ther 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, McDonald RJ, 2000. Amygdala, hippocampus and discriminative fear conditioning to context. Behav. Brain Res 108, 1–19. [DOI] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, Sharma S, 2013. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat 9, 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benford H, Bolborea M, Pollatzek E, Lossow K, Hermans-Borgmeyer I, Liu B, Meyerhof W, Kasparov S, Dale N, 2017. A sweet taste receptor-dependent mechanism of glucosensing in hypothalamic tanycytes. Glia 65, 773–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Mathews S, Ki SH, Wang H, Gao B, 2013. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc 8, 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney EB, Appel SB, Brodie MS, 2001. Electrophysiological effects of cocaethylene, cocaine, and ethanol on dopaminergic neurons of the ventral tegmental area. J. Pharmacol. Exp. Ther 297, 696–703. [PubMed] [Google Scholar]
- Cittadini F, De Giovanni N, Alcalde M, Partemi S, Carbone A, Campuzano O,Brugada R, Oliva A, 2015. Genetic and toxicologic investigation of Sudden Cardiac Death in a patient with Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) under cocaine and alcohol effects. Int. J. Legal Med 129, 89–96. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA, 1996. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J. Comp. Neurol 364, 6–15. [DOI] [PubMed] [Google Scholar]
- Cortez I, Bulavin DV, Wu P, McGrath EL, Cunningham KA, Wakamiya M, Papaconstantinou J, Dineley KT, 2017. Aged dominant negative p38α MAPK mice are resistant to age-dependent decline in adult-neurogenesis and context discrimination fear conditioning. Behav. Brain Res 322, 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, 2008. Alcohol-related neurodegeneration and recovery: mechanisms from animal models. Alcohol Res. Health 31, 377–388. [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, 2003. Alcohol, neural stem cells, and adult neurogenesis. Alcohol Res. Health 27, 197–204. [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Sengupta P, 2016. Men and mice: relating their ages. Life Sci. 152, 244–248. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, MacGregor RR, Wang GJ, Wolf AP, 1992. Alcohol intoxication does not change [11C]cocaine pharmacokinetics in human brain and heart. Synapse 12, 228–235. [DOI] [PubMed] [Google Scholar]
- Garlow SJ, Purselle DC, Heninger M, 2007. Cocaine and alcohol use preceding suicide in African American and white adolescents. J. Psychiatr. Res 41, 530–536. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND, 2004. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia 42, 1447–1458. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Grose AM, 2003. Blood alcohol concentrations after scheduled access in high-alcohol-preferring mice. Alcohol 31, 99–104. [DOI] [PubMed] [Google Scholar]
- Graziani M, Nencini P, Nisticò R, 2014. Genders and the concurrent use of cocaine and alcohol: pharmacological aspects. Pharmacol. Res 87, 60–70. [DOI] [PubMed] [Google Scholar]
- Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El Agha E, Bellusci S, Hajihosseini MK, 2013. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J. Neurosci 33, 6170–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R, 2008. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci 11, 1153–1161. [DOI] [PubMed] [Google Scholar]
- Jones JD, Busse GD, Riley AL, 2006. Strain-dependent sex differences in the effects of alcohol on cocaine-induced taste aversions. Pharmacol. Biochem. Behav 83, 554–560. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH, 2015. Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol 7, a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, DiLeone RJ, Greer CA, Mandyam CD, Eisch AJ, 2007. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J. Neurosci 27, 12623–12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazutkaite G, Soldà A, Lossow K, Meyerhof W, Dale N, 2017. Amino acid sensing in hypothalamic tanycytes via umami taste receptors. Mol. Metab 6, 1480–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, Aja S, Ford E, Fishell G, Blackshaw S, 2012. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci 15, 700–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A, 2014. Adult neural stem cells stake their ground. Trends Neurosci. 37, 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyne J, O’Donoghue B, Clancy M, Kinsella A, O’Gara C, 2010. Concurrent cocaine and alcohol use in individuals presenting to an addiction treatment program. Ir. J. Med. Sci 179, 233–237. [DOI] [PubMed] [Google Scholar]
- Magura S, Rosenblum A, 2000. Modulating effect of alcohol use on cocaine use. Addict. Behav 25, 117–122. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Koob GF, 2012. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 35, 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath EL, Gao J, Kuo YF, Dunn TJ, Ray MJ, Dineley KT, Cunningham KA, Kaphalia BS, Wu P, 2017. Spatial and sex-dependent responses of adult endogenous neural stem cells to alcohol consumption. Stem Cell Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengis MM, Maude-Griffin PM, Delucchi K, Hall SM, 2002. Alcohol use affects the outcome of treatment for cocaine abuse. Am. J. Addict 11, 219–227. [DOI] [PubMed] [Google Scholar]
- Midanik LT, Tam TW, Weisner C, 2007. Concurrent and simultaneous drug and alcohol use: results of the 2000 National Alcohol Survey. Drug Alcohol Depend. 90, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary ME, 2002. Inhibition of HERG potassium channels by cocaethylene: a metabolite of cocaine and ethanol. Cardiovasc. Res 53, 59–67. [DOI] [PubMed] [Google Scholar]
- Ponti G, Farinetti A, Marraudino M, Panzica G, Gotti S, 2018. Sex steroids and adult neurogenesis in the ventricular-subventricular zone. Front. Endocrinol. (Lausanne) 9, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, Kokoeva MV, Placzek M, 2013. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun 4, 2049. [DOI] [PubMed] [Google Scholar]
- Salloum IM, Douaihy A, Ndimbie OK, Kirisci L, 2004. Concurrent alcohol and cocaine dependence impact on physical health among psychiatric patients. J. Addict. Dis 23, 71–81. [DOI] [PubMed] [Google Scholar]
- Salloum IM, Daley DC, Cornelius JR, Kirisci L, Thase ME, 1996. Disproportionate lethality in psychiatric patients with concurrent alcohol and cocaine abuse. Am. J. Psychiatry 153, 953–955. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Bordnick PS, Kearney ML, Fuller SM, Breckenridge JK, 1997. Treatment outcome of cocaine-alcohol dependent patients. Drug Alcohol Depend. 47, 55–61. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW, 2003. Mapping cortical change across the human life span. Nat. Neurosci 6, 309–315. [DOI] [PubMed] [Google Scholar]
- Velasquez MM, von Sternberg K, Mullen PD, Carbonari JP, Kan LY, 2007. Psychiatric distress in incarcerated women with recent cocaine and alcohol abuse. Womens Health Issues 17, 264–272. [DOI] [PubMed] [Google Scholar]
- Xu C, Loh HH, Law PY, 2016. Effects of addictive drugs on adult neural stem/progenitor cells. Cell. Mol. Life Sci 73, 327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM, 2008. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature 451, 1004–1007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


