ABSTRACT
Aims/Introduction
The Japanese diabetes treatment guidelines do not specify the first choice of hypoglycemic agents, unlike those of Western countries. Furthermore, the current situation in diabetes treatment is that the choice of hypoglycemic agents is determined by each physician. Therefore, we aimed to determine the current situation in Miyazaki Prefecture, Japan, in this context. For this, we carried out a questionnaire survey among physicians twice regarding the target value of glycated hemoglobin and the choice of hypoglycemic agents in various cases.
Materials and Methods
We administered an unsigned questionnaire to physicians in Miyazaki Prefecture, Japan, in July 2016 and March 2020. We divided responses into those of diabetologists and those of non‐diabetologists, and analyzed each response. We then compared the results between both years.
Results
In total, 18 diabetologists and 142 non‐diabetologists responded in 2016, and 21 diabetologists and 134 non‐diabetologists responded in 2020. Many diabetologists chose biguanide as the first‐line drug for obese type 2 diabetes patients. In addition, the rate of choice of sodium–glucose cotransporter 2 inhibitor (SGLT2i) among physicians almost increased in 2020. Some non‐diabetologists, and even a few diabetologists, inappropriately chose SGLT2i and biguanide for patients with severe renal dysfunction.
Conclusions
Because SGLT2i became available in 2016 and a few years have passed, both diabetologists and non‐diabetologists seemed to refrain from prescribing SGLT2i. However, with the emergence of various lines of firm evidence regarding the use of SGLT2i, physicians started to prescribe it. However, some diabetologists and non‐diabetologists chose hypoglycemic agents inadequately; therefore, there is a need for novel and precise information.
Keywords: Glycated hemoglobin, Hypoglycemic agent, Sodium–glucose cotransporter 2 inhibitor
We carried out a questionnaire survey among physicians twice regarding the target value of glycated hemoglobin and the choice of hypoglycemic agents in various cases, and divided the responses into those of diabetologists and those of non‐diabetologists. We analyzed each response and compared the results between both years.

INTRODUCTION
According to a consensus report published by the American Diabetes Association and the European Association for the Study of Diabetes, metformin is used as a first‐line hypoglycemic agent for the treatment of type 2 diabetes. In contrast, the Japanese diabetes treatment guidelines do not specify the first choice of hypoglycemic agents. In Japan, only insulin, sulfonylurea (SU) and biguanide (BG) were used as hypoglycemic agents until the development of an α‐glucosidase inhibitor in 1993. Subsequently, thiazolidine (TZD) and glinide became available in 1999, following which dipeptidyl peptidase‐4 inhibitor (DPP‐4i), glucagon‐like peptide‐1 receptor agonist and sodium–glucose cotransporter 2 inhibitor (SGLT2i) became available in 2009, 2010 and 2014, respectively. The choice of hypoglycemic agents is made by each physician considering the patient’s origin, diabetes pathophysiology, associated diabetic complications, age and so on. The Miyazaki Prefecture has a population of approximately 1.09 million people, and diabetes patients account for an estimated 21,000 individuals among the total population. However, there are just 40 diabetologists in Miyazaki Prefecture, and 85% of them reside in Miyazaki City. Under such circumstances, we aimed to clarify the actual situation of diabetes treatment in the Miyazaki Prefecture. For this purpose, we carried out a questionnaire survey twice, in 2016 and 2020, among diabetologists and non‐diabetologists in the Miyazaki Prefecture of Japan regarding the target value of glycated hemoglobin (HbA1c) and the choice of hypoglycemic agents in various cases.
MATERIALS AND METHODS
We administered an unsigned questionnaire to physicians (diabetologists and non‐diabetologists) registered with the Miyazaki Medical Association in July 2016 and March 2020. We defined diabetologists as clinicians with a qualified interest certified by the Japan Diabetes Society (JDS). We analyzed the results and compared the data between diabetologists and non‐diabetologists, and between 2016 and 2020. The contents of the questionnaire included age of the participant, diabetologist or non‐diabetologist, presence of nutritionists, number of diabetes outpatients and four questions. The four questions were as follows. Question A: Value of HbA1c to consider prescribing a hypoglycemic agent for the treatment of type 2 diabetes patients for whom lifestyle intervention was already introduced, and the value of HbA1c to consider administering insulin therapy for the treatment of type 2 diabetes patients for whom hypoglycemic agents were already introduced. Question B (Table 1): In approximately four type 2 diabetes patients (B1–B4), which is the target HbA1c level? The common scenarios in the four cases were that glycemic control was poor with lifestyle management intervention and basal insulin therapy was started 6 months ago. Question C (Table 2): In approximately six type 2 diabetes patients (C1–C6), which of the nine hypoglycemic agents are used as first‐ and second‐line drugs (SU, glinide, TZD, α‐glucosidase inhibitor, BG, DPP‐4i, SGLT2i, glucagon‐like peptide‐1 receptor agonist, insulin or none)? Common scenarios in five (C1–C5) cases were that duration of diabetes was 2 years, and there was no prescription of hypoglycemic agents. Activities of daily living were good. C6 was a 52‐year‐old woman who was a housewife. Her body mass index and HbA1c were 26.4 kg/m2 and 8.9%, respectively. She visited the Miyazaki University Hospital, Miyazaki, Japan, with a chief complaint of low vision and leg edema. After examination, for the first time, she was diagnosed with type 2 diabetes complicated with proliferative diabetic retinopathy, renal failure (serum creatinine level of 2.0 mg/dL; urine protein of 3+) and no liver disease. Question D: The number of type 2 diabetes patients who were prescribed SGLT2i, including in a recent month.
Table 1.
Contents of question B
| Case | Age (years)/sex | Occupation |
Duration of diabetes |
BMI (kg/m2) | Diabetic complication | Characteristic |
|---|---|---|---|---|---|---|
| B1 | 40/Male | Farmer | 2 years | 21.5 | None | None |
| B2 | 79/Female | Unemployed | 5 years | 25.0 | Slight neuropathy | None |
| B3 | 62/Male | Unemployed | 1 year | 19.5 | None | Terminal pancreatic cancer |
| B4 | 80/Female | Unemployed | 15 years | 24.0 |
Proliferative retinopathy, peripheral neuropathy, and nephropathy stage 3 |
eGFR = 40 mL/min/1.732 m2 |
BMI, body mass index; eGFR, estimated glomerular filtration rate.
Table 2.
Contents of question C
| Case | Age (years)/sex | Occupation | BMI (kg/m2) | HbA1c (%) | Diabetic complication | Characteristic |
|---|---|---|---|---|---|---|
| C1 | 46/Female | Housewife | 18.8 | 7.8 | None | No liver disease |
| C2 | 53/Male | Office worker | 30.0 | 7.7 | None | Fatty liver detected on ultrasonography |
| C3 | 56/Female | Housewife | 25.0 | 10.2 | None | No liver disease |
| C4 | 78/Male | Unemployed | 19.2 | 8.2 | None | No liver disease |
| C5 | 76/Male | Unemployed | 29.0 | 8.4 | History of myocardial infarction | No liver disease |
| C6 | 52/Female | Housewife | 26.4 | 8.9 |
PDR Nephropathy |
Serum creatinine 2.0 mg/dL Urine protein 3+ |
BMI, body mass index; HbA1c, glycated hemoglobin; PDR, proliferative diabetic retinopathy.
All statistical analyses were carried out using JMP 14 software (SAS Institute Inc., Cary, NC, USA). Data are presented as the mean ± standard deviation. Comparison between two groups was carried out using unpaired t‐test or two‐way factorial analysis of variance. Statistical significance was set at P < 0.05.
RESULTS
Comparison of the results of the questionnaire between diabetologists and non‐diabetologists (2020 vs 2020)
We received questionnaire responses from 155 of 400 physicians, and the recovery rate was 38.8% (Table 3). Among the 155 physicians, there were 21 diabetologists and 134 non‐diabetologists. The mean age of the participants was 58.3 ± 11.0 years (range 26–89 years). Furthermore, 94.7% and 37.6% of diabetologists and non‐diabetologists worked together with nutritionists, respectively. The number of patients with diabetes in a month was as follows: <30 patients, 22.3%; 30–100 patients, 53.8%; 100–200 patients, 17.7%; and >200 patients, 6.6% among non‐diabetologists; in contrast, the number of patients with diabetes in a month was as follows: <30 patients, 4.3%; 30–100 patients, 14.3%; 100–200 patients, 23.8%; and >200 patients, 57.1% among diabetologists. Diabetologists treated many more patients than non‐diabetologists (P < 0.001).
Table 3.
Characteristics of answerers
| Year | 2016 | 2020 |
|---|---|---|
| n | 160 | 155 |
| Collection rate of questionnaire (%) | 37.8 | 38.8 |
| Age (years) | 58.1 ± 9.3 (37–90) | 58.3 ± 11.0 (26–89) |
| Location (%) |
Miyazaki City: 39.9 Miyakonojo City: 14.7 Nobeoka City: 11.0 Others: 34.4 |
Miyazaki City: 42.7 Miyakonojo City: 15.1 Nobeoka City: 9.9 Others: 32.3 |
| Percentages of diabetologists (%) | 11.3 | 13.5 |
Question A
The HbA1c value that was considered for the administration of any hypoglycemic agent for type 2 diabetes patients for whom lifestyle intervention was already introduced was 7.0 ± 0.4% among non‐diabetologists and 7.0 ± 0.3% among diabetologists (P = 0.96). The HbA1c value that was considered for insulin therapy for type 2 diabetes patients for whom hypoglycemic agents were already introduced was 8.5 ± 0.8% among non‐diabetologists and 8.0 ± 0.5% among diabetologists (P = 0.012).
Question B
B1: Most diabetologists and non‐diabetologists chose a HbA1c value of <6% or 7%, with no significant difference (Table 1; Figure 1). B2: Most diabetologists and non‐diabetologists chose a HbA1c value of <7% or 8%. B3: In this case, 57.1% of diabetologists chose <8% and 9.5% of diabetologists chose <7%. In contrast, 35.6% of non‐diabetologists chose <7%. Non‐diabetologists tended to choose a lower target value of HbA1c (P = 0.09). B4: An approximately fivefold higher proportion of non‐diabetologists chose a HbA1c value of <7% than that chosen by diabetologists. Non‐diabetologists tended to choose a lower target value of HbA1c (P = 0.062).
Figure 1.
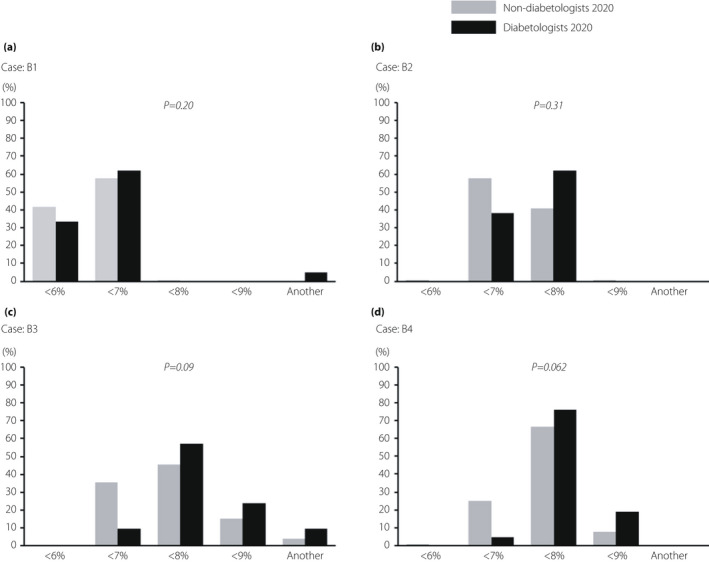
Comparison of the results of question B between diabetologists and non‐diabetologists in 2020. The results of target glycated hemoglobin that diabetologists and non‐diabetologists chose for each of the four cases (B1–B4) in 2020.
Question C
C1: Approximately half of both diabetologists and non‐diabetologists chose DPP‐4i as a first‐line drug, without any significant difference (Table 2; Figure 2). A total of 38.5% of non‐diabetologists and 25.0% of diabetologists chose DPP‐4i, and 25.4% of non‐diabetologists and 30.0% of diabetologists chose BG as a second‐line drug, without any significant difference. C2: Many diabetologists and non‐diabetologists chose BG and SGLT2i as first‐ or second‐line drugs, without any significant difference. C3: In this case, 57.1 and 19.1% of diabetologists chose BG and insulin, respectively, as first‐line drugs, whereas 43.2 and 20.5% of non‐diabetologists chose BG and SGLT2i, respectively, but the difference was not significant. Furthermore, SGLT2i was chosen by 47.6% of diabetologists and 29.9% of non‐diabetologists, and DPP‐4i was chosen by 4.8% of diabetologists and 29.9% of non‐diabetologists, respectively, as second‐line drugs, with a significant difference (P = 0.049). C4: Approximately 60% of both diabetologists and non‐diabetologists chose DPP‐4i as the first‐line drug. As for second‐line drugs, both diabetologists and non‐diabetologists chose various drugs, with no significant difference. C5: Almost all physicians chose BG, DPP‐4i and SGLT2i as first‐line drugs, with no significant difference. As for second‐line drugs, 52.4 and 19.1% of diabetologists chose SGLT2i and BG, respectively. However, 34.1 and 32.6% of non‐diabetologists chose DPP‐4i and SGLT2i, respectively. The choice tended to be significantly different (P = 0.09). C6: As a first‐line drug, almost all diabetologists and non‐diabetologists chose insulin, DPP‐4i or SGLT2i, with no significant difference. As for a second‐line drug, both diabetologists and non‐diabetologists chose various drugs, with no significant difference, as well as second‐line drugs of C4.
Figure 2.
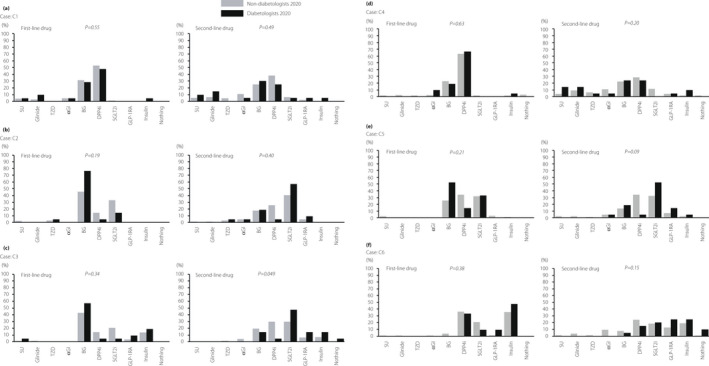
Comparison of the results of question C between diabetologists and non‐diabetologists in 2020. Diabetologists and non‐diabetologists in 2020 chose first‐ and second‐line drugs among nine hypoglycemic agents for each of the six cases (C1–C6). αGI, α‐glucosidase inhibitor; BG, biguanide; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1 RA, glucagon‐like peptide 1 receptor agonist; SGLT2i, sodium–glucose cotransporter 2 inhibitor; SU, sulfonylurea; TZD, thiazolidine.
Question D
Diabetologists prescribed SGLT2i significantly more frequently than non‐diabetologists in a recent month (P < 0.001; Figure 3).
Figure 3.
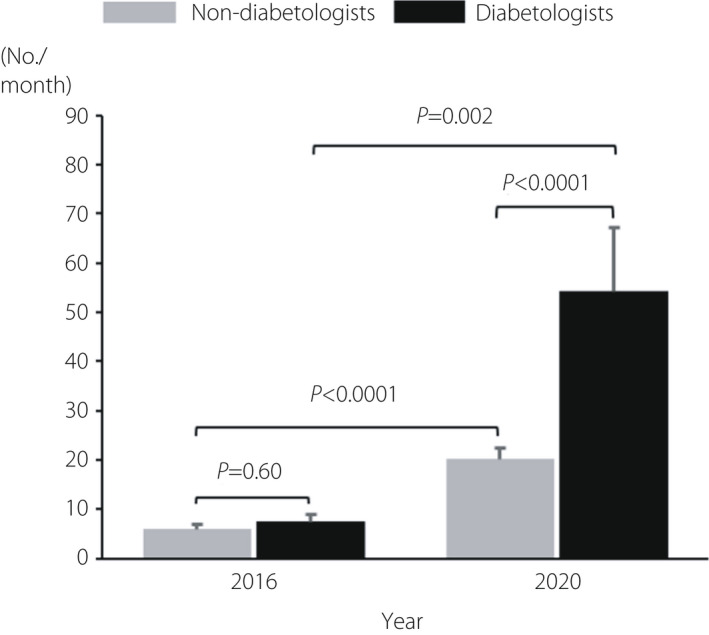
Number of type 2 diabetes patients who were prescribed sodium–glucose cotransporter 2 inhibitor (SGLT2i), including in a recent month.
Comparison of the results between 2016 and 2020 for each diabetologist or non‐diabetologist
In the 2016 questionnaire survey, we received responses from 160 of 423 physicians, and the recovery rate was 37.8% (Table 3). Of the 160 doctors, there were 18 diabetologists and 142 non‐diabetologists. The results of comparison between diabetologists in 2016 (diabetologists‐16) and diabetologists in 2020 (diabetologists‐20), as well as non‐diabetologists in 2016 (non‐diabetologists‐16) and non‐diabetologists in 2020 (non‐diabetologist‐20), are shown below.
Question A
The HbA1c value that was considered for the administration of any hypoglycemic agent for type 2 diabetes patients for whom lifestyle intervention was already introduced was 7.0 ± 0.3% among diabetologists‐16 and 7.0 ± 0.3% among diabetologists‐20 (P = 0.40). In regard to non‐diabetologists, the HbA1c value was 6.9 ± 0.4% among non‐diabetologists‐16 and 7.0 ± 0.4% among non‐diabetologists‐20 (P = 0.49). The value of HbA1c that was considered for insulin therapy for type 2 diabetes patients for whom hypoglycemic agents were already introduced was 7.9 ± 0.7% among diabetologists‐16 and 8.0 ± 0.5% among diabetologists‐20 (P = 0.76). Regarding non‐diabetologists, the HbA1c value was 8.6 ± 1.0% among non‐diabetologists‐16 and 8.5 ± 0.8% among non‐diabetologists‐20 (P = 0.31).
Question B
B1: Most diabetologists and non‐diabetologists chose a HbA1c value of <6% or 7%, with no significant difference (Table 1; Figure 4). B2: Most diabetologists and non‐diabetologists chose a HbA1c value of <7% or 8%. B3: The majority of diabetologists chose <8% or 9% in both questionnaire surveys. Non‐diabetologists‐20 generally chose wide target values compared with non‐diabetologists‐16, with a significant difference (P = 0.032). B4: Both diabetologists and non‐diabetologists chose similar results in 2016 and 2020.
Figure 4.
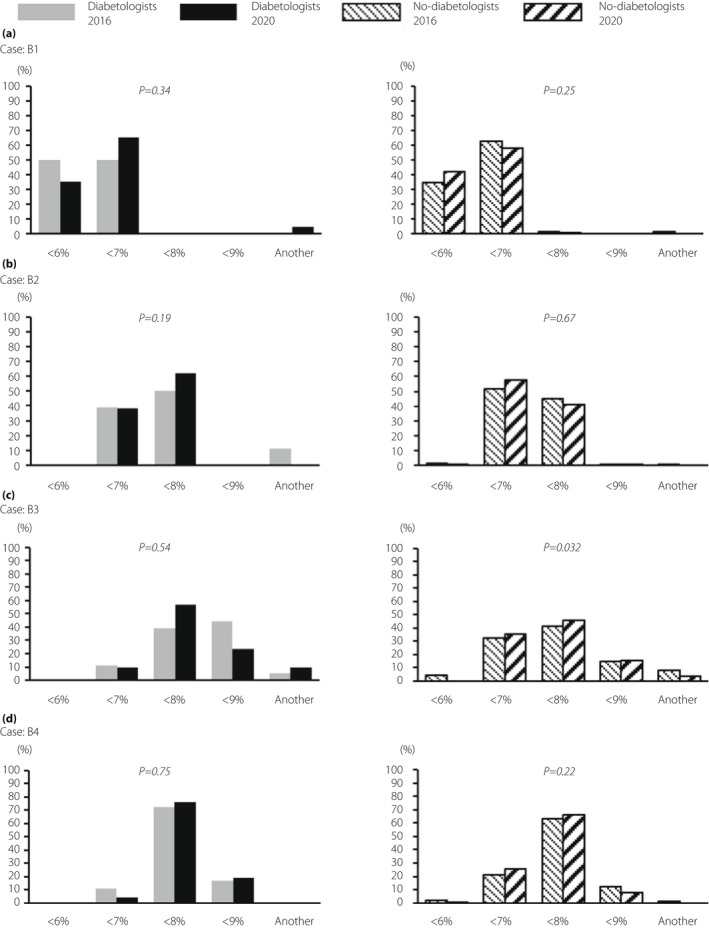
Comparison of the results of question B between 2016 and 2020 for each diabetologists or non‐diabetologists. The results of target glycated hemoglobin that diabetologists and non‐diabetologists chose for each of the four cases (B1–B4) in 2020.
Question C
C1: Approximately 60% of diabetologists‐16 and diabetologists‐20 chose DPP‐4i as a first‐line drug (Table 2, Figure 5). Although 65.4 and 16.9% of non‐diabetologists‐16 chose DPP‐4i and BG, respectively, as a first‐line drug, BG was chosen twice by non‐diabetologists‐20 compared with that by non‐diabetologists‐16. These differences were significant (P = 0.01). Both diabetologists‐16 and diabetologists‐20 chose a variety of second‐line drugs, with no significant difference. Although SU and BG were chosen at fewer frequencies by non‐diabetologists‐20 than by non‐diabetologists‐16, DPP‐4i and SGLT2i were chosen more frequently by non‐diabetologists‐20 than non‐diabetologists‐16. These differences were significant (P = 0.046). C2: More than 70% of both diabetologists‐16 and diabetologists‐20 chose BG as a first‐line drug. Although 46% of both non‐diabetologists‐16 and non‐diabetologists‐20 chose BG as a first‐line drug, non‐diabetologists‐20 chose SGLT2i approximately 1.7‐fold more than non‐diabetologists‐16. These differences were significant (P = 0.044). As a second‐line drug, approximately 56% of both diabetologists chose SGLT2i. Both non‐diabetologists chose a variety of second‐line drugs, with no significant difference. C3: There were few differences in the choice of first‐line drugs between both diabetologists. Furthermore, 43.2 and 20.5% of non‐diabetologists‐20 chose BG and SGLT2i, respectively, as first‐line drugs. The choice of SGLT2i was 2.5‐fold higher among non‐diabetologists‐20, and the choice of DPP‐4i and SU decreased to approximately half of the value. These differences were significant (P = 0.002). As a second‐line drug, diabetologists‐20 chose SGLT2i fourfold more than diabetologists‐16. In contrast, the choice of DPP‐4i decreased significantly (P = 0.004). Non‐diabetologists‐20 chose SGLT2i at a rate 2.3‐fold higher than that of non‐diabetologists‐16, and the use of SU decreased, with a significant difference (P = 0.047). C4: Both diabetologists chose DPP‐4i more frequently than other drugs as a first‐line drug. Non‐diabetologists‐20 chose BG 2.8‐fold more frequently than non‐diabetologists‐16, and prescription of SU decreased to one‐third, with a significant difference (P = 0.022). Regarding second‐line drugs, both diabetologists chose a variety of drugs, with no significant difference. Non‐diabetologists‐20 chose DPP‐4i and SGLT2i at 2.8‐ and 5.0‐fold higher frequency, respectively, than non‐diabetologists‐16. The prescription of SU decreased to one‐quarter. These differences were significant (P < 0.001). C5: As a first‐line drug, half of both diabetologists chose BG, but SGLT2i was chosen more frequently and DPP‐4i was chosen fewer times by diabetologists‐20 than by diabetologists‐16, with a significant difference (P = 0.043). Non‐diabetologists‐20 chose SGLT2i 2.5‐fold more frequently and chose DPP‐4i fewer times than non‐diabetologists‐16, with a significant difference (P = 0.002). As a second‐line drug, diabetologists‐20 chose SGLT2i ninefold more frequently than diabetologists‐16. Prescription of DPP‐4i, glinide and TZD decreased, with significant differences (P = 0.004). Non‐diabetologists‐20 chose SGLT2i at 2.4‐fold higher frequency than non‐diabetologists‐16, and the choice of glinide, TZD, α‐glucosidase inhibitor and BG decreased significantly (P < 0.001). C6: As a first‐line drug, the majority of both the diabetologists and non‐diabetologists chose insulin or DPP‐4i. Although both non‐diabetologists chose a variety of drugs, prescription of SGLT2i increased, and that of SU, glinide and BG decreased, with significant differences (P < 0.001). As second‐line drugs, both diabetologists and non‐diabetologists chose a variety of drugs, with no significant difference.
Figure 5.
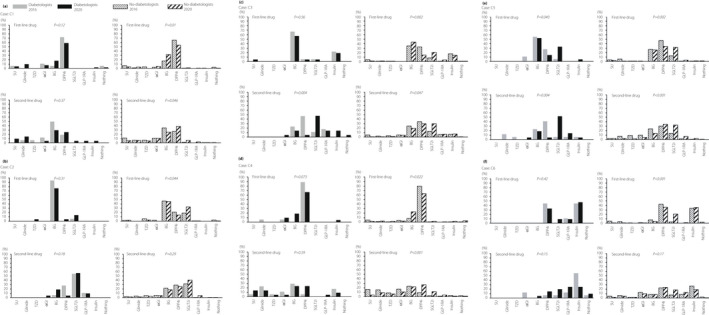
Comparison of the results of question C between 2016 and 2020 for each diabetologists or non‐diabetologists. In 2016 and 2020, diabetologists and non‐diabetologists chose first‐ and second‐line drugs from nine hypoglycemic agents for each of the six cases (C1–C6).
Question D
In 2016, the number of patients with type 2 diabetes who were prescribed SGLT2i in a recent month was small for both diabetologists and non‐diabetologists (Figure 3). However, both diabetologists and non‐diabetologists, especially diabetologists, chose SGLT2i at a significantly higher rate for many patients in 2020 than in 2016.
DISCUSSION
In the present study, we administered a questionnaire survey to diabetologists and general physicians about treatment targets and methods for various diabetes cases. In addition, because we set a 4‐year interval since the same questionnaire survey carried out earlier, some interesting results were obtained.
The number of patients with type 2 diabetes who were prescribed SGLT2i in the recent month was higher in 2020 than in 2016, especially among diabetologists (Figure 3). In addition, diabetologists and non‐diabetologists tended to choose SGLT2i more frequently in 2020 than in 2016, particularly for obese patients (Figure 5b,c,e,f). In Japan, SGLT2i became available in April 2014, and, currently, six types of SGLT2i (empagliflozin, canagliflozin, luseogliflozin, tofogliflozin, ipragliflozin and dapagliflozin) have been certified. In addition to the hypoglycemic effect due to increased urinary glucose excretion, multiple desirable effects 1 , such as weight loss 2 , 3 , 4 , 5 , improved fatty liver 6 , 7 , 8 , antihypertensive effect 9 , 10 and decreasing uric acid, have been proven. Furthermore, empagliflozin and canagliflozin significantly suppressed cardiovascular diseases in patients with a high risk of cardiovascular disease 11 , 12 , and empagliflozin and dapagliflozin decreased the rates of hospitalization due to heart failure 12 , 13 . In addition, empagliflozin suppressed the worsening of diabetic kidney disease and complicated renal events 14 (onset of proteinuria, half of the estimated glomerular filtration rate, renal replacement therapy, kidney disease‐related death), and canagliflozin suppressed the progression of albuminuria and complicated renal events 11 (40% reduction of the estimated glomerular filtration rate, renal replacement therapy, kidney disease‐related death), respectively. Because of these lines of firm evidence, SGLT2i has been used as a second‐line drug for patients with atherosclerotic cardiovascular disease, heart failure, chronic kidney disease or obesity in the American Diabetes Association and the European Association for the Study of Diabetes consensus report. A few years have passed since SGLT2i became available in Japan in 2016, and owing to its adverse effects, such as urinary tract infection and dehydration, physicians, including diabetologists, carefully used SGLT2i for treating diabetes. However, as various lines of evidence became evident, SGLT2i came to be gradually used extensively by not only diabetologists, but also non‐diabetologists. In contrast, some non‐diabetologists chose SGLT2i as first‐ and second‐line drugs for C4, despite C4 being an elderly lean patient; in contrast, none of the diabetologists chose it (Figure 5d). Prescriptions of SGLT2i for elderly and lean patients, such as C4, might cause muscle loss, sarcopenia and dehydration due to bodyweight reduction and polyuria. According to the recommendation given by the JDS, SGLT2i should be used carefully for patients aged ≥75 years and 65–74 years with geriatric syndrome 15 . Therefore, it is necessary to examine the indications of SGLT2i in each case and to disseminate the risks. Although C5 was also an elderly patient, who was obese, SGLT2i is a good indication if physicians pay attention to the issue of dehydration. Some diabetologists and non‐diabetologists chose SGLT2i for C6 (Figure 5f). Although C6 was obese, she also had renal failure (serum creatinine is 2.0 mg/dL). As evidence on whether SGLT2i could reduce cardiorenal events is lacking and the glucose‐lowering effect is limited in patients with severe CKD, such as in case C6, SGLT2i should be avoided in such cases.
As a first‐line drug for obese patients, both diabetologists (C2, C3 and C5) and non‐diabetologists (C2 and C3) chose BG more frequently than other drugs (Figure 5). In the UK prospective diabetes study 34 16 , the metformin‐enhanced therapy group showed a significant reduction of myocardial infarction and diabetes‐related death in obese patients with type 2 diabetes. Since then, metformin has become the first‐line drug for patients with type 2 diabetes in Europe and the USA 17 . According to a retrospective nationwide study carried out in Japan to search for trends in first‐line antidiabetic medication for patients with type 2 diabetes 18 , DPP‐4i was reported as the most common hypoglycemic agent (65.1%), followed by BG (15.9%) and SGLT2i (7.6%). BG was particularly prescribed more commonly in the JDS‐certified facilities (20.8%) than in other facilities (15.3%). In contrast with the trend seen for BG, DPP‐4i was prescribed less commonly in JDS‐certified facilities (61.8%) than in other facilities (65.5%). The hypoglycemic effect can be expected even in non‐obese patients, because the hypoglycemic effect of BG does not depend on body mass index 19 . Although BG is suggested by the JDS to be used carefully for patients aged >75 years, approximately half of the diabetologists chose BG as a first‐line drug for C5 (obese and elderly patients), probably because of his normal renal function. However, one diabetologist and some non‐diabetologists inappropriately chose BG for case C6, despite the presence of renal dysfunction. It is acceptable that there have been many cases where even non‐diabetologists use BG regardless of obesity or non‐obesity, but the common knowledge of patients for whom it should not be used, such as in C6, will be necessary.
The proportion of SU use as the first‐ and second‐line drug for case C6, who had severe renal dysfunction, decreased to almost zero for non‐diabetologists in 2020. According to the recommendation given by the JDS, SU should be used carefully for elderly patients and patients with impaired renal function 20 ; this recommendation should have influenced participant choices of hypoglycemic agents. In cases C4 and C5 (older patients), some non‐diabetologists chose SU as the first‐line drug. SU should be used carefully; however, these choices might decrease over time because of the influence of the recommendation.
As a first‐line drug for lean cases (C1 and C4), both diabetologists and non‐diabetologists chose DPP‐4i more frequently than other drugs (Figure 5a,d). A meta‐analysis reported that the hypoglycemic effect of DPP‐4i in patients with type 2 diabetes is more prominent in Asian individuals than in non‐Asian individuals 21 . In addition, DPP‐4i has an extremely low risk of causing hypoglycemia when used alone. From these viewpoints, the rate of selection of DPP‐4i is high among both diabetologists and non‐diabetologists. In fact, DPP‐4i accounted for 43.5% of the total drug charges ( ¥4,271 billion) for hypoglycemic agents in 2017 22 , according to the surveys carried out by the Japanese Ministry of Health, Labor and Welfare.
Regarding the target value of HbA1c, non‐diabetologists‐20 tended to choose lower HbA1c than diabetologists‐20 for B3 and B4 (Figure 1c,d). B3 was a case of unresectable pancreatic cancer. Regarding glycemic control in patients with advanced cancer, treatment guidelines do not specify the target value of HbA1c, and each physician has to determine the goal 23 . The responses to this question showed that many diabetologists seemed to decide the target HbA1c level according to the prognosis of each patient. Diabetologists have set the target HbA1c level relatively high in this case, because patients with advanced pancreatic cancer often have appetite loss, and reduced energy intake makes diabetes management difficult and increases the risk of severe hypoglycemia. B4 was an elderly patient with progressive diabetic complications. Elderly diabetes patients, especially those aged >80 years, often have dementia. It has been reported that elderly diabetes patients have dementia or mild cognitive impairment at 1.5‐fold higher frequency than elderly non‐diabetes patients 24 . Because mild cognitive impairment and dementia often reduce self‐care adherence, such as insulin injection and medication, diabetes treatment can easily become difficult. In addition, severe and/or frequent hypoglycemia is a risk factor for dementia, falls and bone fractures. From these viewpoints, diabetologists seem to have chosen higher HbA1c levels for elderly patients than non‐diabetologists. Among approximately 49,000 diabetes patients using SU in the USA, the higher the age at prescription, the lower the mean HbA1c was reported 25 . We should consider the goal of glycemic control with the risk of hypoglycemia in each patient, especially for elderly patients 26 .
Regarding question A, the HbA1c value for the consideration of insulin therapy was lower in diabetologists than that in non‐diabetologists in both years. These results were similar to those of a previous study carried out in Japan 27 .
The present study had several limitations. First, not all the respondents of the questionnaire survey were the same between 2016 and 2020. Second, information on the cases presented might not be sufficient for the respondents. Third, because the recovery rate of the questionnaire survey was <40%, the results might not reflect consensus on the target HbA1c value or the treatment method.
In conclusion, we carried out a questionnaire survey among diabetologists and non‐diabetologists in the Miyazaki Prefecture of Japan regarding the target value of HbA1c and choice of hypoglycemic agents for various cases of type 2 diabetes. Diabetologists chose higher target HbA1c levels than non‐diabetologists for patients with advanced cancer and elderly patients with diabetic complications; therefore, it is considered that diabetologists tend to examine the necessity of glycemic control in each case. Both diabetologists and non‐diabetologists chose BG as the first‐line drug for obese patients. In 2020, the choice of SGLT2i use increased in many cases, compared with that in 2016. However, because some inappropriate choices of hypoglycemic agents have been seen in the present study, we must have novel and correct knowledge to ensure proper selection of these drugs.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: NA.
Informed consent: N/A.
Approval date of registry and the registration no. of the study/trial: N/A.
Animal studies: NA.
ACKNOWLEDGMENTS
We thank physicians in Miyazaki Prefecture who responded to this questionnaire.
J Diabetes Investig. 2022; 13: 1011–1020
REFERENCES
- 1. Kashiwagi A, Maegawa H, et al. Metabolic and hemodynamic effects of sodium‐dependent glucose cotransporter 2 inhibitors on cardio‐renal protection in the treatment of patients with type 2 diabetes mellitus. J Diabetes Investig 2017; 8: 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stenlöf K, Cefalu WT, Kim K‐A, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus in adequately controlled with diet and exercise. Diabetes Obes Metab 2013; 15: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double‐blind, placebo‐controlled, phase 3 trial. Diabetes Care 2010; 33: 2217–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roden M, Weng J, Elibracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes mellitus: a randomized, double blind, placebo‐controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013; 1: 208–219. [DOI] [PubMed] [Google Scholar]
- 5. Kashiwagi A, Yoshida S, Nakamura I, et al. Efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes stratified by body mass index: a subgroup analysis of five randomized clinical trials. J Diabetes Investig 2016; 7: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai L‐L, Vethakkan SR, Nik Mustapha NR, et al. Empagliflozin for the treatment of nonalcoholic steatohepatitis in patients with type 2 diabetes mellitus. Dig Dis Sci 2020; 65: 623–631. [DOI] [PubMed] [Google Scholar]
- 7. Sumida Y, Murotani K, Saito M, et al. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non‐alcoholic fatty liver disease: a prospective, single‐arm trial (LEAD trial). Hepatol Res 2019; 49: 64–71. [DOI] [PubMed] [Google Scholar]
- 8. Xing B, Zhao Y, Dong B, et al. Effects of sodium‐glucose cotransporter 2 inhibitors on non‐alcoholic fatty liver disease in patients with type 2 diabetes: a meta‐analysis of randomized controlled trials. J Diabetes Investig 2020; 11: 1238–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inagaki N, Kondo K, Yoshinari T, et al. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24‐week, randomized, double‐blind, placebo‐controlled, phase 3 study. Expert Opin Pharmacother 2014; 15: 1501–1515. [DOI] [PubMed] [Google Scholar]
- 10. Seino Y, Sasaki T, Fukatsu A, et al. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled, phase 3 study. Curr Med Res Opin 2014; 30: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 11. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 12. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 13. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 14. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334. [DOI] [PubMed] [Google Scholar]
- 15.Available from: http://www.fa.kyorin.co.jp/jds/uploads/recommendation_SGLT2.pdf (Japanese only). Accessed January 28, 2022.
- 16. UK prospective diabetes study (UKPDS) Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 17. Buse JB, Wexler DJ, Tsapas A, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 2020: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouchi R, Sugiyama T, Goto A, et al. Retrospective nationwide study on the trends in first‐line antidiabetic medication for patients with type 2 diabetes in Japan. J Diabetes Investig 2021. 10.1111/jdi.13636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeFronzo RA, Barzilai N, Simonson DC, et al. Mechanism of metformin action in obese and lean noninsulin‐dependent diabetic subjects. J Clin Endocrinol Metab 1991; 73: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 20.Available from: https://www.nittokyo.or.jp/uploads/files/recommendation_incretin.pdf (Japanese only). Accessed January 28, 2022.
- 21. Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and non‐Asians : a systematic review and meta‐analysis. Diabetologia 2013; 56: 696–708. [DOI] [PubMed] [Google Scholar]
- 22.Available from: https://www.mhlw.go.jp/bunya/iryouhoken/database/zenpan/dl/cyouzai_doukou_topics_h31_01‐01.pdf (Japanese only). Accessed January 28, 2022.
- 23. King EJ, Haboubi H, Evans D, et al. The management of diabetes in terminal illness related to cancer. QJM 2012; 105: 3–9. [DOI] [PubMed] [Google Scholar]
- 24. Cheng G, Huang C, Deng H, et al. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta‐analysis of longitudinal studies. Intern Med J 2012; 42: 484–491. [DOI] [PubMed] [Google Scholar]
- 25. McCoy RG, Lipska KJ, Van Houten HK, et al. Paradox of glycemic management: multimorbidity, glycemic control, and high‐risk medication use among adults with diabetes. BMJ Open Diabetes Res Care 2020; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Araki E, Haneda M, Kasuga M, et al. New glycemic targets for patients with diabetes from the Japan Diabetes Society. J Diabetes Investig 2017; 8: 123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ishii H, Iwamoto Y, Tajima N, et al. An exploration of barriers to insulin initiation for physicians in Japan: findings from the Diabetes Attitudes, Wishes and Needs (DAWN) Japan study. PLoS One 2012; 6: e36361. [DOI] [PMC free article] [PubMed] [Google Scholar]


