Abstract
Objective:
To test the hypothesis that death with physiological parallels to human cases of sudden unexpected death in epilepsy (SUDEP) can be induced in seizing rats by ictal activation of oxygen-conserving reflexes (OCRs).
Methods:
Urethane-anesthetized female Long-Evans rats were implanted with electrodes for electrocardiography (ECG), electrocorticography (ECoG), and respiratory thermocouple; venous and arterial cannulas; and a laryngoscope guide and cannula or nasal cannula for activation of the laryngeal chemoreflex (LCR) or mammalian diving reflex (MDR), respectively. Kainic acid injection, either systemic or into the ventral hippocampus, induced prolonged acute seizures.
Results:
Reflex challenges during seizures caused sudden death in 18 of 20 rats—all MDR rats (10) and all but two LCR rats (8) failed to recover from ictal activation of OCRs and died within minutes of the reflexes. By comparison, 4 of 4 control (ie, nonseizing) rats recovered from 64 induced diving reflexes (16 per rat), and 4 of 4 controls recovered from 64 induced chemoreflexes (16 per rat). Multiple measures were consistent with reports of human SUDEP. Terminal central apnea preceded terminal asystole in all cases. Heart and respiratory rate fluctuations that paralleled those seen in human SUDEP occurred during OCR-induced sudden death, and mean arterial pressure (MAP) was predictive of death, showing a 17 or 15 mm Hg drop (MDR and LCR, respectively) in the 20 s window centered on the time of brain death. OCR activation was never fatal in nonseizing rats.
Significance:
These results present a method of inducing sudden death in two seizure models that show pathophysiology consistent with that observed in human cases of SUDEP. This proposed mechanism directly informs previous findings by our group and others in the field; provides a repeatable, inducible animal model for the study of sudden death; and offers a potential explanation for observations made in cases of human SUDEP.
Keywords: apnea, diving reflex, laryngeal chemoreflex, seizures, sudden unexpected death in epilepsy
1 |. INTRODUCTION
Sudden unexpected death in epilepsy (SUDEP) is a fatal complication of epilepsy that kills ~12 of every 10 000 epilepsy patients every year.1,2 With ~1% of the US population diagnosed with epilepsy, and a likely similar number worldwide, this is a staggering number of preventable deaths. SUDEP is difficult to study because, although common, it usually occurs unobserved.3–6 The limited clinical data that exist suggest that SUDEP is a cardiorespiratory collapse that occurs immediately following a seizure.6–8 Cardiorespiratory function is largely modulated by the autonomic nervous system (ANS), which is known to be impaired in patients with epilepsy, particularly during or shortly after seizures.9–11 In addition, a sizeable body of clinical evidence has linked SUDEP and other forms of sudden death—such as sudden infant death syndrome (SIDS)12–16; sudden unexpected death in childhood (SUDC)16; and sudden death related to swimming, drowning, and cold water (sudden cardiac death in water, “dry drowning”)17—to a family of reflexes collectively referred to here as “oxygen-conserving reflexes” (OCRs).18–26
In most physiological responses it is rare for both the sympathetic and parasympathetic pathways to be coactivated. A notable exception is the family of OCRs, which strongly coactivate both autonomic pathways. OCRs decrease heart rate and respiration rate (parasympathetic) and constrict peripheral vasculature (sympathetic), pushing blood to the heart, brain, and lungs in order to direct oxygen to key organs during hypoxia.12,13,18,19,21–23,27–29 This unusual coactivation has been linked to possibly fatal cardiac arrhythmias, especially in epilepsy.19,20 The current literature has not confirmed the potential link between OCR responses, seizure activity, and sudden death. In our previous work30 we found that seizing animals would always die after laryngospasm, whereas nonseizing animals would not, leading us to hypothesize that OCR activation during seizure may produce sudden death.
The diving reflex and chemoreflex are the two most widely studied OCRs. They are the most closely implicated in sudden death and the easiest to experimentally study.12,13,16,18,21,22,24,25,28,29,31 The mammalian diving reflex (MDR) has been found in every vertebrate tested.19,21 The MDR has three separate effects: a reduction in heart rate, systemic vasoconstriction, and suppression of respiratory drive.32,33
The laryngeal chemoreflex (LCR) is found in many mammals, including humans, dogs, and rats.12,22,29,30,34 It is most commonly activated in humans by acid reflux.35,36 Acidic fluid, such as gastric secretions, can damage the lungs, so this reflex activates to prevent acid from entering the airways. The chemoreflex induces a decrease in heart rate and blood pressure and, in many cases, obstructive apnea caused by laryngospasm: a spasmic closure of the vocal folds and airway.22 In our previous work we found a possible link between acid reflux and laryngospasm-induced sudden death in an animal model.30
Current literature increasingly supports the hypothesis that OCRs may contribute to types of sudden death.12–16,18,21–23,25,30,37,38 We hypothesize that the neural mechanisms that underlie OCRs, if activated in seizing animals, may induce pathophysiology consistent with that seen in recorded cases of human SUDEP. In this work, we describe two methods by which we reliably induce sudden death with physiological parallels to human SUDEP in two different animal models of temporal lobe epilepsy.
2 |. MATERIALS AND METHODS
2.1 |. Experimental design
Experiments complied with and used procedures approved by the Purdue Animal Care and Use Committee (PACUC). Rats lived under a 12 h:12 h dark/light cycle, with ad libitum access to food and water until induction of anesthesia. In this work, we define “reflex challenge” as the deliberate activation of either the diving reflex or laryngeal chemoreflex by the methods described below. “Preictal reflex challenge” refers to reflex challenges presented prior to administration of kainic acid (KA) to induce seizures; “ictal reflex challenge” and “reflex challenge during seizures” refer to reflex challenges presented after administration of KA during electrographic seizure activity.
In 32 rats, we tested two oxygen-conserving reflexes (or OCRs)—the mammalian diving reflex and the laryngeal chemoreflex—under two epilepsy models—systemic KA and intrahippocampal KA—in 4 experimental groups containing 5 rats per group. These groups were diving reflex, systemic KA; diving reflex, intrahippocampal KA; laryngeal chemoreflex, systemic KA; and laryngeal chemoreflex, intrahippocampal KA. We also had two additional groups of four control rats each—diving reflex control and laryngeal chemoreflex control—which did not have seizures induced. Four additional rats displayed atypical responses to chemoreflex challenges prior to KA administration (discussed in Section 3.3.3) and were not included in any analysis. In a procedure similar to others’ work,26 diving reflex rats were mounted prone in a stereotaxic frame, and a silicone tube was inserted 20 mm into a naris, such that it reached the pharynx. A 2–3 mL ice-water mist was then sprayed for 19 ± 7 s (mean ± SD) for each reflex challenge. For all calculations and analysis, t = 0 was set at the start of the mist. In a procedure similar to prior work,38 chemoreflex rats were mounted supine in a stereotaxic frame with a 3D printed feeding tube inserted into the mouth. A laryngoscope mounted on a GoPro camera modified by the company BackBone visualized the larynx. For each reflex challenge 0.1–0.2 mL tris-buffered HCl (pH = 1.6) was sprayed onto the larynx using a flexible tube. The HCl was suctioned out immediately upon the first attempt at inspiration to minimize the chances of aspiration into the lungs.
For all reflex challenges, a minimum of 5 min (6.1 ± 2.5 min, range 3.3–21.3) was allowed for recovery between challenges, with that time being extended if cardiac and respiratory rates had not returned to approximately pre-challenge levels for ≥2 min before the next reflex challenge. Rarely, this recovery period was reduced between nonseizing challenges if the rat recovered quickly. A maximum of 12 reflex challenges during seizures were performed per rat. In addition, we tested two control groups—diving reflex and chemoreflex—of 4 rats per group. Each of these rats had the same pause between individual reflex challenges, but experienced a total of 16 reflex challenges, matching the maximum possible number of reflex challenges to which an experimental rat may be subjected (4 pre-ictal challenges +12 ictal challenges). These control rats verify that a physiologically normal rat can endure 16 reflex challenges and survive.
2.2 |. Subjects and surgical procedure
Thirty-two female Long-Evans rats (261 ± 16 g; range: 239–300 g; Envigo) were anesthetized with urethane (1.4 g/kg, i.p.). A femoral artery was cannulated for invasive blood pressure (BP) measurement, as was a femoral vein to administer saline (0.9% NaCl, 0.25–0.5 mL bolus, i.v.) as needed to maintain systolic BP >100 mm Hg. In rats that received systemic administrations of KA, this cannula also delivered the systemic KA (10 mg/kg, i.v.). A thermostatically controlled heating blanket maintained rectal temperature at 37°C (TC-1000; CWE Inc.).
The rat was mounted in a stereotaxic frame with the bregma-lambda axis horizontal. Burr holes were drilled in the skull for electrocorticography (ECoG) skull screws 2.5 mm caudal and 0.5 mm rostral to bregma, both 2.0 mm lateral of bregma. In rats that received intrahippocampal administrations of KA, a third burr hole was drilled 5.2 mm caudal, 4.5 mm lateral to bregma, and a Hamilton microsyringe delivered KA 6.8 mm below the cortical surface. Percutaneous needle electrodes in a lead II configuration—inserted at the right shoulder and left caudal thorax—were used to record electrocardiography (ECG), and a thermocouple (5TC-TT-K-36–36, Omega) placed immediately outside the rat’s airway—either outside the nares or in the laryngoscope guide, depending upon whether respiration primarily occurred through the nose or mouth—recorded temperature fluctuations as a proxy for respiratory airflow. When necessary, respiratory effort was inferred from respiration-related electromyography (EMG) recorded from ECG electrodes using the method described previously.39
After at least 10 min of baseline recording, all diving reflex rats and two chemoreflex rats were subjected to four reflex challenges to verify that the anesthetized, but otherwise physiologically normal, rat could successfully recover from the OCR challenges. After one systemic KA and one hippocampal KA chemoreflex rat had undergone these “control” reflexes, we omitted this step for the remainder of the LCR rats after observing that the acid residue in the larynx caused marked inflammation of the tissues and subjectively appeared to impede airflow, which we believed may have adverse effects on seizure development. Following the pre-ictal reflexes, KA (HelloBio HB0355) was administered, and seizures were allowed to progress for 60–90 min prior to initiation of OCR challenges. For systemic KA rats, 10 mg/kg KA was administered intravenously or intraperitoneally. For hippocampal KA rats, 1 μL KA (10 mg/mL) was injected intrahippocampally over 5 min using a Hamilton microsyringe. This experimental timeline is depicted graphically in Figure 1. Data previously acquired by Jefferys et al39 informed the time we allow seizures to progress prior to OCR challenges: Rats that survive the first 48 min post-KA do not spontaneously die of central apnea.
FIGURE 1.
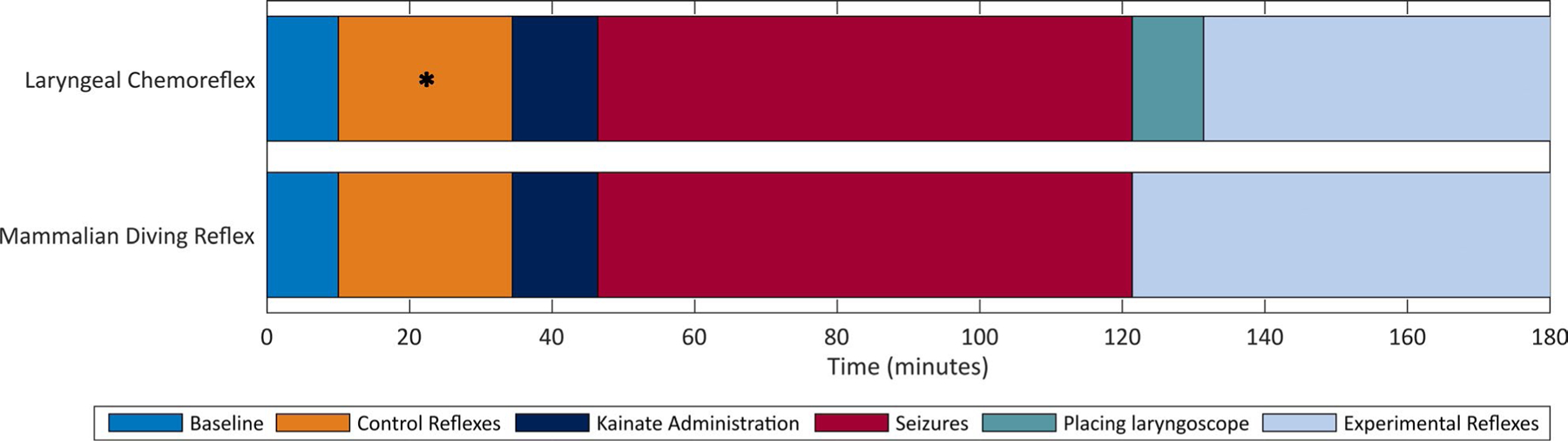
Experimental timeline for laryngeal chemoreflex and mammalian diving reflex experimental groups. “Experimental reflexes” is depicted through 180 min for clarity but may be shorter if death occurs or longer if recovery between reflexes requires longer than the 5-min minimum. The “control reflexes” period indicated by * denotes the period in which the control chemoreflex challenges occurred in two rats. In other chemoreflex experiments, this step was omitted
Data were captured using p511 pre-amplifier (Grass Technologies) and custom-made amplifiers40 and a CED Power3 1401 acquisition unit with Spike2 software (Cambridge Electronic Design). ECG and ECoG were amplified 1000x, high pass filtered at 3 Hz, and sampled at 500 Hz and 2 kHz, respectively. Thermocouple measurements were amplified 50 000x, bandpass filtered between 0.3 and 3 Hz, and sampled at 200 Hz. Analysis was performed in Spike2 and MATLAB. Statistical analyses used MATLAB, Microsoft Excel, and R. Results are expressed as mean ± SD unless otherwise stated. All t tests performed were two-tailed. We pre-selected α = 0.05 for significance. In cases where data from the hippocampal KA and systemic KA responses to a particular reflex are combined, statistical testing was performed to ensure that each data set did not statistically differ from the other.
3 |. RESULTS
3.1 |. Physiological changes during seizure activity
Following hippocampal KA, seizure progression and activity proceeded as described previously.39 In brief, spontaneous electrographic activity is replaced with synchronous field potentials, mean arterial pressure (MAP) increases from 77.0 ± 9.2 to 102.9 ± 20.2 mm Hg (P < .0001, Welch’s t test), and marked tachycardia (269 ± 27.1 bpm rises to 432 ± 53.8 bpm) (P < .0001, Welch’s t test) and tachypnea (81.0 ± 13.3 bpm rises to 146 ± 54.9 bpm) (P < .0001, Welch’s t test) occur. Seizures following systemic KA proceeded as described previously.38,41 In brief, transient marked hypotension occurred during the initial period of continuous seizure activity and was unresponsive to saline. During this time, MAP fell from 75.0 ± 9.9 to 65.1 ± 14.5 mm Hg (P < .001, Welch’s t test). MAP later returned to near baseline, with tachycardia (359 ± 69.1 bpm, up from 303 ± 49.8 bpm at baseline) (P < .001, Welch’s t test) occurring throughout. No significant difference was observed in baseline MAP between hippocampal and systemic KA rats (75.0 ± 9.9 mm Hg and 77.0 ± 9.2 mm Hg, respectively) (P = .43, Welch’s t test). Ictal MAP showed a drastic difference between hippocampal and systemic KA (102 ± 20.2 mm Hg and 65.1 ± 14.5 mm Hg, respectively) (P < .0001, Welch’s t test). We attribute the MAP depression observed in systemic KA rats to the peripheral effects of systemic KA administration.42
3.2 |. Recovery from reflex challenges
For reflex challenges that did not result in death, no statistical difference was observed between control and ictal recovery times from the diving reflex (45.7 ± 11.9 s and 40.8 ± 11.9 s, respectively) (P = .08, Welch’s t test) or chemoreflex (24.4 ± 16.5 s and 18.9 ± 11.0 s, respectively) (P = .29, Welch’s t test). As described in previous literature21,22,26,32,33,43 and consistent with the physiology of the reflex, reflex challenges resulted in transient drops in BP and heart rate coincident with central apnea, which recovered over tens of seconds to baseline levels.
Heart rate (HR) fell from 389 ± 11.4 beats per min to 232 ± 10.2 beats per min (P < .0001, paired t test) during the 30 s following diving reflex and from 401 ± 11.9 beats per min to 343 ± 12.3 beats per min (P < .0001, paired t test) during the 30 s following chemoreflex. HR returned to baseline 170 s after diving reflex and 130 s after chemoreflex (see Figure 2 for all statistical comparisons). Likewise, respiratory rate fell from 144 ± 5.3 breaths per min to 71 ± 6.8 breaths per min (P < 0.0001, paired t test) during the 30 s following diving reflex and from 116 ± 6.7 breaths per min (P < .0001, paired t test) during the 30 s following chemoreflex. Respiratory rate returned to baseline 190 s and 20 s after diving and chemoreflexes, respectively (see Figure 3 for all statistical comparisons).
FIGURE 2.
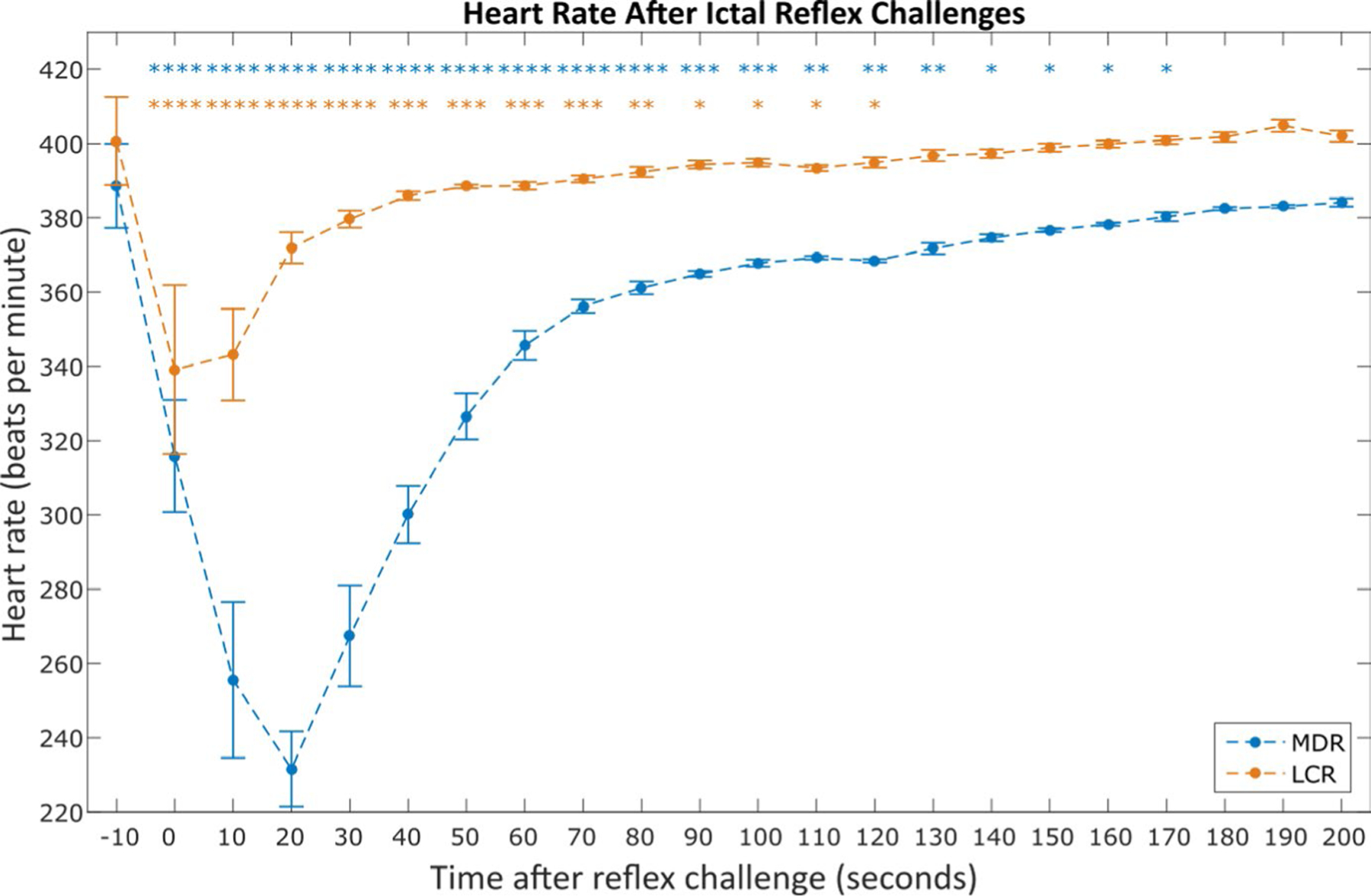
Average heart rate after nonfatal mammalian diving reflex (MDR) and laryngeal chemoreflex (LCR) challenges during seizures. Each point represents the average heart rate in the 10 s window starting at the time indicated on the x-axis (eg, t = 0 represents data for all 0 ≤ t < 10 s). Asterisks indicate a statistically significant difference between the 10 s window and the pre-challenge amplitude (paired t tests). Data were averaged from the total 32 ictal MDR challenges across 10 animals and 69 ictal LCR challenges across 10 animals that did not result in sudden death. *P < .05; **P < .01; ***P < .001; ****P < .0001
FIGURE 3.
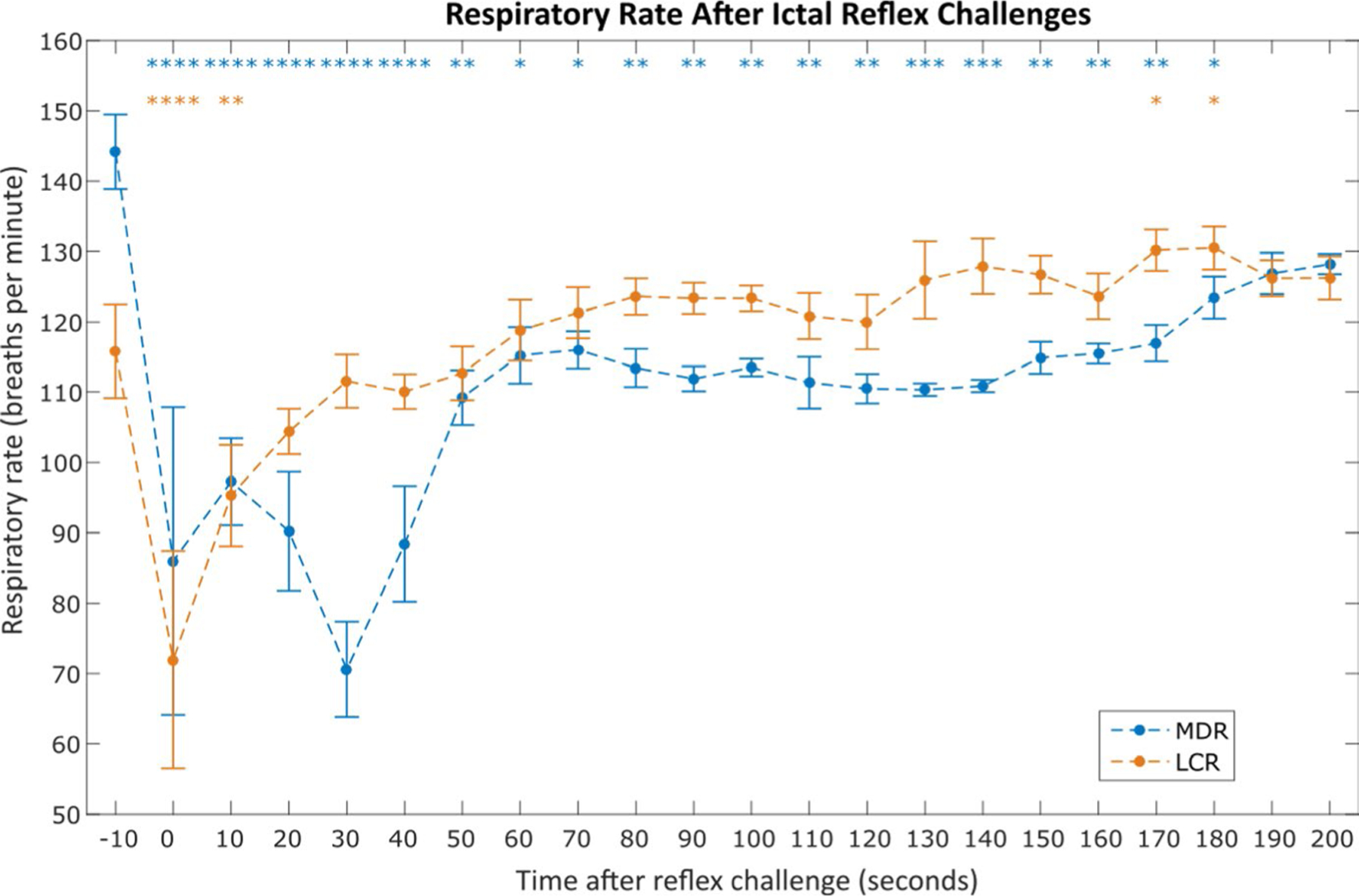
Average respiratory rate after nonfatal mammalian diving reflex (MDR) and laryngeal chemoreflex (LCR) challenges during seizures. Each point represents the average normalized respiratory rate in the 10 s window starting at the time indicated on the x-axis, as in Figure 2. Asterisks indicate statistically significant difference between the 10 s window and the pre-challenge amplitude (paired t tests). Data were averaged from the total 32 ictal MDR challenges across 10 animals and 69 ictal LCR challenges across 10 animals that did not result in sudden death. *P < .05; **P < .01; ***P < .001; ****P < .0001
We observed a statistically significant reduction in electrocorticography (ECoG) amplitude in the 60 s following reflex challenges during seizures for both epilepsy models, as shown in Figure 4. ECoG amplitudes were binned in 10 s windows starting 10 s pre-reflex, and the root mean square (RMS) amplitude of each window was calculated and compared with that of the pre-reflex window. Both reflexes showed multiple points that demonstrated statistically significant reductions in ECoG amplitude. For example, chemoreflex, 10 s post: P < .0001, 20 s post: P < .001; diving reflex, 20 s post: P < .01, 30 s post: P < .0001. Similar postictal EEG suppression is well-documented in both human epilepsy and animal models of epilepsy.
FIGURE 4.
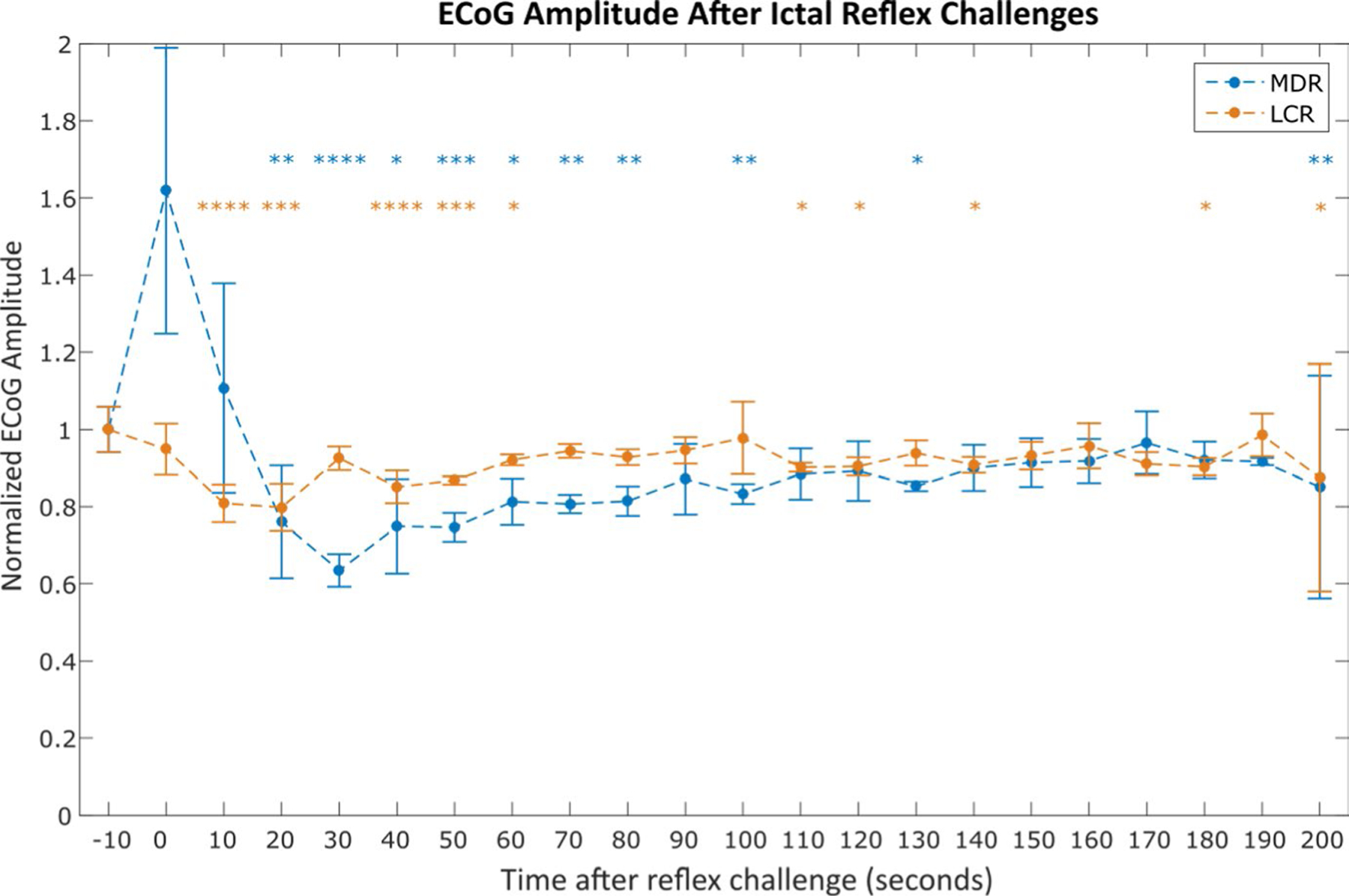
Average electrocorticography (ECoG) amplitude after nonfatal mammalian diving reflex (MDR) and laryngeal chemoreflex (LCR) challenges during seizures. All amplitudes are normalized to the average amplitude in the 10 s window before reflex challenge. Each point represents the average normalized ECoG amplitude in the 10 s window starting at the time indicated on the x-axis and ending at the last measurement acquired before the start of the following point’s time window. Asterisks indicate statistically significant difference between the 10 s window and the pre-challenge amplitude (paired t tests). Data were averaged from the total 32 ictal MDR challenges across 10 animals and 69 ictal LCR challenges across 10 animals that did not result in sudden death. *P < .05; **P < .01; ***P < .001; ****P < .0001
3.3 |. Death by reflex challenge during seizures
OCR challenges triggered sudden death in almost all (18/20) seizing animals, but never (0/8) in nonseizing animals. A 5/5 diving reflex, systemic KA rats and 5/5 diving reflex, hippocampal KA rats experienced sudden death in response to a reflex challenge during seizures (4.2 ± 3.6 ictal challenges prior to death), compared with 0/4 control diving reflex rats dying due to a reflex challenge (P < .005, Barnard’s exact test for both). 4/5 chemoreflex, systemic KA rats and 4/5 chemoreflex, hippocampal KA rats experienced sudden death in response to a reflex challenge during seizures (7.7 ± 4.0 ictal challenges prior to death), compared with 0/4 chemoreflex control rats dying due to a reflex challenge (P < .03, Barnard’s exact test for both). No statistically significant difference was observed between the outcomes of diving reflex experiments overall and the outcomes of chemoreflex experiments overall (P = .22, Barnard’s exact test). In all cases, the terminal apnea following a reflex challenge lacks evidence of continued respiratory effort during cessation of measured airflow, indicating a loss of central respiratory drive rather than obstruction caused by the fluid used to activate the reflex. In addition, terminal apnea preceded terminal asystole in all cases for both reflexes. In fatal diving reflex challenges, terminal apnea occurred 77.4 ± 59.7 s after reflex activation, with terminal cardiac arrest occurring 312.6 ± 52.8 s after reflex activation (P < .0001, paired t test). In fatal chemoreflex challenges, terminal apnea occurred 180 ± 119 s after reflex activation, with terminal cardiac arrest occurring 333 ± 114 s after reflex activation (P < .0005, paired t test). We depict the timeline of events following the fatal reflex challenge for each animal in Figure 5. For brevity, death following as a direct consequence of reflex challenge during seizures will be referred to here as “induced sudden death.”
FIGURE 5.
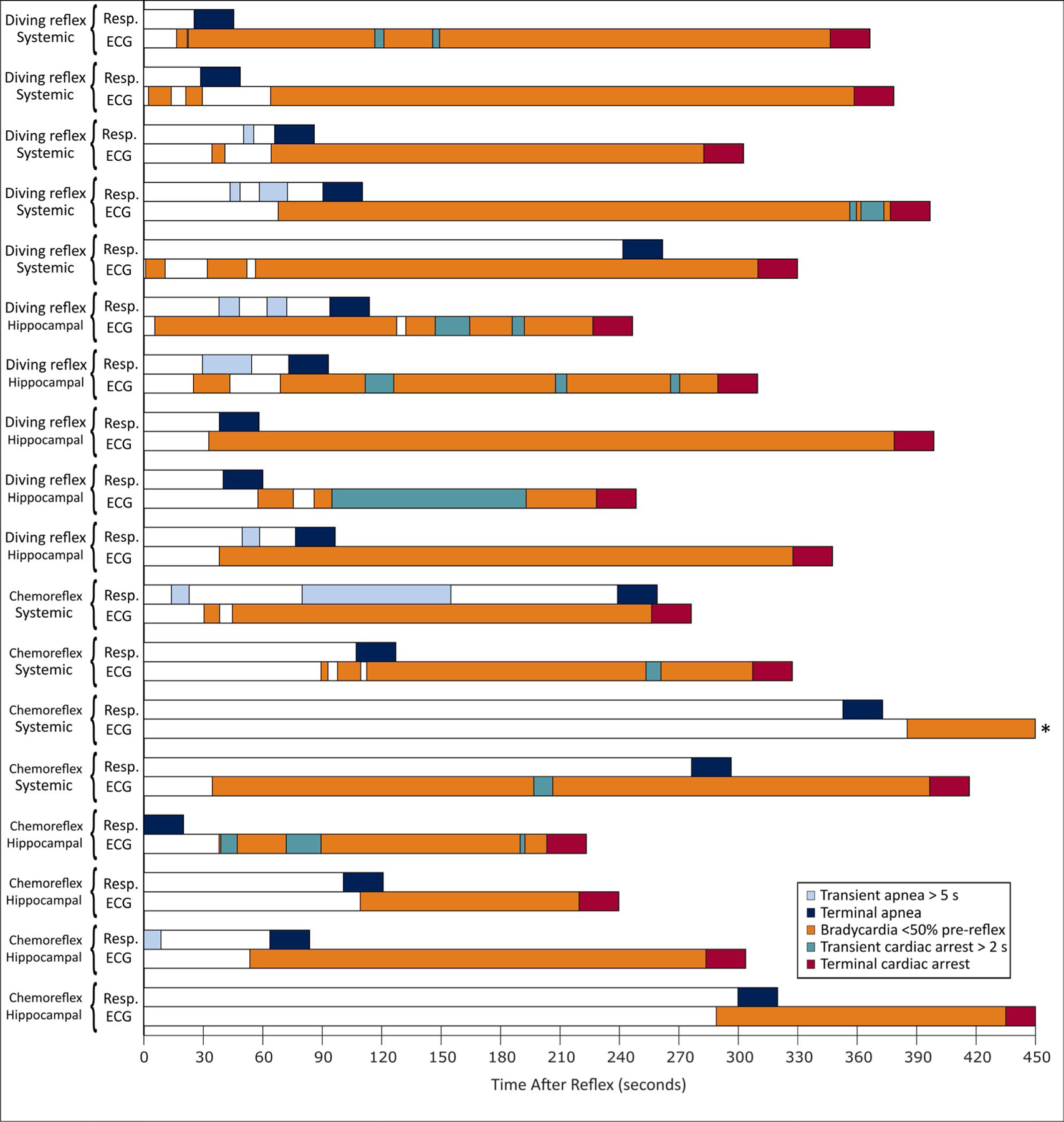
Patterns of cardiorespiratory activity following 10 fatal mammalian diving reflex challenges and 8 fatal laryngeal chemoreflex challenges. Each pair of bars (ie, resp and ECG) represents the data from one rat, with t = 0 indicating the time of reflex challenge. For this analysis, “cardiac arrest” is defined as a lack of measurable change in mean arterial pressure (MAP) for ≥2 s. * indicates a rat for which terminal cardiac arrest occurred at t = 559.4 s, which was not depicted in whole for clarity.
3.3.1 |. Mammalian diving reflex
Diving reflex challenges caused a 38.4% ± 13.0% drop in MAP between 0 and 50 s after the reflex challenge. In baseline and ictal reflexes that did not cause death, MAP returned to within 20% of its pre-challenge amplitude in 65.6 s.
ECoG amplitude was found to predict MAP loss. Brain death (here defined as the RMS amplitude of the ECoG signal falling to within 4 SD of the RMS amplitude of the noise floor of the recording channel) in fatal diving reflex challenges occurred 67.4 ± 8.5 s after reflex challenge and was coincident with a 16.9 ± 5.9 mm Hg drop in MAP within a 20 s window around the time of death. This effect is displayed in Figure 6. No significant differences were observed between systemic KA and hippocampal KA rats for MAP loss around the time of brain death (16.6 ± 8.9 mm Hg and 17.1 ± 2.6 mm Hg, respectively) (P = .90, Welch’s t test), time from reflex challenge to brain death (64.9 ± 9.3 s and 69.9 ± 8.5 s, respectively) (P = .41, Welch’s t test), time from reflex challenge to cardiac arrest (335 ± 34.1 s and 290 ± 58.5 s, respectively) (P = .23, Welch’s t test), or time from reflex challenge to terminal central apnea (90.4 ± 79.5 s and 64.3 ± 21.8 s, respectively) (P = .55, Welch’s t test). Figure 7 depicts the average changes in MAP, heart rate, and respiratory rate for all animals in preictal, nonfatal ictal, and fatal ictal reflex challenges.
FIGURE 6.
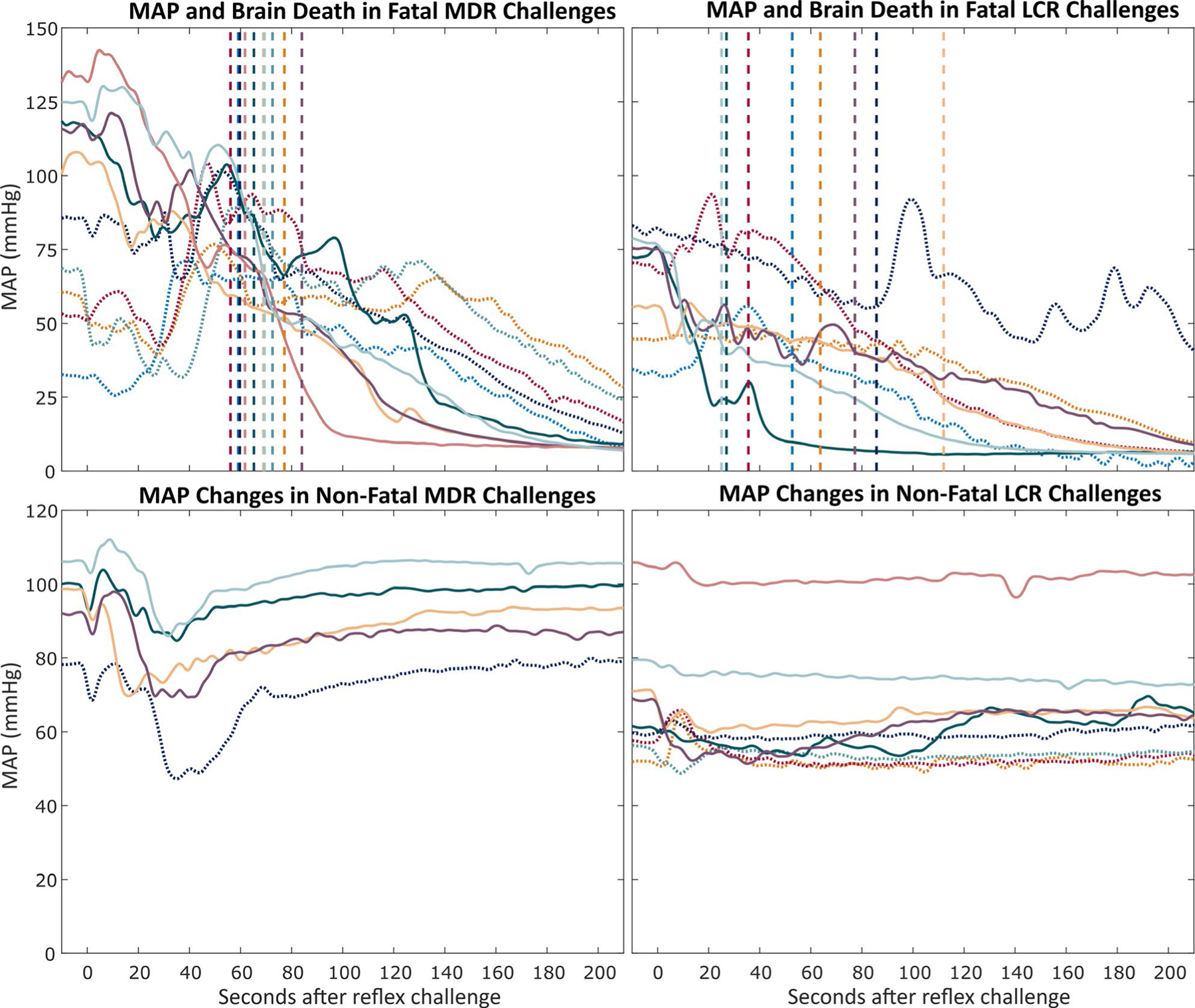
Mean arterial pressure (MAP) during oxygen-conserving reflexes (OCRs) challenges for mammalian diving reflex (MDR) and laryngeal chemoreflex (LCR) challenges. Each color per pair of graphs represents one rat, and colors for the fatal and nonfatal MAP changes from each reflex are consistent between the graphs for that reflex. Solid MAP traces indicate hippocampal kainic acid. Dotted MAP traces indicate systemic kainic acid. The vertical dashed lines indicate the time at which brain death (defined as the root mean square (RMS) amplitude of electrocorticography [ECoG] falling within 4 SDs of the RMS amplitude of the noise floor of the recording channel) occurred. The colors of the vertical lines correspond to the color of the MAP trace for rats in each panel. Top row: MAP changes in fatal reflex challenges during seizures. Traces without a corresponding trace in the nonfatal graph indicate animals that died from the first reflex challenge during seizures. Bottom row: Average MAP changes in nonfatal reflex challenges during seizures. Traces without a corresponding trace in the fatal graph indicate animals that did not die from reflex challenges during seizures. For clarity, confidence intervals are not shown
FIGURE 7.
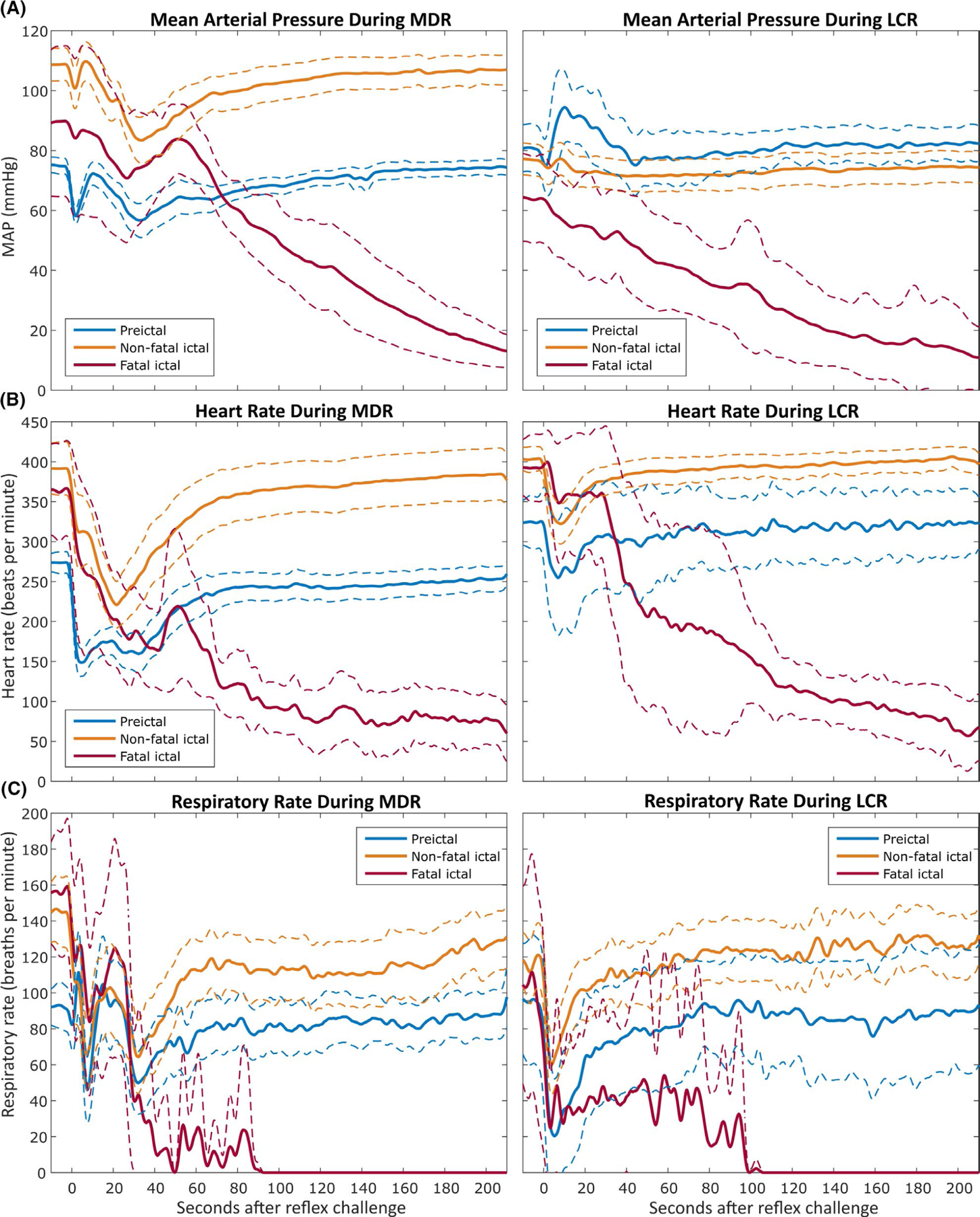
Mean arterial pressure (A), heart rate (B), and respiratory rate (C) measurements during mammalian diving reflex (MDR, left) and laryngeal chemoreflex (LCR, right) challenges. Intrahippocampal and systemic kainic acid data sets have been combined here to permit comparison of reflexes independently from seizure models. The thick lines indicate the average value over time for all trials, with the thinner dashed lines indicating the 95% confidence interval for the values over time across trials. The same legend colors apply to all panels. t = 0 seconds corresponds to the time of reflex challenge initiation. All x-axes are equivalent
3.3.2 |. Laryngeal chemoreflex
Chemoreflex challenges caused a 19.2% ± 14.1% transient drop in MAP between 0 and 50 s after reflex challenge. In nonfatal reflexes, MAP returned to within 20% of its pre-challenge amplitude in 59.0 s.
ECoG amplitude was again observed to correlate with MAP loss. Brain death in fatal chemoreflex challenges occurred 59.9 ± 28.8 s after reflex challenge and was coincident with a 14.8 ± 9.7 mm Hg drop in MAP in a 20 s window around the time of brain death, as shown in Figure 6. Significant differences were observed between systemic KA and hippocampal KA rats for MAP loss in the 20 s window around the time of brain death (6.6 ± 3.8 mm Hg and 23.1 ± 7.4 mm Hg, respectively) (P < .05, Welch’s t test) but not for time from reflex challenge until the onset of brain death (59.4 ± 21.0 s and 60.3 ± 42.1 s, respectively) (P = .97, Welch’s t test), time from reflex challenge to cardiac arrest (380 ± 115 s and 286 ± 91.5 s, respectively) (P = .31, Welch’s t test), or time from reflex challenge until terminal central apnea (243 ± 89.0 s and 116 ± 112 s, respectively) (P = .18, Welch’s t test).
In two of the chemoreflex experiments (one systemic KA and one hippocampal KA), the rat appeared to begin to slowly recover from the reflex challenge, and then died 4–5 min later. The physiological measurements associated with these deaths are consistent with those from deaths immediately following a reflex challenge. We hypothesize two possible explanations for these deaths: trace acid remaining on the larynx or surrounding tissues from the reflex challenge triggered a reflex several min after initial infusion of acid or the rat experienced a spontaneous laryngeal chemoreflex without a readily apparent stimulus. We judge the former to be more likely, since we did not observe spontaneous reflexes in the diving reflex experiments.
3.3.3 |. Near-death events
We also encountered four instances (not included in the 28 animals of the main study) of atypical responses to laryngeal chemoreflex challenges during seizures: In each instance, a systemic KA rat would survive a reflex challenge but never recover to baseline measures. These rats lost any observable ECoG signal and experienced severe bradypnea (<10 breaths per minute) and hypotension during the hour following this reflex challenge. These responses strongly parallel “near SUDEP” or “fatal near SUDEP” defined in the MORTEMUS study.6 We euthanized three of these rats, with the fourth dying spontaneously after approximately 2 h in this state.
4 |. DISCUSSION
4.1 |. Similarities between OCR cardiorespiratory collapse and human SUDEP data
We observe several characteristics of cardiorespiratory collapse due to OCR activation consistent with the clinical observations in SUDEP described by MORTEMUS6. First, terminal apnea preceded terminal asystole by several minutes in every case of induced sudden death, a characteristic common to human cases of SUDEP and numerous animal models that have sought to study SUDEP.44–47 Second, MORTEMUS reports transient asystole, bradycardia, and central apnea coincident with generalized EEG suppression in SUDEP cases. Our cases of induced sudden death show central apnea, bradycardia, and marked hypotension—indicative of severe cardiac dysfunction—coincident with loss of ECoG signal in induced sudden death. Third, human patients who did not die immediately following cardiorespiratory collapse declined progressively for minutes until death, exhibiting atypical respiration and cardiac function during this time, as well as diffuse EEG suppression strongly resembling that seen in SUDEP. In addition to the cases of delayed death addressed in 3.3.2, the atypical responses we describe in 3.3.3 match the description in MORTEMUS of “fatal near SUDEP,” leading us to conclude that the mechanisms underlying de novo human SUDEP and our inducible sudden death are similar. Finally, as we demonstrate in Figure 8, we observe a progression of cardiorespiratory collapse very similar to those reported by MORTEMUS. In Figure 5, we show the latency to terminal apnea and cardiac arrest for each rat, as well as any periods of transient apnea or cardiac arrest that occur prior to the terminal event. These representations further support our observations of similar patterns and timescales of cardiorespiratory collapse between animals, reflexes, and seizure models and of physiological parallels to human SUDEP.
FIGURE 8.
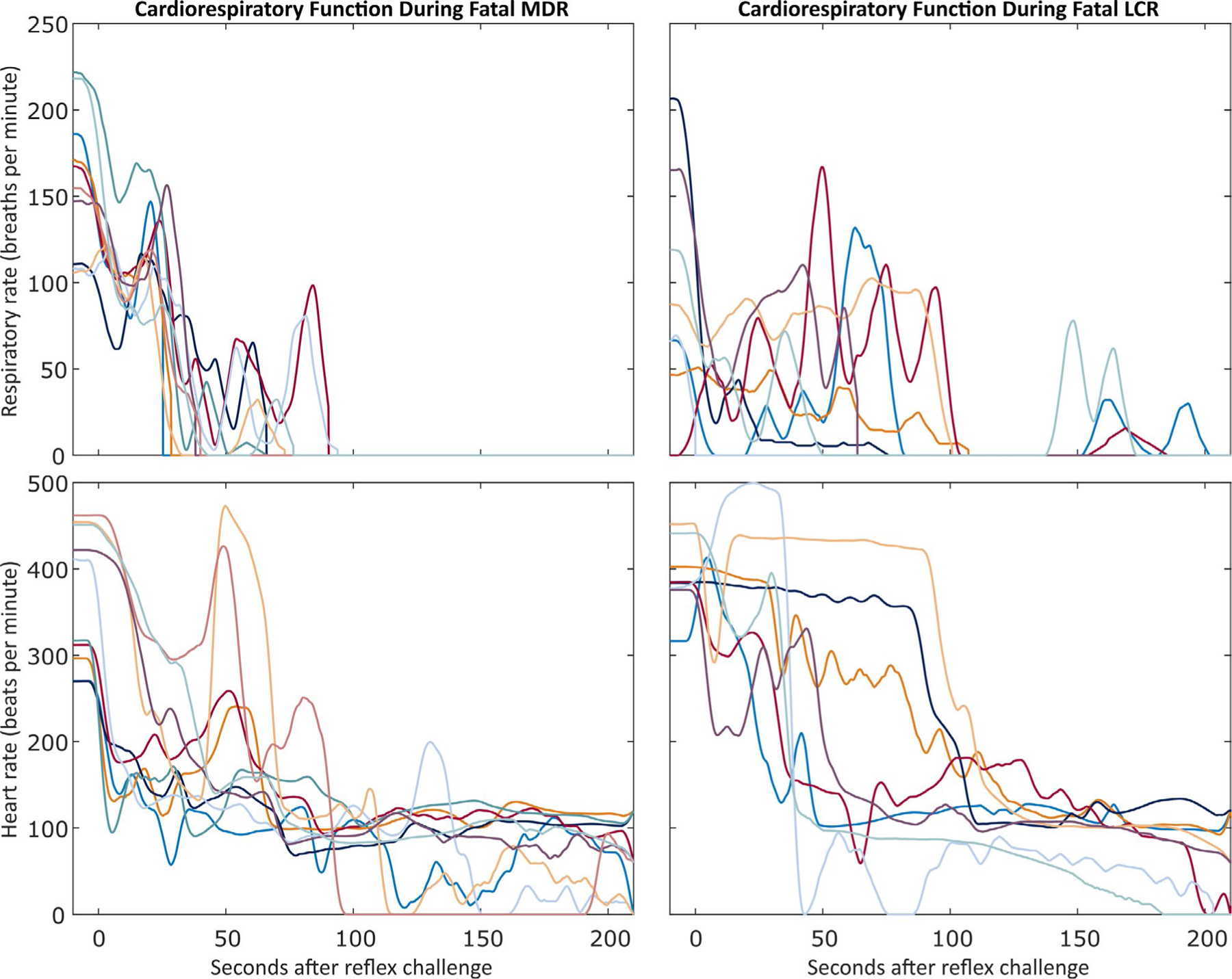
Patterns of respiratory and cardiac rate during the first 3 min following a fatal oxygen-conserving reflex (OCR) challenge. t = 0 seconds corresponds to the time of reflex challenge initiation. Top row: Respiratory rates for the 18 fatal OCR challenges. Each line represents one rat and stops at the beginning of the terminal apnea. Bottom row: Cardiac rates for the 18 fatal OCR challenges. Each line represents one animal and stops at the beginning of the first asystole longer than 5 s if it occurred in the first 210 s after the OCR challenge. Left column: Respiratory and heart rate following 10 fatal mammalian diving reflex (MDR) challenges. Right column: Respiratory and heart rate following 8 fatal laryngeal chemoreflex (LCR) challenges. For each column, each color in both graphs represents the same rat.
4.2 |. Relevance to human SUDEP
Although no deaths during acute status epilepticus in an anesthetized animal can replicate true SUDEP, our findings indicate significant similarities between the physiological changes associated with oxygen-conserving reflexes (or OCRs) and the pathophysiology observed in SUDEP. Specifically, we report that the ictal activation of OCRs presents a significant risk for cardiorespiratory collapse not observed in healthy rats, and we have demonstrated its effectiveness as a method for inducing a SUDEP-like sudden death on-demand. These findings build upon our previous work,30,38 where we showed the potential for acid-induced laryngospasm to cause death in seizing animals, and the work of others,26,46–48 who have documented the shared physiology between spontaneous ictal central apnea and diving reflex-induced central apnea, offering a mechanism that fully encompasses and explains both. Recorded instances of human SUDEP6 show a parallel progression to our findings of bradycardia, followed by terminal apnea preceding terminal asystole. This consistency offers a potential mechanism by which the pathophysiology in recorded human SUDEP may occur and progress. Both OCRs studied in this work could reasonably be experienced by a SUDEP patient: The laryngeal chemoreflex frequently occurs due to acid reflux at night, even in nonepileptic patients.35 Aspiration of saliva during a seizure presents the risk of mechanically triggering a chemoreflex. Spontaneous activation of the diving reflex could be precipitated by a transient ictal apnea causing minor hypoxia.25 We also cannot exclude the contribution of posture to spontaneous OCR activation: Rats breathe most easily while prone; humans do not, and most SUDEP patients are found lying prone.6
In addition, we have shown that our inducible sudden death can be triggered by activation of the laryngeal chemoreflex and causes pathophysiology and death consistent with human SUDEP. This offers a potential explanation for the “secondary mechanism” we hypothesized in our previous work,38 wherein we speculated that, if laryngospasm alone was not the cause of death, it may be one symptom of an underlying neural circuit. Further elucidation of the brainstem pathophysiology, and any potential relationship with changes in brainstem activity observed during spontaneous death in KA studies,39 requires additional direct current (DC)-coupled and single-unit recording49 in the cardiorespiratory control centers. Further physiological studies are also needed to explore the role of central and peripheral chemoreceptors, typically primary initiators of respiratory drive, in OCR regulation and the effects of seizure activity on the brainstem’s processing of their input.
4.3 |. OCR Modulation
The neural circuitry underlying OCRs is conserved across species and functions to provide systemic parasympathetic stimulation to reduce the oxygen demands of the body.19,21,22,24,26,34,43 Quite conversely, generalized seizures preferentially activate the sympathetic nervous system.9 We hypothesize that this combination of competing and contradictory signals causes the centrally mediated cardiorespiratory collapse observed in both our data and human SUDEP data. This suggests a potential opportunity for electroceutical intervention: Electrical stimulation or blocking of a peripheral nerve like the vagus nerve—already an accepted treatment for refractory epilepsy—initiated in response to seizure onset could be implemented to temporarily modulate the parasympathetic input to the heart and lungs.
Key Points.
Activation of oxygen-conserving reflexes (OCRs) during seizures reliably induces sudden death in two rat models of epilepsy.
OCR-induced death shows multiple physiological parallels with recorded cases of human SUDEP.
Terminal central apnea always precedes terminal asystole, suggesting prolonged loss of central respiratory drive after reflex induction.
OCR activation never induces death in control (nonseizing) rats.
Ictal OCR activation provides a novel repeatable, inducible animal model for the study and treatment of sudden death.
ACKNOWLEDGMENTS
We thank Dr. Ed Bartlett for the use of his CED Power3 1401.
Footnotes
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Berg A Mortality in Epilepsy. Epilepsy Curr 2001;1(1):28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thurman David J, Hesdorffer Dale C, French JA. Sudden unexpected death in epilepsy: Assessing the public health burden. Epilepsia 2014;55(10):1479–85. [DOI] [PubMed] [Google Scholar]
- 3.Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: People with nocturnal seizures may be at highest risk. Epilepsia 2012;53(2):253–7. [DOI] [PubMed] [Google Scholar]
- 4.Langan Y, Nashef L, Sander JWAS. Sudden unexpected death in epilepsy: a series of witnessed deaths. J Neurol Neurosurg Psychiatry 2000;68(2):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nobili L, Proserpio P, Rubboli G, Montano N, Didato G, Tassinari CA. Sudden unexpected death in epilepsy (SUDEP) and sleep. Sleep Med Rev 2011;15(4):237–46. [DOI] [PubMed] [Google Scholar]
- 6.Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12(10):966–77. [DOI] [PubMed] [Google Scholar]
- 7.Lacuey N, Vilella L, Hampson JP, Sahadevan J, Lhatoo SD. Ictal laryngospasm monitored by video-EEG and polygraphy: a potential SUDEP mechanism. Epileptic Disorder Int Epilepsy J Videotape 2018;20(2):146–50. [DOI] [PubMed] [Google Scholar]
- 8.Tavee J, Morris H. Severe postictal laryngospasm as a potential mechanism for sudden unexpected death in epilepsy: a near-miss in an EMU. Epilepsia 2008;49(12):2113–7. [DOI] [PubMed] [Google Scholar]
- 9.Devinsky O Effects of seizures on autonomic and cardiovascular function. Epilepsy Curr 2004;4(2):43–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wannamaker BB. Autonomic nervous system and epilepsy. Epilepsia 1985;26(Suppl 1):S31–39. [DOI] [PubMed] [Google Scholar]
- 11.Stewart M Progress in defining autonomic consequences of seizure activity including sudden death. Clin Auton Res 2019;29(2):135–6. [DOI] [PubMed] [Google Scholar]
- 12.Davies AM, Koenig JS, Thach BT. Upper airway chemoreflex responses to saline and water in preterm infants. J Appl Physiol. 1988;64(4):1412–20. [DOI] [PubMed] [Google Scholar]
- 13.Thach BT. Reflux associated apnea in infants: evidence for a laryngeal chemoreflex. Am J Med 1997;103(5A):120S–24S. [DOI] [PubMed] [Google Scholar]
- 14.Xia L, Leiter JC, Bartlett D. Laryngeal reflex apnea in neonates: Effects of CO2 and the complex influence of hypoxia. Respir Physiol Neurobiol 2013;186(1):109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scadding GK, Brock C, Chouiali F, Hamid Q. Laryngeal Inflammation in the Sudden Infant Death Syndrome. Curr Pediatr Rev 2014;10(4):309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Der Velde L, Curran AK, Filiano JJ, Darnall RA, Bartlett D, Leiter JC. Prolongation of the laryngeal chemoreflex after inhibition of the rostral ventral medulla in piglets: a role in SIDS? J Appl Physiol 2003;94(5):1883–95. [DOI] [PubMed] [Google Scholar]
- 17.Shattock MJ, Tipton MJ. ‘Autonomic conflict’: a different way to die during cold water immersion? J Physiol 2012;590(Pt 14):3219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincenzi FF. Sudden unexpected death and the mammalian dive response: catastrophic failure of a complex tightly coupled system. Front Physiol 2019;10:97. 10.3389/fphys.2019.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paton JFR, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Brain Res Rev 2005;49(3):555–65. [DOI] [PubMed] [Google Scholar]
- 20.Mameli O, Caria MA, Melis F, Severino C, Tavera C, Mameli P, et al. Autonomic nervous system activity and life threatening arrhythmias in experimental epilepsy. Seizure 2001;10(4):269–78. [DOI] [PubMed] [Google Scholar]
- 21.Michael PW. The mammalian diving response: an enigmatic reflex to preserve life? Physiology 2013;28(5):284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heman-Ackah YD, Pernell KJ, Goding GS Jr. The laryngeal chemoreflex: An evaluation of the normoxic response. Laryngoscope 2009;119(2):370–9. [DOI] [PubMed] [Google Scholar]
- 23.Marchal F, Corke BC, Sundell H. Reflex apnea from laryngeal chemo-stimulation in the sleeping premature newborn lamb. Pediatr Res 1982;16(8):621–7. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly WT, Bartlett D, Leiter JC. Serotonin in the solitary tract nucleus shortens the laryngeal chemoreflex in anaesthetized neonatal rats. Experiment Physiol 2016;101(7):946–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vega JL. Ictal mammalian dive response: A likely cause of sudden unexpected death in epilepsy. Front Neurol 2018;9:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mooney S, Chin B, Villiere S, Nakase K, Kollmar R, Kim S, et al. Diving responses elicited by nasopharyngeal irrigation mimic seizure-associated central apneic episodes in a rat model. Neurobiol Dis 2019;124:408–15. [DOI] [PubMed] [Google Scholar]
- 27.Schaller B Trigeminocardiac reflex. J Neurol 2004;251(6):658–65. [DOI] [PubMed] [Google Scholar]
- 28.Scholander PF, Hammel HT, LeMessurier H, Hemmingsen E, Garey W. Circulatory adjustment in pearl divers. J Appl Physiol 1962;17(2):184–90. [DOI] [PubMed] [Google Scholar]
- 29.Goding GS. Correlation of laryngeal chemoreflex severity with laryngeal muscle response. Laryngoscope 1998;108(6):863–72. [DOI] [PubMed] [Google Scholar]
- 30.Budde RB, Arafat MA, Pederson DJ, Lovick TA, Jefferys JGR, Irazoqui PP. Acid reflux induced laryngospasm as a potential mechanism of sudden death in epilepsy. Epilepsy Res 2018;148:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGraw MB. Swimming behavior of the human infant. J Pediatr 1939;15:485–90. [Google Scholar]
- 32.Elliott NM, Andrews RD, Jones DR. Pharmacological blockade of the dive response: effects on heart rate and diving behaviour in the harbour seal (Phoca vitulina). J Exp Biol 2002;205(23):3757–65. [DOI] [PubMed] [Google Scholar]
- 33.Murdaugh HV, Cross CE, Millen JE, Gee JBL, Robin ED. Dissociation of bradycardia and arterial constriction during diving in the seal phoca vitulina. Science 1968;162(3851):364–5. [DOI] [PubMed] [Google Scholar]
- 34.Loughlin Christopher J, Koufman James A, Averill David B, Cummins Michelle M, Kim Y, Little John P, et al. Acid-Induced Laryngospasm in a Canine Model. Laryngoscope 2009;106(12):1506–9. [DOI] [PubMed] [Google Scholar]
- 35.Postma GN, Halum SL. Laryngeal and pharyngeal complications of gastroesophageal reflux disease. GI Motility Online; 2006. [Google Scholar]
- 36.Halstead Lucinda A Role of gastroesophageal reflux in pediatric upper airway disorders. Otolaryngol Head Neck Surg 1999;120(2):208–214. 10.1016/s0194-5998(99)70408-0 [DOI] [PubMed] [Google Scholar]
- 37.Yavari P, McCulloch PF, Panneton WM. Trigeminallymediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J Auton Nerv Syst 1996;61(2):195–200. [DOI] [PubMed] [Google Scholar]
- 38.Budde RB, Pederson DJ, Biggs EN, Jefferys JGR, Irazoqui PP. Mechanisms and prevention of acid reflux induced laryngospasm in seizing rats. Epilepsy Behav 2020;111:107188. 10.1016/j.yebeh.2020.107188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jefferys JGR, Arafat MA, Irazoqui PP, Lovick TA. Brainstem activity, apnea, and death during seizures induced by intrahippocampal kainic acid in anaesthetized rats. Epilepsia 2019;60(12):2346–58. [DOI] [PubMed] [Google Scholar]
- 40.Pederson DJ, Quinkert CJ, Arafat MA, Somann JP, Williams JD, Bercich RA, et al. The Bionode: a closed-loop neuromodulation implant. ACM Trans Embed Comput Syst 2019;18(1):1–20.34084098 [Google Scholar]
- 41.Sakamoto K, Saito T, Orman R, Koizumi K, Lazar J, Salciccioli L, et al. Autonomic consequences of kainic acid–induced limbic cortical seizures in rats: Peripheral autonomic nerve activity, acute cardiovascular changes, and death. Epilepsia 2008;49(6):982–96. [DOI] [PubMed] [Google Scholar]
- 42.Zheng X-Y, Zhang H-L, Luo Q, Zhu J. Kainic acid-induced neurodegenerative model: potentials and limitations. J Biomed Biotechnol 2011;2011:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foster GE, Sheel AW. The human diving response, its function, and its control. Scand J Med Sci Sports 2005;15(1):3–12. [DOI] [PubMed] [Google Scholar]
- 44.Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol 2014;592(Pt 19):4395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faingold CL, Randall M, Tupal S. DBA/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy Behav 2010;17(4):436–40. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y, Bravo E, Thirnbeck CK, Smith-Mellecker LA, Kim SH, Gehlbach BK, et al. Severe peri-ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest 2018;128(3): 1141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lertwittayanon W, Devinsky O, Carlen PL. Cardiorespiratory depression from brainstem seizure activity in freely moving rats. Neurobiol Dis 2020;134:104628. [DOI] [PubMed] [Google Scholar]
- 48.Nakase K, Kollmar R, Lazar J, Arjomandi H, Sundaram K, Silverman J, et al. Laryngospasm, central and obstructive apnea during seizures: Defining pathophysiology for sudden death in a rat model. Epilepsy Res 2016;128:126–39. [DOI] [PubMed] [Google Scholar]
- 49.Arafat MA, Rubin LN, Jefferys JGR, Irazoqui PP. A Method of Flexible Micro-Wire Electrode Insertion in Rodent for Chronic Neural Recording and a Device for Electrode Insertion. Ieee Transactions on Neural Systems and Rehabilitation. Engineering 2019;27(9):1724–31. [DOI] [PubMed] [Google Scholar]


