Abstract
Morphological evaluation of the lung is important in the clinical evaluation of pulmonary diseases. However, the disease process, especially in its early phases, may primarily result in changes in pulmonary function without changing the pulmonary structure. In such cases, the traditional imaging approaches to pulmonary morphology may not provide sufficient insight into the underlying pathophysiology. Pulmonary imaging community has therefore tried to assess pulmonary diseases and functions utilizing not only nuclear medicine, but also CT and MR imaging with various technical approaches. In this review, we overview state-of-the art MR methods and the future direction of: (1) ventilation imaging, (2) perfusion imaging and (3) biomechanical evaluation for pulmonary functional imaging.
Introduction
Morphological evaluation of the lung is important for the evaluation of patients with pulmonary diseases because of the close relationship between structural changes of the lung and pathophysiology of such diseases. These correlations between pulmonary morphology and functions are fundamental for diagnostic imaging modalities including chest radiography and CT, which play a significant role in the diagnosis and management of patients with pulmonary disease. However, the underlying disease process, especially in its relatively early phases, may result primarily in changes in pulmonary function without substantial changes in pulmonary morphology or structure. In such cases, the traditional pulmonary imaging approaches may not provide enough insight into the underlying pathological process. Therefore, we have tried to assess pulmonary diseases in terms of gas exchange using nuclear medicine, CT and MR imaging combined with various technical approaches.
The principal function of the lung is gas exchange. For the lung to perform gas exchange effectively, the flow of air to the alveoli (ventilation) and the flow of blood to the capillary (perfusion) have to occur in a coordinated manner. Therefore, pulmonary function can be evaluated as a combination of ventilation and perfusion imaging with reference to spatial and temporal information derived from time-resolved anatomical imaging. A great advantage of pulmonary functional imaging is its ability to examine these processes on a regional basis, which is different from the traditional measures of pulmonary functions, such as spirometry, which evaluates the whole lung as a single unit. As many diseases begin regionally, their functional effects are often masked by normal lung elsewhere until the disease has progressed to involve substantial portions of the lung parenchyma. Pulmonary functional imaging may thus significantly improve our ability to evaluate these diseases before they progress to global changes in function and morphology. In addition to the evaluation of pulmonary disease, functional imaging may have a significant impact upon our understanding of pulmonary physiology. As already stated, many of the traditional methods for the evaluation of physiology lack spatial or temporal resolution. Pulmonary functional imaging, on the other hand, offers new, non-invasive, methods to evaluate regional physiology with high spatial and temporal resolution. In the last a few decades, new techniques for not only CT and MR imaging have been introduced, while new tracers for PET are expected to become available within the next decade, and be tested for clinical relevance for not only radiology, but also pulmonary medicine. Furthermore, MR imaging has long been considered the leading radiological technique in this setting and can be applied as a substitute for nuclear medicine studies, although for routine clinical practice, morphological assessment of lung parenchyma with MR imaging is still considered inferior to CT.
In this review, we describe and discuss state-of-the art MR methods as well as the future direction of (1) ventilation imaging, (2) pulmonary perfusion and hemodynamic imaging, and (3) biomechanical evaluation for pulmonary functional imaging.
Ventilation imaging
Hyperpolarized noble gas MR imaging
Hyperpolarized noble gas MR imaging and oxygen-enhanced (O2-enhanced) MR imaging have been studied as potential MR-based ventilation imaging techniques since the 1990s. 1–4 Hyperpolarized noble gas MR imaging can be performed with helium-3 (3He) and xenon-129 (129Xe). It has been extensively tested for assessment of disease severity and therapeutic effect evaluation of various pulmonary diseases. 5–29 With considering the hyperpolarized noble gas MR techniques, hyperpolarized noble gas MR imaging by using 3He and 129Xe can provide ventilation images as spin-density images and gas molecule diffusion within air space, which is different from oxygen diffusion from alveoli to capillary bed, as diffusion-weighted imaging (DWI). Spin density images during breath-hold represent a snapshot of gas distribution. On the other hands, DWI exploits the high free diffusion of 3He and 129Xe gases. In the context of restriction by the lung microstructure, this allows indirect measurement of the average dimensions of the lung airspaces. When 3He or 129Xe gas is restricted by tissue boundaries, the diffusivity is referred to as the apparent diffusion coefficient (ADC). A semi-quantitative ADC, which is considered as a surrogate for airspace size, map is easily derived. It shows differences in regional ventilation for cystic fibrosis, for asthma and chronic obstructive pulmonary disease (COPD), 5–12 for dynamic gas movement by means of dynamic gradient-echo sequencing 5–9 and for regional gas diffusion to determine alveolar size, 5,6,9,12–15 all with higher spatial resolution or temporal resolution than can be attained with nuclear medicine studies (Figure 1). Moreover, hyperpolarized noble gas MR imaging with 129Xe can also provide additional information such as xenon polarization transfer contrast (XTC) and a depolarization map, which are believed to better reflect the regional lung parenchyma density as can be obtained with hyperpolarized noble gas MR imaging with 3He. 5,6,16 The soluble “dissolved phase” fraction of 129Xe in blood and tissues is ~2% of the total signal after accounting for the lower density of lung tissue and blood in the lungs. The dissolved phase of 129Xe has a different chemical shift frequency than the gas phase. This fact can be exploited to quantify gas exchange. Polarized gas nuclei diffuse rapidly between gas, blood cells, and plasma/tissue compartments, with different chemical shifts in each compartment: 0 ppm, 222 ppm, and 198 ppm, respectively. 5,16 Compartmental modeling of these components has advanced relatively rapidly. 18 Simple and robust single voxel MR spectroscopy readily resolves tissue and blood enables the calculation of blood/tissue ratio as a possible biomarker of diffusion block. 19,20 More quantitative measures such as saturation transfer time allow the kinetics of 129Xe recovery to be modeled. These more advanced method scans provide direct or indirect estimates of average septal wall thickness and alveolar surface area to volume ratio. 16,18–21 Therefore, XTC is considered as one of the advantages of hyperpolarized noble gas MR imaging with 129Xe, when compared with that by 3He. Recently, pO2 mapping by hyperpolarized noble gas MR imaging is introduced as a new promising tool for pulmonary functional MR imaging. 22–24 The paramagnetic effects of oxygen reduce the T1 and T2 of hyperpolarized gases as well as proton signals and can therefore be exploited to provide a quantitative estimate of regional pO2. 22–24 Typically, the same slice is imaged repeatedly at different delay times following gas inhalation to separate RF saturation of signal from signal loss due to local O2 concentration. 25 The measurement is performed within a single breath-hold. 22,26 Modified centric and reverse-centric view orders mitigate effects due to locally variable flip angle (B1-field variation). 26 Gas flow effects within the lungs during a breath-hold using 3He have recently been described, underscoring that pO2 is a mixture of regional pO2 and local collateral ventilation effects. 27–29 Regional pO2 measures can potentially be used to calculate the ratio of ventilation to perfusion (V/Q). 25 In fact, pO2 measurements have recently been used as a marker of disease severity in a study of V/Q heterogeneity in smokers. 27–29 For these reasons, hyperpolarized noble gas MR imaging has been viewed as useful for not only ventilation imaging, but also the quantitative assessment of changes in lung structure in patients with some pulmonary diseases. However, these techniques have not yet been clinically established. Drawbacks of these techniques are their dependence on polarizer and multinuclear technology which is not widely available. 6 The global supply of 3He is also very limited, thus leading to high cost. 17 These drawbacks account for the shift to the more widely available 129Xe nucleus. The technical challenges of this shift have been made easier by advances in 129Xe SEOP polarization. 6 Hyperpolarized 129Xe MR imaging typically uses enriched 129Xe isotope with a natural abundance of 26% to ≤85% to compensate for its lower gyromagnetic ratio and achievable polarization compared to those of 3He gas. 6 However, 129Xe also dissolves in water/blood and therefore lung signal also derives from 129Xe uptake. 6 Moreover, the anesthetic effects of 129Xe is main causes of the limitation for administrating 129Xe gas. All the major types of hyperpolarized 3He MR imaging have now been replicated robustly with enriched hyperpolarized 129Xe MR imaging for future incorporation in the clinical setting, although the above-mentioned limitations of 129Xe have to be considered.
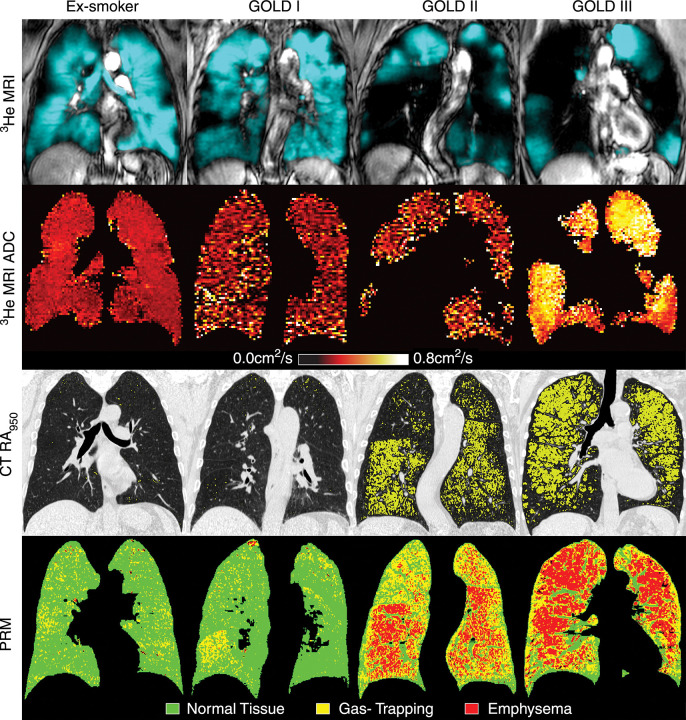
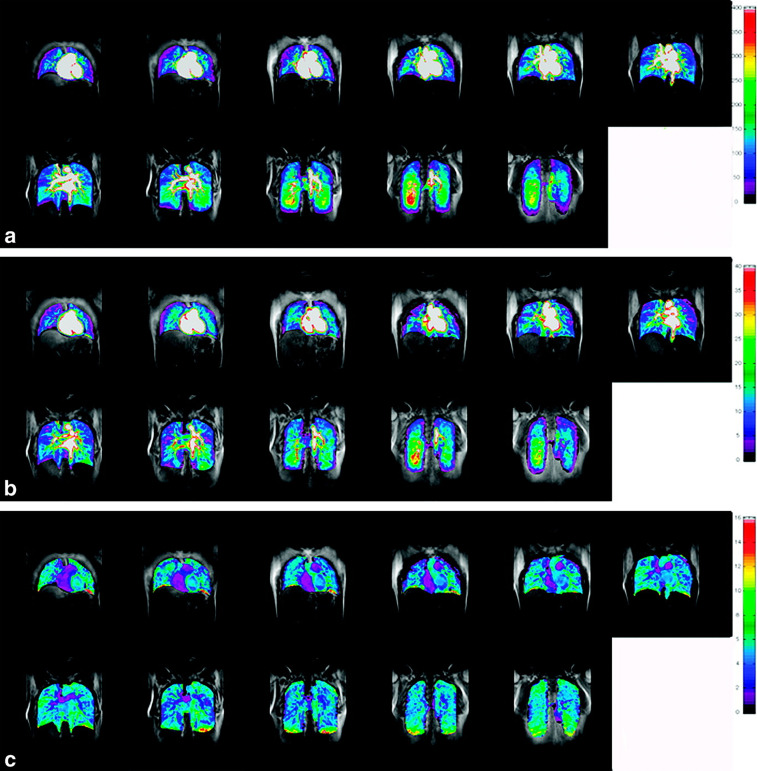
Figure 1.
Ventilation and PRM of (1) a 55-year-old male without COPD (FEV1, 83% of predicted value; FEV1/FVC, 77%; residual volume to total lung capacity ratio [RV/TLC], 45%) (2) a 69-year-old male with GOLD I disease (FEV1, 89% of predicted value; FEV1/FVC, 69%; RV/TLC, 39%; DLCO, 67% of predicted value) (3) an 84-year-old male with GOLD II disease (FEV1, 52% of predicted value; FEV1/FVC, 44%; RV/TLC, 62%; DLCO, 47% of predicted value), and (4) a 67-year-old female with GOLD III disease (FEV1, 33% of predicted value; FEV1/FVC, 39%; RV/TLC, 72%; DLCO, 28% of predicted value). First row: 3He MR images co-registered with 1H MR images (grayscale) show static ventilation (blue areas). Second row: 3He MR imaging ADC maps show that ex-smokers with more advanced COPD (GOLD II/III disease) have elevated ADC values. Third row: CT attenuation masks show areas of less than −950 HU (yellow areas). Fourth row: PRMs show areas of healthy tissue (green), gas trapping (yellow), and emphysema (red). Permission was obtained from reference number 12 (RSNA provide the permission). ADC, apparent diffusion coefficient; COPD, chronic obstructive pulmonary disease; HU, Hounsifield unit; PRM, parametric response map
Oxygen-enhanced MR imaging
O2-enhanced MR imaging has been recommended since 1996 as having potential for MR-based ventilation imaging. 2,6,30–46 The basic feature of the molecular oxygen used for O2-enhanced MR imaging is that it is weakly paramagnetic with a magnetic moment of 2.8 Bohr magnetons. O2 modulates the MR imaging signal of blood and fluid through two different mechanisms: the paramagnetic property of deoxyhemoglobin and the paramagnetic property of molecular oxygen itself. 47,48 Because deoxyhemoglobin is compartmentalized in red blood cells (RBC), tissue water protons do not have access to coordination sites, which is required for the spin-lattice interactions causing T1 shortening. 47,48 Therefore, deoxyhemoglobin with RBC has a T2*-shortening effect with little T1-shortening. 47,48 Because molecular oxygen is paramagnetic, dissolved molecular oxygen will shorten the T1 relaxation time of pulmonary venous blood. Therefore, the areas of the lung affected by pulmonary emphysema are pathologically characterized by a decrease in pulmonary capillary beds, abnormal permanent enlargement of the air spaces distal to the terminal bronchioles by destruction of their walls, while there is no obvious fibrosis. O2-enhanced MR imaging can demonstrate not only regional oxygen enhancement based on oxygen diffusion from alveoli to capillary bed, but also oxygen uptake based on ventilation as well as respiration. For these reasons, it has been suggested as useful for evaluation of various pulmonary diseases such as asthma, COPD, cystic fibrosis, interstitial lung disease, lung cancer 32–46 (Figure 2). O2-enhanced MR imaging is a proton-based technique and thus considered to be more clinically useful than hyperpolarized noble gas MR imaging and one of the key techniques for MR-based ventilation imaging. No other imaging method can demonstrate oxygen uptake based on respiration itself, although O2-enhanced MR imaging is not able to directly visualize O2 itself. However, its drawbacks are changes in low signal intensity due to oxygen inhalation, and multiple acquisitions or increased number of excitations are warranted for compensate low signal intensity change. A certain amont of time is needed for allowing the oxygen to wash in and out, both together resulting in very long acquisition time. It is time consuming to cover the entire lung because the 2D sequence rather than the 3D sequence has been applied in the past studies. Many studies to assess this technique used semi-quantitative assessment, and only a few study studies used quantitative evaluation. 2,32–46 Therefore, further developments of O2-enhanced MR imaging for 3D acquisition are needed to reduce the time needed to cover the entire lung and improve quantitative analysis for oxygen uptake evaluation. Finally, these new techniques should be further investigated to enhance the clinical relevance of O2-enhanced MR imaging.
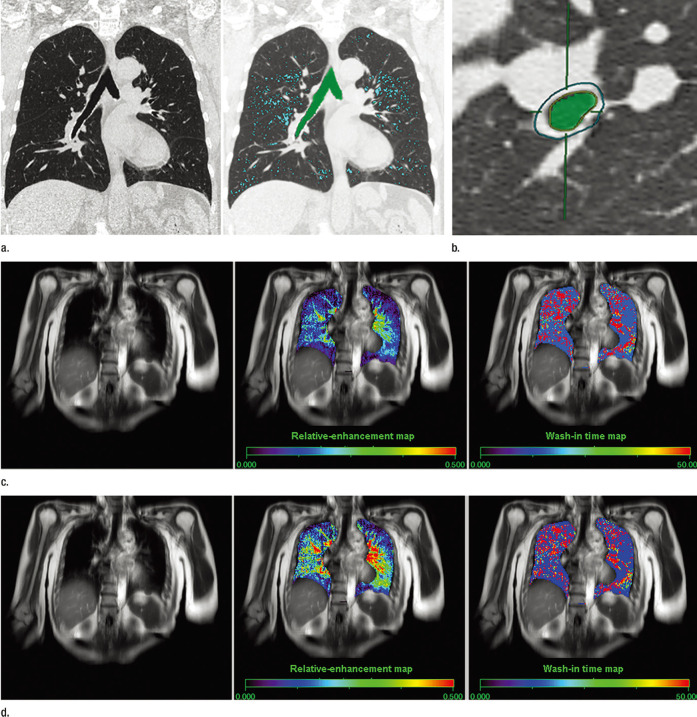
Figure 2.
Images show a 55-year-old female with moderate persistent asthma. (a) Thin-section coronal MPR image (left) shows no emphysema, and MLD was assessed as −890 HU. On the quantitative coronal CT image (right), areas with attenuation values less than −950 HU appear in blue, and the low-attenuation area was assessed as 6.7%. (b) On thin-section CT at the vertical plane to the third level of the bronchus, outer and inner areas of the bronchus are circled and the green area represents the inner lumen area of the bronchus. Quantitatively assessed wall area was 58.6%. (c) Source image of dynamic oxygen-enhanced MR imaging (left), relative enhancement map (center), and wash-in time map (right). Regional distributions of relative enhancement and wash-in time for each pixel were expressed as maps color coded from blue to red. The pretherapeutic mean RER was 0.18, and mean wash-in time was 26.0 s. (d) Source image of dynamic oxygen-enhanced MR imaging (left), relative-enhancement map (center), and wash-in time map (right). Regional distributions of relative enhancement and wash-in time for each pixel were expressed as maps color coded from blue to red. Post-therapeutic mean RER was 0.23, and mean wash-in time was 17.7 s. Permission was obtained from reference number 42 (RSNA provide the permission). HU, Hounsfield unit; MPR, multiplanar reconstruction; RER, relativeenhancement ratio.
Fluorinated gas MR imaging
As part of the continuing developments in hyperpolarized noble gas MR imaging and O2-enhanced MR imaging during the last few decades, some research groups have been testing fluorine-19 (19F) MR imaging as another promising approach for MR-based ventilation imaging. Inert 19F gases, on the other hand, are not hyperpolarized but are still able to be used for ventilation imaging. Fluorinated gases are an attractive choice because the 19F isotope is 100% naturally abundant, with a high gyromagnetic ratio on the order of that of 1H. 6,49 The T1 relaxation is dominated by spin-rotation interactions, T1 ~ T2. 1,50 Both MR decay parameters depend on the concentration of the gas itself and are generally on the order of a few milliseconds for the 19F nucleus. Therefore, signal intensity on 19F MR imaging is directly obtained from 19F itself, and not from hyperpolarization of the gas. Therefore, 19F MR imaging uses inhaled inert fluorinated gases for MR-based ventilation imaging and as such may constitute a more economical alternative to hyperpolarized noble gas MR imaging. 51–54 Inert fluorinated gases are nontoxic, abundant, relatively inexpensive, and the technique can be performed on any MRI scanner with broadband multinuclear imaging capabilities. 51–54 Feasibility studies for human lungs have been conducted and reportedly generated breath-hold images with gas mixtures containing 79% sulfur hexafluoride (SF6) or perfluoropropane (C3F8 or PFP) and 21% oxygen (O2). 52,55 Since they are inert fluorinated gases, SF6 and PFP are non-toxic, abundant, and relatively inexpensive. One report has estimated costs for 3He, 129Xe and 19F gases. 6 The per-liter cost of 3He and enriched 129Xe is much higher than that of 19F gases, but the cost of naturally abundant xenon is similar to that of SF6 and PFP. Since a typical imaging session using 19F MR imaging might use several liters of SF6 or PFP, the total cost of a single study may approach the cost of an enriched hyperpolarized noble gas MR imaging study using 129Xe; however, the advantage of 19F gas MR imaging is that the gases are imaged using thermal equilibrium polarization so that a polarizer system is not required. 53 In addition, 19F gas MR imaging can visualize regional differences in ventilation as well as air trapping and thus may provide functional information similar to that obtained from images acquired with hyperpolarized noble gas MR imaging(Figure 3). 51–54 In addition, 19F MR imaging is also suggested as having the capabilities for demonstration of gas molecule diffusion and ventilation/ perfusion (V/Q) mapping in animal studies. 51,56–59 On the whole, 19F gas MR imaging is expected to complement existing proton-based structural imaging techniques, and the combination of structural and functional lung MR imaging to provide useful outcome measurements for future management of pulmonary diseases based on not only academic, but also clinical findings. However, this technique has to be overcome several aspects such as equipment, scanner time and personnel, etc. Therefore, extensive further investigations are expected to be performed during the next decade or two for clinical application as one of the pulmonary functional imaging techniques used in routine clinical practice.
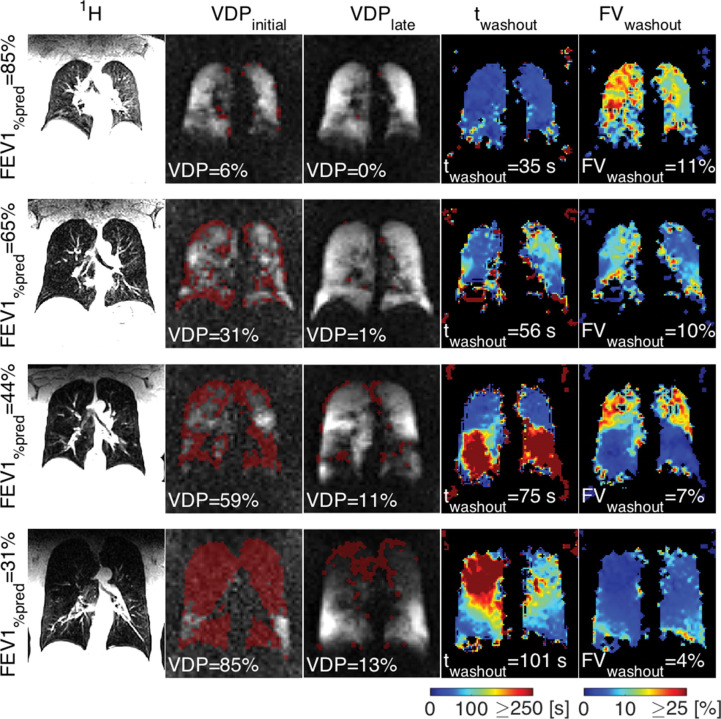
Figure 3.
Images show examples of morphologic imaging, initial and late 19F gas wash-in MR imaging with ventilation defect (red masks), and maps of wash-out times and FV assessed with dynamic 19F gas wash-out MR imaging for the different severities of COPD indicated by percentage predicted FEV1 (FEV1% predicted; corresponding to GOLD stages I–IV). VDP at initial gas wash-in MR imaging and median wash-out time increase and correspondingly median FV decreases with an increase in severity of COPD. Additionally, gas wash-out parameter maps show an increase in heterogeneity of regional lung ventilation with progression of disease. Permission was obtained from reference number 54 (RSNA provides the permission). COPD, chronicobstructive pulmonary disease; FV, fractional ventilation; VDP, volume defectpercentage.
Although we discuss MR-based ventilation imaging such as hyperpolarized noble gas MR imaging, O2-enhanced MR imaging and 19F MR imaging for providing different ventilation-based information, dynamic contrast-enhanced MR imaging based on perfusion MR techniques is currently the most frequently used technique to assess pulmonary ventilation by exploiting the effect of hypoxic vasoconstriction in the lung. 60 With considering this fact, dynamic contrast-enhanced MR imaging with perfusion MR techniques is also applied for not only perfusion, but also ventilation evaluations in patients with various pulmonary diseases in routine clinical practice.
Pulmonary perfusion and hemodynamic imaging
Non-contrast-enhanced and contrast-enhanced MR angiography
As early as the 1990s, it was suggested that non-contrast-enhanced and contrast-enhanced MR angiography are useful for visualization of pulmonary vasculature and blood flow by means of various methods. 61–69 Currently, there are two major established methods for assessment of pulmonary vasculature and blood flow. One is non-contrast-enhanced (CE-) MR examination, which uses a proton within blood as an endogenous tracer, and another is CE-MR examination which uses a gadolinium contrast medium as an intravenously injected tracer. As other methods for MR angiography, it has been suggested that the balanced steady-state free precession (bSSFP) sequence, the 3D electrocardiogram-gated (ECG-gated) fresh blood imaging (3D FBI) using 3D fast spin-echo (SE)–based sequence and the arterial spin labelling technique with SE and gradient-recalled echo (GRE) type sequences are useful for assessment of pulmonary vasculatures 65,66 (Figure 4). Results obtained thus far indicate that these techniques should be used in routine clinical practice for pulmonary vascular disease patients with low renal function or contraindication of gadolinium contrast media.
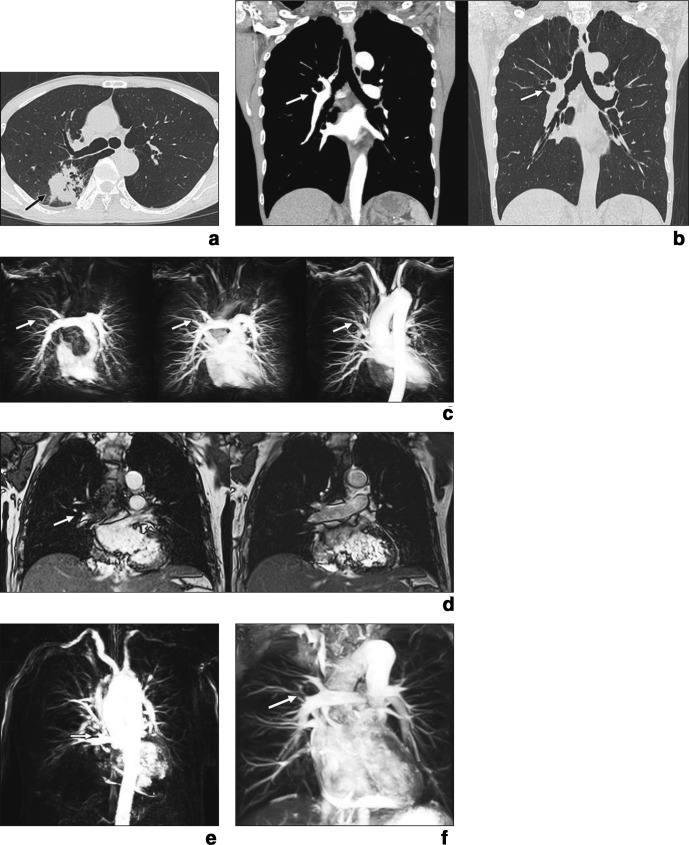
Figure 4.
50-year-old male with adenocarcinoma. (a) Thin-section CT shows lung cancer (arrow) in right posterior segment. (b). Thin-section contrast-enhanced multiplanar reformatted images obtained at mediastinal (left) and lung (right) window settings clearly show that posterior segmental artery (arrows) branches directly from pulmonary arterial trunk (visualization score, 5; variation probability score, 5). (c) Pulmonary arterial phase (left; time, 4.0 s), parenchymal phase (middle; time, 8.0 s), and venous phase (right; time, 12.0 s). 4D contrast-enhanced MR angiography images clearly show that posterior segmental artery (arrows) branches directly from pulmonary arterial trunk (visualization score, 5; variation probability score, 5). (d) Anterior (left) and posterior (right) cine MRI shows that posterior segmental artery (arrow) branches directly from pulmonary arterial trunk (visualization score, 4; variation probability score, 5). (e) Fresh blood imaging clearly shows that posterior segmental artery (arrow) branches directly from pulmonary arterial trunk (visualization score, 5; variation probability score, 5). (f) Time-spatial labeling inversion pulse image clearly shows that posterior segmental artery (arrow) branches directly from pulmonary arterial trunk (visualization score, 5; variation probability score, 5). Permission was obtained from reference number 65 (ARRS provides the permission).
On the other hand, CE-MR angiography examination has been advocated as useful and clinically applicable for diagnosis or assessment of not only pulmonary vascular diseases, but also other diseases including lung cancer during the past two decades. 63–69 This technique is usually known as “time-resolved CE-MR angiography” or “4D CE-MR angiography” after clinical installation of parallel imaging techniques, and is more widely used in routine clinical practice than non-CE-MR angiography. 63–69 Moreover, time-resolved CE-MR angiography is also used as a substitute or in a complementary role for CE-CT angiography and perfusion scanning, SPECT or SPECT/CT, in routine clinical practice because this technique can clearly show not only pulmonary vasculature, but also pulmonary parenchymal perfusion in a single examination. 63–69 Therefore, this examination is considered to be clinically applicable and to be most effective for patients with cardiopulmonary diseases in routine clinical practice.
Non-contrast-enhanced and contrast-enhanced perfusion MR imaging
As for non-CE-perfusion MR imaging, signal targeting with an alternating radiofrequency (STAR) technique with a half-Fourier single shot turbo spin-echo (HASTE) sequence, 70,71 arterial spin tagging imaging, 72 arterial spin labelling imaging such as flow sensitive alternating inversion recovery (FAIR) and FAIR with an extra radiofrequency pulse (FAIRER) imaging, 73 ECG-gated fast-SE perfusion MR imaging generated as subtraction imaging between the systolic and diastolic phases 74,75 were tested using not only healthy volunteers, but also patients with different pulmonary diseases. 66,70–75 In contrast to ECG-gated fast-SE perfusion MR imaging, all other techniques are based on arterial spin labeling (ASL) MR perfusion is an MR perfusion technique which does not require intravenous administration of contrast. Instead, it exploits the ability of MR imaging to magnetically label arterial blood below the imaging slab. ASL images are acquired with MR pulse sequences that have two distinct and independent components, a preparation component and an acquisition component. The preparation component labels the inflowing blood in different magnetic states for the control and label images. Once the blood has been labeled, the acquisition component acquires the data. The acquisition component typically uses a fast acquisition method such as HASTE technique. The distinct and independent nature of the preparation and acquisition components has allowed researchers to choose the combination that is best suited for their specific application. However, all these techniques used 2D acquisition and were time consuming. These techniques have therefore not been used in the clinical setting since they were first introduced.
Since 2009, non-CE perfusion and ventilation assessment of the human lung by means of the Fourier decomposition (FD) technique for proton MRI has been tested using both healthy volunteers and patients with pulmonary diseases. 76–81 FD MR imaging has been proposed as one of the non-CE MR techniques to obtain regional lung perfusion and ventilation-related information during a single acquisition series. 76–81 The method utilizes a rapid free-breathing acquisition of time-resolved MR data combined with the compensation of respiratory motion by non-rigid image registration. Then, the Fourier analysis of the acquired data allows for a spectral separation of respiratory and cardiac signal variations, which are caused by changes of the regional proton density due to the lung parenchyma contraction and by flow-dependent signal dephasing. Thus, FD MR imaging provides an indirect approach for the assessment of ventilation and perfusion. 76–81 Although the FD technique uses 2D acquisition, it can provide perfusion-weighted and ventilation-weighted images at the same time and performs like a proton-based MR technique.
In contrast to the above-mentioned 2D methods, the utility of 3D ECG- and respiratory-gated non-CE-perfusion MR imaging for prediction of post-operative lung function in non-small cell lung cancer patients has been demonstrated and it has shown similar potential for assessment of regional perfusion as dynamic CE-perfusion MR imaging. 82 Although this technique is considered to be only one method for 3D non-CE-perfusion MR imaging, several investigators are currently trying to have it accepted in the clinical setting and argue that it should be used for various pulmonary diseases, since the results of their studies have demonstrated its clinical relevance.
From the semi-quantitative and qualitative assessment of pulmonary circulation assessment, dynamic first-pass CE-perfusion MR imaging was extensively tested for patients with various pulmonary diseases by many investigators during the last two decades. 61–64,66–69,83–91 With the aid of indicator dilution therapy and deconvolution analysis, quantitatively assessed pulmonary blood flow, pulmonary blood volume and mean transit time can be measured on dynamic first-pass CE-perfusion MR imaging. 61–64,66–69,83–91 By using the above-mentioned methods, quantitatively assessed perfusion parameters can be employed as image-based biomarkers, not only for diagnosis, but also for disease severity assessment and patient management in various pulmonary diseases (Figure 5).
Figure 5.
A 30-year-old male volunteer a: Image maps (L to R: ventral to dorsal) of PBF shows regional changes of PBF in the gravitational and isogravitational directions. b: Image maps (L to R: ventral to dorsal) of PBV show regional changes of PBV in the gravitational and isogravitational directions. c: Image maps (L to R: ventral to dorsal) of MTT show regional changes of MTT in the gravitational and isogravitational directions. Permission was obtained from reference number 86 (John Wiley & Son provides the permission). MTT, mean transit time; PBF,pulmonary blood flow; PBV, pulmonary blood volume.
All MR scanners with field strengths equal to or higher than 1.5 Tesla from every vendor can currently perform dynamic first-pass CE-perfusion MR imaging with and without parallel imaging technique. On the other hand, there are no commercially available software for quantitative assessment of pulmonary perfusion parameter, and all previously reported studies used the investigators’ proprietary software. Therefore, vendors should be encouraged to provide appropriate sequences, bolus injection protocols and commercially available software for broadening the clinical application of this technique as soon as they can.
Velocity-encoding MR imaging for pulmonary hemodynamic evaluation
As a substitute for cardiac echography, velocity-encoding (or phase-contrast) MR imaging with velocity sensitizing gradients can be used to quantitatively map the blood flow in the major vessels in the lungs in two or three dimensions. It can thus provide insights into pulmonary vascular resistance and non-steady and turbulent flow in the pulmonary arteries, mainly in pulmonary arterial hypertension due to various cardiopulmonary diseases. 92–96 Recent developments involving 3D view-shared phase contrast methods allow for 3D time-resolved imaging of blood flow in the pulmonary arteries and the acquisition of 4D flow in the pulmonary arteries 94–97 (Figure 6). With applying the 4D velocity-encoded MR technique, 3D pulmonary vascular flow and advanced hemodynamic parameters are able to be assessed, and the degree of vortical flow in the pulmonary trunk may be suggested as one of the key aspects that reflects pulmonary hypertension. 94–97 Therefore, 4D velocity-encoded MR imaging can highlight the value of MR imaging instead of cardiac echogram for the clinical diagnosis and management of patients with pulmonary hypertension.
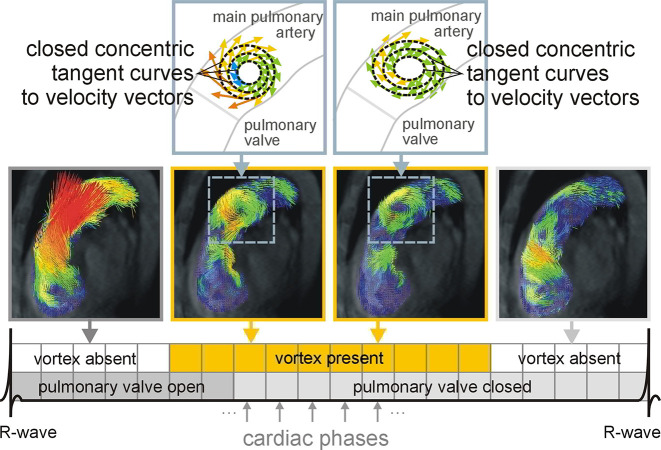
Figure 6.
Process of determination of the duration of vortical blood flow in the main pulmonary artery A vortex of the blood flow in the main pulmonary artery is defined as being present in a cardiac phase if velocity vectors in the pulmonary artery form closed concentric ring-shaped curves that are parallel to the right ventricular outflow tract orientation, which is shown in the middle two images. The duration of the vortical blood flow in the main pulmonary artery is specified as the number of cardiac phases with vortical blood flow divided by all 20 reconstructed cardiac phases (bottom). Permission was obtained from reference number 94 (RSNA provides the permission).
Biomechanical evaluation for pulmonary functional imaging
Recent advances in MR technology have made it possible to assess diaphragmatic and chest wall motion, static and dynamic lung volumes, as well as regional lung function. Even though existing studies have shown major heterogeneity in design and applied methods, it has become evident that MR imaging can visualize pulmonary function as well as diaphragmatic and thoracic wall movement, thus providing new insights into lung physiology.
Several studies were designed to answer basic physiological questions about diaphragmatic configuration and function visualized as static thoracic MR images in sagittal and coronal orientations. 98–101 Lung volumes were calculated from the analyzed contours of thorax and diaphragm and judged to be in good agreement with spirometric measurements. However, all these studies have a limitation, namely, that image acquisition was performed with the subject in a supine position, leaving the question open as to whether the findings would be transferable to a normal upright position. One study addressed this question by using an open MR system to dynamically image subjects in the sitting as well as supine position during slow maximal respiration and showed that diaphragmatic excursion is significantly greater when in the sitting than in the supine position, especially in the posterior region. 102 For static lung volume evaluations, a few studies were conducted to demonstrate methods for estimation of lung volume in normally breathing non-sedated infants with wheezing and bronchitis 103 and emphysema patients scheduled for lung volume reduction surgery as well as healthy volunteers. 104,105
In contrast to static lung volume assessment, dynamic lung volume evaluation could be attained by means of parallel imaging and the use of fast sequences with high temporal resolution and real-time visualization of lung and chest wall motion. 106–109 Compared to healthy controls, patients with emphysema showed a flattened diaphragm and a reduced, irregular respiratory motion of both chest wall and diaphragm. Repetition of dynamic MR imaging after lung volume reduction surgery showed significant improvement in these parameters. Other studies used dynamic MRI to visualize chest wall and diaphragmatic movement for instantaneous spirometric measurements. 107,108 In addition, the influence of tumor location on the local chest wall and diaphragmatic movement was also assessed as evident when this method was used for lung cancer and malignant mesothelioma. 110–114 Further, the introduction of dynamic 3D MR imaging, using an isotropic time-resolved 3D-gradient echo pulse sequence for volumetric assessment of the breathing cycle, 115 improved the correlation between spirometric data and MR-volumetric data. However, the lower temporal resolution of the sequence allowed for a dynamic examination during slow maximum inspiration and expiration. Therefore, 3D sequences were used with parallel imaging techniques to improve correlations between MR-based volumetric evaluations and pulmonary function test results. 115
Pulmonary parenchymal strain and mechanical properties of the lung have been suggested for regional functional assessment, and much effort has been made to study these aspects with the aid of imaging. The thoracic wall and the diaphragm provided sufficient signal to be relatively easy to track. However, motion analysis of these structures is only a substitute for pulmonary motion and function, and approaches to assess pulmonary tissue motion directly are challenging due to the limited signal provided. Nevertheless, it has been possible to evaluate two promising techniques. The first uses grid tagging, a technique widely employed in MR motion analysis of not only heart, but also lung. In the lung, the fast signal decay of the grid due to the large number of susceptibility artefacts at the tissue/air interfaces presents the main challenge. Despite these difficulties, the feasibility of this technique has been shown by several studies. 116–118
The second approach, registration of serial images, has to cope with the limited signal of pulmonary tissue and thus low signal-to-noise ratio, especially for dynamic imaging. So far, this technique has been evaluated in not only healthy volunteers and mice, but also interstitial lung disease. 115,119–123 However, its ability to detect motion changes over time appears promising for serial imaging of patients for the assessment of disease progression. The major limitations of registration methods used for MR imaging remain the poor signal and contrast from solid tissue and blood vessels. If this limitation could be overcome, registration would even be possible on 3D images, which would eliminate the problem of through-plane motion. Another advantage of the registration approach is that it is not dependent on a specific imaging sequence and thus should profit directly from new developments in MR sequences.
Currently, MR-based biomechanical assessment has been mainly attempted for radiation oncology rather than pulmonary functional imaging. The lack of ionizing radiation makes MR-based biomechanical assessment suitable for experimental work with healthy subjects. For this purpose, MR-based biomechanical assessment is just being put into practice and the optimization of the dedicated sequence protocols are strongly encouraged. Moreover, further investigations are warranted in the near future for demonstrating the clinical relevance of this technique for not only motion tracking in radiation oncology, but also pulmonary functional imaging.
Conclusion
This report reviewed state-of-the-art pulmonary ventilation imaging, perfusion imaging and biomechanical evaluation and discussed their future challenges. Imaging techniques are gradually but steadily shifting from nuclear medicine to MR imaging, and from those with qualitative assessment to those with quantitative assessment. In addition, they are still being developed and evaluated for clinical relevance in not only radiology, but also in the multidisciplinary fields of pulmonology, pulmonary physiology, pathophysiology, thoracic surgery, radiation oncology. In addition, the combining of imaging method with various image analyses by means of bioengineering is also important for future clinical settings. Finally, new MR imaging techniques that are under development and/or are being tested can be expected to materialize in the foreseeable future.
Contributor Information
Yoshiharu Ohno, Email: yohno@fujita-hu.ac.jp.
Satomu Hanamatsu, Email: st000mthanmz7@yahoo.co.jp.
Yuki Obama, Email: y-obama@fujita-hu.ac.jp.
Takahiro Ueda, Email: mottoband06@yahoo.co.jp.
Hirotaka Ikeda, Email: ikeda-gif@umin.net.
Hidekazu Hattori, Email: hhattori@fujita-hu.ac.jp.
Kazuhiro Murayama, Email: kmura@fujita-hu.ac.jp.
Hiroshi Toyama, Email: htoyama@fujita-hu.ac.jp.
REFERENCES
- 1. Albert MS, Cates GD, Driehuys B, Happer W, Saam B, Springer CS, et al. Biological magnetic resonance imaging using laser-polarized 129Xe. Nature 1994; 370: 199–201. doi: 10.1038/370199a0 [DOI] [PubMed] [Google Scholar]
- 2. Edelman RR, Hatabu H, Tadamura E, Li W, Prasad PV. Noninvasive assessment of regional ventilation in the human lung using oxygen–enhanced magnetic resonance imaging. Nat Med 1996; 2: 1236–9. doi: 10.1038/nm1196-1236 [DOI] [PubMed] [Google Scholar]
- 3. Kauczor H-U, Surkau R, Roberts T. Mri using hyperpolarized noble gases. Eur Radiol 1998; 8: 820–7. doi: 10.1007/s003300050479 [DOI] [PubMed] [Google Scholar]
- 4. Ramirez MP, Sigaloff KCE, Kubatina LV, Donahue MA, Venkatesh AK, Albert MS. Physiological response of rats to delivery of helium and xenon: implications for hyperpolarized noble gas imaging. NMR Biomed 2000; 13: 253–64. doi: [DOI] [PubMed] [Google Scholar]
- 5. Fain SB, Korosec FR, Holmes JH, O'Halloran R, Sorkness RL, Grist TM. Functional lung imaging using hyperpolarized gas MRI. J Magn Reson Imaging 2007; 25: 910–23. doi: 10.1002/jmri.20876 [DOI] [PubMed] [Google Scholar]
- 6. Kruger SJ, Nagle SK, Couch MJ, Ohno Y, Albert M, Fain SB. Functional imaging of the lungs with gas agents. J Magn Reson Imaging 2016; 43: 295–315. doi: 10.1002/jmri.25002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altes TA, Powers PL, Knight-Scott J, Rakes G, Platts-Mills TAE, de Lange EE, et al. Hyperpolarized3He Mr lung ventilation imaging in asthmatics: preliminary findings. J Magn Reson Imaging 2001; 13: 378–84. doi: 10.1002/jmri.1054 [DOI] [PubMed] [Google Scholar]
- 8. Altes TA, Salerno M. Hyperpolarized gas MR imaging of the lung. J Thorac Imaging 2004; 19: 250–8. doi: 10.1097/01.rti.0000142837.52729.38 [DOI] [PubMed] [Google Scholar]
- 9. Fain SB, Panth SR, Evans MD, Wentland AL, Holmes JH, Korosec FR, et al. Early emphysematous changes in asymptomatic smokers: detection with 3He MR imaging. Radiology 2006; 239: 875–83. doi: 10.1148/radiol.2393050111 [DOI] [PubMed] [Google Scholar]
- 10. Sun Y, O'Sullivan BP, Roche JP, Walvick R, Reno A, Baker D, et al. Using hyperpolarized 3He MRI to evaluate treatment efficacy in cystic fibrosis patients. J Magn Reson Imaging 2011; 34: 1206–11. doi: 10.1002/jmri.22724 [DOI] [PubMed] [Google Scholar]
- 11. Svenningsen S, Kirby M, Starr D, Leary D, Wheatley A, Maksym GN, et al. Hyperpolarized (3) He and (129) Xe MRI: differences in asthma before bronchodilation. J Magn Reson Imaging 2013; 38: 1521–30. doi: 10.1002/jmri.24111 [DOI] [PubMed] [Google Scholar]
- 12. Capaldi DPI, Zha N, Guo F, Pike D, McCormack DG, Kirby M, et al. Pulmonary imaging biomarkers of gas trapping and emphysema in COPD: (3)He MR imaging and CT parametric response maps. Radiology 2016; 279: 597–608. doi: 10.1148/radiol.2015151484 [DOI] [PubMed] [Google Scholar]
- 13. Salerno M, de Lange EE, Altes TA, Truwit JD, Brookeman JR, Mugler JP. Emphysema: hyperpolarized helium 3 diffusion MR imaging of the lungs compared with spirometric Indexes—Initial experience. Radiology 2002; 222: 252–60. doi: 10.1148/radiol.2221001834 [DOI] [PubMed] [Google Scholar]
- 14. van Beek EJR, Dahmen AM, Stavngaard T, Gast KK, Heussel CP, Krummenauer F, et al. Hyperpolarised 3He MRI versus HRCT in COPD and normal volunteers: PHIL trial. Eur Respir J 2009; 34: 1311–21. doi: 10.1183/09031936.00138508 [DOI] [PubMed] [Google Scholar]
- 15. Kirby M, Svenningsen S, Owrangi A, Wheatley A, Farag A, Ouriadov A, et al. Hyperpolarized 3 He and 129 Xe MR Imaging in Healthy Volunteers and Patients with Chronic Obstructive Pulmonary Disease. Radiology 2012; 265: 600–10. doi: 10.1148/radiol.12120485 [DOI] [PubMed] [Google Scholar]
- 16. Ruppert K, Brookeman JR, Hagspiel KD, Mugler JP. Probing lung physiology with xenon polarization transfer contrast (XTC). Magn Reson Med 2000; 44: 349–57. doi: [DOI] [PubMed] [Google Scholar]
- 17. Physics CA. Helium-3 shortage could put freeze on low temperature research. Science 2009; 326: 778–9. [DOI] [PubMed] [Google Scholar]
- 18. Chang YV. MOXE: A model of gas exchange for hyperpolarized 129 Xe magnetic resonance of the lung. Magn Reson Med 2013; 69: 884–90. doi: 10.1002/mrm.24304 [DOI] [PubMed] [Google Scholar]
- 19. Driehuys B, Cofer GP, Pollaro J, Mackel JB, Hedlund LW, Johnson GA. Imaging alveolar-capillary gas transfer using hyperpolarized 129Xe MRI. Proc Natl Acad Sci U S A 2006; 103: 18278–83. doi: 10.1073/pnas.0608458103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaushik SS, Freeman MS, Yoon SW, Liljeroth MG, Stiles JV, Roos JE, et al. Measuring diffusion limitation with a perfusion-limited gas—Hyperpolarized 129 Xe gas-transfer spectroscopy in patients with idiopathic pulmonary fibrosis. J Appl Physiol 2014; 117: 577–85. doi: 10.1152/japplphysiol.00326.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qing K, Ruppert K, Jiang Y, Mata JF, Miller GW, Shim YM, et al. Regional mapping of gas uptake by blood and tissue in the human lung using hyperpolarized xenon-129 MRI. J Magn Reson Imaging 2014; 39: 346–59. doi: 10.1002/jmri.24181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fischer MC, Spector ZZ, Ishii M, Yu J, Emami K, Itkin M, et al. Single-acquisition sequence for the measurement of oxygen partial pressure by hyperpolarized gas MRI. Magn Reson Med 2004; 52: 766–73. doi: 10.1002/mrm.20239 [DOI] [PubMed] [Google Scholar]
- 23. Deninger AJ, Eberle B, Ebert M, Grossmann T, Heil W, Kauczor H, et al. Quantification of regional intrapulmonary oxygen partial pressure evolution during apnea by (3)He MRI. J Magn Reson 1999; 141: 207–16. doi: 10.1006/jmre.1999.1902 [DOI] [PubMed] [Google Scholar]
- 24. Deninger AJ, Eberle B, Bermuth J, Escat B, Markstaller K, Schmiedeskamp J, et al. Assessment of a single-acquisition imaging sequence for oxygen-sensitive3He-MRI. Magn Reson Med 2002; 47: 105–14. doi: 10.1002/mrm.10032 [DOI] [PubMed] [Google Scholar]
- 25. Rizi RR, Baumgardner JE, Ishii M, Spector ZZ, Edvinsson JM, Jalali A, et al. Determination of regional VA/Q by hyperpolarized3He MRI. Magn Reson Med 2004; 52: 65–72. doi: 10.1002/mrm.20136 [DOI] [PubMed] [Google Scholar]
- 26. Miller GW, Mugler JP, Altes TA, Cai J, Mata JF, de Lange EE, et al. A short-breath-hold technique for lung PO2 mapping with 3He MRI. Magn Reson Med 2010; 63: 127–36. doi: 10.1002/mrm.22181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marshall H, Parra-Robles J, Deppe MH, Lipson DA, Lawson R, Wild JM. 3 He pO 2 mapping is limited by delayed-ventilation and diffusion in chronic obstructive pulmonary disease. Magn. Reson. Med. 2014; 71: 1172–8. doi: 10.1002/mrm.24779 [DOI] [PubMed] [Google Scholar]
- 28. Hamedani H, Kadlecek SJ, Ishii M, Emami K, Kuzma NN, Xin Y, et al. A variability study of regional alveolar oxygen tension measurement in humans using hyperpolarized 3 He MRI. Magn Reson Med 2013; 70: 1557–66. doi: 10.1002/mrm.24604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamedani H, Kadlecek SJ, Ishii M, Xin Y, Emami K, Han B, et al. Alterations of regional alveolar oxygen tension in asymptomatic current smokers: assessment with hyperpolarized (3)He MR imaging. Radiology 2015; 274: 585–96. doi: 10.1148/radiol.14132809 [DOI] [PubMed] [Google Scholar]
- 30. Hatabu H, Tadamura E, Chen Q, Stock KW, Li W, Prasad PV, et al. Pulmonary ventilation: dynamic MRI with inhalation of molecular oxygen. Eur J Radiol 2001; 37: 172–8. doi: 10.1016/S0720-048X(00)00298-9 [DOI] [PubMed] [Google Scholar]
- 31. Tadamura E, Hatabu H, Li W, Prasad PV, Edelman RR. Effect of oxygen inhalation on relaxation times in various tissues. J Magn Reson Imaging 1997; 7: 220–5. doi: 10.1002/jmri.1880070134 [DOI] [PubMed] [Google Scholar]
- 32. Ohno Y, Chen Q, Hatabu H. Oxygen-enhanced magnetic resonance ventilation imaging of lung. Eur J Radiol 2001; 37: 164–71. doi: 10.1016/S0720-048X(00)00299-0 [DOI] [PubMed] [Google Scholar]
- 33. Ohno Y, Hatabu H, Takenaka D, Adachi S, Van Cauteren M, Sugimura K. Oxygen-enhanced MR ventilation imaging of the lung: preliminary clinical experience in 25 subjects. AJR Am J Roentgenol 2001; 177: 185–94. [DOI] [PubMed] [Google Scholar]
- 34. Ohno Y, Hatabu H, Takenaka D, Van Cauteren M, Fujii M, Sugimura K. Dynamic oxygen-enhanced MRI reflects diffusing capacity of the lung. Magn Reson Med 2002; 47: 1139–44. doi: 10.1002/mrm.10168 [DOI] [PubMed] [Google Scholar]
- 35. Ohno Y, Hatabu H, Higashino T, Nogami M, Takenaka D, Watanabe H, et al. Oxygen-enhanced MR imaging: correlation with postsurgical lung function in patients with lung cancer. Radiology 2005; 236: 704–11. doi: 10.1148/radiol.2361040005 [DOI] [PubMed] [Google Scholar]
- 36. Ohno Y, Koyama H, Nogami M, Takenaka D, Matsumoto S, Obara M, et al. Dynamic oxygen-enhanced MRI versus quantitative CT: pulmonary functional loss assessment and clinical stage classification of smoking-related COPD. AJR Am J Roentgenol 2008; 190: W93–9. doi: 10.2214/AJR.07.2511 [DOI] [PubMed] [Google Scholar]
- 37. Ohno Y, Iwasawa T, Seo JB, et al. Oxygen-enhanced magnetic resonance imaging versus computed tomography: multicenter study for clinical stage classification of smoking-related chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177: 1095–102. [DOI] [PubMed] [Google Scholar]
- 38. Ohno Y, Koyama H, Matsumoto K, Onishi Y, Nogami M, Takenaka D, et al. Oxygen-enhanced MRI vs. quantitatively assessed thin-section CT: pulmonary functional loss assessment and clinical stage classification of asthmatics. Eur J Radiol 2011; 77: 85–91. doi: 10.1016/j.ejrad.2009.06.027 [DOI] [PubMed] [Google Scholar]
- 39. Ohno Y, Koyama H, Yoshikawa T, Matsumoto K, Aoyama N, Onishi Y, et al. Comparison of capability of dynamic O2-enhanced MRI and quantitative thin-section MDCT to assess COPD in smokers. Eur J Radiol 2012; 81: 1068–75. doi: 10.1016/j.ejrad.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 40. Ohno Y, Nishio M, Koyama H, Yoshikawa T, Matsumoto S, Takenaka D, et al. Oxygen-enhanced MRI, thin-section MDCT, and perfusion SPECT/CT: comparison of clinical implications to patient care for lung volume reduction surgery. AJR Am J Roentgenol 2012; 199: 794–802. doi: 10.2214/AJR.11.8250 [DOI] [PubMed] [Google Scholar]
- 41. Ohno Y, Nishio M, Koyama H, Yoshikawa T, Matsumoto S, Seki S, et al. Oxygen-enhanced MRI for patients with connective tissue diseases: comparison with thin-section CT of capability for pulmonary functional and disease severity assessment. Eur J Radiol 2014; 83: 391–7. doi: 10.1016/j.ejrad.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 42. Ohno Y, Nishio M, Koyama H, Seki S, Yoshikawa T, Matsumoto S, et al. Asthma: comparison of dynamic oxygen-enhanced MR imaging and quantitative thin-section CT for evaluation of clinical treatment. Radiology 2014; 273: 907–16. doi: 10.1148/radiol.14132660 [DOI] [PubMed] [Google Scholar]
- 43. Jobst BJ, Triphan SMF, Sedlaczek O, Anjorin A, Kauczor HU, Biederer J, et al. Functional lung MRI in chronic obstructive pulmonary disease: comparison of T1 mapping, oxygen-enhanced T1 mapping and dynamic contrast enhanced perfusion. PLoS One 2015; 10: e0121520. doi: 10.1371/journal.pone.0121520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Triphan SMF, Breuer FA, Gensler D, Kauczor H-U, Jakob PM. Oxygen enhanced lung MRI by simultaneous measurement of T 1 and T 2 * during free breathing using ultrashort TE. J Magn Reson Imaging 2015; 41: 1708–14. doi: 10.1002/jmri.24692 [DOI] [PubMed] [Google Scholar]
- 45. Triphan SMF, Jobst BJ, Anjorin A, Sedlaczek O, Wolf U, Terekhov M, et al. Reproducibility and comparison of oxygen-enhanced T1 quantification in COPD and asthma patients. PLoS One 2017; 12: e0172479. doi: 10.1371/journal.pone.0172479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fuseya Y, Muro S, Sato S, Tanabe N, Sato A, Tanimura K, et al. Complementary regional heterogeneity information from COPD patients obtained using oxygen-enhanced MRI and chest CT. PLoS One 2018; 13: e0203273. doi: 10.1371/journal.pone.0203273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Young IR, Clarke GJ, Baffles DR, Pennock JM, Doyle FH, Bydder GM. Enhancement of relaxation rate with paramagnetic contrast agents in NMR imaging. Journal of Computed Tomography 1981; 5: 543–7. doi: 10.1016/0149-936X(81)90089-8 [DOI] [PubMed] [Google Scholar]
- 48. Brooks RA, Di Chiro G. Magnetic resonance imaging of stationary blood: a review. Med Phys 1987; 14: 903–13. doi: 10.1118/1.595994 [DOI] [PubMed] [Google Scholar]
- 49. Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JWM, Fluorine BJW. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed 2011; 24: 114–29. doi: 10.1002/nbm.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chang YV, Conradi MS. Relaxation and diffusion of perfluorocarbon gas mixtures with oxygen for lung MRI. J Magn Reson 2006; 181: 191–8. doi: 10.1016/j.jmr.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 51. Adolphi NL, Kuethe DO. Quantitative mapping of ventilation-perfusion ratios in lungs by19F MR imaging ofT1 of inert fluorinated gases. Magn Reson Med 2008; 59: 739–46. doi: 10.1002/mrm.21579 [DOI] [PubMed] [Google Scholar]
- 52. Couch MJ, Ball IK, Li T, Fox MS, Littlefield SL, Biman B, et al. Pulmonary ultrashort echo time 19F MR imaging with inhaled fluorinated gas mixtures in healthy volunteers: feasibility. Radiology 2013; 269: 903–9. doi: 10.1148/radiol.13130609 [DOI] [PubMed] [Google Scholar]
- 53. Couch MJ, Ball IK, Li T, Fox MS, Biman B, Albert MS. 19 F MRI of the lungs using inert fluorinated gases: challenges and new developments. J Magn Reson Imaging 2019; 49: 343–54. doi: 10.1002/jmri.26292 [DOI] [PubMed] [Google Scholar]
- 54. Gutberlet M, Kaireit TF, Voskrebenzev A, Lasch F, Freise J, Welte T, et al. Free-breathing Dynamic 19F Gas MR imaging for mapping of regional lung ventilation in patients with COPD. Radiology 2018; 286: 1040–51. doi: 10.1148/radiol.2017170591 [DOI] [PubMed] [Google Scholar]
- 55. Halaweish AF, Moon RE, Foster WM, Soher BJ, McAdams HP, MacFall JR, et al. Perfluoropropane gas as a magnetic resonance lung imaging contrast agent in humans. Chest 2013; 144: 1300–10. doi: 10.1378/chest.12-2597 [DOI] [PubMed] [Google Scholar]
- 56. Kuethe DO, Caprihan A, Gach HM, Lowe IJ, Fukushima E. Imaging obstructed ventilation with NMR using inert fluorinated gases. J Appl Physiol 2000; 88: 2279–86. doi: 10.1152/jappl.2000.88.6.2279 [DOI] [PubMed] [Google Scholar]
- 57. Ruiz-Cabello J, Pérez-Sánchez JM, Pérez de Alejo R, Rodríguez I, González-Mangado N, Peces-Barba G, et al. Diffusion-Weighted 19F-MRI of lung periphery: influence of pressure and air–SF6 composition on apparent diffusion coefficients. Respir Physiol Neurobiol 2005; 148(1-2): 43–56. doi: 10.1016/j.resp.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 58. Pérez-Sánchez JM, de Alejo RP, Rodríguez I, Cortijo M, Peces-Barba G, Ruiz-Cabello J. In vivo diffusion weighted19F MRI using SF6. Magn Reson Med 2005; 54: 460–3. doi: 10.1002/mrm.20569 [DOI] [PubMed] [Google Scholar]
- 59. Carrero-González L, Kaulisch T, Stiller D. In vivo diffusion-weighted MRI using perfluorinated gases: ADC comparison between healthy and elastase-treated rat lungs. Magn Reson Med 2013; 70: 1761–4. doi: 10.1002/mrm.24627 [DOI] [PubMed] [Google Scholar]
- 60. Biederer J, Heussel CP, Puderbach M, Wielpuetz MO. Functional magnetic resonance imaging of the lung. Semin Respir Crit Care Med 2014; 35: 74–82. [DOI] [PubMed] [Google Scholar]
- 61. Matsuoka S, Hunsaker AR, Gill RR, Jacobson FL, Ohno Y, Patz S, et al. Functional MR imaging of the lung. Magn Reson Imaging Clin N Am 2008; 16: 275–89. doi: 10.1016/j.mric.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 62. Liszewski MC, Hersman FW, Altes TA, Ohno Y, Ciet P, Warfield SK, et al. Magnetic resonance imaging of pediatric lung parenchyma, airways, vasculature, ventilation, and perfusion. Radiol Clin North Am 2013; 51: 555–82. doi: 10.1016/j.rcl.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 63. Ohno Y, Koyama H, Lee HY, Miura S, Yoshikawa T, Sugimura K. Contrast-Enhanced CT- and MRI-based perfusion assessment for pulmonary diseases: basics and clinical applications. Diagn Interv Radiol 2016; 22: 407–21. doi: 10.5152/dir.2016.16123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Johns CS, Swift AJ, Hughes PJC, Ohno Y, Schiebler M, Wild JM. Pulmonary Mr angiography and perfusion imaging—A review of methods and applications. Eur J Radiol 2017; 86: 361–70. doi: 10.1016/j.ejrad.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 65. Ohno Y, Nishio M, Koyama H, Yoshikawa T, Matsumoto S, Seki S, et al. Journal Club: comparison of assessment of preoperative pulmonary vasculature in patients with non-small cell lung cancer by non-contrast- and 4D contrast-enhanced 3-T Mr angiography and contrast-enhanced 64-MDCT. AJR Am J Roentgenol 2014; 202: 493–506. doi: 10.2214/AJR.13.10833 [DOI] [PubMed] [Google Scholar]
- 66. Ohno Y, Yoshikawa T, Kishida Y, Seki S, Karabulut N, Unenhanced KN. Unenhanced and contrast-enhanced Mr angiography and perfusion imaging for suspected pulmonary thromboembolism. AJR Am J Roentgenol 2017; 208: 517–30. doi: 10.2214/AJR.16.17415 [DOI] [PubMed] [Google Scholar]
- 67. Ohno Y, Kawamitsu H, Higashino T, Takenaka D, Watanabe H, van Cauteren M, et al. Time-resolved contrast-enhanced pulmonary MR angiography using sensitivity encoding (sense. J Magn Reson Imaging 2003; 17: 330–6. doi: 10.1002/jmri.10261 [DOI] [PubMed] [Google Scholar]
- 68. Ohno Y, Higashino T, Takenaka D, Sugimoto K, Yoshikawa T, Kawai H, et al. MR angiography with sensitivity encoding (sense) for suspected pulmonary embolism: comparison with MDCT and ventilation-perfusion scintigraphy. AJR Am J Roentgenol 2004; 183: 91–8. doi: 10.2214/ajr.183.1.1830091 [DOI] [PubMed] [Google Scholar]
- 69. Tsuchiya N, Beek EJRvan, Ohno Y, Hatabu H, Kauczor H-U, Swift A, et al. Magnetic resonance angiography for the primary diagnosis of pulmonary embolism: a review from the International workshop for pulmonary functional imaging. World J Radiol 2018; 10: 52–64. doi: 10.4329/wjr.v10.i6.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hatabu H, Tadamura E, Prasad PV, Chen Q, Buxton R, Edelman RR. Noninvasive pulmonary perfusion imaging by STAR-HASTE sequence. Magn Reson Med 2000; 44: 808–12. doi: [DOI] [PubMed] [Google Scholar]
- 71. Chen Q, Siewert B, Bly BM, Warach S, Edelman RR. STAR-HASTE: perfusion imaging without magnetic susceptibility artifact. Magn Reson Med 1997; 38: 404–8. doi: 10.1002/mrm.1910380308 [DOI] [PubMed] [Google Scholar]
- 72. Roberts DA, Gefter WB, Hirsch JA, Rizi RR, Dougherty L, Lenkinski RE, et al. Pulmonary perfusion: Respiratory-triggered three-dimensional MR imaging with arterial spin tagging-preliminary results in healthy volunteers. Radiology 1999; 212: 890–5. doi: 10.1148/radiology.212.3.r99se35890 [DOI] [PubMed] [Google Scholar]
- 73. Mai VM, Berr SS. Mr perfusion imaging of pulmonary parenchyma using pulsed arterial spin labeling techniques: fairer and fair. J Magn Reson Imaging 1999; 9: 483–7. doi: [DOI] [PubMed] [Google Scholar]
- 74. Tadamura E, Hatabu H. Assessment of pulmonary perfusion using a subtracted haste image between diastole and systole. Eur J Radiol 2001; 37: 179–83. doi: 10.1016/S0720-048X(00)00297-7 [DOI] [PubMed] [Google Scholar]
- 75. Ogasawara N, Suga K, Zaki M, Okada M, Kawakami Y, Matsunaga N. Assessment of lung perfusion impairment in patients with pulmonary artery-occlusive and chronic obstructive pulmonary diseases with noncontrast electrocardiogram-gated fast-spin-echo perfusion MR imaging. J Magn Reson Imaging 2004; 20: 601–11. doi: 10.1002/jmri.20150 [DOI] [PubMed] [Google Scholar]
- 76. Bauman G, Puderbach M, Deimling M, Jellus V, Chefd'hotel C, Dinkel J, et al. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of Fourier decomposition in proton MRI. Magn Reson Med 2009; 62: 656–64. doi: 10.1002/mrm.22031 [DOI] [PubMed] [Google Scholar]
- 77. Bauman G, Scholz A, Rivoire J, Terekhov M, Friedrich J, de Oliveira A, et al. Lung ventilation- and perfusion-weighted Fourier decomposition magnetic resonance imaging: In vivo validation with hyperpolarized 3 He and dynamic contrast-enhanced MRI. Magn Reson Med 2013; 69: 229–37. doi: 10.1002/mrm.24236 [DOI] [PubMed] [Google Scholar]
- 78. Bauman G, Puderbach M, Heimann T, Kopp-Schneider A, Fritzsching E, Mall MA, et al. Validation of Fourier decomposition MRI with dynamic contrast-enhanced MRI using visual and automated scoring of pulmonary perfusion in young cystic fibrosis patients. Eur J Radiol 2013; 82: 2371–7. doi: 10.1016/j.ejrad.2013.08.018 [DOI] [PubMed] [Google Scholar]
- 79. Sommer G, Bauman G, Koenigkam-Santos M, Draenkow C, Heussel CP, Kauczor H-U, et al. Non-contrast-enhanced preoperative assessment of lung perfusion in patients with non-small-cell lung cancer using Fourier decomposition magnetic resonance imaging. Eur J Radiol 2013; 82: e879–87. doi: 10.1016/j.ejrad.2013.06.030 [DOI] [PubMed] [Google Scholar]
- 80. Schönfeld C, Cebotari S, Voskrebenzev A, Gutberlet M, Hinrichs J, Renne J, et al. Performance of perfusion-weighted Fourier decomposition MRI for detection of chronic pulmonary emboli. J Magn Reson Imaging 2015; 42: 72–9. doi: 10.1002/jmri.24764 [DOI] [PubMed] [Google Scholar]
- 81. Voskrebenzev A, Gutberlet M, Klimeš F, Kaireit TF, Schönfeld C, Rotärmel A, et al. Feasibility of quantitative regional ventilation and perfusion mapping with phase‐resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn. Reson. Med 2018; 79: 2306–14. doi: 10.1002/mrm.26893 [DOI] [PubMed] [Google Scholar]
- 82. Ohno Y, Seki S, Koyama H, Yoshikawa T, Matsumoto S, Takenaka D, et al. 3D ECG- and respiratory-gated non-contrast-enhanced (Ce) perfusion MRI for postoperative lung function prediction in non-small-cell lung cancer patients: a comparison with thin-section quantitative computed tomography, dynamic CE-perfusion MRI, and perfus. J Magn Reson Imaging 2015; 42: 340–53. doi: 10.1002/jmri.24800 [DOI] [PubMed] [Google Scholar]
- 83. Hatabu H, Gaa J, Kim D, Li W, Prasad PV, Edelman RR. Pulmonary perfusion and angiography: evaluation with breath-hold enhanced three-dimensional fast imaging steady-state precession MR imaging with short TR and te. AJR Am J Roentgenol 1996; 167: 653–5. doi: 10.2214/ajr.167.3.8751673 [DOI] [PubMed] [Google Scholar]
- 84. Hatabu H, Gaa J, Kim D, Li W, Prasad PV, Edelman RR. Pulmonary perfusion: qualitative assessment with dynamic contrast-enhanced MRI using ultra-shortTE and inversion recovery turbo flash. Magn Reson Med 1996; 36: 503–8. doi: 10.1002/mrm.1910360402 [DOI] [PubMed] [Google Scholar]
- 85. Levin DL, Chen Q, Zhang M, Edelman RR, Hatabu H. Evaluation of regional pulmonary perfusion using ultrafast magnetic resonance imaging. Magn Reson Med 2001; 46: 166–71. doi: 10.1002/mrm.1172 [DOI] [PubMed] [Google Scholar]
- 86. Ohno Y, Hatabu H, Higashino T, Takenaka D, Watanabe H, Nishimura Y, et al. Dynamic perfusion MRI versus perfusion scintigraphy: prediction of postoperative lung function in patients with lung cancer. AJR Am J Roentgenol 2004; 182: 73–8. doi: 10.2214/ajr.182.1.1820073 [DOI] [PubMed] [Google Scholar]
- 87. Ohno Y, Hatabu H, Murase K, Higashino T, Kawamitsu H, Watanabe H, et al. Quantitative assessment of regional pulmonary perfusion in the entire lung using three-dimensional ultrafast dynamic contrast-enhanced magnetic resonance imaging: preliminary experience in 40 subjects. J Magn Reson Imaging 2004; 20: 353–65. doi: 10.1002/jmri.20137 [DOI] [PubMed] [Google Scholar]
- 88. Ohno Y, Hatabu H, Murase K, Higashino T, Nogami M, Yoshikawa T, et al. Primary pulmonary hypertension: 3D dynamic perfusion MRI for quantitative analysis of regional pulmonary perfusion. AJR Am J Roentgenol 2007; 188: 48–56. doi: 10.2214/AJR.05.0135 [DOI] [PubMed] [Google Scholar]
- 89. Ohno Y, Koyama H, Matsumoto K, Onishi Y, Nogami M, Takenaka D, et al. Dynamic Mr perfusion imaging: capability for quantitative assessment of disease extent and prediction of outcome for patients with acute pulmonary thromboembolism. J Magn Reson Imaging 2010; 31: 1081–90. doi: 10.1002/jmri.22146 [DOI] [PubMed] [Google Scholar]
- 90. Ohno Y, Koyama H, Yoshikawa T, Nishio M, Matsumoto S, Matsumoto K, et al. Contrast-Enhanced multidetector-row computed tomography vs. time-resolved magnetic resonance angiography vs. contrast-enhanced perfusion MRI: assessment of treatment response by patients with inoperable chronic thromboembolic pulmonary hypertension. J Magn Reson Imaging 2012; 36: 612–23. doi: 10.1002/jmri.23680 [DOI] [PubMed] [Google Scholar]
- 91. Ohno Y, Nishio M, Koyama H, Seki S, Tsubakimoto M, Fujisawa Y, et al. Solitary pulmonary nodules: comparison of dynamic first-pass contrast-enhanced perfusion area-detector CT, dynamic first-pass contrast-enhanced MR imaging, and FDG PET/CT. Radiology 2015b; 274: 563–75. doi: 10.1148/radiol.14132289 [DOI] [PubMed] [Google Scholar]
- 92. Sanz J, Kuschnir P, Rius T, Salguero R, Sulica R, Einstein AJ, et al. Pulmonary arterial hypertension: noninvasive detection with phase-contrast MR imaging. Radiology 2007; 243: 70–9. doi: 10.1148/radiol.2431060477 [DOI] [PubMed] [Google Scholar]
- 93. Hopkins SR, Wielpütz MO, Kauczor H-U. Imaging lung perfusion. J Appl Physiol 2012; 113: 328–39. doi: 10.1152/japplphysiol.00320.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Reiter G, Reiter U, Kovacs G, Olschewski H, Fuchsjäger M. Blood flow vortices along the main pulmonary artery measured with MR imaging for diagnosis of pulmonary hypertension. Radiology 2015; 275: 71–9. doi: 10.1148/radiol.14140849 [DOI] [PubMed] [Google Scholar]
- 95. Reiter U, Reiter G, Fuchsjäger M. MR phase-contrast imaging in pulmonary hypertension. Br J Radiol 2016; 89: 20150995. doi: 10.1259/bjr.20150995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nagao M, Yamasaki Y, Abe K, Hosokawa K, Kawanami S, Kamitani T, et al. Energy efficiency and pulmonary artery flow after balloon pulmonary angioplasty for inoperable, chronic thromboembolic pulmonary hypertension: analysis by phase-contrast MRI. Eur J Radiol 2017; 87: 99–104. doi: 10.1016/j.ejrad.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 97. Markl M, Schnell S, Wu C, Bollache E, Jarvis K, Barker AJ, et al. Advanced flow MRI: emerging techniques and applications. Clin Radiol 2016; 71: 779–95. doi: 10.1016/j.crad.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Paiva M, Verbanck S, Estenne M, Poncelet B, Segebarth C, Macklem PT. Mechanical implications of in vivo human diaphragm shape. J Appl Physiol 1992; 72: 1407–12. doi: 10.1152/jappl.1992.72.4.1407 [DOI] [PubMed] [Google Scholar]
- 99. Gauthier AP, Verbanck S, Estenne M, Segebarth C, Macklem PT, Paiva M. Three-Dimensional reconstruction of the in vivo human diaphragm shape at different lung volumes. J Appl Physiol 1994; 76: 495–506. doi: 10.1152/jappl.1994.76.2.495 [DOI] [PubMed] [Google Scholar]
- 100. Gierada DS, Hakimian S, Slone RM, Yusen RD. Mr analysis of lung volume and thoracic dimensions in patients with emphysema before and after lung volume reduction surgery. AJR Am J Roentgenol 1998; 170: 707–14. doi: 10.2214/ajr.170.3.9490958 [DOI] [PubMed] [Google Scholar]
- 101. Cluzel P, Similowski T, Chartrand-Lefebvre C, Zelter M, Derenne JP, Grenier PA. Diaphragm and chest wall: assessment of the inspiratory pump with MR imaging-preliminary observations. Radiology 2000; 215: 574–83. doi: 10.1148/radiology.215.2.r00ma28574 [DOI] [PubMed] [Google Scholar]
- 102. Takazakura R, Takahashi M, Nitta N, Murata K. Diaphragmatic motion in the sitting and supine positions: healthy subject study using a vertically open magnetic resonance system. J Magn Reson Imaging 2004; 19: 605–9. doi: 10.1002/jmri.20051 [DOI] [PubMed] [Google Scholar]
- 103. Chapman B, O'Callaghan C, Coxon R, Glover P, Jaroszkiewicz G, Howseman A, et al. Estimation of lung volume in infants by echo planar imaging and total body plethysmography. Arch Dis Child 1990; 65: 168–70. doi: 10.1136/adc.65.2.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gierada DS, Curtin JJ, Erickson SJ, Prost RW, Strandt JA, Goodman LR. Diaphragmatic motion: fast gradient-recalled-echo MR imaging in healthy subjects. Radiology 1995; 194: 879–84. doi: 10.1148/radiology.194.3.7862995 [DOI] [PubMed] [Google Scholar]
- 105. Gierada DS, Hakimian S, Slone RM, Yusen RD. Mr analysis of lung volume and thoracic dimensions in patients with emphysema before and after lung volume reduction surgery. AJR Am J Roentgenol 1998; 170: 707–14. doi: 10.2214/ajr.170.3.9490958 [DOI] [PubMed] [Google Scholar]
- 106. Qanadli SD, Orvoen-Frija E, Lacombe P, Di Paola R, Bittoun J, Frija G. Estimation of gas and tissue lung volumes by MRI: functional approach of lung imaging. J Comput Assist Tomogr 1999; 23: 743–8. doi: 10.1097/00004728-199909000-00020 [DOI] [PubMed] [Google Scholar]
- 107. Suga K, Tsukuda T, Awaya H, Takano K, Koike S, Matsunaga N, et al. Impaired respiratory mechanics in pulmonary emphysema: evaluation with dynamic breathing MRI. J Magn Reson Imaging 1999; 10: 510–20. doi: [DOI] [PubMed] [Google Scholar]
- 108. Suga K, Tsukuda T, Awaya H, Matsunaga N, Sugi K, Esato K. Interactions of regional respiratory mechanics and pulmonary ventilatory impairment in pulmonary emphysema: assessment with dynamic MRI and xenon-133 single-photon emission CT. Chest 2000; 117: 1646–55. doi: 10.1378/chest.117.6.1646 [DOI] [PubMed] [Google Scholar]
- 109. Kondo T, Kobayashi I, Taguchi Y, Ohta Y, Yanagimachi N. A dynamic analysis of chest wall motions with MRI in healthy young subjects. Respirology 2000; 5: 19–25. doi: 10.1046/j.1440-1843.2000.00221.x [DOI] [PubMed] [Google Scholar]
- 110. Plathow C, Ley S, Fink C, Puderbach M, Heilmann M, Zuna I, et al. Evaluation of chest motion and volumetry during the breathing cycle by dynamic MRI in healthy subjects: comparison with pulmonary function tests. Invest Radiol 2004; 39: 202–9. doi: 10.1097/01.rli.0000113795.93565.c3 [DOI] [PubMed] [Google Scholar]
- 111. Plathow C, Fink C, Ley S, Puderbach M, Eichinger M, Schmähl A, et al. Measurement of diaphragmatic length during the breathing cycle by dynamic MRI: comparison between healthy adults and patients with an intrathoracic tumor. Eur Radiol 2004; 14: 1392–9. doi: 10.1007/s00330-004-2336-y [DOI] [PubMed] [Google Scholar]
- 112. Plathow C, Ley S, Fink C, Puderbach M, Hosch W, Schmähl A, et al. Analysis of intrathoracic tumor mobility during whole breathing cycle by dynamic MRI. Int J Radiat Oncol Biol Phys 2004; 59: 952–9. doi: 10.1016/j.ijrobp.2003.12.035 [DOI] [PubMed] [Google Scholar]
- 113. Plathow C, Schoebinger M, Fink C, Ley S, Puderbach M, Eichinger M, et al. Evaluation of lung volumetry using dynamic three-dimensional magnetic resonance imaging. Invest Radiol 2005; 40: 173–9. doi: 10.1097/00004424-200503000-00007 [DOI] [PubMed] [Google Scholar]
- 114. Plathow C, Klopp M, Schoebinger M, Thieke C, Fink C, Puderbach M, et al. Monitoring of lung motion in patients with malignant pleural mesothelioma using two-dimensional and three-dimensional dynamic magnetic resonance imaging: comparison with spirometry. Invest Radiol 2006; 41: 443–8. doi: 10.1097/01.rli.0000208222.03256.ba [DOI] [PubMed] [Google Scholar]
- 115. Kolb C, Wetscherek A, Buzan MT, Werner R, Rank CM, Kachelrie M, et al. Regional lung ventilation analysis using temporally resolved magnetic resonance imaging. J Comput Assist Tomogr 2016; 40: 899–906. doi: 10.1097/RCT.0000000000000450 [DOI] [PubMed] [Google Scholar]
- 116. Chen Q, Mai VM, Bankier AA, Napadow VJ, Gilbert RJ, Edelman RR. Ultrafast Mr grid-tagging sequence for assessment of local mechanical properties of the lungs. Magn Reson Med 2001; 45: 24–8. doi: [DOI] [PubMed] [Google Scholar]
- 117. Napadow VJ, Mai V, Bankier A, Gilbert RJ, Edelman R, Chen Q. Determination of regional pulmonary parenchymal strain during normal respiration using spin inversion tagged magnetization MRI. J Magn Reson Imaging 2001; 13: 467–74. doi: 10.1002/jmri.1068 [DOI] [PubMed] [Google Scholar]
- 118. Cai J, Sheng K, Benedict SH, Read PW, Larner JM, Mugler JP, et al. Dynamic MRI of grid-tagged hyperpolarized helium-3 for the assessment of lung motion during breathing. Int J Radiat Oncol Biol Phys 2009; 75: 276–84. doi: 10.1016/j.ijrobp.2009.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gee J, Sundaram T, Hasegawa I, Uematsu H, Hatabu H. Characterization of regional pulmonary mechanics from serial magnetic resonance imaging data. Acad Radiol 2003; 10: 1147–52. doi: 10.1016/S1076-6332(03)00329-5 [DOI] [PubMed] [Google Scholar]
- 120. Sundaram TA, Gee JC. Towards a model of lung biomechanics: pulmonary kinematics via registration of serial lung images. Med Image Anal 2005; 9: 524–37. doi: 10.1016/j.media.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 121. Sundaram TA, Avants BB, Gee JC. Towards a dynamic model of pulmonary parenchymal deformation: evaluation of methods for temporal reparameterization of lung data. Med Image Comput Comput Assist Interv 2005; 8(Pt 2): 328–35. doi: 10.1007/11566489_41 [DOI] [PubMed] [Google Scholar]
- 122. Kolb C, Wetscherek A, Buzan MT, Werner R, Rank CM, Kachelrie M, et al. Regional lung ventilation analysis using temporally resolved magnetic resonance imaging. J Comput Assist Tomogr 2016; 40: 899–906. doi: 10.1097/RCT.0000000000000450 [DOI] [PubMed] [Google Scholar]
- 123. Biederer J. Using lung MRI and elastic registration to assess pulmonary fibrosis. Radiology 2019; 291: 493–4. doi: 10.1148/radiol.2019190233 [DOI] [PubMed] [Google Scholar]