Abstract
Central nervous system (CNS) insults elevate endogenous toxins and alter levels of indicators of metabolic disorder. These contribute to neurotrauma, neurodegenerative diseases and chronic pain and are possible targets for pharmaceutical treatment. Microdialysis samples substances in the extracellular space for chemical analysis. It has demonstrated that toxic levels of glutamate are released and that toxic levels of the reactive species , H2O2, HO· NO and HOONO are generated upon CNS injury. Agent administration by microdialysis can also help elucidate mechanisms of damage and protection, and to identify targets for clinical application. Microdialysis sampling indicates that circuits descending from the brain to the spinal cord transmit and modulate pain signals by releasing neurotransmitter amines and amino acids. Efforts are under way to develop microdialysis into a technique for intensive care monitoring and predicting outcomes of brain insults. Finally, microdialysis sampling has demonstrated in vivo elevation of glial cell line-derived neurotrophic factor following grafting of primed fetal human neural stem cells into brain-injured rats, the first in vivo demonstration of the release of a neurotrophic factor by grafted stem cells. This increased release correlated with significantly improved spatial learning and memory.
Keywords: Clinical application, Descending control, Excitotoxicity, Glutamate, Hydrogen peroxide, Hydroxyl radical, Microdialysis, Pain, Spinal cord injury, Stem cells, Superoxide, Traumatic brain injury
1. Introduction
Release of neurochemical messengers is central to intercellular signaling, but excess release of such agents also contributes substantially to neurological disorders. This secondary damage to the central nervous system (CNS) contributes to functional impairments, chronic pain and cell death; to ameliorate these outcomes, it is important to identify the agents of such damage. Characterizing their release is necessary to defining the roles of CNS messengers in disease as well as in health. We here review studies of the in vivo release of neurotoxic agents and the damage they cause. In vivo release is characterized by microdialysis (Ungerstedt, 1984; Benveniste, 1989; McAdoo et al., 1997), which samples dissolved molecules that diffuse across a semipermeable membrane into a probe with fluid flowing through its lumen. The dialysis zone of the probe is placed to sample substances released in the site of interest. Probes can either be parallel vertical inlet and outlet tubes connected to the dialysis membrane or porous linear fibers coated except at a short dialysis zone. Analysis of fluid collected from the probe outlet provides information regarding changes in the chemical composition of the extracellular milieu in the site of sampling. Microdialysis can also be used to administer candidate damaging and/or pharmacological agents into pertinent areas to characterize their actions. We will describe release of endogenous damaging substances by CNS trauma and the consequences revealed by microdialysis and related sampling methods in animal models (Ungerstedt, 1984; Benveniste, 1989; Liu and McAdoo, 1993a) and in the clinic (Tisdall and Smith, 2006; Hillered et al., 2005, 2006), during the transmission of pain signals (Sorkin et al., 1988; Willis, 1982; Malmberg and Yaksh, 1995) and by stem cells transplanted into injured rat brains (Gao et al., 2006). We will also describe experimental approaches to elucidating the mechanisms of secondary damage in CNS insults. Applications to both animal models and humans will be presented to convey the range of the application of microdialysis to investigating CNS insults.
CNS traumas are accompanied by the release of high concentrations of neurotransmitters and other substances. Some of these, particularly elevated concentrations of the excitatory amino acids (EAAs) glutamate and aspartate and free radicals, are toxic at the levels of their release, thereby causing secondary damage. Others reflect degrees of metabolic derangements. To establish that a substance released in response to an insult produces secondary damage, it must be demonstrated that the substance is released and is damaging at the levels released following insult (Liu and McAdoo, 1995). Microdialysis coupled with chemical analysis can provide such data (Liu, 1993; Liu et al., 1999c, 2004; Liu and McAdoo, 1993b; Xu et al., 2004, 2005). A model combining microdialysis, neurochemistry, histology and electrophysiology has been developed for characterizing the roles of suspected damaging agents (Liu and McAdoo, 1993a). It provides estimates of the concentration of substances released and establishes the consequences of applying the substance at that level. Much of this review describes applications based on this model.
Two unique characteristics, self-renewal and multipotential differentiation, render stem cells an attractive and presumably unlimited source of cells to treat neurological disorders, including CNS traumas. Stem cell-mediated functional improvements in various models of neurotrauma and neurodegeneration are attributed to functioning of neural cells differentiated from stem cells or release of neurotrophic factors by grafted cells. Here we include a description of the use of microdialysis to detect the release of trophic factors and neurotransmitters from grafted stem cells or their differentiated progeny. These agents may enhance recovery from CNS trauma.
2. Microdialysis sampling and calibration
As stated in the Introduction, microdialysis involves sampling of substances dissolved in the extracellular fluid by perfusing a fluid across a semipermeable membrane in a probe inserted into the area of interest. Concentrations obtained by microdialysis sampling both in vitro and in vivo are substantially lower than those in the sampled fluid, necessitating probe calibration to determine levels released. The ratio of the concentration in the samples collected from the probe to the unperturbed concentration outside the probe is termed the relative recovery. The trans-membrane concentration ratio depends on the flow rate through the probe, the area of the dialysis membrane, the temperature, the molecular weight of the solute and the tortuosity of the surrounding fluid (Benveniste, 1989). The inside/outside ratio is generally below 1.0 as the result of the slow rate of diffusion across the cell membrane and drainage of solute molecules from the extracellular fluid by diffusion into and removal by the flowing dialysis fluid. This significantly lowers the concentrations of sampled substances over substantial distances from the dialysis membrane (Jacobson et al., 1985; Lindefors et al., 1989; Sorkin et al., 1988; Benveniste, 1989; Bungay et al., 1990; Xu et al., 2004).
To establish that a substance contributes to a disorder requires demonstrating that the substance is present at a concentration that produces an appropriate effect in response to an insult of interest (Liu and McAdoo,1995). To accomplish this by microdialysis, it is necessary to determine the concentration released and to establish that the agent has the appropriate effects when that concentration is administered by microdialysis; this in turn requires calibrating the gradients across the fiber membrane during both sampling and administration. Microdialysis can be used to determine the concentration of a substance released and then to determine the effects of administering that substance into the site of interest at the concentration released.
Several methods of relating the concentrations of substances sampled in the fluid surrounding the probe to the concentrations in the collected fluid, i.e., calibrating the probe, have been developed: 1) estimating the recovery at zero flow rate by taking samples at a series of flow rates and extrapolating the concentrations in those samples to the concentration at zero flow. At zero flow, the concentration in the perfusion fluid becomes equal to the concentration in the fluid outside the probe (Ståhle et al., 1991; Menacherry et al., 1992; Parsons and Justice, 1992). 2) Dialysis at a very low flow rate at which the concentration inside and that outside approach each other can also be used to estimate the concentrations surrounding the probe (Liu et al., 1991; Menacherry et al., 1992). 3) Inserting a parallel collecting probe to determine the concentration generated through an administering probe to relate the concentrations inside and outside the administering probe (Liu and McAdoo, 1993a; Liu et al., 1999c; Xu et al., 2004). 4) Passing a series of concentrations of a substance through the dialysis probe and measuring the concentrations in the recovered fluid. When the concentration perfused through the probe is less than that outside of the probe, the substance will enter the probe and the concentration in the collected fluid will be higher than that in the fluid surrounding the probe. Conversely, when the concentration inside the probe is greater than that outside, the concentration in the collected fluid will be lower than that pumped into the probe. When the concentrations inside and outside the probe are equal, there will be no net flux in either direction, i.e., the concentration in the tissue surrounding the probe equals the concentration in the fluid being pumped through the probe. The differences between the concentration in the recovered fluid and the concentration in the fluid pumped into the probe are plotted to locate the no net flux point, that is, the concentration around the probe (Hamberger et al., 1988; Ståhle et al., 1991; Parsons and Justice, 1992).
The ability of microdialysis to administer known concentrations of substances into tissue can be used to administer a substance in vivo to simulate release in response to a trauma (Liu et al., 1991, 1999c; Xu et al., 2004). In vivo calibration (see following) of the outside/inside concentration ratio for the collecting fiber and the inside/outside ratio for the administering fiber enables determination of the concentration produced over time by release in vivo in the site of injury, and in turn to mimic that release by administration by microdialysis. This combination has been used to establish the role of glutamate (Liu et al., 1991, 1999c; Xu et al., 2004, 2005) and of reactive species (Liu and McAdoo, 1993a; Liu et al., 1994, 1998) in spinal cord injury (SCI).
In experiments performed to determine whether concentrations of glutamate released following SCI are damaging, collecting fibers were calibrated to estimate concentrations of glutamate released into the spinal cord by 1) determining the ratio of the concentration inside the fiber to the concentration outside the fiber by the no net flux method in the normal cord, and 2) by extrapolating the recovery to zero flow. The concentrations attained in microdialysis samples collected during injury are multiplied by the outside/inside ratio to estimate the glutamate concentrations present in the extracellular space over the course of the injury.
The glutamate concentrations to be applied through the administering probe to mimic those released following SCI can be estimated by gluing an administering fiber and a sampling fiber together side by side such that a portion of the glutamate administered through the first is recovered in the second. The two fibers are prepared and placed such that their dialysis zones are of equal length and aligned with each other. Given that the permeability of the dialysis material is the same for the two fibers, the probability of a given molecule leaving one probe is equal to that of it entering the other, if the two dialysis zones have the same length (area). Under these circumstances, the inside:outside ratio of the collecting fiber will equal the outside:inside ratio for the administering fiber,
where GA is the concentration in the administering fiber, GB is the concentration recovered in the collecting fiber, G is the concentration in the space between the fibers and GA/G and G/GB are the respective and equal outside/inside and inside/outside concentration ratios for the two fibers. This ratio, in combination with the concentration released upon injury is used to establish the concentration to administer to imitate release upon injury. We have found eight-fold concentration ratios (outside:inside) across microdialysis fiber walls at a flow rate of 2 μl/min across a 2 mm dialysis zone (Liu et al., 1999c; Xu et al., 2004).
Based on the preceding calibrations, administration of 4 mM glutamate through a microdialysis fiber gave an estimated glutamate concentration in the white matter outside the fiber of 500 μM (Xu et al., 2004), the concentration released upon SCI at an average distance slightly greater than 110 μM from the administering probe, i.e., overlapping the location of the collecting fiber (0–220 μ). Cell counts were made at 100–300 μm from the fiber track to determine the densities (neurons/unit area) of surviving neurons or oligodendrocytes. The density of neurons was decreased by about 75% at this distance in spinal cord tissue taken 24 h after exposure to glutamate (Liu et al.,1999c). Similarly administering 4.0 mM into a dialysis probe placed in the white matter gave a 57% reduction in oligodendrocyte densities (Xu et al., 2004). Therefore glutamate release following SCI contributes substantially to the damage caused by SCI.
3. Microdialysis and the role of glutamate in spinal cord injury
3.1. A model for characterizing spinal cord injury
SCI has been studied by an assortment of experimental models, differing chiefly in the way in which the cord is injured. In all models, the animal is anesthetized and the cord exposed. In the most common approach, the exposed cord is injured by being struck by a piston dropped from a known height, usually while recording impact parameters (Gruner, 1992), or driven by a motor that provides a preset force and duration of contact (Scheff et al., 2003). The animal may then be maintained under anesthesia for physiological measurements, microdialysis sampling and/or treatment with an agent, or allowed to awaken for assessment of function and/or later tissue sampling.
In studies of SCI, a microdialysis probe is constructed from a dialysis fiber (Lindefors et al., 1989, Skilling et al., 1988; Sorkin et al., 1988). In our lab, these fibers (18 kD molecular weight cutoff; Spectrum Industries) are coated with a thin layer of silicon rubber (Dow Chemical 3140 RTN) except for a 0.5–2 mm dialysis zone. Coated fibers have maximum diameters of 210–230 μm. A small dissecting pin is glued into the lumen at one end of the fiber. The fiber is placed in the cord by inserting the pin into one side of the exposed cord and pushing the pin and the trailing fiber through the cord. The pin is then cut off, the dialysis zone is placed at the desired location in the cord and pumping fluid through the fiber at a desired flow rate is begun. The cord surface is impacted directly above the dialysis zone (Fig. 1).
Fig. 1.
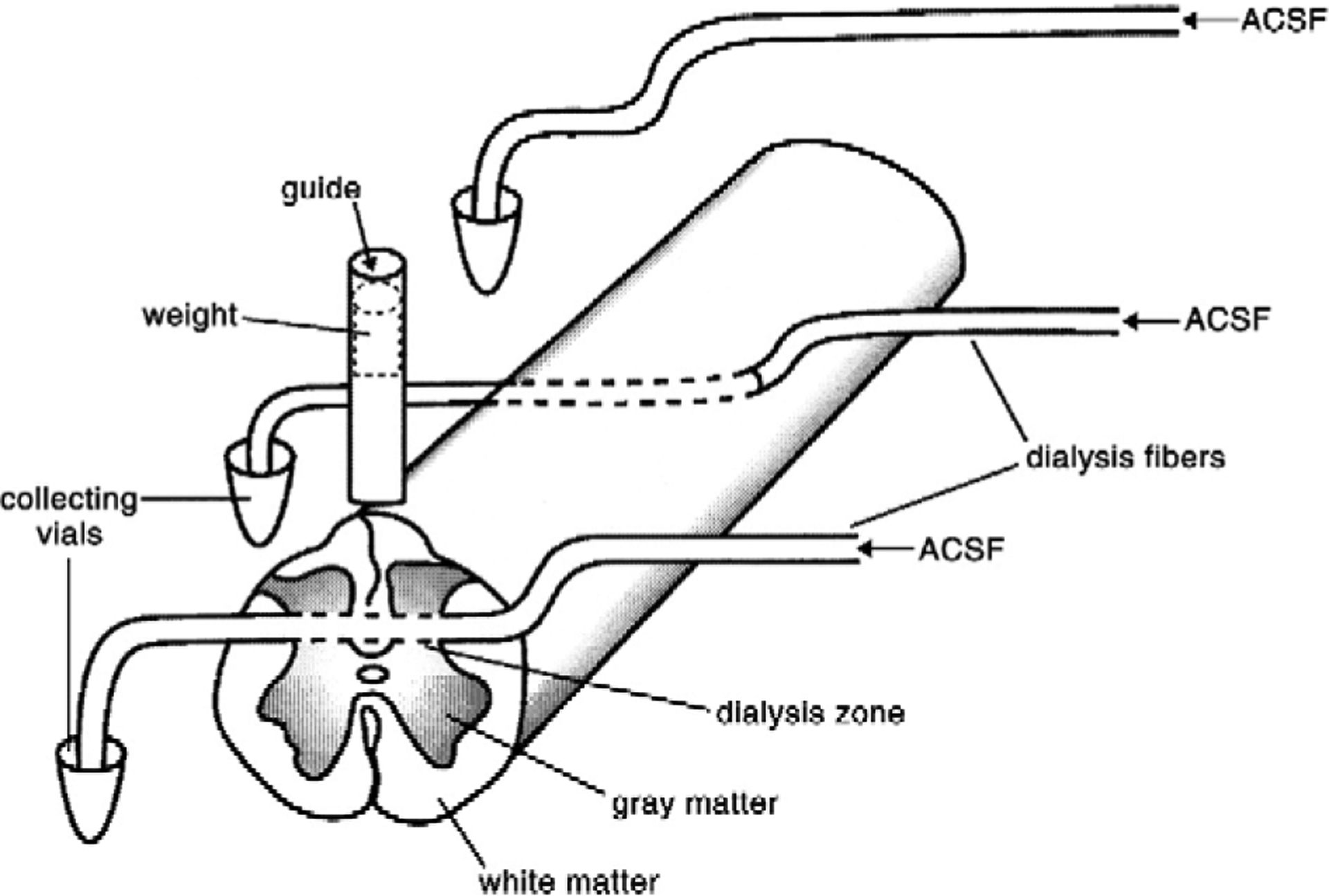
Setup for sampling the production of HO· following spinal cord injury. ACSF containing salicylic acid was perfused through a fiber with its dialysis zone in the site of injury to collect dihydroxybenzoic acids to provide a measure of hydroxyl radical formation. A second microdialysis fiber was passed through the cord 2 cm rostral to the site of injury to provide the basal level of HO· formation. A third fiber was passed directly from the syringe pump supplying the ACSF/DHBA and collecting vials to assess background HO· formation (From de Castro et al., 2004).
3.2. Toxicity of glutamate to neurons
Starting in 1969, Olney and coworkers demonstrated that acidic amino acids, then known to excite neurons, are neurotoxic (Olney,1969; Olney et al; 1971). Choi et al. (1987) subsequently showed that moderate concentrations of glutamate are lethal to cultured cortical neurons. Glutamatergic agonists are generally neurotoxic (Choi et al.,1987; Faden and Simon, 1988; Yezierski et al., 1993). Additionally, a variety of glutamate receptor blockers protect neurons from glutamate toxicity (Choi,1988; Faden and Simon, 1988; Wrathall et al., 1994, 1996; Agrawal and Fehlings, 1997; Liu et al., 1997). Microdialysis experiments demonstrate that EAA concentrations increase manyfold following SCI (Panter et al., 1990; Liu et al., 1991, 1999c; McAdoo et al., 1999) and traumatic brain injury (Faden et al., 1989; Bullock et al., 1998).
Time courses of glutamate release in the SCI epicenter and at 3 mm away are shown in Fig. 2. This figure shows that in the contusion injury model glutamate release increases and then decreases rapidly at the epicenter and returns to near baseline within 20 min at 3 mm from the epicenter. Thus released glutamate itself does not cause damage very far from the site of impact in a contusion model.
Fig. 2.
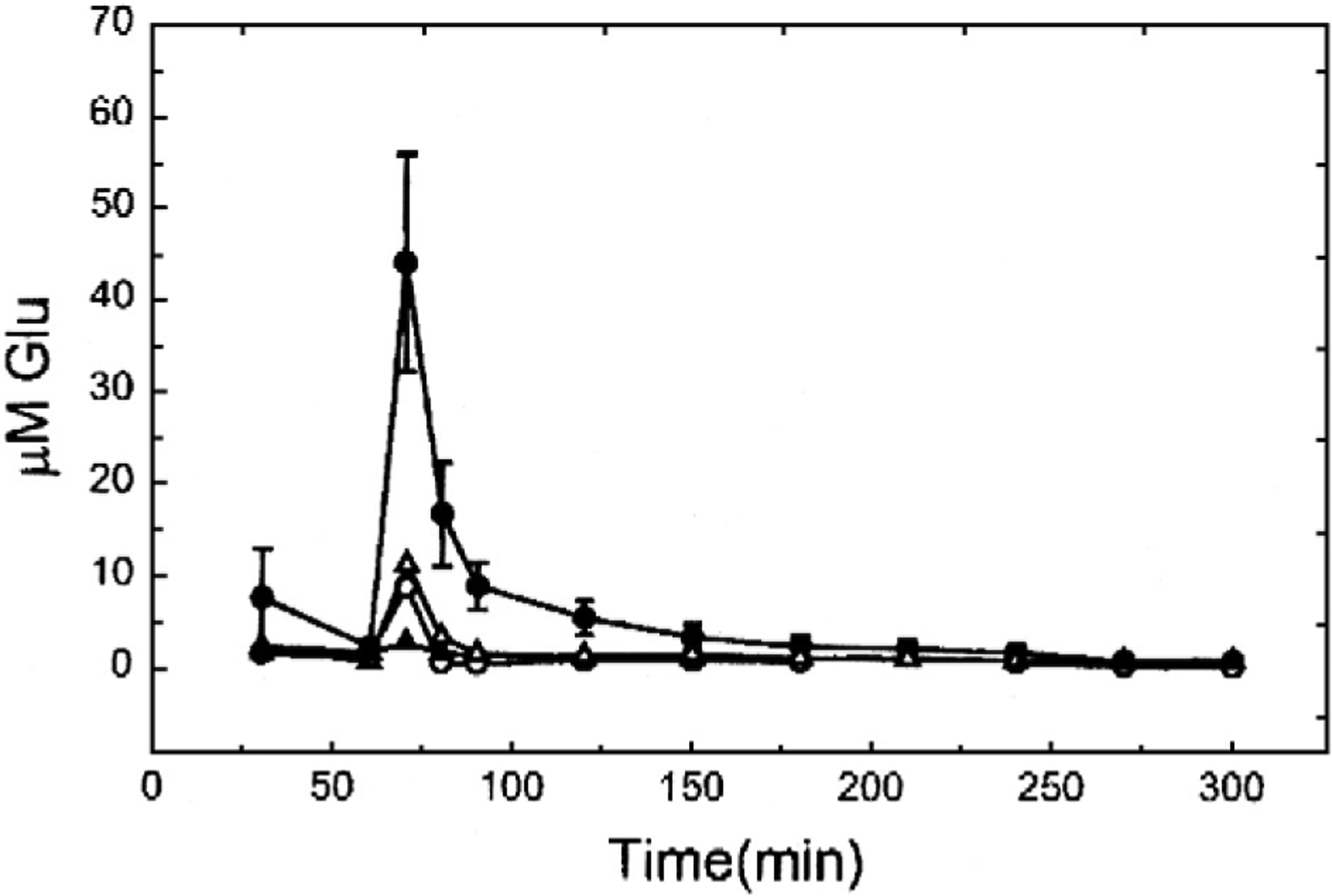
Glutamate concentrations in samples from a microdialysis fiber through the center of a contusion injury (tall peak, average from 3 animals) and simultaneously through another fiber at a point 3 mm away (traces with small peaks, individual animals) illustrating the rapid fall off of the concentration with distance from the site of injury (From McAdoo et al., 1999).
In vivo toxicity of glutamate was hard to demonstrate in early experiments, presumably due to efficient uptake of administered glutamate (Mangano and Schwarcz, 1983). However, use of microdialysis to administer glutamate at concentrations and for times simulating glutamate release following SCI demonstrated that the level of glutamate released following SCI is toxic to neurons (Liu et al., 1991, 1999c).
3.3. Toxicity of glutamate to oligodendrocytes
Oligodendrocytes as well as neurons are important in signal transmission because the myelin sheath they form around axons speeds axonal conduction. Antagonists of glutamate receptors reduce destruction of oligodendrocytes by SCI (Rosenberg et al., 1999; Grossman et al., 2001) and glutamate (Xu et al., 2004). These agents also reduce the loss of white matter (Wrathall et al., 1994) and improve recovery from functional deficits caused by SCI (Wrathall et al., 1992, 1994, 1996). Therefore, the release of glutamate in the white matter of the spinal cord and its effects on oligodendrocytes were investigated (Xu et al., 2004). In those experiments, a 0.5 mm dialysis zone (the width of the white matter) was placed in the white matter. Calibration as described above estimated the maximum glutamate concentration reached in the white matter post-SCI to be 550±80 μM, within experimental error of the concentration reached in the white matter following SCI (Xu et al., 2004). Administration of ACSF, 4.0 mM glutamate or 10 mM glutamate through the fiber reduced oligodendrocyte density by 22%, 57% and 74% respectively at 24 h post-injury (Xu et al., 2004), so damage to oligodendrocytes as well as to neurons by glutamate released following SCI likely contributes to the crippling caused by SCI. Also, the reduction of oligodendrocyte density by ACSF administration demonstrates that the fiber itself causes some damage. Administration of the AMPA glutamate receptor antagonist NBQX reduced glutamate-induced oligodendrocyte death, demonstrating the involvement of AMPA receptors in the glutamate-produced loss of oligodendrocytes (Xu et al., 2004). Loss of oligodendrocytes was largely finished by 24 h after exposure to glutamate (Xu et al., 2004; Xu et al., in press).
3.4. Effect of glutamate on spinal cord function
Administering the concentration of glutamate released upon SCI into the spinal cord by microdialysis produces functional impairments (Xu et al., 2005) in addition to cell death (Liu et al., 1999c; Xu et al., 2004), further demonstrating its toxicity.
The effects on locomotor function of glutamate administration by microdialysis were assessed (Fig. 3) utilizing the Basso–Beattie–Bresnahan rating scale (Basso et al., 1995) and the Photobeam Activity System (PAS; San Diego Instruments, Inc, San Diego, CA) (Mills et al., 2001; Xu et al., 2005). The BBB assessment is standard in SCI (Basso et al., 1995); in it hindlimb positions and movements are evaluated to rate the degree of impairment on a 0–21 point scale, 21 being normal (Fig. 3).
Fig. 3.
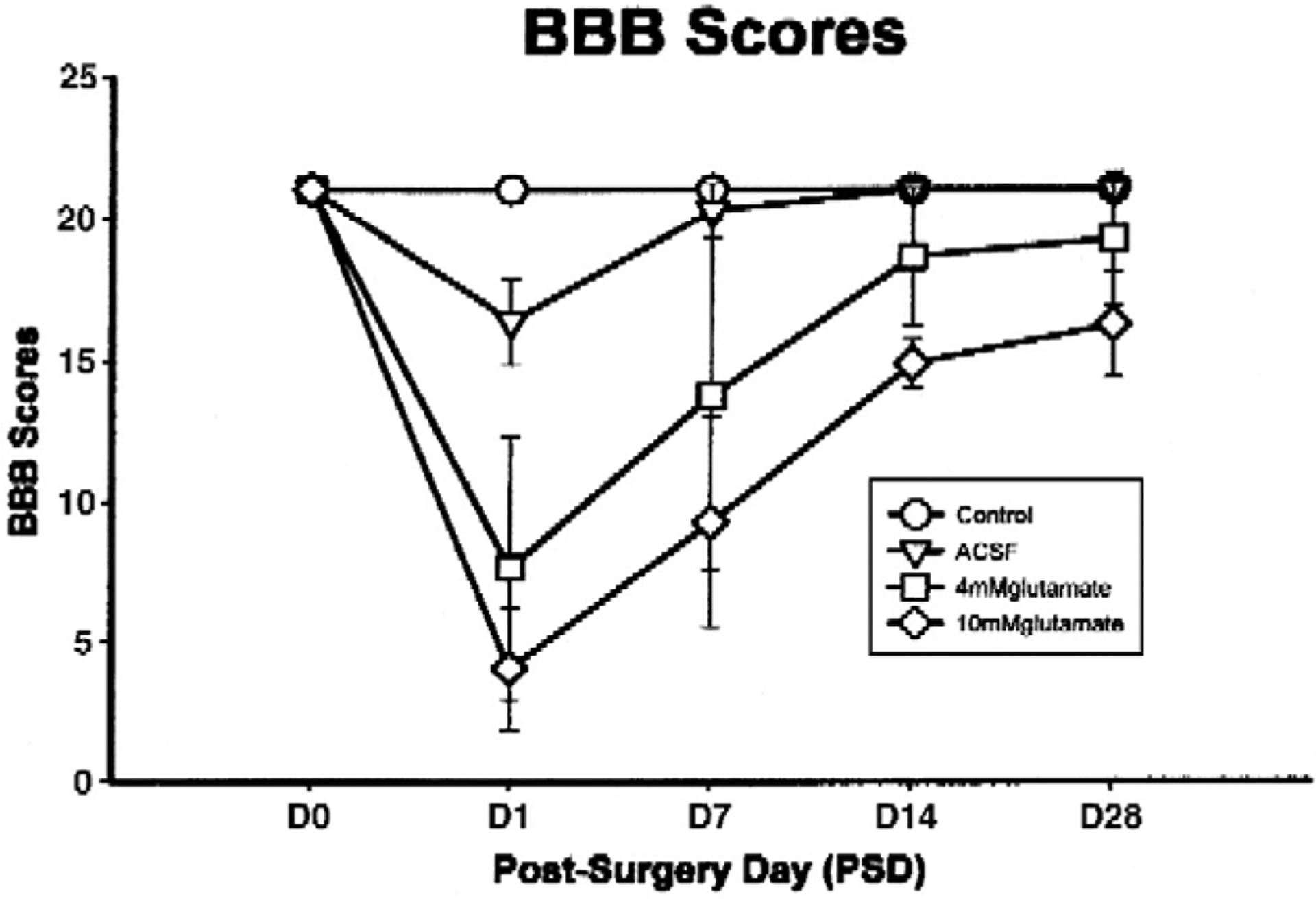
Basso–Beattie–Bresnahan scores from normal rats, rats receiving ACSF, 4 mM glutamate or 10 mm glutamate administered into their spinal cords (concentrations are in the fiber lumen). This illustrates the ability to determine the effects of agents of interest by administering them into target tissue by microdialysis (From Xu et al., 2005).
The PAS evaluates movement in the X, Y and Z directions. X and Y movements are recorded based on breaks in laser beams directed in the X and Y direction at 4 cm above the floor, and movement in the Z direction is recorded from breaking of horizontal beams 12 cm above the floor. The PAS software transforms the beam-break records into 6 parameters: distance traveled, rearing events, rearing time, beam breaks, active time and resting time. Administration of ACSF, 4 mM glutamate and 10 mM glutamate inside the microdialysis fiber reduced distance traveled by rats in the order listed relative to normal controls at two days post-surgery, but by 28 days all of these parameters except the effects on them of 10 mM glutamate were nearly back to normal (Xu et al., 2005). However, impairment due to the 10 mM glutamate treatment was quite substantial, even at the termination of the experiment at 28 days. Numbers of rearing events and rearing time were similarly affected. The effect of the treatments on active time was less substantial, but the effects of 10 mM glutamate inside the fiber on active time were significant at nearly all times relative to the other treatments. Thus the levels of glutamate released upon SCI can cause both cell death and long-term loss of function.
3.5. Mechanisms of glutamate release in neurotrauma
Microdialysis can yield information on the mechanisms of glutamate release following SCI. SCI probably raises glutamate levels by a combination of simple rupture of cells, influx from the circulation (McAdoo et al., 1997, 2005; Xu et al., 1998), synaptic release elicited by neuronal firing, and blockade and reversal of transport systems (McAdoo et al., 2000). The last is probably caused by extreme depolarization of cell membranes following SCI (Eidelberg et al., 1975; Young et al., 1982). The reversal of transport was assessed by determining the effects of dihydrokainate (DHK), a blocker of the GLT1 glutamate transporter, on the elevation of glutamate following SCI (McAdoo et al., 2000). DHK administration reduced glutamate release, a characteristic of reversed glutamate transport, so that observation supports release by reversed transport. Whether transport reversal releases amino acid neurotransmitters normally is controversial because to achieve sufficient membrane depolarization would require abnormally high levels of stimulation (Timmerman and Westerink, 1997). Under normal circumstances, blocking glutamate reuptake increases glutamate concentrations sampled by microdialysis, as would be expected from reduced removal (Timmerman and Westerink, 1997).
It has been hypothesized that the excitation and damage caused by the initial glutamate release following trauma produces further glutamate release followed by further damage (Doble, 1995; Liu et al., 1997). Operation of the following feedback cascade involving activation of glutamate receptors: trauma→glutamate release→secondary damage→glutamate release→etc. has been hypothesized to produce further release of glutamate and secondary damage. Therefore, operation of this cascade was investigated by determining whether administering glutamate receptor antagonists and Na+-channel blockers by microdialysis following SCI to interrupt this cascade reduced the post-SCI release of glutamate (McAdoo et al., 2005). The effects of administering the NMDA glutamate receptor blockers MK-801 and memantine, the AMPA/kainate glutamate receptor blockers NBQX and GYKI 52466, the AMPA receptor desensitization blocker cyclothiazide and the Na+-channel blockers riluzole, mexiletine and QX-314 on post-SCI glutamate release were characterized. None of the agents detectably affected glutamate release following SCI. Glutamate receptor and Na+-channel blockers should have reduced glutamate release if the hypothesized cascade involves synaptic release of glutamate. Thus, consistent with the conclusion that microdialysis does not sample synaptically released glutamate (Obrenovitch and Urenjack, 1997; Timmerman and Westerink, 1997), these experiments provide no evidence for synaptic release of glutamate following SCI. However, since glutamate administered at the level released by SCI is toxic (Liu et al., 1991, 1999c), it must reach targets, most likely receptors in synapses, that mediate its toxicity. Administering the glutamate transport blocker DHK indicated that about 34% of SCI-induced glutamate release occurs by transport reversal (see above, McAdoo et al., 2000). Other probable contributors to glutamate release are mechanical damage to cells in the impact area and leakage from the circulation through a compromised blood-brain barrier (McAdoo et al., 1997, Xu et al., 1998).
4. Highly reactive species and spinal cord injury
4.1. Reactive species
Highly reactive oxygen and nitrogen species are additional candidates to contribute to secondary damage following neurotraumas and in neurodegenerative diseases (Halliwell, 1992), offering possible further targets for intervention in those disorders. Reactive species include the hydroxyl radical (HO·), hydrogen peroxide (H2O2), superoxide (O2·−) and peroxynitrite (HOONO). Among these species, HO· is the most reactive and most damaging. A brief summary of the reactions of these species is:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
Given the hypothesized importance of reactive species in many CNS disorders, measurements of their levels and assessments of the damage caused at those levels are needed. Microdialysis combined with reactions with trapping agents can meet the first need, and generating the reactive species by reactants perfused into the CNS allows meeting the second, as will be described in the following sections.
4.2. Hydroxyl radical (HO·)
HO· can be detected by passing a trapping agent, e.g., salicylic acid (Chiueh et al., 1992) or phenylalanine (Liu, 1993), through a microdialysis probe with its dialysis zone placed in the site of interest. As the trapping agent passes through the dialysis zone, it diffuses out of the probe, reacts as follows with HO· outside the probe to form 2,3-and 2,5-dihydroxybenzoic acids (DHBAs), which then diffuse back into the probe. Fluid passing through the lumen of the probe is collected and analyzed for the reaction products, typically by high performance liquid chromatography (HPLC) with electrochemical detection (Chiueh et al., 1992; Liu, 1993).
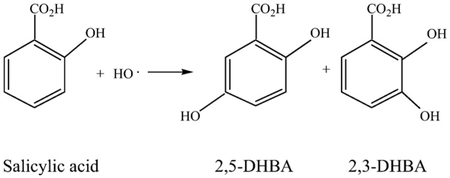
The use of salicylic acid as a trapping agent (see preceding) has demonstrated that SCI produces HO· (Liu et al., 2004). Administering H2O2 and FeCl2/EDTA into the spinal cord through separate fibers to generate HO· (Reaction 3 above) revealed that HO· can produce substantial damage (Liu et al., 1994). The time course of HO· production following SCI was established by implanting a perfusion cannula formed into a loop into the intrathecal space of the cord, pumping salicylic acid through the loop and measuring the levels of 2,3 and 2,5 DHBA in the effluent from the loop (Liu et al., 2004). This loop was prepared by making holes in the wall of a 26 cm catheter at 0.5 cm intervals to perfuse salicylic acid into the intrathecal space. A wire was first inserted longitudinally through the lumen of the catheter. The ends of the catheter were attached to a push–pull pump via inflow and outflow lines and the catheter was bent into a loop. This loop was inserted through the cisternal opening on the dorsal cervix down to the lumbar level of the spinal cord in the intrathecal space (Liu et al., 2004; Marsala et al., 1995). ACSF was push-pulled through the loop for 1 h to allow baselines to stabilize followed by perfusing the loop with 50 mM salicylate in ACSF. The cord was then injured by dropping a weight down a guide tube onto the cord. Perfusion through the loop was continued until 5 min, 1, 3 or 6 h post-injury. After sampling, the cord was frozen in situ with liquid nitrogen to prevent oxidative damage when the tissue was removed. The pieces of cord were homogenized, centrifuged and filtered, followed by analysis of 2,3- and 2,5-DHBA to characterize the generation of HO· following spinal cord injury. 2,3-DHBA was significantly elevated in the spinal cord only from 5 min to 3 h after SCI (Liu et al., 2004).
To explore the role of HO· in SCI, the effects of generating HO· inside the spinal cord were assessed by placing the loop together with a microdialysis fiber in the gray matter of the spinal cord. H2O2 was perfused through the loop and FeCl2 (10 μM)/EDTA (25 μM) was administered through the microdialysis fiber in Ringer’s solution such that the two agents mixed to generate HO·. Administering 5 mM H2O2 through the cannula gave a H2O2 concentration in the fiber estimated to be comparable to that estimated following SCI by analyzing DHBAs. After administering H2O2 and FeCl2/EDTA for 4 h, the incision was closed, the animal allowed to wake up and finally it was reanesthetized at 24 h after exposure to HO·. At that time, the animal was fixed by transcardial perfusion with 0.9% saline followed by 4% paraformaldehyde in ACSF. The cord segment centered on the dialysis zone was then removed, prepared and examined histologically. Cell losses determined by counting cells near the fiber tracks in stained sections were significant in tissue taken 24 h later. The detection of HOP production and cell death following its generation is strong evidence for involvement of HO· in the secondary damage that contributes to the damage in SCI.
Catalytic activity attributed to iron was elevated for less than 30 min after SCI in samples collected with the cannula described above (Liu et al., 2003), and HOP was elevated for only 3 h following SCI (Liu et al., 2004). In contrast, (Liu et al., 1998) and H2O2 (Liu et al., 1999a) were elevated for ca. 10 h post-SCI. Taken together, the time courses do not support the belief that reduction of Fe3+ to Fe2+ by and H2O2 (Liu et al., 2004) is important in SCI. In addition, separate microdialysis sampling failed to detect an increase in low molecular weight forms of iron following SCI (de Castro et al., 1999), further evidence that little iron is released following SCI. What discrepancy there is among the results for iron ions may be due to the cannula collecting bound, high molecular weight forms of iron versus microdialysis collecting only low molecular weight species (cutoff, 18 kDa). Large molecules can be sampled with a cannula that exchanges solutes through a hole(s) in its wall much larger in diameter than the pores in a dialysis membrane (Yan et al., 2006), similar to the cannulas used as described here to administer agents for generating reactive species.
4.3. Superoxide
Superoxide can be analyzed by monitoring the reduction of cytochrome-c (an adaptation of a widely used assay for (Leslie and Allen, 1987). To accomplish this, oxidized cytochrome-c dissolved in ACSF is perfused through a cannula with holes in its wall placed in the site of interest (Liu et al., 1998; see above). A perforated cannula is used to maximize passage of cytochrome-c out of and into the cannula because the amount of cytochrome-c entering a microdialysis fiber may be reduced by adsorption of the peptide to the walls of the probe (Maidment et al., 1991). Electron transfer from reduces cytochrome-c, in the process shifting the spectrophotometric absorbance maximum of cytochrome-c. Measurement of the absorption of collected samples at 550 nm and comparison of the results to a standard curve gives the level of present in the extracellular fluid. This approach has been used to sample superoxide production following SCI (Liu et al., 1998) and in a rat model of amyotrophic lateral sclerosis (Liu et al., 1999b) with a mutation in the human Cu, Zn-superoxide dismutase gene (Deng et al., 1993; Rosen et al., 1993). A twofold elevation of superoxide for 6 h following SCI was observed.
4.4. Spinal cord injury, neutrophils and reactive oxygen species
The infiltration of immune cells led by neutrophils contributes to post-SCI inflammation (Taoka et al., 1997; Carlson et al., 1998). Neutrophils contain high levels of NADPH-oxidase (Rossi et al., 1986), enabling them to generate large quantities of . may contribute to the adverse consequences of SCI directly or by conversion to HO· (Liu et al., 2004; see above). Samples were collected by microdialysis at 24 h post-injury, the time of maximum neutrophil infiltration. Sampling of HO· (by salicylic acid infusion) and H2O2 (analyzed by addition of salicylic acid and FeCl2 to collected samples to convert H2O2 to DHBs followed by HPLC analysis-electrochemical detection (Liu et al., 1999a)) indicated no significant differences in the levels of HO· or H2O2 between injured spinal cords from normal animals and injured spinal cords in animals depleted of neutrophils by systemic treatment with an antibody to neutrophils (de Castro et al., 2004). While this could be attributable to HO· only being generated and reacting intracellularly, this would not be the case with H2O2, which readily crosses cell membranes. These results raise a question as to whether infiltrating neutrophils cause damage by releasing reactive oxygen species following SCI, or perhaps indicate that synthesis of is regulated in immune cells so as to occur only under circumscribed conditions.
4.5. Reactive nitrogen species in spinal cord injury
There is substantial evidence that reactive nitrogen species (RNS), chiefly peroxynitrite (reaction 6 above), cause secondary damage in CNS afflictions. This may be due to oxidation reactions and protein damage by nitration of tyrosine. Microdialysis studies analogous to those described above have been used to explore the mechanisms of damage by RNS following SCI (Liu et al., 2000, 2005). RNS formation was assessed by perfusing tyrosine through a microdialysis fiber implanted in the site of injury and measuring nitrotyrosine in the collected dialysates. The nitrotyrosine maximized at about 40 min and then decreased slowly. In addition, based on immunohistochemistry, the number of nitrotyrosine stained cells rose to 3.5 times the basal level following injury, maximizing at 12–24 h post-injury. Thus, both analysis of nitrotyrosine and immunohistochemistry demonstrate production of RNS following SCI.
Combined administration of superoxide dismutase and nitro-l-arginine blocked post-contusion SCI production of nitrotyrosine, consistent with the production of RNS from and NO (Liu et al., 2000). Nitric oxide levels measured with an NO.-specific electrode implanted into the site of injury rose quickly and then returned to baseline by about 1 h post-injury. In other experiments, administration of 3-morpholinosydnonimine (a precursor to NO, and HOONO) by microdialysis at the concentration estimated to produce levels of HONOO equivalent to those present following SCI elevated the membrane lipid peroxidation products malondialdehyde and 4-hydroxynonenal, demonstrating that peroxynitrite also causes membrane lipid peroxidation (Liu et al., 2005).
5. Applications of microdialysis to human brain injury
5.1. Background
Considerable efforts to develop microdialysis into a useful tool for investigating brain injury and predicting outcomes for neurointensive care monitoring in the clinic have been pursued beginning with the pioneering efforts of Persson and Hillered (1992). Microdialysis sampling has been utilized in clinical studies of traumatic brain injury (Tisdall and Smith, 2006; Hillered et al., 2005, 2006), infarction (Bosche et al., 2003; Dohmen et al., 2007), aneurismal sub-arachnoid hemorrhage (Schulz et al., 2000; Staub et al., 2000) and epilepsy (Carlson et al., 1992; During and Spencer, 1993; Hillered et al., 2005). We will focus on brain injury due to its being studied most extensively and the similarities among results from all of these syndromes, e.g., elevated excitatory amino acid release and glucose metabolism. Studies of spinal cord injury are not included because very few such studies have utilized microdialysis.
Microdialysis sampling has been used in efforts to improve the treatment of TBI patients by monitoring changes in post-TBI biochemistry. Microdialysis has also been utilized in attempts to further the understanding of secondary damage in TBI, that is, damage caused by processes secondary to the primary trauma, such as hypoxia and neurotransmitter release. Studies have involved human patients and animal models. Tisdall and Smith (2006) summarize the current status of the clinical application of microdialysis as follows, “Cerebral MD is increasingly used as a bedside monitor to provide on-line analysis of brain tissue biochemistry during neurointensive care”. The reader is referred to recent reviews (Tisdall and Smith, 2006; Hillered et al., 2005, 2006) for more extensive coverage of the application of microdialysis to TBI patients than that provided here.
Substances analyzed in samples collected from TBI patients by microdialysis post-TBI include glutamate, lactate, pyruvate, glucose and glycerol. Table 1 lists biochemical responses to head injury revealed by microdialysis sampling and the damaging processes to which the changes are believed to contribute (Tisdall and Smith, 2006).
Table 1.
Results of analyses of microdialysates following TBI and their interpretation
| Biochemical | Change consequences |
|---|---|
| Increased glutamate | Hypoxia/ischemia, excitotoxicity |
| Low glucose | Hypoxia/ischemia; reduced cerebral glucose supply, cerebral glycolysis |
| Increased lactate | Hypoxia/ischemia |
| Increased lactate/pyruvate ratio | Hypoxia/ischemia; reduced cellular redox state, reduced cerebral glucose supply, mitochondrial dysfunction |
| Increased glycerol | Hypoxia, ischemia; cell membrane degradation |
5.2. Model for clinical applications to human head injury
In clinical studies, commercially available microdialysis probes (CMA70 Brain Catheter, CMA/Microdialysis AB, Stockholm, Sweden) are inserted into regions of interest in the brain of the TBI patient. These are conventionally designed microdialysis probes with parallel inlet and outlet tubes. The probe is attached to a pump with biocompatible polyurethane tubing. Fluid is pumped into the probe through a polyurethane shaft which is connected to a cylindrical tip constructed with a semipermeable polyamide membrane across which exchange takes place (Hillered et al., 2005; Tisdall and Smith, 2006). Samples are collected in small tubes with an automatic sample collector. Molecular weight cutoffs of 20 kDa or 100 kDa are available (Hutchinson et al., 2005). These probes have gold tips for location by computed tomography. Basic procedures and principles of microdialysis are described in other sections of this contribution and elsewhere in this volume, so they are described here only briefly with regard to use in clinical studies.
Microdialysis results are made more meaningful by simultaneously measuring assorted physiological parameters. These measurements include brain oxygen pressure, intracranial pressure, cerebral perfusion pressure, pH, venous oxygen saturation (Hlatky et al., 2004), patient outcome according to the Glasgow Outcome Scale, and electrophysiological recording (Alves et al., 2005). In the last, a recording electrode is attached to a microdialysis probe and the combination is inserted into the brain. This approach demonstrated that elevated glutamate and lactate and lowered glucose concentrations in the injured hemisphere were accompanied by suppressed neuron firing and EEGs in rats. Firing rates were also found to be slowed in human TBI patients (Alves et al., 2005).
5.3. TBI release of glutamate
Experiments with rats established that TBI causes severity-dependent release of glutamate and other amino acids (Faden et al., 1989; Nilsson et al., 1990b). Extension of microdialysis sampling to humans also revealed elevated glutamate in TBI patients (Persson and Hillered, 1992; Bullock et al, 1998), which raised the possibility of treating TBI by administering glutamate antagonists. Characterizing the actions of glutamate is important to understanding the injury process itself, to identifying agents for treatment and to defining parameters for clinical monitoring. Microdialysis sampling demonstrates that TBI generates a variety of patterns of amino acid release in humans (Bullock et al., 1998). In contrast to results from animal models (Faden et al., 1989; Obrenovitch and Urenjak, 1997), glutamate concentrations remain quite high for at least as long as monitored (up to 4 days) in many post-TBI patients (Zauner and Bullock, 1995; Bullock et al., 1998). The different time courses of glutamate release between TBI humans and animal models raises issues as to whether results can be reliably extrapolated from animal models to humans. To be confident of the validity of so extrapolating, the origin of the differences between the two needs to be determined. This could also provide invaluable insight into the mechanisms of secondary damage in human brain injury. Inactivation of amino acid transport systems is a suggested cause of prolonged glutamate elevation in TBI patients (Bullock et al., 1998).
High dialysate amino acid concentrations in damaged brain correlate most strongly with focal contusion and secondary ischemic events — low cerebral perfusion pressure, raised intracranial pressure, hypoxemia and reduced cerebral blood flow (Bullock et al., 1998). High EAA concentrations in microdialysates from TBI patients either tend downward as the patient recovers (starting from 6–20 times normal) or increase steadily from 20–30 times normal glutamate levels as the patient progresses from uncontrollable ICP to brain death (Zauner and Bullock, 1995). Sustained reduction of cerebral blood flow below the threshold for ischemic neuronal damage generates massive EAA release. This glutamate elevation correlated with adverse outcomes (death, vegetative state, severe disability), outcomes being worst among patients with highest dialysate amino acid concentrations (Bullock et al., 1998).
NMDA receptor antagonists demonstrably improve the outcome of brain injury in rats (Faden et al., 1989; McIntosh et al., 1990, 1998). Unfortunately, all glutamate antagonists tested to date for clinical use on TBI patients have side effects restricting their use (Zauner and Bullock, 1995; Nicholson et al., 2007) or have proven not to be efficacious in clinical trials (Doppenberg et al., 1997; Narayan et al., 2002), and the role of synaptic release of glutamate in TBI has been challenged (Obrenovitch and Urenjak, 1997). These problems have diminished interest in administering glutamatergic antagonists to reduce secondary damage in TBI and related syndromes.
Obrenovitch and Urenjak (1997) questioned conventional thinking regarding the mechanisms of damage by glutamate following TBI. One source of their skepticism was that extracellular glutamate is cleared within 5 min after moderate TBI in rats (Nilsson et al., 1994), whereas glutamate receptor antagonists and glutamate synaptic release inhibitors can be effective when administered 30 min to 3 h after trauma in animal models. However, this disconnect between duration of release and the time window of agent effectiveness is not compelling evidence against excitotoxicity because, as already noted, glutamate is elevated for about an hour following severe experimental CNS trauma (Faden et al., 1989) and for days following human TBIs (Zauner and Bullock, 1995; Bullock et al., 1998). Thus the reality of the difference between the duration of release and the time window of agent effectiveness needs to be determined by comparing the two under the same conditions.
High concentrations of non-vesicular amino acids including aspartate are released in addition to glutamate upon TBI (Bullock et al., 1998). Such release presumably occurs primarily by non-synaptic mechanisms (Obrenovitch and Urenjak, 1997; Timmerman and Westerink, 1997). However, this does not rule out the possibility of damage to target neurons by actions of elevated glutamate on its receptors, as glutamate is probably toxic even in the presence of high concentrations of non-toxic amino acids. Damage may be due to glutamate actions on the NMDA receptor with a deficient Mg2+-block, and it has been argued that beneficial effects of glutamate antagonists may occur by processes other than blocking activation of glutamate receptors (Obrenovitch and Urenjak, 1997). Furthermore, operation of other processes simultaneously with glutamate receptor activation are not out of the question, and these mechanisms may be reciprocally reinforcing. Much of the glutamate released by neurotraumas likely results from mechanical damage, other cell death processes, breach of the blood-brain barrier and reversal of glutamate uptake systems (McAdoo et al., 2005). Glutamate released by these processes may also reach and activate synaptic glutamate receptors and thereby cause cell death that may still be blocked by actions of glutamate antagonists on glutamate receptors.
5.4. Co-release of zinc and glutamate
Zinc, an excitotoxin and modulator of glutamatergic neurotrans-mission (Choi and Koh, 1998; Frederickson et al., 2005), is stored with glutamate in presynaptic terminals of specific “gluzinergic” cerebrocortical neurons (Frederickson et al., 2006). This colocalization generated the hypothesis that Zn2+ and glutamate are co-released following a CNS insult, and that synergistic actions between them enhance their individual toxicities. Microdialysis sampling in a rat model of brain ischemia demonstrated that free zinc and glutamate are released together, providing the first demonstration of the co-release of glutamate and Zn2+ during ischemia and reperfusion (Frederickson et al., 2006). This is consistent with Zn2+ and glutamate contributing jointly to excitotoxicity. However, Zn2+ was also released without glutamate during reperfusion following ischemia, so sometimes zinc release alone may cause damage.
5.5. Glucose in TBI
Glucose utilization usually first increases post-injury and then decreases (Vespa et al., 2005). During hypoxia, glucose is metabolized to pyruvate, which is then reduced to lactate. This less efficient utilization of glucose increases its consumption and can reduce its concentration after TBI. Glucose, lactate and pyruvate concentrations in microdialysis samples reflect the extent of glycolysis, and a high lactate/glucose ratio can be an indicator of tissue hypoxia/ischemia and of elevated anaerobic glycolysis. The intracellular redox state (Persson and Hillered, 1992) can be related to the extracellular lactate/pyruvate ratio by the following equation (Ståhl et al., 2001),
where KLDH is the lactate dehydrogenase equilibrium constant (Siesjö, 1978).
Glucose concentrations predominantly increase following TBI, increases which when extreme are associated with decreased survival and more frequent vegetative state outcomes (Lam et al., 1991). Very low levels of glucose do occur in areas of high glucose consumption in some victims following TBI; levels below 0.10 mM post-TBI are also associated with poor outcomes (Vespa et al., 2003). However, the correlation between ischemia defined by positron emission tomography imaging and low microdialysate glucose concentration was poor. Furthermore, under these circumstances reduction of elevated glucose levels by treatment with insulin did not improve functional outcome (Vespa et al., 2006). At this point, if they are to be useful, efforts to treat TBI by controlling glucose levels are in need of substantially increased understanding and development.
5.6. Lactate, pyruvate and the lactate/pyruvate ratio in TBI
TBI in rats usually elevates lactate (Krishnappa et al., 1999; Nilsson et al., 1990b) as well as glucose. Ischemia is thought to be the single most important cause of brain damage in TBI (Persson and Hillered, 1992; Poca et al., 2007). Under post-TBI conditions of hypoxia/ischemia, anaerobic glycolysis, i.e., conversion of glucose to lactate, provides a significant source of ATP. Substantially elevated brain lactate has been considered to indicate a poor prognosis in TBI patients (Chen et al., 2000). The lowering of the pH associated with intense lactate production as well as less efficient energy metabolism may directly contribute to secondary damage post-SCI. Lactate levels alone may not be good measures of levels of ischemia. This was further demonstrated by comparing post-TBI cerebral lactate concentrations to arteriojugular differences in lactate concentrations (Poca et al., 2007). Samples for this were obtained from TBI patients with a cannula in the jugular bulb and a microdialysis probe in their less damaged hemisphere. The results, increased lactate in 81% of arteriovenus measurements versus in 3.1% of brain lactate measurements, demonstrated that arteriojugular venous differences in lactate concentrations do not reflect increased cerebral lactate production and therefore are not reliable indicators of brain ischemia. This has led to the conclusion that the use of lactate measurements should be reconsidered (Poca et al., 2007). Attendees at a consensus meeting regarding the use of microdialysis in monitoring TBI and subarachnoid hemorrhage patients concluded that lactate analysis alone is not a reliable marker of brain ischemia (Bellander et al., 2004).
For reproducible results, it is essential that the microdialysis zone be placed appropriately and consistently relative to the site of damage and brain structures. Compensation for variable recovery by determining the lactate/pyruvate ratio in microdialysates (Persson and Hillered, 1992) can give a good measure of ischemia as compared to that obtained simultaneously by PET (Enblad et al., 1996). However, several recent microdialysis studies of human patients gave lactate/pyruvate ratios that did not reliably measure brain ischemia or failing energy metabolism (Vespa et al., 2005; Samuelsson et al., 2007), so measuring lactate/pyruvate ratios does not reliably solve the problems with lactate measurements. Longitudinal microdialysis data from patients with severe TBI revealed a 25% incidence of metabolic crisis (defined as a lactate/pyruvate ratio >40), but only a 2.4% incidence of ischemia (one patient), despite increased lactate/pyruvate ratios in the remaining patients in the study (Vespa et al., 2005). Ischemia was assessed based on the oxygen extraction fraction and cerebral venous oxygen content derived from positron emission tomography in the region sampled by microdialysis. Thus non-ischemic metabolic crises occurred in these patients. Oxidative metabolism was reduced in conjunction with altered glucose metabolism.
5.7. Evaluation of treatment strategies
A potentially important application of cerebral microdialysis to neurointensive care is to define better the effects that treatment strategies have on the injured brain. Glucose utilization increases in the first week post-TBI, and normalization of elevated serum glucose is generally recommended for the critically ill neurological patient. However, in one study of 19 TBI patients, a 70% reduction of microdialysis glucose by treatment with insulin did not change the rate of glucose metabolism, nor did it improve functional outcome after TBI (Vespa et al., 2006). This treatment did generate markers of cellular distress: elevated glutamate, an elevated lactate/pyruvate ratio and lowered glucose in microdialysates. Also, lowering glucose did not improve survival or 6 month outcome. Thus this procedure will at least require further evaluation and development to be applicable to TBI.
5.8. Assessment of clinical applications of microdialysis
The present state of the application of microdialysis to brain-injured patients has been summarized by Tisdall and Smith, 2006 as being a research tool to be used in specialist centers. To make microdialysis a standard tool for monitoring the TBI patient, the precision of results needs to be improved. To accomplish this, origins of variations between microdialysis measures and the state of the patient need to be clarified. For example, the same pattern of glucose and its metabolites in microdialysates can reflect different metabolic states in TBI, clouding the meaning of such results. Thus developing microdialysis into a routine clinical tool still faces substantial challenges, including whether it is possible.
6. Microdialysis studies of pain signaling and its modulation
6.1. Pain transmission in the spinal cord
Pain is another significant clinical problem in which understanding the actions of participating intercellular messengers is important to developing treatments. Chronic pain is an adverse outcome of SCI (Hulsebosch et al., 2000; Sidall et al., 2002) and many other neurological disorders. Sampling the extracellular fluid and agent administration by microdialysis have been used to identify neurotransmitters released in response to nociceptive inputs (stimuli expected to cause pain). Neurotransmitters involved in descending control (regulation of pain transmission by neurons that descend from the brain) of pain pathways in the spinal cord have also been characterized by microdialysis (Sorkin et al., 1988, 1993; Cui et al., 1999).
Initial microdialysis investigations of nociceptive signaling were performed on rats (Skilling et al., 1988), cats and monkeys (Sorkin et al., 1988). Skilling and coworkers used microdialysis sampling to demonstrate increases in extracellular concentrations of amino acids in response to intradermal injection of 5% formalin into the hindlimb (a model of acute pain). Their experiments were conducted on awake, freely-moving animals. Formalin elevated extracellular glutamate and aspartate, consistent with these EAAs transmitting nociceptive signals. Both groups used a coated (except for a 2 mm dialysis zone) dialysis fiber inserted transversely through the spinal cord. Sorkin et al. (1988) developed methods for recording the electrical activity of individual neurons while sampling the extracellular fluid with a microdialysis fiber. In some experiments on monkeys, hook electrodes were placed on the sural nerve, and the cord dorsum potential was monitored with a platinum ball electrode contacting the dorsal surface of the cord over the fiber. Single unit potentials were recorded in the vicinity of the dialysis zone with carbon fiber microelectrodes.
Willis and coworkers demonstrated by microdialysis sampling that electrical stimulation of the sciatic nerve strongly enough to activate c-fibers therein released glutamate, aspartate, asparagine, serine, glycine, threonine, alanine and taurine in the dorsal horn of the spinal cord, but such release was not observed at a stimulus strength that activated only A fibers (Paleckova et al., 1992). This suggested that the weaker stimulus activated only one of two pathways. Addition of CNQX, an AMPA/kainate receptor blocker, to the perfusing medium prevented the release of the amino acids upon stimulation. This suggests that AMPA/kainate receptors operate in the first synapse made by primary afferent c-fibers in the spinal cord and that their blockade prevents activation of interneurons in the dorsal horn. Complete block of amino acid release by the infusion of TTX into the dorsal horn suggests that increased neuronal activity was involved in the release of amino acids in these experiments, although this release may not have been synaptic (Timmerman and Westerink, 1997).
Sampling with a microdialysis loop implanted into the intrathecal space over the spinal cord has been used to elucidate the roles of excitatory amino acids, prostaglandin E2 and nitric oxide in the effects of morphine on responses to a noxious stimulus, 5% formalin injection (Malmberg and Yaksh, 1995). Formalin injection evokes biphasic flinching of the injected paw. During phase 1, there was a significant increase in the release of glutamate, aspartate, taurine, glycine, citrulline, and PGE2. Citrulline is a metabolic co-product of nitric oxide, so changes in citrulline were taken as measures of the production of nitric oxide (Sorkin, 1993), although sources of citrulline in addition to co-production with nitric oxide may compromise these experiments. Only citrulline and PGE2 were significantly increased in the second phase of flinching. Morphine injection had no effect on the resting release, but significantly reduced formalin-induced behavior and release of glutamate, aspartate, taurine, glycine, citrulline, and PGE2. These reductions were reversed by pretreatment with the opiate antagonist naloxone. Both types of results implicate involvement of an opioid receptor in the behaviors and amino acid release.
6.2. Descending control of nociceptive transmission
Stimulation in several medial brainstem structures both produces analgesia and inhibits nociceptive neurons in the spinal cord by pathways that descend to the spinal cord (Willis, 1982; Besson and Chaouch, 1987). Substantial evidence, including results of microdialysis sampling, indicates that one of these structures, the nucleus raphe magnus (NRM) mediates these effects by releasing 5-HT in the spinal cord (Sorkin et al., 1988, 1993). Electrical stimulation within the NRM of the cat during sampling by microdialysis in the lumbar dorsal horn released 5-HT and several amino acids in the cord. Stimulation of the ipsilateral dorsolateral funiculus evoked release of 5-HT. Stimulation in the NRM while applying mechanical stimuli of different intensities to the hindpaw [brush (innocuous), press, pinch, squeeze (clearly noxious)] reduced electrical responses of a spinothalamic tract (STT) cell (a neuron that transmits nociceptive signals from the spinal cord to the thalamus) in conjunction with an increase in 5-HT release near the STT cell, evidence for a contribution of 5-HT release to the inhibition of transmission of nociceptive signals. Administration of strychnine, a glycinergic antagonist, through the microdialysis fiber antagonized NRM-induced inhibition when 5-HT release was not detected, but did not block inhibition during increased 5-HT release. In addition to a serotonergic pathway, these results point to a non-serotonergic, descending raphe-spinal inhibitory pathway that includes a glycine-mediated stage. Thus sampling and agent administration by microdialysis together with electrophysiological recording can help identify the neurochemical messengers utilized by pain and other pathways.
Stimulation in the periaqueductal gray (PAG) produces analgesia by activation of pathways that descend to the cord through the NRM. Microdialysis sampling demonstrated that the release of 5-HT, norepinephrine, glutamate, glycine and aspartate in the dorsal horn of the spinal cord increases during PAG stimulation (Cui et al., 1999). The time course of inhibition of dorsal horn neurons generated by long-lasting PAG stimulation matched that of the release of the neurotransmitters, suggesting that the released neurotransmitters mediate the inhibition produced in the dorsal horn by PAG stimulation.
6.3. Microdialysis studies of supraspinal involvement in pain transmission
Pain transmission also involves release of neurotransmitters by supraspinal systems related to the descending control systems. Stiller et al. (2003) have reviewed the use of microdialysis in pain research, including supraspinal studies, so we give a short summary of supraspinal investigations here.
Injecting formalin into the hindpaw of the rat produces Ca2+ and nerve impulse dependent release of glutamate, arginine, and aspartate in PAG dialysates (Silva et al., 2000). Handling, pinching or saline injection into the hind paw did not increase glutamate levels, suggesting that its release helped mediate painful and persistent noxious stimulation. Following similar formalin injection into the rat hind paw, microdialysis sampling by Sajedianfard et al. (2005) demonstrated that norepinephrine is released in the locus coeruleus, a noradrenergic center. This was accompanied by a substantial increase in nociceptive behavior.
Injection of prostaglandin E2 into the plantar area of the rat paw caused thermal hypersensitivity (Smith et al., 2006). Microdialysis sampling in the same animals revealed an accompanying 230% increase in extracellular 5-HT levels in the rostral ventromedial medulla, a serotonergic center. Capsaicin injection in the same area in the same studies similarly increased thermal sensitivity and extracellular 5-HT levels. Morphine attenuated PGE2-induced thermal hypersensitivity and lowered extracellular levels of 5-HT. These observations suggest that serotonin release in the Rostral ventral medial medulla (RVM) contributes to thermal hypersensitivity in the RVM.
Goettl et al. (2002) found by microdialysis sampling in the thalamus that ligating spinal roots L5 and L6 to induce neuropathic pain significantly decreased extracellular 5-HT levels contralaterally but not ipsilaterally. This is consistent with release of 5-HT in the thalamus reducing neuropathic pain.
In summary, microdialysis sampling has identified a number of neurotransmitters involved supraspinally in pain perception.
6.4. Post-surgery back pain
Microdialysis studies of injury to spinal nerves approached clinical applicability in a recently described model of failed back surgery (Rooney et al., 2004). It was hypothesized that post-surgery chronic back pain arises from damage caused by an increase in the extracellular concentrations of excitatory amino acids in the dorsal horn due to unintentional distortion of dorsal spinal structures during surgery. It was further hypothesized that application of a pharmacological nerve block prior to an insult to dorsal root neurons in rats would prevent the release of amino acids and consequently the development of chronic pain. Microdialysis sampling in the dorsal horn revealed significant increases in the concentrations of the EAAs aspartate and glutamate and non-neurotransmitter amino acids in the dorsal horn upon bilateral severing of dorsal roots L4 and L5. Topical application of Lidocaine, a Na+-channel blocker that suppresses electrical activity, starting 10 min before the nerves were severed prevented the increase in EAA release. Behavior tests demonstrated the development of mechanical allodynia (conversion of perception of a normally non-noxious stimulus to perception as noxious) in untreated rats with cut nerves; this was attenuated by the application of Lidocaine. This correlation demonstrates that conduction block may provide a treatment to prevent the development of chronic pain following back surgery.
7. Microdialysis and neural repair by stem and other grafted cells
7.1. Stem cells in neural repair
Exciting current advances in the stem cell field include isolation and propagation of human embryonic stem cells (hESCs) (Thomson et al., 1998) and germ cells (hEGCs) (Shamblott et al., 1998). These cells are pluripotent since they can become any cell type in the human body, including neurons. Along this line, many groups have been able to generate neural progenitor cells from cultured hESCs (Benzing et al., 2006; Carpenter et al., 2001; Reubinoff et al., 2001; Shin et al., 2006; Zhang et al., 2001). Another source for neural cells, multipotent neural stem cells, has also been isolated successfully from the fetal (Carpenter et al., 1999; Svendsen et al., 1998; Uchida et al., 2000; Vescovi et al., 1999; Villa et al., 2000) and the adult (Johansson et al., 1999; Palmer et al., 2001; Roy et al., 2000) human CNS.
Evidence accumulated using rodent, monkey and human stem cells suggests that stem cell transplantation may be a way to effectively regenerate traumatically injured or chronically degenerated CNS tissue, such as that damaged during spinal cord or brain injury, stroke, Parkinson’s disease, amyotrophic lateral sclerosis, Huntington’s disease and Alzheimer’s disease (for reviews see Bjorklund and Lindvall, 2000; Enzmann et al., 2006; Fisher, 1997; Gage, 2000; Goldman, 2005; Isacson, 2003; Keirstead, 2001; Le Belle and Svendsen, 2002; Lindvall and Kokaia, 2006; McKay, 1997). Potential applications of stem cells to the amelioration of neurotrauma or neurodegeneration include 1) replacing lost neurons to relay electrical impulses, 2) replacing lost oligodendrocytes for re-myelination, 3) providing normal functional astrocytes, and 4) providing trophic factors to protect injured or surviving normal neurons from secondary toxic insults such as oxidative stress, inflammation and excitotoxicity. Understanding the efficacy of stem cell grafting and the mechanisms underlying stem cell-mediated neural regeneration has been achieved by morphological, electrophysiological, neurochemical and behavioral analyses. Microdialysis was recently used to detect trophic factors or neurotransmitters released by grafted stem cells or their differentiated progeny (Gao et al., 2006; Rodriguez-Gomez, 2007). We describe the use of microdialysis to explore the role of the release of protective substances by grafted cells in improving recovery from neurotrauma.
7.2. Sampling of neurotrophic factors released from grafted cells
Grafted stem cells ameliorate functional deficits in various disease models, even when stem cell neuronal differentiation and integration are lacking. One possible mechanism underlying such beneficial effects is the release of neurotrophic factors by grafts, which then protect host neurons (Borlongan et al., 2004; Kerr et al., 2003; Llado et al., 2004; McKay, 1997; Nieto-Sampedro et al., 1987; Ourednik et al., 2002; Shear et al., 2004). Consequently, reciprocal interactions between grafts and host have received increasing attention, primarily from the pioneer work done by Ourednik (Ourednik et al., 1993; Ourednik et al., 2002) and Snyder (Teng et al., 2002). Such graft-host interactions most likely have great impact not only on the fate of grafted cells, but also on host self-repair plasticity (Ourednik and Ourednik, 2004a; Ourednik and Ourednik, 2004b). Along this line, Snyder and colleagues were the first to report that an immortalized NSC line (C17.2) from mouse cerebellum secretes glial cell line-derived neurotrophic factor (GDNF) in vitro (Llado et al., 2004; Ourednik et al., 2002). However, Kerr et al. (2003) found that human embryonic germ cells secrete brain-derived neurotrophic factor (BDNF) and transforming growth factor-alpha (TGFα) in vitro. Although conceptually advanced, these studies did not provide direct evidence for secretion of neurotrophic factors from grafted cells in an in vivo setting.
Recently, we used microdialysis to detect increased secretion of GDNF in vivo following transplantation of human fetal neural stem cells (hNSCs) into injured rat brains (Gao et al., 2006). These hNSCs were originally derived from an 8-week human fetal forebrain (Svendsen et al., 1998) and long-term expanded in vitro by exposure to a combination of three growth factors, epidermal growth factor (EGF), basic fibroblast growth factor (bFGF or FGF-2) and lympho-cyte inhibitory factor (LIF) (Tarasenko et al., 2004). When cultured in suspension, hNSCs grow into neurospheres containing a heterogeneous population of cells. These long-term cultured hNSCs (at least 95 passages over three years) retain a normal diploid karyotype and have proliferation and differentiation patterns indistinguishable from cells of earlier passages (unpublished observations). To increase the capacity of hNSCs to differentiate into neurons, we developed a priming procedure by exposing adhesively cultured hNSCs in bFGF, heparin and laminin for 4–5 days prior to transplantation (Wu et al., 2002). Primed hNSCs were then grafted into the rat hippocampal region near the fluid percussion injury site at 24 h post-injury. We found that this acute hNSC grafting prevented traumatic brain injury-caused impairment of spatial learning and memory in rats within 2 weeks post-injury (Fig. 4).
Fig. 4.
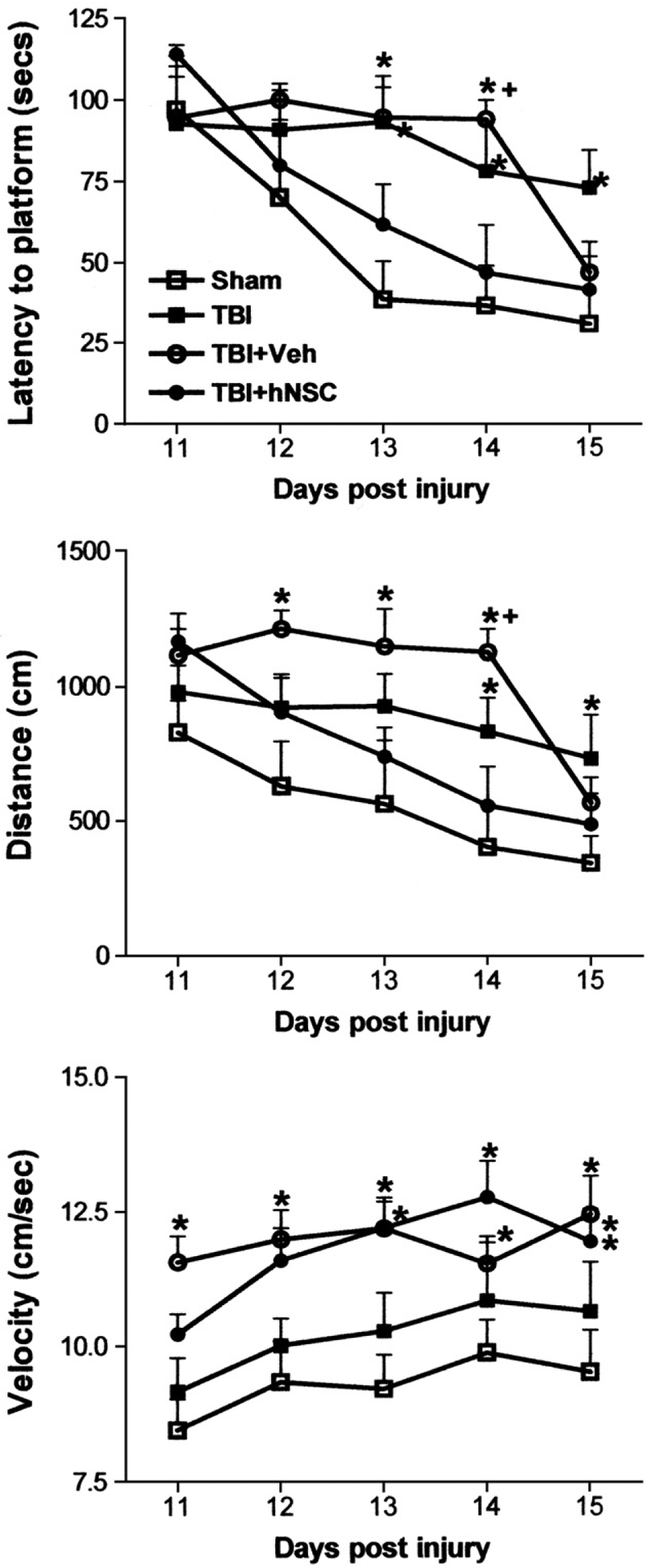
Results of performing the Morris Water Maze (MWM) test on rats following traumatic brain injury (TBI) and human neural stem cell (hNSC) grafting. The TBI was a moderate (2.0 atm) fluid percussion injury; groups were Sham, craniotomy without injury; Veh, vehicle injection; hNSC, graft with hNSCs. Group differences in MWM performance across all days were analyzed by a repeated measures mixed model ANOVA of SAS/STAT® 9.1. Groups were compared on each day using one-way ANOVA followed by the Tukey–Kramer post-hoc test: *, significantly different from the sham group (p<0.05);+, significantly different from both sham and hNSC-grafted groups (p<0.05) (from Gao et al., 2006).
Although primed hNSCs differentiated into neurons in the hippocampus, the acute effect of hNSC transplantation was probably not due to the functional integration of hNSC-derived neurons. To explore whether grafted hNSCs aided host regeneration by secreting trophic factors, we assessed expression of several trophic factors at mRNA and protein levels and found that hNSCs expressed and secreted significant amounts of GDNF in vitro. Furthermore, we were the first group to apply microdialysis techniques to directly measure secretion of GDNF from hNSC after transplantation in vivo. This was done by implanting CMA12 probes (Microdialysis Inc., North Chelmsford, MA) into the hippocampal area of live animals and collecting artificial cerebrospinal fluid running through the probe continuously at 2 μl/min for 6 h. The collected samples were then subjected to GDNF ELISA measurements. We found that hNSC grafting significantly increased GDNF secretion in injured hippocampi when compared with controls (brain injury alone or injury plus vehicle injection) (Fig. 5). Thus, both in vitro studies and in vivo microdialysis sampling suggested that hNSC-secreted GDNF contributes to protecting host hippocampal neurons from secondary damage such as that by reactive oxygen species and glutamate, and thus prevents brain injury-generated cognitive deficits. Interestingly, vehicle injection alone was also followed by a trend toward increased GDNF secretion, although not statistically significantly when compared to TBI alone (Fig. 5). Therefore, it is possible but not demonstrated that an injection-mediated increase of endogenous GDNF contributes to the delayed improvement of Morris Water Maze performance observed in vehicle-injected rats (Fig. 4).
Fig. 5.
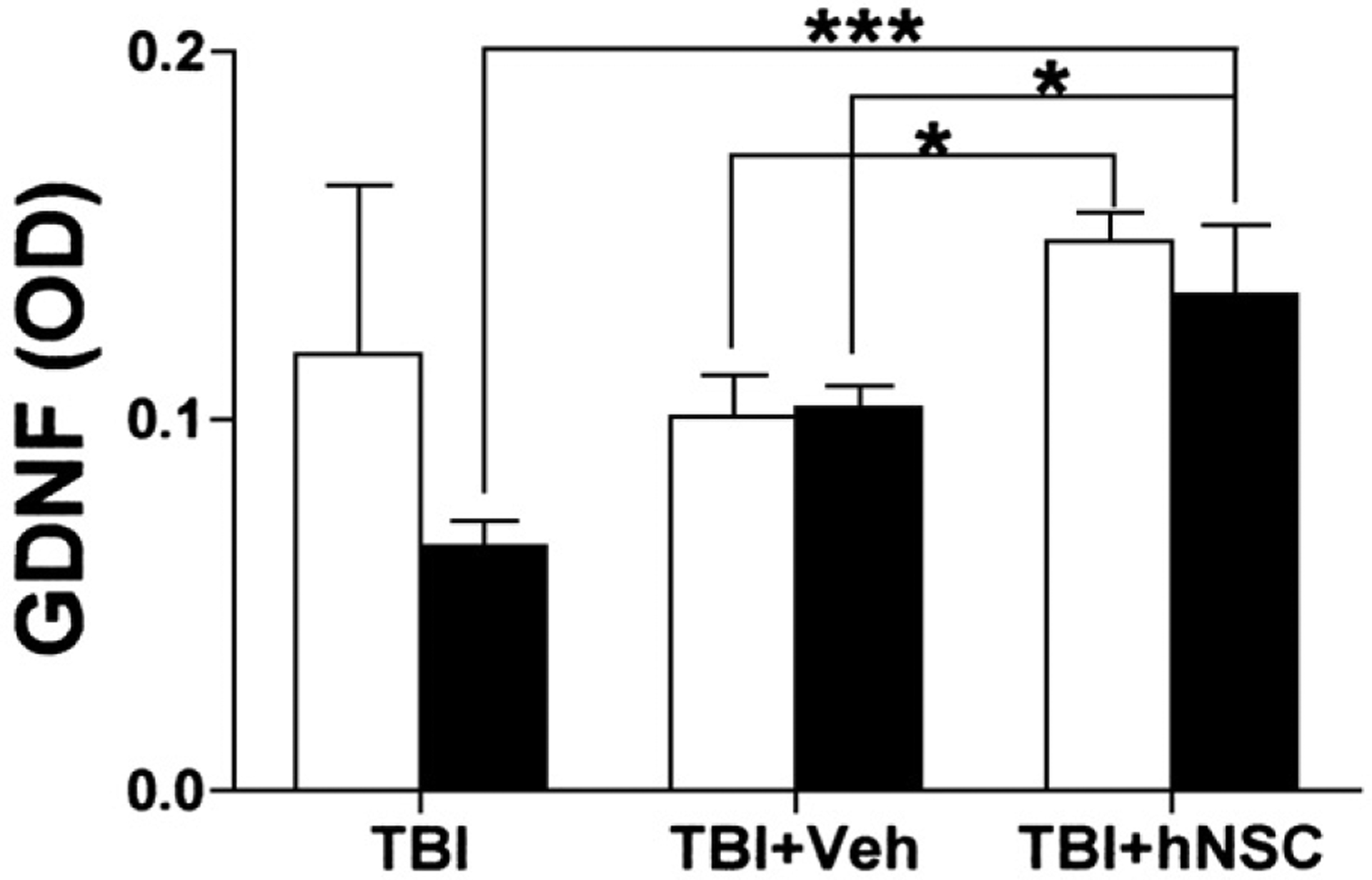
GDNF secretion in the rat hippocampus one week after traumatic brain injury with or without human neural stem cell (hNSCs) grafting. Microdialysis samples were collected for 6 h from both intact (empty bars) and injured/grafted (solid bars) hippocampi in each rat 7 days after injury. Samples were concentrated and assayed by ELISA. TBI, fluid percussion model of traumatic brain injury; TBI+Vel, vehicle injection into the TBI site of rat brain; TBI+hNSC, hNSC graft into the TBI site. Note hNSC grafting resulted in significant increases in GDNF secretion in TBI hippocampi when compared with controls (TBI alone and TBI plus vehicle injection). Values (N=4 for TBI, and 3 for other groups) are means±s.e.m. *** p<0.001 and * p<0.05; one-way ANOVA with the Tukey–Kramer post-hoc test (From Gao et al., 2006).
7.3. Detection of neurotransmitters released from grafted cells
Loss of specific types of neurons and their neurotransmitters occurs in both neurodegenerative diseases and neurotrauma. For example, cholinergic neurons [acetylcholine (ACh) releasers] degenerate in the basal forebrain in Alzheimer’s disease and in the spinal cord in amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease), while dopaminergic neurons [dopamine (DA) releasers] die in the substantia nigra of the midbrain in Parkinson’s disease. Attempts pioneered by Perlow et al. (1979) and Bjorklund and Stenevi (1979) to replace lost neurons in various animal models of neural diseases by grafting embryonic neural tissues initially and later embryonic stem cells or neural stem/progenitor cells are ongoing. Accumulated evidence supports cell transplantation being a promising approach to restoring neural function.
Microdialysis has been widely used since the early 1990’s to assess neurotransmitter release from grafted neurons in vivo to determine the efficacy of grafting embryonic/fetal neural tissues. Such neurotransmitters include ACh, DA, 5-HT, noradrenaline (NA) and gamma aminobutyric acid (GABA). Lesion of the fimbria-fornix cholinergic pathway depletes ACh in the hippocampal formation. Grafting of fetal rat septal tissues (containing cholinergic neurons) into the hippocampus restored the basal level of ACh in microdialysis samples analyzed by HPLC with a post-column enzyme reaction and electrochemical detection (Nilsson et al., 1990a; Hilgert et al., 2000). Furthermore, the graft-derived ACh release was responsive to pharmacological stimulants such as citalopram or fenfluramine (Hilgert et al., 2000), to electrical stimulation of the lateral habenula (Nilsson et al., 1990a), or to behavioral activation, including sensory stimulation by handling, immobilization stress and swimming motor stimulation (Nilsson and Bjorklund, 1992). Grafting fetal rat ventral spinal cord tissues into the rat lumbar spinal cord with motor neuron loss induced by perinatal injection of volkensin restored ACh output to near the normal level (Gulino et al., 2007).
In an animal model mimicking Parkinson’s disease, 6-hydroxydopamine (a neurotoxin) is frequently used to kill DA neurons on one side of the brain. Amphetamine (a monoamine releaser by transport exchange) increases DA release on the uninjured side and thus produces a distinct rotation ipsilateral to the lesioned side. Grafting fetal DA neurons into the DA-depleted striatum counteracted amphetamine-induced ipsilateral rotation, which was correlated to the increased striatal release of DA, as analyzed by microdialysis and HPLC (Kondoh and Low, 1994). Amphetamine or dihydrokainic acid (a glutamate uptake inhibitor) increased DA release from grafts, further indicating that grafted DA neurons became functionally integrated into the host circuitry (Kondoh and Low, 1994). Besides ACh and DA, microdialysis coupled with HPLC has been used to detect 5-HT (Cenci and Kalen, 2000; Daszuta et al., 1989), NA (Kalen et al., 1991; Leanza et al., 1999) and GABA (Campbell et al., 1993) released from grafted fetal neurons, as well as ACh and DA released from grafted fibroblasts that were genetically modified to express critical enzymes for ACh or DA synthesis (Chang et al., 2002; Fisher et al., 1993; Horellou et al., 1990; Leff et al., 1998; Uchida et al., 1992; Wachtel et al., 1997).
There is only one report of using microdialysis to monitor neurotransmitter release from grafted stem cell-derived neurons. The study used the unilateral 6-OHDA lesioned rodent model for Parkinson’s disease (Rodriguez-Gomez et al., 2007). Prior to grafting, mouse embryonic stem cells (ESCs) were guided toward DA neuronal differentiation through a five-step in vitro process. The process includes 1) proliferation of ESCs; 2) generation of ESC aggregates called embryoid bodies; 3) selection of nestin-positive neural progenitor cells; 4) expansion of progenitor cells in the presence of FGF-8 and Sonic hedgehog (Shh); and 5) a brief differentiation step (2 days), withdrawing FGF-8 and Shh. These ESC-derived immature DA neurons were then grafted into the DA-depleted striatum at 2 weeks post-lesion. ESC-derived DA neurons survived and ameliorated amphetamine-induced ipsilateral rotation for at least 32 weeks. Functional recovery was correlated to increased extracellular levels of DA and its metabolites, 3,4-dihydroxyphenylacetic acid and homovanillic acid in the grafted striatum, as determined by microdialysis and HPLC. Furthermore, ESC-derived neuron grafts increased DA release in response to pharmacological challenges in vivo, including K+-induced depolarization, nomifensine-induced inhibition of DA reuptake and amphetamine-induced DA release. These microdialysis studies indicate that grafted ESC-neurons have a high affinity DA reuptake system similar to that in normal DA neurons and can release DA in an activity-dependent manner.
In summary, microdialysis offers a highly sensitive way to monitor trophic factors, growth factors, neurotransmitters and cytokines released from grafted stem cells in vivo. It will be used more widely in the foreseeable future, not only to evaluate the efficacy of stem cell grafting, but also to define the mechanisms underlying interactions between stem cells and host cells through released factors. The latter will provide insights to develop better strategies to maximize stem cell graft-mediated neural regeneration.
8. Summary
Elevated release of endogenous damaging substances contributes to many neurological problems, including neurotrauma, stroke, epilepsy, neurodegenerative diseases and chronic pain. Identifying endogenous toxins that contribute to a particular syndrome and characterizing their release or generation is important to identifying potential targets for pharmaceutical blockade. Microdialysis provides a means of sampling substances released in vivo for chemical analysis. It has been shown by this means that the level of glutamate released upon SCI is toxic to neurons and oligodendrocytes, and that such glutamate contributes to permanent loss of function. This release is probably due mostly to mechanical damage, increased leakage of the blood-spinal cord barrier and reversal of transport. Sometimes the deleterious effects of glutamate are likely amplified by the co-release of zinc. There is good evidence that excess release of glutamate contributes to all of the above syndromes. Microdialysis sampling demonstrates the generation of the reactive species , H2O2, HO·, NO, HOONO and Fe2+ following trauma. Furthermore, damage done by generating reactive species in situ utilizing microdialysis and related techniques demonstrates that they are cytotoxic. Sampling metabolites of oxidation post-TBI is a potentially useful tool for monitoring the state of the patient. Pain perception can be diminished by activating control systems that descend to the spinal cord; microdialysis sampling indicates that release of neurotransmitter amines and amino acids are involved in such control. Microdialysis also provides evidence that the release of excitatory amino acids upon damaging spinal nerves and the spinal cord generates chronic pain. Finally, microdialysis sampling can also be used to assess treatments for neurological problems. Microdialysis sampling demonstrated that release of glial cell line-derived neurotrophic factor increased following the grafting of primed fetal human neural stem cells into brain-injured rats, the first in vivo demonstration of the release of a neurotrophic factor in conjunction with stem cell grafting. This release was accompanied by significant improvement of spatial learning and memory (Gao et al., 2006). In summary, microdialysis has and will make useful contributions to understanding the role of substances released in a wide variety of neurological disorders.
Acknowledgments
We thank NIH [NS11255 (DM), NS39161(DM), NS46025 (PW)] and Mission Connect of the TIRR Foundation for the financial support, Debbie Pavlu for her help with manuscript preparation and Michael Hughes for his help with the figures.
Abbreviations:
- ACh
acetyl choline
- ACSF
artificial cerebrospinal fluid
- ALS
amyotrophic lateral sclerosis
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- CCP
cerebral perfusion pressure
- CNS
central nervous system
- DA
dopamine
- DHBA
dihydroxybenzoic acid
- DHK
dihydrokainate
- EAA
excitatory amino acids
- EGF
epithelial growth factor
- ELISA
enzyme-linked immunosorbent assay
- GABA
gamma aminobutyric acid
- GDNF
glial cell line-derived neurotrophic factor
- hEGCs
human embryonic germ cells
- hESCs
human embryonic stem cells
- 5-HT
5-hydroxytryptamine or serotonin
- ICP
intracranial pressure
- NA
noradrenalin
- GDNF
glial cell line-derived neurotrophic factor
- hESCs
human embryonic stem cells
- hNSCs
human fetal neural stem cells
- HPLC
high performance liquid chromatography
- LIF
leukocyte inhibitory factor
- NRM
nucleus raphe magnus
- NSCs
neural stem cells
- PAG
periaqueductal gray
- PAS
photobeam activity system
- PbtO2
brain tissue oxygen tension
- PET
positron emission tomography
- RNS
reactive nitrogen species
- RVM
rostral ventromedial medulla
- SCI
spinal cord injury
- Shh
Sonic hedgehog
- SjvO2
jugular venous oxygen saturation
- STT
spinothalamic tract
- TBI
traumatic brain injury
- TGFα
transforming growth factor-alpha
- TTX
tetrodotoxin
- VPLc
ventral basal lateral region of the thalamus
References
- Agrawal SK, Fehlings MG. Role of NMDA and non-NMDA ionotropic glutamate receptors in traumatic spinal cord axonal injury. J Neurosci 1997;17:1055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves OL, Bullock R, Clausen T, Reinert M, Reeves TM. Concurrent monitoring of cerebral electrophysiology and metabolism after traumatic brain injury: an experimental and clinical study. J Neurotrauma 2005;22:733–49. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating for open field testing in rats. J. Neurotrauma 1995;12:1–21. [DOI] [PubMed] [Google Scholar]
- Bellander BM, Cantais E, Enblad P, Hutchinson P, Nordström CH, Robertson C, et al. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med 2004;30:2166–9. [DOI] [PubMed] [Google Scholar]
- Benveniste H Brain microdialysis. J Neurochem 1989;52:1667–79. [DOI] [PubMed] [Google Scholar]
- Benzing C, Segschneider M, Leinhaas A, Itskovitz-Eldor J, Brustle O. Neural conversion of human embryonic stem cell colonies in the presence of fibroblast growth factor-2. Neuroreport 2006;17:1675–81. [DOI] [PubMed] [Google Scholar]
- Besson JM, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev 1987;67:67–86. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res 1979;177:555–60. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci 2000;3:537–44. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 2004;35:2385–9. [DOI] [PubMed] [Google Scholar]
- Bosche B, Dohmen C, Graf R, Neveling M, Staub F, Kracht L, Sobesky J, et al. Extracellular concentrations of non-transmitter amino acids in peri-infarct tissue of patients predict malignant middle cerebral artery infarction. Stroke 2003;34:2908–15. [DOI] [PubMed] [Google Scholar]
- Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, et al. Factors affecting excitatory amino acid release following severe human head injury. J Neurosurg 1998;89:507–18. [DOI] [PubMed] [Google Scholar]
- Bungay PM, Morrison PF, Dedrick RL. Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci 1990;46:105–19. [DOI] [PubMed] [Google Scholar]
- Campbell K, Kalen P, Wictorin K, Lundberg C, Mandel RJ, Bjorklund A. Characterization of GABA release from intrastriatal striatal transplants: dependence on host-derived afferents. Neuroscience 1993;53:403–15. [DOI] [PubMed] [Google Scholar]
- Carlson H, Ronne-Engström E, Ungerstedt U, Hillered L. Seizure related elevations of extracellular amino acids in human focal epilepsy. Neurosci Lett 1992;140:30–2. [DOI] [PubMed] [Google Scholar]
- Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol 1998;151:77–88. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Cui X, Hu ZY, Jackson J, Sherman S, Seiger A, et al. In vitro expansion of a multipotent population of human neural progenitor cells. Exp Neurol 1999;158: 265–78. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol 2001;172:383–97. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Kalen P. Serotonin release from mesencephalic raphe neurons grafted to the 5,7-dihydroxytryptamine-lesioned rat hippocampus: effects of behavioral activation and stress. Exp Neurol 2000;164:351–61. [DOI] [PubMed] [Google Scholar]
- Chang JW, Lee WY, Milstien S, Kang UJ. A site-specific mutation of tyrosine hydroxylase reduces feedback inhibition by dopamine in genetically modified cells grafted in parkinsonian rats. J Neurochem 2002;83:141–9. [DOI] [PubMed] [Google Scholar]
- Chen T, Qian YZ, Di X, Rice A, Zhu JP, Bullock R. Lactate/glucose dynamics after rat fluid percussion brain injury. J Neurotrauma 2000;17:135–42. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Krishna G, Tulsi P, Obata T, Lang K, Huang SJ, et al. Intracranial microdialysis of salicylic acid to detect hydroxyl radial generation through dopamine autooxidation in the caudate nucleus: Effects of MPP. Free Radic Biol Med 1992;13:581–3. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988;1:623–34. [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci 1998;21:347–75. [DOI] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci 1987;7:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Feng Y, McAdoo DJ, Willis WD. Periaqueductal gray stimulation-induced inhibition of nociceptive dorsal horn neurons in rats is associated with the release of norepinephrine, serotonin, and amino acids. J Pharm Exp Ther 1999;289:868–76. [PubMed] [Google Scholar]
- Daszuta A, Kalen P, Strecker RE, Brundin P, Bjorklund A. Serotonin neurons grafted to the adult rat hippocampus. II. 5-HT release as studied by intracerebral microdialysis. Brain Res 1989;498:323–32. [DOI] [PubMed] [Google Scholar]
- de Castro R Jr, Alcock NW, McAdoo DJ. Sampling of low molecular weight iron by microdialysis following spinal cord injury. J Neurosci Res 1999;57:735–9. [PubMed] [Google Scholar]
- de Castro R Jr, Hughes MG, Xu GY, Clifton C, Calingasan NY, Gelman BB, et al. Evidence that infiltrating neutrophils do not release reactive oxygen species in the site of spinal cord injury. Exp Neurol 2004;190:414–24. [DOI] [PubMed] [Google Scholar]
- Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, et al. Anyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science 1993;261:1047–51. [DOI] [PubMed] [Google Scholar]
- Doble A Excitatory amino acid receptors and neurodegeneration. Therapie 1995;50: 319–37. [PubMed] [Google Scholar]
- Dohmen C, Bosche B, Graf R, Reithmeier T, Ernestus RI, Brinker G, et al. Identification and clinical impact of impaired cerebrovascular autoregulation in patients with malignant middle cerebral artery infarction. Stroke 2007;38:56–61. [DOI] [PubMed] [Google Scholar]
- Doppenberg EMR, Choi SC, Bullock R. Clinical trials in traumatic brain injury. What can we learn from previous studies? In: Slikker E Jr, Trembly B, editors. Neuroprotective Agents. New York Acad Sci; 1997. p. 305–22. New York. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 1993;341:1607–10. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Sulivan J, Brigham A. Immediate consequences of spinal cord injury: possible role of potassium in axonal conduction block. Surg Neurol 1975;3: 317–21. [PubMed] [Google Scholar]
- Enblad P, Valtysson J, Andersson J, Lilja A, Valind S, Antoni G, et al. Simultaneous intracerebral microdialysis and positron emission tomography in the detection of ischemia in patients with subarachnoid hemorrhage. J Cereb Blood Flow Metab 1996;16:637–44. [DOI] [PubMed] [Google Scholar]
- Enzmann GU, Benton RL, Talbott JF, Cao Q, Whittemore SR. Functional considerations of stem cell transplantation therapy for spinal cord repair. J Neurotrauma 2006;23: 479–95. [DOI] [PubMed] [Google Scholar]
- Faden AI, Simon RP. A potential role for excitotoxins in the pathophysiology of spinal cord injury. Ann Neurol 1988;23:623–6. [DOI] [PubMed] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 1989;244:798–800. [DOI] [PubMed] [Google Scholar]
- Fisher LJ. Neural precursor cells: applications for the study and repair of the central nervous system. Neurobiol Dis 1997;4:1–22. [DOI] [PubMed] [Google Scholar]
- Fisher LJ, Raymon HK, Gage FH. Cells engineered to produce acetylcholine: therapeutic potential for Alzheimer’s disease. Ann N Y Acad Sci 1993;695:278–84. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nature Rev 2005;6:449–62. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Muelle RN, Zeng Y, et al. Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp Neurol 2006;198:285–93. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science 2000;287:1433–8. [DOI] [PubMed] [Google Scholar]
- Gao J, Prough DS, McAdoo DJ, Grady JJ, Parsley MO, Ma L, et al. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp Neurol 2006;201:281–92. [DOI] [PubMed] [Google Scholar]
- Goettl VM, Huang Y, Hakshaw KV, Stephens RL Jr. Reduced basal release of serotonin from the ventrobasal thalamus of the rat in a model of neuropathic pain. Pain 2002;99:359–66. [DOI] [PubMed] [Google Scholar]
- Stem Goldman S. and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol 2005;23:862–71. [DOI] [PubMed] [Google Scholar]
- Grossman SD, Rosenberg LJ, Wrathall JR. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol 2001;168:27382. [DOI] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma 1992;9:123–8. [DOI] [PubMed] [Google Scholar]
- Gulino R, Cataudella T, Casamenti F, Pepeu G, Stanzani S, Leanza G. Acetylcholine release from fetal tissue homotopically grafted to the motoneuron-depleted lumbar spinal cord. An in vivo microdialysis study in the awake rat. Exp Neurol 2007;204:326–38. [DOI] [PubMed] [Google Scholar]
- Halliwell B Reactive oxygen species and the central nervous system. J Neurochem 1992;59:1609–23. [DOI] [PubMed] [Google Scholar]
- Hamberger A, Jacobson I, Lehmann A, Sandberg M. Dynamics of the cellular and extracellular compartment of brain amino acids — visions and reality of the dialysis approach. NATA ASI Series 1988;H20:81–90. [Google Scholar]
- Hilgert M, Buchholzer M, Jeltsch H, Kelche C, Cassel JC, Klein J. Serotonergic modulation of hippocampal acetylcholine release after long-term neuronal grafting. Neuroreport 2000;11:3063–5. [DOI] [PubMed] [Google Scholar]
- Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma 2005;22:3–41. [DOI] [PubMed] [Google Scholar]
- Hillered L, Persson L, Nilsson P, Ronne-Engstrom E, Enblad P. Continuous monitoring of cerebral metabolism in traumatic brain injury: a focus on cerebral microdialysis. Curr Opin Crit Care 2006;12:112–8. [DOI] [PubMed] [Google Scholar]
- Hlatky R, Valadka AB, Goodman JC, Robertson CS. Evolution of brain tissue injury after evacuation of acute traumatic subdural hematomas. Neurosurgery 2004;55: 1318–24. [DOI] [PubMed] [Google Scholar]
- Horellou P, Brundin P, Kalen P, Mallet J, Bjorklund A. In vivo release of dopa and dopamine from genetically engineered cells grafted to the denervated rat striatum. Neuron 1990;5:393–402. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma 2000;17:1205–17. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, O’Connell MTO, Nortje J, Smith P, Al-Rawi PG, Gupta AK, et al. Cerebral microdialysis methodology — evaluation of 20 kDa and 100 kDa catheters. Physiol Meas 2005;26:423–8. [DOI] [PubMed] [Google Scholar]
- Isacson O The production and use of cells as therapeutic agents in neurodegenerative diseases. Lancet Neurol 2003;2:417–24. [DOI] [PubMed] [Google Scholar]
- Jacobson I, Sandberg M, Hamberger A. Mass transfer in brain dialysis devices — a new method for the estimation of extracellular amino acids concentration. J Neurosci Meth 1985;15:263–8. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J. Neural stem cells in the adult human brain. Exp Cell Res 1999;253:733–6. [DOI] [PubMed] [Google Scholar]
- Kalen P, Cenci MA, Lindvall O, Bjorklund A. Host brain regulation of fetal locus coeruleus neurons grafted to the hippocampus in 6-hydroxydopamine-treated rats. An intracerebral microdialysis study. Eur J Neurosci 1991;3:905–18. [DOI] [PubMed] [Google Scholar]
- Keirstead HS. Stem cell transplantation into the central nervous system and the control of differentiation. J Neurosci Res 2001;63:233–6. [DOI] [PubMed] [Google Scholar]
- Kerr DA, Llado J, Shamblott MJ, Maragakis NJ, Irani DN, Crawford TO, et al. Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J Neurosci 2003;23:5131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh T, Low WC. Glutamate uptake blockade induces striatal dopamine release in 6-hydroxydopamine rats with intrastriatal grafts: evidence for host modulation of transplanted dopamine neurons. Exp Neurol 1994;127:191–8. [DOI] [PubMed] [Google Scholar]
- Krishnappa IK, Contant CF, Robertson CS. Regional changes in cerebral extracellular glucose and lactate concentrations following severe cortical impact injury and secondary ischemia in rats. J Neurotrauma 1999;16:213–24. [DOI] [PubMed] [Google Scholar]
- Lam AM, Winn HR, Cullen BF, Sundling N. Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg 1991;75:545–51. [DOI] [PubMed] [Google Scholar]
- Le Belle JE, Svendsen CN. Stem cells for neurodegenerative disorders: where can we go from here? BioDrugs 2002;16:389–401. [DOI] [PubMed] [Google Scholar]
- Leanza G, Cataudella T, Dimauro R, Monaco S, Stanzani S. Release properties and functional integration of noradrenergic-rich tissue grafted to the denervated spinal cord of the adult rat. Eur J Neurosci 1999;11:1789–99. [DOI] [PubMed] [Google Scholar]
- Leff SE, Rendahl KG, Spratt SK, Kang UJ, Mandel RJ. In vivo l-DOPA production by genetically modified primary rat fibroblast or 9L gliosarcoma cell grafts via coexpression of GTPcyclohydrolase I with tyrosine hydroxylase. Exp Neurol 1998;151: 249–64. [DOI] [PubMed] [Google Scholar]
- Leslie RGQ, Allen R. Evaluation and improvements of a rapid microassay for measuring superoxide anion production by phagocytes. 2. Biochemical aspects 1987;103:261–6. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Amberg G, Ungerstedt U. Intracerebral microdialysis: I. Experimental studies of diffusion kinetics. J Pharmacol 1989;22:141–56. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature 2006;441:1094–6. [DOI] [PubMed] [Google Scholar]
- Generation Liu D. and detection of hydroxyl radical in vivo in rat spinal cord by microdialysis administration of Fenton’s reagents and microdialysis sampling. J Biochem Biophys Methods 1993;27:281–91. [DOI] [PubMed] [Google Scholar]
- Liu D, McAdoo DJ. An experimental model combining microdialysis with electrophysiology, histology, and neurochemistry for exploring mechanisms of secondary damage in spinal cord injury: effects of potassium. J Neurotrauma 1993a;10:349–62. [DOI] [PubMed] [Google Scholar]
- Liu D, McAdoo DJ. Methylprednisolone reduces excitatory amino acid release following experimental spinal cord injury. Brain Res 1993b;609:293–7. [DOI] [PubMed] [Google Scholar]
- Liu D, McAdoo DJ. A microdialysis model for identifying agents of secondary damage in spinal cord injury. In: Ohnishi ST, Ohnishi T, editors. CRC Series in Membrane-Linked Diseases Central Nervous System Trauma Research Techniques. New York: CRC Press; 1995. p. 405–13. [Google Scholar]
- Liu D, Thangnipon W, McAdoo DJ. Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res 1991;547:344–8. [DOI] [PubMed] [Google Scholar]
- Liu D, Yang R, Yan X, McAdoo DJ. Hydroxyl radicals generated in vivo kill neurons in the rat spinal cord: electrophysiological, histological, and neurochemical results. J Neurochem 1994;62:37–44. [DOI] [PubMed] [Google Scholar]
- Liu S, Ruenes GL, Yezierski RP. NMDA and non-NMDA receptor antagonists protect against excitotoxic injury in the rat spinal cord. Brain Res 1997;756:160–7. [DOI] [PubMed] [Google Scholar]
- Liu D, Sybert TE, Qian H, Liu J. Superoxide production after spinal injury detected by microperfusion of cytochrome. Free Rad Biol Med. 1998;25:298–304. [DOI] [PubMed] [Google Scholar]
- Liu D, Liu J, Wen J. Elevation of hydrogen peroxide after spinal cord injury detected by using the Fenton reaction. Free Rad Biol Med. 1999a;27:478–82. [DOI] [PubMed] [Google Scholar]
- Liu D, Wen J, Liu J, Li L. The roles of free radicals in amyotrophic lateral sclerosis: reactive oxygen species and elevated oxidation of protein, DNA, and membrane phospholipids. FASEB J 1999b;13:2318–28. [DOI] [PubMed] [Google Scholar]
- Liu D, Xu GY, Pan E, McAdoo DJ. Neurotoxicity of glutamate at the concentration released upon spinal cord injury. Neuroscience 1999c;93:1383–9. [DOI] [PubMed] [Google Scholar]
- Liu D, Ling X, Wen J, Liu J. The role of reactive nitrogen species in secondary spinal cord injury: formation of nitric oxide, peroxynitrite, and nitrated protein. J Neurochem 2000;75:2144–54. [DOI] [PubMed] [Google Scholar]
- Liu D, Liu J, Sun D, Alcock NW, Wen J. Spinal cord injury increases iron levels: catalytic production of hydroxyl radicals. Free Rad Biol Med 2003;34:64–71. [DOI] [PubMed] [Google Scholar]
- Liu D, Liu J, Sun D, Wen J. The time course of hydroxyl radical formation following spinal cord injury: the possible role of the iron-catalyzed Haber–Weiss reaction. J Neurotrauma 2004;21:805–16. [DOI] [PubMed] [Google Scholar]
- Liu D, Bao F, Prough DS, DeWitt DS. Peroxynitrite generated at the level produced by spinal cord injury induces peroxidation of membrane phospholipids in normal rat cord: reduction by a metalloporphyrin. J Neurotrauma 2005;22:1123–33. [DOI] [PubMed] [Google Scholar]
- Llado J, Haenggeli C, Maragakis NJ, Snyder EY, Rothstein JD. Neural stem cells protect against glutamate-induced excitotoxicityand promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci 2004;27: 322–31. [DOI] [PubMed] [Google Scholar]
- Maidment NT, Siddall BJ, Rudolph VR, Erdelyl E, Evans CJ. Dual determination of extracellular cholecystokinin and neurotensin fragments in rat forebrain: microdialysis combined with a sequential multiple antigen radioimmunoassay. Neuroscience 1991;45:81–93. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. The effect of morphine on formalin-evoked behaviour and spinal release of excitatory amino acids and prostaglandin E2 using microdialysis in conscious rats. Br J Pharm 1995;114:1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano RM, Schwarcz R. Chronic infusion of endogenous excitatory amino acids into rat striatum and hippocampus. Brain Res Bull 1983;10:47–51. [DOI] [PubMed] [Google Scholar]
- Marsala M, Malmberg AB, Yaksh TL. The spinal loop dialysis catheter: characterization of use in the unanesthetized rat. J Neurosci Meth 1995;62:43–53. [DOI] [PubMed] [Google Scholar]
- McAdoo DJ, Hughes MG, Xu GY, Robak G, de Castro R Jr. Microdialysis studies of the role of chemical agents in secondary damage upon spinal cord injury. J Neurotrauma 1997;14:507–15. [DOI] [PubMed] [Google Scholar]
- McAdoo DJ, Xu GY, Robak G, Hughes MG. Changes in amino acid concentrations over time and space around an impact injury and their diffusion through the rat spinal cord. Exp Neurol 1999;159:538–44. [DOI] [PubMed] [Google Scholar]
- McAdoo DJ, Xu GY, Robak G, Hughes MG, Price EM. Evidence that reversed glutamate uptake contributes significantly to glutamate release following experimental injury to the rat spinal cord. Brain Res 2000;865:283–5. [DOI] [PubMed] [Google Scholar]
- McAdoo DJ, Hughes MG, Nie L, Shah B, Clifton C, Fullwood S, et al. The effect of glutamate receptor blockers on glutamate release following spinal cord injury. Lack of evidence for an ongoing feedback cascade of damage→glutamate release→damage→glutamate release→etc. Brain Res 2005;1038:92–9. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Soares H, Hayes R, Simon R. Effect of noncompetitive blockade of N-methyl-d-aspartate receptors on the neurochemical sequelae of experimental brain injury. J. Neurochem 1990;55:1170–9. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Juhler M, Wieloch T. Novel pharmacologic strategies in the treatment of experimental traumatic brain injury. J. Neurotrauma 1998;15:731–69. [DOI] [PubMed] [Google Scholar]
- McKay R Stem cells in the central nervous system. Science 1997;276:66–71. [DOI] [PubMed] [Google Scholar]
- Menacherry S, Hubert W, Justice JB Jr. In vivo calibration of microdialysis probes for exogenous compounds. Anal Chem 1992;64:577–83. [DOI] [PubMed] [Google Scholar]
- Mills CD, Grady JJ, Hulsebosch CE. Changes in exploratory behavior as a measure of chronic central pain following spinal cord injury. J Neurotrauma 2001;18:1091–105. [DOI] [PubMed] [Google Scholar]
- Narayan RK, et al. Clinical trials in head injury. J. Neurotrauma 2002;19:503–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KL, Mansbach RS, Menniti FS, Balster RL. The phencyclidine-like discriminative stimulus effects and reinforcing properties of the NR2B-selective N-methyl-d-aspartate antagonist CP-101 606 in rats and rhesus monkeys. Behavioural Pharmacol 2007;18:731–43. [DOI] [PubMed] [Google Scholar]
- Nieto-Sampedro M, Kesslak JP, Gibbs R, Cotman CW. Effects of conditioning lesions on transplant survival, connectivity, and function. Role of neurotrophic factors. Ann N Y Acad Sci 1987;495:108–19. [DOI] [PubMed] [Google Scholar]
- Nilsson OG, Bjorklund A. Behaviour-dependent changes in acetylcholine release in normal and graft-reinnervated hippocampus: evidence for host regulation of grafted cholinergic neurons. Neuroscience 1992;49:33–44. [DOI] [PubMed] [Google Scholar]
- Nilsson OG, Kalen P, Rosengren E, Bjorklund A. Acetylcholine release from intrahippocampal septal grafts is under control of the host brain. Proc Natl Acad Sci U S A 1990a;87:2647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson P, Hillered L, Pontén U, Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J Cerebral Blood Flow Metab 1990b;10:631–7. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Ronne-Engström E, Flink R, Ungerstedt U, Carlson H, Hillered L. Epileptic seizure activity in the acute phase following cortical impact trauma in rat. Brain Res 1994;637:227–32. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP, Urenjak J. Is high extracellular glutamate the key to excitotoxicity in traumatic brain injury? J Neurotrauma 1997;14:677–98. [DOI] [PubMed] [Google Scholar]
- Olney JW. Glutamate-induced retinal degeneration in neonatal mice. Electron microscopy of the acutely evolving lesion. J Neuropath Exp Neurol 1969;28:455–74. [DOI] [PubMed] [Google Scholar]
- Olney JW, Ho OL, Rhee V. Cytotoxic effects of acidic and sulphur containing amino acids on the infant mouse central nervous system. Exp Brain Res 1971;14:61–76. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik V. Graft-induced plasticity in the mammalian host CNS. Cell Transplant 2004a;3:307–18. [DOI] [PubMed] [Google Scholar]
- Ourednik V, Ourednik J. Multifaceted dialogue between graft and host in neurotrans-plantation. J Neurosci Res 2004b;76:193–204. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik W, Van der LH. Do foetal neural grafts induce repair by the injured juvenile neocortex? Neuroreport 1993;5:133–6. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol 2002;20:1103–10. [DOI] [PubMed] [Google Scholar]
- Paleckova V, Palecek J, McAdoo DJ, Willis WD. The non-NMDA antagonist CNQX prevents release of amino acids into the rat spinal cord dorsal horn evoked by sciatic nerve stimulation. Neurosci Lett 1992;148:19–22. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH. Cell culture. Progenitor cells from human brain after death. Nature 2001;411:42–3. [DOI] [PubMed] [Google Scholar]
- Panter SS, Yum SW, Faden AI. Alteration in extracellular amino acids after traumatic spinal cord injury. Ann Neurol 1990;27:96–9. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB Jr. Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis. J Neurochem 1992;58:212–8. [DOI] [PubMed] [Google Scholar]
- Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science 1979;204:643–7. [DOI] [PubMed] [Google Scholar]
- Persson L, Hillered L. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J Neurosurg 1992;76:72–80. [DOI] [PubMed] [Google Scholar]
- Poca MA, Sahuquillo J, Vilalta A, Garnacho A. Lack of utility of arteriojugular venous differences of lactate as a reliable indicator of increased brain anaerobic metabolism in traumatic brain injury. J Neurosurg 2007;106:530–7. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol 2001;19:1134–40. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gomez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, et al. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells (Dayt) 2007;25:918–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney BA, Crown ED, Hulsebosch CE, McAdoo DJ. Preemptive analgesia with lidocaine prevents failed back surgery syndrome. Exp Neurol 2004;204:589–96. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993;362:59–62. [DOI] [PubMed] [Google Scholar]
- Rosenberg LJ, Teng YD, Wrathall JR. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f) quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci 1999;19:464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F, Bianca VD, Grzeskowiak LZ. Mechanisms of oxygen free radicals production in granulocytes. In: Novelli Ursini, editor. Oxygen Free Radicals in Shock. Florence, Karger, Basel; 1986. p. 15–28. [Google Scholar]
- Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med 2000;6:271–7. [DOI] [PubMed] [Google Scholar]
- Sajedianfard J, Khatami S, Semnanian S, Naghdi N, Jorjani M. In vivo measurement of noradrenaline in the locus coeruleus of rats during the formalin test: a microdialysis study. Eur J Pharmacol 2005;512:153–6. [DOI] [PubMed] [Google Scholar]
- Samuelsson C, Hillered L, Zetterling M, Enblad P, Hesselager G, Pyttlefors M, et al. Cerebral glutamine and glutamate levels in relation to compromised energy metabolism: a microdialysis study in subarachnoid hemorrhage patients. J Cereb Blood Flow Metab 2007;27:1309–17. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fuggacia I, Main JA, Lump JE. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma 2003;20:179–93. [DOI] [PubMed] [Google Scholar]
- Schulz MK, Wang LP, Tange M, Bjerre P. Cerebral microdialysis monitoring: determination of normal and ischemic cerebral metabolisms in patients with aneurismal subarachnoid hemorrhage. J Neurosurg 2000;93:808–14. [DOI] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. PNAS 1998;95:13726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear DA, Tate MC, Archer DR, Hoffman SW, Hulce VD, Laplaca MC, et al. Neural progenitor cell transplants promote long-term functional recovery after traumatic brain injury. Brain Res 2004;1026:11–22. [DOI] [PubMed] [Google Scholar]
- Shin S, Mitalipova M, Noggle S, Tibbitts D, Venable A, Rao R, et al. Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells (Dayt) 2006;24:125–38. [DOI] [PubMed] [Google Scholar]
- Sidall PJ, Yezierski RP, Loeser JD. Taxonomy and epidemiology of spinal cord injury pain. In:Yezierski RP, Burchiel KJ, editors. Spinal cord injury pain. Seattle: IASP Press; 2002. p. 9–24. [Google Scholar]
- Siesjö BK. Brain energy metabolism. New York: John Wiley & Sons; 1978. p. 193. [Google Scholar]
- Silva E, Hernandez L, Contreras Q, Guerrero F, Alba G. Noxious stimulation increases glutamate and arginine in the periaqueductal gray matter in rats: a microdialysis study. Pain 2000;87:131–5. [DOI] [PubMed] [Google Scholar]
- Skilling SR, Smullin DH, Beitz AJ, Larson AA. Extracellular amino acid concentrations in the dorsal spinal cord of freely moving rats following veratridine and nociceptive stimulation. J Neurochem 1988;51:127–32. [DOI] [PubMed] [Google Scholar]
- Smith VA, Beyer CE, Brandt MR. Neurochemical changes in the RVM associated with peripheral inflammatory stimuli. Brain Res 2006;1095:65–72. [DOI] [PubMed] [Google Scholar]
- Sorkin LS. NMDA evokes an l-NAME sensitive spinal release of glutamate and citrulline. NeuroReport 1993;4:479–82. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Steinman JL, Hughes MG, Willis WD, McAdoo DJ. Microdialysis recovery of serotonin released in spinal cord dorsal horn. J Neurosci Methods 1988;23:131–8. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, McAdoo DJ, Willis WD. Raphe magnus stimulation-induced antinociception in the cat is associated with release of amino acids as well as serotonin in the lumbar dorsal horn. Brain Res 1993;618:95–108. [DOI] [PubMed] [Google Scholar]
- Ståhle L, Segersvard S, Ungerstedt U. A comparison between three methods for estimation of extracellular concentrations of oxogenous and endogenous compounds by microdialysis. J Pharmacol Methods 1991;25:41–52. [DOI] [PubMed] [Google Scholar]
- Ståhl N, Ungerstedt U, Nordström CH. Brain energy metabolism during controlled reduction of cerebral perfusion pressure in severe head injuries. Intensive Care Med 2001;27:1215–23. [DOI] [PubMed] [Google Scholar]
- Staub F, Graf R, Gabel P, Köchling M, Klug N, Heiss WD. Multiple interstitial substances measured by microdialysis in patients with subarachnoid hemorrhage. Neurosurgery 2000;47:1106–16. [DOI] [PubMed] [Google Scholar]
- Stiller CO, Taylor BK, Linderoth B, Gustafsson H, Afrah AW, Brodin E. Adv Drug Deliv Rev 2003;55:1065–79. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods 1998;85:141–52. [DOI] [PubMed] [Google Scholar]
- Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, et al. Role of neutrophils in spinal cord injury in the rat. Neuroscience 1997;79:1177–82. [DOI] [PubMed] [Google Scholar]
- Tarasenko YI, Yu Y, Jordan PM, Bottenstein J, Wu P. Effect of growth factors on proliferation and phenotypic differentiation of human fetal neural stem cells. J Neurosci Res 2004;78:625–36. [DOI] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A 2002;99:3024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–7. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BHC. Brain microdialysis of GABA and glutamate: what does it signify? Synapse 1997;27:242–61. [DOI] [PubMed] [Google Scholar]
- Tisdall MM, Smith M. Cerebral microdialysis: research technique or clinical tool. Br J Anaesthesia 2006;97:18–25. [DOI] [PubMed] [Google Scholar]
- Uchida K, Tsuzaki N, Nagatsu T, Kohsaka S. Tetrahydrobiopterin-dependent functional recovery in 6-hydroxydopamine-treated rats by intracerebral grafting of fibroblasts transfected with tyrosine hydroxylase cDNA. Dev Neurosci 1992;4:173–80. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A 2000;97:14720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U Measurement of neurotransmitter release by intracranial dialysis. In: Marsden CA, editor. Measurements of Neurotransmitter Release In Vivo. New York: John Wiley & Sons; 1984. p. 81–105. [Google Scholar]
- Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, et al. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol 1999;156:71–83. [DOI] [PubMed] [Google Scholar]
- Vespa PM, McArthur D, O’Phelan K, Glenn T, Etchepare M, Kelly D, et al. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J Cereb Blood Flow Metab 2003;23:865–77. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab 2005;25:763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P, Boonyyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, et al. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med 2006;34:850–6. [DOI] [PubMed] [Google Scholar]
- Villa A, Snyder EY, Vescovi A, Martinez-Serrano A. Establishment and properties of a growth factor-dependent, perpetual neural stem cell line from the human CNS. Exp Neurol 2000;161:67–84. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, Bencsics C, Kang UJ. Role of aromatic l-amino acid decarboxylase for dopamine replacement by genetically modified fibroblasts in a rat model of Parkinson’s disease. J Neurochem 1997;69:2055–63. [DOI] [PubMed] [Google Scholar]
- Willis WD. Control of nociceptive transmission in the spinal cord. In: Ottoson D, editor. Sens Physiol, vol. 3. Berlin: Springer–Verlag; 1982. p. 1–159. [Google Scholar]
- Wrathall JR, Teng YD, Choiniere D, Mundt DJ. Evidence that local non-NMDA receptors contribute to functional deficits in contusive spinal cord injury. Brain Res 1992;586:140–3. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Choiniere D, Teng YD. Dose-dependent reduction of tissue loss and functional impairment after spinal cord trauma with the AMPA/kainite antagonist NBQX. J Neurosci 1994;14:6598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrathall JR, Teng YD, Choiniere D. Amelioration of functional deficits from spinal cord trauma with systemically administered NBQX, an antagonist of non-N-methyl-d-aspartate receptors. Exp Neurol 1996;137:119–26. [DOI] [PubMed] [Google Scholar]
- Wu P, Tarasenko YI, Gu YP, Huang LYM, Coggeshall RE, Yu YJ. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rat. Nat Neurosc 2002;5:1271–8. [DOI] [PubMed] [Google Scholar]
- Xu GY, McAdoo DJ, Hughes MG, Robak G, de Castro R Jr. Considerations in the determination by microdialysis of resting extracellular amino acid concentrations and release upon spinal cord injury. Neuroscience 1998;86:1011–21. [DOI] [PubMed] [Google Scholar]
- Xu GY, Hughes MG, Ye Z, Hulsebosch CE, McAdoo DJ. Concentrations of glutamate released following spinal cord injury kill oligodendrocytes in the spinal cord. Exp Neurol 2004;187:329–36. [DOI] [PubMed] [Google Scholar]
- Xu GY, Hughes MG, Zhang L, Cain L, McAdoo DJ. Administration of glutamate into the spinal cord at extracellular concentrations reached post-injury causes functional impairements. Neurosci Lett 2005;384:271–6. [DOI] [PubMed] [Google Scholar]
- Xu GY, Liu S, Hughes MG, McAdoo DJ. Glutamate-induced losses of oligodendrocytes and neurons and activation of caspase-3 in the rat spinal cord. Neuroscience in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan JY, Sun RQ, Hughes MG, McAdoo DJ, Willis WD. Intradermal injection of capsaicin induces acute substance P release from rat spinal cord dorsal horn. Neurosci Lett 2006;410:183–6. [DOI] [PubMed] [Google Scholar]
- Yezierski RP, Santana M, Park SH, Madsen PW. Neuronal degeneration and spinal cavitation following intraspinal injections of quisqualic acid in the rat. J Neurotrauma 1993;10:445–56. [DOI] [PubMed] [Google Scholar]
- Young W, Koreh I, Yen V, Lindsay A. Effect of sympathectomy on extracellular potassium ionic activity and blood flow in experimental spinal cord contusion. Brain Res 1982;253:115–24. [DOI] [PubMed] [Google Scholar]
- Zauner A, Bullock R. The role of excitatory amino acids in severe brain trauma: opportunities for therapy: A review. J Neurotrauma 1995;12:547–54. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 2001;19:1129–33. [DOI] [PubMed] [Google Scholar]


