Abstract
Aim
To investigate (1) the association of lifestyle changes and living and working conditions with glycemic control and (2) whether treatment was intensified appropriately in patients with diabetes under the first COVID‐19 state of emergency in Japan.
Materials and Methods
A total of 321 participants were included. Participants completed a questionnaire regarding lifestyle changes, including diet, physical activity, and living and working conditions during the COVID‐19 pandemic. The change in hemoglobin A1c (HbA1c) levels was estimated before (June 1, 2019 to August 31, 2019) and during (June 1, 2020 to August 31, 2020) the pandemic. Factors associated with changes in HbA1c levels were examined by multiple linear regression analysis. The proportion of patients who received treatment intensification for diabetes was compared between before and during the pandemic.
Results
There was no significant change in HbA1c levels before the pandemic and during the pandemic (7.13 ± 0.98% vs 7.18 ± 1.01%, P = 0.186). Teleworking (estimate 0.206, P = 0.004) and living with a dog (estimate −0.149, P = 0.038) were significantly associated with changes in HbA1c levels after adjusting for covariates. There was no significant difference in the proportion of patients who received treatment intensification for diabetes during the pandemic and before the pandemic in either the elderly or non‐elderly patients.
Conclusions
Overall glycemic control did not worsen during the pandemic. Nonetheless, environmental factors, including telework, were found to influence glycemic control in patients with diabetes. Further studies are needed to clarify whether the COVID‐19 pandemic could affect treatment intensification for diabetes.
Keywords: COVID‐19, Glycemic control, Lifestyle changes
The study investigated (1) the association of lifestyle changes and living and working conditions with glycemic control and (2) whether treatment was intensified appropriately in diabetes patients under the first COVID‐19 state of emergency in Japan. Teleworking and living with a dog were positively and negatively associated with changes in HbA1c levels, respectively. The proportion of patients who received treatment intensification for diabetes during the pandemic was slightly lower than that before the pandemic, but the difference did not reach statistical significance.
INTRODUCTION
Coronavirus disease 2019 (COVID‐19) 1 has spread worldwide from December 2019, leading to an ongoing pandemic. Diabetes has been reported to be independently associated with an increased risk of morbidity and mortality, and poor glycemic control is a predictor of severity and mortality in patients with diabetes 2 , 3 , 4 , 5 , 6 , 7 . With the spread of COVID‐19, each country went into lockdown to prevent further spread of the disease. Since the first case of COVID‐19 was reported in January 2020 in Japan, the Japanese government ordered all schools closed and introduced telework in March 2020 8 . The government first declared a state of emergency for seven prefectures on April 7 and expanded it to the whole nation on April 16. It asked citizens to stay at home and to avoid closed spaces, crowded places, and close‐contact settings. This was without coercion or lockdown, which was different from the response in other countries. The state of emergency was then lifted on May 25, 2020.
Under lockdown or the state of emergency, people’s lifestyles, including eating habits and physical activity, were changed 9 , 10 , 11 , 12 , 13 . There is concern that non‐communicable diseases, such as diabetes, are worsened by lifestyle changes, including the introduction of telework. Previous studies have shown that teleworking increases sedentary time 14 , 15 . However, evidence regarding the relationship between working conditions and glycemic control is scarce. Furthermore, the COVID‐19 pandemic has affected the frequency of medical visits and the quality of medical care; thus, treatment intensification may not have been properly implemented in diabetes patients. Therefore, we performed a retrospective observational study to investigate (1) the association of lifestyle changes and living and working conditions with glycemic control and (2) whether treatment was intensified appropriately in patients with diabetes under the first COVID‐19 state of emergency in Japan.
MATERIALS AND METHODS
Study design and participant selection
In this retrospective observational study, patients with diabetes, aged ≥20 years, who had been treated regularly at the National Center for Global Health and Medicine (NCGM) Hospital prior to January 1, 2019, were considered eligible. The number of diabetic patients whose HbA1c was available from June 1, 2020, to August 31, 2020, was 1,600, using the Japan Diabetes compREhensive database project based on an Advanced electronic Medical record System (JDREAMS) 16 . Four hundred sixteen patients were screened from November 1, 2020, to March 31, 2021. Patients with a history of admission from April 1 to May 31, 2019, those with severe cognitive impairment, those with cancer with a performance status of 2 or higher, those with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, those with severe liver cirrhosis, and those who the researchers considered inappropriate for study participation were excluded (Figure 1). Patients without laboratory data from June 1, 2019, to August 31, 2019, and those who declined to participate after enrolment were also excluded.
Figure 1.
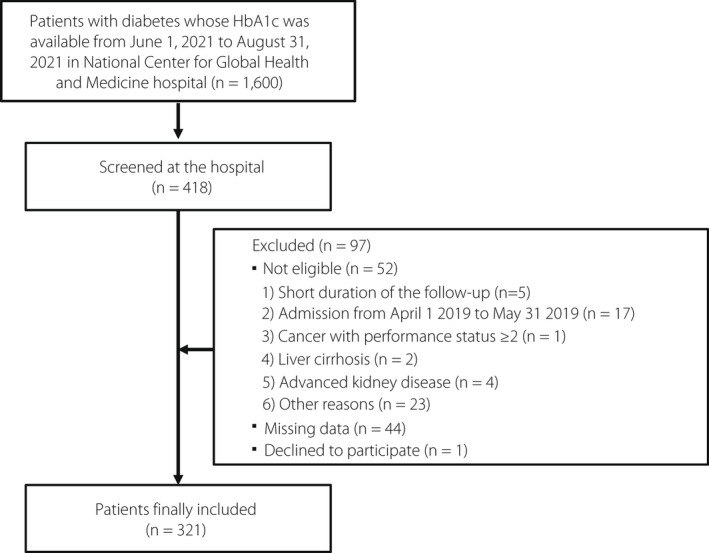
Flowchart of study patients.
Clinical and laboratory data
Clinical and laboratory data were obtained from medical records between June 1, 2019, and August 31, 2019, and between June 1, 2020, and August 31, 2020. Data included age, sex, type of diabetes, duration of diabetes, diabetic retinopathy, history of cardiovascular disease (CVD), height, weight, body mass index (BMI), systolic and diastolic blood pressure (SBP and DBP, respectively), hemoglobin, plasma glucose, HbA1c, triglycerides, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ‐glutamyl transpeptidase (γ‐GTP), creatinine, uric acid, urinary albumin‐to‐creatinine ratio (ACR), and information on medication. The HbA1c concentration was measured using high‐performance liquid chromatography. eGFR was calculated using the equation for the Japanese 17 . After enrolment in the study, participants completed a questionnaire regarding changes in their lifestyles during the COVID‐19 pandemic. These included smoking and drinking status, sleep duration, number of cohabitants, living with or without a dog, working environment (work with commuting, telework, or unemployed), household income, type of insurance, dietary intake, physical activity, and exercise. The patients answered the questionnaire from November 12, 2020 to March 19, 2021.
Outcomes
The primary outcome was the change in HbA1c levels before (June 1, 2019 to August 31, 2019) and during (June 1, 2020 to August 31, 2020) the COVID‐19 pandemic. Changes in laboratory and anthropometric data were also determined. Data on events of acute metabolic emergencies, including diabetic ketoacidosis (DKA), hyperglycemic hyperosmolar state (HHS), and severe hypoglycemia, from April 1, 2020, to August 31, 2020, and the use of telemedicine that started during the COVID‐19 pandemic were obtained. The diagnoses of DKA and HHS were based on medical records. In this study, severe hypoglycemia was defined as an event requiring the assistance of another person to actively administer carbohydrates, glucagon, or take other corrective actions.
We further investigated whether treatment for diabetes was intensified appropriately before and during the pandemic. The HbA1c target in Japan is basically set at <53.0 mmol/mol (7.0%) for preventing diabetic complications 18 , while that for the elderly is set individually according to age, activities of daily living, cognitive function, comorbidities, and the use of drugs potentially associated with severe hypoglycemia 19 . Accordingly, we first calculated the proportion of patients who received treatment intensification in patients aged <65 years old. Then, among those aged 65 years or older, patients with drugs associated with a risk for severe hypoglycemia and those aged 85 years or older who were at an extremely high risk for both physical disability and dementia 20 , 21 , 22 were excluded and the same calculation was done.
We defined patients who required treatment intensification (1) before and (2) during the pandemic as (1) those who did not change diabetes treatment between December 2018 and May 2019 and kept their HbA1c levels 53.0 mmol/mol or higher between March 2019 and August 2019 and (2) those who did not change diabetes treatment between December 2019 and May 2020 and kept their HbA1c levels 53.0 mmol/mol or higher between March 2020 and August 2020, respectively. Among these patients, we calculated the proportion of patients who received treatment intensification between June 2019 and August 2019 (before the pandemic) and between June 2020 and August 2020 (during the pandemic).
Statistical analysis
Data are presented as mean ± SD, median with interquartile range (IQR), or percentage according to data distribution. First, for the descriptive analysis, data before (June 1, 2019 to August 31, 2019) and during (June 1, 2020 to August 31, 2020) the COVID‐19 pandemic were compared by paired‐t test, Wilcoxon signed‐rank test, McNemar’s test, or McNemar‐Bowker test. Second, to determine the association between the change in HbA1c and variables, the t‐test or analysis of variance (anova) were used. The Tukey‐Kramer method was used for post hoc analysis after anova. Two‐way anova was used to determine the interaction between age and telework. Multiple linear regression analysis with forward stepwise entry was performed to clarify the factors associated with the change in HbA1c and other laboratory and anthropometric data. The variables used in the analysis were age, sex, type of diabetes, duration of diabetes, history of CVD, presence of proliferative diabetic retinopathy (PDR), variables listed above before the COVID‐19 pandemic, medications before the COVID‐19 pandemic, smoking and drinking status, living environment (with or without cohabitants and with or without a dog), working environment (telework vs others), household income, changes in dietary intake, physical activity, and exercise during the pandemic. Statistical analyses were performed using IBM SPSS, version 24.0 (IBM Corp., Armonk, NY, USA). A P value <0.05 was considered statistically significant.
RESULTS
A total of 321 participants (72.5% male) were included. The characteristics of the patients are shown in Table 1. There was no significant change in HbA1c levels before and during the COVID‐19 pandemic (54.4 ± 10.7 vs 55.0 ± 11.1 mmol/mol, P = 0.186). As shown in Figure S1a, there were very few patients whose HbA1c levels were markedly changed during the pandemic (median with interquartile range; 0.1 [−0.30 to 0.37]). Also, the distribution of change in HbA1c levels was almost comparable between patients aged <65 years and those aged 65 years or older (Figure S1b,c) without a significant difference between the groups (0.5 ± 9.3 vs 0.6 ± 7.0 mmol/mol, P = 0.872). SBP significantly increased as weight, BMI, and uric acid decreased during the pandemic. Regarding medication, the prescription of sodium‐glucose transport protein 2 inhibitors, angiotensin receptor blockers, and statins were significantly increased during the COVID‐19 pandemic.
Table 1.
Patient characteristics before and during the COVID‐19 pandemic
| June 1–Aug 31, 2019 | June 1–Aug 31, 2020 | P value † | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Anthropometry (n = 321) | |||||
| Age (years) | 67.8 | 11.8 | |||
| Sex (% male) | 72.5 | ||||
|
Type of diabetes (number) (type1/type2/other) |
29/276/16 | ||||
| Duration of diabetes (years) | 15.6 | 10.6 | |||
| Retinopathy (NDR/SDR/PDR) | 77.1/14.1/8.8 | ||||
| History of CVD (%) | 20.9 | ||||
| Height (cm) | 164.1 | 9.1 | |||
| Weight (kg) | 69.2 | 14.9 | 68.9 | 14.6 | 0.046 |
| Body mass index (kg/m2) | 25.6 | 4.5 | 25.4 | 4.4 | 0.045 |
| Systolic blood pressure (mmHg) | 125 | 13 | 126 | 15 | 0.041 |
| Diastolic blood pressure (mmHg) | 70 | 11 | 71 | 10 | 0.183 |
| Laboratory data | |||||
| Hb (g/dL) | 14.3 | 1.6 | 14.4 | 1.7 | 0.191 |
| Glucose (mmol/L) | 7.8 | 2.4 | 8.0 | 2.8 | 0.226 |
| HbA1c (mmol/mol) | 54.4 | 10.7 | 55.0 | 11.1 | 0.186 |
| HbA1c (%) | 7.13 | 0.98 | 7.18 | 1.01 | 0.186 |
| Triglycerides (mmol/L) | 1.78 | 1.34 | 1.80 | 1.30 | 0.783 |
| HDL cholesterol (mmol/L) | 1.44 | 0.41 | 1.44 | 0.42 | 0.954 |
| TG/HDL cholesterol ratio | 1.45 | 1.40 | 1.48 | 1.50 | 0.636 |
| LDL cholesterol (mmol/L) | 2.52 | 0.70 | 2.50 | 0.71 | 0.727 |
| AST (U/L) | 25 | 19 | 25 | 14 | 0.573 |
| ALT (U/L) | 28 | 25 | 26 | 21 | 0.140 |
| γ‐GTP (U/L) | 44 | 52 | 42 | 56 | 0.284 |
| eGFR (mL/min/1.73 m2) | 65.3 | 18.7 | 64.9 | 18.7 | 0.310 |
| Uric acid (μmol/L) | 335 | 78 | 327 | 79 | 0.020 |
| Log urinary ACR (n = 84) | 1.24 | 0.76 | 1.31 | 0.71 | 0.188 |
| Medication | Percentage | Percentage | P value ‡ |
|---|---|---|---|
| Sulfonylureas | 20.0 | 18.1 | 0.070 |
| Biguanides | 54.4 | 56.3 | 0.109 |
| Thiazolidines | 7.5 | 6.3 | 0.289 |
| α‐Glucosidase inhibitors | 19.7 | 19.4 | 1.000 |
| Glinides | 7.5 | 8.1 | 0.774 |
| DPP4 inhibitors | 53.8 | 55.9 | 0.248 |
| SGLT2 inhibitors | 30.0 | 38.4 | <0.001 |
| GLP‐1 receptor agonists | 13.4 | 15.3 | 0.4146 |
| Insulin | 30.6 | 28.7 | 0.070 |
| Calcium channel blockers | 39.7 | 41.3 | 0.227 |
| ARBs | 40.9 | 43.8 | 0.035 |
| ACE inhibitors | 8.4 | 7.8 | 0.625 |
| α‐Blockers | 1.9 | 1.3 | 0.625 |
| β‐Blockers | 11.9 | 13.1 | 0.219 |
| MR blockers | 1.9 | 1.9 | 1.000 |
| Diuretics | 9.4 | 10.0 | 0.727 |
| Statins | 50.9 | 54.1 | 0.021 |
| Fibrates | 5.3 | 5.0 | 1.000 |
| Ezetimib | 6.3 | 6.9 | 0.625 |
| UA‐lowering agents | 14.7 | 15.6 | 0.581 |
| Antiplatelets | 19.4 | 19.1 | 1.000 |
ACE, angiotensin‐converting enzyme; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; CVD, cardiovascular disease; DPP4, dipeptidyl peptidase 4; GLP‐1, glucagon‐like peptide‐1; GTP, glutamyl transpeptidase; Hb, hemoglobin; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MR, mineral‐corticoid receptor; NDR, non‐diabetic retinopathy; PDR, proliferative diabetic retinopathy; SD, standard deviation; SDR, simple diabetic retinopathy; SGLT2, sodium‐glucose cotransporter 2; UA, uric acid.
Paired t‐test.
McNemar’s test.
Before the pandemic, no episodes of acute metabolic emergencies were observed among the patients. During the pandemic, one patient (0.3%) experienced DKA, and no episode of HHS or severe hypoglycemia was observed. Twenty‐five patients (7.8%) started using telemedicine in 2020.
Lifestyle changes during the pandemic are presented in Table 2. The number of cigarettes did not change and the percentage of drinkers was significantly decreased, however, ethanol consumption by the drinkers was significantly increased. During the pandemic, 29.2% of the patients were living alone, 9.6% lived with a dog, 11.7% were introduced to telework, and 11.2% were welfare recipients. As shown in Figure 2, there was a significant difference in the change in HbA1c levels among workers who commuted, teleworkers, and unemployed patients (P = 0.038 by anova). Furthermore, post‐hoc analysis revealed that teleworkers had significantly increased HbA1c levels compared with workers who commuted (Figure 2a). The change in HbA1c levels in patients living with a dog tended to be slighter than that in patients without a dog (Figure 2b). There was no significant difference in changes in HbA1c levels during the pandemic among household income categories (Figure 2c). As age may have affected the impact of teleworking on the change in HbA1c, a sensitivity analysis was done according to the age (<65 years old vs ≥65 years old) (Figure S2). Twenty‐four out of 108 and six out of 213 were teleworking during the pandemic in the non‐elderly and in the elderly group. The change in HbA1c was significantly higher in patients with teleworking than that in patients without (P = 0.014) and no significant interaction was observed between age and telework (P = 0.916 for interaction by two‐way anova).
Table 2.
Lifestyle changes during the COVID‐19 pandemic
| Lifestyle (n = 307) | June 1–Aug 31, 2019 | June 1–Aug 31, 2020 | P value ‡ |
|---|---|---|---|
| Percentage | Percentage | ||
| Smoking (no. of cigarettes) | |||
| None | 79.7 | 79.9 | 0.980 |
| <10 | 5.2 | 5.2 | |
| 10–19 | 9.2 | 9.0 | |
| 20–40 | 5.6 | 5.2 | |
| ≥40 | 0.3 | 0.7 | |
| Drinking | |||
| Yes (%) | 47.1 | 42.3 | <0.001 |
| Ethanol (g/day) in the drinkers | 11.4 [4.5–34.3] † | 13.9 [5.0–39.5] † | 0.023 |
| Ethanol ≥40 g/day in male | 13.0 | 13.9 | 0.727 |
| Ethanol ≥20 g/day in female | 8.5 | 8.4 | 1.000 |
| Sleep duration (hours) (mean ± SD) | 7.28 ± 1.46 | 7.36 ± 1.50 | 0.124 |
| Percentage | |
|---|---|
| Living environment (n = 318) | |
| No. of cohabitants including the patient | |
| One | 29.2 |
| Two | 42.1 |
| Three | 18.3 |
| Four | 7.5 |
| Five or more | 2.8 |
| Living with a dog (yes) | 9.6 |
| Working environment (n = 256) | |
| Work with commuting | 50.0 |
| Telework | 11.7 |
| Unemployed | 38.3 |
| Household income (Yen/month) (n = 251) | |
| Welfare recipients | 11.2 |
| <200,000 Yen/month | 20.3 |
| 200,000–399,999 Yen/month | 31.9 |
| 400,000–599,999 Yen/month | 16.3 |
| 600,000–799,999 Yen/month | 8.0 |
| ≥800,000 Yen/month | 12.4 |
Median with interquartile range
Paired t‐test, Wilcoxon signed‐rank test, or McNemar’s test, McNemar‐Bowker test as appropriate.
Figure 2.
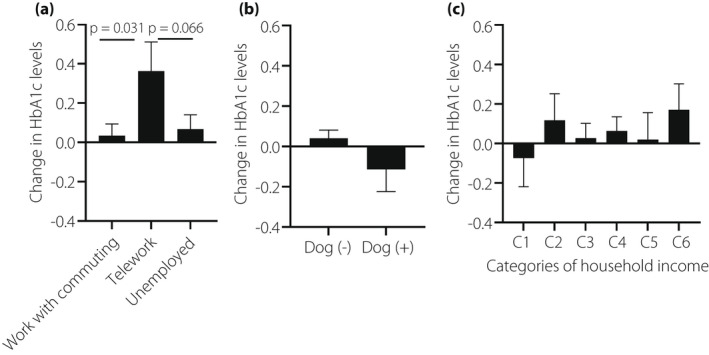
Change in HbA1c levels according to working condition (a), living with or without a dog (b), and household income (c). C1, welfare recipients; C2, household income <200,000 Yen/month; C3, household income 200,000–399,999 Yen/month; C4, household income 400,000–599,999 Yen/month; C5, household income 600,000–799,999 Yen/month; C6, household income ≥800,000 Yen/month.
Regarding dietary intake (Table 3), the frequency of eating out/banquets decreased in more than 70% of the patients. Conversely, the intake of fruits, sweets, and snacks increased in 20–30% of the patients. Both physical activity and exercise decreased in more than 40% of the patients. Patients with decreased snacks had significantly decreased HbA1c levels. Adding milk/sugar to coffee/tea significantly contributed to an increase in HbA1c levels during the pandemic (Figure 3). As shown in Figure 4, patients with decreased physical activity and exercise showed elevated HbA1c levels compared with those with stable or increased levels. However, there were no significant differences in the HbA1c level changes by either physical activity (P = 0.412) or exercise (P = 0.147). The change in HbA1c was comparable by type of insurance and was not correlated with change in sleep time (data not shown).
Table 3.
Changes in eating and exercise behaviors during the COVID‐19 pandemic among patients with diabetes
| Decreased (%) | Unchanged (%) | Increased (%) | |
|---|---|---|---|
| Dietary intake (n = 314) | |||
| Carbohydrates | 12.7 | 72.0 | 15.3 |
| Sodium | 9.2 | 83.4 | 7.3 |
| Meat | 9.9 | 80.5 | 9.6 |
| Fish | 10.5 | 79.9 | 9.6 |
| Vegetables | 6.4 | 77.0 | 16.6 |
| Fruits | 9.3 | 69.6 | 21.2 |
| Sweets | 15.9 | 56.4 | 27.7 |
| Snacks | 17.5 | 57.6 | 24.8 |
| Eating out/banquets | 71.1 | 26.3 | 2.6 |
| Cooking for myself | 4.9 | 73.4 | 21.7 |
| Convenience store lunch/home delivery meal | 17.8 | 60.9 | 21.2 |
| Adding milk/sugar to coffee/tea | 15.3 | 77.6 | 7.1 |
| Physical activity (n = 303) | 42.1 | 51.6 | 6.3 |
| Exercise (n = 288) | 42.4 | 47.2 | 10.3 |
Figure 3.
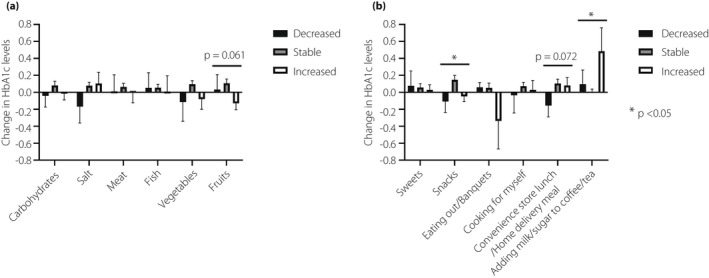
Change in HbA1c levels according to nutrients (a) and eating behaviors (b).
Figure 4.
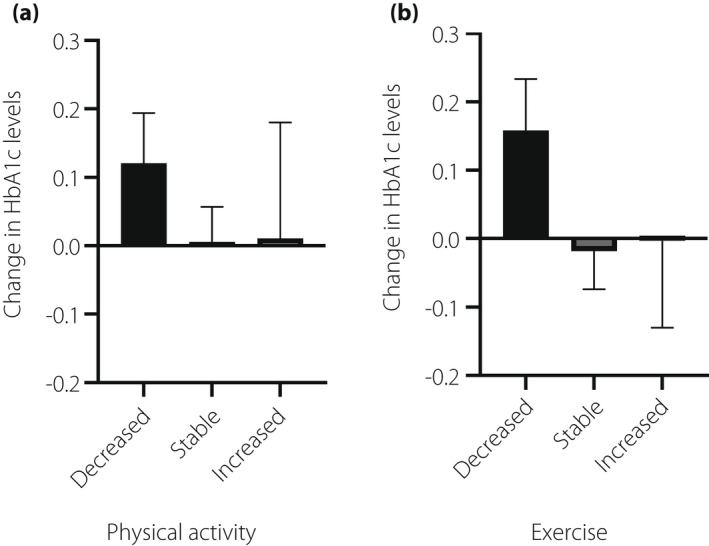
Change in HbA1c levels according to physical activity (a) and exercise (b).
In a multiple linear regression analysis, teleworkers were shown to be at a significantly high risk of worsening glycemic control (Table 4). Furthermore, living with a dog was negatively and independently associated with change in HbA1c level. We considered the possibility that living with a dog and teleworking could affect physical activity and exercise. Thus, we calculated the proportions of categories (decreased, stable, or increased) of physical activity and exercise between patients living with and without a dog (Table S1) and among those commuting to work, teleworkers, and unemployed patients (Table S2). Patients living with a dog tended to have low proportions of decreased physical activity and exercise (P = 0.149 for physical activity and P = 0.133 for exercise) compared with those living without a dog. Unexpectedly, teleworkers showed high proportions of both decreased and increased physical activity and exercise compared with those commuting to work or unemployed (P = 0.046 for physical activity and P = 0.002 for exercise). There was no significant difference in the change in dietary intake between living with/without a dog or working condition (data not shown).
Table 4.
Multiple linear regression analysis to investigate factors associated with changes in HbA1c (mmol/mol) levels during the COVID‐19 pandemic
| Estimate | Standard error | t value | P value | |
|---|---|---|---|---|
| Intercept | 7.555 | 2.325 | 3.250 | 0.001 |
| Telework (ref: work with commuting or unemployed) | 4.242 | 1.459 | 2.908 | 0.004 |
| Living with a dog (ref: living without a dog) | −3.153 | 1.588 | −1.985 | 0.048 |
| HbA1c prior to the COVID‐19 pandemic (mmol/mol) | −0.133 | 0.042 | −3.140 | 0.002 |
| Presence of proliferative diabetic retinopathy | −2.690 | 1.603 | −1.678 | 0.095 |
| History of cardiovascular disease | 2.112 | 1.177 | 1.794 | 0.074 |
HbA1c, hemoglobin A1c.
Next, we investigated factors associated with changes in weight, BMI, SBP, and uric acid levels (Table S3). These were significantly changed during the pandemic (Table 1). We also investigated factors associated with changes in lipid metabolism, liver enzyme, and renal function variables (Table S4). In terms of exercise‐related behavioral changes, decreased exercise was associated with increased weight and SBP. Furthermore, decreased physical activity was associated with an increased BMI and albuminuria. Regarding dietary‐related changes, fish intake was associated with increased uric acid levels; vegetable intake with increased HDL cholesterol levels; and convenience store lunch/home delivery meal and carbohydrate intake were positively and negatively, respectively, associated with LDL cholesterol levels. Regarding the working environment, teleworking was associated with increased ALT and γ‐GTP levels. Furthermore, living with a dog was associated with decreased LDL cholesterol levels.
Finally, we investigated the proportion of patients who required treatment intensification for diabetes and those who received treatment intensification before and during the COVID‐19 pandemic in the non‐elderly patients (aged <65 years) (Figure S3). The proportion of patients who required treatment intensification were 31.4% and 39.1% before and during the pandemic (P = 0.258). Among these, 18.8% and 8.3% were intensified diabetes treatment before and during the pandemic (P = 0.206), respectively. Among the elderly patients (aged ≥65 years) except those who aged 85 years or older and those with drugs potentially associated with severe hypoglycemia (Figure S4), the proportion of patients who required treatment intensification for diabetes were 9.3% and 12.4% before and during the pandemic (P = 0.344). Among these, 11.1% and 25.0% were intensified diabetes treatment before and during the pandemic (P = 0.270), respectively.
DISCUSSION
In this study, it was demonstrated that HbA1c levels were not significantly changed during the COVID‐19 pandemic in Japanese patients with diabetes. Teleworking and living with a dog were positively and negatively, respectively, associated with worsening diabetes. Lastly, the proportion of patients who received treatment intensification for diabetes during the pandemic was slightly lower than that before the pandemic, but the difference did not reach statistical significance.
Diabetes and hyperglycemia are reported to be associated with morbidity and mortality in patients with COVID‐19 2 , 3 , 4 , 5 , 6 , 7 . Therefore, it is important to identify diabetic patients whose HbA1c levels are increased during the pandemic. The change in HbA1c levels during the COVID‐19 pandemic in patients with diabetes reported from Japan is controversial 23 , 24 , 25 , 26 , 27 , 28 , 29 ; some reports show an increase in HbA1c 24 , 25 , 26 , while others show a decrease 23 , 27 , 28 . The differences in these results may be due to regional differences in COVID‐19 prevalence and socioeconomic status, as well as differences in the definition of HbA1c change in each study. Lifestyle factors associated with an increased HbA1c in these studies were reduced exercise, increased calorie intake, and snacking. In this study, patients with reduced physical activity or exercise had increased HbA1c levels compared with those with unchanged or increased physical activity or exercise. Further large‐scale nationwide studies are warranted.
As elderly patients with diabetes are considered to have HbA1c targets and therapeutic approaches for diabetes management tailored to each individual and there may be differences in working status between the elderly and non‐elderly patients during the pandemic, we focused on the analysis separately for patients aged <65 years old and those over the age of 65 years old. The distribution of change in HbA1c levels was almost comparable between patients aged <65 years old and those aged 65 years or older, and was consistent with the result of a previous study 27 .
This study is unique in investigating the association of living and working environments, in addition to changes in dietary intake, physical activity, and exercise, with changes in glycemic control in patients with diabetes during the COVID‐19 pandemic. Telework is excellent in terms of infection control 30 ; thus, it is recommended by the Centers for Disease Control and Prevention (CDC) 31 and the Ministry of Health, Labor and Welfare in Japan 8 . This study revealed that telework was positively and living with a dog was negatively associated with worsening glycemic control during the pandemic. The impact of telework was not affected by age. Besides our report, there is only one study evaluating working status among patients with diabetes 32 , consistent with our findings, they also showed that telework was associated with a deterioration of glycemic control. Initially we thought that glycemic control in teleworkers with diabetes might have worsened due to decreased physical activity and changes in eating habits; however, this was not the case. Rather, the proportion of patients who increased physical activity and exercise during the pandemic was higher among teleworkers than that among patients who commuted to work or those unemployed. It is possible that the amount of physical activity accompanied by commuting was lost due to the introduction of telework, leading to impaired glycemic control. In contrast, as expected, the proportion of patients with decreased physical activity and exercise tended to be low among patients living with a dog compared with that among those living without a dog. This suggests that the dog owners continued to walk with their dogs during the pandemic. Therefore, their physical activity and exercise did not drop, and their glycemic control remained good.
Aside from glycemic control, decreased physical activity was associated with an increased BMI and albuminuria. Moreover, exercise was associated with increased weight and SBP. These findings are consistent with those of previous observational and interventional studies 33 , 34 , 35 , 36 , 37 suggesting that the COVID‐19 pandemic could increase the risk of obesity, hypertension, and chronic kidney disease through lifestyle changes related to physical activity/exercise. Monitoring and intervention of physical activity is necessary to improve the quality of care in patients with diabetes during the pandemic. We also found that telework was associated with elevated liver enzymes. The reason for this is unclear; however, there was no significant difference in the change in alcohol consumption with working condition (data not shown). This suggests that teleworkers are more likely to develop non‐alcoholic fatty liver disease than other groups and to have elevated liver enzyme levels during the pandemic.
Fish and vegetable intake were positively associated with changes in uric acid and HDL cholesterol levels, respectively. A previous report showed that fish meat such as bonito contains large amounts of purines 38 and that seafood intake contributed to hyperuricemia 39 . These findings are similar to those of the current study. Since a previous meta‐analysis showed a negative association between vegetable intake and HDL cholesterol levels 40 , some confounding factors may exist in our study. In the same study, the reduction in LDL cholesterol levels through a vegetarian diet was much greater than that in HDL cholesterol levels. Therefore, eating sufficient vegetables to control lipid metabolism during the COVID‐19 pandemic is a reasonable strategy. As for eating habits, convenience store lunch/home delivery meals were positively associated with LDL cholesterol levels. Convenience foods and deliveries may contain high levels of cholesterol. The inverse association between carbohydrate consumption and LDL cholesterol levels was consistent with the results of previous studies 41 , 42 . In some clinical trials 43 , 44 , 45 , LDL cholesterol levels increased significantly when carbohydrate intake was decreased by a simple exchange for fat, especially saturated fatty acids, or when fiber intake was decreased. Further studies are needed to clarify the mechanism by which dietary intake and eating habits exacerbate lipid metabolism during the COVID‐19 pandemic.
Although the proportion of patients who received treatment intensification for diabetes both before and during the pandemic seems to be low in this study, it is compatible with a previous systematic review 46 . The proportion of treatment intensification for diabetes within 3 months in patients with HbA1c levels of 53.0 mmol/mol or higher was shown to be 5–60%. The probability of receiving treatment intensification was decreased with increased age and the number of oral antidiabetic agents 47 . In this study, more than half of the patients were aged 65 years or older and more than 60% of the patients received three or more oral antidiabetic agents and/or GLP‐1RA and/or insulin both before and during the pandemic (data not shown). These results may have affected the proportion of treatment intensification for diabetes. Under the first COVID‐19 state of emergency in Tokyo, medical services were disrupted. Consequently, the number of hospital visits and medical care 48 were decreased, similar to other countries with lockdowns 49 . Thus, we initially hypothesized that the pandemic may have reduced the opportunity for patients with diabetes to receive a treatment intensification, but the proportion of intensified treatment was not decreased during the pandemic. As the study included patients whose laboratory data were available before and during the pandemic, it is possible that some patients who discontinued or delayed regular visits during the pandemic were excluded, presumably attenuating the impact of the COVID‐19 pandemic on treatment intensification for diabetes. Given these, compared with the pre‐pandemic period, it would be necessary to carefully monitor whether diabetes treatment was intensified appropriately during the pandemic.
In this study, 7.8% of the patients received telemedicine during the pandemic to reduce the risk of contracting COVID‐19. Telemedicine is useful in keeping patients on track with their diabetes treatment 50 and has been reported to improve glycemic control in a retrospective study from Japan 51 . However, in Japan, both self‐monitoring of blood glucose and continuous glucose monitoring are covered by insurance almost exclusively for patients receiving insulin or glucagon‐like peptide 1 receptor agonist (GLP‐1RA). Therefore, it is impossible to monitor glycemic control without blood sampling at clinics/hospitals in patients without insulin/GLP‐1RA treatment. This may lead to an inadequate intensification of diabetes treatment when telemedicine is introduced. In situations such as the COVID‐19 pandemic where access to medical care is limited, a system that allows all patients with diabetes to monitor their glycemic control remotely would be desirable.
This study has some limitations. First, this was a single‐center retrospective study conducted at a National Center Hospital and with a relatively small sample size; thus, the generalizability of the results is limited. Second, this study used a brief questionnaire to assess the dietary and exercise‐related behavioral changes; therefore, it was impossible to quantify dietary intake nor physical activity. Third, to date (until July 2021), the Japanese government has already declared a state of emergency four times; therefore, it will be necessary to verify whether the findings in this study can be replicated. Fourth, the COVID‐19 pandemic may have affected the frequency of medical visits and some patients may have dropped out from regular visits; however, we were unable to include these patients in this study because only patients whose HbA1c levels were available prior to and during the pandemic were included. Finally, as information on physical activity and cognitive function was unavailable, precise categorization of health status in the elderly to set a HbA1c target was impossible in this study.
In conclusion, overall glycemic control did not worsen during the pandemic. Nonetheless, environmental factors, including teleworking, were found to influence glycemic control in patients with diabetes. Further studies are needed to clarify whether the COVID‐19 pandemic could affect treatment intensification for diabetes.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: This study was approved by the Institutional Review Board of the NCGM (approval number, NCGM‐G‐ 004033‐00; approval date, November 09, 2020).
Informed consent: Written informed consent was obtained from all patients.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Table S1 | Changes in physical activity and exercise in patients with diabetes living with and without a dog during the COVID‐19 pandemic
Table S2 | Changes in physical activity and exercise in patients with diabetes according to their working environments during the COVID‐19 pandemic
Table S3 | Factors associated with changes in weight, body mass index, systolic blood pressure, and uric acid levels
Table S4 | Factors associated with changes in lipid metabolism, liver enzyme levels, and renal function
Figure S1 | Histogram of the changes in HbA1c levels in patients with diabetes during the COVID‐19 pandemic.
Figure S2 | Comparison of the impact of teleworking on the change in HbA1c levels (mmol/mol) according to the age.
Figure S3 | Proportion of patients aged <65 years old who required treatment intensification and those who received treatment intensification before and during the pandemic.
Figure S4 | Proportion of patients aged 65 years or older who required treatment intensification and those who received treatment intensification before and during the pandemic except those who aged 85 years or older or those who were treated with drugs potentially associated with severe hypoglycemia.
ACKNOWLEDGMENTS
We thank all the staff members at the Department of Diabetes, Endocrinology, and Metabolism at the NCGM Hospital for their contribution to patient enrolment in this study. This study was supported by the Health and Labour Sciences Research Grant (21CA2021). The funding agency had no role in the design or conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
J Diabetes Investig. 2022; 13: 1094–1104
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐19) situation reports. Available from: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports. Accessed July 10, 2021.
- 2. Varikasuvu SR, Dutt N, Thangappazham B, et al. Diabetes and COVID‐19: a pooled analysis related to disease severity and mortality. Prim Care Diabetes 2021; 15: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mantovani A, Byrne CD, Zheng M‐H, et al. Diabetes as a risk factor for greater COVID‐19 severity and in‐hospital death: a meta‐analysis of observational studies. Nutr Metab Cardiovasc Dis 2020; 30: 1236–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID‐19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180: 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dennis JM, Mateen BA, Sonabend R, et al. Type 2 diabetes and COVID‐19‐related mortality in the critical care setting: a national cohort study in England, March–July 2020. Diabetes Care 2021; 44: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID‐19‐related mortality in England: a whole‐population study. Lancet Diabetes Endocrinol 2020; 8: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holman N, Knighton P, Kar P, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol 2020; 8: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ministry of Internal Affairs, Japan . Coronavirus disease 2019 (COVID‐19): MIC ICT policy. Available from: https://www.soumu.go.jp/main_sosiki/joho_tsusin/eng/COVID‐19/index.html. Accessed July 9, 2021.
- 9. Grant F, Scalvedi ML, Scognamiglio U, et al. Eating habits during the COVID‐19 lockdown in Italy: the nutritional and lifestyle side effects of the pandemic. Nutrients 2021; 13: 2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dobrowolski H, Włodarek D. Body mass, physical activity and eating habits changes during the first COVID‐19 pandemic lockdown in Poland. Int J Environ Res Public Health 2021; 18: 5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prete M, Luzzetti A, Augustin LSA, et al. Changes in lifestyle and dietary habits during COVID‐19 lockdown in Italy: results of an online survey. Nutrients 2021; 13: 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruiz‐Roso MB, Knott‐Torcal C, Matilla‐Escalante DC, et al. COVID‐19 lockdown and changes of the dietary pattern and physical activity habits in a cohort of patients with type 2 diabetes mellitus. Nutrients 2020; 12: E2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruissen MM, Regeer H, Landstra CP, et al. Increased stress, weight gain and less exercise in relation to glycemic control in people with type 1 and type 2 diabetes during the COVID‐19 pandemic. BMJ Open Diabetes Res Care 2021; 9: e002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fukushima N, Machida M, Kikuchi H, et al. Associations of working from home with occupational physical activity and sedentary behavior under the COVID‐19 pandemic. J Occup Health 2021; 63: e12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koohsari MJ, Nakaya T, McCormack GR, et al. Changes in workers' sedentary and physical activity behaviors in response to the COVID‐19 pandemic and their relationships with fatigue: longitudinal online study. JMIR Public Health Surveill 2021; 7: e26293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sugiyama T, Miyo K, Tsujimoto T, et al. Design of and rationale for the Japan Diabetes compREhensive database project based on an Advanced electronic Medical record System (J‐DREAMS). Diabetol Int 2017; 8: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 18. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig 2020; 11: 1020–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes . Glycemic targets for elderly patients with diabetes. Geriatr Gerontol Int 2016; 16: 1243–1245. [DOI] [PubMed] [Google Scholar]
- 20. Yoshida D, Ninomiya T, Doi Y, et al. Prevalence and causes of functional disability in an elderly general population of Japanese: the Hisayama study. J Epidemiol 2012; 22: 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakurai T, Iimuro S, Sakamaki K, et al. Risk factors for a 6‐year decline in physical disability and functional limitations among elderly people with type 2 diabetes in the Japanese elderly diabetes intervention trial. Geriatr Gerontol Int 2012; 12(Suppl 1): 117–126. [DOI] [PubMed] [Google Scholar]
- 22.Gender Equality Bureau Cabinet Office. Available from: https://www.gender.go.jp/about_danjo/whitepaper/h30/zentai/html/zuhyo/zuhyo01‐00‐43.html. Accessed July 09, 2021.
- 23. Masuda M, Tomonaga O. Study on the effects of changes in lifestyle of patients with diabetes on glycaemic control before and after the declaration of the state of emergency in Japan. Diabetol Int 2021; 20: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanji Y, Sawada S, Watanabe T, et al. Impact of COVID‐19 pandemic on glycemic control among outpatients with type 2 diabetes in Japan: a hospital‐based survey from a country without lockdown. Diabetes Res Clin Pract 2021; 176: 108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munekawa C, Hosomi Y, Hashimoto Y, et al. Effect of coronavirus disease 2019 pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: a cross‐section and retrospective cohort study. Endocr J 2021; 68: 201–210. [DOI] [PubMed] [Google Scholar]
- 26. Hosomi Y, Munekawa C, Hashimoto Y, et al. The effect of COVID‐19 pandemic on the lifestyle and glycemic control in patients with type 1 diabetes: a retrospective cohort study. Diabetol Int 2022; 13: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka N, Hamamoto Y, Kurotobi Y, et al. Lifestyle changes as a result of COVID‐19 containment measures: bodyweight and glycemic control in patients with diabetes in the Japanese declaration of a state of emergency. J Diabetes Investig 2021; 12: 1718–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aso Y, Iijima T, Tomaru T, et al. No negative impact of a national state of emergency by COVID‐19 outbreak on hemoglobin A1c levels in patients with type 2 diabetes living in semi‐rural Japan. Am J Med Sci 2021; 362: 104–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watanabe T, Temma Y, Okada J, et al. Influence of the stage of emergency declaration due to the coronavirus disease 2019 outbreak on plasma glucose control of patients with diabetes mellitus in the Saku region of Japan. J Rural Med 2021; 16: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fisher KA, Olson SM, Tenforde MW, et al. Telework before illness onset among symptomatic adults aged ≥18 years with and without COVID‐19 in 11 outpatient health care facilities – United States, July 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1648–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. CDC . Community, work, and school. Centers for Disease Control and Prevention, February 11, 2020. Available from: https://www.cdc.gov/coronavirus/2019‐ncov/community/high‐risk‐workers.html. Accessed July 9, 2021.
- 32. Kishimoto M, Ishikawa T, Odawara M. Behavioral changes in patients with diabetes during the COVID‐19 pandemic. Diabetol Int 2020; 12: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saco‐Ledo G, Valenzuela PL, Ruiz‐Hurtado G, et al. Exercise reduces ambulatory blood pressure in patients with hypertension: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc 2020; 9: e018487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heberle I, de Barcelos GT, Silveira LMP, et al. Effects of aerobic training with and without progression on blood pressure in patients with type 2 diabetes: a systematic review with meta‐analyses and meta‐regressions. Diabetes Res Clin Pract 2021; 171: 108581. [DOI] [PubMed] [Google Scholar]
- 35. Wadén J, Forsblom C, Thorn LM, et al. Physical activity and diabetes complications in patients with type 1 diabetes: the Finnish Diabetic Nephropathy (FinnDiane) study. Diabetes Care 2008; 31: 230–232. [DOI] [PubMed] [Google Scholar]
- 36. Ikizler TA, Robinson‐Cohen C, Ellis C, et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol 2018; 29: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dunkler D, Kohl M, Heinze G, et al. Modifiable lifestyle and social factors affect chronic kidney disease in high‐risk individuals with type 2 diabetes mellitus. Kidney Int 2015; 87: 784–791. [DOI] [PubMed] [Google Scholar]
- 38. Kaneko K, Aoyagi Y, Fukuuchi T, et al. Total purine and purine base content of common foodstuffs for facilitating nutritional therapy for gout and hyperuricemia. Biol Pharm Bull 2014; 37: 709–721. [DOI] [PubMed] [Google Scholar]
- 39. Aihemaitijiang S, Zhang Y, Zhang LI, et al. The association between purine‐rich food intake and hyperuricemia: a cross‐sectional study in Chinese adult residents. Nutrients 2020; 12: E3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yokoyama Y, Levin SM, Barnard ND. Association between plant‐based diets and plasma lipids: a systematic review and meta‐analysis. Nutr Rev 2017; 75: 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Song S, Song WO, Song Y. Dietary carbohydrate and fat intakes are differentially associated with lipid abnormalities in Korean adults. J Clin Lipidol 2017; 11: 338–347.e3. [DOI] [PubMed] [Google Scholar]
- 42. Fechner E, Smeets E, Schrauwen P, et al. The effects of different degrees of carbohydrate restriction and carbohydrate replacement on cardiometabolic risk markers in humans – a systematic review and meta‐analysis. Nutrients 2020; 12: E991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ebbeling CB, Leidig MM, Feldman HA, et al. Effects of a low‐glycemic load vs low‐fat diet in obese young adults: a randomized trial. JAMA 2007; 297: 2092–2102. [DOI] [PubMed] [Google Scholar]
- 44. Vidon C, Boucher P, Cachefo A, et al. Effects of isoenergetic high‐carbohydrate compared with high‐fat diets on human cholesterol synthesis and expression of key regulatory genes of cholesterol metabolism. Am J Clin Nutr 2001; 73: 878–884. [DOI] [PubMed] [Google Scholar]
- 45. De Natale C, Annuzzi G, Bozzetto L, et al. Effects of a plant‐based high‐carbohydrate/high‐fiber diet versus high‐monounsaturated fat/low‐carbohydrate diet on postprandial lipids in type 2 diabetic patients. Diabetes Care 2009; 32: 2168–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab 2018; 20: 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mata‐Cases M, Franch‐Nadal J, Real J, et al. Therapeutic inertia in patients treated with two or more antidiabetics in primary care: factors predicting intensification of treatment. Diabetes Obes Metab 2018; 20: 103–112. [DOI] [PubMed] [Google Scholar]
- 48. Ikesu R, Miyawaki A, Sugiyama T, et al. Trends in diabetes care during the COVID‐19 outbreak in Japan: an observational study. J Gen Intern Med 2021; 36: 1460–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Forde R, Arente L, Ausili D, et al. The impact of the COVID‐19 pandemic on people with diabetes and diabetes services: a pan‐European survey of diabetes specialist nurses undertaken by the Foundation of European Nurses in Diabetes survey consortium. Diabet Med 2021; 38: e14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Patel SY, McCoy RG, Barnett ML, et al. Diabetes care and glycemic control during the COVID‐19 pandemic in the United States. JAMA Intern Med 2021; 181: 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Onishi Y, Yoshida Y, Takao T, et al. Diabetes management by either telemedicine or clinic visit improved glycemic control during the coronavirus disease 2019 pandemic state of emergency in Japan. J Diabetes Investig 2021. 10.1111/jdi.13546 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Changes in physical activity and exercise in patients with diabetes living with and without a dog during the COVID‐19 pandemic
Table S2 | Changes in physical activity and exercise in patients with diabetes according to their working environments during the COVID‐19 pandemic
Table S3 | Factors associated with changes in weight, body mass index, systolic blood pressure, and uric acid levels
Table S4 | Factors associated with changes in lipid metabolism, liver enzyme levels, and renal function
Figure S1 | Histogram of the changes in HbA1c levels in patients with diabetes during the COVID‐19 pandemic.
Figure S2 | Comparison of the impact of teleworking on the change in HbA1c levels (mmol/mol) according to the age.
Figure S3 | Proportion of patients aged <65 years old who required treatment intensification and those who received treatment intensification before and during the pandemic.
Figure S4 | Proportion of patients aged 65 years or older who required treatment intensification and those who received treatment intensification before and during the pandemic except those who aged 85 years or older or those who were treated with drugs potentially associated with severe hypoglycemia.


