ABSTRACT
Aims/Introduction
Diabetes is associated with poor clinical outcomes of coronavirus disease 2019 (COVID‐19). However, the impact of newly diagnosed diabetes on prognosis has not been clarified. The objective of this study was to show the features and outcome of COVID‐19 patients with newly diagnosed diabetes in Japan.
Materials and Methods
We retrospectively analyzed 62 patients with diabetes hospitalized for COVID‐19 between 1 April and 18 August 2021 at the National Center for Global Health and Medicine in Tokyo, Japan. We evaluated the worst severity of COVID‐19 and plasma blood glucose levels in patients with newly diagnosed diabetes or pre‐existing diabetes.
Results
This study included 62 confirmed COVID‐19 patients with diabetes, including 19 (30.6%) patients with newly diagnosed diabetes and 43 (69.4%) patients with pre‐existing diabetes. Patients with newly diagnosed diabetes significantly progressed to a critical condition more frequently during hospitalization than patients with pre‐existing diabetes (52.6% vs 20.9%, P = 0.018). In addition, patients with newly diagnosed diabetes had significantly higher average plasma blood glucose levels for the first 3 days after admission than those with pre‐existing diabetes.
Conclusions
Our study suggests that the proportion of COVID‐19 patients who are newly diagnosed with diabetes is high, and they have an increased risk of developing severe disease than those with pre‐existing diabetes. It might be advisable that at the point of COVID‐19 diagnosis, blood glucose and glycated hemoglobin levels be assessed in all patients.
Keywords: COVID‐19, Diabetes, Hyperglycemia
We retrospectively analyzed 62 patients with diabetes hospitalized for coronavirus disease 2019 at the National Center for Global Health and Medicine in Japan. Our study showed that patients with newly diagnosed diabetes had a higher severity of coronavirus disease 2019 and had difficulty in managing glycemic control compared with patients with pre‐existing diabetes.

INTRODUCTION
The coronavirus disease 2019 (COVID‐19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), mainly penetrates the respiratory tract and lungs, resulting in acute respiratory distress syndrome and multiple organ failure in severe cases 1 . Evidence is emerging that diabetes is associated with worse clinical outcomes and higher mortality in patients with COVID‐19 2 , 3 . Recently, several studies have suggested that patients with newly diagnosed diabetes on hospital admission have a higher risk of serious conditions and mortality due to COVID‐19 4 . However, the causes of their poor prognosis are not clearly understood. In addition, the actual situation in Japan, which has an insurance system with a well‐developed public health checkup system, has not been previously reported.
Poor glycemic control during hospitalization has been associated with severe conditions and death in patients with COVID‐19 5 , while glycemic control in COVID‐19 patients with diabetes is extremely difficult. SARS‐CoV‐2 can not only potentially cause impaired insulin secretion, but can lead to severe insulin resistance 6 , 7 . In particular, after the addition of glucocorticoids to the treatment protocol for severe COVID‐19, the difficulty in glycemic control was reported to be even more pronounced 8 . Untreated diabetes patients who were identified as having diabetes for the first time on admission are predicted to have difficulty in managing hyperglycemia after admission, but the detailed features of glucocorticoid‐induced hyperglycemia in patients with newly diagnosed diabetes have not been shown to date.
Therefore, we carried out a retrospective observational study to show the features and outcomes of COVID‐19 patients with newly diagnosed diabetes in Japan. In addition, we examined the characteristics of plasma blood glucose trends in patients after glucocorticoid initiation.
MATERIALS AND METHODS
Study design and participants
The present retrospective observational study was carried out at the National Center for Global Health and Medicine (NCGM) in Tokyo, Japan, which is one of the national designated institutions where severe COVID‐19 patients were gathered during the pandemic. We retrospectively analyzed 62 patients with diabetes hospitalized for COVID‐19 between 1 April and 18 August 2021 at the NCGM. The diagnosis of COVID‐19 was based on the positivity of SARS‐CoV‐2 in real‐time reverse transcription polymerase chain reaction.
The protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The study protocol was approved by the institutional review board of the National Center for Global Health and Medicine, Tokyo, Japan (approval number: NCGM‐S‐004328‐00).
Data collection
We collected the following information from electronic medical records: demographic data, comorbidities, vital signs and laboratory findings at admission. Laboratory findings included blood count, liver and kidney function tests, glycated hemoglobin (HbA1c) levels, coagulation profile, and inflammatory markers. We also recorded trends of biomarkers (lactate dehydrogenase [LDH] and C‐reactive protein [CRP]) during the first 7 days of hospitalization. Other data included prognosis (death, discharge or hospital transfer), treatment regimens of COVID‐19 (systemic corticosteroids, remdesivir, tocilizumab, baricitinib and heparin) and respiratory support (e.g., nasal or mask, high‐flow nasal cannula, non‐invasive positive pressure ventilation and invasive ventilation). Systemic corticosteroid regimens, including methylprednisolone therapy (intravenous, 2 mg/kg–1000 mg daily for 3–5 consecutive days with gradual tapering) and dexamethasone (oral or intravenous, 6 mg daily for 7–10 consecutive days), were chosen by the specialist in infection and respiratory care based on the severity of respiratory failure. Methylprednisolone therapy is often used in critical cases.
Definition of COVID‐19 severity
The primary outcome was that of the worst severity of COVID‐19 during hospitalization. The severity of COVID‐19 was categorized into four levels as critical, severe, moderate and mild. Specifically, patients were defined as in a critical condition if they required non‐invasive positive pressure ventilation or invasive ventilation 9 , and as in a severe condition if they had clinical signs of pneumonia (fever, dyspnea, cough and tachypnea) accompanied by one of the following: respiratory rate >30 breaths/min, severe respiratory distress or oxygen saturation (SpO2) <90% on room air. A moderate condition referred to the presence of fever and respiratory symptoms with radiological findings of pneumonia. A mild condition was defined as mild clinical symptoms without any sign of pneumonia on imaging. We also evaluated the clinical outcomes, including death, alive discharge and hospital transfer.
Definition of diabetes and glycemic control
In the present study, diabetes was categorized into two types: newly diagnosed diabetes and pre‐existing diabetes. Newly diagnosed diabetes was defined as having both HbA1c ≥6.5% (48 mmol/mol) and fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or random blood glucose ≥200 mg/dL (10.0 mmol/L) on admission with no prior history of diabetes. Pre‐existing diabetes was defined based on self‐reported history of diabetes, prior medical records before admission, reporting a diagnosis of diabetes or treatment with glucose‐lowering medications.
We measured plasma glucose levels before each meal and at bedtime with a glucometer. Based on these data during the first week of hospitalization, we calculated the percentage of time, including time per day, within the target glucose range (time in range [TIR] 70–180 mg/dL; 3.9 ‐ 10.0 mmol/L) and time above target glucose range (time above range [TAR] >180 mg/dL; >10.0 mmol/L). The validity of TIR and TAR using intermittent blood glucose measurements were reported previously 10 . We also collected data on preadmission and in‐hospital glycemic management practices. In‐hospital glycemic control was carried out by diabetologists through daily assessment of glycemic patterns, and adjusting basal, nutritional and correctional insulin doses accordingly. Depending on the decision of the diabetologist, continuous intravenous insulin infusion and/or glucose‐lowering medications were used.
Statistical analysis
Continuous variables were reported as the mean ± standard deviation or median (interquartile range), and categorical variables were reported as percentages. Differences in clinical characteristics between groups were assessed by the Student's t‐test or χ2‐test for continuous and categorical variables, respectively. Statistical significance was set at P < 0.05. Statistical analyses were carried out using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) 11 . Dynamic graphs of plasma glucose levels, LDH and CRP were drawn using Python (version 3.8, Python Software Foundation, Wilmington, DE, USA) programming language modules: Pandas, NumPy, Matplotlib and Seaborn.
RESULTS
Population characteristics
The present study included 62 confirmed COVID‐19 patients with diabetes, including 19 patients with newly diagnosed diabetes and 43 patients with pre‐existing diabetes. Amongst the latter, most patients with pre‐existing diabetes had type 2 diabetes (n = 39). There were two patients with type 1 diabetes, and one case each of gestational and pancreatic diabetes. The general characteristics of the study population are given in Table 1. Patients with newly diagnosed diabetes were significantly younger, and had fewer comorbidities of hypertension and dyslipidemia than those with pre‐existing diabetes. There were no significant differences in sex or body mass index. From the laboratory examination at admission, patients with newly diagnosed diabetes had significantly higher mean values of HbA1c levels, aspartate aminotransferase, alanine aminotransferase, LDH and CRP, and lower mean values of serum albumin than patients with pre‐existing diabetes. The proportion of patients with newly diagnosed diabetes was higher in the younger age groups of both sexes than in the older age groups (Figure S1).
Table 1.
Patient characteristics
| Total | Newly diagnosed diabetes | Pre‐existing diabetes | P | |
|---|---|---|---|---|
| (n = 62) | (n = 19) | (n = 43) | ||
| Age (years) | 58.0 ± 14.2 | 51.5 ± 13.3 | 60.8 ± 13.8 | 0.015 |
| Sex male, n (%) | 47 (75.8) | 15 (78.9) | 32 (74.4) | 1 |
| BMI (kg/m2) | 28.2 ± 5.7 | 28.2 ± 6.0 | 28.2 ± 5.6 | 0.998 |
| Comorbidities, n (%) | ||||
| Hypertension | 36 (58.1) | 4 (21.1) | 32 (74.4) | <0.001 |
| Dyslipidemia | 29 (46.8) | 3 (15.8) | 26 (60.5) | 0.002 |
| Coronary artery disease | 5 (8.1) | 0 (0.0) | 5 (11.6) | 0.312 |
| Cerebrovascular disease | 5 (8.1) | 0 (0.0) | 5 (11.6) | 0.312 |
| Past or current smoking | 24 (38.7) | 8 (42.1) | 16 (37.2) | 0.781 |
| Laboratory at admission | ||||
| Plasma glucose, mg/dL | 233.0 ± 144.6 | 232.2 ± 94.7 | 233.4 ± 162.8 | 0.977 |
| HbA1c (%) | 8.1 ± 1.7 | 8.9 ± 2.2 | 7.8 ± 1.3 | 0.018 |
| HbA1c (mmol/mol) | 65 ± 19 | 74 ± 24 | 62 ± 14 | 0.018 |
| Serum albumin (g/dL) | 3.3 ± 0.5 | 3.1 ± 0.4 | 3.4 ± 0.6 | 0.045 |
| AST (U/L) | 60.4 ± 36.8 | 75.2 ± 28.3 | 53.9 ± 38.5 | 0.035 |
| ALT (U/L) | 48.7 ± 32.2 | 61.2 ± 32.6 | 43.2 ± 30.8 | 0.041 |
| LDH (U/L) | 424.9 ± 215.7 | 589.3 ± 256.6 | 352.3 ± 147.5 | <0.001 |
| Serum creatinine (μmol/L) | 97.3 ± 61.9 | 97.3 ± 70.7 | 79.6 ± 44.2 | 0.360 |
| Lymphocyte count (/μL) | 940.5 ± 415.3 | 1049.6 ± 439.4 | 892.3 ± 400.0 | 0.171 |
| Neutrophil count (/μL) | 4795.7 ± 2675.2 | 5510.6 ± 2890.6 | 4479.7 ± 2545.6 | 0.164 |
| CRP (mg/dL) | 8.3 ± 6.7 | 11.1 ± 7.4 | 7.1 ± 6.2 | 0.033 |
| D‐dimer (μg/mL) | 8.0 ± 29.7 | 5.2 ± 15.9 | 9.2 ± 34.2 | 0.629 |
| Parameters at admission | ||||
| Systolic blood pressure (mmHg) | 127.2 ± 20.2 | 131.3 ± 18.2 | 125.3 ± 21.0 | 0.286 |
| Body temperature (degrees) | 37.7 ± 1.0 | 38.0 ± 1.0 | 37.5 ± 0.9 | 0.068 |
| Oxygen saturation (%) | 92.2 ± 5.8 | 92.5 ± 6.2 | 92.1 ± 5.7 | 0.826 |
| Oxygen concentration (L) | 3.7 ± 4.5 | 6.0 ± 4.8 | 2.7 ± 4.0 | 0.009 |
Data are expressed as number and percentage or mean ± standard deviation.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C‐reactive protein; BMI, body mass index; HbA1c, glycated hemoglobin; LDH, lactate dehydrogenase.
Clinical outcomes of COVID‐19
Table 2 presents the treatment and outcomes. Overall, 93.5% of the hospitalized patients at our institution were grouped as critical or with severe conditions and with the worst severity of COVID‐19, just 6.5% were moderate, and no cases were mild. The mortality rate in the present study population was 9.6%. Patients with newly diagnosed diabetes progressed significantly more frequently to critical conditions during their hospitalization than patients with pre‐existing diabetes (52.6% vs 20.9%, P = 0.018). In the analysis of only patients with type 2 diabetes, as shown in Tables S1 and S2, the background of patients and clinical outcomes were similar to that of the overall population. Patients with newly diagnosed diabetes were also more frequently treated with methylprednisolone therapy and tocilizumab than patients with pre‐existing diabetes.
Table 2.
Coronavirus disease 2019 therapies and outcomes
| Total | Newly diagnosed diabetes | Pre‐existing diabetes | P | |
|---|---|---|---|---|
| (n = 62) | (n = 19) | (n = 43) | ||
| Worst severity classification of COVID‐19, n (%) | ||||
| Critical | 19 (30.6) | 10 (52.6) | 9 (20.9) | 0.018 |
| Severe | 39 (62.9) | 9 (47.4) | 30 (69.8) | 0.153 |
| Moderate | 4 (6.5) | 0 (0) | 4 (9.3) | 0.303 |
| Outcomes, n (%) | ||||
| Death | 6 (9.6) | 2 (10.5) | 4 (9.3) | 1 |
| Discharged alive | 52 (83.9) | 16 (84.2) | 36 (83.7) | 1 |
| Transferred | 4 (6.5) | 1 (5.3) | 3 (7.0) | 1 |
| Worst route of oxygen delivery, n (%) | ||||
| No oxygen demand | 4 (6.5) | 0 (0.0) | 4 (9.3) | 0.303 |
| Nasal or mask | 26 (41.9) | 6 (31.6) | 20 (46.5) | 0.403 |
| High flow nasal cannula | 13 (21.0) | 3 (15.8) | 10 (23.3) | 0.737 |
| Non‐invasive positive pressure ventilation | 14 (22.6) | 9 (47.4) | 5 (11.6) | 0.006 |
| Invasive ventilation | 5 (8.1) | 1 (5.3) | 4 (9.3) | 1 |
| Glucocorticoids | ||||
| Methylprednisolone therapy | 32 (51.6) | 14 (73.7) | 18 (41.9) | 0.028 |
| Dexamethasone | 30 (48.4) | 5 (26.3) | 25 (58.1) | 0.028 |
| Others, n (%) | ||||
| Remdesivir | 53 (85.5) | 17 (89.5) | 36 (83.7) | 0.709 |
| Tocilizumab | 18 (29.0) | 10 (52.6) | 8 (18.6) | 0.013 |
| Baricitinib | 14 (22.6) | 4 (21.1) | 10 (23.3) | 1 |
| Heparin | 51 (82.3) | 18 (94.7) | 33 (76.7) | 0.149 |
Data are expressed as number and percentage.
COVID‐19, coronavirus 2019.
As shown in Figure 1, both LDH and CRP levels at admission were higher in patients with newly diagnosed diabetes than in patients with pre‐existing diabetes. Thereafter, the LDH level of patients with newly diagnosed diabetes gradually decreased, whereas the LDH level of patients with pre‐existing diabetes remained high and subsequently exceeded that of the former in the latter half of the initial 2 weeks of admission. The CRP levels consistently decreased in patients in both groups.
Figure 1.
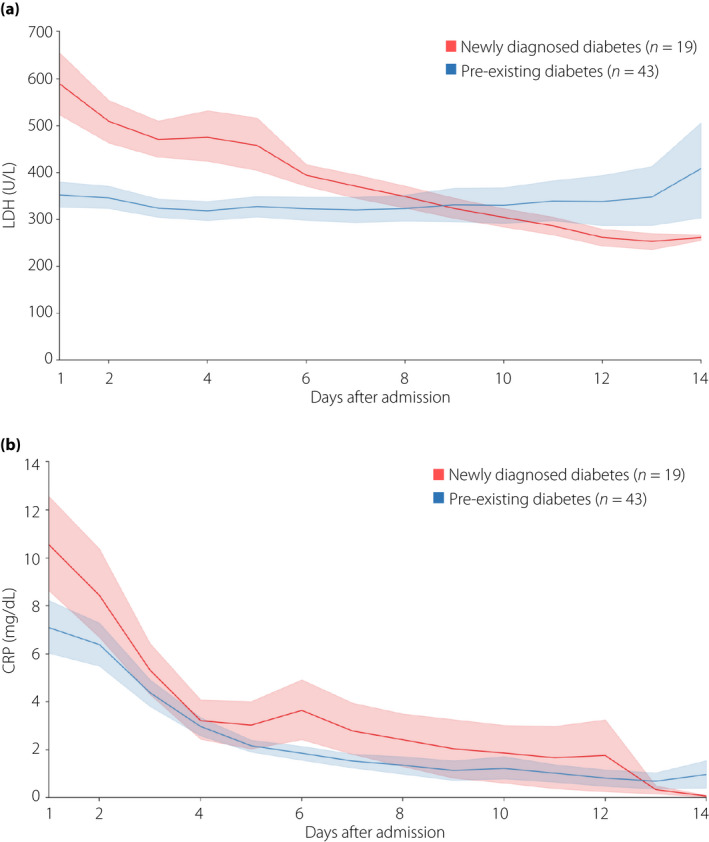
Dynamic trajectories of median (a) lactate dehydrogenase (LDH) and (b) C‐reactive protein (CRP) levels during the initial 2 weeks of hospitalization, with interquartile ranges by shaded regions, in patients with newly diagnosed diabetes (red) or patients with pre‐existing diabetes (blue).
Glycemic control
As shown in Table 3, patients with newly diagnosed diabetes had significantly higher average plasma blood glucose for the initial 3 days after admission, higher TAR (>180 mg/dL; 10.0 mmol/L) for the first 5 days, and lower TIR (target range 70–180 mg/dL; 3.9–10.0 mmol/L) for the first 5 days than those with pre‐existing diabetes. As shown in Figure 2, the dynamics of blood glucose levels showed a pattern of postprandial hyperglycemia exacerbation throughout the first week of hospitalization. During the initial 3 days after admission, the fasting blood glucose level was extremely difficult to control, especially in patients with newly diagnosed diabetes.
Table 3.
Glycemic control throughout the first week of hospitalization
| Newly diagnosed diabetes | Pre‐existing diabetes | P | |
|---|---|---|---|
| (n = 19) | (n = 43) | ||
| Average plasma glucose (mg/dL) | |||
| 3 days | 267.6 ± 48.3 | 233.0 ± 64.2 | 0.040 |
| 5 days | 249.2 ± 41.2 | 229.0 ± 49.8 | 0.126 |
| 7 days | 233.6 ± 39.5 | 220.2 ± 43.0 | 0.252 |
| TAR >180 mg/dL (10.0 mmol/L), % | |||
| 3 days | 88.9 ± 13.5 | 67.5 ± 28.3 | 0.003 |
| 5 days | 80.3 ± 18.5 | 67.5 ± 24.6 | 0.047 |
| 7 days | 72.8 ± 19.7 | 64.2 ± 22.9 | 0.162 |
| TIR 70–180 mg/dL (3.9–10.0 mmol/L), % | |||
| 3 days | 11.1 ± 13.5 | 33.8 ± 29.4 | 0.002 |
| 5 days | 19.7 ± 18.5 | 33.2 ± 24.9 | 0.039 |
| 7 days | 27.6 ± 20.1 | 35.9 ± 23.3 | 0.184 |
Data are expressed as mean ± standard deviation.
PG, plasma glucose level; TAR, time above range; TIR, time in range.
Figure 2.
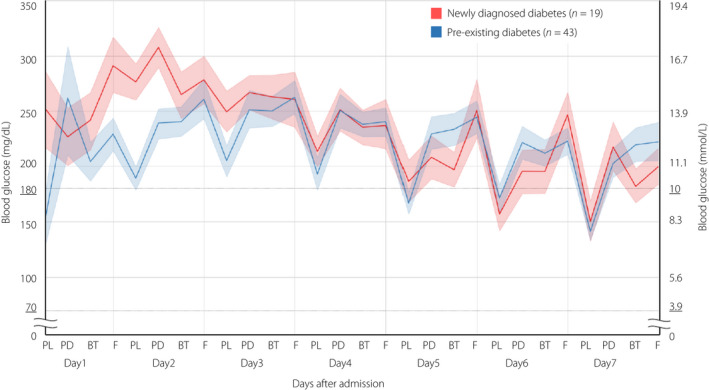
Dynamic trajectories of median blood glucose levels throughout the first week of hospitalization, with interquartile ranges by shaded regions, in patients with newly diagnosed diabetes (red) or patients with pre‐existing diabetes (blue). Point‐of‐care glucose levels were checked at fasting (F), prelunch (PL), predinner (PD) and bedtime (BT). The target range of blood glucose levels was between 70 and 180 mg/dL; 3.9 and 10.0 mmol/L (underlined).
As for the treatment of diabetes, 16.7% of patients with pre‐existing diabetes received insulin injections (Table S3). After admission, the total daily insulin units were not significantly different between newly‐diagnosed and pre‐existing diabetes, and for both groups the mean dose was >0.3 units/kg/day during the first week of hospitalization (Table S4). Continuous intravenous insulin infusion was carried out only in patients with pre‐existing diabetes and not in patients with newly diagnosed diabetes.
DISCUSSION
The present study showed that among COVID‐19 patients with diabetes who were admitted to our hospital, >30% were newly diagnosed with diabetes after admission, and they had a higher severity of COVID‐19 and had difficulty in managing glycemic control during the first week of hospitalization compared with patients with pre‐existing diabetes. We speculated that patients with newly diagnosed diabetes did not have an opportunity to receive treatment for diabetes before hospitalization; undiagnosed diabetes caused untreated exposure to hyperglycemia that might have contributed to the progression of organ damage and respiratory failure induced by the virus.
COVID‐19 infection leads to decreased expression of angiotensin‐converting enzyme 2 (ACE2), consequently leading to increased inflammation, cell damage and respiratory failure 12 . Chronic hyperglycemia leads to decreased ACE2 expression, which has a protective effect against inflammation, making cells more vulnerable to the inflammatory damage of the virus 6 . Hyperglycemia also worsens the respiratory system due to its effect on lung volume and diffusing capacity 13 . We found that patients with newly diagnosed diabetes had higher levels of HbA1c and inflammatory markers, including CRP and LDH, and required more oxygen on admission than patients with pre‐existing diabetes. Overall, the present results were consistent with previous reports showing that newly diagnosed diabetes is associated with a higher risk of patients being critically ill with COVID‐19 4 , 13 , 14 , 15 .
In our population, patients with pre‐existing diabetes had a significantly lower HbA1c than patients with newly‐diagnosed diabetes, suggesting that their relatively good glycemic control due to the previous treatment for diabetes might have influenced the relatively mild severity of COVID‐19. Even though we had many patients in critical condition and although vaccination was not yet widely available during the study period, the deterioration in patient outcome was fortunately lower than that reported in previous studies, with fewer deaths 16 , 17 . Presumably, one of the reasons for fewer deaths is that our hospital is a nationally designated medical institution for infectious diseases and has many specialists with extensive experience, including glucocorticoid therapy for severe or critical cases of COVID‐19.
The association between hyperglycemia and poor prognosis of COVID‐19 has been reported in several studies 5 . Although several reports have shown that COVID‐19 patients with diabetes, especially those treated with glucocorticoids, have difficulty with glycemic control after hospitalization and require a large amount of insulin 18 , 19 , 20 , we focused on newly diagnosed diabetes and showed for the first time the detailed features of glucocorticoid‐induced hyperglycemia in COVID‐19 patients with newly diagnosed diabetes.
We experienced great difficulty in managing hyperglycemia during the first week of hospitalization in patients with newly diagnosed diabetes. TIR and TAR in patients with newly diagnosed diabetes were very low and high, respectively, even though these indicators are known to be lower when assessed by the point‐of‐care than by continuous glucose monitoring 10 . There are several possible reasons for this finding. First, patients with newly diagnosed diabetes had higher HbA1c levels on admission than patients with pre‐existing diabetes, and their hyperglycemia was left untreated just before the diagnosis of diabetes, resulting in a severe state of glucotoxicity. Glucotoxicity might have caused high insulin resistance and progressive loss of pancreatic β‐cell function in patients with newly diagnosed diabetes 21 . Second, high‐dose glucocorticoid therapy administered to patients with more than moderate conditions of COVID‐19 caused marked hyperglycemia. The dynamics of blood glucose levels that showed postprandial hyperglycemia were typical of glucocorticoid‐induced hyperglycemia. Third, the diabetogenic influence of COVID‐19 might also be considered 4 , 22 . SARS‐CoV‐2 might cause acute impairment of insulin secretion by binding to ACE2 receptors on β‐cells, and might also damage β‐cells by increasing the levels of inflammatory cytokines 7 . As the ACE2 receptor is expressed in the liver, adipose tissue and skeletal muscle, binding of SARS‐CoV‐2 to these receptors might impair the response to insulin 7 . In the present study, we did not collect data on insulin secretion or resistance, although we certainly required a very large amount of insulin. These findings highlight the difficulty of achieving good glycemic control at the initial stage of hospitalization; therefore, it is desirable to develop better methods of glycemic control for critical COVID‐19 patients.
Under the pandemic with limited medical resources, methods of glycemic control that can reduce the direct contact with COVID‐19 patients are required 18 , so we mainly used subcutaneous injections of basal and bolus insulin. We monitored glycemic patterns to adjust the basal and bolus insulin, according to food intake and use of glucocorticoids. The degree of blood glucose elevation due to the direct effects of COVID‐19 and glucocorticoids varied greatly among individuals, making it difficult to create a fixed regimen 22 . Several protocols for the control of dexamethasone‐induced hyperglycemia and the use of remote continuous glucose monitoring have been attempted 20 , 23 .
Finally, in patients with newly diagnosed diabetes, the delay in recognizing the presence of diabetes delayed diabetologists’ intervention, which might have affected their poor glycemic control, presumably leading to the severe condition of COVID‐19.
In the present study, the proportion of patients with newly diagnosed diabetes was 32.8% of hospitalized COVID‐19 patients with type 2 diabetes. All patients with newly diagnosed diabetes presented with HbA1c ≥6.5%, which means that they most likely had undiagnosed diabetes, rather than being affected by COVID‐19 infection per se, acute metabolic stress or medications after admission. In a meta‐analysis of eight studies, the proportion of newly diagnosed diabetes was 14.4% 4 , which was 31.4% in another Mexican study 16 .
As Japan has a well‐developed health screening system and a universal health insurance system that covers all its citizens, thus achieving the longest healthy life expectancy 24 , 25 , it is surprising that many patients with newly diagnosed diabetes were found. In addition, it is worth noting that there were more patients with newly diagnosed diabetes among younger people of both sexes. In Japan, the proportion of people who failed to visit a hospital despite being diagnosed with glucose intolerance during a health checkup or interrupted a hospital visit, is high among young people, according to research by the Japan Diabetes Intervention Trail‐2 (J‐DOIT 2) and the Ministry of Health, Labor and Welfare 26 , 27 . Therefore, we consider it essential to encourage patients to visit a hospital promptly to prevent worsening COVID‐19 prognosis.
The present study had some limitations. Because our hospital had the role of an advanced medical institution under the pandemic, many severe and critical cases were accumulated; thus, this study did not include the data of patients with mild cases. In addition, as we did not deal with data from patients without diabetes, we could not compare the data between patients with and without diabetes.
In conclusion, the present study suggests that the proportion of COVID‐19 patients who are newly diagnosed with diabetes is high, and that they have a higher risk of developing severe disease than those with pre‐existing diabetes. It might be plausible that at the point of COVID‐19 diagnosis, blood glucose and HbA1c levels first be assessed in all patients.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The study protocol was approved by the Institutional Review Board of the National Center for Global Health and Medicine, Tokyo, Japan (approval number: NCGM‐S‐004328‐00, approval date: 21 October 2021).
Informed consent: We applied the opt‐out method to obtain consent for this study.
Approval date of registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Figure S1 | Proportion of patients with newly diagnosed and pre‐existing diabetes classified by sex and age in the 100% stacked column graph.
Table S1 | Patient characteristics (only patients with type 2 diabetes).
Table S2 | Coronavirus disease 2019 therapies and outcomes (only patients with type 2 diabetes).
Table S3 | Preadmission treatments, n (%).
Table S4 | In‐hospital management of diabetes.
ACKNOWLEDGMENTS
We thank all the patients and medical staff who participated in this study. We also appreciate Dr Maho Taguchi, Dr Erika Sugito, Dr Koko Ishizuka, Dr Aiko Terakawa and Dr Koji Maruyama for their continuous support.
No funding was used for the completion of this paper.
J Diabetes Investig. 2022; 13: 1086–1093
REFERENCES
- 1. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA 2020; 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Cui Y, Shen M, et al. Association of diabetes mellitus with disease severity and prognosis in COVID‐19: a retrospective cohort study. Diabetes Res Clin Pract 2020; 165: 108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sathish T, Kapoor N, Cao Y, et al. Proportion of newly diagnosed diabetes in COVID‐19 patients: a systematic review and meta‐analysis. Diabetes Obes Metab 2021; 23: 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu L, She Z‐G, Cheng XU, et al. Association of blood glucose control and outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab 2020; 31: 1068–1077.e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID‐19. Lancet Diabetes Endocrinol 2020; 8: 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sathish T, Tapp RJ, Cooper ME, et al. Potential metabolic and inflammatory pathways between COVID‐19 and new‐onset diabetes. Diabetes Metab 2021; 47: 101204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saand AR, Flores M, Kewan T, et al. Does inpatient hyperglycemia predict a worse outcome in COVID‐19 intensive care unit patients? J Diabetes 2021; 13: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Clinical Management of COVID‐19: Interim Guidance. World Health Organization, 2020; 13–15. [Google Scholar]
- 10. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019; 42: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fadini GP, Morieri ML, Boscari F, et al. Newly‐diagnosed diabetes and admission hyperglycemia predict COVID‐19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract 2020; 168: 108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farag AA, Hassanin HM, Soliman HH, et al. Newly diagnosed diabetes in patients with COVID‐19: different types and short‐term outcomes. Trop Med Infect Dis 2021; 6: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li H, Tian S, Chen T, et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID‐19. Diabetes Obes Metab 2020; 22: 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vargas‐Vázquez A, Bello‐Chavolla OY, Ortiz‐Brizuela E, et al. Impact of undiagnosed type 2 diabetes and pre‐diabetes on severity and mortality for SARS‐CoV‐2 infection. BMJ Open Diabetes Res Care 2021; 9: e002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laurenzi A, Caretto A, Molinari C, et al. Pre‐existing diabetes and COVID‐associated hyperglycaemia in patients with COVID‐19 pneumonia. Biology 2021; 10: 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Korytkowski M, Antinori‐Lent K, Drincic A, et al. A pragmatic approach to inpatient diabetes management during the COVID‐19 pandemic. J Clin Endocrinol Metab 2020; 105: 3076–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu L, Girgis CM, Cheung NW. COVID‐19 and diabetes: insulin requirements parallel illness severity in critically unwell patients. Clin Endocrinol 2020; 93: 390–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asiri AA, Alguwaihes AM, Jammah AA, et al. Assessment of the effectiveness of a protocol to manage dexamethasone‐induced hyperglycemia among hospitalized patients with COVID‐19. Endocr Pract 2021; 27: 1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaiser N, Leibowitz G, Nesher R. Glucotoxicity and beta‐cell failure in type 2 diabetes mellitus. J Pediatr Endocrinol Metab 2003; 16: 5–22. [DOI] [PubMed] [Google Scholar]
- 22. Khunti K, Del Prato S, Mathieu C, et al. COVID‐19, hyperglycemia, and new‐onset diabetes. Diabetes Care 2021; 44: 2645–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davis GM, Faulds E, Walker T, et al. Remote continuous glucose monitoring with a computerized insulin infusion protocol for critically Ill patients in a COVID‐19 medical ICU: proof of concept. Diabetes Care 2021; 44: 1055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hosokawa R, Ojima T, Myojin T, et al. Associations between healthcare resources and healthy life expectancy: a descriptive study across secondary medical areas in Japan. Int J Environ Res Public Health 2020; 17: 6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakatani H. Population aging in Japan: policy transformation, sustainable development goals, universal health coverage, and social determinates of health. Glob Health Med 2019; 1: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayashino Y, Suzuki H, Yamazaki K, et al. A cluster randomized trial on the effect of a multifaceted intervention improved the technical quality of diabetes care by primary care physicians: The Japan Diabetes Outcome Intervention Trial‐2 (J‐DOIT2). Diabetes Med 2016; 33: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ministry of Health, Labour and Welfare . National Health and Nutrition Survey, Ministry of Health, Japan 2016; 31–32 (Japanese).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Proportion of patients with newly diagnosed and pre‐existing diabetes classified by sex and age in the 100% stacked column graph.
Table S1 | Patient characteristics (only patients with type 2 diabetes).
Table S2 | Coronavirus disease 2019 therapies and outcomes (only patients with type 2 diabetes).
Table S3 | Preadmission treatments, n (%).
Table S4 | In‐hospital management of diabetes.


