Abstract
Objectives:
Acute invasive fungal sinusitis (AIFS) is a rapidly progressive disease, whose delayed identification results in poor outcomes, especially in immunocompromised individuals. A surge in of AIFS in the wake of the COVID-19 pandemic has lent additional morbidity and mortality to an already precarious clinical scenario. Early detection of AIFS in individuals who are symptomatic/ at risk can allow early therapy, enabling better patient outcomes. Our study aims to determine optimal soft-tissue markers on CT for the early detection of AIFS.
Methods:
In this case–control study, 142 patients with equal distribution of subjects were chosen based on histopathological diagnosis of AIFS; and their non-contrast CT scans were retrospectively assessed to determine the diagnostic utility of specific soft-tissue markers that would enable diagnosis of AIFS.
Results:
A total of nine markers with adequate sensitivity and specificity were identified, including pterygopalatine and sphenopalatine fossae, inferior orbital fissure and nasolacrimal duct involvement, premaxillary thickening, retro-antral and orbital stranding, and infratemporal muscle oedema. It was determined that the combined occurrence of any three out of nine markers was 91.5% sensitive and 95.9% specific for diagnosis of AIFS (p < 0.005).
Conclusion:
Early, accurate detection of AIFS in predisposed individuals is possible with identification of soft-tissue markers on NECT, enabling early intervention.
Advances in knowledge:
Being the aggressive disease that it is, AIFS may be managed early if the index of suspicion is held high via CT imaging; which our diagnostic checklist aims at enabling.
Introduction
Fungal sinusitis is a serious but often misdiagnosed condition of the nose and paranasal sinuses, with certain forms of fungal sinusitis having a high mortality rate of up to 50–80%. The most recent and widely accepted classification of fungal sinusitis is two-fold: invasive sinusitis and noninvasive sinusitis. 1 Invasive fungal sinusitis is characterized by the presence of hyphae in the mucosa, submucosa, bone, or blood vessels of paranasal sinuses, while noninvasive sinusitis is characterized by the absence of such features. 1,2 Invasive fungal sinusitis is further categorized into acute invasive fungal sinusitis, chronic invasive fungal sinusitis, and chronic granulomatous invasive fungal sinusitis. 1 Among these, acute invasive fungal sinusitis (AIFS) is considered the most aggressive form, 1,3 and is more common in immunocompromised individuals and in individuals with poorly controlled diabetes. 4 AIFS in patients with poorly controlled diabetes is predominantly caused by fungi belonging to the order Zygomycetes (up to 80%), which includes Rhizopus, Rhizomucor, Absidia, and Mucor; these infections are known to progress rapidly due to the high virulence of the organism and to the decreased immune response of the affected patients. AIFS in immunocompromised patients with severe neutropenia is caused by Aspergillus species fungi and are called fulminant-invasive sinusitis or neutropenic sinusitis. 5
The typical clinical presentation of individuals with AIFS is fever, facial pain or numbness, nasal congestion, serosanguineous nasal discharge, and epistaxis. 1 Local extension beyond the sinuses can cause orbital, intracranial, and maxillofacial involvement, and presents with subperiosteal/orbital abscesses, bulbar palsy, altered mental state, facial nerve palsy, palatal necrosis, nodularity and ulceration, proptosis, ophthalmoplegia, and vision loss. 6 AIFS has a very high mortality rate of up to 50%, 7 and intracranial extension of the infection can increase mortality to as high as 73%. 8 Given the rapidly progressive nature of AIFS infection, delay in diagnosis and late initiation of treatment can increase mortality further and lead to treatment difficulties. However, with diligent surveillance, patients with risk factors for AIFS can have a mortality rate as low as 18%, thus underscoring the importance of early detection and intervention. 8,9
Diagnosis of AIFS is based on histopathological demonstration of fungal hyphae in the mucosa, submucosa, bones, and blood vessels. 10,11 Patients with limited infection and infection not involving the anterior nasal tissue require surgical biopsy. 12 Coagulopathy and other comorbidities can pose a significant risk to patients with AIFS during the intraoperative and postoperative periods. 9
Imaging plays a vital role in AIFS diagnosis. Per American College of Radiology (ACR) appropriateness criteria, non-contrast computed tomography (CT) is deemed to be appropriate for investigating acute or chronic fungal rhinosinusitis with or without invasion. 13 Although magnetic resonance imaging (MRI) is superior in assessing intracranial and intraorbital extension, 1 this technique is more time-consuming and requires a cooperative, stationary, and prone patient.
In this post-COVID-19 (SARS-CoV-2) era, there has been a markedly increased incidence of AIFS in at-risk patients with COVID-19 compared to non-COVID-19 patients. 3 Given the need for rapid screening of at-risk patients, we retrospectively analysed soft tissue findings in non-contrast CT performed on patients with biopsy-proven AIFS. Through this study, we aim to find reliable CT soft-tissue markers to diagnose or exclude AIFS.
Aim
To identify reliable CT soft-tissue markers to diagnose or exclude AIFS.
Objective
To use a set of predefined CT soft-tissue markers to differentiate AIFS from non-invasive fungal sinusitis for accurate AIFS diagnosis.
To evaluate the diagnostic capability of each soft-tissue marker and to determine those which are capable of differentiating AIFS from noninvasive sinusitis.
Methods
Appropriate ethical clearance was obtained from our institutional ethics committee. The need for written informed consent was waived due to the retrospective design of the study.
Study design and patient selection
A case–control study in the form of retrospective analysis of non-contrast CT was performed on a group of patients who had a clinical history or features suggestive of sinusitis. Patients who had undergone treatment for AIFS in our institution from January 2012 to April 2021 were included in the study. The cases and controls were selected based on the following criteria:
Inclusion criteria for cases
Duration of symptoms suggestive of sinusitis (including but not limited to sudden onset facial pain, epistaxis, nasal congestion, serosanguineous discharge, and fever) less than four weeks from time of presentation,
Histopathological confirmation of AIFS following functional endoscopic sinus surgery (FESS), and
CT scan following the onset of symptoms or features of sinusitis using our institution’s standard paranasal (PNS) imaging protocol.
Exclusion criteria for cases
All sinuses not adequately visualized by CT,
Image degradation due to artefacts, and
Histopathologically proven cases of AIFS displaying overt destruction of sinus walls.
Inclusion criteria for controls
Clinical history or features of sinusitis,
Histopathological confirmation of noninvasive sinusitis following FESS, and
CT scan following the onset of symptoms or features of sinusitis using our institution’s standard PNS imaging protocol.
Exclusion criteria for controls
All sinuses not adequately visualized by CT and
Image degradation due to artefacts.
Image acquisition
Images were acquired using GE BrightSpeed 32-slice and GE Optima 128slice multidetector CT scanners (GE Healthcare, Chicago, IL, USA) using our institution’s standard PNS imaging protocol. Non-contrast CT images were acquired at 150 mAs and 120 kV, with 5 mm thickness and interval spacing of 0.5 mm, that were later reformatted to 0.625 mm sections with sagittal and coronal reconstruction. The images were accessed via our Picture Archiving and Communication System and analysed in both the bone window and soft tissue window.
Image analysis
Images were reviewed by two radiologists (DS and KS) with 5 years and 3 years of experience, respectively, in head and neck imaging. The reviewers were blinded to the clinical details and histopathology reports of the patients. In case of any discrepancy in findings between the reviewers, the opinion of the expert reviewer (SC or SK) with 12 and 14 years of experience, respectively, in reviewing head and neck images was taken as final. Reviewer findings and their associated definitions can be seen in Table 1. All findings were recorded for the ipsilateral side of the sinus involved.
Table 1.
CT Markers with definition
| CT MARKER | DEFINITION |
|---|---|
| Premaxillary thickening | Soft tissue oedema or thickening anterior to the maxillary sinus |
| Retro-antral fat stranding | Soft tissue edema in the retro-antral plane |
| Bone rarefaction | Thinned out sinus wall with or without features of bone erosion |
| Pterygopalatine fossa (PPF) soft-tissue thickening with or without widening | Soft tissue component in the PPF, with or without widening |
| Sphenopalatine foramen (SPF) soft-tissue thickening with or without widening | Soft tissue component in the SPF, with or without widening |
| Lacrimal sac and nasolacrimal duct inflammatory changes | Soft tissue inflammatory changes in the lacrimal sac or nasolacrimal duct |
| Infratemporal muscle edema (Temporalis and Pterygoid muscles) | Increase in size or loss of striations in the temporalis or pterygoid muscle. |
| Extraconal fat stranding | Fat stranding in the extraconal space with or without edema of ocular muscle |
| Intraconal fat stranding | Fat stranding in the intraconal space |
| Preseptal thickening | Features of edema or swelling in the preseptal region |
| CT density of sinus content (measured in Hounsfield Units, HU) | The density of content within the sinus cavity was measured |
| Alveolar process involvement | Marrow emphysema and/ or erosion within the maxillary alveolar processes |
| Inferior orbital fissure involvement | Soft tissue component in the inferior orbital fissure, with or without widening |
| Unilateral involvement | Unilateral, or ≥2 sinuses involved (>50% occlusion) with soft tissue changes, or unilateral nasal mucosal thickening. |
Data analysis
Statistical analyses were performed using the STATA v. 16 software. The sensitivity, specificity, positive predictive value, negative predictive value, and likelihood ratios for each CT marker were calculated by performing diagnostic analysis. A chi-square test was performed for categorical variables. Logistic regression analysis was performed to find the association between CT findings and mortality.
Results
A total of 142 patients were selected for the study, with 71 patients each in the case and control groups. Forty-three patients in the case group had diabetes, 16 patients had active COVID-19 with a history of diabetes and steroid use, 4 patients had aplastic anaemia, 2 patients had idiopathic thrombocytopenic purpura (ITP), 2 patients had acute myelocytic leukemia (AML), 2 patients had multiple myeloma, and 2 patients were renal transplant recipients on immunosuppressants. Fifty-nine patients in the control group had diabetes, 6 patients had active COVID-19 with a history of diabetes and steroid use, 1 patient had AML, 1 patient had aplastic anaemia, and 4 patients had no underlying condition. Mucor and Rhizopus were the two most commonly isolated organisms in our patients.
A total of 14 CT markers were reviewed and recorded for each patient. Nine out of 14 markers had a specificity of greater than 90%; these markers were inferior orbital fissure involvement (98.6%), intraconal fat stranding (97.3%), infratemporal muscle edema (96%), extraconal fat stranding (94.6%), pterygopalatine fossa involvement (94.6%), lacrimal sac/nasolacrimal duct involvement (93.2%), retro-antral fat stranding (93.2%), proptosis (92%), and premaxillary thickening (90.5%). Four out of the 14 markers had a sensitivity of greater than 80%; these markers were bone rarefaction (84.5%), retro-antral fat stranding (86%), sphenopalatine foramen involvement (86%), and pterygopalatine fossa involvement (80.3%). Maximum interobserver agreement was seen with retro-antral fat stranding, intraconal fat stranding, pterygopalatine fossa widening and inferior orbital fissure involvement (κ statistic of 0.81, 0.77, 0.74, and 0.70, respectively). The third expert reviewer was consulted for 23 cases (15%), in view of discordant results from the initial reviewers. The sensitivity, specificity, positive predictive value, and negative predictive value are summarized in Figures 1 and 2.
Figure 1.
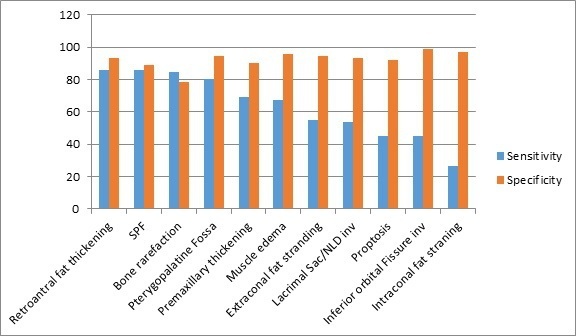
Sensitivity and specificity of the CT findings assessed in this study
Figure 2.
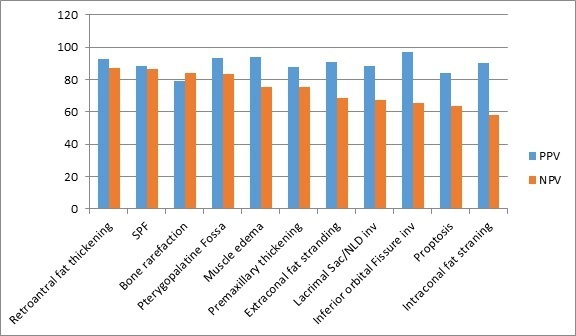
Positive predictive value (PPV) and negative predictive value (NPV) of the CT findings assessed in this study
The positive likelihood ratio for all the CT markers was calculated as the general prevalence of the findings could influence the positive and negative predictive values. A total of nine CT markers with a positive likelihood ratio of more than seven were shortlisted; these were inferior orbital fissure involvement (33.4), infratemporal muscle edema (16.7), pterygopalatine fossa involvement (14.9), retro-antral fat stranding (12.7), extraconal fat stranding (10.2), intraconal fat stranding (9.9), sphenopalatine foramen involvement (7.95), lacrimal sac and nasolacrimal duct involvement (7.9) and premaxillary thickening (7.3). Out of the nine shortlisted markers, if ≥2 markers were identified, the sensitivity and specificity were 96 and 87%, respectively; if ≥3 markers were identified, the sensitivity and specificity were 92 and 96%, respectively; and if ≥4 were identified, the sensitivity and specificity were 85 and 97.3%, respectively. As revealed by statistical analysis, the number of markers showing the optimal derived sensitivity and specificity was found to be 3. In considering more than three markers, the individual sensitivities of each marker was seen to impede the overall efficiency of the diagnostic assessment.
Discussion
AIFS is an extremely aggressive infection with high morbidity and mortality that is most often seen in immunocompromised patients and is rarely encountered in immunocompetent individuals. 5,14 The primary risk factor for AIFS are impaired neutrophilic response, 15,16 which could be due to underlying hematological malignancy; aplastic anaemia; or immunosuppressed states (immunosuppressant therapy, chemotherapy, or uncontrolled diabetes). 17 AIFS progression is determined by the degree of immunosuppression, 18 with an absolute neutrophil count less than 500 predisposing patients to an increased risk of AIFS and other opportunistic infections. 5,16–18 Early diagnosis, when the infection is more localized, has been linked to improved prognosis. 5,16,19,20 Hence, early diagnosis is of paramount importance to initiate timely treatment, which includes recovery from the neutropenic state, initiation of antifungal medication, and surgical debridement. 19–22
Early CT imaging of the paranasal sinuses is helpful in both AIFS diagnosis and in guiding subsequent surgical debridement. 5,16 However, histopathological evaluation is required for diagnostic confirmation of AIFS. 17 Previous studies 9 have compared the sensitivity of CT with MRI for diagnosing AIFS and have concluded MRI to have higher sensitivity than CT, given the superior soft tissue resolution of the former. However, the specificity of CT (82%) and MRI (83%) were very similar. 9,23 As per the ACR appropriateness criteria, both non-contrast CT and contrast-enhanced MRI were found to be appropriate for diagnostic imaging of patients with suspected AIFS. 13 Given the utility of non-contrast CT, we with this study we intended to identify markers that could further improve the sensitivity and specificity of CT in the diagnosis of AIFS.
Non-contrast CT is suitable for the evaluation of bony dehiscence. 1 However, relying on this imaging modality alone as an important criterion for accurate AIFS diagnosis could delay the much-needed early management of AIFS, as bone destruction occurs in a more advanced stage of the infection. 9 Peri-antral soft-tissue involvement has been reported as one of the earliest signs of AIFS. 24 Soft-tissue involvement can occur due to direct extension through bony dehiscence or via perivascular extension of infection through penetrating vessels in the bone or along nerves. 8,25 Given the importance of the soft-tissue findings, we used multiple CT soft tissue findings as markers to enhance the sensitivity of CT in diagnosing AIFS.
Based on a review of previous studies, we selected 14 CT markers that could help differentiate AIFS from noninvasive sinusitis. 8,26,27 In our study, we found bone rarefaction to have higher sensitivity (84%) and lower specificity (78%) than shown by Middlebrooks et al.. 27 Inferior orbital fissure involvement was found to be the most specific feature for AIFS with a specificity of 98.6%, albeit with a lower sensitivity (45.1%). Similar to the results of Middlebrooks et al, 27 we observed the soft-tissue findings to have very high specificity but low sensitivity, with only four markers having a sensitivity of more than 80% (i.e., retro-antral fat stranding [86%], sphenopalatine foramen involvement [86%], bone rarefaction [84.5%], and pterygopalatine fossa involvement [80.3%]). This could be due to the prevalence of each marker in our selected cases; as a result, we chose markers with a likelihood ratio of ≥7 and performed a diagnostic analysis. Out of the nine shortlisted markers (Table 2), (Figures 3 and 4), if ≥3 findings were identified, the sensitivity and specificity for the diagnosis of AIFS were 91.5 and 95.9%, respectively. We recommend using this criterion, as it lends a greater sensitivity and specificity compared to previous research. 27
Table 2.
9 Point CT checklist for AIFS
| 9 POINT CT CHECKLIST |
|---|
| Pterygopalatine fossa involvement (Figure 3a) |
| Premaxillary thickening (Figure 3b) |
| Retro-antral fat stranding (Figure 3b) |
| Sphenopalatine foramen involvement (Figure 3a) |
| Infratemporal muscle edema (ipsilateral pterygoid and temporalis muscle) (Figure 3c) |
| Inferior orbital fissure involvement (Figure 3d) |
| Extraconal fat stranding (Figure 4a) |
| Intraconal fat standing (Figure 4a) |
| Lacrimal sac and nasolacrimal duct involvement (Figure 4b and c) |
Figure 3.
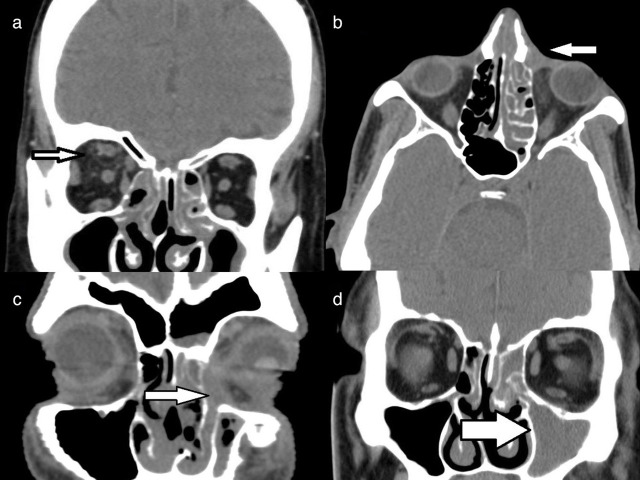
Axial non-contrast CT of paranasal sinuses in soft tissue window, demonstrating: (a) Soft tissue in the left pterygopalatine fossa (black arrow) and sphenopalatine foramen (white arrow); (b) Right premaxillary superficial soft tissue edema (white arrow) and retro-antral extension of inflammation seen as fat stranding (black arrow); (c) Edematous muscles of the left infratemporal fossa, with soft-tissue inflammation, and (d) Soft tissue in the inferior orbital fissure, indicating orbital spread (black arrow)
Figure 4.
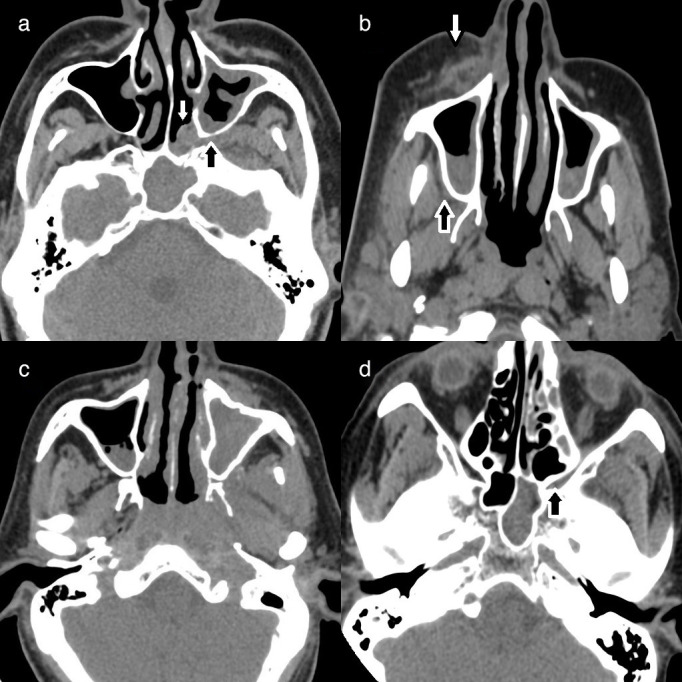
Axial non-contrast CT of paranasal sinuses in soft tissue window, demonstrating (highlighted by white arrows): (a) Intraconal and extraconal fat stranding involving the right orbit white; (b) Soft tissue thickening involving the left nasolacrimal gland; (c) Soft tissue thickening of the left nasolacrimal duct, with extensive left orbital inflammation, and (dD) Opacification of left ethmoid and maxillary sinuses with left hemifacial soft-tissue inflammation
We considered concurrent involvement of the nasal cavity and ipsilateral paranasal sinus as “unilateral” disease. In doing so, we found unilateral involvement to be common among patients with AIFS (76.6%), which is similar to the findings of previous studies. 27,28 There was no significant relationship between the CT density of sinus content (measured in Hounsfield Units, HU) and AIFS, and we could not determine a significant density for diagnosis of the same.
The most common underlying aetiology for AIFS in our study population was diabetes (60.5%), which could explain the high prevalence of Mucor and Rhizopus species of fungi in our study population. However, our study findings could be used for screening all AIFS patients irrespective of the causative organism, as previous studies did not find significant imaging differences between Mucor and Aspergillus-caused AIFS. 27,28 The mortality in our case group was 26%, lower than the mortality (50%–80%) reported in previous studies. 4,5,20,21 No specific marker in our study was observed to influence patient prognosis, as was found by Middlebrooks et al. 27
A possible limitation to this study may be the manner of patient inclusion: selection bias is inevitable in retrospective studies, as AIFS is seen in individuals who are more often than not already critically ill; this may lead to overestimation of mortality. However, considering the emergent nature of this study in the wake of the spate of AIFS infections following the COVID-19 pandemic, we have chosen not to afford this issue too much attention. Measurement bias may also be expected, and we have tried to minimize this to the extent possible by having two independent, blinded observers analyse study images.
Conclusion
There is an urgent need for a rapid screening tool for AIFS, particularly in light of the surge of AIFS incidence in the wake of the COVID-19 pandemic, secondary to the use of corticosteroids. 29 Careful monitoring of at-risk patients is required to ensure optimal outcome. Non-contrast CT may be performed for symptomatic or asymptomatic patients with other features of fungal infection (including invasive pulmonary fungal infection or positive serum galactomannan). 30 Our list of nine relevant-point CT markers may be a useful imaging-based screening tool for AIFS, following which nasal endoscopy/ biopsy may be performed if imaging suggests the presence of infection.
Footnotes
Contributors: Conceptualization: Deepa Susan John, Karthik Shyam, Dhilip Andrew. Data acquisition: Deepa Susan John, Karthik Shyam, Dhilip Andrew. Data curation: Deepa Susan John, Karthik Shyam, Dhilip Andrew. Formal analysis: Karthik Shyam, Dhilip Andrew. Methodology: Deepa Susan John, Karthik Shyam, Dhilip Andrew, Soumya Cicilet, Saikanth Reddy Deepalam. Project administration: Soumya Cicilet, Saikanth Reddy Deepalam. Software: Karthik Shyam, Dhilip Andrew. Supervision: Soumya Cicilet, Saikanth Reddy Deepalam. Validation: Soumya Cicilet, Saikanth Reddy Deepalam. Visualization: Deepa Susan John, Karthik Shyam, Dhilip Andrew. Writing-original draft: Deepa Susan John, Karthik Shyam, Dhilip Andrew. Writing-review & editing: Deepa Susan John, Karthik Shyam, Dhilip Andrew, Soumya Cicilet, Saikanth Reddy Deepalam.
Contributor Information
Deepa Susan John, Email: deepa.susanne18@gmail.com.
Karthik Shyam, Email: dr.karthikshyam@gmail.com.
Dhilip Andrew, Email: dhilipandrew@gmail.com.
Soumya Cicilet, Email: soumyac01@gmail.com.
Saikanth Reddy Deepalam, Email: saisouparna@gmail.com.
REFERENCES
- 1. Aribandi M, McCoy VA, Bazan C. Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiographics 2007; 27: 1283–96. doi: 10.1148/rg.275065189 [DOI] [PubMed] [Google Scholar]
- 2. Bakhshaee M, Bojdi A, Allahyari A, Majidi MR, Tavakol S, Najafzadeh MJ, et al. Acute invasive fungal rhinosinusitis: our experience with 18 cases. Eur Arch Otorhinolaryngol 2016; 273: 4281–87. doi: 10.1007/s00405-016-4109-z [DOI] [PubMed] [Google Scholar]
- 3. Ismaiel WF, Abdelazim MH, Eldsoky I, Ibrahim AA, Alsobky ME, Zafan E, et al. The impact of covid-19 outbreak on the incidence of acute invasive fungal rhinosinusitis. Am J Otolaryngol 2021; 42: 103080. doi: 10.1016/j.amjoto.2021.103080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waitzman AA, Birt BD. Fungal sinusitis. J Otolaryngol 1994; 23: 244–49. [PubMed] [Google Scholar]
- 5. Gillespie MB, O’Malley BW Jr, Francis HW. An approach to fulminant invasive fungal rhinosinusitis in the immunocompromised host. Arch Otolaryngol Head Neck Surg 1998; 124: 520–26. doi: 10.1001/archotol.124.5.520 [DOI] [PubMed] [Google Scholar]
- 6. El-Kholy NA, El-Fattah AMA, Khafagy YW. Invasive fungal sinusitis in post covid-19 patients: a new clinical entity. Laryngoscope 2021; 131: 2652–58. doi: 10.1002/lary.29632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner JH, Soudry E, Nayak JV, Hwang PH. Survival outcomes in acute invasive fungal sinusitis: a systematic review and quantitative synthesis of published evidence. Laryngoscope 2013; 123: 1112–18. doi: 10.1002/lary.23912 [DOI] [PubMed] [Google Scholar]
- 8. DelGaudio JM, Swain RE, Kingdom TT, Muller S, Hudgins PA. Computed tomographic findings in patients with invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg 2003; 129: 236–40. doi: 10.1001/archotol.129.2.236 [DOI] [PubMed] [Google Scholar]
- 9. Groppo ER, El-Sayed IH, Aiken AH, Glastonbury CM. Computed tomography and magnetic resonance imaging characteristics of acute invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg 2011; 137: 1005–10. doi: 10.1001/archoto.2011.170 [DOI] [PubMed] [Google Scholar]
- 10. deShazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Gardner L, Swain R. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg 1997; 123: 1181–88. doi: 10.1001/archotol.1997.01900110031005 [DOI] [PubMed] [Google Scholar]
- 11. Ghadiali MT, Deckard NA, Farooq U, Astor F, Robinson P, Casiano RR. Frozen-section biopsy analysis for acute invasive fungal rhinosinusitis. Otolaryngol Head Neck Surg 2007; 136: 714–19. doi: 10.1016/j.otohns.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 12. Gillespie MB, Huchton DM, O’Malley BW. Role of middle turbinate biopsy in the diagnosis of fulminant invasive fungal rhinosinusitis. Laryngoscope 2000; 110: 1832–36. doi: 10.1097/00005537-200011000-00013 [DOI] [PubMed] [Google Scholar]
- 13. Cornelius RS, Martin J, Wippold FJ, Aiken AH, Angtuaco EJ, Berger KL, et al. ACR appropriateness criteria sinonasal disease. J Am Coll Radiol 2013; 10: 241–46. doi: 10.1016/j.jacr.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 14. Valera FCP, do Lago T, Tamashiro E, Yassuda CC, Silveira F, Anselmo-Lima WT. Prognosis of acute invasive fungal rhinosinusitis related to underlying disease. Int J Infect Dis 2011; 15: e841-4. doi: 10.1016/j.ijid.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 15. Chopra H, Dua K, Malhotra V, Gupta RP, Puri H. Invasive fungal sinusitis of isolated sphenoid sinus in immunocompetent subjects. Mycoses 2006; 49: 30–36. doi: 10.1111/j.1439-0507.2005.01170.x [DOI] [PubMed] [Google Scholar]
- 16. Gillespie MB, O’Malley BW. An algorithmic approach to the diagnosis and management of invasive fungal rhinosinusitis in the immunocompromised patient. Otolaryngol Clin North Am 2000; 33: 323–34. doi: 10.1016/s0030-6665(00)80008-0 [DOI] [PubMed] [Google Scholar]
- 17. Melancon CC, Clinger JD. The use of frozen section in the early diagnosis of acute invasive fungal sinusitis. Otolaryngol Head Neck Surg 2017; 157: 314–19. doi: 10.1177/0194599817697279 [DOI] [PubMed] [Google Scholar]
- 18. Ferguson BJ. Mucormycosis of the nose and paranasal sinuses. Otolaryngol Clin North Am 2000; 33: 349–65. doi: 10.1016/s0030-6665(00)80010-9 [DOI] [PubMed] [Google Scholar]
- 19. Foshee J, Luminais C, Casey J, Farag A, Prestipino A, Iloreta AM, et al. An evaluation of invasive fungal sinusitis outcomes with subsite analysis and use of frozen section analysis. Int Forum Allergy Rhinol 2016; 6: 807–11. doi: 10.1002/alr.21714 [DOI] [PubMed] [Google Scholar]
- 20. DelGaudio JM, Clemson LA. An early detection protocol for invasive fungal sinusitis in neutropenic patients successfully reduces extent of disease at presentation and long term morbidity. Laryngoscope 2009; 119: 180–83. doi: 10.1002/lary.20014 [DOI] [PubMed] [Google Scholar]
- 21. Kennedy CA, Adams GL, Neglia JP, Giebink GS. Impact of surgical treatment on paranasal fungal infections in bone marrow transplant patients. Otolaryngol Head Neck Surg 1997; 116: 610–16. doi: 10.1016/S0194-59989770236-5 [DOI] [PubMed] [Google Scholar]
- 22. Green KK, Barham HP, Allen GC, Chan KH. Prognostic factors in the outcome of invasive fungal sinusitis in a pediatric population. Pediatr Infect Dis J 2016; 35: 384–86. doi: 10.1097/INF.0000000000001015 [DOI] [PubMed] [Google Scholar]
- 23. Howells RC, Ramadan HH. Usefulness of computed tomography and magnetic resonance in fulminant invasive fungal rhinosinusitis. Am J Rhinol 2001; 15: 255–61. doi: 10.1177/194589240101500407 [DOI] [PubMed] [Google Scholar]
- 24. Silverman CS, Mancuso AA. Periantral soft-tissue infiltration and its relevance to the early detection of invasive fungal sinusitis: ct and mr findings. AJNR Am J Neuroradiol 1998; 19: 321–25. [PMC free article] [PubMed] [Google Scholar]
- 25. Orguc S, Yücetürk AV, Demir MA, Goktan C. Rhinocerebral mucormycosis: perineural spread via the trigeminal nerve. J Clin Neurosci 2005; 12: 484–86. doi: 10.1016/j.jocn.2004.07.015 [DOI] [PubMed] [Google Scholar]
- 26. Finkelstein A, Contreras D, Pardo J, Cruz JP, Gonzalez C, Constanza Beltrán M, et al. Paranasal sinuses computed tomography in the initial evaluation of patients with suspected invasive fungal rhinosinusitis. Eur Arch Otorhinolaryngol 2011; 268: 1157–62. doi: 10.1007/s00405-011-1561-7 [DOI] [PubMed] [Google Scholar]
- 27. Middlebrooks EH, Frost CJ, De Jesus RO, Massini TC, Schmalfuss IM, Mancuso AA. Acute invasive fungal rhinosinusitis: a comprehensive update of ct findings and design of an effective diagnostic imaging model. AJNR Am J Neuroradiol 2015; 36: 1529–35. doi: 10.3174/ajnr.A4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slonimsky G, McGinn JD, Goyal N, Crist H, Hennessy M, Gagnon E, et al. A model for classification of invasive fungal rhinosinusitis by computed tomography. Sci Rep 2020; 10(1): 12591. doi: 10.1038/s41598-020-69446-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehta S, Pandey A. Rhino-orbital mucormycosis associated with covid-19. Cureus 2020; 12: e10726. doi: 10.7759/cureus.10726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fernandez IJ, Crocetta FM, Demattè M, Farneti P, Stanzani M, Lewis RE, et al. Acute invasive fungal rhinosinusitis in immunocompromised patients: role of an early diagnosis. Otolaryngol Head Neck Surg 2018; 159: 386–93. doi: 10.1177/0194599818765744 [DOI] [PubMed] [Google Scholar]