Abstract
Dissolution is a pivotal tool for oral formulations. Dissolution could be used to either reduce the risk of product failure through quality control or predict and understand in vivo performance of drug formulations. The latter is always challenging because multiple factors such as selection of media, gastrointestinal components, physiological factors, consideration of fasted and fed state are involved. Previously published dissolution methods such as one-step dissolution in individual simulated gastric fluid, simulated intestinal fluid, or phosphate buffer saline did not signify the realistic gastrointestinal transit effect. Docetaxel (DTX), a poorly water-soluble drug, is commercially available only as injectable dosage forms, and thus many publications studied the development of oral DTX formulations. In our previous report, we developed oral lipid-based DTX granules that showed higher oral absorption in rats compared to DTX powder. However, one-step dissolution in simulated gastric fluid showed no difference between DTX granules and DTX powder. Therefore, the present study aimed to develop new two-step biorelevant dissolution methods for DTX oral formulations. In the study, new two-step biorelevant dissolution methods in fasted or fed states with pancreatin were developed and compared with other previously reported dissolution methods. The new two-step biorelevant dissolution methods successfully discriminated the difference of dissolution between DTX granules and DTX powder, which reflected the in vivo difference of absorption of these two formulations. Moreover, food effects were confirmed for DTX. The new dissolution methods have the potential to be used to predict and understand in vivo performance of oral solid dosage forms.
Keywords: dissolution, biorelevant media, oral delivery, poorly water-soluble drugs, docetaxel
INTRODUCTION
Dissolution testing is a pivotal analytical parameter for oral solid dosage forms and is considered throughout product development and manufacturing. Dissolution testing could be used to either reduce the risk of product failure by matching quality control requirements or predict and understand in vivo performance. The latter is always a challenging task because multiple factors such as selection of appropriate media, components, physiological factors, consideration of fasted and fed conditions are involved in the design. However, irrespective of the purpose, compendial media that mimick only a pH range (from 1.2 to 7.4) of gastrointestinal fluids do not evaluate oral formulations in physiologic conditions. These media include simulated gastric fluid (SGF), simulated intestinal fluid (SIF), or phosphate buffer saline (PBS) with or without surfactants (1, 2). For poorly water-soluble drugs, adding surfactants such as Tween-80, sodium lauryl sulfate, and Triton-X 100 in the dissolution media is recommended (2, 3). However, these media are not physiologically relevant and often result in over or underestimation of drug release that leads to inapt prediction for in vivo performance (4).
To overcome these limitations and to predict the in vivo performance of drug formulations more closely, novel biorelevant dissolution methods have been introduced since last few years to simulate stomach and intestinal environment in terms of contents, in vivo hydrodynamics, pH, buffer capacity, surface tension, and osmolality (5, 6). Biorelevant dissolution methods could predict and help to understand in vivo performance, reduce the risk of drugs or formulations being incorrectly rejected, and render physiologically relevant results (7). In biorelevant media, fasted and fed state conditions need to be considered to understand the impact of meals on drug release. Taking physiological aspects, gastrointestinal transit (from low pH to high pH) after oral administration also should be considered for dissolution method development. Hence, performing dissolution in individual SGF, SIF, PBS, or biorelevant media as one step would not represent the realistic transit effect. A two-step dissolution, in which a formulation is initially put into gastric fluid and after certain time-points, physiological components are added into the same vessel to mimick intestinal fluid, is employed to closely resemble the gastrointestinal transit.
Among developed and under pipeline chemical entities, poorly water-soluble drugs own nearly 40% of the entire drugs, thus creating ample challenges for the development of effective oral formulations (8). Lipid-based formulations have been widely used to solve the challenges (9). Dissolution method development for these lipid-based formulations is highly critical. Because of limited solubility in the dissolution media, complex in vivo aspects such as rate of precipitation, digestion of lipids in the presence of enzymes, formation of different colloidal phases, and drug disposition have to be considered (10, 11). Docetaxel (DTX), a widely used anticancer drug, is a poorly water-soluble drug. It is commercially available only as intravenous formulations. Several investigations have been published on oral DTX formulations (12–16). Among few published reports, dissolution was performed as one-step either in a hydroalcoholic mixture or in PBS (pH 7.4) containing Tween-80 (12, 15, 16). These dissolution media do not mimic in vivo environment because Tween-80 or hydroalcoholic media are not physiologically present. Moreover, higher solubilizing effects produced by these media would mislead the prediction of in vivo performance. For example, in our previous study, we have developed lipid-based DTX granules that produce in situ self-assembled nanoparticles (ISNPs) upon contact with water. We tested the dissolution of DTX granules and DTX powder in SGF (pH 1.2) as well as in vivo absorption in rats (17). DTX powder and DTX granules had similar dissolution profiles, although the DTX granules produced over 10-fold higher liver absorption compared to DTX powder in rats (17). DTX granules produce lipid-based nanoparticles that could generate a supersaturated condition to facilitate DTX dissolution in vivo. Indeed, in our previous studies, DTX granules were given to mice at 5 mg/kg. At this dose, the concentration of DTX granules in the mouse stomach (~250 ug/mL) was over the saturated solubility of DTX. Therefore, for lipid-based formulations of poorly water-soluble drugs, new two-step biorelevant dissolution methods are especially warranted to compare different formulations and understand in vivo performance.
The present study aimed to develop new two-step biorelevant dissolution methods for DTX oral formulations. We developed new two-step biorelevant dissolution methods and compared them with previously reported dissolution methods for oral DTX formulations. Five different dissolution media studied for DTX granules and DTX powder included (i) one-step dissolution in PBS with 0.5% Tween-80, (ii) one-step dissolution in SGF, (iii) two-step dissolution in SGF and SIF with pancreatin, (iv) two-step biorelevant dissolution in fasted SGF (FaSGF) and fasted SIF (FaSIF) with pancreatin, and (v) two-step biorelevant dissolution in fed SGF (FeSGF) and fed SIF (FeSIF) with pancreatin. Among them, media (i) and (ii) are general compendial dissolution media given to guide the development of dissolution methods for specific drugs. In the past, very few reports were published wherein biorelevant dissolution media has been validated and used for in vitro simulation (4, 7). To the best of our knowledge, this is the first report wherein two-step biorelevant dissolution methods were developed and compared with compendial dissolution media for oral DTX formulations.
MATERIALS AND METHODS
Materials
DTX was purchased from LC Laboratories (Woburn, MA). Sodium taurocholate was purchased from Merck Millipore (Billerica, MA). Potassium dihydrogen phosphate, disodium hydrogen phosphate, potassium chloride, calcium chloride, and tris-hydroxymethyl aminomethane (TRIS) maleate were purchased from Acros Organics (Fair Lawn, NJ). Tween-80 was obtained as a gift from BASF (Florham Park, NJ). Pepsin and acetonitrile (HPLC grade) were purchased from Fisher Scientific (Pittsburgh, PA). Sodium chloride, sodium hydroxide, hydrochloric acid, and acetic acid were purchased from Sigma-Aldrich (St. Louis, MO). Pancreatin powder (from porcine pancreas) was purchased from Thermo Scientific (Fail Lawn, NJ). Powdered lecithin was purchased from MP biomedicals (Solon, OH). Whole milk manufactured by Great Value was purchased commercially from Walmart store.
The media were prepared according to the literature (3, 4, 18, 19), and the composition of each medium is summarized in Table I. To prepare pancreatin solution, 20% of pancreatin powder were dispersed in distilled water and centrifuged for 15 min at 5000 rpm at 5°C. The clear supernatant was collected as the pancreatin solution used in the experiments below. The pancreatin solution was prepared freshly and stored in a refrigerator until used. Lecithin and taurocholate need a long time to dissolve. Thus, FaSIF and FeSIF were prepared 1 day before the experiments and stored in a refrigerator until used. For FeSGF, a solution containing acetate buffer and sodium chloride was mixed with milk at a 1:1 ratio on the day of the experiments. The medium was adjusted to pH 5.0 to make the final FeSGF.
Table I.
| Composition (mM) | PBS pH 7.4 Original | SGF pH 1.2 Original | FaSGF pH 1.6 Original | FeSGF pH 5.0 Original | SIF pH 6.8 Original/concentrateda | FaSIF pH 6.5 Original/concentrateda | FeSIF pH 5.8 Original/concentrateda |
|---|---|---|---|---|---|---|---|
| Sodium chloride | 137 | 34.3 | 34.2 | 237 | - | 68.6/ 137 | 126/ 251 |
| Potassium chloride | 2.70 | - | - | - | - | - | - |
| Sodium hydroxide | - | - | - | - | qs to pH | qs to pH | qs to pH |
| Potassium dihydrogen phosphate | 1.80 | - | - | - | 49.9/ 99.9 | - | - |
| Disodium hydrogen phosphate | 10.0 | - | - | - | - | - | - |
| Hydrochloric acid | - | qs to pH | qs to pH | qs to pH | - | - | - |
| Acetic acid | - | - | - | 17.1 | - | - | - |
| Sodium acetate | - | - | - | 29.8 | - | - | - |
| Whole milk | - | - | - | 1:1b | - | - | - |
| Pepsin | - | 0.092 | 0.0029 | - | - | - | - |
| TRIS maleate | - | - | - | - | - | 2/ 4 | 50/ 100 |
| Calcium chloride | - | - | - | - | - | 1.87/ 3.74 | 5/ 10 |
| Sodium taurocholate | - | - | 0.08 | - | - | 3/ 6 | 10/ 20 |
| Lecithin | - | - | 0.02 | - | - | 0.75/ 1.5 | 2/ 4 |
| Tween-80 | 3.82 | - | - | - | - | - | - |
TRIS tris-hydroxymethyl aminomethane, PBS phosphate buffer saline, SGF simulated gastric fluid, FaSGF fasted state simulated gastric fluid, FeSGF fed state simulated gastric fluid, SIF simulated intestinal fluid, FaSIF fasted state simulated intestinal fluid, FeSIF fed state simulated intestinal fluid
Concentrated SIF, FaSIF, and FeSIF media were added after completion of the first step at 30 min to adjust the media for the second step in two-step biorelevant dissolution
Sodium chloride, acetic acid, and sodium acetate were dissolved in 1 L distilled water and mixed with whole milk at a 1:1 ratio to get final FeSGF
Preparation of DTX Formulations for Dissolution
DTX granules were prepared as reported previously (17). Briefly, DTX powder was added into a mixture of Miglyol 812 and TPGS (1:1 ratio) and mixed on a hot plate for 20 min at 50°C. Aeroperl 300 was then added into this mixture, allowed to mix and cool down at room temperature to obtain DTX granules. DTX powder was prepared by mixing free DTX with Avicel pH. The drug loading of DTX powder and DTX granules was 9.3%. For each dissolution study, 50 mg of DTX powder or DTX granules, equivalent to 4.6 mg of DTX, was added into the dissolution medium.
Size Measurement of DTX Granules
DTX granules were placed in Milliq water, vortexed for 30 s, and centrifuged at 14,000 rpm for 5 min at room temperature. To measure particle size, the supernatant was diluted with Milli-Q water and measured by Zetasizer Ultra (Malvern Panalytical, Malvern, UK).
Solubility Measurements of DTX in Dissolution Media
The solubility of DTX was measured in dissolution media. Briefly, in a glass vial, excess of DTX was added into 5 mL of individual dissolution medium. Vials were placed on a digital block heater (Thermo Scientific, Waltham, MA) for overnight mixing at 37°C. After 12 h, the suspension was centrifuged at 14,000 rpm for 5 min, and 0.1 mL of supernatant was diluted with 1.4 mL of methanol, which was further vortexed at 3000 rpm for 1 min and centrifuged at 14,000 rpm for 5 min to precipitate impurities produced after the addition of methanol. After centrifugation, DTX concentration in the supernatant was measured by a previously reported HPLC method (17). The solubility measurement was conducted three times for each medium.
In Vitro Lipolysis in FaSIF
Lipolysis of DTX granules in FaSIF (pH 7.4) in the presence and absence of pancreatin was performed to characterize the digestion of lipid in DTX granules as previously described with modification (19, 20). FaSIF (36 mL) was taken in a glass beaker under continuous stirring at 300 rpm at 37°C. DTX granules (50 mg) were added to the glass vessel. After dispersion for 10 min, 4 mL of pancreatin solution were added into the vessel to initiate digestion. Free fatty acids (FFAs) generated during lipolysis were titrated against 0.05 M NaOH to achieve the endpoint (pH 7.4) using a pH-stat titrator (Easy pH, Mettler Toledo). To measure particle size, 0.5 mL of samples was withdrawn before the addition of pancreatin and after the endpoint, and particle size was measured by Zetasizer Ultra. The experiments were conducted three times.
One-Step Dissolution in PBS with 0.5% Tween-80
Dissolution of DTX powder and DTX granules in PBS (pH 7.4) containing 0.5% Tween-80 was studied over 4 h. In our previous studies, DTX granules were given to mice at 5 mg/kg. At this dose, the concentration of DTX granules in the mouse stomach (~250 ug/mL) was over the saturated solubility of DTX. To mimic the condition and understand the oral absorption of DTX granules, the dissolution was not performed in a sink condition. Briefly, DTX powder or DTX granules (equivalent to 4.6 mg of DTX) were placed in 36 mL of PBS with 0.5 Tween-80 in a glass beaker and stirred at 100 rpm at 37°C for 4 h. At predetermined time intervals, 1 mL of sample was withdrawn and centrifuged at 14,000 rpm for 5 min to obtain clear supernatant. One part of this supernatant (0.2 mL) was diluted with methanol and vortexed at 3000 rpm for 1 min and further centrifuged at 14,000 rpm for 5 min to remove impurities produced after the addition of methanol. After centrifugation, DTX concentration in the supernatant was measured by HPLC as previously described (17). The other part of the supernatant (0.2 mL) was diluted with Milli-Q water to measure particle size by Zetasizer Ultra. The experiments were conducted three times.
One-Step Dissolution in SGF
Dissolution of DTX powder and DTX granules was performed in SGF (pH 1.2) as described above for the dissolution in PBS with 0.5% Tween 80. Instead of using PBS with 0.5% Tween 80, SGF was used as the dissolution medium. The experiments were conducted three times.
Two-Step Dissolution in SGF and SIF with Pancreatin
Dissolution of DTX powder and DTX granules in two-step dissolution media containing SGF (pH 1.2) and SIF (pH 6.8) in the presence of pancreatin was performed over 4.5 h. Briefly, DTX powder or DTX granules were placed in a glass vessel with 21 mL of SGF under continuous stirring at 100 rpm at 37°C. At 10, 20, and 30 min, 1 mL of sample was withdrawn and centrifuged to obtain clear supernatant. At 30 min, 18 mL of concentrated SIF was added into the same vessel to get 36 mL of total volume, and 0.2 mL of 2 M NaOH was added to change the pH to 6.8. Pancreatin solution (4 mL) was then added to the vessel to initiate digestion. Samples were continually withdrawn at 45, 60, 120, 180, 210, and 270 min. Concentrations of DTX in samples were measured by HPLC, and particle size was measured by Zetasizer Ultra as described above for the dissolution in PBS with 0.5% Tween 80. The experiments were conducted three times.
Two-Step Dissolution in FaSGF and FaSIF with Pancreatin
Dissolution of DTX powder and DTX granules in two-step dissolution media containing FaSGF (pH 1.6) and FaSIF (pH 6.5) in the presence of pancreatin was performed over 4.5 h. Briefly, DTX powder or DTX granules were placed in a glass beaker with 21 mL of FaSGF under continuous stirring at 100 rpm at 37°C. After 30 min, 18 mL of concentrated FaSIF was added into the same vessel to get 36 mL of total volume, and 0.18 mL of 2 M NaOH was added to change the pH to 6.5. Pancreatin solution (4 mL) was then added to the vessel to initiate digestion. Sampling time points and sample measurement were performed as described above for the two-step dissolution in SGF and SIF with pancreatin. The experiments were conducted three times.
Two-Step Dissolution in FeSGF and FeSIF with Pancreatin
Dissolution of DTX powder and DTX granules in two-step dissolution media containing FeSGF (pH 5.0) and FeSIF (pH 5.8) in the presence of pancreatin was performed over 4.5 h. Instead of using FaSGF and FaSIF as described above, FeSGF and FeSIF were used to conduct the experiments. Sampling time points and sample measurements were performed as described above for the two-step dissolution in SGF and SIF with pancreatin. The experiments were conducted three times.
Statistical Analysis
Each measurement was performed in triplicate. The results were reported as mean ± standard deviation (n = 3). The data were analyzed for statistical significance (p < 0.05) using an unpaired two-tailed Student t test at 95% confidence level.
RESULTS
DTX Granules and Nanoparticle Formation
DTX granules were prepared by mixing DTX, Miglyol 812, TPGS, and Aeroperl 300. Upon contact with water, Miglyol 812 and TPGS were released from Aeroperl 300 and formed ISNPs that encapsulated DTX. DTX ISNPs had particle size around 150 nm with narrow size distribution indicated by polydispersity index (PI.) below 0.3.
Solubility Measurements of DTX in Dissolution Media
The solubility of DTX in the compendial and biorelevant dissolution media was determined as shown in Table II. In case of the gastric fluids, solubility of DTX followed the order as FaSGF < SGF < FeSGF (1.56 μg/mL < 3.61 μg/mL < 20.3 μg/mL). Thus, the small amounts of sodium taurocholate and lecithin added in FaSGF did not increase the solubility compared to SGF. Solubility of DTX in the intestinal fluids was in the order of SIF < FaSIF < FeSIF (4.86 μg/mL < 5.93 μg/mL < 9.04 μg/mL with pancreatin or 3.87 μg/mL < 5.59 μg/mL < 7.02 μg/mL without pancreatin).
Table II.
Solubility of Docetaxel in the Dissolution Media (n=3)
| Dissolution media | Solubility at 37°C (μg/mL) |
|---|---|
| PBS with 0.5% Tween-80 | 32.5 ± 0.57 |
| SGF pH 1.2 | 3.61 ± 0.52 |
| FaSGF pH 1.6 | 1.56 ± 0.47 |
| FeSGF pH 5.0 | 20.3 ± 0.60 |
| SIF pH 6.8 | 3.87 ± 0.26 |
| SIF pH 6.8 + pancreatin | 4.86 ± 0.36 |
| FaSIF pH 6.5 | 5.59 ± 0.38 |
| FaSIF pH 6.5 + pancreatin | 5.93 ± 0.42 |
| FeSIF pH 5.8 | 7.02 ± 0.54 |
| FeSIF pH 5.8 + pancreatin | 9.04 ± 0.10 |
| SGF pH 1.2 + SIF pH 6.8 | 6.43 ± 0.08 |
| SGF pH 1.2 + SIF pH 6.8 + pancreatin | 7.88 ± 0.57 |
| FaSGF pH 1.6 + FaSIF pH 6.5 | 2.58 ± 0.50 |
| FaSGF pH 1.6 + FaSIF pH 6.5 + pancreatin | 4.63 ± 0.27 |
| FeSGF pH 5.0 + FeSIF pH 5.8 | 41.3 ± 1.23 |
| FeSGF pH 5.0 + FeSIF pH 5.8 + pancreatin | 47.9 ± 0.65 |
In general, adding pancreatin increased the solubility of DTX in the medium (p < 0.05), except for FaSIF because the solubility of DTX in FaSIF and FaSIF with pancreatin had no significant difference (p > 0.05). Without pancreatin, solubility of DTX in FaSIF (5.59 μg/mL) was significantly higher than that in SIF (3.87 μg/mL) (p < 0.05); however, with pancreatin they had no difference (4.86 μg/mL vs 5.93 μg/mL) (p > 0.05). Among the single medium, the solubility of DTX in PBS with 0.5% Tween-80 was the highest (32.5 μg/mL) followed by 20.3 μg/mL in FeSGF. The combination of FaSGF and FaSIF did not significantly increase solubility compared to the single medium. However, the combination of FeSGF and FeSIF significantly increased solubility compared to either FeSGF or FeSIF. In all tested media, the combination of FeSGF and FeSIF with pancreatin gave the highest solubility (47.9 μg/mL).
In Vitro Lipolysis of DTX Granules
In vitro lipolysis of DTX granules in FaSIF (pH 7.4) in the presence of pancreatin was performed. As shown in Figure 1A, before the addition of pancreatin, monodispersed DTX ISNPs (127.9 nm) generated by DTX granules were observed. After the addition of pancreatin for 60 min, a subpopulation at 50 nm was detected and particles became multidispersed.
Fig. 1.
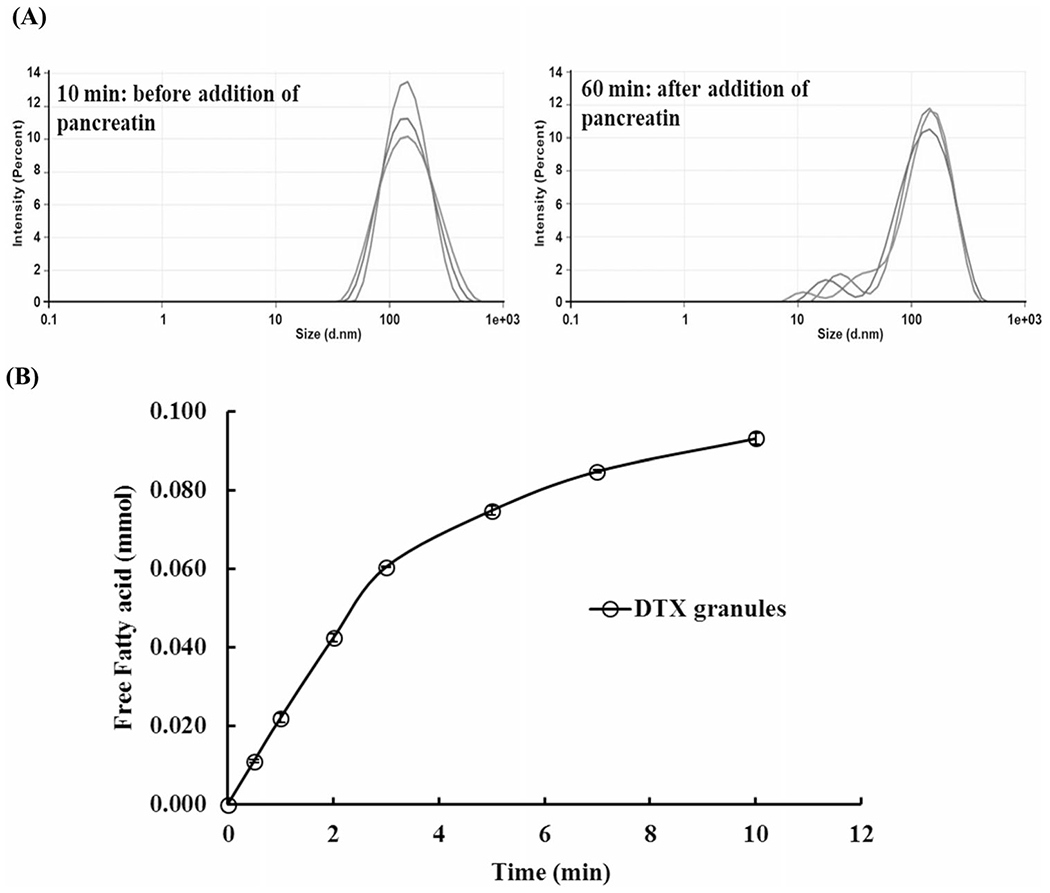
In vitro lipolysis of DTX granules in FaSIF (pH 7.4). A Particle size in the medium with DTX granules at 10 min before the addition of pancreatin and at 60 min at the end of lipolysis. B The amount of free fatty acid produced during the lipolysis of DTX granules in the presence of pancreatin (n=3)
The initial pH of FaSIF was 7.4. Right after the addition of pancreatin, pH was dropped to 6.87, indicating the production of fatty acid by lipid digestion. The endpoint of pH 7.4 was achieved within 10 min with the titration using 0.05 M NaOH. Totally, 0.093 mmol free fatty acid was released during the study (Figure 1B). As a control, DTX granules without pancreatin maintained the pH of FaSIF at 7.4 over 60 min, indicating there was no lipid digestion in the control.
One-Step Dissolution in PBS with 0.5% Tween-80
PBS with 0.5% Tween-80 produced 13.8 nm of monodispersed particles (Figure 2A) because the critical micelle concentration of Tween-80 is around 0.3% (21). However, the presence of Tween-80 did not affect the particle size of DTX ISNPs. As shown in Figure 2A, the particle size of DTX ISNPs did not significantly change over 4 h (p > 0.05).
Fig. 2.
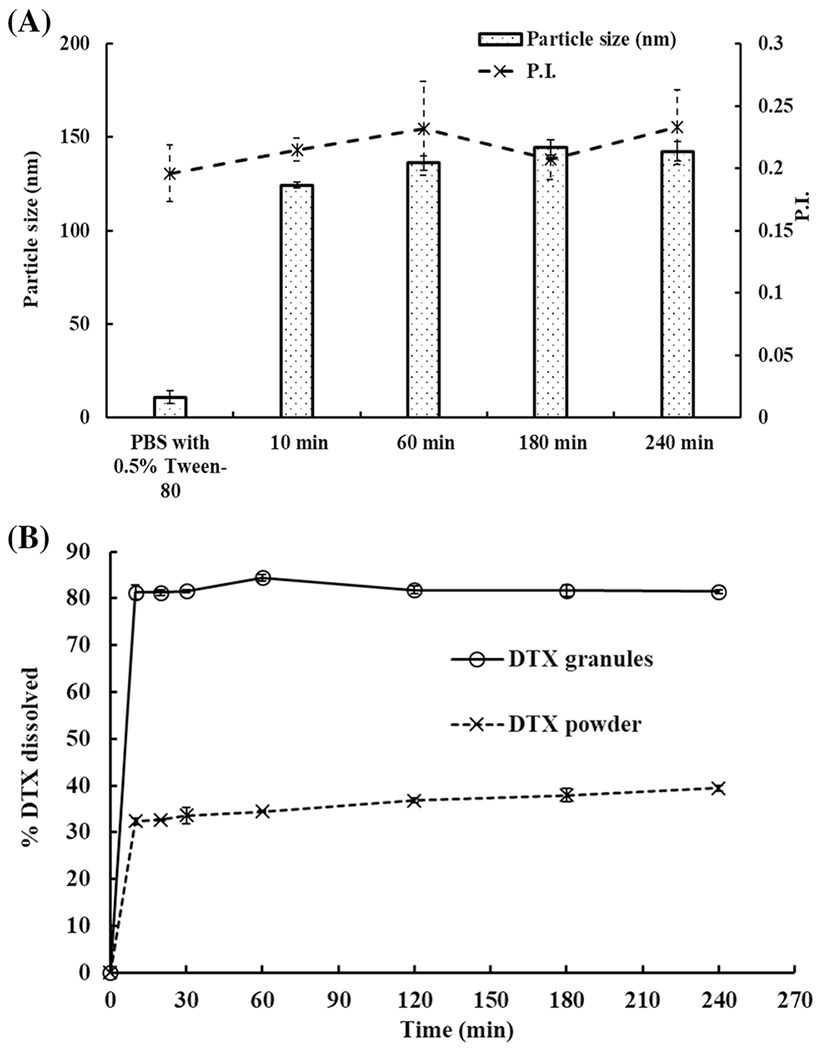
One-step dissolution of DTX granules and DTX powder in PBS (pH 7.4) with 0.5% Tween-80. A Particle size in the medium with DTX granules at 10 min, 60 min, 180 min and 240 min (n=3). B One-step dissolution profiles of DTX granules and DTX powder in PBS with 0.5% Tween-80 over 4 h (n=3)
Dissolution of DTX granules and DTX powder in PBS with 0.5% Tween-80 showed 80% and 32% of DTX release within 10 min, respectively, and then kept constant for 4 h (Figure 2B).
One-Step Dissolution in SGF
Dissolution of DTX granules and DTX powder in SGF was performed over 2 h. As per Figure 3A, DTX granules in SGF showed intact NPs over 2 h and particle size was below 150 nm. The result demonstrated the stability of DTX ISNPs in the SGF for 2 h, and it was in line with our previously reported size stability study on DTX granules (17).
Fig. 3.
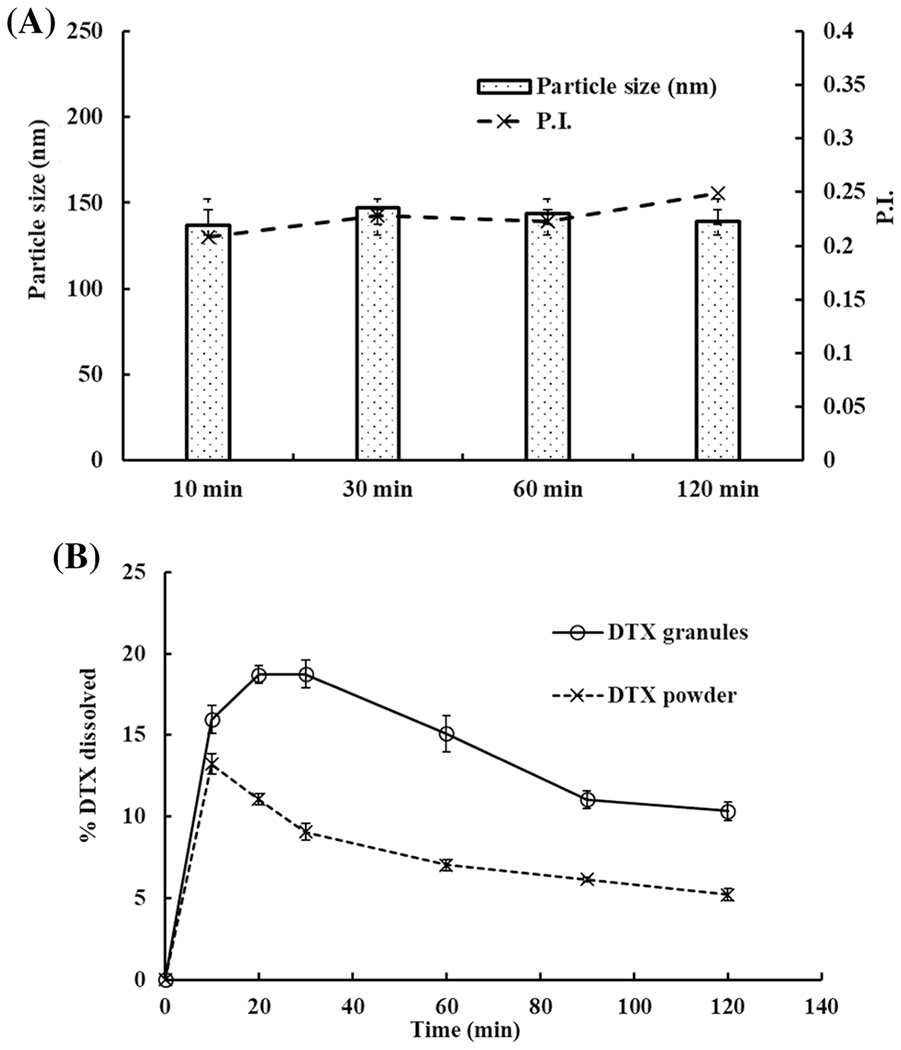
One-step dissolution of DTX granules and DTX powder in SGF (pH 1.2). A Particle size in the medium with DTX granules at 10 min, 30 min, 60 min, and 120 min (n=3). B One-step dissolution profiles of DTX granules and DTX powder measured over 2 h (n=3)
As per Figure 3B, within 20 min 18% of DTX were released from DTX granules, and then DTX release was declined to 10% at 2 h. In contrast, DTX powder showed 13% DTX release at 10 min after which DTX release gradually decreased to 5% at 2 h. The decline from both groups could be as a result of precipitation of initially dissolved DTX (within the first 20 min) in SGF due to poor water solubility. However, there was a significant difference (p < 0.05) between both the groups at each time point.
Two-Step Dissolution in SGF and SIF with Pancreatin
Two-step dissolution of DTX granules and DTX powder in SGF for 30 min followed by SIF over 4.5 h in the presence of pancreatin was performed. As per Figure 4A, DTX granules showed intact ISNPs in SGF. After switching to SIF with pancreatin, ISNPs remained intact over 4.5 h. The data demonstrated the size stability of the DTX ISNPs in the SGF and SIF media in the presence of pancreatin.
Fig. 4.
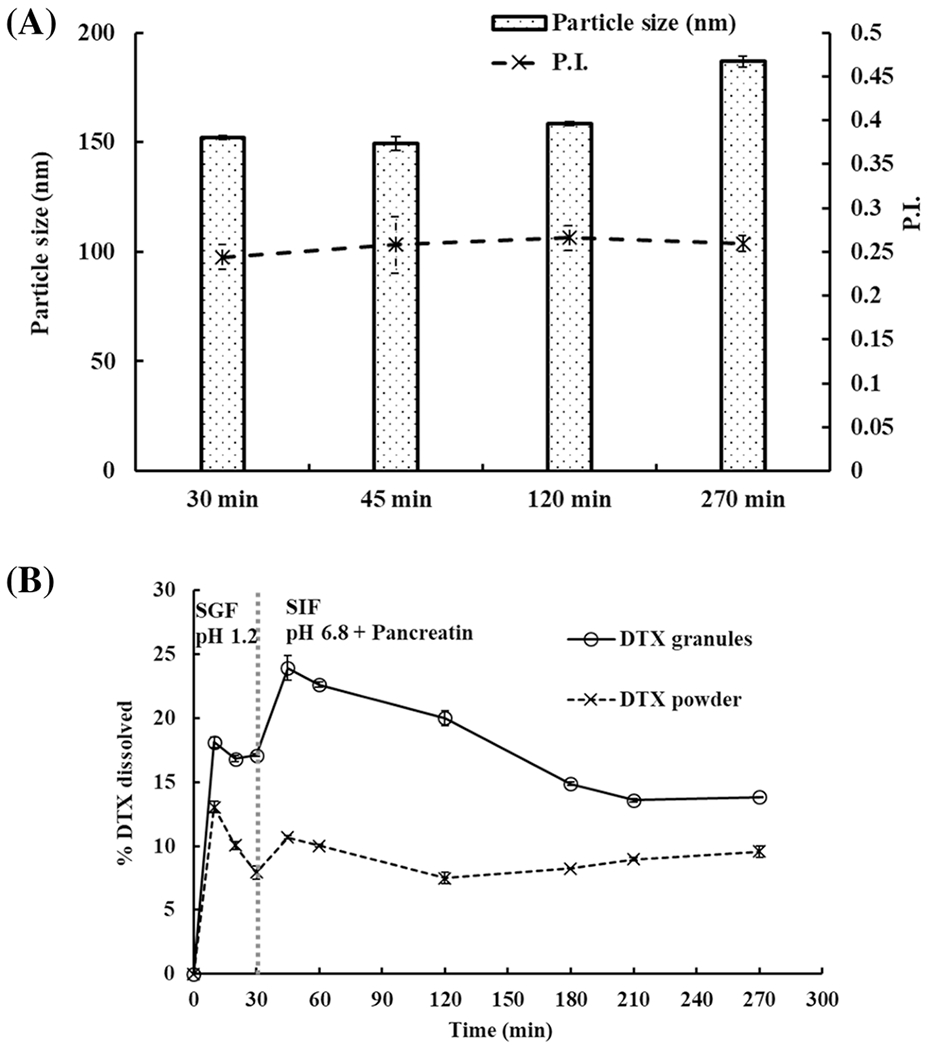
Two-step dissolution of DTX granules and DTX powder in SGF (pH 1.2) for 30 min and in SIF (pH 6.8) with pancreatin for 4.5 h. A Particle size in the medium with DTX granules at 30 min, 45 min, 120 min, and 270 min. B Two-step dissolution profiles of DTX granules and DTX powder measured over 4.5 h (n=3)
In SGF, 18% of DTX were released from DTX granules within 10 min (Figure 4B). After switching to SIF with pancreatin, DTX release from DTX granules increased to 24% at 45 min (Figure 4B). For DTX powder, the highest release was observed at 10 min in SGF. After changing to SIF with pancreatin, the release did not increase. DTX release from granules and powder in two-step dissolution showed a significant difference at all time points (p < 0.05).
Two-Step Dissolution in FaSGF and FaSIF with Pancreatin
Two-step dissolution of DTX granules and DTX powder in fasted state biorelevant media containing FaSGF for 30 min followed by FaSIF in the presence of pancreatin over 4.5 h was performed. Figure 5A shows the particle size in the media before and after dissolution. Surfactants such as sodium taurocholate and lecithin that could form colloid particles were added into the media; thus, FaSGF and FaSIF contained particles at 300 nm and 100 nm, respectively. Adding pancreatin increased particle size to 550 nm. During the dissolution study, the particle size of the media was maintained at 100 nm.
Fig. 5.
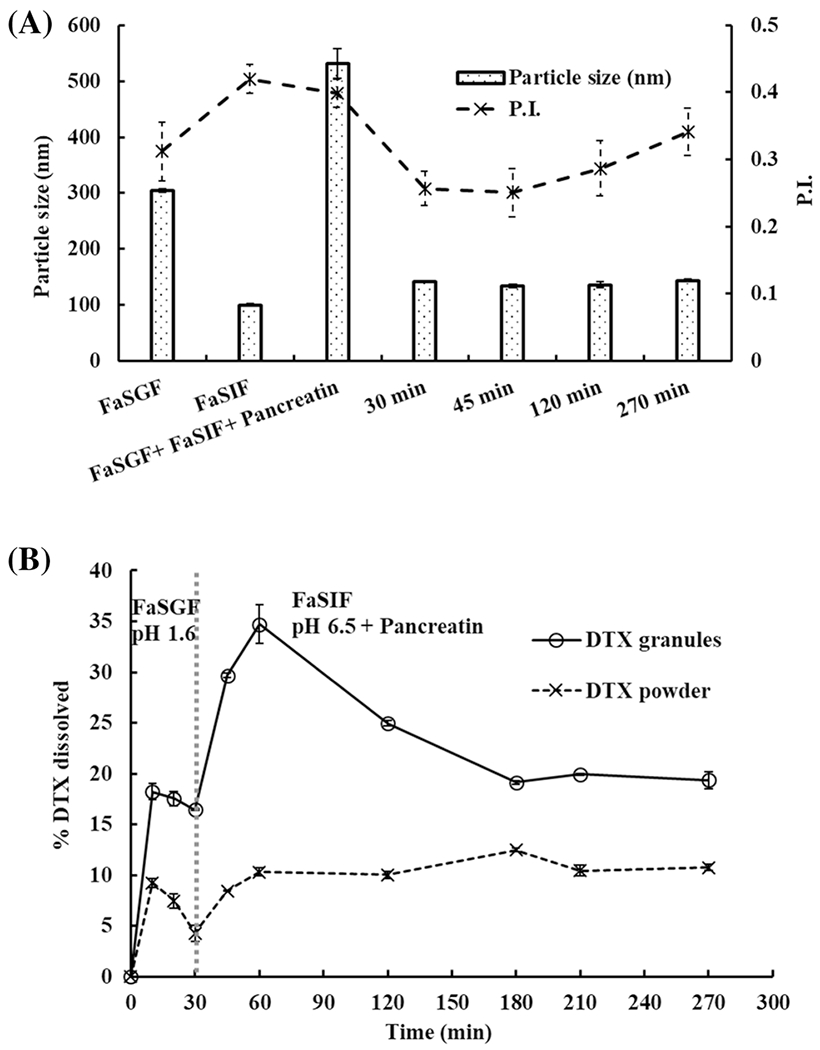
Two-step dissolution of DTX granules and DTX powder in FaSGF (pH 1.6) for 30 min and in FaSIF (pH 6.5) for 4.5 h with pancreatin. A Particle size in the medium with DTX granules at 30 min, 45 min, 120 min, and 270 min. B Two-step dissolution profiles of DTX granules and DTX powder measured over 4.5 h (n=3)
As shown in Figure 5B, DTX was released from DTX granules up to 18% at 10 min in the FaSGF. After switching to FaSIF with pancreatin, DTX further released from DTX granule to 33% at 1 h and then declined to 20% at 3 h. The maximum release of DTX from DTX powder was 10% in both FaSGF and FaSIF. There was a significant difference (p < 0.05) between the release of DTX from granules and powder in the fasted state dissolution at all the time points
Two-Step Dissolution in FeSGF and FeSIF with Pancreatin
Two-step biorelevant dissolution of DTX granules and DTX powder in a fed state containing FeSGF for 30 min followed by FeSIF in the presence of pancreatin over 4.5 h was performed. In FeSGF, milk and surfactants were added into the media, which generated colloid particles. Thus, the blank media contained particles with a size around 300 nm (Figure 6A). During the dissolution, although particle size in the medium with DTX granules did not change, particle size distribution was various as indicated by P.I. > 0.3. After the addition of pancreatin, particle size became multidispersed.
Fig. 6.
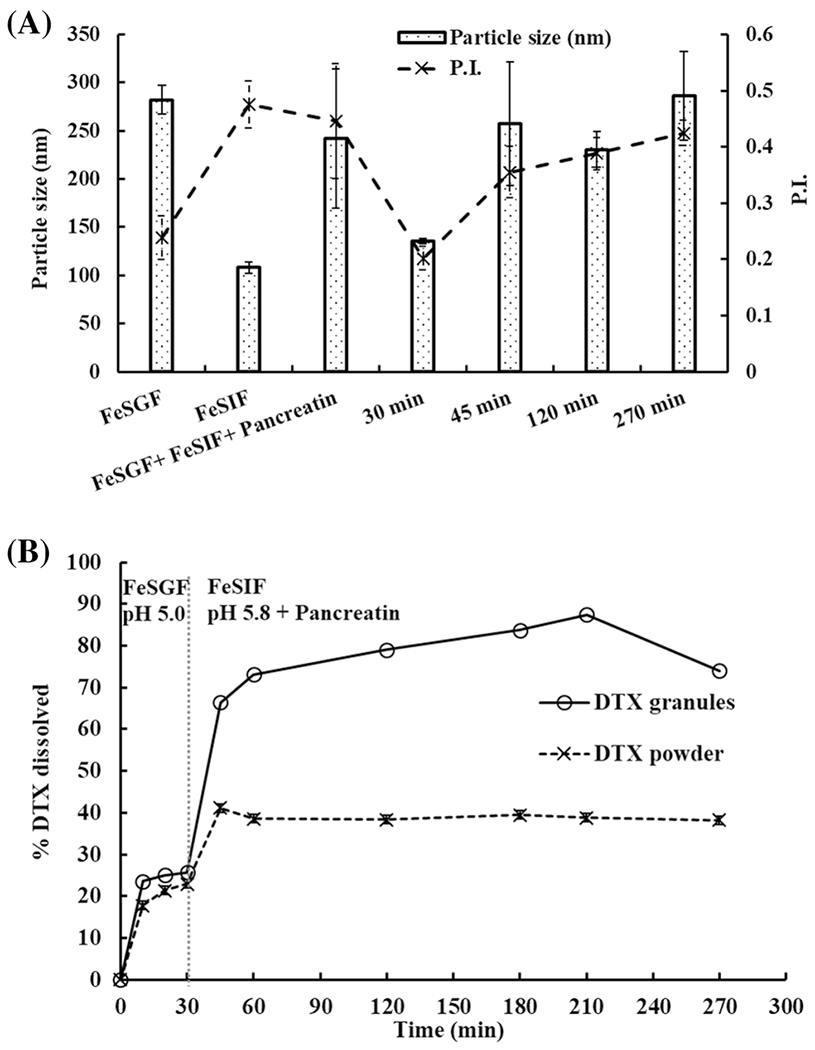
Two-step dissolution of DTX granules and DTX powder in FeSGF (pH 5.0) for 30 min and in FeSIF (pH 5.8) with pancreatin for 4.5 h. A Particle size in the medium with DTX granules at 30 min, 45 min, 120 min, and 270 min. B Two-step dissolution profiles of DTX granules and DTX powder measured over 4.5 h (n=3)
As shown in Figure 6B, in the FeSGF, 25% of DTX were released from DTX granules within 10 min and 22% of DTX was released from DTX powder within 20 min. After switching to FeSIF with pancreatin at 30 min, DTX quickly released from DTX granules to 66% at 45 min and reached a maximum release of 88% at 3.5 h, while DTX powder reached 40% release at 45 min and maintained up to 4.5 h. There was a significant difference (p < 0.05) between the release of DTX from granules and powder in the fed state dissolution at all the time points.
DISCUSSION
Dissolution has been used by research laboratories, industries, and regulatory agencies for various purposes such as formulation development, batch-to-batch consistency, and approval of solid oral dosage forms. Dissolution also is used to compare different formulations to select an optimal formulation, give guidance for in vivo evaluation, and understand oral absorption. For BCS IV compounds such as DTX, due to their poor solubility and poor permeability, it is often challenging to select a dissolution method that could predict in vivo performance of drug formulations. Dissolution of the poorly soluble drugs has been performed in compendial media such as SGF, SIF, and PBS with or without a surfactant (3, 18). In addition, for poorly soluble drugs, it has been recommended to study dissolution using the biorelevant test media to mimic the transaction of drug formulations in the entire gastrointestinal tract (4, 7). Here, we tested five different dissolution methods to compare DTX granules and DTX powder to develop two-step biorelevant dissolution methods.
Solubility is a key driving force for drug dissolution. The solubility of DTX in each tested medium was measured. The addition of different components in dissolution media significantly impacted drug solubility in the media. As expected, surfactants such as Tween-80, lecithin, and bile salts increased DTX solubility in the media (Table II). Moreover, food components such as milk and TRIS maleate greatly increased DTX solubility, indicating there would be food effects for this drug. Interestingly, the addition of pancreatin also increased DTX solubility. Milk and surfactants produced colloid particles in the fasted and fed state media (Figures 5A and 6A), which could further increase solubility (22, 23). However, because of colloid particles in blank media, monitoring particle size of drug nanoparticles during the dissolution testing is impossible by using current experimental techniques. It needs caution when one tries to correlate the dissolution of drug nanoparticles with nanoparticle size. A two-step biorelevant dissolution involves both gastric and intestinal fluids. When two fed state media (FeSGF and FeSIF) were combined, milk, bile salt, and surfactant from each medium were combined in one medium. Also, pancreatin digested milk to produce other components in media. These materials added together produced more colloid particles and solubilization powder than those in a single medium, which significantly increased DTX solubility. As shown in Figure 6A, stable colloid particles were detected in the media in the course of two-step dissolution. Thus, synergistic effects were observed as the combination caused higher solubility than any single medium (Table II). Moreover, DTX granules and DTX powder showed different impacts on DTX solubility. For DTX powder, the final concentration of DTX in the dissolution testing was similar to its solubility in the corresponding medium. However, for DTX granules, the final concentration of DTX in the dissolution testing was 2-fold higher than its solubility in the medium, indicating DTX granules generated a supersaturated condition during the dissolution that facilitated DTX release. Surfactant and lipid in DTX granules could be attributed to this supersaturated condition observed in the dissolution studies.
According to our previous animal studies, DTX granules increased 10-fold drug absorption in the liver compared to DTX powder (17). In this previous publication, we measured the dissolution of DTX granules and DTX powder in a concentration close to a sink condition using USP paddles at 75 rpm and did not observe a significant difference in dissolution profiles between DTX granules and DTX powder (17). In this study, more concentration of DTX was used in SGF dissolution. Although there was a significant difference when comparing the dissolution at each time point (p < 0.05), the total release of DTX granules and DTX powder in SGF were similar, 18% vs 13% (Figure 3B). This means SGF is not a suitable medium to compare DTX granules and DTX powder to understand in vivo performance. Although PBS with 0.5% Tween-80 differentiated DTX granules and DTX powder (Figure 2), this medium is irrelevant to physiological conditions and cannot be used to predict and understand in vivo performance. In fact, media with surfactants are normally used for quality control during drug manufacturing to control batch-to-batch consistency (24). Oral drug absorption involves two steps—in the stomach and small intestine. To mimic the physiological environment in drug absorption, two-step biorelevant dissolution is preferred. Without considering food effects, the two-step dissolution was performed in SGF for 30 min and then in SIF with pancreatin for 3.5 h (Figure 4B). In this method, DTX granules showed 10% higher release than DTX powder. However, less than 25% of DTX was released from DTX granules, which could not explain the good absorption of DTX granules we observed in animal studies. The two-step dissolution in fasted condition had similar results with the two-step dissolution in SGF and SIF (Figures 4B and 5B). When food effects were considered, the combination of fed state media significantly increased the solubility of DTX (Table II) because of the synergistic effects; thus, the DTX release increased to 88% (Figure 6B). Compared to DTX powder, DTX granules increased 40% of release in the two-step fed state dissolution. The overall results demonstrated that DTX had food effects on oral absorption. Comparing DTX granules and DTX powder for food effects, food had a higher impact on DTX granules than DTX powder, because the increase of dissolution caused by food was 50% for DTX granules and 30% for DTX powder, respectively (Figures 5B and 6B). DTX is a BCS IV drug. Food components in gastric and intestinal fluids increased the solubility of DTX (Table II). Thus, there were food effects observed for DTX powder. DTX granules were lipid-based granules. Miglyol 812, the lipid in DTX granules, was continually digested by pancreatin to produce free fatty acid just like a lipid in food (Figure 1). It is known that lipid digestion stimulates the body to produce bile salts that facilitate drug dissolution and absorption (4, 7). Thus, DTX granules showed more food effects than DTX powder. In our previous animal studies, we did not control food consumption. Indeed, we observed more variation in absorption in animals treated with DTX granules than in those treated with DTX powder. Now, the different variation previously observed in the two treatment groups is explained by the dissolution results here. For future animal studies about oral absorption of DTX formulations, controlling food consumption is recommended.
CONCLUSIONS
While there are several dissolution methods reported, methods for poorly soluble and poorly permeable drugs that simulate in vivo performance have remained a huge challenge in the area of oral drug delivery. By using DTX oral formulations as a model BCS IV drug formulation, two-step biorelevant dissolution methods including fasted and fed conditions were developed. DTX granules spontaneously produced DTX ISNPs upon contact with water. Particle size measurement of DTX ISNPs was interrupted by colloid particles that were produced by food components and surfactants in the dissolution media. Food significantly increased the solubility of DTX. The two-step dissolution studies in fasted condition and fed condition further confirmed the food effects on DTX. DTX granules were more impacted by food effects than DTX powder, because of the lipid component in DTX granules. The two-step biorelevant dissolution results explained the variation observed in previous animal studies in which food consumption was not controlled. In summary, the two-step biorelevant dissolution methods have the potential to be used to predict and understand in vivo performance of oral solid dosage forms.
Funding
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R35GM138225 to Dong, X.
Footnotes
Conflict of Interest The authors declare no competing interests.
References
- 1.Gray VA, Rosanske TW. Dissolution. 2nd; Elsevier; 2020. [Google Scholar]
- 2.Shohin I, Grebenkin DY, Malashenko EA, Stanishevskii YM, Ramenskaya GV. Dissolution technologies. 2016;23(3):6–11. 10.14227/DT230316P6. [DOI] [Google Scholar]
- 3.Jantratid E, Janssen N, Reppas C, Dressman JB. Pharm Res. 2008;25(7):1663–76. 10.1007/s11095-008-9569-4. [DOI] [PubMed] [Google Scholar]
- 4.Klein S AAPS J. 2010;12(3):397–406. 10.1208/s12248-010-9203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koziolek M, Schneider F, Grimm M, Modebeta C, Seekamp A, Roustom T, Siegmund W, Weitschies W. J Control Release. 2015;220(Pt A):71–8. 10.1016/j.jconrel.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Mehtani D, Seth A, Sharma P, Maheshwari R, Abed SN, Deb PK, Chougule MB, Tekade RK. Dissolution profile consideration in pharmaceutical product development, vol. 1. Academic Press; 2018. [Google Scholar]
- 7.Mann J, Dressman J, Rosenblatt K, Ashworth L, Muenster U, Frank K, Hutchins P, Williams J, Klumpp L, Wielockx K, Berben P, Augustijns P, Holm R, Hofmann M, Patel S, Beato S, Ojala K, Tomaszewska I, Bruel JL, Butler J. Mol Pharm. 2017;14(12):4192–201. 10.1021/acs.molpharmaceut.7b00198. [DOI] [PubMed] [Google Scholar]
- 8.Khan J, Rades T, Boyd B. Pharm Res. 2016;33(3):548–62. 10.1007/s11095-015-1829-5. [DOI] [PubMed] [Google Scholar]
- 9.Feeney OM, Crum MF, McEvoy CL, Trevaskis NL, Williams HD, Pouton CW, Charman WN, Bergstrom CAS, Porter CJH. Adv Drug Deliv Rev. 2016;101:167–94. 10.1016/j.addr.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Kalepu S, Manthina M, Padavala V. Acta Pharm Sin B. 2013;3(6):361–72. 10.1016/j.apsb.2013.10.001. [DOI] [Google Scholar]
- 11.Mu H, Holm R, Mullertz A. Int J Pharm. 2013;453(1):215–24. 10.1016/j.ijpharm.2013.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Akhtartavan S, Karimi M, Karimian K, Azarpira N, Khatami M, Heli H. Biomed Pharmacother. 2019;109:2427–33. 10.1016/j.biopha.2018.11.110. [DOI] [PubMed] [Google Scholar]
- 13.Cho HJ, Park JW, Yoon IS, Kim DD. Int J Nanomed. 2014;9:495–504. 10.2147/IJN.S56648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang G, Tang B, Chao Y, Zhang Y, Xua H, Tang X. RSC Adv. 2015;5:96437–47. 10.1039/C5RA14588K. [DOI] [Google Scholar]
- 15.Liu D, Liu Z, Wang L, Zhang C, Zhang N. Colloids Surf B: Biointerfaces. 2011;85(2):262–9. 10.1016/j.colsurfb.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Yin YM, Cui FD, Mu CF, Choi MK, Kim JS, Chung SJ, Shim CK, Kim DD. J Control Release. 2009;140(2):86–94. 10.1016/j.jconrel.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Le S, Chang CM, Nguyen T, Liu Y, Chen YA, Hernandez E, Kapur P, Hsieh JT, Johnston K, Dong X. J Biomed Nanotechnol. 2020;16(5):583–93. 10.1166/jbn.2020.2925. [DOI] [PubMed] [Google Scholar]
- 18.Boyd BJ, Bergstrom CAS, Vinarov Z, Kuentz M, Brouwers J, Augustijns P, Brandl M, Bernkop-Schnurch A, Shrestha N, Preat V, Mullertz A, Bauer-Brandl A, Jannin V. Eur J Pharm Sci. 2019;137:104967. 10.1016/j.ejps.2019.104967. [DOI] [PubMed] [Google Scholar]
- 19.Stillhart C, Durr D, Kuentz M. J Pharm Sci. 2014;103(4):1194–203. 10.1002/jps.23892. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim F, Gershkovich P, Sivak O, Wasan EK, Wasan KM. Eur J Pharm Sci. 2012;46(5):323–8. 10.1016/j.ejps.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Chen LR, Wesley JA, Bhattachar S, Ruiz B, Bahash K, Babu SR. Pharm Res. 2003;20(5):797–801. 10.1023/a:1023493821302. [DOI] [PubMed] [Google Scholar]
- 22.Fatouros DG, Walrand I, Bergenstahl B, Mullertz A. Pharm Res. 2009;26(2):361–74. 10.1007/s11095-008-9750-9. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Z, Dunn C, Khadra I, Wilson CG, Halbert GW. Mol Pharm. 2017;14(12):4132–44. 10.1021/acs.molpharmaceut.7b00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun DD, Wen H, Taylor LS. J Pharm Sci. 2016;105(9):2477–88. 10.1016/j.xphs.2016.03.024. [DOI] [PubMed] [Google Scholar]


