Abstract
Initial adhesion of fungi to plasticized polyvinyl chloride (pPVC) may determine subsequent colonization and biodeterioration processes. The deteriogenic fungus Aureobasidium pullulans was used to investigate the physicochemical nature of adhesion to both unplasticized PVC (uPVC) and pPVC containing the plasticizers dioctyl phthalate (DOP) and dioctyl adipate (DOA). A quantitative adhesion assay using image analysis identified fundamental differences in the mechanism of adhesion of A. pullulans blastospores to these substrata. Adhesion to pPVC was greater than that to uPVC by a maximum of 280% after a 4-h incubation with 108 blastospores ml−1. That plasticizers enhance adhesion to PVC was confirmed by incorporating a dispersion of both DOA and DOP into the blastospore suspension. Adhesion to uPVC was increased by up to 308% in the presence of the dispersed plasticizers. Hydrophobic interactions were found to dominate adhesion to uPVC because (i) a strong positive correlation was observed between substratum hydrophobicity (measured by using a dynamic contact angle analyzer) and adhesion to a range of unplasticized polymers including uPVC, and (ii) neither the pH nor the electrolyte concentration of the suspension buffer, both of which influence electrostatic interactions, affected adhesion to uPVC. In contrast, adhesion to pPVC is principally controlled by electrostatic interactions. Enhanced adhesion to pPVC occurred despite a relative reduction of 13° in the water contact angle of pPVC compared to that of uPVC. Furthermore, adhesion to pPVC was strongly dependent on both the pH and electrolyte concentration of the suspension medium, reaching maximum levels at pH 8 and with an electrolyte concentration of 10 mM NaCl. Plasticization with DOP and DOA therefore increases adhesion of A. pullulans blastospores to pPVC through an interaction mediated by electrostatic forces.
Major problems of substratum damage occur when plasticized polyvinyl chloride (pPVC) is colonized by microorganisms in many different environmental situations. It has long been established that this susceptibility results from the presence of plasticizers, commonly organic acid esters such as dioctyl phthalate (DOP) and dioctyl adipate (DOA), added to modify physical or mechanical properties of the polymer (7). Since these early studies, degradation of ester-based plasticizers has been demonstrated among both bacteria (4, 5, 14) and fungi (2, 45). Loss of plasticizers from pPVC due to microbial degradation results in brittleness, shrinkage, and ultimately failure of the PVC in its intended application.
Although no detailed quantitative studies have been published, fungi are reported to be the principal deteriogenic organisms in structural and outdoor applications of pPVC (3, 18). However, despite widespread commercial use of pPVC and considerable economic losses due to its biodeterioration, mechanisms of fungal attachment to pPVC have not previously been examined.
Microbial adhesion is the first in a series of events that occur during the colonization of a solid substratum. Adhesion to inert materials such as plastics or glass is known to be controlled by nonspecific interactions between the cell surface and the substratum. Research has focused on bacterial adhesion, where hydrophobicity both of the substratum (8, 35, 46) and of the cell surface (33, 40, 44) and electrostatic charge on the cell surface (17, 34, 43) are important factors in adhesion to inert substrata.
While bacterial adhesion to surfaces has been studied extensively, the nonspecific adhesion of fungi has received comparatively little attention. Most fungal adhesion studies have focused on adhesion of the opportunistic pathogen Candida albicans to synthetic materials used for medical prostheses. Increased adhesion of C. albicans to plastics has been associated with increased hydrophobicity both of the fungal surface (30, 32) and of the substratum (27). Electrostatic forces have also been demonstrated to influence adhesion of C. albicans to hydrophilic glass (25), although they are thought to be of minor importance in adhesion to more hydrophobic plastics (27). Therefore, similar physicochemical characteristics appear to control adhesion of both bacteria and C. albicans.
Nothing is known about the physicochemical factors controlling adhesion of fungi that colonize and deteriorate plastics within the environment. This study reports on the mechanisms of adhesion of the deuteromycete A. pullulans because it was found to be the dominant fungus causing deterioration of pPVC films during outdoor exposure trials in Florida (18). A. pullulans is ubiquitous within the environment and is known to colonize many habitats (9). It is one of relatively few fungi that can colonize living leaf surfaces (1) and is also the principal colonizer of painted wood surfaces (15).
As part of a long-term study of microbial colonization processes occurring on pPVC, the initial adhesion of the deteriogenic fungus A. pullulans has been studied in vitro. We have investigated the effect of incorporating the plasticizers DOP and DOA into PVC on the adhesion of A. pullulans blastospores. Physicochemical parameters influencing the nonspecific adhesion of blastospores to both unplasticized PVC (uPVC) and pPVC have also been investigated.
MATERIALS AND METHODS
A. pullulans (de Bary) Arnaud.
A. pullulans (IMI70103) was maintained on malt extract agar (Oxoid, Unipath, Ltd., Basingstoke, United Kingdom), and to produce blastospores, cultures were incubated for 5 days at 25°C in the dark. Under these conditions, only the mycelial and blastospore morphotypes of A. pullulans developed. For long-term storage, blastospores were frozen at −80°C in 20% (vol/vol) glycerol solution (BDH, Poole, United Kingdom).
Growth of A. pullulans on pPVC as the sole carbon source was used as a test method to confirm the ability of this isolate to deteriorate pPVC. A piece of pPVC (4 by 4 cm) was sandwiched between two layers of 12 g of bacteriological agar liter−1 (Oxoid) in deionized water. The upper layer of agar was seeded with approximately 105 blastospores of A. pullulans ml−1 before pouring. A control agar plate was prepared that contained blastospores, but without a piece of pPVC incorporated. Both plates were incubated at 25°C for 7 days, after which time, growth of A. pullulans could clearly be seen in agar immediately above the pPVC. Absence of growth on the control plate confirmed the ability of A. pullulans to obtain a source of carbon from the pPVC.
Adhesion assay.
pPVC sheets (0.5 mm thick) were formulated that contained the following (parts per hundred resin): EP 6779 (PVC resin) [European Vinyls Corporation (UK) Ltd.], 75; Vinnolit C65V (PVC resin), 25; DOP (plasticizer) (Exxon Chemicals, Ltd.), 25; DOA (plasticizer) (Exxon Chemicals, Ltd.), 25; Lankromark LN138 (calcium-zinc stabilizer) (AKCROS), 2; and Lankroflex ED63 (epoxidized oleate ester), 3. uPVC sheets (0.2 mm thick) were obtained from Goodfellow, Ltd., Cambridge, United Kingdom.
Discs, 4 mm in diameter, were cut from sheets by using a pair of punch pliers (RS components). Discs were cleaned in 2% Lipsol detergent (LIP, Shipley, England) and rinsed thoroughly in deionized water. Handled by the edges only and with forceps, discs were placed in rows of five into the wells of a 96-well assay plate (Costar).
A. pullulans blastospores were harvested in phosphate-buffered saline (PBS) containing 8.0 g of NaCl liter−1, 0.2 g of KCl liter−1, 1.15 g of Na2HPO4 liter−1, 0.2 g of KH2PO4 liter−1, and 0.1 g of MgCl2 liter−1 at pH 7.3 and separated from hyphae by filtration through three layers of lens tissue paper. Blastospores were centrifuged for 8 min at 3,600 × g, washed three times, and resuspended in PBS to an optical density at 540 nm of 0.59 (108 blastospores ml−1). Aliquots of 200 μl of the blastospore suspension were placed into wells containing pPVC discs by using a multichannel pipette (Treff Lab). Following incubation at 25°C, the blastospore suspension was removed by pipette, and discs were washed three times by sequentially adding and removing 200-μl aliquots of PBS. During the washing procedure, pipette tips were inserted into the base of wells so that liquid crossed the surfaces of discs evenly and with minimal variation in shear forces. Spores were then fixed for 15 min by the addition of 100 μl of 20% (vol/vol) formaldehyde to each well and washed once in PBS. Discs were allowed to air dry for 1 h before being stained with Gram crystal violet (Difco). Stain (100 μl) was applied to each well for 5 s and quickly removed, and discs were washed a further three times in PBS. Stained discs were then transferred from wells to a microscope slide for image analysis.
Blastospores on pPVC discs were visualized by using a Leica Medilux microscope equipped with an automated stage for image analysis. Digital images of the pPVC surface were captured under bright-field illumination with a charge-coupled device camera (Sony XC-75CE), and the percentage of surface covered with attached blastospores was quantified with image analysis software (Quantimet Qwin 570, version 01.00; Leica, Ltd. Cambridge, United Kingdom).
Reproducibility of adhesion data.
To determine disc-to-disc (intrabatch) variation within the adhesion assay, rows of five discs of both uPVC and pPVC were exposed to a suspension of 108 blastospores ml−1 derived from a single plate. Five rows were sampled for each material, and adhesion was quantified after a 4-h incubation period. To determine whether different batches of blastospores gave different levels of adhesion (interbatch variation), adhesion was measured by exposing rows of five pPVC and uPVC discs to blastospores harvested from five different cultures. Inter- and intrabatch variation in adhesion were statistically assessed by analysis of variance.
Kinetics of adhesion to uPVC and pPVC.
To determine the time period required for maximal adhesion to pPVC, a time course experiment was carried out over a 10-h period with a suspension of 108 blastospores ml−1. After each sample time, five pPVC discs were removed, washed, and stained, and the mean percentage of surface cover with blastospores for the discs was determined. The influence of blastospore concentration on numbers of blastospores attaching was determined by exposing rows of five discs to blastospore concentrations in the range 2 × 107 to 5 × 108 blastospores ml−1 for a 4-h adhesion period.
Influence of plasticizers on adhesion to uPVC.
A mixed dispersion of both DOP and DOA was created within PBS suspension buffer to examine the influence of plasticizers on adhesion of A. pullulans blastospores to uPVC. Five milliliters of each plasticizer was added to 400 ml of PBS. Both DOP and DOA are immiscible with water but are completely miscible with each other and form an organic phase over the PBS. The entire volume was homogenized (Ystral D-7801; 260 W; 25,000 rpm; Dottingen) for 1 min and centrifuged for 10 min at 3,600 × g in order to remove large droplets of plasticizer. The resulting dispersion was separated from liquid DOP and DOA remaining on the surface of the PBS by running the volume through a glass separation funnel fitted with a tap. The eluent from the column was considered to be the 100% plasticizer concentration. Dilutions were prepared to contain relative plasticizer concentrations in the range 0 to 100% of this undiluted dispersion. Blastospores were suspended to 108 blastospores ml−1 in each dilution of the dispersion and applied to rows of five discs of uPVC for 4 h. Plasticizer concentrations in PBS were kept constant throughout the washing procedure.
Both DOP and DOA exhibit low water solubility of up to 1 mg liter of H2O−1 (Chemical Abstract Service no. 117-84-0 for DOP and 103-23-1 for DOA). To determine the effect of low levels of dissolved plasticizer on adhesion, dispersed plasticizers in PBS were ultracentrifuged at 80,000 × g for 30 min to remove undissolved plasticizer. The resulting clear solution was separated from the remaining liquid DOP and DOA by using a separation funnel, and the eluent from the column was considered to be the 100% dissolved plasticizer concentration. Dilutions were prepared to contain relative dissolved plasticizer concentrations in the range 0 to 100% of the undiluted solution. Rows of five discs of uPVC were exposed for 4 h to blastospores suspended to 108 blastospores ml−1 in each dilution. Dissolved plasticizer concentrations in PBS were kept constant throughout the washing procedure.
Effect of substratum hydrophobicity on adhesion.
Contact angle measurements were made on a range of polymers and glass to examine the influence of substratum hydrophobicity on adhesion. The polymers used were polyethylene tetraphthalate (PET), polypropylene, polytetrafluoroethylene, and uPVC (Goodfellow Ltd.); polyethylene and fluoroethylenepropylene (Fluorplast, Raamsdonkveer, The Netherlands); and tissue culture-treated PET (Thermanox) (Agar Scientific, Ltd., Stansted, United Kingdom). Glass microscope slides were obtained from Chance Proper, Ltd., Warley, England.
Surface hydrophobicity of materials was determined with a DCA-312 dynamic contact angle analyzer (Cahn Instruments, Madison, Wis.) (11). This equipment uses the principle of the Wilhelmy balance and has an advantage over sessile drop methods, in that larger surface areas may be sampled (28). Samples (2 by 2 cm) were cut from sheets of each polymer, and the contact angle was measured by immersion to a depth of 1 cm at a stage speed of 19.6 μm s−1. The wetting fluid used was deionized water (Elix 3; Millipore Corp., Watford, United Kingdom). Advancing contact angles (θa) were determined on five replicate samples of each material.
Six replicate discs of each polymer were prepared as described for pPVC and uPVC. Fragments of glass small enough to be inserted into plate wells were prepared by breaking microscope slides under tissue paper. Adhesion to the different materials was measured at a blastospore concentration of 108 cells ml−1 incubated with discs for 4 h.
Influence of pH on adhesion to pPVC and uPVC.
Blastospores were suspended to 108 blastospores ml−1 in PBS adjusted to pH values in the range 2 to 13. Rows of five discs of both pPVC and uPVC were exposed for 4 h to blastospores at each pH value. pH values in the PBS solutions were kept constant throughout the washing procedure.
Influence of electrolyte concentration on adhesion to pPVC and uPVC.
Blastospores were suspended to 108 blastospores ml−1 in deionized water containing the electrolyte NaCl in the concentration range 0 to 100 mM. uPVC and pPVC discs in rows of five were exposed to blastospores at each NaCl concentration for 4 h. NaCl electrolyte concentrations were kept constant throughout the washing procedure.
Microelectrophoresis.
Zeta potentials, a measure of the net charge on the surface of the blastospores, were measured in PBS at a range of pH values at room temperature with a Lazer Zee Meter 501 (PenKem), which uses the scattering of incident laser light to detect cells at relatively low magnifications. The absolute electrophoretic mobilities can be derived directly from the velocities of the organisms in the applied electric field, the applied voltage, and the dimensions of the electrophoresis chamber (21). Electrophoretic mobilities were measured for blastospores suspended to a concentration of ≈1 × 107 blastospores ml−1 and converted into zeta potentials on the basis of the Helmholtz-Smoluchowski equation (20).
LTSEM.
For low-temperature scanning electron microscopy (LTSEM), pPVC and uPVC discs were incubated with 108 blastospores ml−1 for 4 h before undergoing washing, fixation, and crystal violet staining as under normal assay conditions. The discs were then rapidly frozen by being plunged into nitrogen slush and transferred to a Cambridge 200 scanning electron microscope.
Once inside the microscope, ice on the surface of the discs was sublimed at −65°C until all visible ice crystals had disappeared. The discs were withdrawn into the prechamber and sputter coated with gold. The specimen stub was returned to the cold stage set at −170°C and observed.
RESULTS
Adhesion assay.
To determine the reproducibility of the adhesion assay, adhesion values were compared statistically by analysis of variance. Disc-to-disc (intrabatch) variation was quantified for both uPVC and pPVC by comparison of adhesion values derived from discs incubated with the same batch of A. pullulans blastospores. No significant disc-to-disc variation (P > 0.05) occurred for either material among the means of five rows of five discs incubated in the wells of a tissue culture plate. The individual mean and standard deviation values for percentage surface cover with blastospores among the five rows ranged between 32.3% ± 2.3% and 39.5% ± 2.7% for pPVC and between 2.5% ± 0.4% and 3.4% ± 1.4% for uPVC.
However, for both uPVC and pPVC, significant interbatch variation (P < 0.001) occurred among mean adhesion values from five separate batches of blastospores. Individual mean and standard deviation values for percentage surface cover with blastospores among each of the five batches ranged between 31.5% ± 3.1% and 43.2% ± 3.0% for pPVC and between 2.4% ± 0.4% and 15.9% ± 3.0% for uPVC. To eliminate this source of variation, each subsequent adhesion experiment was completed with blastospores from a single batch.
Kinetics of adhesion to pPVC and uPVC.
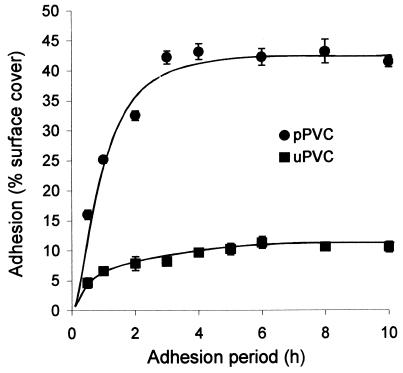
The kinetics of adhesion of A. pullulans blastospores to pPVC and uPVC were examined by monitoring the percentage of surface cover of discs with adhered blastospores over a 10-h period (Fig. 1). Maximum adhesion to pPVC was 280% greater than that to uPVC at 4 h. Adhesion to uPVC rose quickly to 7% in 1 h and then more slowly to reach a plateau of 11.3% by 6 h. In contrast, the percentage of the pPVC disc surface covered with attached blastospores increased rapidly up to 3 h and reached a maximum of 43% surface cover after 4 h of incubation. Regular checks for blastospore germination on both uPVC and pPVC surfaces were made throughout the 10-h incubation period, and no incidence of germination was observed. A second time course experiment investigated the possibility that the adhesion plateau observed on pPVC was an artifact of the settling process which occurred during incubation and that more blastospores could potentially attach. After a 6-h adhesion period, unbound spores were washed from discs and replaced with a fresh suspension of 108 blastospores ml−1 in PBS. Adhesion was then monitored for an additional 6-h period. Replacement of the spore suspension resulted in no further increase in adhesion (data not shown), and the percentage of surface cover did not increase beyond a maximum of 43%, suggesting that saturation of binding sites on the pPVC had occurred. Since maximal adhesion on pPVC occurred after 4 h, this time was subsequently chosen as the incubation period.
FIG. 1.
Time course of adhesion of A. pullulans IMI70103 blastospores to uPVC and pPVC. Error bars show ±1 standard error of the mean.
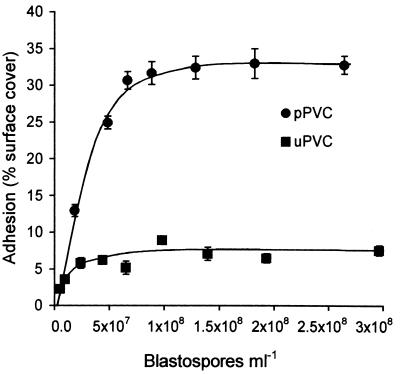
In order to determine the effect of increasing spore concentration on adhesion, the percentage of cover of pPVC and uPVC discs was determined after 4 h at spore concentrations in the range 5.2 × 106 to 3 × 108 blastospores ml−1 (Fig. 2). The isotherm for binding of A. pullulans to pPVC showed that as the concentration of unbound spores increased to 7 × 107 blastospores ml−1, adhesion to pPVC increased quickly to 30.6% surface cover, slowed over the concentration range 7 × 107 to 1.3 × 108 blastospores ml−1, and reached a maximum of 33.0% surface cover at a concentration of 1.8 × 108 blastospores ml−1. The percentage of cover of the pPVC surface with blastospores did not exceed 33% (1.1 × 105 blastospores mm−2) in this experiment. Adhesion to uPVC increased rapidly to 5.8% surface cover in the concentration range 5.2 × 106 to 2.3 × 107 blastospores ml−1 and reached a plateau of 7.6% surface cover with blastospore concentrations of 1.0 × 108 ml−1 and above.
FIG. 2.
Influence of blastospore concentration on adhesion of A. pullulans to uPVC and pPVC. Error bars show ±1 standard error of the mean.
Influence of plasticizers on adhesion.
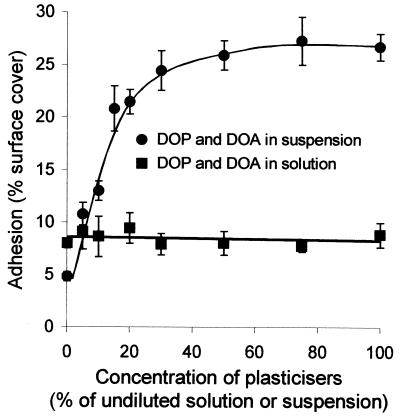
To determine whether the increased adhesion to pPVC relative to uPVC was due to an attractive interaction between blastospores and the plasticizers, adhesion to uPVC was measured with blastospores suspended in a range of concentrations of a mixed dispersion of DOP and DOA (Fig. 3). Adhesion of A. pullulans blastospores to uPVC was increased by up to 308% by incorporation of the plasticizers into the suspension medium. Adhesion increased most rapidly within the plasticizer concentration range 0 to 15% of the undiluted dispersion, where the percentage of surface cover of the uPVC with blastospores increased from 6.2% to 20.7%. The rate of increase in adhesion slowed during the plasticizer concentration range of 15 to 50%, reaching a plateau in adhesion of 25.4% surface cover with blastospores when the relative concentration of plasticizers was 50% of the undiluted dispersion. While adhesion to uPVC was strongly dependent on the concentration of dispersed plasticizers present in PBS, dissolved plasticizers remaining after removal of the dispersion did not influence the attachment of blastospores to the plastic (Fig. 3). Adhesion remained constant at 8% surface cover across the relative dissolved plasticizer concentration range of 0 to 100% of the undiluted solution.
FIG. 3.
Influence of the plasticizers DOP and DOA both as a suspension and dissolved in PBS on adhesion of A. pullulans blastospores to uPVC. Error bars show ±1 standard error of the mean.
Substratum hydrophobicity.
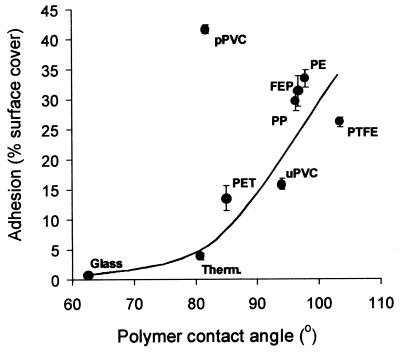
The importance of substratum hydrophobicity in blastospore adhesion was investigated (Fig. 4) by comparing levels of adhesion to a range of materials with different water contact angles. The lowest adhesion was to the relatively hydrophilic glass, where the percentage of cover of the surface with blastospores did not exceed 1%. The highest adhesion levels of 41% surface cover were observed on pPVC. A clear relationship existed between increasing water contact angle of the surface and increased adhesion of blastospores for the majority of materials tested, except pPVC. Adhesion to pPVC was approximately 660% higher than would be expected due to its hydrophobicity. In contrast, adhesion of blastospores to uPVC fitted the relationship between adhesion and hydrophobicity. Despite a relative reduction of 13° in the water contact angle of pPVC compared to that of uPVC, adhesion to pPVC was 163% greater than to uPVC.
FIG. 4.
Effect of increasing surface contact angle on adhesion of A. pullulans IMI70103 to different substrata. Therm., Thermanox (tissue culture-treated PET); PTFE, polytetrafluoroethylene; PP, polypropylene; FEP, fluoroethylene polypropylene; PE, polyethylene. Error bars show ±1 standard error of the mean.
Influence of pH and electrolyte concentration.
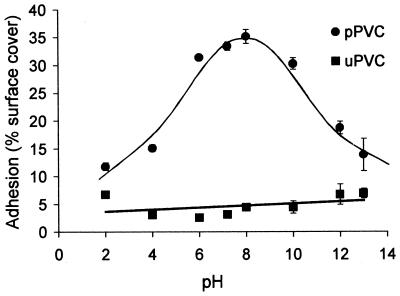
To investigate whether incorporation of plasticizers into PVC influences electrostatic interactions between blastospores and the substratum, levels of adhesion to both pPVC and uPVC were compared at a range of different pHs and electrolyte concentrations of the suspension buffer. Adhesion to pPVC was strongly influenced by pH (Fig. 5), with maximum adhesion occurring in the pH range 6 to 10. The percentage of surface cover with blastospores rose from 12% to 35% as pH increased from 2 to 8, reducing to 14% surface cover at pH 13. In contrast, adhesion of blastospores to uPVC was unaffected by changing the pH of the suspension buffer.
FIG. 5.
Influence of suspension buffer pH on adhesion of A. pullulans blastospores to pPVC and uPVC. Error bars show ±1 standard error of the mean.
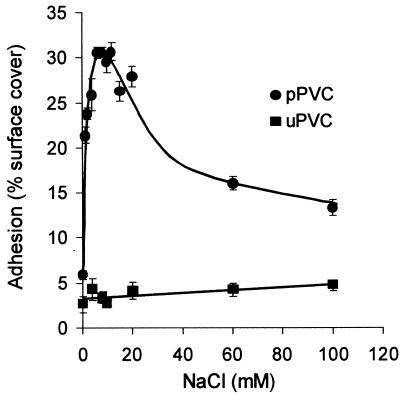
Increasing molarities of NaCl were added to suspensions of blastospores in deionized water prior to incubation with pPVC discs (Fig. 6). The percentage of cover of disc surfaces with attached blastospores increased from 6% with no electrolyte to a maximum of 30% across the optimal concentration range of 6 to 12 mM NaCl. A subsequent reduction in adhesion to 13% surface cover occurred as the electrolyte concentration was further increased to 100 mM. Adhesion to uPVC was unaffected by the electrolyte concentration within the suspension buffer.
FIG. 6.
Effect of electrolyte (NaCl) concentration on adhesion of A. pullulans IMI70103 to pPVC and uPVC. Error bars show ±1 standard error of the mean.
Influence of pH on blastospore cell surface charge.
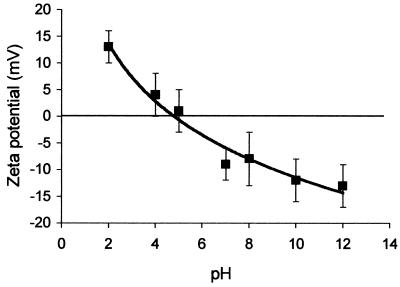
To study the effect of pH the on cell surface electrostatic properties of A. pullulans blastospores, the zeta potentials of the blastospores were measured in PBS as a function of pH (Fig. 7). The blastospores demonstrated pH-dependent zeta potentials and possessed an isoelectric point within the pH range used, approximately at pH 5. Zeta potentials ranged from +13 mV (pH 2) to −13 mV (pH 12).
FIG. 7.
Zeta potentials of A. pullulans IMI70103 blastospores in PBS as a function of pH. Data points represent averages for duplicate zeta potential measurements. Error bars show ±1 standard deviation of the mean.
LTSEM of pPVC and uPVC with attached blastospores.
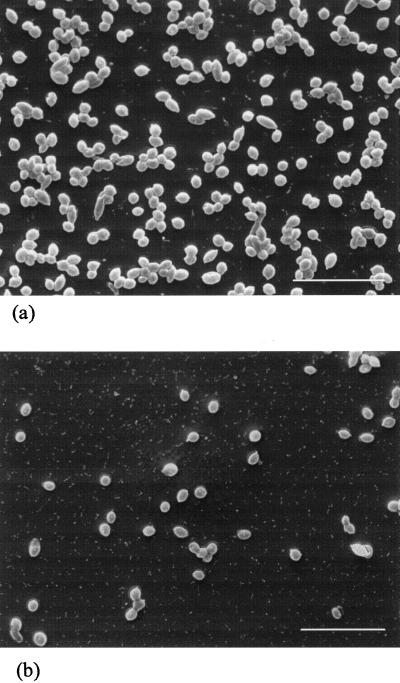
Blastospores were attached to uPVC and pPVC discs by incubation with 108 blastospores ml−1 for 4 h. Discs were examined under LTSEM subsequent to the normal washing, fixation, and staining procedures within the adhesion assay (Fig. 8). Blastospores were observed to be randomly dispersed on the surface of the discs and occurred as either single cells or aggregated into clumps. However, there was a far greater density of blastospores on the surface of pPVC (Fig. 8a) than on that of uPVC (Fig. 8b). The surfaces of both the uPVC and pPVC discs appeared smooth under LTSEM.
FIG. 8.
LTSEM of blastospores attached to pPVC (a) and uPVC (b) discs following incubation with 108 blastospores ml−1 for 4 h. Discs were washed, fixed, and stained as normal for the adhesion assay. Bar, 20 μm.
DISCUSSION
The plasticizers DOP and DOA clearly enhance adhesion of A. pullulans blastospores to PVC, and the physicochemical basis for this enhanced adhesion has been elucidated by using a rapid and quantitative adhesion assay. The plasticizers increased adhesion of blastospores to uPVC by a maximum of 308% when presented as a colloidal suspension (Fig. 3), indicating that there is an affinity of the blastospores for DOA and DOP. Equally high levels of adhesion occurred when blastospores were exposed to uPVC discs pretreated with plasticizer suspension for 1 h (data not shown). Thus, the plasticizers are probably coating the uPVC surface, resulting in an increase in adhesion of blastospores mediated by DOP and DOA in a concentration-dependent manner. On the basis of these observations, subsequent experiments were designed to investigate the nature of the interaction of blastospores with both uPVC and pPVC.
Plasticizers can be utilized as a carbon source by A. pullulans. Consequently, DOA and DOP could increase adhesion indirectly by stimulating metabolic activity and the synthesis of adhesive cell surface structures. The ability of some fungi to attach to substrata is affected by their exposure to respiration inhibitors (26, 38). However, 20 mM sodium azide, a mitochondrial respiration inhibitor, had no apparent effect on the adhesion of A. pullulans blastospores to pPVC but caused a 100% reduction in viability of the blastospores (data not shown). Therefore, use of plasticizers as a carbon source by A. pullulans is unlikely to contribute to the increased levels of adhesion observed in the presence of plasticizers.
Plasticizers could also influence adhesion indirectly by leaching from the pPVC and dissolving in the liquid phase. Leached plasticizers could alter the physicochemical properties of the blastospore cell surface or of the suspension medium, for example, by acting as surfactants. The ability of plasticizers to leach from pPVC into an aqueous environment is well established (31, 47). However, dissolved plasticizers did not influence adhesion of A. pullulans to uPVC (Fig. 3). Furthermore, the water contact angle on uPVC, which would be greatly reduced in the presence of a surfactant, was not influenced by the incorporation of a mixed dispersion of DOP and DOA into the wetting fluid (data not shown). Therefore, the plasticizers do not act as surfactants, and the quantities of plasticizer that may leach from the surface of the pPVC are insufficient to cause the observed difference in adhesion to pPVC and uPVC. Thus, it is likely that plasticizers enhance adhesion by directly influencing physicochemical interactions, such as hydrophobic or electrostatic forces, between blastospores and the PVC substratum.
Hydrophobic interactions control adhesion of A. pullulans blastospores to the unplasticized polymers studied, including uPVC. This is evident from the strong positive correlation between increasing substratum hydrophobicity and adhesion observed among the range of polymers tested. Furthermore, electrostatic interactions do not play a detectable role in the adhesion of A. pullulans blastospores to uPVC, because adhesion was not influenced by either the pH or the electrolyte concentration of the suspension medium. The importance of substratum hydrophobicity in fungal adhesion to polymers is now well recognized. Increased substratum hydrophobicity has also been shown to correlate with increased adhesion of ungerminated conidia of plant pathogenic fungi (12, 38, 41) and of yeast cells of C. albicans (19, 27) to various substrata. However, attachment of blastospores to pPVC clearly did not fit the relationship between substratum hydrophobicity and adhesion, suggesting that electrostatic interactions may play an additional role in blastospore adhesion to pPVC.
Evidence for the involvement of electrostatic forces in adhesion to pPVC comes from the effect of pH on adhesion. pH influences the cell surface charge of blastospores (Fig. 7) and also exerts a major effect on their adhesion to pPVC, which was optimal at pH 8 and minimal at pHs 2 and 13. pPVC containing the plasticizers DOP and DOA has been shown to have a net negative surface charge at pH 7.4 in PBS (23). Therefore, reduced adhesion at pH values above 8 is presumably due to electrostatic repulsion between negatively charged blastospores and a negatively charged pPVC substratum. Support for this hypothesis comes from the observation that adhesion increased concurrently with a reduction in the negative zeta potential of the blastospores in the pH range 6 to 12. However, high levels of adhesion were also predicted at pH values in the range 2 to 5 due to electrostatic attraction between positively charged blastospores and the pPVC surface. The observed inhibition of adhesion at low pH values suggests that other factors controlling adhesion, such as the electrostatic charge of the substratum, are also influenced by the changes in pH of the suspension medium. Reduced adhesion at both high and low pH values has previously been described in the adhesion of bacteria to stainless steel (39, 42) and was interpreted to be caused by changes in surface charge of both the bacterial cell and the substratum. For example, it is possible that surface charge may be altered by hydrolysis of the plasticizers to their free acids at extremes of pH. Furthermore, measurements of the zeta potential at the surface of capillary tubes have demonstrated that several polymers, including PVC, may acquire a net positive charge at low pH values (37). In our study, it is possible that both the spores and the pPVC are protonated at low pH and that adhesion is inhibited due to electrostatic repulsion between the two positively charged surfaces.
Further evidence in support of electrostatic interactions between blastospores and pPVC is provided by the effect of the electrolyte concentration on adhesion. A major reduction in adhesion was observed when the blastospores were suspended in deionized water. This reduction was presumed to be due to electrostatic repulsion, which is more pronounced in solutions of low ionic strength. Similar inhibition of adhesion at low ionic strength has previously been demonstrated among both bacteria (35, 39) and C. albicans (25, 27). Maximum adhesion to pPVC occurred at 0.01 M NaCl, at which point electrostatic repulsion between the blastospore and the pPVC was presumed to be at a minimum. The subsequent decrease in adhesion at NaCl concentrations above 0.01 M may result from reduced electrostatic interaction caused by high concentrations of electrolyte. Depending on the characteristics of the adhesive interaction, electrolytes may inhibit adhesion by screening short-range electrostatic attraction between oppositely charged groups (16) or by modifying the conformation of cell surface molecules involved in adhesion (36). Either process could be responsible for the observed reduction in adhesion of blastospores to pPVC, although further study would be required to understand in detail the exact nature of the electrostatic interaction.
The adhesion assay identified significant batch-to-batch variability in adhesion of A. pullulans blastospores to PVC. While a number of assays to quantify adhesion of fungal conidia to polymer surfaces have previously been described (6, 12, 29), none have fully investigated the reproducibility of adhesion to the substrata tested. Interbatch variation has previously been determined in studies of bacterial adhesion to polymer surfaces (22, 24), but this study is the first to highlight the fact that similar variation can occur among levels of adhesion of fungal conidia to different substrata. The reasons for variation in adhesion levels between batches are poorly understood, but could be due to slight fluctuations in environmental conditions during growth and development of the blastospores. In practice, since adhesion levels of A. pullulans blastospores were consistent within batches, valid comparisons of adhesion data could be made within each batch.
The kinetics of adhesion of A. pullulans to uPVC and pPVC suggest that both of these surfaces contain a finite number of binding sites which become saturated at blastospore concentrations above 108 blastospores ml−1. SEM observations of blastospores on pPVC and uPVC indicate that these sites are distributed evenly over the substratum. Adhesion data are frequently interpreted in terms of the number of sites which are available for a microorganism to attach to a surface (10, 13). However, saturation of the surface with blastospores may also imply negative cooperativity, i.e., that the presence of attached blastospores reduces the probability of others attaching in their vicinity. Negative-negative charge interactions that would occur between pPVC and blastospores and between blastospores and blastospores would be expected to create a condition of negative cooperative binding. Negative cooperativity of this nature has previously been shown to occur in the adhesion of C. albicans to PET coverslips (27).
In summary, we have demonstrated that incorporation of plasticizers into PVC enhances adhesion of blastospores of the deteriogenic fungus A. pullulans through an interaction that is mediated by electrostatic forces. In contrast, adhesion to uPVC is controlled principally by hydrophobic attraction. The implication of these results is that plasticizers may accelerate the biodeterioration processes occurring on pPVC by enhancing fungal adhesion. Such information should be taken into account in the design of novel PVC formulations that utilize surface chemistry to reduce microbial attachment.
ACKNOWLEDGMENTS
This work was supported by a BBSRC CASE award in collaboration with Zeneca Biocides, Blackley, Manchester, United Kingdom.
We thank Ron Swart and David Hodge, Zeneca Specialties, Blackley, Manchester, United Kingdom, and Malcolm Jones, School of Biological Sciences, University of Manchester, for helpful discussion about the data and manuscript.
REFERENCES
- 1.Andrews J H, Harris R F, Spear R N, Gee W L, Nordheim E V. Morphogenesis and adhesion of Aureobasidium pullulans. Can J Microbiol. 1994;40:6–17. [Google Scholar]
- 2.Berk S, Ebert H, Teitell L. Utilization of plasticizers and related organic compounds by fungi. Ind Eng Chem. 1957;49:1115–1124. [Google Scholar]
- 3.Bessems E. The biodeterioration of plasticised PVC and its prevention. J Vinyl Technol. 1988;10:3–6. [Google Scholar]
- 4.Booth G H, Cooper A W, Robb J A. Bacterial degradation of plasticised PVC. J Appl Bacteriol. 1968;31:305–310. [Google Scholar]
- 5.Booth G H, Robb J A. Bacterial degradation of plasticised PVC—effect of some physical properties. J Appl Chem. 1968;18:194. [Google Scholar]
- 6.Braun E J, Howard R J. Adhesion of Cochliobolus heterostrophus conidia and germlings to leaves and artificial surfaces. Exp Mycol. 1994;18:211–220. [Google Scholar]
- 7.Brown A E. Problem of fungal growth on resins, plastics and plasticisers. Report no. 6067. Washington, D.C: U.S. Office of Scientific Research and Development; 1945. [Google Scholar]
- 8.Busscher H J, Sjollema J, van der Mei H C. Relative importance of surface free energy as a measure of hydrophobicity in bacterial adhesion to solid surfaces. In: Doyle R J, Rosenberg M, editors. Microbial cell surface hydrophobicity. Washington, D.C: American Society for Microbiology; 1990. pp. 335–359. [Google Scholar]
- 9.Cooke W B. An ecological life history of Aureobasidium pullulans (de Bary) Arnaud. Mycopathol Mycol Appl. 1959;12:1–45. doi: 10.1007/BF02118435. [DOI] [PubMed] [Google Scholar]
- 10.Cowan M M. Kinetic analysis of microbial adhesion. Methods Enzymol. 1995;253:179–188. doi: 10.1016/s0076-6879(95)53018-5. [DOI] [PubMed] [Google Scholar]
- 11.Domingue J. Probing the surface of the solid/liquid interface. Am Lab. 1990;21:1–4. [Google Scholar]
- 12.Doss R P, Potter S W, Chastagner G A, Christian J K. Adhesion of nongerminated Botrytis cinerea conidia to several substrata. Appl Environ Microbiol. 1993;59:1786–1791. doi: 10.1128/aem.59.6.1786-1791.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle R J. Strategies in experimental microbial adhesion research. In: Mozes N, Handley P S, Busscher H J, Rouxhet P G, editors. Microbial cell surface analysis: structural and physicochemical methods. New York, N.Y: VCH Publishers; 1991. pp. 291–318. [Google Scholar]
- 14.Eaton R W, Ribbons D W. Utilization of phthalate esters by micrococci. Arch Microbiol. 1982;132:185–188. doi: 10.1007/BF00508728. [DOI] [PubMed] [Google Scholar]
- 15.Eveleigh D E. The disfigurement of painted surfaces by fungi, with special reference to Phoma violacea. Ann Appl Biol. 1961;49:403–411. [Google Scholar]
- 16.Fletcher M. Adherence of marine microorganisms to smooth surfaces. In: Beachey E H, editor. Bacterial adherence. London, United Kingdom: Chapman & Hall, Ltd.; 1980. pp. 345–374. [Google Scholar]
- 17.Gilbert P, Evans D J, Evans E, Duguid I D, Brown M R W. Surface characteristics and adhesion of Escherichia coli and Staphylococcus epidermidis. J Appl Bacteriol. 1991;71:72–77. [PubMed] [Google Scholar]
- 18.Hamilton N F. Biodeterioration of flexible polyvinyl chloride films by fungal organisms. In: Oxley T A, Barry S, editors. Biodeterioration 5. Chichester, United Kingdom: John Wiley & Sons, Ltd.; 1983. pp. 663–678. [Google Scholar]
- 19.Hazen K C. Participation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cells. Infect Immun. 1989;57:1894–1900. doi: 10.1128/iai.57.7.1894-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiemenz P C. Electrophoresis and other related electrokinetic phenomena. In: Lagowski J J, editor. Principles of colloid and surface chemistry. New York, N.Y: Marcel Dekker; 1977. pp. 452–487. [Google Scholar]
- 21.James A M. Charge properties of microbial cell surfaces. In: Mozes N, Handley P S, Busscher H J, Rouxhet P G, editors. Microbial cell surface analysis: structural and physicochemical methods. New York, N.Y: VCH Publishers; 1991. pp. 221–262. [Google Scholar]
- 22.John S F, Hillier V F, Handley P S, Derrick M R. Adhesion of staphylococci to polyurethane and hydrogel-coated polyurethane catheters assayed by an improved radiolabelling technique. J Med Microbiol. 1995;43:133–140. doi: 10.1099/00222615-43-2-133. [DOI] [PubMed] [Google Scholar]
- 23.Jones C R. The effect of biocides on the adhesion and bioluminescence of Pseudomonas fluorescens. Ph.D. thesis. Manchester, United Kingdom: University of Manchester; 1997. [Google Scholar]
- 24.Jones C R, Handley P S, Robson G D, Eastwood I M, Greenhalgh M. Biocides incorporated into plasticized polyvinylchloride reduce adhesion of Pseudomonas fluorescens BL146 and substratum hydrophobicity. J Appl Bacteriol. 1996;81:1–9. [Google Scholar]
- 25.Jones L, O’Shea P. The electrostatic nature of the surface of Candida albicans: a role in adhesion. Exp Mycol. 1994;18:111–120. [Google Scholar]
- 26.Jones M J, Epstein L. Adhesion of Nectria haematococca macroconidia. Physiol Mol Plant Pathol. 1989;35:453–461. [Google Scholar]
- 27.Klotz S A, Drutz D J, Zajic J E. Factors governing adherence of Candida species to plastic surfaces. Infect Imm. 1985;50:97–101. doi: 10.1128/iai.50.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lander M L, Siewierski L M, Brittain W J, Vogler E A. A systematic comparison of contact angle measurements. Langmuir. 1993;9:2237–2239. [Google Scholar]
- 29.Mercure E W, Leite B W, Nicholson R L. Adhesion of ungerminated conidia of Colletotrichum graminicola to artificial hydrophobic surfaces. Physiol Mol Plant Pathol. 1994;45:421–440. [Google Scholar]
- 30.Miyake Y, Fujita Y, Minagi S, Suginaka H. Surface hydrophobicity and adherence of Candida to acrylic surfaces. Microbios. 1986;46:7–14. [PubMed] [Google Scholar]
- 31.Murase A, Sugiura M, Araga T. An infrared spectroscopic study of the migration of a plasticiser in poly(vinyl chloride) resins. Polymer Degrad Stability. 1994;43:415–422. [Google Scholar]
- 32.Panagoda G J, Ellepola A N B, Samaranayake L P. Adhesion to denture acrylic surfaces and relative cell-surface hydrophobicity of Candida parapsilosis and Candida albicans. APMIS. 1998;106:736–742. [PubMed] [Google Scholar]
- 33.Pascual A, Fleer A, Westerdaal N A C, Verhoef J. Modulation of adherence of coagulase-negative staphylococci to teflon catheters in-vitro. Eur J Clin Microbiol. 1986;5:518. doi: 10.1007/BF02017694. [DOI] [PubMed] [Google Scholar]
- 34.Rijnaarts H H M, Norde W, Lyklema J, Zehnder A J B. The isoelectric point of bacteria as an indicator for the presence of cell surface polymers that inhibit adhesion. Colloids Surf. 1995;4:191–197. [Google Scholar]
- 35.Rönner U, Husmark U, Henriksson A. Adhesion of bacillus spores in relation to hydrophobicity. J Appl Bacteriol. 1990;69:550–556. doi: 10.1111/j.1365-2672.1990.tb01547.x. [DOI] [PubMed] [Google Scholar]
- 36.Rutter P R, Abbott A. A study of the interaction between oral streptococci and hard surfaces. J Gen Microbiol. 1978;105:219–226. doi: 10.1099/00221287-105-2-219. [DOI] [PubMed] [Google Scholar]
- 37.Schützner W, Kenndler E. Electrophoresis in synthetic organic polymer capillaries: variation of electroosmotic velocity and zeta-potential with pH and solvent composition. Anal Chem. 1992;64:1991–1995. [Google Scholar]
- 38.Sela-Buurlage M B, Epstein L, Rodriguez R J. Adhesion of ungerminated Colletotrichum musae conidia. Physiol Mol Plant Pathol. 1991;39:345–352. [Google Scholar]
- 39.Stanley P M. Factors affecting the irreversible attachment of Pseudomonas aeruginosa to stainless steel. Can J Microbiol. 1983;29:1493–1499. doi: 10.1139/m83-230. [DOI] [PubMed] [Google Scholar]
- 40.Stenström T A. Bacterial hydrophobicity, an overall parameter for the measurement of adhesion potential to soil particles. Appl Environ Microbiol. 1989;55:142–147. doi: 10.1128/aem.55.1.142-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terhune B T, Hoch H C. Substrate hydrophobicity and adhesion of Uromyces urediospores and germlings. Exp Mycol. 1993;17:241–252. [Google Scholar]
- 42.Vanhaecke E, Remon J-P, Moors M, Raes F, De Rudder D, Van Peteghem A. Kinetics of Pseudomonas aeruginosa adhesion to 304 and 316-L stainless steel: role of cell surface hydrophobicity. Appl Environ Microbiol. 1990;56:788–795. doi: 10.1128/aem.56.3.788-795.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Loosdrecht M C M, Lyklema J, Norde W, Schraa G, Zehnder A J B. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl Environ Microbiol. 1987;53:1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Loosdrecht M C M, Lyklema J, Norde W, Schraa G, Zehnder A J B. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987;53:1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitney P J. A comparison of two methods for testing defined formulations of PVC for resistance to fungal colonization with two methods for the assessment of their biodegradation. Int Biodeterior Biodegrad. 1996;37:205–213. [Google Scholar]
- 46.Wiencek K M, Fletcher M. Effects of substratum wettability and molecular topography on the initial adhesion of bacteria to chemically defined substrata. Biofouling. 1997;11:293–311. [Google Scholar]
- 47.Wilson A S, editor. Plasticisers: principles and practice. London, United Kingdom: Institute of Materials; 1995. [Google Scholar]