Abstract
This commentary reviews the contribution of imaging by CT and MRI to functional assessment in chronic obstructive pulmonary disease (COPD). CT can help individualize the assessment of COPD by quantifying emphysema, air trapping and airway wall thickening, potentially leading to more specific treatments for these distinct components of COPD. Longitudinal changes in these metrics can help assess progression or improvement. On hyperpolarized gas MRI, the apparent diffusion coefficient of provides an index of airspace enlargement reflecting emphysema. Perfusion imaging and measurement of pulmonary vascular volume on non-contrast CT provide insight into the contribution of pulmonary vascular disease to pulmonary impairment. Functional imaging is particularly valuable in detecting early lung dysfunction in subjects with inhalational exposures.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disease of the airways, often associated with bronchiolar narrowing and obstruction, emphysematous destruction of alveoli and vascular destruction. It is the fourth leading cause of death in the US, and the eighth leading cause of disability, as measured by disability adjusted life-years. 1 Although COPD is defined by physiologic airflow obstruction, physiologic assessment is limited by the fact that it provides only a global index of pulmonary function, and often cannot distinguish among subtypes of COPD (predominant large airway inflammation, small airway obstruction or predominant emphysema). Imaging is pivotal in identifying and characterizing the relative components of these subtypes of COPD, offering the opportunity for individualized treatment of patients with this condition. Other potential roles for functional imaging in COPD include characterization of vascular dysfunction, and detection of early lung injury.
Comparison of CT and MRI for functional imaging in COPD
Advantages of CT for functional imaging in COPD include excellent contrast resolution and ease of quantification of emphysema and air trapping using density-based metrics. However, because of radiation dose considerations, it is not usual to perform true dynamic imaging. Major advantages of MRI are the ability to directly quantify ventilation, identify progression and reversibility with bronchodilators, to directly measure airspace dimensions, and to quantify pulmonary perfusion. 2 For quantifying ventilation, the ventilation defect percentage can be computed. For airspace dimensions, the apparent diffusion coefficient (ADC) is the most commonly used metric. For determining perfusion, pulmonary microvascular blood flow can be measured using gadolinium-enhanced MRI. 3
Opportunities for functional imaging in COPD
Emphysema
Emphysema is characterized by irreversible destruction of alveolar walls, which results in decreased lung attenuation on CT. CT metrics that can be used to quantify this effect include the % of lung voxels with low attenuation (e.g., ≤−950 Hounsfield units (HUs), expressed as %LAA-950), or the 15th percentile of the lung attenuation histogram (PERC 15). Although the %LAA-950 (often imprecisely expressed as % emphysema) is more intuitive, the percentile measurement has the advantage that the HU value can be directly converted to lung density, which can be also adjusted for inspired lung volume (volume-adjusted lung density or VALD). 4 VALD is particularly useful for analysis of emphysema progression over time, and is often relatively independent of physiologic measurement. 4
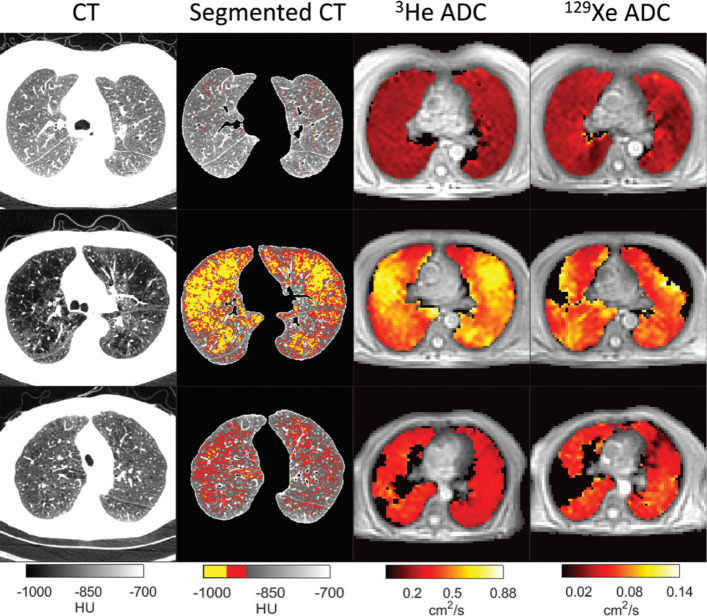
On MRI, alveolar destruction is assessed by hyperpolarized gas imaging. ADC obtained from diffusion-weighted MRI of inhaled hyperpolarized helium 3 (3He) or xenon 129 (129Xe) provide a relative measure of alveolar size. 5 129Xe is more widely available than 3He, and can also directly evaluate gas exchange. A recent study of 10 healthy volunteers, 16 with mild or moderate COPD, and 10 with severe COPD showed that 129Xe provided very similar ADC results to 3He, with progressively increasing ADC in subjects with increasing severity of COPD (Figure 1). ADC values correlated more strongly than %LAA-950 with lung diffusing capacity (Spearman rank correlation coefficient 0.80 for 129Xe compared with −0.61 for %LAA-950) . 5
Figure 1.
Functional imaging in a normal individual (top row), and in two patients with moderate (GOLD Stage III) COPD (middle and bottom rows). The first column shows axial CT lung images, the second column shows, segmented CT images with voxels <-950 HU and −910 HU displayed in yellow and red, respectively. The third column shows corresponding 3He ADC maps, and the fourth column shows 129Xe ADC maps. In the top row, CT images show uniformly dense parenchyma throughout the lung and uniformly low ADC values in both 3He and 129Xe ADC maps. In the middle row, CT depicts large areas of lung parenchyma with low attenuation coefficients. Clear visual concordance is present between these areas in CT image and elevated ADC values in corresponding 3He and 129Xe ADC maps. In the bottom row, there are relatively few low attenuation pixels on CT, but both ADC maps show elevated values. Image reproduced with permission from Tafti et al. Radiology doi: 10.1148/radiol.2020192804. Published online August 11, 2020. © Radiological Society of North America. ADC, apparent diffusion coefficient; COPD, chronic obstructive pulmonary disease; HU Hounsfield unit.
Airflow obstruction
Airflow obstruction in COPD is usually due to a combination of emphysematous air trapping and small airways obstruction, with small airways being defined as airways less than 2 mm in internal diameter. 6 On CT scanning, substantial functional information can be derived from measuring the degree of air-trapping on end-expiratory imaging. The patient is instructed to expire either to residual volume (RVCT) or to functional respiratory capacity (FRCCT). Accepted metrics for air trapping on expiratory CT include the percentage of voxels with attenuation ≤ −856 HUs (LAA-856exp), ratio of expiratory lung volume to inspiratory lung volume (TLCCT) (RVCT/TLCCT or FRCCT/TLCCT), and ratio of inspiratory/expiratory lung attenuation. These metrics all provide strong correlation with airflow obstruction measured by the ratio of forced expiratory volume in 1 s to forced vital capacity (FEV1/FVC ratio). 7 More recently, techniques of inspiratory–expiratory co-registration have helped to distinguish between gas trapping due to emphysema, where lung attenuation is reduced on inspiration, and gas trapping due to functional small airways disease (fSAD), where inspiratory lung attenuation is normal. 8 The extent of fSAD is a predictor of subsequent progression of airflow obstruction and development of emphysema in cigarette smokers. 8 Other registration-derived biomechanical metrics include Jacobian determinants and strain tensors, which offer improved correlation with spirometry, and predict physiologic decline. 9
Dynamic expiratory CT is acquired during a forced expiration, and is most useful in documenting expiratory tracheal collapse, which may contribute to physiologic impairment in COPD, and is often not identified on static end-expiratory CT. 10
MRI can also be used to assess severity of airflow limitation in COPD. For example, the ventilation defect percentage (expressed as a percentage of total lung volume) may be followed over time, and change in VDP correlates quite well with change in FEV1 (Spearman correlation coefficient −0.70, p = 0.03). 2
Large airway changes
In COPD, bronchial wall thickening is thought be due to a combination of bronchial inflammation and remodeling, and is best assessed with CT. From thin section volumetric CT scans, analytic software can generate high quality three-dimensional (3D) maps of the airway tree, down to subsegmental level. A number of resultant metrics can be used to evaluate bronchial wall thickening. A widely used summary measure calculated from the extracted airways is the square root of wall area of a hypothetical airway with internal perimeter of 10 mm (Pi10). This metric correlates significantly with physiologic measures, 6 min walk distance and quality of life measures. 11 Smoking cessation results in a significant decrease in Pi10, suggesting that it is a useful metric of smoking-related inflammation. Additionally, Pi10 is substantially higher in current or former smokers than in never smokers. 11
Total airway count (TAC) is a novel metric for chronic airways disease. Using careful methodology to develop a full 3D CT model of the airway tree, Kirby et al showed that the total number of airways identified is significantly reduced in mild COPD compared with never smokers. 12 TAC correlated with measures of airflow obstruction, and was associated with subsequent progression of airflow obstruction. 12 More recently, the same group has shown that TAC correlates with the count of terminal bronchioles on micro-CT, supporting the concept that airway dropout is an important pathogenetic feature of COPD.
Pulmonary vascular dysfunction
Pulmonary vascular dysfunction in COPD may be due to a combination of factors including vascular destruction, endothelial dysfunction, and hypercoagulability, 13 culminating in the development of pulmonary hypertension, which is an important predictor of morbidity and mortality in COPD. Pulmonary perfusion may be quantified directly using dual energy CT, and indirectly by evaluating pulmonary vascular volume. On MRI, pulmonary microvascular blood flow may be measured by first-pass imaging after gadolinium administration.
Dual energy CT may also be used to assess heterogeneity of pulmonary perfusion and vasodilator responsiveness. In a study of smokers with emphysema, they were shown to have decreased heterogeneity of pulmonary perfusion following administration of sildenafil, suggesting that these subjects had heterogeneously increased vascular resistance which improved following sildenafil. 14
Measurements of pulmonary vascular volume on CT provide an indirect measure of pulmonary perfusion on CT. Total blood volume may provide useful information, but the volume of blood vessels with cross-sectional area <5 mm2, an index of vascular pruning, is more informative. In a study of 2388 participants in the Framingham Heart Study, decrease in this metric was associated with evidence of pulmonary functional impairment, even in subjects with normal spirometry. 13
One of the simplest measurements of pulmonary vascular function on CT is the diameter of the main pulmonary artery, which is often normalized by expressing it as a ratio to the diameter of the adjacent ascending aorta. An abnormal PA/A ratio (>1) is associated with increased prevalence of exacerbations of COPD, with right ventricular dysfunction, and with increased mortality. 15
Identifying subtle lung injury
Narrowing and disappearance of small conducting airways is an early sign of lung injury in cigarette smokers, but may not be detected on routine physiologic tests. Physiologic measurements provide only a global index of pulmonary abnormality, and the range of normal is quite wide. Subtle or regional abnormalities may not be apparent. For example, about 25% of cigarette smokers with normal spirometry have significant symptoms, and their quality of life is often impaired. 16 Functional imaging can detect impairment when physiology is normal. For example, on MRI, smokers without COPD have higher intraindividual standard deviation of ADC than non-smokers, indicating increased heterogeneity of airspace size, particularly in the anterior lung. 17 Abnormalities of He-3 diffusion have also been demonstrated in subjects exposed to second-hand smoke. 18
Vascular indices may be affected in early COPD: in one study of 30 patients with mild COPD, pulmonary microvascular blood flow measured on first-pass gadolinium-enhanced MRI was reduced by 30% compared with those without COPD. 3 In smokers with mild COPD and emphysema, decreased pulmonary microvascular blood flow is associated with cellular markers of endothelial injury. 19
Current utility of functional imaging metrics
Most of the metrics described here are primarily of value in research on the pathophysiology of COPD, and are not yet available for clinical use. For clinical purposes, FDA-approved software available at some academic centers in the US can provide CT-based measurements of emphysema, airway thickening and expiratory air trapping. These metrics will likely become more widely applicable when targeted treatments become available for emphysema and large and small airways disease. These CT-based metrics may also be used in cigarette smokers undergoing lung cancer screening to provide quantitative metrics of smoking-related diseases including emphysema, airways disease, coronary calcification and osteoporosis. 20
Necessary further developments in functional imaging techniques will include standardization of measurement techniques, determination of intraindividual repeatability, validation in large diverse populations of cigarette smokers and other forms of inhalational injury, and determination of prognostic significance and sensitivity to longitudinal change.
Conclusion
Given the limitations of physiologic measurements, functional imaging has great potential to supplant physiologic measurements by identifying subtle lung injury, providing more precise, individualized subtyping of patterns of lung injury in COPD, and facilitating longitudinal measurements, which will become more important as treatments for COPD subtypes become available.
REFERENCES
- 1. Bhatt SP, Washko GR, Hoffman EA, Newell JD, Bodduluri S, Diaz AA, et al. Imaging advances in chronic obstructive pulmonary disease. insights from the genetic epidemiology of chronic obstructive pulmonary disease (COPDGene) study. Am J Respir Crit Care Med 2019; 199: 286–301. doi: 10.1164/rccm.201807-1351SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirby M, Mathew L, Wheatley A, Santyr GE, McCormack DG, Parraga G. Chronic obstructive pulmonary disease: longitudinal hyperpolarized (3)He MR imaging. Radiology 2010; 256: 280–9. doi: 10.1148/radiol.10091937 [DOI] [PubMed] [Google Scholar]
- 3. Hueper K, Vogel-Claussen J, Parikh MA, Austin JHM, Bluemke DA, Carr J, et al. Pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. The MESA COPD study. Am J Respir Crit Care Med 2015; 192: 570–80. doi: 10.1164/rccm.201411-2120OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pompe E, Strand M, van Rikxoort EM, Hoffman EA, Barr RG, Charbonnier JP, et al. Five-year progression of emphysema and air trapping at CT in smokers with and those without chronic obstructive pulmonary disease: results from the COPDGene study. Radiology 2020; 295: 218–26. doi: 10.1148/radiol.2020191429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tafti S, Garrison WJ, Mugler JP, Shim YM, Altes TA, Mata JF, et al. Emphysema index based on hyperpolarized 3He or 129Xe diffusion MRI: performance and comparison with quantitative CT and pulmonary function tests. Radiology 2020; 297: 201–10. doi: 10.1148/radiol.2020192804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011; 365: 1567–75. doi: 10.1056/NEJMoa1106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schroeder JD, McKenzie AS, Zach JA, Wilson CG, Curran-Everett D, Stinson DS, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol 2013; 201: W460–70. doi: 10.2214/AJR.12.10102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Labaki WW, Gu T, Murray S, Hatt CR, Galbán CJ, Ross BD, et al. Voxel-Wise longitudinal parametric response mapping analysis of chest computed tomography in smokers. Acad Radiol 2019; 26: 217–23. doi: 10.1016/j.acra.2018.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhatt SP, Bodduluri S, Hoffman EA, Newell JD, Sieren JC, Dransfield MT, et al. Computed tomography measure of lung at risk and lung function decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017; 196: 569–76. doi: 10.1164/rccm.201701-0050OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Donnell CR, Bankier AA, O'Donnell DH, Loring SH, Boiselle PM. Static end-expiratory and dynamic forced expiratory tracheal collapse in COPD. Clin Radiol 2014; 69: 357–62. doi: 10.1016/j.crad.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charbonnier J-P, Pompe E, Moore C, Humphries S, van Ginneken B, Make B, et al. Airway wall thickening on CT: relation to smoking status and severity of COPD. Respir Med 2019; 146: 36–41. doi: 10.1016/j.rmed.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirby M, Tanabe N, Tan WC, Zhou G, Obeidat Ma'en, Hague CJ, et al. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression. findings from a population-based study. Am J Respir Crit Care Med 2018; 197: 56–65. doi: 10.1164/rccm.201704-0692OC [DOI] [PubMed] [Google Scholar]
- 13. Synn AJ, Li W, San José Estépar R, Zhang C, Washko GR, O'Connor GT, et al. Radiographic pulmonary vessel volume, lung function and airways disease in the Framingham heart study. Eur Respir J 2019; 54: 1900408 12 09 2019. doi: 10.1183/13993003.00408-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iyer KS, Newell JD, Jin D, Fuld MK, Saha PK, Hansdottir S, et al. Quantitative dual-energy computed tomography supports a vascular etiology of smoking-induced inflammatory lung disease. Am J Respir Crit Care Med 2016; 193: 652–61. doi: 10.1164/rccm.201506-1196OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LaFon DC, Bhatt SP, Labaki WW, Rahaghi FN, Moll M, Bowler RP, et al. Pulmonary artery enlargement and mortality risk in moderate to severe COPD: results from COPDGene. Eur Respir J 2020; 55: 1901812 12 02 2020. doi: 10.1183/13993003.01812-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JHM, Grenier PA, et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med 2015; 175: 1539–49. doi: 10.1001/jamainternmed.2015.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swift AJ, Wild JM, Fichele S, Woodhouse N, Fleming S, Waterhouse J, et al. Emphysematous changes and normal variation in smokers and COPD patients using diffusion 3He MRI. Eur J Radiol 2005; 54: 352–8. doi: 10.1016/j.ejrad.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 18. Wang C, Mugler JP, de Lange EE, Patrie JT, Mata JF, Altes TA. Lung injury induced by secondhand smoke exposure detected with hyperpolarized helium-3 diffusion Mr. J Magn Reson Imaging 2014; 39: 77–84. doi: 10.1002/jmri.24104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomashow MA, Shimbo D, Parikh MA, Hoffman EA, Vogel-Claussen J, Hueper K, et al. Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. The multi-ethnic study of atherosclerosis chronic obstructive pulmonary disease study. Am J Respir Crit Care Med 2013; 188: 60–8. doi: 10.1164/rccm.201209-1697OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Regan EA, Lowe KE, Make BJ, Lynch DA, Kinney GL, Budoff MJ, et al. Identifying smoking-related disease on lung cancer screening CT scans: increasing the value. Chronic Obstr Pulm Dis 2019; 6: 233–45. doi: 10.15326/jcopdf.6.3.2018.0142 [DOI] [PMC free article] [PubMed] [Google Scholar]