Abstract
Aims/Introduction
To assess the impact of baseline characteristics on the efficacy and safety of oral semaglutide in Japanese patients with type 2 diabetes.
Materials and Methods
In the Peptide InnOvatioN for Early diabEtes tReatment (PIONEER) 9 and 10 trials, Japanese patients were randomized to once‐daily oral semaglutide (3, 7, or 14 mg) or a comparator (placebo or once‐daily subcutaneous liraglutide 0.9 mg in PIONEER 9; once‐weekly subcutaneous dulaglutide 0.75 mg in PIONEER 10) for 52 weeks, with 5 weeks of follow up. An exploratory analysis grouped patients in each trial according to baseline glycated hemoglobin (HbA1c; ≤8.0, >8.0–≤9.0, or >9.0%), body mass index (<25, ≥25–<30, or ≥30 kg/m2) and, for PIONEER 10 only, by background medication (sulfonylurea, glinide, thiazolidinedione, α‐glucosidase inhibitor, sodium‐glucose cotransporter 2 inhibitor). Efficacy (changes from baseline to week 26 in HbA1c and bodyweight) and safety were assessed.
Results
Seven hundred and one patients were included (PIONEER 9: N = 243; PIONEER 10: N = 458). In both trials, HbA1c reductions increased as baseline HbA1c increased; there were no other apparent patterns between the variables investigated and HbA1c or bodyweight changes. There was one statistically significant subgroup interaction between baseline HbA1c and estimated treatment differences in bodyweight change for oral semaglutide 14 mg versus placebo in PIONEER 9 (P = 0.0286). Baseline HbA1c, baseline body mass index and background medication did not appear to affect the proportions of patients reporting adverse events.
Conclusions
Oral semaglutide is effective across a range of baseline subgroups of Japanese patients with type 2 diabetes, with no unexpected safety findings.
Keywords: Glucagon‐like peptide‐1 analog, Glycemic control, Type 2 diabetes
This subgroup analysis of the PIONEER 9 and PIONEER 10 studies assessed the impact of baseline characteristics on the efficacy and safety of oral semaglutide in Japanese patients with type 2 diabetes. In both trials, glycated hemoglobin (HbA1c) reductions increased as baseline HbA1c increased; there were no other apparent patterns between the variables investigated and HbA1c changes, bodyweight changes, or adverse events. These data suggest that oral semaglutide is effective across a range of baseline subgroups of Japanese patients with type 2 diabetes, with no unexpected safety findings.

INTRODUCTION
Semaglutide is the first glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) available in an oral formulation for the treatment of type 2 diabetes. For oral administration, semaglutide is co‐formulated with an absorption enhancer, sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate, in a once‐daily tablet. The efficacy and safety of three doses of oral semaglutide (3, 7, and 14 mg) were investigated in patients with type 2 diabetes in the phase IIIa Peptide InnOvatioN for Early diabEtes tReatment (PIONEER) program, which comprised eight global and two Japanese trials 1 . Based on the PIONEER program, oral semaglutide has been approved for the treatment of type 2 diabetes in Japan, North America and much of Europe 2 , 3 , 4 .
The clinical characteristics of East Asian people with type 2 diabetes, such as those from Japan, differ compared with global populations 5 . For example, type 2 diabetes tends to develop at a lower body mass index (BMI) and at a younger age in East Asian individuals compared with those of European descent 6 , 7 . Furthermore, prediabetes and early‐stage type 2 diabetes are characterized by greater levels of β‐cell dysfunction in East Asian populations compared with Caucasians 8 .
The efficacy and safety of once‐weekly subcutaneous (s.c.) semaglutide have been investigated in Japanese individuals with type 2 diabetes 9 , 10 , and Japanese patients were included in several of the multinational PIONEER trials 11 , 12 , 13 , 14 . To assess the dose–response, efficacy and safety of oral semaglutide in Japanese patients with type 2 diabetes, the PIONEER program also comprised two Japan‐specific trials, PIONEER 9 and PIONEER 10 15 , 16 . PIONEER 9 was a monotherapy trial that showed significant, dose‐dependent reductions in glycated hemoglobin (HbA1c) with oral semaglutide compared with placebo. At the 14 mg dose, oral semaglutide also significantly reduced HbA1c compared with liraglutide (0.9 mg) 15 . PIONEER 10 assessed the safety and efficacy of oral semaglutide in patients receiving oral glucose‐lowering therapy, and showed significant reductions in HbA1c at the 14 mg dose, and in bodyweight at the 7 and 14 mg doses, compared with dulaglutide (0.75 mg) 16 . In both trials, oral semaglutide was well tolerated, and the safety profile was consistent with that of other GLP‐1RAs 15 , 16 .
While the efficacy and safety of oral semaglutide have been shown in the overall populations of the PIONEER 9 and 10 trials 15 , 16 , individual patients can respond to treatments differently based on their demographic and clinical characteristics 17 . Indeed, treatment guidelines for type 2 diabetes specify that treatment should be tailored to the individual 18 , 19 , 20 , 21 . In order to do this appropriately, it is important to understand the effect that different characteristics can have on a patient’s response to a treatment. For this reason, exploratory analyses of the PIONEER 9 and 10 trials were performed to investigate the efficacy and safety of oral semaglutide in subgroups of Japanese patients with type 2 diabetes defined by baseline HbA1c, baseline BMI and background medication.
MATERIALS AND METHODS
Trial designs
PIONEER 9 (NCT03018028) was a 52‐week, phase II/IIIa, multicenter, randomized, placebo‐ and active‐controlled trial conducted at 16 sites in Japan. PIONEER 10 (NCT03015220) was a 52‐week, phase III, multicenter, open‐label, parallel‐group, active‐controlled trial conducted at 36 sites in Japan (Figure S1a,b).
Both trials comprised 52‐week treatment periods with an additional 5 weeks of follow up for safety assessments. Once‐daily oral semaglutide (3, 7, and 14 mg) was compared with placebo (PIONEER 9), once‐daily s.c. liraglutide 0.9 mg (PIONEER 9) and once‐weekly s.c. dulaglutide 0.75 mg (PIONEER 10).
Both trials were conducted in accordance with ICH Good Clinical Practice guidelines 22 , the Declaration of Helsinki and applicable regulatory requirements. The trial protocols were approved by local independent ethics committees and institutional review boards at each trial site.
Full methods for each trial have been published previously 15 , 16 .
Patient population
PIONEER 9 included Japanese adults aged ≥20 years, diagnosed with type 2 diabetes at least 30 days before screening. Patients were required to have an HbA1c of 6.5–9.5% if also receiving background oral glucose‐lowering medication as monotherapy (washed out before randomization), or 7.0–10.0% if treated with medical nutrition therapy and exercise alone.
PIONEER 10 included Japanese adults aged ≥20 years, diagnosed with type 2 diabetes at least 60 days before screening, and with an HbA1c of 7.0–10.5%. Background therapy (sulfonylurea [SU], glinide, thiazolidinedione [TZD], alpha‐glucosidase inhibitor [α‐GI], or sodium‐glucose cotransporter‐2 inhibitor [SGLT2i]) was continued throughout the trial at the stable pre‐trial dose and frequency, unless it needed to be changed for safety reasons.
Patients were required to provide written informed consent before any trial‐related activities took place. The full eligibility criteria are provided in the primary publications for each trial 15 , 16 .
Subgroup analyses
The subgroup analyses were exploratory and conducted post‐hoc. Baseline HbA1c (≤8.0, >8.0–≤9.0, and >9.0%) and baseline BMI (<25, ≥25–<30, and ≥30 kg/m2) measured at randomization, and background medication at screening (SU, glinide, TZD, α‐GI, or SGLT2i, as background oral glucose‐lowering monotherapy) were chosen to define the subgroups as they are key indicators of disease status.
End‐points and assessments
The primary end‐point in PIONEER 9 was change in HbA1c from baseline to week 26, with change in bodyweight from baseline to week 26 as a supportive secondary efficacy end‐point. Safety end‐points included the number of treatment‐emergent adverse events (AEs) and the number of severe (defined according to the American Diabetes Association classification 23 ) or blood glucose‐confirmed (defined as <3.1 mmol/L [56 mg/dL]) symptomatic hypoglycemic episodes up to week 57.
In PIONEER 10, the primary end‐point was the number of treatment‐emergent AEs up to week 57, with the number of severe or blood glucose‐confirmed hypoglycemic episodes up to week 57 as a supportive secondary safety end‐point. The supportive secondary efficacy end‐points were changes in HbA1c and bodyweight from baseline to week 26.
These end‐points were also used for this subgroup analysis.
Statistical analysis
Data from all participants of PIONEER 9 and 10 were included in the subgroup analyses. Efficacy analyses were based on the full analysis set, which included all randomized patients, and safety assessments used the safety analysis set, which included all patients who were exposed to at least one dose of the trial product.
The efficacy analyses for PIONEER 9 and 10 were based on two estimands 24 . For these subgroup analyses, the treatment effect was assessed using the treatment policy estimand (regardless of premature trial product discontinuation or rescue medication use). The changes from baseline in HbA1c and bodyweight were analyzed using a pattern mixture model with analysis of covariance (ancova)‐based multiple imputation to impute missing data. After imputation, the complete datasets were analyzed using an ancova model with treatment, stratification, subgroup, and interaction between treatment and subgroup as categorical fixed effects and the baseline value as a covariate; the results were combined using Rubin’s rule 25 . No adjustments for multiplicity were performed.
The safety end‐points were analyzed descriptively.
RESULTS
Patient disposition and baseline characteristics
Across both trials, a total of 701 patients were included in the analysis. In PIONEER 9, 243 patients received either oral semaglutide 3 mg (N = 49), 7 mg (N = 49), or 14 mg (N = 48), liraglutide 0.9 mg (N = 48), or placebo (N = 49). In PIONEER 10, 458 patients received either oral semaglutide 3 mg (N = 131), 7 mg (N = 132), or 14 mg (N = 130), or dulaglutide 0.75 mg (N = 65). All randomized patients received at least one dose of trial product and were included in the analyses.
Baseline characteristics by trial and subgroup are shown in Table 1. Patient numbers in some of the baseline HbA1c and baseline BMI subgroups were low, particularly in PIONEER 9, where some subgroups included fewer than 50 patients in total.
Table 1.
Baseline characteristics by subgroup
| Patients, N | Females, n (%) | Age, years | HbA1c, % | Duration of diabetes, years | Bodyweight, kg | BMI, kg/m2 | eGFR, mL/min/1.73 m2 | |
|---|---|---|---|---|---|---|---|---|
| PIONEER 9 | ||||||||
| Overall | 243 | 52 (21.4) | 59 (9) | 8.2 (0.9) | 7.6 (5.6) | 71.1 (13.3) | 25.9 (4.3) | 97 (12) |
| Baseline HbA1c, % | ||||||||
| ≤8.0 | 128 | 31 (24.2) | 59 (9) | 7.5 (0.3) | 7.0 (5.2) | 71.6 (13.8) | 26.3 (4.4) | 97 (12) |
| >8.0–≤9.0 | 68 | 10 (14.7) | 61 (9) | 8.5 (0.3) | 8.2 (5.8) | 69.7 (13.2) | 25.3 (4.2) | 95 (11) |
| >9.0 | 47 | 11 (23.4) | 59 (11) | 9.7 (0.5) | 8.3 (6.3) | 71.8 (12.4) | 25.5 (3.7) | 99 (12) |
| Baseline BMI, kg/m2 | ||||||||
| <25 | 108 | 23 (21.3) | 64 (8) | 8.3 (1.0) | 9.4 (5.9) | 61.4 (7.7) | 22.4 (1.7) | 94 (12) |
| ≥25–<30 | 100 | 16 (16.0) | 58 (9) | 8.2 (0.8) | 6.7 (5.3) | 74.8 (7.4) | 27.0 (1.3) | 98 (12) |
| ≥30 | 35 | 13 (37.1) | 52 (9) | 8.0 (1.0) | 4.5 (3.4) | 90.6 (13.6) | 33.5 (3.6) | 104 (11) |
| PIONEER 10 | ||||||||
| Overall | 458 | 117 (25.5) | 58 (10) | 8.3 (0.9) | 9.4 (6.3) | 72.1 (15.6) | 26.2 (4.8) | 97 (13) |
| Baseline HbA1c, % | ||||||||
| ≤8.0 | 206 | 53 (25.7) | 59 (10) | 7.5 (0.3) | 9.0 (6.7) | 72.1 (15.4) | 26.2 (4.4) | 95 (13) |
| >8.0–≤9.0 | 144 | 35 (24.3) | 58 (11) | 8.5 (0.3) | 9.4 (6.0) | 71.5 (14.9) | 26.0 (4.6) | 97 (14) |
| >9.0 | 108 | 29 (26.9) | 57 (10) | 9.7 (0.4) | 10.0 (5.9) | 73.0 (17.0) | 26.7 (5.7) | 99 (13) |
| Baseline BMI, kg/m2 | ||||||||
| <25 | 207 | 56 (27.1) | 62 (9) | 8.3 (0.9) | 11.1 (6.9) | 61.5 (8.3) | 22.7 (1.7) | 94 (13) |
| ≥25–<30 | 180 | 39 (21.7) | 57 (10) | 8.4 (0.9) | 8.1 (5.3) | 75.3 (9.1) | 27.2 (1.4) | 98 (14) |
| ≥30 | 71 | 22 (31.0) | 52 (10) | 8.4 (1.0) | 7.5 (5.4) | 94.8 (17.3) | 34.4 (5.3) | 103 (12) |
| Background medication | ||||||||
| SU | 147 | 27 (18.4) | 60 (10) | 8.5 (1.0) | 10.8 (6.8) | 70.2 (13.3) | 25.4 (4.1) | 96 (13) |
| Glinide | 77 | 21 (27.3) | 59 (10) | 8.4 (0.9) | 8.9 (5.3) | 71.2 (18.2) | 26.3 (6.3) | 95 (14) |
| TZD | 79 | 20 (25.3) | 60 (10) | 8.3 (0.9) | 8.8 (5.4) | 73.8 (13.6) | 27.2 (4.6) | 96 (12) |
| α‐GI | 77 | 28 (36.4) | 57 (11) | 8.2 (1.0) | 7.6 (6.6) | 72.8 (17.0) | 26.4 (4.4) | 97 (15) |
| SGLT2i | 78 | 21 (26.9) | 57 (10) | 8.2 (0.8) | 9.4 (6.3) | 74.3 (17.3) | 26.7 (4.8) | 99 (13) |
Data are the mean (SD) unless otherwise specified. Data are for all treatment arms combined for each subgroup in each trial. α‐GI, alpha‐glucosidase inhibitor; BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; SD, standard deviation; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
Efficacy by subgroup
Effect by baseline HbA1c (PIONEER 9 and PIONEER 10)
In both trials, HbA1c reductions appeared generally greater in the higher baseline HbA1c subgroup relative to the other subgroups (Figure 1). HbA1c reductions were dose‐dependent with oral semaglutide and appeared to be greater with oral semaglutide 14 mg versus placebo, liraglutide 0.9 mg, and dulaglutide 0.75 mg in all subgroups across both trials, except in the >8.0–≤9.0% subgroup versus liraglutide in PIONEER 9 (Figure 1, Figure S2). However, in both trials, there were no statistically significant treatment‐by‐subgroup interactions between baseline HbA1c and the change in HbA1c for oral semaglutide versus the comparators (Figure 1, Figure S2).
Figure 1.
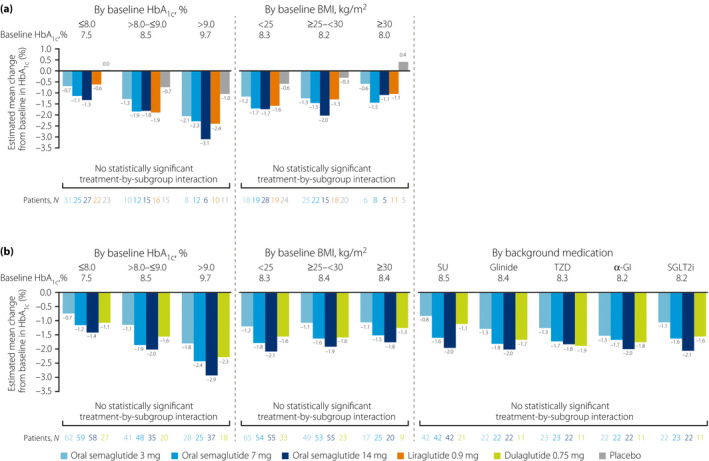
Change from baseline in HbA1c at week 26 by subgroup in (a) PIONEER 9 and (b) PIONEER 10. Baseline values are for all treatment arms combined for each subgroup in each trial. For all analyses, missing values were imputed by a pattern mixture model using multiple imputation. The pattern was defined by randomized treatment arm and treatment status (premature trial product discontinuation or initiation of rescue medication, or both), and imputation was carried out within groups defined by trial product and treatment status. For the subgroup analyses, the estimated changes from baseline were analyzed using an ancova model with treatment, strata, subgroup, and interaction between treatment and subgroup as categorical fixed effects, and baseline HbA1c as a covariate. The statistical analyses were not controlled for multiplicity. α‐GI, alpha‐glucosidase inhibitor; ancova, analysis of covariance; BMI, body mass index; HbA1c, glycated hemoglobin; N, number of patients contributing to the analysis; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
Across the subgroups, there was no consistent relationship between baseline HbA1c and change from baseline in bodyweight for any treatment arm (Figure 2). However, for oral semaglutide 7 and 14 mg in both trials, and for the active comparators, bodyweight was reduced from baseline in the ≤8.0% subgroup, but had either increased from baseline, or decreased to a smaller extent, in the >9.0% subgroup. Across the subgroups, changes in bodyweight with oral semaglutide appeared to be dose‐dependent, with bodyweight reductions generally being greater (or bodyweight increases generally being smaller) with oral semaglutide 7 and 14 mg than with liraglutide 0.9 mg and dulaglutide 0.75 mg (Figure 2, Figure S3). There was one statistically significant treatment‐by‐subgroup interaction between baseline HbA1c and the change in bodyweight, which was for the 14 mg dose versus placebo in PIONEER 9 (P = 0.0286; Figure 2, Figure S3).
Figure 2.
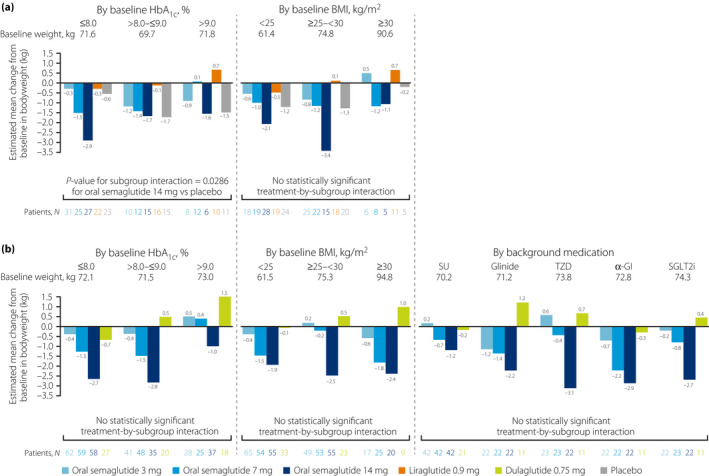
Change from baseline in bodyweight at week 26 by subgroup in (a) PIONEER 9 and (b) PIONEER 10. Baseline values are for all treatment arms combined for each subgroup in each trial. For all analyses, missing values were imputed by a pattern mixture model using multiple imputation. The pattern was defined by randomized treatment arm and treatment status (premature trial product discontinuation or initiation of rescue medication, or both), and imputation was carried out within groups defined by trial product and treatment status. For the subgroup analyses, the estimated changes from baseline were analyzed using an ancova model with treatment, strata, subgroup, and interaction between treatment and subgroup as categorical fixed effects, and baseline bodyweight as a covariate. The statistical analyses were not controlled for multiplicity. The P‐value is for the unadjusted two‐sided test of treatment by subgroup interaction. α‐GI, alpha‐glucosidase inhibitor; ancova, analysis of covariance; BMI, body mass index; HbA1c, glycated hemoglobin; N, number of patients contributing to the analysis; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
Effect by baseline BMI (PIONEER 9 and PIONEER 10)
For the effect by baseline BMI, reductions in HbA1c were generally dose‐dependent with oral semaglutide (Figure 1). In each subgroup, HbA1c reductions with oral semaglutide 7 and 14 mg appeared to be generally greater than reductions with placebo in PIONEER 9, and were similar to reductions with liraglutide 0.9 mg, except in the ≥25–<30 kg/m2 group, where reductions with oral semaglutide 14 mg were greater (Figure 1, Figure S2). In PIONEER 10, HbA1c reductions generally appeared greater with oral semaglutide 14 mg compared with dulaglutide 0.75 mg across the BMI subgroups. In both trials, there were no statistically significant treatment‐by‐subgroup interactions between baseline BMI and the change in HbA1c for oral semaglutide versus the comparators (Figure 1, Figure S2).
Similar to the change in HbA1c, there did not appear to be a consistent relationship between the change in bodyweight and baseline BMI for any treatment (Figure 2). Bodyweight reductions with oral semaglutide 14 mg appeared to be greater than those with liraglutide 0.9 mg and dulaglutide 0.75 mg, both of which were associated with increased bodyweight in some subgroups, across all of the baseline BMI subgroups (Figure 2, Figure S3). In both trials, there were no statistically significant treatment‐by‐subgroup interactions between baseline BMI and the change in bodyweight for oral semaglutide versus the comparators (Figure 2, Figure S3).
Effect by background medication (PIONEER 10)
In PIONEER 10, HbA1c was reduced from baseline with all treatments in all background medication subgroups and there was no discernible pattern in HbA1c reductions by background medication (Figure 1b). The HbA1c reductions with oral semaglutide 7 and 14 mg were generally similar to those with dulaglutide 0.75 mg in all subgroups, except the background SU subgroup, where the reductions in HbA1c appeared greater with these doses of oral semaglutide, and with oral semaglutide 14 mg in the background SGLT2i subgroup (Figure 1b, Figure S2b). There were no statistically significant treatment‐by‐subgroup interactions between background medication and the change in HbA1c for oral semaglutide versus dulaglutide 0.75 mg (Figure 1b, Figure S2b).
For bodyweight, the changes in bodyweight were generally larger with oral semaglutide 14 mg compared with dulaglutide 0.75 mg across background medication subgroups (Figure 2b, Figure S3b). There were no statistically significant treatment‐by‐subgroup interactions between background medication and the change in bodyweight for oral semaglutide versus dulaglutide 0.75 mg (Figure 2b, Figure S3b).
Safety outcomes
The proportions of patients reporting AEs were similar between treatments in individual HbA1c and BMI subgroups across both trials, and there was no discernible effect of baseline HbA1c or BMI on the incidence of AEs (Table 2). In PIONEER 10, the safety profile of oral semaglutide compared with dulaglutide did not appear to be affected by background medication. Serious AEs were infrequent, generally occurring in ≤10% of patients in any treatment group for any baseline HbA1c, baseline BMI, or background medication subgroup.
Table 2.
On‐treatment adverse events up to week 57 in PIONEER 9 and PIONEER 10 by subgroup
| PIONEER 9 | PIONEER 10 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Oral semaglutide 3 mg | Oral semaglutide 7 mg | Oral semaglutide 14 mg | Liraglutide 0.9 mg | Placebo | Oral semaglutide 3 mg | Oral semaglutide 7 mg | Oral semaglutide 14 mg | Dulaglutide 0.75 mg | |
| By baseline HbA1c, % | |||||||||
| Patients, N | |||||||||
| ≤8.0 | 31 | 25 | 27 | 22 | 23 | 62 | 59 | 58 | 27 |
| >8.0–≤9.0 | 10 | 12 | 15 | 16 | 15 | 41 | 48 | 35 | 20 |
| >9.0 | 8 | 12 | 6 | 10 | 11 | 28 | 25 | 37 | 18 |
| Any AEs, n (%) | |||||||||
| ≤8.0 | 23 (74.2) | 21 (84.0) | 19 (70.4) | 17 (77.3) | 16 (69.6) | 44 (71.0) | 53 (89.8) | 51 (87.9) | 23 (85.2) |
| >8.0–≤9.0 | 9 (90.0) | 8 (66.7) | 10 (66.7) | 11 (68.8) | 14 (93.3) | 35 (85.4) | 34 (70.8) | 30 (85.7) | 15 (75.0) |
| >9.0 | 5 (62.5) | 8 (66.7) | 5 (83.3) | 4 (40.0) | 9 (81.8) | 22 (78.6) | 19 (76.0) | 30 (81.1) | 15 (83.3) |
| SAEs, n (%) | |||||||||
| ≤8.0 | 1 (3.2) | 1 (4.0) | 0 | 0 | 0 | 3 (4.8) | 2 (3.4) | 5 (8.6) | 1 (3.7) |
| >8.0–≤9.0 | 1 (10.0) | 1 (8.3) | 0 | 0 | 1 (6.7) | 4 (9.8) | 2 (4.2) | 1 (2.9) | 0 |
| >9.0 | 0 | 1 (8.3) | 0 | 0 | 2 (18.2) | 2 (7.1) | 0 | 1 (2.7) | 0 |
| GI AEs, n (%) | |||||||||
| ≤8.0 | 9 (29.0) | 7 (28.0) | 8 (29.6) | 10 (45.5) | 4 (17.4) | 17 (27.4) | 27 (45.8) | 31 (53.4) | 12 (44.4) |
| >8.0–≤9.0 | 5 (50.0) | 4 (33.3) | 5 (33.3) | 4 (25.0) | 2 (13.3) | 14 (34.1) | 15 (31.3) | 19 (54.3) | 8 (40.0) |
| >9.0 | 3 (37.5) | 7 (58.3) | 3 (50.0) | 4 (40.0) | 4 (36.4) | 9 (32.1) | 9 (36.0) | 20 (54.1) | 6 (33.3) |
| By baseline BMI, kg/m2 | |||||||||
| Patients, N | |||||||||
| <25 | 18 | 19 | 28 | 19 | 24 | 65 | 54 | 55 | 33 |
| ≥25–<30 | 25 | 22 | 15 | 18 | 20 | 49 | 53 | 55 | 23 |
| ≥30 | 6 | 8 | 5 | 11 | 5 | 17 | 25 | 20 | 9 |
| Any AEs, n (%) | |||||||||
| <25 | 15 (83.3) | 14 (73.7) | 20 (71.4) | 11 (57.9) | 20 (83.3) | 50 (76.9) | 44 (81.5) | 51 (92.7) | 28 (84.8) |
| ≥25–<30 | 16 (64.0) | 16 (72.7) | 11 (73.3) | 12 (66.7) | 15 (75.0) | 38 (77.6) | 41 (77.4) | 45 (81.8) | 18 (78.3) |
| ≥30 | 6 (100) | 7 (87.5) | 3 (60.0) | 9 (81.8) | 4 (80.0) | 13 (76.5) | 21 (84.0) | 15 (75.0) | 7 (77.8) |
| SAEs, n (%) | |||||||||
| <25 | 0 | 2 (10.5) | 0 | 0 | 3 (12.5) | 4 (6.2) | 2 (3.7) | 2 (3.6) | 1 (3.0) |
| ≥25–<30 | 0 | 1 (4.5) | 0 | 0 | 0 | 5 (10.2) | 2 (3.8) | 5 (9.1) | 0 |
| ≥30 | 2 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GI AEs, n (%) | |||||||||
| <25 | 6 (33.3) | 7 (36.8) | 12 (42.9) | 8 (42.1) | 5 (20.8) | 19 (29.2) | 25 (46.3) | 35 (63.6) | 12 (36.4) |
| ≥25–<30 | 7 (28.0) | 8 (36.4) | 3 (20.0) | 4 (22.2) | 4 (20.0) | 17 (34.7) | 20 (37.7) | 25 (45.5) | 9 (39.1) |
| ≥30 | 4 (66.7) | 3 (37.5) | 1 (20.0) | 6 (54.5) | 1 (20.0) | 4 (23.5) | 6 (24.0) | 10 (50.0) | 5 (55.6) |
| By background medication † | |||||||||
| Patients, N | |||||||||
| SU | – | – | – | – | – | 42 | 42 | 42 | 21 |
| Glinide | – | – | – | – | – | 22 | 22 | 22 | 11 |
| TZD | – | – | – | – | – | 23 | 23 | 22 | 11 |
| α‐GI | – | – | – | – | – | 22 | 22 | 22 | 11 |
| SGLT2i | – | – | – | – | – | 22 | 23 | 22 | 11 |
| Any AEs, n (%) | |||||||||
| SU | – | – | – | – | – | 36 (85.7) | 34 (81.0) | 38 (90.5) | 19 (90.5) |
| Glinide | – | – | – | – | – | 18 (81.8) | 19 (86.4) | 21 (95.5) | 10 (90.9) |
| TZD | – | – | – | – | – | 19 (82.6) | 16 (69.6) | 15 (68.2) | 8 (72.7) |
| α‐GI | – | – | – | – | – | 11 (50.0) | 18 (81.8) | 17 (77.3) | 8 (72.7) |
| SGLT2i | – | – | – | – | – | 17 (77.3) | 19 (82.6) | 20 (90.9) | 8 (72.7) |
| SAEs, n (%) | |||||||||
| SU | – | – | – | – | – | 3 (7.1) | 2 (4.8) | 2 (4.8) | 0 |
| Glinide | – | – | – | – | – | 1 (4.5) | 1 (4.5) | 1 (4.5) | 0 |
| TZD | – | – | – | – | – | 3 (13.0) | 0 | 1 (4.5) | 0 |
| α‐GI | – | – | – | – | – | 1 (4.5) | 1 (4.5) | 2 (9.1) | 1 (9.1) |
| SGLT2i | – | – | – | – | – | 1 (4.5) | 0 | 1 (4.5) | 0 |
| GI AEs, n (%) | |||||||||
| SU | – | – | – | – | – | 16 (38.1) | 20 (47.6) | 26 (61.9) | 11 (52.4) |
| Glinide | – | – | – | – | – | 4 (18.2) | 7 (31.8) | 13 (59.1) | 8 (72.7) |
| TZD | – | – | – | – | – | 13 (56.5) | 7 (30.4) | 9 (40.9) | 2 (18.2) |
| α‐GI | – | – | – | – | – | 2 (9.1) | 8 (36.4) | 9 (40.9) | 1 (9.1) |
| SGLT2i | – | – | – | – | – | 5 (22.7) | 9 (39.1) | 13 (59.1) | 4 (36.4) |
The on‐treatment observation period started at the date of first dose of trial product, included the period after initiation of rescue medication (if any), and excluded the period after trial product discontinuation (if applicable). α‐GI, alpha‐glucosidase inhibitor; AE, adverse event; BMI, body mass index; GI, gastrointestinal; HbA1c, glycated hemoglobin; n, number of patients with at least one event; N, number of patients contributing to the analysis; SAE, serious adverse event; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
Only data from PIONEER 10 were analyzed by background medication.
Nasopharyngitis was generally the most frequently reported AE in all treatment arms across all subgroups and in both trials (Table S1). There did not appear to be a clear relationship between baseline HbA1c, baseline BMI, or background medication subgroups, and the occurrence of gastrointestinal AEs. In both trials, events of diabetic retinopathy did not appear to occur more frequently in any of the subgroups (Table S2).
Two patients in PIONEER 9 (liraglutide 0.9 mg, n = 2) and 10 patients in PIONEER 10 (oral semaglutide 3 mg, n = 3; oral semaglutide 7 mg, n = 3; oral semaglutide 14 mg, n = 4) experienced blood glucose‐confirmed symptomatic hypoglycemic episodes, and none of these episodes were severe. Most (9 out of 10) of the hypoglycemic episodes in PIONEER 10 occurred in patients receiving background SU. There was no clustering of events in any subgroup when analyzed by baseline HbA1c or baseline BMI in either trial.
DISCUSSION
In the overall trial populations, oral semaglutide 14 mg was more effective than liraglutide (in PIONEER 9) and dulaglutide (in PIONEER 10) for reducing HbA1c 15 , 16 . The current analyses suggest that these findings were consistent across subgroups of baseline HbA1c, BMI, and background medication. In PIONEER 9 and 10, HbA1c reductions tended to be greater with higher baseline HbA1c in all treatment arms. There was no clear relationship between baseline BMI or background medication and the changes in HbA1c with any treatment. Oral semaglutide 14 mg reduced bodyweight more than liraglutide or dulaglutide in the overall trial populations 15 , 16 , and this was seen consistently across most subgroups analyzed. Only one statistically significant subgroup interaction was identified, which was between baseline HbA1c and the treatment differences in the change in bodyweight for oral semaglutide 14 mg versus placebo in PIONEER 9. For subgroups of baseline HbA1c, BMI, and background medication, the safety profile of oral semaglutide appeared to be consistent.
The findings of this analysis in Japanese patients suggest that, while baseline HbA1c did not appear to affect the comparative efficacy of oral semaglutide on glycemic control, the change from baseline in HbA1c increases as baseline HbA1c increases. However, this could partly be attributed to regression toward the mean since similar effects were also observed in the placebo group. A subgroup analysis of the global PIONEER 1–5, 7, and 8 trials, which used the same HbA1c cut‐offs as the present analysis, also found that HbA1c reductions from baseline were greater in patients with higher baseline HbA1c compared with lower baseline HbA1c 26 . This pattern was also observed in a subgroup analysis of once‐weekly s.c. semaglutide in a global population, although that analysis had a greater number of HbA1c subgroups 27 . Furthermore, similar findings were also observed in subgroup analyses of Japanese patients who received other GLP‐1RAs, specifically dulaglutide 0.75 mg 28 and lixisenatide 20 μg 29 . These findings are in line with the known glucose‐dependent mechanism of action of GLP‐1RAs, which activate the GLP‐1 receptor only in the presence of elevated levels of glucose, leading to stimulation of insulin secretion and suppression of glucagon secretion (known as the incretin effect) 30 , 31 . However, this pattern of greater HbA1c reductions in patients with higher baseline HbA1c has also been reported with SGLT2is 32 , 33 , 34 , suggesting this result might not be solely due to the incretin‐based mechanism of action of GLP‐1RAs.
The current analysis did not identify any consistent relationship between baseline HbA1c and treatment differences in bodyweight changes with oral semaglutide versus dulaglutide or liraglutide. These findings are consistent with results from similar analyses of oral semaglutide versus comparators in the global PIONEER trials 26 . Reductions in bodyweight from baseline with oral semaglutide, liraglutide, and dulaglutide in PIONEER 9 and 10 did appear to be greater in the lowest baseline HbA1c subgroup than in the highest subgroup in our analysis. This result was not consistent with subgroup analyses of the global PIONEER trials, in which there were no apparent patterns between the change in bodyweight and baseline HbA1c 26 . For once‐weekly s.c. semaglutide, baseline HbA1c did significantly affect the change from baseline in bodyweight in a global population, with weight loss decreasing as baseline HbA1c increased 27 . In addition, the subgroup analysis of Japanese patients receiving dulaglutide 0.75 mg also found that lower baseline HbA1c was significantly associated with greater bodyweight changes from baseline 28 .
Baseline BMI did not appear to affect the HbA1c and bodyweight reductions achieved, nor the differences in the reductions between oral semaglutide and comparators, in PIONEER 9 and 10. While corresponding analyses of the global PIONEER trials are not yet available, an analysis of global once‐weekly s.c. semaglutide trials also found no significant subgroup interactions between treatment and BMI for change in HbA1c 35 . Furthermore, a meta‐analysis of global liraglutide trials found that changes in bodyweight from baseline with either liraglutide or placebo were independent of baseline BMI 36 . It should be noted that the numbers of patients with a BMI of >30 kg/m2 in PIONEER 9 and 10 were low, which makes interpreting the findings from the present analysis difficult. The possibility of low patient numbers in some subgroups is a known challenge of these types of exploratory analyses 37 . In the case of the present analysis, the small number of patients in the highest baseline BMI subgroup was not surprising, considering Japanese patients with type 2 diabetes tend to have lower BMI compared with patients of European ancestry 6 , 7 . Reassuringly, patients in the <25 kg/m2 subgroup did not appear to be at greater risk of AEs than patients in other baseline BMI subgroups in PIONEER 9 and 10.
In terms of background medication, there were some variations in weight loss across the subgroups, with the smallest reductions occurring in patients who were receiving background SU. This may be because SUs are associated with weight gain 22 . No statistically significant interactions were identified between background medication and comparative weight loss in the present analysis. This is consistent with an exploratory subgroup analysis of five of the global PIONEER trials (PIONEER 3–5 and 7–8), which also did not identify any such interactions with oral semaglutide 14 mg or flexibly dosed, although smaller reductions in weight were observed in patients on background SU compared with other subgroups 38 . This analysis – which included patients who were receiving metformin, insulin, SU, SGLT2i or combinations as background medication – revealed greater reductions in HbA1c and bodyweight for oral semaglutide versus comparators (except for liraglutide, which was accompanied by similar reductions in HbA1c) irrespective of background medication 38 . The only significant treatment‐by‐subgroup interaction was in PIONEER 8, where a greater reduction in HbA1c was seen with oral semaglutide in patients receiving background insulin compared with those receiving insulin plus metformin 38 . The PIONEER trials included in this subgroup analysis enrolled almost 500 Japanese patients in total (11.1%, 10.5%, and 26.5% of the enrolled patients in PIONEER 3, 4, and 8, respectively) 39 . Overall, therefore, this analysis of global PIONEER trials supports the use of oral semaglutide in combination with other commonly used glucose‐lowering agents, including metformin and insulin 38 .
All treatments were well tolerated, and background medication did not appear to affect safety in PIONEER 10, with the exception of hypoglycemia, where 9 of the 10 episodes were observed in the small number of patients who were receiving background SU. However, this is not unexpected considering hypoglycemia is a known side‐effect of SU treatment 20 . Indeed, it is recommended to reduce the dose of background SU when starting treatment with oral semaglutide 2 , 3 , 4 . Since the incidence of external adjudication committee‐confirmed events of interest was low in the trials 15 , 16 , with no more than two events in any treatment group, these events were not analyzed by subgroups.
These exploratory analyses had several limitations. Firstly, the trials were not powered for subgroup analyses and reliably identify potential relationships between baseline variables and treatment effects of oral semaglutide. Furthermore, while subgroup analyses can provide useful information that can help guide treatment decisions in specific groups of patients, they should always be interpreted with caution because of the small number of patients in each subgroup and the multiple comparisons being made, which can result in false positive findings 37 . Indeed, the patient numbers were low in some subgroups for PIONEER 9 and 10, which makes it difficult to interpret the results of our analysis. Because there is a risk of over‐interpreting subgroup analyses, more data would be required before firmer conclusions can be drawn on any potential patterns by baseline variables. Finally, PIONEER 10 did not include patients receiving metformin as background medication, which may reduce the generalizability of the findings to non‐Japanese populations in which metformin is used as first‐line treatment.
In conclusion, these data suggest that oral semaglutide can be used for the treatment of type 2 diabetes in Japanese patients and is effective across a range of baseline HbA1c, baseline BMI, and background medication subgroups.
DISCLOSURES
DY has received consulting or speaker fees from MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., and Novo Nordisk Pharma Ltd.; and has also received clinically commissioned/joint research grants from Arklay Co. Ltd., Eli Lilly and Company, MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co. Ltd., and Taisho Pharmaceutical Co. Ltd. HK has received consulting or speaker fees from Astellas, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Mitsubishi Tanabe Pharma, MSD, Novartis, Novo Nordisk, Sanofi, Taisho Pharma, and Takeda Pharmaceutical Company; and research grants or endowments from Astellas, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Mitsubishi Tanabe Pharma, MSD, Novartis, Novo Nordisk, Ono Pharmaceutical, Otsuka Pharmaceutical, Sanofi, Sumitomo Dainippon Pharma, Taisho Pharmaceutical, and Takeda Pharmaceutical Company. YT has received honorarium for serving on advisory boards for Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Mitsubishi Tanabe Pharma, MSD, Novo Nordisk A/S, Sanofi, and Teijin; speaker’s fees from Astellas Pharma, AstraZeneca, Bayer Yakuhin, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Mitsubishi Tanabe Pharma, MSD, Novo Nordisk A/S, Ono Pharmaceutical Co. Ltd., Sanofi, Sanwa Kagaku Kenkyusho, Shionogi, Sumitomo Dainippon Pharma, and Takeda; and research support from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, MSD, Novartis, Novo Nordisk A/S, Ono Pharmaceutical Co. Ltd., Sanofi, Sanwa Kagaku Kenkyusho, Shionogi, Sumitomo Dainippon Pharma, and Takeda. YY has received consulting or speaker fees from Daiichi Sankyo, Mitsubishi Tanabe Pharma, MSD, Novo Nordisk Pharma, Ono Pharmaceutical, Sanofi, Sumitomo Dainippon, and Takeda Pharmaceutical Company; and research grants from Daiichi Sankyo, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho, and Takeda Pharmaceutical Company. NI has received honoraria for lectures from ARKRAY Marketing, Astellas Pharma, AstraZeneca, Bayer Yakuhin, Chugai Pharmaceutical, Daiichi Sankyo, Eli Lilly Japan, FUJIFILM Corporation, Hokkaido University, International Life Sciences Institute Japan, Iwate Prefectural Hospital Association, Japan Tobacco, Japanese Red Cross Wakayama Medical Center, Kissei Pharmaceutical, Kowa Company, Kyowa Kirin, Mainichi ga Hakken, Medtronic Japan, Mitsubishi Tanabe Pharma, MSD, MSD Life Science Foundation, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Olympus Corporation, Ono Pharmaceutical, Pfizer Japan, Public Interest Incorporated Foundation, Saishin Igaku Sha, Sanofi, Sanwa Kagaku Kenkyusho, Scohia Pharma, Sumitomo Dainippon Pharma, SUNSTAR Foundation, Taisho Pharma, Takeda Pharmaceutical, Terumo Corporation, The Sankei Shimbun, Toyooka Hospital, Tsumura & Co, University of Occupational and Environmental Health, and Wakayama Medical University; has received clinically commissioned and joint research grants from Astellas Pharma, AstraZeneca, Daiichi Sankyo, Eli Lilly Japan, Kyowa Kirin, Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Novo Nordisk Pharma, Ono Pharmaceutical, Sanofi, Sumitomo Dainippon Pharma, Taisho Pharma, and Tsumura & Co.; and scholarship grants from Astellas Pharma, Daiichi Sankyo, Eli Lilly Japan, Japan Tobacco, Kissei Pharmaceutical, Kyowa Kirin, LifeScan, Mitsubishi Tanabe Pharma Corporation, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Ono Pharmaceutical, Pfizer Japan, Sanofi, Sanwa Kagaku Kenkyusho, Shionogi & Co., Sumitomo Dainippon Pharma, Taisho Pharma, Takeda Hospital Group, Takeda Pharmaceutical, Teijin Pharma, The Japan China Medical Association, and Tsumura & Co. SD and TBJ are employees of Novo Nordisk A/S, the sponsor of this trial. SD is a shareholder of Novo Nordisk A/S. HH is an employee of, and holds shares in, Novo Nordisk Pharma Ltd.
Approval of the research protocol: The protocols for PIONEER 9 and PIONEER 10 were approved by local independent ethics committees and institutional review boards at each trial site and conform to the provisions of the Declaration of Helsinki as described previously 15 , 16 .
Informed consent: Written informed consent was obtained from all patients.
Registry and the registration no. of the trial: The trials are registered with the United States National Library of Medicine (http://www.clinicaltrials.gov). PIONEER 9: NCT03018028 (registered on 11 January 2017). PIONEER 10: NCT03015220 (registered on 9 January 2017).
Animal studies: N/A.
Supporting information
Table S1 | Most frequent on‐treatment adverse events in PIONEER 9 and PIONEER 10 by subgroup.
Table S2 | In‐trial adverse events of special interest in PIONEER 9 and PIONEER 10 by subgroup.
Figure S1 | Trial designs of (a) PIONEER 9 and (b) PIONEER 10.
Figure S2 | Estimated treatment differences in the change from baseline in HbA1c at week 26 by subgroup in (a) PIONEER 9 and (b) PIONEER 10.
Figure S3 | Estimated treatment differences in the change from baseline in bodyweight at week 26 by subgroup in (a) PIONEER 9 and (b) PIONEER 10.
ACKNOWLEDGMENTS
These trials were funded by Novo Nordisk A/S, Søborg, Denmark. The authors thank the subjects participating in this trial, and the investigators, all trial site staff, and all Novo Nordisk employees involved in these trials. The authors would also like to thank Kazushiro Fujiwara PhD of Novo Nordisk Pharma Ltd. for reviewing the manuscript, and Sophie Walton MSc of Axis, a division of Spirit Medical Communications Group Limited, for assistance with medical writing and editorial support (funded by Novo Nordisk A/S).
J Diabetes Investig. 2022; 13: 975–985
Clinical Trial Registry
United States National Library of Medicine
PIONEER 9: NCT03018028.
PIONEER 10: NCT03015220.
DATA AVAILABILITY STATEMENT
Data will be shared with researchers submitting a research proposal approved by the independent review board. Access request proposals can be found on the Novo Nordisk Trials website. Individual participant data will be shared in data sets in a de‐identified and anonymized format, with no limitations on how the data can be used.
REFERENCES
- 1. Thethi TK, Pratley R, Meier JJ. Efficacy, safety and cardiovascular outcomes of once‐daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab 2020; 22: 1263–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Product for type 2 diabetes, oral GLP‐1 receptor agonist semaglutide (genetic recombination) [Internet]. Pharmaceuticals and Medical Devices Agency, 2021. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/620023_2499014F1021_1_03. Accessed February 17, 2022. (Japanese).
- 3. RYBELSUS® semaglutide tablets prescribing information [Internet]. Food and Drug Administration, 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213182s000,213051s001lbl.pdf. Accessed June 10, 2020.
- 4. RYBELSUS® summary of product characteristics [Internet]. European Medicines Agency, 2020. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/rybelsus. Accessed June 10, 2020.
- 5. Ma RCW, Chan JCN. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013; 1281: 64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoon K‐H, Lee J‐H, Kim J‐W, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006; 368: 1681–1688. [DOI] [PubMed] [Google Scholar]
- 7. International Diabetes Federation . IDF Diabetes Atlas, 9th edn. Brussels: International Diabetes Federation, 2019. Available from: https://www.diabetesatlas.org. Accessed June 10, 2020. [Google Scholar]
- 8. Yabe D, Seino Y, Fukushima M, et al. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep 2015; 15: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaku K, Yamada Y, Watada H, et al. Safety and efficacy of once‐weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: a randomized trial. Diabetes Obes Metab 2018; 20: 1202–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seino Y, Terauchi Y, Osonoi T, et al. Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes Metab 2018; 20: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care 2019; 42: 1724–1732. [DOI] [PubMed] [Google Scholar]
- 12. Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA 2019; 321: 1466–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double‐blind, phase 3a trial. Lancet 2019; 394: 39–50. [DOI] [PubMed] [Google Scholar]
- 14. Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care 2019; 42: 2262–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamada Y, Katagiri H, Hamamoto Y, et al. Dose‐response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52‐week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol 2020; 8: 377–391. [DOI] [PubMed] [Google Scholar]
- 16. Yabe D, Nakamura J, Kaneto H, et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open‐label, randomised, active‐controlled, phase 3a trial. Lancet Diabetes Endocrinol 2020; 8: 392–406. [DOI] [PubMed] [Google Scholar]
- 17. Wijn SRW, Rovers MM, Le LH, et al. Guidance from key organisations on exploring, confirming and interpreting subgroup effects of medical treatments: a scoping review. BMJ Open 2019; 9: e028751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig 2018; 9: 657–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 executive summary. Endocr Pract 2020; 26: 107–139. [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes‐2021. Diabetes Care 2021; 44(Suppl 1): S111–S124. [DOI] [PubMed] [Google Scholar]
- 22. Structure and content of clinical study reports E3 [Internet]. International Council for Harmonisation, 1995. Available from: https://www.ich.org/page/efficacy‐guidelines. Accessed June 10, 2020.
- 23. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36: 1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aroda VR, Saugstrup T, Buse JB, et al. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: the PIONEER 1 randomized clinical trial as an example. Diabetes Obes Metab 2019; 21: 2203–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York, NY: John Wiley & Sons, 1987. [Google Scholar]
- 26. Meier JJ, Bauer R, Blicher TM, et al. Efficacy of oral semaglutide according to baseline HbA1c: an exploratory subgroup analysis of the PIONEER trial programme. Oral presentation #51. Diabetologia 2019; 62(Suppl 1): Abstract 51. [Google Scholar]
- 27. Aroda VR, Capehorn MS, Chaykin L, et al. Impact of baseline characteristics and beta‐cell function on the efficacy and safety of subcutaneous once‐weekly semaglutide: a patient‐level, pooled analysis of the SUSTAIN 1–5 trials. Diabetes Obes Metab 2020; 22: 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Onishi Y, Oura T, Nishiyama H, et al. Subgroup analysis of phase 3 studies of dulaglutide in Japanese patients with type 2 diabetes. Endocr J 2016; 63: 263–273. [DOI] [PubMed] [Google Scholar]
- 29. Seino H, Onishi Y, Naito Y, et al. Lixisenatide improves glycemic outcomes of Japanese patients with type 2 diabetes: a meta‐analysis. Diabetol Metab Syndr 2016; 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drucker DJ. Mechanisms of action and therapeutic application of glucagon‐like peptide‐1. Cell Metab 2018; 27: 740–756. [DOI] [PubMed] [Google Scholar]
- 31. Holst JJ. The incretin system in healthy humans: the role of GIP and GLP‐1. Metabolism 2019; 96: 46–55. [DOI] [PubMed] [Google Scholar]
- 32. Wilding JPH, Blonde L, Leiter LA, et al. Efficacy and safety of canagliflozin by baseline HbA1c and known duration of type 2 diabetes mellitus. J Diabetes Complications 2015; 29: 438–444. [DOI] [PubMed] [Google Scholar]
- 33. Liu J, Tarasenko L, Terra SG, et al. Efficacy of ertugliflozin in monotherapy or combination therapy in patients with type 2 diabetes: a pooled analysis of placebo‐controlled studies. Diab Vasc Dis Res 2019; 16: 415–423. [DOI] [PubMed] [Google Scholar]
- 34. Johnson JF, Parsa R, Bailey RA. Real‐world clinical outcomes among patients with type 2 diabetes receiving canagliflozin at a specialty diabetes clinic: subgroup analysis by baseline HbA1c and age. Clin Ther 2017; 39: 1123–1131. [DOI] [PubMed] [Google Scholar]
- 35. Viljoen A, Frias JP, Gondolf T, et al. 998‐P: efficacy and safety of semaglutide by baseline BMI in SUSTAIN 1–5 and 7. Diabetes 2019; 68(Suppl 1): Abstract 998‐P. [Google Scholar]
- 36. Montanya E, Fonseca V, Colagiuri S, et al. Improvement in glycated haemoglobin evaluated by baseline body mass index: a meta‐analysis of the liraglutide phase III clinical trial programme. Diabetes Obes Metab 2016; 18: 707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guideline on the investigation of subgroups in confirmatory clinical trials [Internet]. European Medicines Agency, 2019. Available from: https://www.ema.europa.eu/en/investigation‐subgroups‐confirmatory‐clinical‐trials. Accessed June 10, 2020.
- 38. Buse JB, Crowley M, Eriksson JW, et al. 957‐P: efficacy of oral semaglutide according to background medication: an exploratory subgroup analysis of the PIONEER trial program. Diabetes 2020; 69(Suppl 1): Abstract 957‐P. [Google Scholar]
- 39. Rasmussen MF. The development of oral semaglutide, an oral GLP‐1 analog, for the treatment of type 2 diabetes. Diabetol Int 2020; 11: 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Most frequent on‐treatment adverse events in PIONEER 9 and PIONEER 10 by subgroup.
Table S2 | In‐trial adverse events of special interest in PIONEER 9 and PIONEER 10 by subgroup.
Figure S1 | Trial designs of (a) PIONEER 9 and (b) PIONEER 10.
Figure S2 | Estimated treatment differences in the change from baseline in HbA1c at week 26 by subgroup in (a) PIONEER 9 and (b) PIONEER 10.
Figure S3 | Estimated treatment differences in the change from baseline in bodyweight at week 26 by subgroup in (a) PIONEER 9 and (b) PIONEER 10.
Data Availability Statement
Data will be shared with researchers submitting a research proposal approved by the independent review board. Access request proposals can be found on the Novo Nordisk Trials website. Individual participant data will be shared in data sets in a de‐identified and anonymized format, with no limitations on how the data can be used.


