Abstract
Objectives:
To validate reliability of slice-encoding for metal artefact correction (SEMAC)-MRI findings in prosthesis loosening detection by comparing them to surgical outcomes (gold standard) in symptomatic patients following hip arthroplasties. To evaluate periprosthetic anatomical structures in symptomatic patients to identify an alternative cause of hip symptoms.
Methods:
We prospectively followed 47 symptomatic patients (55 hips, 39 painful hips – group P and 16 control hips – group C) at our institution from 2011 to 2016. We acquired 1.5 T MRI conventional and SEMAC-MRI images for all patients. Two consultants scored MRI for osteolysis and marrow oedema zone-wise using predefined signal characteristics and settled scoring variations by consensus. We used Spearman Rank-Order Correlation for correlation analysis and used OMERACT (Outcome Measures in Rheumatology) filter pillars to validate SEMAC-MRI findings.
Results:
Eleven patients needed revision surgery, all from group P. None from group C required revision surgery. Remaining 28 hips in the group P were managed conservatively pain completely resolved in 21 hips, eight hips had trochanteric bursitis, eight had extraarticular cause and the remaining five hips had spontaneous pain resolution. We found moderate-to-weak correlation between SEMAC-MRI findings for prosthesis loosening and revision surgery outcomes. Sensitivity, Specificity, PPV and NPV in Group P were (72.7, 64.3, 44.4, 85.7%) in T1W-SEMAC, (90.9, 46.4, 40.0, 92.9%) in STIR-SEMAC and (36.3, 78.5, 40.0, 75.8%) in PDW-SEMAC.
Conclusion:
Negative SEMAC-MRI results can effectively exclude prosthesis loosening confirmed on revision surgery and SEMAC-MRI can detect alternative cause of hip pain accurately.
Advances in knowledge:
Negative SEMAC-MRI in painful THA patients can effectively exclude prosthesis loosening as a cause.
Introduction
According to the 17th Annual report of National Joint Registry, UK, a total of 1,191, 253 total hip arthroplasty (THA) procedures have been carried out in the last 16 years (2003–2019). 1 THA is a well -established surgical procedure for alleviating pain and increasing mobility in patients with symptomatic end-stage hip arthritis. 2 Its longevity is well documented, and studies have described 10-year and 25-year survival rates following THA to be 95 and 80%, respectively. 3,4 The risk of requiring revision is significantly higher in young patients who are being increasingly offered THA. 5 This is in part due to the increased life expectancy and in part due to higher activity levels and type of activity young patients undertake. Aseptic loosening was the most common indication for revision surgery. 1 Its timely diagnosis is necessary to reduce further osteolysis making revision technically more challenging. At times, radiographs and blood tests can be normal in aseptic loosening. 6
Use of magnetic resonance imaging (MRI) following THA has remarkably increased for implant imaging in recent years. 7 MRI can diagnose changes associated with failure of prosthesis fixation, such as synovitis, periprosthetic osteolysis, fractures and muscle injuries, and can contribute in diagnosis of infection suspected clinically. 8,9 Its diagnostic capability, unfortunately, is hampered by implant-related artefacts produced from magnetic field inhomogeneities more pronounced at higher magnetic field strength 10,11 from magnetic susceptibility differences between the metal implant and the surrounding tissue 12 reducing its diagnostic yield.
In recent times, sophisticated MRI acquisition techniques have made artefact reduction possible improving periprosthetic tissues assessment.. 13 Several studies have documented metal artefact reduction sequence (MARS) effectiveness following THA, 14–18 which generates a combined image using various acquisition parameters, reducing implant-related artefacts. Slice encoding for metal artefact correction (SEMAC), similar to MARS, is a novel technique which uses two-dimensional slice selective excitations but then phase-encodes each slice in the through-plane dimension and combines them to form a composite image. 19,20
Although there are many reports about the efficacy of SEMAC-MRI, 19–21 after extensive literature search, we found no study clarifying the relationship between SEMAC-MRI findings and clinical outcomes; namely, THA prosthesis loosening requiring revision surgery. Hence, our prime objective was to assess the diagnostic performance of SEMAC-MRI in diagnosing prosthesis loosening by validating imaging findings intraoperatively using OMERACT criteria to check criterion and construct validity. Additional objectives were to highlight the key MRI characteristics aiding detection of implant loosening and evaluate attributable soft tissue pathologies in symptomatic patients.
Methods
Patient selection
Following regional health research authority and institutional review board approval, we invited patients without and with pain following THA to participate in the study from our arthroplasty follow-up clinics. We selected patients with Marathon acetabular cup and Corail cementless stem (Depuy Synthes, Warsaw, IN, metal on polyethylene articulation) implanted during THA between January 2011 and October 2016 via a posterior approach. We termed those without pain a control group (Group C) and those with painful THA in group P. All patients received a thorough clinical assessment including detailed history taking, clinical examination of the affected and the contralateral hip as well as both knees and spine to rule out any obvious cause. Additionally, all patients had had a standardised hematological workup including inflammatory markers, such as white cell counts, ESR and C-reactive proteins. Prior to the study cohort formation, we excluded individuals with painful THA following trauma and the patients in whom infection was suspected clinically making our study population design selective for patients with possible periprosthetic osteolysis and aseptic loosening. The following additional exclusion criteria were used: pregnancy, contraindication to MRI and unwilling to participate further in the study.
Image acquisition
We acquired various conventional and SEMAC MRI sequences using 1.5 Tesla MRI scanner (MAGNETOM® Avanto, Siemens, Erlangen, Germany) in all patients. The obtained MRI sequences included conventional T1W and T2W axial, T2W sagittal, and T1W, PD and STIR coronal sequences followed by T1W, PD and STIR coronal sequences using SEMAC parameters, which are mentioned in the Table 1.
Table 1.
MRI parameters
| Sequence | T1W axial | T2W axial | T2W sagittal | T1W coronal | PDW coronal | STIR coronal | T1W-SEMAC coronal | PDW-SEMAC coronal | STIR-SEMAC coronal |
|---|---|---|---|---|---|---|---|---|---|
| TR (ms) | 684 | 3000 | 3000 | 833 | 3500 | 3000 | 833 | 3500 | 3000 |
| TE (ms) | 9.1 | 68 | 78 | 8.8 | 30 | 47 | 8.8 | 32 | 47 |
| TI (ms) | - | - | - | - | - | 160 | - | - | 160 |
| ETL | 39 | 29 | 34 | 35 | 13 | 42 | 35 | 21 | 42 |
| FOV (mm) | 360 | 360 | 360 | 420 | 400 | 400 | 420 | 400 | 400 |
| Slice thickness (mm) | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 4 | 5 |
| Slices | 21 | 21 | 22 | 24 | 25 | 26 | 24 | 25 | 26 |
| Base resolution | 448 | 448 | 448 | 384 | 333 | 384 | 384 | 333 | 384 |
| NSA | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 |
| Bandwidth (Hz/Px) | 620 | 744 | 620 | 723 | 610 | 651 | 723 | 543 | 651 |
| Acquisition time (minutes) | 4:04 | 2:59 | 3:29 | 3:58 | 3:48 | 4:17 | 3:58 | 7:26 | 4:17 |
(TR – Repetition time; TE – Echo time; TI – Time to invert; ETL – echo train length; FOV – field of view; NSA – Number of signal average; ms – miliseconds; mm – milimeters; and Hz/Px – Hertz/Pixel).
Image interpretation
Two consultants (25 and 15 years of professional experience) reviewed both conventional and SEMAC MRIs in all patients for periprosthetic signal changes (Figure 1) in form of high STIR signal, low signal on T1 and iso to high signal on PD-weighted images characterising osteolysis, fluid infiltration and bone marrow oedema (BME) in ‘Gruen Zones’ around the femoral component and ‘Delee and Charnley Zones’ around acetabular components (Figure 2). 22,23 An area of focal low intensity in T1W and/or high intensity on PDW that displayed an absence of cancellous markings is termed as osteolysis, a focal area of iso-intensity in PDW images just beneath the prosthesis is considered as peri-prosthetic fluid infiltration and diffuse high intensity on STIR around the prosthesis with preserved cancellous bony trabeculae is defined as BME. We have further assessed anatomic structures around the hip prosthesis such as psoas tendon insertion and muscle/tendon of gluteus medius, gluteus minimus, obturator internus and obturator externus to identify alternative causes of symptomatic implanted hips such as fluid collection in the soft tissues, trochanteric bursitis, joint effusion and synovitis.
Figure 1.
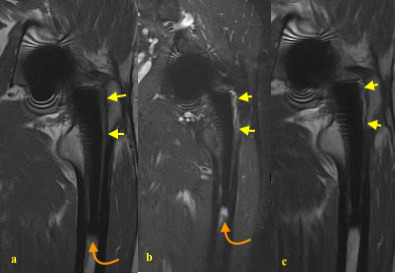
68-year-old male with left hip pain following THA. (a). T1 SEMAC sequence - absent cancellous markings in Gruen zone 1 and 2 suggesting osteolysis (yellow arrows), (b). STIR-SEMAC sequence – Gruen zone 1 and 2 indicates the fluid infiltration around the femoral component (yellow arrows) and increased signal at zone four around the femoral stem tip not following fluid signal is consistent with the BME (curved amber arrows), (c). PDW-SEMAC sequence. Fluid signal corresponding to area of osteolysis (yellow arrows) – please note the sensitivity of STIR-SEMAC as compared to PDW-SEMAC.
Figure 2.
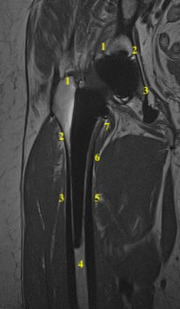
76-year-old female with right hip pain following THA. T1W-SEMAC image demonstrating ‘Delee and Charnley zones’ for acetabular component and Gruen’s zone for femoral components.
Quantitative analysis
The components were consensus scored for diagnostic quality on five-point scale: (0) definitely non-diagnostic; (1) probably non-diagnostic; (2) possibly diagnostic; (3) probably diagnostic; (4) definitely diagnostic. Abnormalities were assessed on MRI using a four-point scale: (0) none; (1) mild; (2) moderate; (3) severe.
The findings were recorded and scored, zone-wise in a standardised manner by experienced consultants who were blinded to conventional and SEMAC-MRI sequences (Figure 3) and to clinical findings and radiographs whilst evaluating the MR images. We adjudicated interobserver variations by consensus to rectify observer bias.
Figure 3.
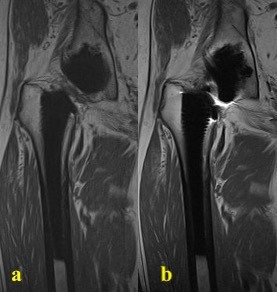
80-year-old male with right hip pain following THA. (a). T1-weighted image with SEMAC and (b). T1W without SEMAC acquisition parameters demonstrating effective metal artefact suppression on T1w sequences.
Reference standard
Prosthesis loosening or revision surgery due to loosening during the follow-up periods acted as a primary endpoint. A standardised proforma was used by surgeons to record the surgical findings including following questions – whether implant was loose or not?, If loose, which anatomical area – femur or acetabulum – were involved?, was there any associated osteolysis or not?, if present, which part of the implant was affected by the osteolysis? And was it correlating with the MRI findings or not?
Statistical analysis
We used statistical software (SPSS for windows, v.21, Chicago, IL) for statistical analysis. We employed Spearman Rank-Order Correlation to ascertain the correlation between SEMAC-MRI findings of prosthesis loosening and primary endpoint – prosthesis loosening on revision surgery. We also analysed factors that may predict the revision surgery and significance of abnormal findings on SEMAC-MRI between revised and non-revised cases using logistic regression analysis. p value < 0.05 was considered significant. Additionally, we calculated sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of various SEMAC-MRI sequences to predict prosthesis loosening or revision surgery.
Results
We included a total of 47 patients (24 males, 23 females) in our study and investigated 55 hips (39 unilateral, eight bilateral) in the study population. The mean patient age at the time of MRI was 65.0 (SD: 12.1, range: 37–82) years, average time between index procedure - THA and MRI was 4.9 (SD: 4.5, range: 1–15) years and we have followed them up for 1.9 (SD: 1.1, range: 1–4) years after MRI. There were 16 hips in Group C and 39 hips in Group P. Out of the cohort of 39 hips in Group P, 11 hips required revision surgery and aseptic implant loosening was confirmed in all the cases at the time of surgery.
Visualisation of bone-cement/bone-prosthesis interface with and without SEMAC
Visualisation for bone-cement or bone-prosthesis junctions were significantly better in STIR-SEMAC than conventional STIR suggesting diagnostic superiority. Similarly, the diagnostic quality of T1W-SEMAC for acetabular component and STIR-SEMAC for femoral components was statistically higher, whereas that of T1W-SEMAC for femurs and PDW-SEMAC for acetabulum and femur and conventional MRIs has shown no statistical difference. Diagnostic quality scores on T1W and PDW images were statistically adequate for femurs on both conventional and SEMAC MRIs (Table 2).
Table 2.
Visualisation of interface between bone and cement/prosthesis (coronal images)
| STIR CONV | STIR SEMAC | P-value | T1 CONV | T1 SEMAC | P-value | PD CONV | PD SEMAC | P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Acetabulum | Zone.1 | 0.64 (1.3) | 2.0 (1.1) | 0.0047 | 0.73 (1.1) | 2.4 (1.2) | 0.0053 | 0.75 (1.5) | 2.3 (0.5) | NS |
| Zone.2 | 0.73 (1.3) | 2.2 (1.1) | 0.0048 | 0.73 (1.1) | 2.6 (1.2) | 0.0057 | 0.75 (1.5) | 2.8 (0.5) | NS | |
| Zone.3 | 0.73 (1.3) | 2.3 (1.1) | 0.0048 | 0.73 (1.1) | 2.6 (1.2) | 0.0057 | 0.75 (1.5) | 2.8 (0.5) | NS | |
| Femur | Zone.1 | 1.6 (1.5) | 3.2 (0.8) | 0.013 | 3.3 (0.9) | 3.7 (0.9) | 0.018 | 3.8 (0.5) | 4.0 (0.0) | NS |
| Zone.2 | 2.5 (1.1) | 3.4 (0.5) | 0.011 | 3.5 (0.9) | 3.8 (0.4) | NS | 3.8 (0.5) | 3.8 (0.5) | NS | |
| Zone.3 | 2.5 (1.4) | 3.5 (0.5) | 0.0044 | 3.5 (0.9) | 3.8 (0.4) | NS | 3.8 (0.5) | 3.8 (0.5) | NS | |
| Zone.4 | 2.6 (1.4) | 3.5 (0.5) | 0.050 | 3.7 (0.5) | 3.9 (0.3) | NS | 3.7 (0.5) | 3.9 (0.3) | NS | |
| Zone.5 | 2.7 (1.4) | 3.5 (0.5) | NS | 3.8 (0.4) | 3.9 (0.3) | NS | 3.8 (0.5) | 3.8 (0.5) | NS | |
| Zone.6 | 2.8 (1.2) | 3.5 (0.5) | 0.033 | 3.8 (0.4) | 3.9 (0.3) | NS | 3.5 (1.0) | 4.0 (0.0) | NS | |
| Zone.7 | 1.8 (1.4) | 3.2 (0.6) | 0.0077 | 3.1 (0.7) | 3.8 (0.5) | 0.019 | 2.8 (1.9) | 4.0 (0.0) | NS |
CONV, conventional; NS, not significant.
Paired T test was used, and data are expressed as mean (standard deviation).
Abnormal findings in cases with and without prosthesis loosening
There was a moderate statistical correlation between osteolysis on MRI and surgical validation of prosthesis loosening in both acetabular (correlation coefficient, r = 0.655) and femoral (correlation coefficient, r = 0.667) components and we could not establish strong association between MRI appearances and surgical outcomes (Table 3).
Table 3.
Abnormal findings in cases with and without prosthesis loosening (coronal images)
| STIR SEMAC | T1 SEMAC | PD SEMAC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stable | Loosening | P-value | Stable | Loosening | P-value | Stable | Loosening | P-value | ||
| Acetabulum | Zone.1 | 0.071 (0.26) | 0.09 (0.30) | NS | 0.071 (0.26) | 0.09 (0.30) | NS | 0.0 (0.0) | 0.0 (0.0) | NS |
| Zone.2 | 0.11 (0.31) | 0.09 (0.30) | NS | 0.071 (0.26) | 0.09 (0.30) | NS | 0.0 (0.0) | 0.0 (0.0) | NS | |
| Zone.3 | 0.18 (0.39) | 0.27 (0.47) | NS | 0.071 (0.26) | 0.18 (0.40) | NS | 0.071 (0.26) | 0.09 (0.30) | NS | |
| Femur | Zone.1 | 0.25 (0.44) | 0.73 (0.47) | 0.0049 | 0.18 (0.39) | 0.18 (0.40) | NS | 0.0 (0.0) | 0.0 (0.0) | NS |
| Zone.2 | 0.14 (0.36) | 0.09 (0.30) | NS | 0.071 (0.26) | 0.09 (0.30) | NS | 0.0 (0.0) | 0.0 (0.0) | NS | |
| Zone.3 | 0.071 (0.26) | 0.09 (0.30) | NS | 0.0 (0.0) | 0.09 (0.30) | NS | 0.071 (0.26) | 0.09 (0.30) | NS | |
| Zone.4 | 0.18 (0.39) | 0.18 (0.40) | NS | 0.0 (0.0) | 0.09 (0.30) | NS | 0.0 (0.0) | 0.0 (0.0) | NS | |
| Zone.5 | 0.25 (0.97) | 0.0 (0.0) | NS | 0.0 (0.0) | 0.09 (0.30) | NS | 0.0 (0.0) | 0.0 (0.0) | NS | |
| Zone.6 | 0.11 (0.31) | 0.09 (0.30) | NS | 0.036 (0.19) | 0.0 (0.0) | NS | 0.071 (0.26) | 0.09 (0.30) | NS | |
| Zone.7 | 0.11 (0.31) | 0.55 (0.52) | 0.0027 | 0.11 (0.31) | 0.36 (0.50) | NS | 0.0 (0.0) | 0.0 (0.0) | NS | |
NS, not significant.
Student’s T test was used, and data are expressed as median (standard deviation).
Visualisation of surrounding anatomic structures
We found that gluteus medius, gluteus minimus, obturator intenus and obturator externus were significantly better visualised on STIR-SEMAC than conventional STIR, and there was no statistical difference in the diagnostic yield of T1W and PDW images in both acquisition techniques. All sequences on both conventional and SEMAC have shown similar diagnostic performance visualising psoas tendon insertion (Table 4).
Table 4.
Visualisation of anatomic structures
| STIR CONV |
STIR SEMAC |
P-value | T1 CONV |
T1 SEMAC |
P-value | PD CONV | PD SEMAC | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Psoas tendon insertion | 2.3 (1.6) | 3.4 (0.5) | NS | 3.8 (0.4) | 3.9 (0.3) | NS | 3.8 (0.5) | 4.0 (0.0) | NS |
| Gluteus medius | 3.1 (0.8) | 3.9 (0.3) | 0.011 | 3.7 (0.6) | 3.9 (0.3) | NS | 3.5 (0.6) | 3.8 (0.5) | NS |
| Gluteus minimus | 1.5 (1.4) | 3.1 (1.2) | 0.0077 | 3.0 (1.5) | 3.3 (1.2) | NS | 1.8 (1.7) | 3.0 (0.8) | NS |
| Obturator intenus | 1.8 (1.3) | 3.6 (0.7) | 0.0048 | 3.6 (0.5) | 3.8 (0.4) | NS | 2.3 (1.7) | 3.8 (0.5) | NS |
| Obturator externus | 2.1 (1.0) | 3.3 (0.7) | 0.012 | 3.7 (0.5) | 3.8 (0.4) | NS | 3.0 (0.8) | 3.3 (1.0) | NS |
CONV, conventional; NS, not significant.
Paired T test was used, and data are expressed as mean (standard deviation).
All 11 hips requiring revision surgery belonged to group P, whereas no hip in Group C has undergone revision surgery. Remaining 28 hips in the group P were managed conservatively among which pain had recovered in 21 hips on follow-up – eight hips had trochanteric bursitis treated with steroid injections, eight had extraarticular cause for hip pain consisting of 5 with degenerative lumbar disease and three with either knee or ankle OA – confirmed on further imaging and the remaining five hips had spontaneous pain resolution. Among hips having persistent symptoms on follow-up, one had trochanteric bursitis, and three had degenerative lumbar disease contributing to referred hip pain (Figure 4). It was clear that there was no radiological evidence of prosthesis loosening or subsidence in cases showing pain resolution on follow-up.
Figure 4.
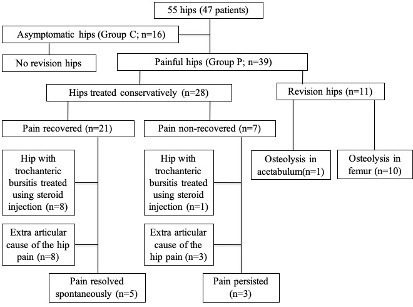
Patient flow during the study
Logistic regression analysis showed that significant differences between hips with and without revision surgery in Group P were a positive finding of T1W-SEMAC in stem zone one and a positive finding of PDW-SEMAC in stem zone seven corresponding to a positive finding of STIR-SEMAC in stem zone 1 (Odds ratio; 8.00; 95% confidence interval 1.65–38.8, p = 0.0098) and a positive finding of STIR-SEMAC in stem zone 7 (Odds ratio; 10.0; 95% confidence interval 1.85–54.0, p = 0.0074).
Using Spearman Rank-Order Correlation, we found that the statistical correlation was moderate (r = 0.415) on T1W-SEMAC and weak (r = 0.35) on STIR-SEMAC between the SEMAC-MRI findings and the primary endpoint – revision surgery.
Furthermore, we have provided sensitivity, specificity, PPV and NPV of all three SEMAC-MRI sequences in identification of aseptic loosening in group P (Table 5).
Table 5.
Sensitivity, Specificity, PPV and NPV of SEMAC-MRI to detect loosening of the prosthesis
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| T1-SEMAC | 72.7 | 64.3 | 44.4 | 85.7 |
| STIR-SEMAC | 90.9 | 46.4 | 40.0 | 92.9 |
| PD-SEMAC | 36.3 | 78.5 | 40.0 | 75.8 |
NPV: Negative predictive value;PPV: Positive predictive value.
Discussion
Our study demonstrated that following THA, (1) STIR-SEMAC achieved the best metallic artefact reduction making it the most sensitive sequence to identify implant loosening if present which can be validated on revision surgery, (2) presence of positive MRI findings in the zone one on T1W-SEMAC and in the zone seven on PDW-SEMAC could predict implant loosening and (3) SEMAC-MRI have also found causes contributing to patient symptoms in group P who did not require revision surgery on follow-up such as trochanteric bursitis and degenerative spine disease which were managed conservatively.
Sutter et al concluded that STIR and T1W sequences were significantly better for both qualitative and quantitative imaging. Albeit clinically relevant artefact reduction was achieved only on STIR images, almost half of the abnormal imaging findings were missed due to increased noise on conventional STIR compared to STIR-SEMAC. Our study echoes these findings.. 21
We concluded that better metallic artefact suppression can be achieved by SEMAC-MRI which can be multifactorial 24 and our study provides similar results to those of Tartaglino and colleagues in postoperative spine imaging 25 and Olsen et al, in periprosthetic soft tissues assessment following hip replacement. 10 SEMAC-MRI can significantly improve periprosthetic soft tissue evaluation by reducing metallic artefacts in turn improving prosthesis-related complications including infections, fractures and tendinopathies in painful hips as described by Agten et al 14 and detecting periprosthetic fluid collections and masses as per Hart et al. 15
SEMAC-MRI can also detect adverse reaction to metal debris including pseudotumours and muscle atrophy, monitor at-risk hips and exclude prosthesis loosening in painful hips following MOM-THA. 16,17,26,27 Furthermore, MRI can be less sensitive than CT in detecting periprosthetic osteolysis in MOM-THA and may demonstrate abnormalities in asymptomatic patients regardless of bearing type 28–30 and one should not revise MOM-THA based on MRI abnormalities alone. However, these findings are less relevant to our study given differential prosthesis selection.
Fehring et al 30 stated that MRI abnormalities are commonly seen regardless of bearing type, can be nonspecific and should not be used for decision making and in partial disagreement to these findings, we concluded that abnormal T1W or PDW SEMAC-MRI findings of the proximal femur in painful hip patients could predict implant loosening. Additionally, due to its high sensitivity, NPV and moderate correlation between positive MRI findings and implant loosening, STIR-SEMAC can help diagnose periprosthetic osteolysis, or BME once-reliable fat saturation achieved. 5 In other words, when there are no findings in STIR-SEMAC, the risk for revision due to prosthesis loosening is supposed to be low and other causes for pain should be investigated.
There are certain limitations to our study. We have only evaluated the osteolysis or fluid infiltration around the prosthesis as joint effusion following ceramic-on-polyethylene (COP) THA are common in asymptomatic patients. 31 Furthermore, we have not customised SEMAC-MRI for each patient and implant material but such SEMAC optimisation increases diagnostic quality according to Deligianni and colleagues. 32 Hence, we might have achieved different results, had we used tailored SEMAC. As we have evaluated patients with MOP-THA only, our results should not be compared to those following MOM-THA directly. Having described that COP and MOM-THA have a similar incidence of periprosthetic fluid collections on MRI 33 whereas asymptomatic patients with MOM-THA have shown more MRI abnormalities than those with MOP-THA. 18 We have not evaluated the efficacy of SEMAC-MRI combined with ultrasound where the latter can detect local tissue reaction in asymptomatic functioning prostheses earlier. 34,35 From a technical aspect, we have not evaluated off-resonance suppression for SEMAC-MRI which may further reduce artefacts. 35 All of these can act as points of interest for future studies.
Beyond above-mentioned limitations, this was the first study to clarify the relationship between SEMAC-MRI findings and prosthesis loosening validated on revision surgery. STIR-SEMAC sequence was proven exceptionally useful for implant loosening exclusion given its high negative predictive value.
Conclusion
In painful hips following THA, SEMAC-MRI is a useful tool in differentiating aseptic implant loosening from other soft tissue and extraarticular causes. It will help the surgeons counsel the patients and treat their symptoms appropriately. STIR-SEMAC demonstrates high sensitivity and negative predictive value in detecting periprosthetic fluid and marrow oedema, surrogate markers for prosthesis loosening and can help excluding prosthesis loosening with high certainty.
Footnotes
Acknowledgements: The research was conducted at the National Institute for Health Research (NIHR) Leeds Biomedical Research Centre; however, not funded by the NIHR grant. This article presents independent research supported by the National Institute for Health Research (NIHR) Leeds Biomedical Research Centre (BRC). (R&D Number: RR12/10615 and REC: 12/YH/0555). Professor Pandit is a National Institute for Health Research (NIHR) Senior Investigator. We express our gratitude to Dr Daniel Skrzypiec for his valuable contribution to the study, Dr Steve Tanners from Medical Physics for developing the MR protocol and Ms. Emily Dye for her help with MRI parameters.
Disclosure: The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Contributor Information
Tsuneari Takahashi, Email: tsuneari9@jichi.ac.jp.
Siddharth Thaker, Email: siddharth.thaker@nhs.net.
Giovanni Lettieri, Email: G.lettieri@leeds.ac.uk.
Anthony Redmond, Email: a.redmond@leeds.ac.uk.
Michael R. Backhouse, Email: Michael.backhouse@warwick.ac.uk.
Martin Stone, Email: martin.stone@hotmail.com.
Hemant Pandit, Email: h.pandit@leeds.ac.uk.
Philip O'Connor, Email: philip.oconnor@nhs.net.
REFERENCES
- 1. Reed M, Wilton MT. Executive summary. In The National Joint Registry 17th Annual Report 2020 [Internet]. National Joint Registry; 2020. [PubMed] [Google Scholar]
- 2. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370: 1508–19. doi: 10.1016/S0140-6736(07)60457-7 [DOI] [PubMed] [Google Scholar]
- 3. Prime MS, Palmer J, Khan WS. The national joint registry of england and wales. Orthopedics 2011; 34: 107–10. doi: 10.3928/01477447-20101221-21 [DOI] [PubMed] [Google Scholar]
- 4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the united states from 2005 to 2030. The Journal of Bone and Joint Surgery-American Volume 2007; 89: 780–85. doi: 10.2106/00004623-200704000-00012 [DOI] [PubMed] [Google Scholar]
- 5. Bayliss LE, Culliford D, Monk AP, Glyn-Jones S, Prieto-Alhambra D, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet 2017; 389: 1424–30. doi: 10.1016/S0140-6736(17)30059-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ben-Shlomo Y, Blom A, Boulton C, Brittain R, Clark E, et al. Outcomes after joint replacement 2003 to 2019. InThe National Joint Registry 17th Annual Report 2020 [Internet]. National Joint Registry; 2020. [Google Scholar]
- 7. Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States 2000-2010. US Department of Health and Human Services, Centers for Disease Control and Prevention. National Center for Health Statistics; 2015. [Google Scholar]
- 8. Twair A, Ryan M, O’Connell M, Powell T, O’Byrne J, et al. MRI of failed total hip replacement caused by abductor muscle avulsion. AJR Am J Roentgenol 2003; 181: 1547–50. doi: 10.2214/ajr.181.6.1811547 [DOI] [PubMed] [Google Scholar]
- 9. Olsen RV, Munk PL, Lee MJ, Janzen DL, MacKay AL, et al. Metal artifact reduction sequence: early clinical applications. Radiographics 2000; 20: 699–712. doi: 10.1148/radiographics.20.3.g00ma10699 [DOI] [PubMed] [Google Scholar]
- 10. Matsuura H, Inoue T, Ogasawara K, Sasaki M, Konno H, et al. Quantitative analysis of magnetic resonance imaging susceptibility artifacts caused by neurosurgical biomaterials: comparison of 0.5, 1.5, and 3.0 tesla magnetic fields. Neurol Med Chir (Tokyo) 2005; 45: 395–98. doi: 10.2176/nmc.45.395 [DOI] [PubMed] [Google Scholar]
- 11. Jungmann PM, Agten CA, Pfirrmann CW, Sutter R. Advances in mri around metal. J Magn Reson Imaging 2017; 46: 972–91. doi: 10.1002/jmri.25708 [DOI] [PubMed] [Google Scholar]
- 12. Naraghi AM, White LM. Magnetic resonance imaging of joint replacements. Semin Musculoskelet Radiol 2006; 10: 98–106. doi: 10.1055/s-2006-934220 [DOI] [PubMed] [Google Scholar]
- 13. Agten CA, Sutter R, Pfirrmann CWA. CT and mri of hip arthroplasty. Radiologe 2014; 54: 715–25. doi: 10.1007/s00117-014-2693-8 [DOI] [PubMed] [Google Scholar]
- 14. Hart AJ, Sabah S, Henckel J, Lewis A, Cobb J, et al. The painful metal-on-metal hip resurfacing. J Bone Joint Surg Br 2009; 91: 738–44. doi: 10.1302/0301-620X.91B6.21682 [DOI] [PubMed] [Google Scholar]
- 15. Wynn-Jones H, Macnair R, Wimhurst J, Chirodian N, Derbyshire B, et al. Silent soft tissue pathology is common with a modern metal-on-metal hip arthroplasty: early detection with routine metal artifact-reduction mri scanning. Acta Orthop 2011; 82: 301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sabah SA, Mitchell AWM, Henckel J, Sandison A, Skinner JA, et al. Magnetic resonance imaging findings in painful metal-on-metal hips: a prospective study. J Arthroplasty 2011; 26: 71–76. doi: 10.1016/j.arth.2009.11.008 [DOI] [PubMed] [Google Scholar]
- 17. Mistry A, Cahir J, Donell ST, Nolan J, Toms AP. MRI of asymptomatic patients with metal-on-metal and polyethylene-on-metal total hip arthroplasties. Clin Radiol 2011; 66: 540–45. doi: 10.1016/j.crad.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 18. Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: slice encoding for metal artifact correction in mri. Magn Reson Med 2009; 62: 66–76. doi: 10.1002/mrm.21967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agten CA, Del Grande F, Fucentese SF, Blatter S, Pfirrmann CWA, et al. Unicompartmental knee arthroplasty mri: impact of slice-encoding for metal artefact correction mri on image quality, findings and therapy decision. Eur Radiol 2015; 25: 2184–93. doi: 10.1007/s00330-015-3596-4 [DOI] [PubMed] [Google Scholar]
- 20. Sutter R, Ulbrich EJ, Jellus V, Nittka M, Pfirrmann CWA. Reduction of metal artifacts in patients with total hip arthroplasty with slice-encoding metal artifact correction and view-angle tilting mr imaging. Radiology 2012; 265: 204–14. doi: 10.1148/radiol.12112408 [DOI] [PubMed] [Google Scholar]
- 21. Gruen TA, Poggie RA, Lewallen DG, Hanssen AD, Lewis RJ, et al. Radiographic evaluation of a monoblock acetabular component: a multicenter study with 2- to 5-year results. J Arthroplasty 2005; 20: 369–78. doi: 10.1016/j.arth.2004.12.049 [DOI] [PubMed] [Google Scholar]
- 22. Alm JJ, Mäkinen TJ, Lankinen P, Moritz N, Vahlberg T, et al. Female patients with low systemic bmd are prone to bone loss in gruen zone 7 after cementless total hip arthroplasty. Acta Orthop 2009; 80: 531–37. doi: 10.3109/17453670903316801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pusey E, Lufkin RB, Brown RK, Solomon MA, Stark DD, et al. Magnetic resonance imaging artifacts: mechanism and clinical significance. Radiographics 1986; 6: 891–911. doi: 10.1148/radiographics.6.5.3685515 [DOI] [PubMed] [Google Scholar]
- 24. Tartaglino LM, Flanders AE, Vinitski S, Friedman DP. Metallic artifacts on mr images of the postoperative spine: reduction with fast spin-echo techniques. Radiology 1994; 190: 565–69. doi: 10.1148/radiology.190.2.8284417 [DOI] [PubMed] [Google Scholar]
- 25. Siddiqui IA, Sabah SA, Satchithananda K, Lim AK, Cro S, et al. A comparison of the diagnostic accuracy of mars mri and ultrasound of the painful metal-on-metal hip arthroplasty. Acta Orthop 2014; 85: 375–82. doi: 10.3109/17453674.2014.908345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ebreo D, Bell PJ, Arshad H, Donell ST, Toms A, et al. Serial magnetic resonance imaging of metal-on-metal total hip replacements. follow-up of a cohort of 28 mm ultima tps thrs. Bone Joint J 2013; 95-B: 1035–39. doi: 10.1302/0301-620X.95B8.31377 [DOI] [PubMed] [Google Scholar]
- 27. Waldstein W, Schmidt-Braekling T, Boettner F. MRI does not detect acetabular osteolysis around metal-on-metal birmingham tha. Arch Orthop Trauma Surg 2014; 134: 1009–15. doi: 10.1007/s00402-014-2005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robinson E, Henckel J, Sabah S, Satchithananda K, Skinner J, et al. Cross-sectional imaging of metal-on-metal hip arthroplasties. can we substitute mars mri with ct? Acta Orthop 2014; 85: 577–84. doi: 10.3109/17453674.2014.964618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fehring TK, Fehring K, Odum SM. Metal artifact reduction sequence mri abnormalities occur in metal-on-polyethylene hips. Clin Orthop Relat Res 2015; 473: 574–80. doi: 10.1007/s11999-014-3873-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jennings JM, Martin JR, Kim RH, Yang CC, Miner TM, et al. Metal artifact reduction sequence mri abnormalities in asymptomatic patients with a ceramic-on-polyethylene total hip replacement. J Bone Joint Surg Am 2017; 99: 593–98. doi: 10.2106/JBJS.16.00910 [DOI] [PubMed] [Google Scholar]
- 31. Deligianni X, Bieri O, Elke R, Wischer T, Egelhof T. Optimization of scan time in mri for total hip prostheses: semac tailoring for prosthetic implants containing different types of metals. Rofo 2015; 187: 1116–22. 10.1055/s-0041-104893 [DOI] [PubMed] [Google Scholar]
- 32. Bisseling P, de Wit BWK, Hol AM, van Gorp MJ, van Kampen A, et al. Similar incidence of periprosthetic fluid collections after ceramic-on-polyethylene total hip arthroplasties and metal-on-metal resurfacing arthroplasties: results of a screening metal artefact reduction sequence-mri study. Bone Joint J 2015; 97-B: 1175–82. doi: 10.1302/0301-620X.97B9.35247 [DOI] [PubMed] [Google Scholar]
- 33. Matharu GS, Mansour R, Dada O, Ostlere S, Pandit HG, et al. Which imaging modality is most effective for identifying pseudotumours in metal-on-metal hip resurfacings requiring revision: ultrasound or mars-mri or both? Bone Joint J 2016; 98-B: 40–48. doi: 10.1302/0301-620X.98B1.36746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robinson DJ, Lee S, Marks P, Schneider ME. Ultrasound screening for adverse local tissue reaction after hip arthroplasty. Ultrasound Med Biol 2017; 43: 1549–56. doi: 10.1016/j.ultrasmedbio.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 35. den Harder JC, van Yperen GH, Blume UA, Bos C. Off-resonance suppression for multispectral mr imaging near metallic implants. Magn Reson Med 2015; 73: 233–43. doi: 10.1002/mrm.25126 [DOI] [PubMed] [Google Scholar]


