Abstract
With increasing subspecialised experience in radical cytoreductive surgery and intra-abdominal chemotherapy for peritoneal malignancy, outcomes have improved significantly in selected patients. The surgery and the treatment regimens are radical and therefore correct patient selection is critical. The radiologist plays a central role in this process by estimating, as precisely as possible, the pre-treatment disease burden. Because of the nature of the disease process, accurate staging is not an easy task. Tumour deposits may be very small and in locations where they are very difficult to detect. It must be acknowledged that no form of modern day imaging has the capability of detecting the smallest peritoneal nodules, which may only be visible to direct inspection or histopathological evaluation. Nonetheless, it behoves the radiologist to be as exact and precise as possible in the reporting of this disease process. This is both to select patients who are likely to benefit from radical treatment, and just as importantly, to identify patients who are unlikely to achieve adequate cytoreductive outcomes. In this review, we outline the patterns of spread of disease and the anatomic basis for this, as well as the essential aspects of reporting abdominal studies in this patient group. We provide an evidence-based update on the relative strengths and limitations of our available multimodality imaging techniques namely CT, MRI and positron emission tomography/CT.
Introduction
Peritoneal malignancy has traditionally been associated with poor outcomes. In the prospective multicentre EVOCAPE study published in 2000, the mean and median survival in patients who did not receive treatment was 6.0 and 3.1 months respectively. 1 Cytoreductive surgery (CRS) and heated intraperitoneal chemotherapy (HIPEC) or early post-operative intraperitoneal chemotherapy (EPIC) have become available in specialist centres and significant improvements in disease-free progression and long-term survival are being seen. 2,3 CRS is major surgery, and the chemotherapy regimens are intensive and can be associated with significant morbidity and mortality. 4,5 Careful patient selection is crucial, both to identify patients who are likely to achieve complete cytoreduction and to avoid causing morbidity to patients who are unlikely to benefit. 3,6,7 A knowledge of the disease process and its pattern of spread is essential if the radiologist is to correctly interrogate abdominal studies. Furthermore, the unique distribution of peritoneal cancer requires an understanding of the anatomical spaces of the abdomen and pelvis. Whilst CT imaging is the cornerstone of imaging in peritoneal malignancy, both positron emission tomography (PET)/CT imaging and MRI have advantages to offer in some cases.
Anatomy of the peritoneal cavity
The mesentery is now recognised as one organ in which all abdominal digestive organs develop, and which maintains these in systemic continuity throughout life. 8
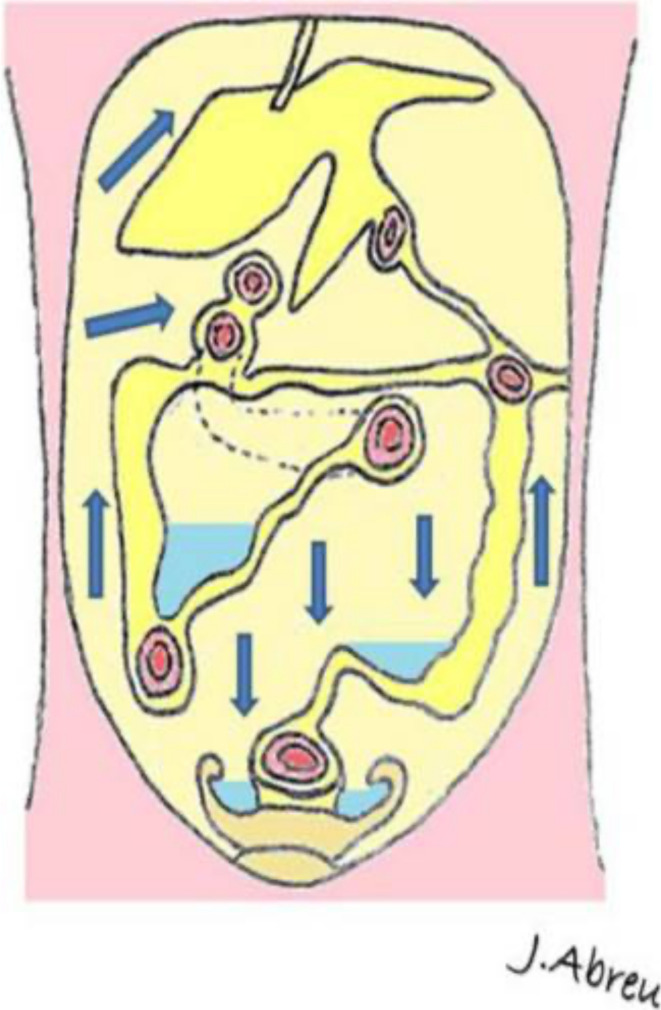
Peritoneal folds begin as a dorsal and ventral mesentery in utero, supporting the primitive gut. The dorsal mesentery connects intra-abdominal organs to the posterior abdominal wall, while the ventral mesentery connects the stomach to the anterior wall. As the embryo develops, the dorsal and ventral mesentery form ligaments, mesenteries, omenta and potential spaces from the resulting reflections. Theses reflections form pathways for spread of disease from one potential space to the next, and a knowledge of these communications helps understand the spread of peritoneal disease 9 (Figure 1).
Figure 1.
Pathways of ascitic fluid and potential spread of peritoneal disease. Reproduced with permission from Krishnamurthy et al.
The peritoneal cavity is the potential space that lies between the two abdominal peritoneal layers. The parietal peritoneum covers the deep surface of the abdominal wall, the diaphragm superiorly, the retroperitoneum and the pelvis. The visceral peritoneum partially or completely covers the bowel, abdominal and pelvic organs. In normal health, the layers are separated only by a small amount of lubricating fluid that allows smooth movement of the abdominal contents, within the limits of their ligamentous and mesenteric attachments. The peritoneum allows absorption of fluid and also has immunologic properties. Fluid in the peritoneal space flows cephalad, influenced by respiration and peristalsis, and the pattern of movement dictates the spread of peritoneal disease. The pattern of fluid circulation is dictated by the peritoneal folds. 10
In males, the peritoneal sac is fully closed. In females, it is penetrated by the lateral fallopian tubes, providing a communication with the extraperitoneal gynaecologic tract and allowing a potential pathway for the retrograde spread of disease to the peritoneal cavity.
Mechanisms of spread of peritoneal cancer
Intraperitoneal Seeding
Cancer cells detach from the primary tumour into the peritoneal fluid, transmigrate to distant peritoneum, attaching to peritoneum and invading the subperitoneal tissues. This pattern of spread is characteristic of pseudomyxoma perionei and ovarian cancer. 11,12
Distribution occurs by peristaltic movement of peritoneal fluid and by gravity. These steps are under the influence of multiple molecules including various growth factors, cytokines and chemokines. 10
On the left side, cephalad flow of fluid in the paracolic gutter is impeded by the phrenicocolic ligament. 9 On the right side, fluid flows without impediment to the right upper quadrant and around the liver, before being absorbed by subdiaphragmatic lymphatics (Figure 2). This accounts for the propensity of pelvic tumours to spread to the perihepatic and right subdiaphragmatic area. Dependent peritoneal recesses are potential locations for fluid accumulation by gravity such as the superior mesocolon, inferior part of mesentery, ileocolic junction, pouch of Douglas and paracolic gutters (Figures 2–4).
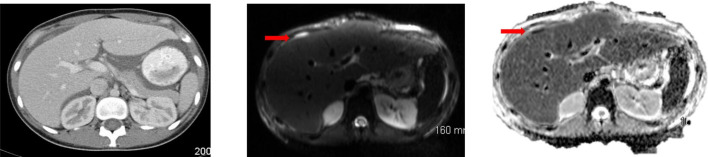
Figure 2.
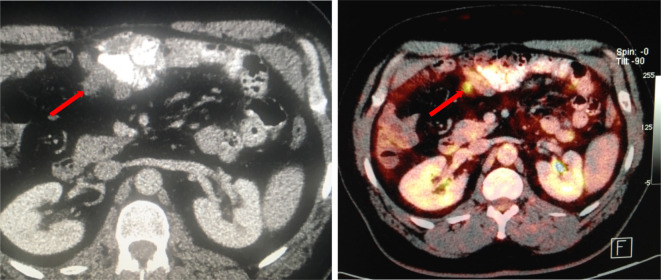
In a 21-year-old patient with underlying peritoneal mesothelioma, a subtle serosal deposit at the anterior surface of the liver is seen on CT. MRI-DWI helps increase conspicuity and confidence that this is indeed a metastatic deposit. DWI, diffusion-weighted imaging.
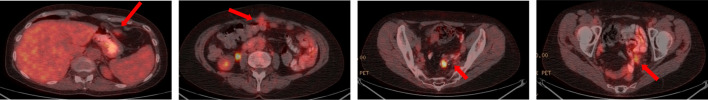
Figure 3.
A 54-year-old female with appendiceal Ca (pseudomyxoma). Here, we can see how ascitic fluid comes to lay dependently within the right and left paracolic gutters. We can also see how PET/CT can help in differentiating between reactive and malignant ascites by the presence of subtle peritoneal implants demonstrating increased metabolic activity on PET/CT. PET, positron emission tomography.
Figure 4.
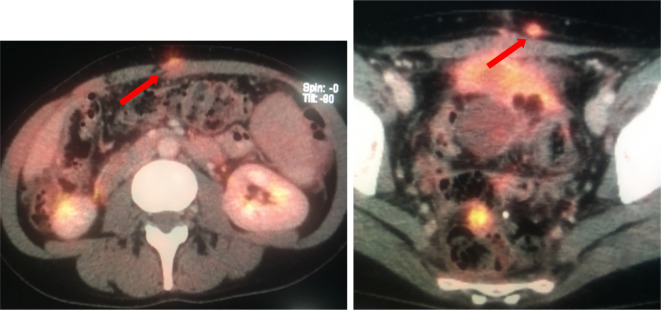
A 43-year-old female with peritoneal disease secondary to a moderately differentiated adenocarcinoma of colonic origin. PET/CT demonstrates metabolically active disease implants in unfavourable sites around the epigastrium, infiltrating the anterior abdominal wall and within the deep pelvis involving bowel loops. PET, positron emission tomography.
Lymphatic spread
Disease may spread along the extensive lymphatic system of the greater omentum and via extensive right subdiaphragmatic lymphatics, into the anterior mediastinal lymphatic chains and on to the superior vena cava. Aggregates of lymphoid tissues on the peritoneal surface are recognisable surgically as ‘milky spots’ and contain numerous lymphatic orifices through which macrophages migrate to the peritoneal cavity. 13 These orifices, or stomata, are the gateways from the peritoneal surface to underlying lymphatic capillary networks through which cancer cells can pass relatively easily. 14 Stomata are primarily located in the greater omentum, diaphragm, small bowel mesentery, pelvic peritoneum and falciform ligament. This pattern of spread is seen in a small percentage of peritoneal malignancy (PM) and is seen in lymphomas especially non-Hodgkin’s lymphoma. 15
Direct invasion
Malignancy may spread by direct contiguous invasion into and along the leaves of the mesentery, along vessels and into adjacent ligaments (Figure 5). This pattern of spread has been described in pancreatic, gastric, biliary, colonic, hepatic, splenic and ovarian cancers. 15 Small bowel carcinoid has a predilection for infiltrating the small bowel mesentery. Invasion is also promoted by the secretion of various proteinases and growth factors by the cancer cells. 10
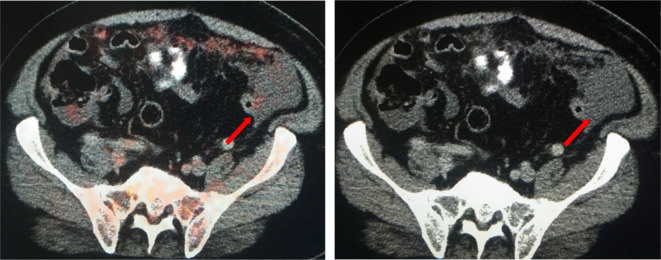
Figure 5.
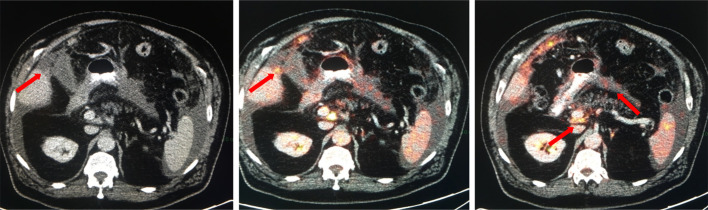
A 73-year-old male with diagnosis of atypical mesothelioma with peritoneal disease and mesenteric disease. While solid omental disease may be easily detected at CT and more conspicuous at PET/CT, small volume mesenteric deposits can be difficult to detect (arrows) on CT. FDG uptake within these small deposits on PET/CT increases our confidence that these represent metastatic implants. FDG, fludeoxyglucose; PET, positron emission tomography
Haematogenous spread
This occurs when high-grade tumours invade into blood vessels and are delivered to, and implant on, the peritoneal surface aided by the secretion of vascular permeability factors. These deposits implant on the antimesenteric border of the bowel. 16 PM arising from breast cancer, lung cancer and melanoma spread in this manner. 17
Definitions
As the term ‘peritoneal carcinomatosis’ only refers to one cell lineage (epithelial), PM has broadly replaced its use in the literature. PPM encompasses three distinct cell lineages and thus diseases. These are epithelial (carcinomatosis), lymphoid (lymphomatosis) and mesenchymal (sarcomatosis). The vast majority of PM is epithelial.
Patient selection for cytoreductive surgery
Patient selection for surgery is a multidisciplinary effort and involves clinical assessment, histopathological evaluation and calculation of the Peritoneal Cancer Index (PCI) using imaging. Patients are assessed clinically using the Eastern Cooperative Oncology Group scoring system. An ECOG score of <2 is required for a patient to be considered suitable for treatment with CRS and HIPEC/EPIC. Histopathological evaluation of the disease is also required prior to selection for treatment. The histology of the peritoneal disease is an important determinant of outcome. Patients with pseudomyxoma peritonei (PMP), carefully selected patients with colorectal cancer peritoneal metastases, and patients with advanced epithelial ovarian cancers have been associated with good outcomes, 6,18,19 whereas metastatic gastric cancer and malignant mesotheliomas are associated with a worse prognosis. 20 The most common non-gynaecologic cases undergoing CRS are PMP and metastatic appendiceal cancer, followed by other sites of colorectal carcinoma and, more rarely, primary malignant mesothelioma.
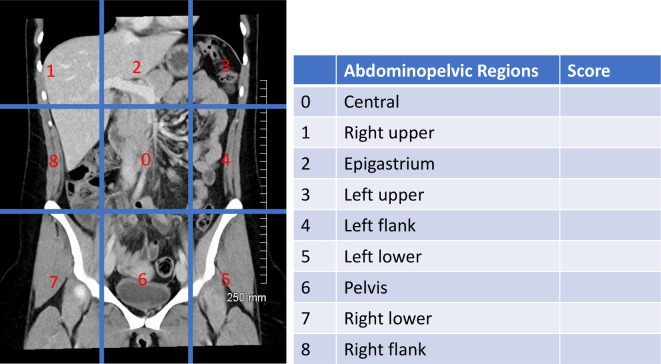
The PCI was originally a surgical method of quantifying peritoneal disease in the peritoneal cavity. 21 This is now determined pre-operatively by imaging. The PCI is intended to be an objective score of disease burden and distribution which aids in patient selection and estimation of prognosis. CT, MRI and PET/CT can all used to determine disease burden. 22–38 The radiologic PCI is calculated by scoring each of 13 abdominal locations 1–3 depending on size of largest deposit (<5 mm, 5 mm–5cm; >5 cm) giving a score out of a maximum of 39. There are nine equal anatomic divisions of the peritoneal cavity and four additional sites of small bowel and mesentery to be evaluated (Figure 6). The final PCI is determined at the time of laparotomy for intended CRS. Despite a favourable radiologic PCI, some patients may be found to be unsuitable candidates at the time of laparotomy.
Figure 6.
PCI Score. The score from each region is added to give a total score. The central region is subdivided into regions 9–12; the upper jejenum, lower jenenum, upper ileum, lower ileum. Reproduced with permission from Fehniger et al. 25
Types of cytoreductive surgery
CRS usually requires both peritonectomy and visceral resection. 39
The surgical goal is to remove all macroscopic disease (2.5 mm or greater), with HIPEC used to tackle microscopic disease. Removal of adjacent viscera may be required. The type of surgery required is determined by the site of disease. Various different peritonectomy procedures are listed in Table 1. 40
Table 1.
Types of peritonectomy procedures and resections (reproduced with permission from Sugarbaker et al 40 )
| Peritonectomy procedures | Resections |
|---|---|
| Anterior parietal peritonectomy | Old abdominal incisions, umbilicus, epigastric fat pad |
| Left upper quadrant peritonectomy | Greater omentum and spleen |
| Right upper quadrant peritonectomy | Glissons capsule deposits |
| Pelvic peritonectomy | Uterus, ovaries and rectosigmoid colon |
| Omental bursectomy | Gallbladder fossa and lesser omentum |
Radiologic findings in peritoneal malignancy (PM)
The abnormalities which may be found in PM have previously been extensively described including presence of ascites, omental disease, mesenteric disease and serosal involvement of bowel, liver or other organs. Ascites occurs secondary to blocked lymphatics or secondary to excess fluid production secondary to increased capillary permeability 41,42 and is associated with a poorer prognosis. 43 Involvement of the omentum ranges from tiny nodules that are too small to detect on CT to larger deposits and eventually large masses known as omental caking. Mesenteric invasion may be deduced by anomalous fixation of loops of small bowel, increased mesenteric density on CT and the presence of a stellate mass or nodules. Serosal implants may be nodular or plaque-like with diffuse thickening which demonstrates enhancement greater than that of liver, especially on delayed fat-saturated MRI imaging. Common findings in peritoneal lymphomatosis include homogenous masses causing bulky omental caking with this homogenous and smooth thickening diffusely infiltrating the peritoneum and mesentery. 44 Features suggesting peritoneal sarcomatosis include deforming deposits, that are often vascular, and the variable presence of ascites. 45 A knowledge of these potential differential diagnoses is important, particularly in the case of peritoneal lymphomatosis, as it is treated non-surgically. In the case of sarcomatous involvement, knowledge of the underlying classification of abdominal sarcomatous disease is also important, as only some show metastatic involvement of the peritoneum. 46
Radiological and hybrid imaging
CT remains the mainstay of pre-operative evaluation and staging of patients with peritoneal malignancy. A contrast-enhanced multidetector CT with reformatting in sagittal and coronal planes is a reliable, reproducible, fast, available and relatively inexpensive examination available in every modern radiology department. However, its limitations must be acknowledged. CT frequently underestimates PCI. A multicentre study showed that it underestimated disease in 33% of patients. 47 CT has been found to have a sensitivity of only 11% for nodules under 5 mm 48 and has particular difficulty in detecting lesions on the small bowel. 28 In patients with little intraabdominal fat, and in the absence of ascites, it can be particularly difficult on CT to identify subtle serosal lesions.
However, a more recent prospective study of 80 patients by Ahmed et al found that CT and laparoscopy were equally effective in pre-operative peritoneal malignancy categorisation and that a PCI of <20 on CT was accurate in the prediction of optimal cytoreduction. 49
PET/CT exploits the increased glucose metabolism of tumours to improve tumour detection. PET/CT has been shown to be a more accurate imaging modality than CT or PET alone in estimating the extent of peritoneal disease. 30,50 Figures 2, 3 and 7 demonstrate the increased conspicuity of peritoneal disease with PET/CT vs CT alone. In a recent systematic review and meta-analysis looking at the diagnostic accuracy of PET/CT in detecting peritoneal malignancy, PET/CT showed overall pooled sensitivity of 0.87 [95% CI (0.77–0.93)] and pooled specificity of 0.92 [95% CI; (0.89–0.94)] (14 studies, n – 671 pts). 34
Figure 7.
A 41-year-old female with diagnosis metastatic colorectal cancer. Small volume isolated abdominal wall disease implants may be difficult to identify on CT due to post-surgical changes but is well seen on PET/CT due to it’s increased metabolic activity. Although this extra-peritoneal disease makes CRS more difficult, it may be amenable to resection with the help of plastic surgeons depending on small lesion size and disease location. CRS, cytoreductive surgery; PET, positron emission tomography.
PET/CT offers the ability to detect even very small lesions, but only if the disease is metabolically active (Figures 5 and 8). In general, low-grade tumours are associated with low-grade metabolic activity and are, therefore, more difficult to detect. In addition, PET/CT actually may help predict tumour grade in patients with PMP, with high-grade PMP lesions demonstrating significantly higher SUVMax values compared with low-grade lesions. 33 Furthermore, the authors concluded that PET/CT also demonstrated the ability to predict disease-free survival, with significantly lower disease-free survival in patient with lesions demonstrating more avid FDG uptake (higher histologic grade) using an SUVMax cut-off of 2.7.
Figure 8.
A 54-year-old gentleman with a history of prior hemicolectomy for colorectal cancer re-presenting for consideration for CRS and HIPEC. CT demonstrates a small implant involving the small bowel and mesentery. Given similar density to the adjacent small bowel, this implant could easily have been missed. Due to its increased metabolic activity, it is readily identified on PET/CT. In general, when present, disease involving the small bowel is usually more diffuse than visualised on imaging and would preclude surgery. However, focal small bowel disease such as in the case may be amenable to advanced surgical treatment with CRS and HIPEC. CRS, cytoreductive surgery; HIPEC, heated intraperitoneal chemotherapy; PET, positron emission tomography.
In many of the reported PET/CT studies, i.v. contrast was not administered. 29,30,51 One group who reported 97% sensitivity for PET/CT (for disease detection) used i.v. contrast. 32
PET/CT has the added advantage of detecting previously unrecognised metastatic disease outside the abdomen which may influence patient selection for HIPEC. PET/CT can be useful in the setting of ascites. PET/CT can be used to assess for subtle peritoneal disease, which can aid differentiation between a reactive and malignant aetiology (Figure 3). Abdominal wall involvement can be a relative contraindication to CRS. Disease is often seen at port/drain sites or scars. Small volume isolated abdominal wall disease may be difficult to identify on CT due to post-surgical changes but is well seen on PET/CT due to increased metabolic activity (Figure 7). PET/CT is useful in the setting of prior surgery. In the post-op patient, post-operative anatomy and scarring can limit interpretation on CT. PET/CT can show FDG-avid soft tissue thickening that would be missed on CT (Figure 9).
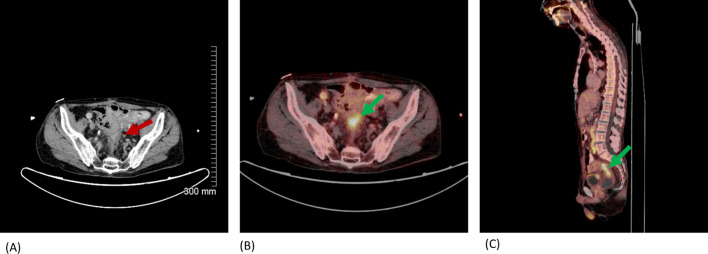
Figure 9.
Axial CT abdomen with i.v. + PO contrast (A) & fused axial (B) and sagittal (C) PET/CT. A 57-year-old male with history of rectal cancer with Hartmann’s resection, CRS and HIPEC. Increasing soft tissue thickening is seen at pelvic stump on CT (red arrow). FDG avid soft tissue on PET/CT with mild luminal narrowing (green arrows). Rectal stump & serosal recurrence confirmed on histology. CRS, cytoreductive surgery; HIPEC, heated intraperitoneal chemotherapy; FDG, fludeoxyglucose; PET, positron emission tomography.
MRI is significantly improved with both diffusion-weighted imaging (DWI) and delayed post-contrast imaging 22,23 (Figures 2 and 10). In a study of 34 patients performed by Low et al, 38 they found a sensitivity of 88%, specificity 74% with a diagnostic accuracy 84% for peritoneal disease. In 2015, in a study of 22 patients, Low et al found that MRI correctly predicted PCI score in 91%. 22
Figure 10.
A 21-year-old female with extensive peritoneal malignancy secondary to peritoneal mesothelioma. MRI-DWI images (B) show diffuse subtle deposits on the pelvic peritoneal reflections which are much more conspicuous than on the corresponding contrast enhanced CT (A). DWI, diffusion-weighted imaging.
Peritoneal enhancement greater than liver is abnormal. This is easier to see on MRI than CT. The high contrast conspicuity of fat-suppressed T 1W delayed gadolinium-enhanced MRI can depict lesions less than 1 cm and also detect lesions in areas which are anatomically difficult on CT (subphrenic, mesenteric and bowel serosa). 22 Furthermore, MRI-DWI has been shown to correlate well with surgical findings in patients with PM due to colorectal and ovarian peritoneal malignancy. 35,36 The presence of disease is characterised by intense high signal on DWI and low signal on the ADC map (Figures 2 and 10). DWI images are most reliably interpreted when cross-referenced with conventional MRI images. Accurate MRI interpretation correlates with reader experience level but the reliability of an inexperienced reader can be greatly improved with a 6 month period of training. 52 Whereas the protocol for CT in most institutions is standard, the MRI techniques described in the literature are quite variable. Some studies did not use gadolinium contrast for MRI 28 and earlier studies did not evaluate DWI. Low et al have published extensively on MRI in PM and describe a detailed imaging protocol. 23 The results of this group report higher sensitivity and specificity for MRI than any other published studies. The added benefit of DWI has been established. 22,28 Delayed MR contrast imaging has also been shown to aid detection or peritoneal infiltration. 24
Comparative studies
Several studies have attempted to compare the accuracy of different imaging techniques for predicting PCI (Table 2). This is a very difficult task and individual studies are extremely varied in design and aims. Some studies evaluated only the presence or absence of disease, 28,32 some studies evaluated the ability of imaging to classify patients into broad groups based on degree of disease burden (PCI <20 or>20) 52 and other studies evaluated accuracy in great detail by quantifying disease in each of the 13 possible sites. 23,30
Table 2.
Studies comparing the relative efficacy of CT, PET/CT and MRI in the diagnosis of peritoneal carcinomatosis
| Study | Type | No. patients | Study design | CT | MRI-DWI | PET/CT sensitivity | Study observations | Study limitations |
|---|---|---|---|---|---|---|---|---|
| Low 2012 38 | Retrospective | 34 | Evaluated ability of MRI to detect individual lesions | Sensitivity 87% (62% without DWI) | ||||
| Low 2015 23 | Retrospective | 22 | Compared ability of CT and MRI to categorise tumour burden into low, moderate or high | Per site sensitivity 55% | Per site sensitivity 95% | MRI significantly more accurate than CT Results are much better for MRI than other published studies |
||
| Soussan 2012 30 | Retrospective | 30 | Evaluated ability of PET/CT and MRI-DWI to detect disease in one of three sites | 84% per patient sensitivity 74% per site sensitivity |
84% per patient sensitivity 63% per site sensitivity |
MRI-DWI more sensitive than PET/CT for perihepatic lesions | Some patients did not have surgical confirmation of disease | |
| Fehniger 2016 25 | Prospective | 27 | Evaluated ability of CT and MRI-DWI to detect disease in 12 sites | 25–95% average 64% | 0–94.1% average 48% | CT and MRI-DWI equivalent for most sites MRI-DWI superior for detection of diaphragmatic disease |
||
| Pasqual 2014 27 | Retrospective | 47 | Compared CT and PET/CT to detect disease in 13 sites | 69% | 71% | No significant difference between CT and PET/CT: both had low accuracy especially for small bowel involvement | Unclear if a radiologist was involved in study | |
| Torkzad 2015 52 | Prospective | 39 | Evaluated ability of CT and MRI to categorise tumour burden into two groups – low and high | Experienced reader: Per patient k = 1 Per site k = .8 Inexperienced reader: Per patient k = .73 Per site k = .6 With training k = .7 |
Experienced reader: Per patient k = 1 Per site k = .77 Inexperienced reader: Per patient k = .58 Per site k = .39 With training k = .9 |
CT and MRI were equivalent when read by an experienced radiologist CT had better accuracy for the inexperienced radiologist Training and experience improved accuracy of MR interpretation |
||
| Dirisamer 2008 32 | Retrospective | 62 | Evaluated ability of CT and PET/CT to detect disease | 83% sensitivity | PET/CT 97% sensitivity (PET 81%) |
PET/CT better than CT alone or PET alone | Did not evaluate the ability to accurately quantify disease | |
| Satoh 2011 28 | Retrospective | 237 | Evaluated ability of CT, MRI-DWI and PET/CT to detect disease | 76% sensitivity | 84% sensitivity (56% without DWI) | 94% sensitivity | DWI better than MRI alone Of CT, MRI-DWI and PET/CT, PET/CT most useful, followed by MRI-DWI |
Did not evaluate ability to accurately quantify disease Contrast not used in MRI |
| Dohan 2017 53 | Retrospective | 28 | Evaluated CT alone and CT + MRI DWI in detection of disease burden | Average sensitivity 53% | Average sensitivity = 81% when read with CT | CT and MRI significantly increased sensitivity compared to CT alone | ||
| Laghi 37 2016 | Systematic Review and Meta-Analysis | 22 articles, 934 patients | Pooled analysis comparing CT and MRI in detection of disease | Sensitivity 83%, specificity 86% | Sensitivity 86%, specificity 88% | Sensitivity 82%, specificity 93% | One of only meta-analyses and systematic reviews. Shows comparable results across three modalities. | Low numbers of available studies limits volume of evidence-based information relating to MRI |
| Kim 34 | Systematic Review and Meta-Analysis | 14 studies, 671 patients | Pooled analysis looking at diagnostic accuracy of PET/CT for detection of peritoneal carcinomatosis | Sensitivity 87%, specificity 92% | Largest meta-analysis looking at PET/CT | Larger data sets needed to examine PET/CT diagnostic accuracy in different cancers | ||
| Engbersen 2019 36 | Prospective | 25 patients | Diagnostic accuracy of MRI-DWI vs surgical PCI | intraclass correlation coefficient (ICC) of 0.86 | MRI-PCI equally accurate to surgical inspection (S-PCI) to select patients in which a complete resection could be achieved. | Only looked at patients with ovarian cancer | ||
| Abdalla Ahmed 49 2019 | Prospective | 80 | Diagnostic accuracy of CT vs surgical PCI | Overall agreement of CT in peritoneal carcinomatosis categorisation was good (K = 0.70–0.80) | Both laparoscopy and CT are equally effective in preoperative peritoneal carcinomatosis categorisation | Only looked at patients with ovarian cancer | ||
| Sant Van’t 35 2018 | Prospective | 56 | DWI-MRI vs surgical PCI | intraclass correlation coefficient (ICC) of 0.83 and 0.88 for two readers | MRI-PCI had a stronger correlation with surgical PCI (ICC 0⋅83–0⋅88) than did CT-PCI (ICC 0⋅39–0⋅44). | Only looks at colorectal cancer | ||
| Sant Van’t 54 2020 | Meta-analysis | 24 studies, 2302 patients | Pooled analysis comparing CT, MRI and PET/CT diagnostic accuracy | Sensitivity 0.68, specificity 0.88 | Sensitivity 0.91, specificity 0.85 | Sensitivity 0.79, specificity 0.90 | DWI-MRI shows highest sensitivity for detection of PC from GI or ovarian origin | Study heterogeneity in MR – different protocols, use of contrast and reader professions |
DWI, diffusion-weighted imaging; GI, gastrointestinal; PC, prostate cancer; PCI, Peritoneal Cancer Index; PET, positron emission tomography.
A 2016 meta-analysis of 22 studies whose primary end point was to assess diagnostic accuracy of CT and MRI in detecting PC concluded that CT should be the preferred diagnostic imaging modality because of the robustness of the data. 37 Low’s group (who describe a very rigorous MRI technique) have reported very high accuracy for MRI, 23 quoting a 95% sensitivity for detection of disease on a per site basis. They found that contrast MRI-DWI was significantly superior to CT. Other studies have also emphasised the superiority of MRI-DWI over both CT and PET/CT in detecting disease in the right supramesocolic region, in particular perihepatic and subdiaphragmatic disease. 30,55 Overall, CT combined with MRI have been shown to be more accurate than CT alone. 53
We have found only two studies which compared all three imaging modalities. Michielsen et al concluded that MRI-DWI was superior to both PET/CT and contrast-enhanced CT for detection of mesenteric and serosal deposits for characterisation of primary tumour in a small study of 32 patients with ovarian carcinoma. 55 In one of the largest studies of imaging in PM (237 patients), Satoh et al concluded that PET/CT was most accurate followed by MRI-DWI. 28 A limitation of Satoh’s study was that PET/CT and CT/MRI were performed in two different patient populations and so were not compared directly. Furthermore, patients undergoing PET/CT did not receive i.v. contrast. Van’t Sant et al has attempted to compare the three modalities by performing a meta-analysis which included 24 articles with 37 data sets, but did not include Satoh’s study. 56 Van’t Sant’s group concluded that (DW)MRI and PET/CT were comparable in terms of detection of PM for ovarian and gastrointestinal tumours and further suggested that since it is more widely available that MRI, PET/CT should be the imaging modality of choice in staging these patients. Their findings, with the pooled sensitivities and specificities of the three modalities, can be seen in the table below.
Ultrasound is often the first line imaging investigation in suspected gynaecological malignancy. For this reason, it is important to be familiar with the appearances of peritoneal disease on ultrasound. Important areas to assess on ultrasound include the pouch of Douglas, diaphragmatic surfaces, the paracolic gutters, and the regions of the mesentery and omentum. Suggested ultrasound assessment of the peritoneum involves initial assessment of the entire abdomen and pelvic cavity using a standard-frequency transducer (3.5–5 MHz) followed by use of a higher frequency probe to more closely interrogate lesions. 57 Lesions of the parietal peritoneum will not move with gravity or breathing manoeuvres, whereas lesions of the visceral peritoneum, mesentery, or omentum usually will. 57
There currently is not enough evidence, looking at the sensitivity and specificity of ultrasound in diagnosing peritoneal malignancy, however, De Blasis et al reviewed three prospective studies focusing on the specific role of ultrasound and found high sensitivity (range 81.4–91%) and specificity (range 88–96%) in the diagnosis of peritoneal carcinomatosis, as well as in omental involvement (sensitivity 67–94%, specificity 90%). 58
It should be stated, that overall, there is a paucity of evidence for the use of ultrasound as a primary imaging modality in the diagnosis of peritoneal malignancy and that it is not routinely used in clinical practice.
18F-FDG PET/MRI has been gradually transitioning from a research-based imaging modality to increasing clinical use in sites across the world. 59 It offers better T-categorisation for primary bone, head and neck, and soft-tissue tumours and a higher accuracy for metastatic lesion detection in the brain, liver, and bone when compared to PET/CT. 60–62 PET/CT, however, offers better delineation of margins within lung parenchyma.
In a 2021 study of 34 patients looking at 18F-FDG PET/MRI and diffusion-weighted MRI for estimating the extent of PM in ovarian and endometrial cancer, PET/MRI was found to be superior in estimating the spread of PM. 63 It was particularly advantageous in those with a high tumour burden, which could prove important in deciding about operability of a gynaecological cancer and save an unnecessary diagnostic laparotomy.
Despite encouraging research in the field, it’s use is still quite limited, primarily due to high costs and lack of availability.
68Ga-FAPI PET/CT (gallium-68 fibroblast-activation-protein inhibitor (FAPI)) is a novel PET tracer that shows exciting potential for the future. Fibroblast-activation-protein is overexpressed by cancer cells of various tumour histological subtypes. Kratochwil et al assessed tracer uptake in 28 different cancers and found high uptake and image contrast in many cancers, including common causes of peritoneal malignancy such as colorectal and ovarian cancer. 64 In the setting of ovarian cancer, lower intestinal/peritoneal uptake might offer better sensitivity in the detection of peritoneal deposits. Another study of 75 patients who underwent both 18F-FDG PET/CT and [68Ga]Ga-DOTA-FAPI-04 found a superior diagnostic efficacy with [68Ga]Ga-DOTA-FAPI-04 for diagnosis of primary tumours (Sensitivity 82.1vs 98.2%) and in the setting of peritoneal malignancy with higher rates of tracer uptake seen [standardised uptake value (SUV) median 2.14 vs 5.74]. 65
These studies show the great potential in [68Ga]Ga-DOTA-FAPI-04 in future PM imaging with its use likely to increase as more research is performed.
Unfavourable signs on imaging
Even if the burden of peritoneal disease is low, disease at a surgically critical site may add significant risk and or difficulty to surgery. Chandramohan et al 66 have proposed a modification of the radiologic PCI reporting system that gives weight to findings with U0 indicating no unfavourable sites of disease, U1 indicating involved sites that may provide a challenge to CRS and U2 indicating disease at sites that may preclude adequate cytoreductive surgery (Table 3).
Table 3.
Review areas for unfavourable sites of involvement (reproduced with permission from Chandramohan et al 66 )
| U1 sites | U2 sites |
|---|---|
|
|
U1 sites increases surgical complexity and may need, e.g. gastrectomy, Whipple’s procedure, nephrectomy, ureteric re-implantation, cystectomy, or prostatectomy.
U2 sites reduce the likelihood of complete cytoreduction.
U1 sites include epigastric disease, such as within the lesser omentum, the lesser sac and along the ligaments of the liver. Disease along the spleen, stomach encasement, or within a peripancreatic or paraduodenal distribution would also be classified as a U1 site (Figure 4). U1 sites in the retroperitoneum include hydronephrosis with ureteric involvement, or disease within the muscles of the retroperitoneum. Pelvic U1 sites include the bladder trigone, seminal vesicles, prostate or disease encasing the external iliac vessels.
Sites of disease that may preclude adequate cytoreductive surgery, or U2 sites, include biliary obstruction due to tumour, supracoeliac, periportal, epiphrenic or retroperitoneal nodal involvement (Figure 5 – FDG Avid portocaval lymph node). Mesenteric or small bowel disease at the root of the mesentery, the ligament of Treitz, at the DJ flexure or disease causing small bowel obstruction are further U2 sites. U2 disease within the pelvis includes side-wall or nodal disease, disease involving the sacrum, or frozen pelvis from previously treated rectal cancer (Figure 9).
Other important findings to report include ascites (Figure 3), abdominal wall involvement (Figure 7), small bowel and mesenteric involvement and extraperitoneal metastases. 67,68
While scoring systems, such as that proposed by Chandramohan, are useful, the critical point is that when these sites of disease are present, the radiologist must make this clear to the treating oncologists and surgeons as this impacts patient treatment options.
Limitations of imaging
The limitations of all imaging modalities available to us must be understood (Table 1). Although it remains the workhorse on oncologic imaging, CT can be poor at detecting plaque-like mesenteric involvement, and detects small bowel and mesenteric disease in <25%, 53 and appears to be the least sensitive and specific. Physiologic uptake in the stomach and liver, bowel and genitourinary tract may lead to difficulty interpreting these areas on PET/CT. The limited spatial resolution and the long acquisition time of the PET component of PET/CT may lead to blurring and underestimation of FDG uptake. Lesions under 5 mm are below the threshold for detection on PET. Lesions which do not take up FDG, or are not sufficiently metabolically active, such as mucinous colorectal and ovarian lesions may not be visible on PET. The added radiation dose of PET/CT is quoted as a potential disadvantage, although in this patient group this is not a serious consideration if the test is useful. MRI-DWI has been shown to be less accurate at diagnosing abnormal lymph nodes than PET/CT as these will have high signal on DWI images regardless of biologic behaviour. 28,69 Susceptibility artefact from gas in the GI tract may lead to false positives on MRI-DWI. Bowel movement occurring over the prolonged examination time, despite administration of a smooth muscle relaxant, can cause image blurring. The quality of MR images is highly protocol- and patient-dependent. The technique described by Low et al, who have published very good results for MRI-DWI includes oral and rectal contrast, i.v. Hyoscine Sulphate, and double dose gadolinium with immediate and delayed (5 min) imaging, as well as portal venous phase imaging in tumours which metastasize to liver parenchyma. 21 This is a rigorous protocol which is not carried out in most centres.
A particular area of difficulty is detection of inframesocolic disease. Soussan et al reported less than 50% sensitivity for small (<1 cm) implants in the inframesocolic region for both PET/CT and MRI-DWI. 29 They found that sensitivity depended on lesion size for both modalities. MRI-DWI was more sensitive than any other modality for disease around the liver with the low signal of the liver contrasting against the high signal of disease on DWI (Figure 2); however, it has been found that in areas such as the spleen, the combination of CT and MRI did not significantly improve the detection of disease, when compared to CT alone. 70 This is due to the fact that the spleen has intrinsically high signal on DWI. Furthermore, physiologic uptake by the liver may hamper interpretation of serosal disease.
Conclusion
The most important thing for the radiologist to recognise is the importance of finding disease in unfavourable locations that would make a patient unlikely to benefit from the onerous undertaking of CRS and HIPEC. In most cases, a good quality CT scan interpreted by an experienced radiologist will identify such findings, if present. It seems reasonable that in the relatively small percentage of patient who ‘qualify’ for CRS and HIPEC, that they would undergo additional imaging with either MRI and/or PET-CT depending on tumour biology and location of disease (Table 4 4). MRI with delayed contrast and DWI is sensitive for peritoneal deposits and liver surface disease, while PET/CT is useful for high-grade tumours and detecting unsuspected metastatic disease.
Table 4.
Advantages and disadvantages of CT, PET/CT and MRI in assessment of peritoneal malignancy
| Modality | Advantages | Disadvantages |
|---|---|---|
| CT |
|
|
| PET/CT |
|
|
| MRI |
|
|
DWI, diffusion-weighted imaging; FDG, fludeoxyglucose; GU, genitourinary; PET, positron emission tomography.
Contributor Information
Jack W Power, Email: jackwpower1@gmail.com.
Philip J Dempsey, Email: philipjdempsey@gmail.com.
Andrew Yates, Email: andrewyates@mater.ie.
Helen Fenlon, Email: hfenlon@mater.ie.
Jurgen Mulsow, Email: jmulsow@mater.ie.
Conor Shields, Email: cshields@mater.ie.
Carmel G Cronin, Email: ccronin@mater.ie.
REFERENCES
- 1. Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000; 88: 358–63. doi: [DOI] [PubMed] [Google Scholar]
- 2. Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003; 21: 3737–43. doi: 10.1200/JCO.2003.04.187 [DOI] [PubMed] [Google Scholar]
- 3. Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008; 15: 2426–32. doi: 10.1245/s10434-008-9966-2 [DOI] [PubMed] [Google Scholar]
- 4. Esquivel J, Elias D, Baratti D, Kusamura S, Deraco M. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol 2008; 98: 263–7. doi: 10.1002/jso.21053 [DOI] [PubMed] [Google Scholar]
- 5. Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol 2011; 104: 692–8. doi: 10.1002/jso.22017 [DOI] [PubMed] [Google Scholar]
- 6. Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol 2006; 24: 4011–9. doi: 10.1200/JCO.2006.07.1142 [DOI] [PubMed] [Google Scholar]
- 7. Hesdorffer ME, Chabot JA, Keohan ML, Fountain K, Talbot S, Gabay M, et al. Combined resection, intraperitoneal chemotherapy, and whole abdominal radiation for the treatment of malignant peritoneal mesothelioma. Am J Clin Oncol 2008; 31: 49–54. doi: 10.1097/COC.0b013e3180684181 [DOI] [PubMed] [Google Scholar]
- 8. Coffey JC, Walsh D, Byrnes KG, Hohenberger W, Heald RJ. Mesentery — a ‘New’ organ. Emerging Topics in Life Sciences 2020; 4: 191–206. [DOI] [PubMed] [Google Scholar]
- 9. Kastelein AW, Vos LMC, de Jong KH, van Baal JOAM, Nieuwland R, van Noorden CJF, et al. Embryology, anatomy, physiology and pathophysiology of the peritoneum and the peritoneal vasculature. Semin Cell Dev Biol 2019; 92: 27–36. doi: 10.1016/j.semcdb.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 10. Gore RM, Newmark GM, Gore MD. Chapter 112 - Ascites and Peritoneal Fluid Collections. In: Gore R. M, Levine M. S, eds. Textbook of Gastrointestinal Radiology. (Third Edition). Philadelphia: W.B. Saunders; 2008. pp. 2119–33. [Google Scholar]
- 11. Sugarbaker PH. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg 1994; 219: 109–11. doi: 10.1097/00000658-199402000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010; 177: 1053–64. doi: 10.2353/ajpath.2010.100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beelen RH, Fluitsma DM, Hoefsmit EC. The cellular composition of omentum milky spots and the ultrastructure of milky spot macrophages and reticulum cells. J Reticuloendothel Soc 1980; 28: 585–99. [PubMed] [Google Scholar]
- 14. Shimotsuma M, Shields JW, Simpson-Morgan MW, Sakuyama A, Shirasu M, Hagiwara A, et al. Morpho-physiological function and role of omental milky spots as omentum-associated lymphoid tissue (OALT) in the peritoneal cavity. Lymphology 1993; 26: 90–101. [PubMed] [Google Scholar]
- 15. Karaosmanoglu D, Karcaaltincaba M, Oguz B, Akata D, Ozmen M, Akhan O. CT findings of lymphoma with peritoneal, omental and mesenteric involvement: peritoneal lymphomatosis. Eur J Radiol 2009; 71: 313–7. doi: 10.1016/j.ejrad.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 16. Shaw MS, Healy JC, Reznek RH. Imaging the peritoneum for malignant processes. Imaging 2000; 12: 21–33. [Google Scholar]
- 17. Le O. Patterns of peritoneal spread of tumor in the abdomen and pelvis. World J Radiol 2013; 5: 106–12. doi: 10.4329/wjr.v5.i3.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012; 30: 2449–56. doi: 10.1200/JCO.2011.39.7166 [DOI] [PubMed] [Google Scholar]
- 19. Van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. New England Journal of Medicine 2018; 378: 230–40. [DOI] [PubMed] [Google Scholar]
- 20. Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009; 27: 6237–42. doi: 10.1200/JCO.2009.23.9640 [DOI] [PubMed] [Google Scholar]
- 21. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996; 82: 359–74. doi: 10.1007/978-1-4613-1247-5_23 [DOI] [PubMed] [Google Scholar]
- 22. Low RN, Sebrechts CP, Barone RM, Muller W. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings--a feasibility study. AJR Am J Roentgenol 2009; 193: 461–70. doi: 10.2214/AJR.08.1753 [DOI] [PubMed] [Google Scholar]
- 23. Low RN, Barone RM, Lucero J. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol 2015; 22: 1708–15. doi: 10.1245/s10434-014-4041-7 [DOI] [PubMed] [Google Scholar]
- 24. Low RN. MR imaging of the peritoneal spread of malignancy. Abdom Imaging 2007; 32: 267–83. doi: 10.1007/s00261-007-9210-8 [DOI] [PubMed] [Google Scholar]
- 25. Fehniger J, Thomas S, Lengyel E, Liao C, Tenney M, Oto A. A prospective study evaluating diffusion weighted magnetic resonance imaging (DW-MRI) in the detection of peritoneal carcinomatosis in suspected gynaecologic malignancies. GynecolOncol 2016; 142: 169–75. [DOI] [PubMed] [Google Scholar]
- 26. Fujii S, Matsusue E, Kanasaki Y, Kanamori Y, Nakanishi J, Sugihara S, et al. Detection of peritoneal dissemination in gynecological malignancy: evaluation by diffusion-weighted MR imaging. Eur Radiol 2008; 18: 18–23. doi: 10.1007/s00330-007-0732-9 [DOI] [PubMed] [Google Scholar]
- 27. Pasqual EM, Bertozzi S, Bacchetti S, Londero AP, Basso SMM, Santeufemia DA, et al. Preoperative assessment of peritoneal carcinomatosis in patients undergoing hyperthermic intraperitoneal chemotherapy following cytoreductive surgery. Anticancer Res 2014; 34: 2363–8. [PubMed] [Google Scholar]
- 28. Satoh Y, Ichikawa T, Motosugi U, Kimura K, Sou H, Sano K, et al. Diagnosis of peritoneal dissemination: comparison of 18F-FDG PET/CT, diffusion-weighted MRI, and contrast-enhanced MDCT. AJR Am J Roentgenol 2011; 196: 447–53. doi: 10.2214/AJR.10.4687 [DOI] [PubMed] [Google Scholar]
- 29. Patel CM, Sahdev A, Reznek RH. CT, MRI and PET imaging in peritoneal malignancy. Cancer Imaging 2011; 11: 123–39. doi: 10.1102/1470-7330.2011.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soussan M, Des Guetz G, Barrau V, Aflalo-Hazan V, Pop G, Mehanna Z, et al. Comparison of FDG-PET/CT and MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal malignancy. Eur Radiol 2012; 22: 1479–87. doi: 10.1007/s00330-012-2397-2 [DOI] [PubMed] [Google Scholar]
- 31. Li J, Yan R, Lei J, Jiang C. Comparison of PET with PET/CT in detecting peritoneal carcinomatosis: a meta-analysis. Abdom Imaging 2015; 40: 2660–6. doi: 10.1007/s00261-015-0418-8 [DOI] [PubMed] [Google Scholar]
- 32. Dirisamer A, Schima W, Heinisch M, Weber M, Lehner HP, Haller J, et al. Detection of histologically proven peritoneal carcinomatosis with fused 18F-FDG-PET/MDCT. Eur J Radiol 2009; 69: 536–41. doi: 10.1016/j.ejrad.2007.11.032 [DOI] [PubMed] [Google Scholar]
- 33. Hotta M, Minamimoto R, Gohda Y, Igari T, Yano H. Impact of a modified peritoneal cancer index using FDG-PET/CT (PET-PCI) in predicting tumor grade and progression-free survival in patients with pseudomyxoma peritonei. Eur Radiol 2019; 29: 5709–16. doi: 10.1007/s00330-019-06102-1 [DOI] [PubMed] [Google Scholar]
- 34. Kim S-J, Lee S-W. Diagnostic accuracy of 18F-FDG PET/CT for detection of peritoneal carcinomatosis; a systematic review and meta-analysis. Br J Radiol 2018; 91: 20170519. doi: 10.1259/bjr.20170519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sant Van 't I, van Eden WJ, Engbersen MP, NFM K, Woensdregt K, Lambregts DMJ. Diffusion-weighted MRI assessment of the peritoneal cancer index before cytoreductive surgery. BJS 2019; 106: 491–8. [DOI] [PubMed] [Google Scholar]
- 36. Engbersen MP, Van' T Sant I, Lok C, Lambregts DMJ, Sonke GS, Beets-Tan RGH, et al. MRI with diffusion-weighted imaging to predict feasibility of complete cytoreduction with the peritoneal cancer index (PCI) in advanced stage ovarian cancer patients. Eur J Radiol 2019; 114: 146–51. doi: 10.1016/j.ejrad.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 37. Laghi A, Bellini D, Rengo M, Accarpio F, Caruso D, Biacchi D, et al. Diagnostic performance of computed tomography and magnetic resonance imaging for detecting peritoneal metastases: systematic review and meta-analysis. Radiol Med 2017; 122: 1–15. doi: 10.1007/s11547-016-0682-x [DOI] [PubMed] [Google Scholar]
- 38. Low RN, Barone RM. Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol 2012; 19: 1394–401. doi: 10.1245/s10434-012-2236-3 [DOI] [PubMed] [Google Scholar]
- 39. Mehta SS, Bhatt A, Glehen O. Cytoreductive surgery and Peritonectomy procedures. Indian J Surg Oncol 2016; 7: 139–51. doi: 10.1007/s13193-016-0505-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995; 221: 29–42. doi: 10.1097/00000658-199501000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saif MW, Siddiqui IAP, Sohail MA. Management of ascites due to gastrointestinal malignancy. Ann Saudi Med 2009; 29: 369–77. doi: 10.4103/0256-4947.55167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang DK, Kim JW, Kim BK, Lee KL, Song CS, Han JK, et al. Clinical significance of CT-defined minimal ascites in patients with gastric cancer. World J Gastroenterol 2005; 11: 6587–92. doi: 10.3748/wjg.v11.i42.6587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sangisetty SL, Miner TJ. Malignant ascites: a review of prognostic factors, pathophysiology and therapeutic measures. World J Gastrointest Surg 2012; 4: 87–95. doi: 10.4240/wjgs.v4.i4.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cabral FC, Krajewski KM, Kim KW, Ramaiya NH, Jagannathan JP. Peritoneal lymphomatosis: CT and PET/CT findings and how to differentiate between carcinomatosis and sarcomatosis. Cancer Imaging 2013; 13: 162–70. doi: 10.1102/1470-7330.2013.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oei TN, Jagannathan JP, Ramaiya N, Ros PR. Peritoneal Sarcomatosis versus peritoneal carcinomatosis: imaging findings at MDCT. AJR Am J Roentgenol 2010; 195: W229–35. doi: 10.2214/AJR.09.3907 [DOI] [PubMed] [Google Scholar]
- 46. Levy AD, Manning MA, Al-Refaie WB, Miettinen MM. Soft-tissue sarcomas of the abdomen and pelvis: Radiologic-Pathologic features, part 1-Common sarcomas: from the radiologic pathology archives. Radiographics 2017; 37: 462–83. doi: 10.1148/rg.2017160157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Esquivel J, Chua TC, Stojadinovic A, Melero JT, Levine EA, Gutman M, et al. Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol 2010; 102: 565–70. doi: 10.1002/jso.21601 [DOI] [PubMed] [Google Scholar]
- 48. Koh J-L, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 2009; 16: 327–33. doi: 10.1245/s10434-008-0234-2 [DOI] [PubMed] [Google Scholar]
- 49. Abdalla Ahmed S, Abou-Taleb H, Ali N, M Badary D. Accuracy of radiologic- laparoscopic peritoneal carcinomatosis categorization in the prediction of surgical outcome. Br J Radiol 2019; 92: 20190163. doi: 10.1259/bjr.20190163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pfannenberg C, Königsrainer I, Aschoff P, Oksüz MO, Zieker D, Beckert S, et al. (18)F-FDG-PET/CT to select patients with peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2009; 16: 1295–303. doi: 10.1245/s10434-009-0387-7 [DOI] [PubMed] [Google Scholar]
- 51. Rubini G, Altini C, Notaristefano A, Merenda N, Rubini D, Ianora AAS, et al. Role of 18F-FDG PET/CT in diagnosing peritoneal carcinomatosis in the restaging of patient with ovarian cancer as compared to contrast enhanced CT and tumor marker Ca-125. Rev Esp Med Nucl Imagen Mol 2014; 33: 22–7. doi: 10.1016/j.remn.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 52. Torkzad MR, Casta N, Bergman A, Ahlström H, Påhlman L, Mahteme H. Comparison between MRI and CT in prediction of peritoneal carcinomatosis index (PCI) in patients undergoing cytoreductive surgery in relation to the experience of the radiologist. J Surg Oncol 2015; 111: 746–51. doi: 10.1002/jso.23878 [DOI] [PubMed] [Google Scholar]
- 53. Dohan A, Hoeffel C, Soyer P, Jannot AS, Valette P-J, Thivolet A, et al. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br J Surg 2017; 104: 1244–9. doi: 10.1002/bjs.10527 [DOI] [PubMed] [Google Scholar]
- 54. van 't Sant I, Engbersen MP, Bhairosing PA, Lambregts DMJ, Beets-Tan RGH, van Driel WJ, et al. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis. Eur Radiol 2020; 30: 3101–12. doi: 10.1007/s00330-019-06524-x [DOI] [PubMed] [Google Scholar]
- 55. Michielsen K, Vergote I, Op de Beeck K, Amant F, Leunen K, Moerman P, et al. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: a clinical feasibility study in comparison to CT and FDG-PET/CT. Eur Radiol 2014; 24: 889–901. doi: 10.1007/s00330-013-3083-8 [DOI] [PubMed] [Google Scholar]
- 56. van 't Sant I, Engbersen MP, Bhairosing PA, Lambregts DMJ, Beets-Tan RGH, van Driel WJ, et al. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis. Eur Radiol 2020; 30: 3101–12. doi: 10.1007/s00330-019-06524-x [DOI] [PubMed] [Google Scholar]
- 57. Hanbidge AE, Lynch D, Wilson SR. US of the peritoneum. Radiographics 2003; 23: 663–85. doi: 10.1148/rg.233025712 [DOI] [PubMed] [Google Scholar]
- 58. De Blasis I, Moruzzi MC, Moro F, Mascilini F, Cianci S, Gueli Alletti S, et al. Role of ultrasound in advanced peritoneal malignancies. Minerva Med 2019; 110: 292–300. doi: 10.23736/S0026-4806.19.06103-2 [DOI] [PubMed] [Google Scholar]
- 59. Fendler WP, Czernin J, Herrmann K, Beyer T. Variations in PET/MRI operations: results from an international survey among 39 active sites. J Nucl Med 2016; 57: 2016–21. doi: 10.2967/jnumed.116.174169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Heacock L, Weissbrot J, Raad R, Campbell N, Friedman KP, Ponzo F, et al. PET/MRI for the evaluation of patients with lymphoma: initial observations. AJR Am J Roentgenol 2015; 204: 842–8. doi: 10.2214/AJR.14.13181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Fürst S, Martinez-Möller A, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med 2012; 53: 845–55. doi: 10.2967/jnumed.111.098608 [DOI] [PubMed] [Google Scholar]
- 62. Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G. Oncologic PET/MRI, part 2: bone tumors, soft-tissue tumors, melanoma, and lymphoma. J Nucl Med 2012; 53: 1244–52. doi: 10.2967/jnumed.112.109306 [DOI] [PubMed] [Google Scholar]
- 63. Jónsdóttir B, Ripoll MA, Bergman A, Silins I, Poromaa IS, Ahlström H, et al. Validation of 18F-FDG PET/MRI and diffusion-weighted MRI for estimating the extent of peritoneal carcinomatosis in ovarian and endometrial cancer -a pilot study. Cancer Imaging 2021; 21: 34. doi: 10.1186/s40644-021-00399-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med 2019; 60: 801–5. doi: 10.2967/jnumed.119.227967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging 2020; 47: 1820–32. doi: 10.1007/s00259-020-04769-z [DOI] [PubMed] [Google Scholar]
- 66. Chandramohan A, Thrower A, Smith SA, Shah N, Moran B. "PAUSE": a method for communicating radiological extent of peritoneal malignancy. Clin Radiol 2017; 72: 972–80. doi: 10.1016/j.crad.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 67. Chandramohan A, Thrower A, Shah N, Mohamed F. Radiological predictors of complete cytoreduction in 59 patients with peritoneal mesothelioma treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy at a UK referral centre. Br J Radiol 2017; 90: 20170361. doi: 10.1259/bjr.20170361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aherne EA, Fenlon HM, Shields CJ, Mulsow JJ, Cronin CG. What the radiologist should know about treatment of peritoneal malignancy. AJR Am J Roentgenol 2017; 208: 531–43. doi: 10.2214/AJR.16.16646 [DOI] [PubMed] [Google Scholar]
- 69. Ono K, Ochiai R, Yoshida T, Kitagawa M, Omagari J, Kobayashi H, et al. Comparison of diffusion-weighted MRI and 2-[fluorine-18]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for detecting primary colorectal cancer and regional lymph node metastases. J Magn Reson Imaging 2009; 29: 336–40. doi: 10.1002/jmri.21638 [DOI] [PubMed] [Google Scholar]
- 70. Berthelin M-A, Barral M, Eveno C, Rousset P, Dautry R, Pocard M, et al. Preoperative assessment of splenic involvement in patients with peritoneal carcinomatosis with CT and MR imaging. Eur J Radiol 2019; 110: 60–5. doi: 10.1016/j.ejrad.2018.11.022 [DOI] [PubMed] [Google Scholar]