Abstract
Excretory urography (EU) had been the most frequently performed imaging modality for uroradiology in the past. With the advances in ultrasonography, and development of cross-sectional urography with CT and MRI, EU is now seldom performed. Consequently, there has been a decline of expertise in this technique. However, EU has multiple advantages such as dynamic nature, easy availability, low cost and radiation burden. These render it potentially very valuable in specific indications like congenital anomalies, urothelial lesions and urinary leaks. This review intends to emphasize the current day relevance of excretory urography, outline the key points of the technique, and describe the pearls and pitfalls of interpretation.
Introduction
Once a cornerstone in the evaluation of urinary tract diseases, Excretory urography (EU) has now been surpassed by newer diagnostic modalities such as ultrasonography, CT, MRI and radionuclide imaging. 1 EU is also known as intravenous urography (IVU) or intravenous pyelography (IVP). However, the newer modalities have their own limitations. 1,2 Ultrasonography is operator dependent and may not be able to visualize large portions of urinary tract with bowel gas acting as an important limitation. MRI is expensive, not widely available and may not be able to demonstrate calcifications. In pediatric patients, MRI requires sedation. Renal scintigraphy is a highly sensitive method. Dynamic radionuclide renography with 99mTc MAG3/DTPA is indicated in the assessment of renal perfusion, differential renal function, suspected urinary tract obstruction and renovascular hypertension. 3 DMSA static renography is also very sensitive in assessing renal shape, size, location and is indicated in functional as well as morphological evaluation like cortical scars, pyelonephritis, congenital abnormalities of kidney and urinary tract. 3 However, spatial resolution remains a limiting factor in radionuclide imaging.
Some of the disadvantages of EU that led to it being replaced by CT urography are:
Limited evaluation of the upper urinary tract due to overlying bowel gas and feces. 4
Non-opacification of urinary segments. 4
Low sensitivity of 50–75% and low accuracy in detection and characterization of urothelial lesions compared to CT urography. 5
Lack of ability to visualize the renal parenchyma, ureteric and bladder wall and surrounding structures. CT urography is a better modality than EU to detect alternate possible diagnosis. 6
However, there are some advantages of EU, that make it a relevant procedure in specific urological indications till this date.
EU is preferable in situations where CT urography cannot be performed such as urinary tract assessment in an unstable patient who cannot be shifted to the CT room or intraoperative assessment of urinary tract. 7
There are many rural, remote or less-developed areas especially in low income countries where CT is unavailable and EU can be performed with the easily available basic radiography equipment. 8–10
EU is still the gold-standard method to detect the earliest changes of genitourinary tuberculosis till date. 11,12
EU is a dynamic imaging method in which the contrast can be followed at sequential time intervals with only a small additional radiation dosage. A particular disadvantage of CT urography is seen in the setting of asymmetric excretion, where inadequate opacification of the pelvicalyceal system on the obstructed side can occur due to lack of sequential imaging. 13
In the present scenario, due to the reduced number of procedures, there has been a constant loss of expertise in performing and interpreting the conventional uroradiological procedures particularly in the younger radiologists.
Indications
EU does not form a part of the current routine urological imaging protocols for evaluating renal/flank pain and has been essentially replaced by NCCT KUB for diagnosis of urinary tract calculous disease.
According to the American College of Radiology (ACR) guidelines, currently EU is indicated in the following situations when cross-sectional imaging techniques such as USG, CT or MRI are either unavailable or are considered inappropriate for the clinical circumstance 7 :
In patients with suspected or known ureteral obstruction.
In suspected urinary tract congenital anomalies when EU is considered more appropriate than cross-sectional imaging modalities.
For assessment of upper urinary tract in patients with hematuria due to suspected urothelial lesions such as tuberculosis.
Assessment of urinary tract to identify abnormalities that may predispose to infection such as duplex ureter.
Evaluation of integrity of urinary tract after therapeutic interventions or trauma such as in a case of trauma patient who is unstable to be shifted to the CT room, EU can be performed in the operating room.
In the follow-up of patients with recurrent renal/ureteral calculi where limited number of images are acquired to reduce the overall radiation burden.
To detect the early changes of genitourinary tuberculosis. 11,12
Technique
The urographic sequence is designed to capture the optimally opacified parts of the urinary tract at appropriate timings according to physiology of iodinated water soluble contrast medium uptake and excretion. The urographic contrast once injected into blood stream, undergoes renal uptake through afferent arteriole in the nephron. Subsequent process of glomerular filtration and tubular concentration leads to nephrographic opacification. Further, it is excreted into the collecting tubules and later into the calyceal system.
Any pathology at the level of renal blood flow, glomerular filtration rate, intra- or post-renal obstruction or venous outflow leads to alteration in urographic timing and appearance.
An outline of imaging technique is presented in Table 1 4,14–18
Table 1.
Imaging sequence and their significance
| Plain X-ray | Assess calculus, bowel preparation, exposure factors |
|---|---|
| 1 min nephrographic phase | 1 min post-injection film to assess renal parenchyma and contour |
| 5 min radiograph collimated to kidneys | 5 min post-injection, shows the pelvicalyceal system and upper ureters. Compression is applied at 5 min and released at 15 min post-injection |
| 10 min Pyelographic Phase | Collimated to kidneys, 5 min post-compression (10 min post injection), shows well-distended pelvicalyceal system |
| 15 min KUB radiograph | 15 min post-injection (immediately after compression release), shows outline of ureters and bladder |
| Full bladder and post-void images | To assess bladder shape, outline, residual volume. |
KUB, kidney ureter bladder.
Pearls and pitfalls in imaging technique:
Plain film: bowel preparation was historically advised. However, current recommendation is that it tends to dehydrate the patient without adding diagnostic value. A low tube potential of 65–75kVp maximizes contrast resolution for visualization of calcifications [Figure 1a]. 18 Field of view should include suprarenal area to pubic symphysis, centering being done on the iliac crests. Bladder or urethral calculi may be missed if entire kidney ureter bladder (KUB) region is not included in plain film. In a tall patient, separate pelvic radiograph may be required to include the region of symphysis pubis. Lower ureteric calculi can be obscured by sacrum. If no calculus is visualized in a patient with definite history of flank pain, oblique radiographs should be obtained to remove bony overlap.
Adequate collimation as per the examination phase and use of grid.
Any contraindications to iodinated contrast media must be ruled out. Intravenous contrast bolus should be administered rapidly within 30–60 s to improve density of opacification.
Compression helps to distend the pelvicalyceal system adequately and should be used after review of 5 min KUB radiograph (Figure 1c). 19–21 Abdominal compression is contraindicated in cases of severe abdominal pain, recent abdominal surgery or trauma, abdominal aortic aneurysm, intraabdominal mass, renal transplant or urinary diversion surgery and presence of caliceal dilatation on 5 min radiograph. A radiograph collimated to the renal areas is acquired 5 min after compression is applied (Figure 1d). 22
A 15 min post-injection or 10 min post-compression KUB radiograph acquired just after the release of compression helps in adequate visualization of ureters (Figure 2a). 23,24
Prone views can be obtained for visualization of lower ureters and anterior pelvicalyceal system (PCS) (Figure 2b). 23,24
Oblique views for ureters can be obtained to better visualize the level of obstruction by projecting them away from the spine (Figure 2c). Optional oblique views can also be obtained for renal areas to delineate any obscured or overlapping parts of pelvicalyceal system.
Lateral views are useful in suspected urinary fistulas like vesicovaginal fistula.
If pelvicalyceal system does not get opacified till the 15 min post-injection radiograph, then further process depends upon the presence or absence of nephrogram. If nephrogram has been visualized, then delayed images are acquired at increasing intervals of 2, 4, 8, 16 and 24 h till opacification is seen (Figure 3a and b). 25 If no opacification is seen at radiograph taken 24 h post-contrast injection, the kidney can be labelled as non-excreting. However, if nephrogram is absent, it indicates a lack of contrast uptake and delayed images are not required.
Nephrotomograms can be acquired at any stage of the procedure for better anatomical information.
Intravenous furosemide can be used in suspected ureteropelvic junction (UPJ) obstruction. UPJ pathology is suspected if contrast fails to clear the pelvicalyceal system 10 min after furosemide injection.
Figure 1.
a) Plain KUB radiograph shows bilateral renal and psoas muscle outlines against the retroperitoneal fat density. A mid ureteric calculus is seen at the level of L3-L4 intervertebral disc. (b) 1 min radiograph showing urographic nephrogram. Bilateral prompt and homogenous renal uptake is observed. Renal size is best measured in this phase. (c) 5 min radiograph shows timely and simultaneous bilateral renal excretion. (d) 10 min post-injection or 5 min post-compression radiograph shows adequate distension and clear anatomical depiction of the pelvicalyceal system. KUB, kidney ureter bladder.
Figure 2.
a) 15 min supine KUB radiograph after release of compression captures contrast passing through bilateral ureters. Note the normal partial opacification of left ureter due to peristalsis. Right ureter is opacified till the calculus (arrow). (b) 15 min KUB radiograph in prone position in the same patient as (a). Contrast is seen to flow into the right lower ureter in prone position. (c) Left posterior oblique view in another patient with ureteric calculus. Position of calculus in the course of ureter is confirmed in this view (arrow). Left proximal hydroureteronephrosis is seen. KUB, kidney ureter bladder.
Figure 3.
Interpretation of nephrogram in flank pain. (a, b) 30-year-old male patient with chronic left flank pain. (a) 1 min radiograph showing left renal pelvic calculus and upper polar calculus with small lower polar secondary calculi. Peripheral shell-like outline of uptake in severely thinned out left renal cortex is seen suggestive of “rim nephrogram” sign. This is characteristic of severe hydronephrosis. (b) Delayed radiograph at 2 h shows persistent dense contrast excretion into the left pelvicalyceal system. (c) Post-void radiograph in another patient with left flank pain shows a pseudoureterocoele due to a left-sided VUJ calculus. Also noted is the increasingly dense persistent nephrogram of left kidney suggesting acute obstruction with Grade 4 hydroureteronephrosis.
Pearls to reduce radiation dosage:
Usage of gonadal shields.
Nephrographic 1 min film can be skipped and directly 5 min KUB radiograph can be obtained which shows both nephrogram and pelvicalyceal system well.
Full bladder and post-void evaluation can be done by ultrasound.
Interpretation
A) Plain radiograph is used to assess exposure factors, detect calculi and calcification due to tuberculosis, papillary necrosis or nephrocalcinosis. Any incidental bony anomalies in spine or sacrum should be observed in suspected congenital renal anomalies.
B) Nephrographic phase has two parts; the angiographic or cortical nephogram and urographic or parenchymal nephrogram. 25
In angiographic techniques, contrast within the renal microvasculature gives rise to angiographic or cortical nephrogram. Abnormalities of this phase are indicator of disturbance in blood flow to kidney.
Contrast material filtered from the bloodstream into the nephrons by glomerular filtration reaches maximum density at 1 min after injection (Figure 1b) to produce the urographic nephrogram. There is homogenous density of entire renal parenchyma. A normal nephrogram thus requires normal blood flow, functional and structural integrity of nephrons and unobstructed flow of infiltrate through the tubules.
Renal size, outline and position can be assessed in this radiograph.
The normal kidney may range from 9 to 13 cm in cephalocaudal length. Abnormality can be suspected if right kidney is longer by ≥1.5 cm than left or left kidney is longer by ≥2 cm than right.
Renal outline should be smooth. Variations like dromedary hump, persistent fetal lobulation can be clarified using interpapillary line. Renal scarring or irregularity can be seen in chronic pyelonephritis or reflux nephropathy. Focally anormal contour suggests cysts or neoplasms.
Normal renal position is in the flank from L1 to L3 vertebral levels, with the orientation parallel to psoas outline.
Patterns of abnormal nephrogram
Immediate, faint and persistent nephrogram
It is seen in severe impairment of glomerular function, e.g. chronic glomerular disease, atheroembolic renal disease, e.g. in renal artery stenosis as shown in Figure 4.
Figure 4.
28-year-old hypertensive male patient. (a) Nephrogram phase shows small left kidney with smooth outline. Renal uptake is faint compared to right side, which can be explained by decreased blood flow and GFR. (b) 5 min radiograph shows no contrast excretion in left side. (c) 10 min radiograph shows delayed and faint opacification of left renal PCS. GFR, glomerular filtration rate; PCS, pelvicalyceal system.
Increasingly dense nephrogram
It is faint to begin with, but gets increasingly dense over hours to days. It occurs due to acute post-renal obstruction, acute tubular necrosis, intratubular obstruction, e.g. acute urate or myeloma nephropathy (Figure 3).
Immediate, dense and persistent nephrogram
It is characterized by early appearance of nephrogram which persists or slightly increases in density over time. Acute tubular necrosis is the most common cause of this pattern. Sever acute pyelonephritis usually causes unilateral abnormality.
Striated parenchymal nephrogram
There is inhomogeneity characterized by alternating bands of lucency and density oriented in direction similar to tubules or collecting ducts. It can be seen in acute post-renal obstruction and severe acute pyelonephritis.
Patchy parenchymal nephrogram
Inhomogeneous nephrograms are seen in obliterative diseases of microvasculature, e.g. polyarteritis nodosa, scleroderma, necrotizing angiitis due to random occlusive events in microvasculature.
Deformed nephrogram
Loss of renal structural integrity can be assessed by identifying disruption of homogeneity, e.g. processes that cause loss of tissue (reflux nephropathy, infarction); displacement of parenchyma (simple cyst, polycystic kidney disease, medullary cystic kidney, focal hydronephrosis); replacement of parenchyma (neoplasm or inflammatory mass) can lead to deformation of nephrogram (Figure 5a). 25
Figure 5.
Congenital anomalies of renal tubular origin. (a, b) EU in a 45-year-old male patient with ADPKD. (a) 5 min and b) 15 min radiographs show bilaterally enlarged kidneys, small radiolucent defects in nephrogram (black arrows) and thinned out, splayed calyces giving the “spider leg” appearance (white arrows). (c, d) EU in medullary sponge kidney. (c) Plain radiograph shows multiple small calcifications (white arrow) seen in left kidney. (d) 5 min radiograph shows dilated intramedullary tubules (black arrow) known as “paint brush” appearance. ADPKD, autosomal recessive polycystic kidney disease; EU, excretory urography
C) 5-min radiograph shows the pelvicalyceal system and is used to determine the temporal symmetry in the excretion of the contrast (Figure 1c).
D) Pyelographic phase. Pelvicalyceal system is best demonstrated on 10 min post-compression radiograph (Figure 1d). 18 Renal pelvis divides into 2–3 major calyces, each major calyx branches into 4–5 minor calyces. Each minor calyx is related to a renal papilla which forms a concave impression on the calyceal outline with sharp forniceal angles normally. Hydronephrosis can be graded into four types based on appearance of calyces (Figure 6). 18 Caliceal filling defects can be due to radiolucent calculi, blood clots, neoplasm, sloughed-off papillae, fungal ball, gas or iatrogenic foreign bodies. Interpapillary line can be drawn which helps detect any renal mass or calyceal diverticulum (Figure 7).
Figure 6.
Grades of hydronephrosis. (a) Normal PCS shows deep papillary impressions and acute forniceal angles. (b) Grade 1. Slight blunting of forniceal angles. (c) Grade 2. Obvious blunting of forniceal angles with preserved papillary impressions. (d) Grade 3. Obvious blunting of forniceal angles and loss of papillary impressions. (e) Grade 4. Ballooning of PCS. (f) The PCS appears grossly dilated. However, papillary impressions are still preserved. Hence, this is Grade 2 hydronephrosis. PCS, pelvicalyceal system.
Figure 7.
Interpapillary line and its deviation. (a) Interpapillary line is the line joining the tips of papillae. Normal interpapillary line should run parallel to the renal outline. Any deviation in renal contour requires explanation. Sequential radiographs show incomplete calyceal opacification in 5-min radiograph (b) followed by gradual progressive filling and enlargement (c, d) of a dilated structure connected to upper polar minor calyx. The structure lies outside the interpapillary line and retains contrast on delayed radiograph (e) suggestive of calyceal diverticulum.
E) Ureter bladder image. 15-min KUB radiograph delineates the calibre, position and outline of ureters and shows any calculus missed on plain film as filling defect.
Full bladder image is used to assess the bladder distension, shape, outline and any intraluminal filling defects.
Post-void image is used to assess residual volume and bladder mucosa.
EU patterns in urinary tract pathology
Congenital abnormalities of the kidney and urinary tract (CAKUT) 26,27 can be classified into three major types - abnormalities in the renal parenchymal development, aberrant embryonic migration and abnormalities of the collecting system.
EU offers the advantage of phased opacification of urinary tract and visualization of entire KUB region in single view.
The various conditions are enumerated in Table 2, many of which can be detected easily on EU.
Table 2.
Classification of congenital abnormalities of the kidney and urinary tract
| Renal parenchymal form anomalies | Aberrant embryonic migration | Anomalous collecting system |
|---|---|---|
|
|
|
Ectopic kidney is characterized by location of kidneys outside the flank region (L1–L3 vertebral levels). It can be simple ectopia or crossed ectopia when it is located contralateral to its ureteric orifice. 27–29 Both can be unilateral or bilateral. Crossed ectopia can show fusion in various interesting configurations 27–32 (Figure 8). Radionuclide imaging can help in localizing a poorly functioning ectopic kidney, however, the anatomical details of ureteropelvic system are not well delineated. 3,33–35
Figure 8.
Congenital anomalies of renal fusion. (a, b) Cross-fused ectopic kidney. (a)5 min radiograph shows opacification of the left kidney where lower pole could not be made out separately and faint opacification of right kidney (black arrows) which is located on the left side of spine and fused to lower pole of left kidney. (b) 15 min radiograph shows malrotated left kidney with pelvis pointing to the left and ureter displaced laterally. The right ureter is seen to cross the midline. (c) Horseshoe kidney. A 32-year-old male patient shows horseshoe kidney with duplication of right pelvicalyceal system and ureter.
Horseshoe kidney 27,36–38 is the most common renal congenital anomaly which can be present in 1:400 adults. It occurs due to the fusion of two distinct functioning kidneys across the midline by an isthmus of functioning renal parenchyma or fibrous tissue. During ascent, the isthmus gets hooked beneath the inferior mesenteric artery, leading to inferior location and malrotation of kidneys (Figure 8c).
Duplex collecting system may be complete when two separate ureteric buds arise from a single Wolffian duct or incomplete due to abnormal ureteric bud bifurcation. Based on the degree of fusion, it can present as bifid renal pelvis, partial ureteric duplication (Y-shaped ureter), and incomplete ureteric duplication with ureters joining near or in bladder wall (V-shaped ureter) and complete ureteric duplication with separate ureteric orifices (Figure 9). 39–41 Double ureters follow Weigert-Meyer rule. Ureter of the upper moiety inserts ectopically inferior and medial to the that of the lower pole moiety. Upper moiety often ends in an ureterocoele with obstruction (Figure 9b and c) and lower moiety shows reflux. 42,43 “Drooping lily” sign is a classic urographic sign which refers to the inferolateral displacement of the opacified lower pole moiety due to an obstructed and unopacified upper pole moiety (Figure 9b and c). Upper pole moiety ureter can insert into the vaginal vault in females and variably into the prostate, seminal vesicle, urethra in males.
Figure 9.
Congenital anomalies of PCS multiplication. (a–c) Left renal duplex system. Serial EU images show (a) nephrographic phase, (b) 5 min radiograph (c) 10-min radiograph. Left upper polar calyx is not opacified (black arrow). Mid and lower pole calyces are slightly displaced inferiorly. The left sided visualized ureter and pelvicalyceal system are not dilated. A large ureterocele (white arrow) is seen appearing as a filling defect, likely arising from the terminal part of upper moiety which is not opacified due to obstruction. (d) Polycalycosis. EU in a 25-year-old male shows increased number of calyces on right sided with a mosaic multifaceted appearance suggestive of polycalycosis. EU, excretory urography; PCS, pelvicalyceal system.
Medullary sponge kidney also known as renal tubular ectasia is a developmental defect in formation of collecting tubules. It is generally detected in middle-aged adults. The pathology is restricted to renal pyramids. 44–46 Bilateral renal involvement is more common. Numerous small calculi of varying shapes are seen in the region of pyramids on plain radiograph (Figure 5c). Multiple cyst like cavities can be seen opacified with contrast in the medullary region, which obscure the calculi. The “paint brush” or “bouquet of flowers” appearance of ectatic tubules is characteristic (Figure 5d). 44
Polycystic kidney disease can be autosomal recessive (ARPKD) and autosomal dominant (ADPKD). Renal cysts originate as diverticulae from wall of proximal or distal tubule. With tubular epithelial proliferation and fluid expansion, they separate from the tubule. ARPKD can be diagnosed antenatally. Post-natal diagnosis can be made on ultrasound as bilateral enlarged kidneys which are uniformly echogenic due very small cyst size. 47,48 EU shows a streaked nephrogram with no significant distinguishing feature. ADPKD usually presents after third decade. 49–51 On EU, the “spiderleg pyelogram” is characteristic. (Figure 5a and b).
Congenital megacalycosis and polycalycosis is caused by abnormal development of renal medulla leading to hypoplastic pyramids. 52 There is non-obstructive dilatation and malformation of calyces and occasionally, the number of calcyces is increased to 12 or more, when it is termed polycalycosis (Figure 9d). The infundibuli and pelvis are normal and there is no anatomical or functional progression.
Retrocaval ureter or circumcaval ureter is a rare abnormality resulting from anomalous persistence of posterior cardinal vein during development of inferior vena cava (IVC). Right ureter passes behind the IVC and then crosses first medially and then anterior to IVC to circumvent the IVC leading to an S-shaped curve and compression of ureter at this point (Figure 10). 53 Calculi occasionally coexist. It presents as increasing hydronephrosis in middle aged adults. EU is relevant because the abnormality can be overlooked on NCCT or CT urography especially on 3D reconstruction or multiplanar reformatted images due to poor contrast opacification of distal ureter. 54
Figure 10.
Retrocaval ureter. Typical fishhook deformity is seen in the right midureter with proximal hydroureteronephrosis.
Urinary tract calculi
At present, the investigation of choice for urinary calculi is NCCT, which has 100% sensitivity to calculi, including those which are lucent on radiographs. 55,56 However, considering the radiation burden, EU can be used in the follow up of recurrent calculi. 57 The findings of obstructive uropathy due to calculi have been covered in the section on nephrographic patterns (Figure 3).
Urinary leaks and fistulas
Due to its dynamic nature, EU can be helpful in demonstrating pelvicalyceal leaks in cases of trauma, and fistulous communications like renoalimentary fistulae and vesicovaginal fistulae (Figure 11).
Figure 11.
Vesicovaginal fistula. Lateral 15 min radiograph in an EU series done for calculus disease shows opacification of vagina (black arrow) through a vesicovaginal fistula. EU, excretory urography.
Urothelial lesions
EU is a sensitive technique to detect urothelial lesions due to inherent high spatial resolution.
Genitourinary tuberculosis
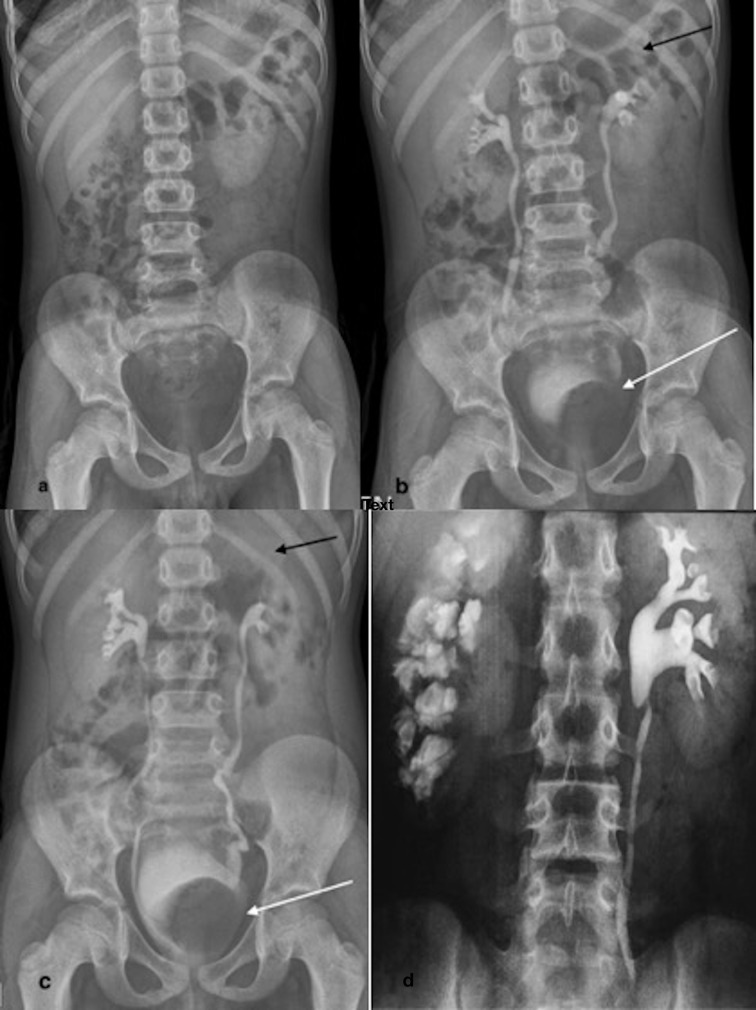
Plain film may detect calcifications. Characteristic “putty-like” calcification of uniform density of more than 1 cm diameter may be detected. A lobar pattern with calcific rims outlining the periphery of distorted renal lobes, is pathognomonic of tuberculosis (TB) (Figure 12a). “Scarred calculi” conforming to the shape of deformed calyces are quite suggestive of renal tuberculosis. Ureteric calcification, if detected, is also pathognomic. Other tell-tale signs of calcified abdominal nodes or calcified hepatosplenic granulomas may be seen.
Figure 12.
Various phases of EU in different patients depicting urinary tract tuberculosis. (a) Plain radiograph shows characteristic “putty like” calcification of left kidney. Right DJ stent is noted. (b) 10-min radiograph shows “egg-in-cup” left lower polar papillary necrosis (black arrow). Note is made of calcified retroperitoneal nodes. (c) 15-min radiograph shows a bizarre shaped calculus (white arrow) in left mid ureter with upstream dilatation. Contrast opacification in left upper polar papillary region suggestive of papillary necrosis (black arrow). (d) Full bladder phase radiograph. Small capacity “thimble bladder” is observed with smooth outline. Also noted are beaded appearance of left lower third ureter with proximal hydroureteronephrosis and increasingly dense nephrogram. A stricture is present at lower end of right ureter causing proximal hold-up of contrast.
The earliest urographic changes are seen in minor calyces which lose sharpness of the outline. With disease progression, the calyceal outline becomes fuzzy, irregular and subsequently moth-eaten in appearance.
In practice, however, papillary necrosis is usually the earliest sign (Figure 12c). It can be central (due to ischemia) or forniceal type (due to direct erosion).
Cavitation (Figure 12b) can be obstructive or non-obstructive. Obstructive cavity is not opacified on retrograde pyelography (RGP), while faint opacification may be seen on EU. Non-obstructive cavity is well opacified on RGP. “Egg in cup” appearance is typical of papillary necrosis (Figure 12b).
Other typical features like hiked up renal pelvis (Figure 12b), strictures/scars, mass lesions and autonephrectomy (Figure 12a) are seen in late stage of disease due to fibrosis and scarring.
Long segment strictures most commonly involve the distal third of ureter (Figure 12d). Other typical appearances are beaded or corkscrew appearance (Figure 12d) and pipe stem ureter.
Thimble bladder is a small capacity bladder due to scarring. Its smooth outline differentiates it from small capacity neurogenic bladder which has a thickened trabeculated wall (Figure 12d). Vesicoureteric reflux can be seen due to fibrosis in trigone region. 11,12,58–61
Urothelial carcinoma
Previously indicated for screening of the upper urinary tract in cases of urinary bladder carcinoma, EU is no longer utilized for this purpose. CT urography is more accurate than EU in detection and localization of urothelial carcinoma. 5 However, single or multiple pelvicalyceal filling defects or irregular mucosal outline may be detected in superficial spread. “Stipple sign”, “phantom calyx”, “amputated calyx” have been described in literature, although they are not specific for carcinoma. 62
Conclusion
Once considered the cornerstone of uroimaging, EU has now been replaced by advanced cross-sectional urography methods. However, the simplicity and the dynamic nature of this technique, cost effectiveness, availability and lower radiation burden are some of its irrefutable advantages.
Knowledge of the clinical situations where it is relevant even today, as well as the pearls and pitfalls of its technique and interpretation, is essential so that a valuable technique does not become a forgotten art.
Contributor Information
Prateek Kumar Madaan, Email: madaan1991@gmail.com.
Rohini Gupta Ghasi, Email: rohini1912@gmail.com.
REFERENCES
- 1. Amis ES. Epitaph for the urogram (editorial). Radiology 1999; 213: 639–40. doi: 10.1148/radiology.213.3.r99dc47639 [DOI] [PubMed] [Google Scholar]
- 2. Becker JA, Pollack HM, McClennan BL. Urography survives (letter). Radiology 2001; 218: 299–300. doi: 10.1148/radiology.218.1.r01ja11299 [DOI] [PubMed] [Google Scholar]
- 3. ACR-SPR practice parameter for the performance of Renal Scintigraphy [Internet]. acr.org]. 2017. Available from: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/RenalScint.pdf
- 4. Hattery RR, Williamson B, Hartman GW, LeRoy AJ, Witten DM. Intravenous urographic technique. Radiology 1988; 167: 593–99. doi: 10.1148/radiology.167.3.3363117 [DOI] [PubMed] [Google Scholar]
- 5. Jinzaki M, Matsumoto K, Kikuchi E, Sato K, Horiguchi Y, Nishiwaki Y, et al. Comparison of ct urography and excretory urography in the detection and localization of urothelial carcinoma of the upper urinary tract. AJR Am J Roentgenol 2011; 196: 1102–9. doi: 10.2214/AJR.10.5249 [DOI] [PubMed] [Google Scholar]
- 6. Homer JA, Davies-Payne DL, Peddinti BS. Randomized prospective comparison of non-contrast enhanced helical computed tomography and intravenous urography in the diagnosis of acute ureteric colic. Australas Radiol 2001; 45: 285–90. doi: 10.1046/j.1440-1673.2001.00922.x [DOI] [PubMed] [Google Scholar]
- 7. ACR-SAR practice parameter for the performance of Excretory Urography [Internet]. acr.org]. 2019. Available from: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/ExcretoryUrog.pdf
- 8. Fleet R, Audette L-D, Marcoux J, Villa J, Archambault P, Poitras J. Comparison of access to services in rural emergency departments in quebec and british columbia. CJEM 2014; 16: 437–48. doi: 10.1017/s1481803500003456 [DOI] [PubMed] [Google Scholar]
- 9. Bergeron C, Fleet R, Tounkara FK, Lavallée-Bourget I, Turgeon-Pelchat C. Lack of ct scanner in a rural emergency department increases inter-facility transfers: a pilot study. BMC Res Notes 28, 2017; 10(1): 772. doi: 10.1186/s13104-017-3071-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shetty DB, Shetty PB. Tele radiology services between rural districts and state capital bangalore, india, a cost effective, sustainable health care project for rural areas. Ann Glob Health August 20, 2016; 82: 557. RESOURCE> .. doi: 10.1016/j.aogh.2016.04.498 [DOI] [Google Scholar]
- 11. Merchant S, Bharati A, Merchant N. Tuberculosis of the genitourinary system-urinary tract tuberculosis: renal tuberculosis-part i. Indian J Radiol Imaging 2013; 23: 46–63: 46. doi: 10.4103/0971-3026.113615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Merchant S, Bharati A, Merchant N. Tuberculosis of the genitourinary system-urinary tract tuberculosis: renal tuberculosis-part ii. Indian J Radiol Imaging 2013; 23: 64–77. doi: 10.4103/0971-3026.113617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O’Connor OJ, Maher MM. CT urography. AJR Am J Roentgenol 2010; 195: W320-4. doi: 10.2214/AJR.10.4198 [DOI] [PubMed] [Google Scholar]
- 14. Friedenberg RM, Harris RD. (n.d.). Excretory urography in the adult.
- 15. Dunnick NR, Sandler CM, Newhouse JH, Amis ES. Textbook of uroradiology. . 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 16. Amis ES. Excretory urography. In: Taveras JM, Ferrucci JT, eds. Radiology: diagnosis-imaging- intervention. Philadelphia: Lippincott- Raven; 1997, pp. 1–12. [Google Scholar]
- 17. Zagoria RJ, Donati DL, Chen MYM, Gelfand DW, Ott DJ, Dyer RB. Cost-effective filming sequence for intravenous urography. South Med J 1994; 87: 899–901. doi: 10.1097/00007611-199409000-00007 [DOI] [PubMed] [Google Scholar]
- 18. Dyer RB, Chen MYM, Zagoria RJ. Intravenous urography: technique and interpretation. Radiographics 2001; 21: 799–821. doi: 10.1148/radiographics.21.4.g01jl26799 [DOI] [PubMed] [Google Scholar]
- 19. Daughtridge TG. Ureteral compression device for excretory urography. Am J Roentgenol Radium Ther Nucl Med 1965; 95: 431–38. doi: 10.2214/ajr.95.2.431 [DOI] [PubMed] [Google Scholar]
- 20. Mawhinney RR, Gregson RHS. Is ureteric compression still necessary? Clin Radiol 1987; 38: 179–80. doi: 10.1016/s0009-9260(87)80026-0 [DOI] [PubMed] [Google Scholar]
- 21. Pfister RC, Shea TE. Nephrotomography: performance and interpretation. Radiol Clin North Am 1971; 9: 62. [PubMed] [Google Scholar]
- 22. Hughes TH, Hine AL. The most advantageous timing of external ureteric compression during intravenous urography. Br J Radiol 1991; 64: 314–17. doi: 10.1259/0007-1285-64-760-314 [DOI] [PubMed] [Google Scholar]
- 23. Lowe LH, Zagoria RJ. Fluoroscopic evaluation of the ureters during intravenous urography. South Med J 1994; 87: 627–30. doi: 10.1097/00007611-199406000-00010 [DOI] [PubMed] [Google Scholar]
- 24. Pfister RC, Newhouse JH. Radiology of ureter. Urology 1978; 12: 15–39. doi: 10.1016/0090-4295(78)90364-3 [DOI] [PubMed] [Google Scholar]
- 25. Davidson AJ, Hartman DS, Choyke PL, Wagner BJ. (n.d.). Davidson’s radiology of the kidney and genitourinary tract. . 3rd ed. Philadelphia: Saunders;1999, pp. 41–72. [Google Scholar]
- 26. Ramanathan S, Kumar D, Khanna M, Al Heidous M, Sheikh A, Virmani V, et al. Multi-modality imaging review of congenital abnormalities of kidney and upper urinary tract. World J Radiol 28, 2016; 8: 132–41. doi: 10.4329/wjr.v8.i2.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Houat AP, Guimarães CTS, Takahashi MS, Rodi GP, Gasparetto TPD, Blasbalg R, et al. Congenital anomalies of the upper urinary tract: a comprehensive review. Radiographics 2021; 41: 462–86. doi: 10.1148/rg.2021200078 [DOI] [PubMed] [Google Scholar]
- 28. Solanki S, Bhatnagar V, Gupta AK, Kumar R. Crossed fused renal ectopia: challenges in diagnosis and management. J Indian Assoc Pediatr Surg 2013; 18: 7–10. doi: 10.4103/0971-9261.107006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Babu Csr, Sharma V, Gupta OP. Renal fusion anomalies: a review of surgical anatomy. Anat Physiol 2015; s5: s5. doi: 10.4172/2161-0940.S5-001 [DOI] [Google Scholar]
- 30. Akdogan L, Oguz AK, Ergun T, Ergun I. The rarest of the rare: crossed fused renal ectopia of the superior ectopia type. Case Rep Nephrol 2015; 2015: 742419: e742419. doi: 10.1155/2015/742419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhattar R, Maheshwari A, Tomar V, Yadav SS. Crossed fused ectopic kidney: a case report. J Clin Diagn Res 2017; 11: PD11–12. doi: 10.7860/JCDR/2017/26944.10434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mudoni A, Caccetta F, Caroppo M, Musio F, Accogli A, Zacheo MD, et al. Crossed fused renal ectopia: case report and review of the literature. J Ultrasound 2017; 20: 333–37. doi: 10.1007/s40477-017-0245-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Njeh I, Chtourou K, BenHamida A. Relative ectopic kidney function quantification using dmsa tomoscintigraphy modality. J Healthc Eng 2020; 2020: 6092305. doi: 10.1155/2020/6092305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moon EH, Kim M-W, Kim YJ, Sun IO. Crossed fused renal ectopia: presentations on 99mtc-mag3 scan, 99mtc-dmsa spect, and multidetector ct. Clin Nucl Med 2015; 40: 835–37. doi: 10.1097/RLU.0000000000000937 [DOI] [PubMed] [Google Scholar]
- 35. Natarajan A, Agrawal A, Purandare N, Shah S, Rangarajan V. Rare case of thoracic kidney detected by renal scintigraphy. Indian J Nucl Med 2016; 31: 219–21. doi: 10.4103/0972-3919.181863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shah HU, Ojili V. Multimodality imaging spectrum of complications of horseshoe kidney. Indian J Radiol Imaging 2017; 27: 133–40. doi: 10.4103/ijri.IJRI_298_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dyer RB, Chen MY, Zagoria RJ. Classic signs in uroradiology. Radiographics 2004; 24 Suppl 1: S247-80. doi: 10.1148/rg.24si045509 [DOI] [PubMed] [Google Scholar]
- 38. Je B-K, Kim HK, Horn PS. Incidence and spectrum of renal complications and extrarenal diseases and syndromes in 380 children and young adults with horseshoe kidney. AJR Am J Roentgenol 2015; 205: 1306–14. doi: 10.2214/AJR.15.14625 [DOI] [PubMed] [Google Scholar]
- 39. Share JC, Lebowitz RL. The unsuspected double collecting system on imaging studies and at cystoscopy. AJR Am J Roentgenol 1990; 155: 561–64. doi: 10.2214/ajr.155.3.2117358 [DOI] [PubMed] [Google Scholar]
- 40. Fernbach SK, Feinstein KA, Spencer K, Lindstrom CA. Ureteral duplication and its complications. Radiographics 1997; 17: 109–27. doi: 10.1148/radiographics.17.1.9017803 [DOI] [PubMed] [Google Scholar]
- 41. Berrocal T, López-Pereira P, Arjonilla A, Gutiérrez J. Anomalies of the distal ureter, bladder, and urethra in children: embryologic, radiologic, and pathologic features. Radiographics 2002; 22: 1139–64. doi: 10.1148/radiographics.22.5.g02se101139 [DOI] [PubMed] [Google Scholar]
- 42. Doery AJ, Ang E, Ditchfield MR. Duplex kidney: not just a drooping lily. J Med Imaging Radiat Oncol 2015; 59: 149–53. doi: 10.1111/1754-9485.12285 [DOI] [PubMed] [Google Scholar]
- 43. Didier RA, Chow JS, Kwatra NS, Retik AB, Lebowitz RL. The duplicated collecting system of the urinary tract: embryology, imaging appearances and clinical considerations. Pediatr Radiol 2017; 47: 1526–38. doi: 10.1007/s00247-017-3904-z [DOI] [PubMed] [Google Scholar]
- 44. PALUBINSKAS AJ. Medullary sponge kidney. Radiology 1961; 76: 911–19. doi: 10.1148/76.6.911 [DOI] [PubMed] [Google Scholar]
- 45. Koraishy FM, Ngo T-TT, Israel GM, Dahl NK. CT urography for the diagnosis of medullary sponge kidney. Am J Nephrol 2014; 39: 165–70. doi: 10.1159/000358496 [DOI] [PubMed] [Google Scholar]
- 46. Pisani I, Giacosa R, Giuliotti S, Moretto D, Regolisti G, Cantarelli C, et al. Ultrasound to address medullary sponge kidney: a retrospective study. BMC Nephrol October 12, 2020; 21(1): 430. doi: 10.1186/s12882-020-02084-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lonergan GJ, Rice RR, Suarez ES. Autosomal recessive polycystic kidney disease: radiologic-pathologic correlation. Radiographics 2000; 20: 837–55. doi: 10.1148/radiographics.20.3.g00ma20837 [DOI] [PubMed] [Google Scholar]
- 48. Dillman JR, Trout AT, Smith EA, Towbin AJ. Hereditary renal cystic disorders: imaging of the kidneys and beyond. Radiographics 2017; 37: 924–46. doi: 10.1148/rg.2017160148 [DOI] [PubMed] [Google Scholar]
- 49. Rahbari-Oskoui F, Mittal A, Mittal P, Chapman A. Renal relevant radiology: radiologic imaging in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2014; 9: 406–15. doi: 10.2215/CJN.08940813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gradzik M, Niemczyk M, Gołębiowski M, Pączek L. Diagnostic imaging of autosomal dominant polycystic kidney disease. Pol J Radiol 2016; 81: 441–53. doi: 10.12659/PJR.894482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gaur P, Gedroyc W, Hill P. ADPKD-what the radiologist should know. Br J Radiol 2019; 92: 1098: 20190078. doi: 10.1259/bjr.20190078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kalaitzis C, Patris E, Deligeorgiou E, Sountoulides P, Bantis A, Giannakopoulos S, et al. Radiological findings and the clinical importance of megacalycosis. Res Rep Urol 2015; 7: 153–55. doi: 10.2147/RRU.S81519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pitt DC. Retrocaval ureter. Radiology April 1965; 84: 699–702. RESOURCE> .. doi: 10.1148/84.4.699 [DOI] [PubMed] [Google Scholar]
- 54. Hassan R, Aziz AA, Mohamed SKC. Retrocaval ureter: the importance of intravenous urography. Malays J Med Sci 2011; 18: 84–87. [PMC free article] [PubMed] [Google Scholar]
- 55. Fulgham PF, Assimos DG, Pearle MS, Preminger GM. Clinical effectiveness protocols for imaging in the management of ureteral calculous disease: aua technology assessment. J Urol 2013; 189: 1203–13: S0022-5347(12)05259-7. doi: 10.1016/j.juro.2012.10.031 [DOI] [PubMed] [Google Scholar]
- 56. Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis) ACR Appropriateness criteria [Internet]. acr.org]. 2015. Available from: https://acsearch.acr.org/docs/69362/Narrative/
- 57. Goldman SM, Sandler CM. Genitourinary imaging: the past 40 years. Radiology 2000; 215: 313–24. doi: 10.1148/radiology.215.2.r00ma29313 [DOI] [PubMed] [Google Scholar]
- 58. Gibson MS, Puckett ML, Shelly ME. Renal tuberculosis. Radiographics 2004; 24: 251–56. doi: 10.1148/rg.241035071 [DOI] [PubMed] [Google Scholar]
- 59. Jung YY, Kim JK, Cho K-S. Genitourinary tuberculosis: comprehensive cross-sectional imaging. AJR Am J Roentgenol 2005; 184: 143–50. doi: 10.2214/ajr.184.1.01840143 [DOI] [PubMed] [Google Scholar]
- 60. Dhangar DSP, Kothawala DIH, Patil DS, Kumar DA, Whatkar DA. Radiologic signs of genitourinary tuberculosis: an aid for earlier diagnosis. Int J Med Res 2016; 1: 01–08. [Google Scholar]
- 61. Radwan A, Menias CO, El-Diasty MT, Etchison AR, Elshikh M, Consul N, et al. Multimodality imaging of genitourinary tuberculosis. Curr Probl Diagn Radiol [Internet] 2020. [DOI] [PubMed] [Google Scholar]
- 62. Browne RFJ, Meehan CP, Colville J, Power R, Torreggiani WC. Transitional cell carcinoma of the upper urinary tract: spectrum of imaging findings. Radiographics 2005; 25: 1609–27. doi: 10.1148/rg.256045517 [DOI] [PubMed] [Google Scholar]